Abstract
Background: Recent evidence indicates that social contact is related to similarities in weight gain over time. However, no studies have examined this effect in a twin design, in which genetic and other environmental effects can also be estimated.
Objective: We determined whether the frequency of social contact is associated with similarity in weight change from young adulthood (mean age: 20 y) to middle age (mean age: 41 y) in twins and quantified the percentage of variance in weight change attributable to social contact, genetic factors, and other environmental influences.
Design: Participants were 1966 monozygotic and 1529 dizygotic male twin pairs from the Vietnam-Era Twin Registry. Regression models tested whether frequency of social contact and zygosity predicted twin pair similarity in body mass index (BMI) change and weight change. Twin modeling was used to partition the percentage variance attributable to social contact, genetic, and other environmental effects.
Results: Twins gained an average of 3.99 BMI units, or 13.23 kg (29.11 lb), over 21 y. In regression models, both zygosity (P < 0.001) and degree of social contact (P < 0.02) significantly predicted twin pair similarity in BMI change. In twin modeling, social contact between twins contributed 16% of the variance in BMI change (P < 0.001), whereas genetic factors contributed 42%, with no effect of additional shared environmental factors (1%). Similar results were obtained for weight change.
Conclusion: Frequency of social contact significantly predicted twin pair similarity in BMI and weight change over 21 y, independent of zygosity and other shared environmental influences.
INTRODUCTION
Obesity is a major public health problem (1). Sixty-four percent of the US population is estimated to be overweight or obese [body mass index (BMI; in kg/m2) ≥25] (2) and is at increased risk of weight-related comorbidities, including coronary artery disease, diabetes, and certain cancers. As such, understanding factors that contribute to weight gain is critical.
Social networks have increasingly been shown to affect weight gain and risk of obesity (3–5). In a landmark study, Christakis and Fowler (3) showed that a person's chance of becoming obese increases 57% if a friend becomes obese, 40% if a sibling becomes obese, and 37% if a spouse becomes obese. Moreover, the risk of obesity spreads through networks of social contact. Criticisms have been raised about potential confounders, such as the potential for individuals to select friends of similar weight (homophily) and the need to control for other aspects of the environment that are shared (6). Increasing evidence suggests, however, that the effect of social networks on obesity is robust (4, 7–9).
At the same time, BMI is highly heritable in twin studies, with heritability estimates ranging from 0.45 to 0.87 (10–13). A few studies in the literature have also found significant genetic effects on weight change over time (11, 13–15). Effects of environmental factors that contribute to similarity between twins (shared environment) on BMI and weight change have rarely been detected in twin samples, particularly in adults (10–15). One cross-sectional twin study found significant, albeit modest, correlations between twin-pair social contact and twin similarity in BMI (r = 0.10–0.14) (10), although the percentage of the variance in BMI attributable to social contact was not formally estimated in the context of other genetic and environmental influences in twin modeling.
The purpose of this study was to examine the extent to which frequency of social contact between members of a twin pair influences their similarity in changes in BMI and weight from young adulthood to middle age. We use the unique features of the twin design to quantify the relative effect of degree of social contact, heritable genetic effects, other shared environmental effects, and nonshared environmental effects on variation in weight and BMI change.
SUBJECTS AND METHODS
Sample
The Vietnam-Era Twin (VET) Registry is a nationally distributed US cohort consisting of male-male twin pairs born between 1939 and 1957 in which both siblings served on active military duty during the Vietnam War era (16). Zygosity was determined by using a questionnaire and blood group typing method that achieved 95% accuracy (16). Registry members are representative of all twins who served in the military during the Vietnam Era on a variety of sociodemographic and other variables (17, 18). The data used in the present study were from military induction (modal year 1968) and the National Heart, Lung, and Blood Institute (NHLBI) Survey (1990). A total of 3495 (1966 monozygotic and 1529 dizygotic) male-male Vietnam-Era twin pairs with complete data at the NHLBI survey were included in analyses. These participants were a community-dwelling sample; they were not a patient or Department of Veterans Affairs sample. Although they served during the Vietnam era, the large majority was not in combat or in Vietnam. Demographic and health comparisons based on a randomly selected subset of participants indicate that they are largely representative of American men in their age range (19, 20).
All participants gave verbal informed consent at the time of the interviews. The current data analysis was approved by the Miriam Hospital Institutional Review Board and procedures that were followed were in accordance with The Miriam Hospital Guidelines.
Measures
Measured height and weight at the time of military induction were extracted from military records (modal year, 1968). Height and weight were measured by self-report in 1990. Previous studies have shown that self-report of weight is a valid measure of actual weight with an average error of only 0.5–1 kg (1–2 lb) (21). In 1990, participants also responded to the following questions: 1) In the past 5 y, how frequently have you and your twin gotten together? and 2) In the past 5 y, how frequently have you and your twin talked on the telephone or written to each another? Possible responses to both questions were as follows: 1) almost daily, 2) 1–4 times/wk, 3) 1–3 times/mo, 4) occasionally during the year, 5) less than once per year, and 6) not applicable, twin deceased (these pairs were omitted). Because these measures were highly correlated (r = 0.71, P < 0.001), they were combined, and the frequency of twin-pair social contact was defined as the most frequent contact (either in person or via phone or mail) rated by each twin averaged across twins within a pair.
Before the analyses, height at each age was compared for discrepancies that may influence BMI. If the discrepancy between adult heights was >10 cm (4 in), these values were checked by hand and outliers were excluded. If the discrepancy between adult heights was ≥5 cm (2 in) but ≤10 cm (4 in), the average of the adult heights was used. Finally, twin-pair differences in BMI were checked at each time point, and one outlier >10 SDs above the mean was excluded from further analysis.
BMI at each time point was calculated as weight (in kg)/height squared (in m). BMI and weight change were defined as the residuals in 1990 once the respective measures from young adulthood were statistically covaried.
Statistical analyses
Two complementary approaches to statistical analysis were used. First, pairwise linear multiple regression was used to examine the association between twin-pair zygosity, social contact, and twin-pair similarity in BMI and weight change from young adulthood to middle age. Standardized β weights and the associated P values were used to characterize the effects of the independent variables (ie, zygosity and social contact) on the dependent variables (ie, twin-pair differences in weight and BMI). A P value of 0.05 denoted significance. We also tested for interactions between zygosity and social contact on twin-pair differences in change in weight and BMI, which would suggest that the genetic effects on BMI or weight change were augmented or diminished by twin-pair social contact. Each twin pair was entered once into the analyses. Regression analyses were conducted in SPSS (version 14; IBM Corporation, Somers, NY).
The linear regression models were complemented by twin structural modeling to determine the amount of variance in change in BMI and weight attributable to social contact when genetic and other shared environmental effects are taken into account. The traditional twin model explains the observed total phenotypic variation and covariation between monozygotic and dizygotic twins in terms of orthogonal latent factors due to additive (A) and dominant (D) genetic effects and shared/common (C) and nonshared (E) environmental effects, including measurement error (22). Monozygotic twins correlate 1.0 on additive genetic (A) effects, whereas dizygotic twins correlate 0.5, because they share, on average, 50% of their segregating genes. Shared environmental (C) influences correlate 1.0 across twins, regardless of zygosity, whereas nonshared environmental influences, by definition, do not correlate across twins. Because genetic dominance effects did not contribute significantly to variance in change in BMI or weight [Δχ2 (1) = 0, P = 1.0 for both models], we focused on “ACE” models.
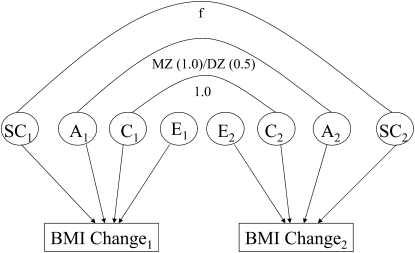
Advances in structural equation modeling over the past decade now make it possible to model measures reflecting similarity of environments as a specific variance component in traditional ACE models and to provide CIs on the potential magnitude of the effect of twin similarity (23). Following this example, to determine the potential role of twin-pair frequency of social contact in accounting for variance in change in BMI and weight, we extended the traditional ACE model to include a fourth source of variance: twin-pair social contact. The twin-pair score (ie, averaged across twins, see above) for social contact was included in the model as a direct measure of correlation of the latent social contact (eg, SC factors; Figure 1). The values of frequency of social contact (above) were recoded to vary from 0 to 1, with 0 denoting less than yearly contact, 0.25 yearly contact, 0.50 monthly contact, 0.75 weekly contact, and 1.0 daily contact. Thus, the social contact influence predicts that twins who have more social contact will be more highly correlated for BMI than twins who had less social contact. The full model is depicted in Figure 1.
FIGURE 1.
Twin structural equation model for social contact (SC), additive genetic effects (A), shared environmental effects (C), and nonshared environmental effects (E) on BMI change in twins 1 and 2 within a pair. MZ, monozygotic twin pair; DZ, dizygotic twin pair; f, frequency of contact between twins by phone, mail, or in person (0, less than yearly contact; 0.25, yearly contact; 0.50, monthly contact; 0.75, weekly contact; 1.0, daily contact).
The significance of frequency of social contact was determined by comparing the fit of models omitting the parameter with the fit of a full model, by using a Δχ2 statistic based on the difference in −2 log likelihoods between the 2 models with df equal to the difference in the number of parameters between the full and reduced models. All twin models and maximum likelihood parameter estimates were calculated by using the raw data capabilities of the Mx program (24).
The standard underlying assumptions of twin modeling include lack of assortative mating, lack of gene-environment correlation, and lack of gene × environment interaction. Without parental data, we were unable to test for assortative mating; thus, estimates of shared environment may be upwardly biased. Following the traditional ACE model, we assumed that the A,C, and E influences are orthogonal, so we did not directly test for latent gene-environment correlation. We tested for gene × social contact interaction by determining whether the impact of zygosity on twin pair similarity in weight change appeared to vary by social contact in regression analyses. As nothing suggestive was identified in regression analyses, we did not formally test gene x social contact interaction in twin modeling. A final important assumption is the equal environments assumption, that is, the assumption that monozygotic and dizygotic twins are equally correlated for environmental factors of relevance to the trait of interest (25, 26). Because we anticipated that social contact may produce a violation of this assumption, we designed our twin modeling to include the social contact parameter as a fourth latent factor orthogonal to the additive genetic, shared environmental and nonshared environmental latent factors. Thus, the residual additive genetic, shared environmental and nonshared environmental factors were independent of variability in social contact, that is, the estimates of A, C, and E were independent of violations of the equal environments assumption as measured by greater social contact in monozygotic twins than in dizygotic twins. Moreover, our model allowed us to directly estimate the effects of violations of the equal environments assumption on variation in BMI and weight change.
RESULTS
Demographic and descriptive statistics
Demographic information and descriptive statistics for BMI and social contact are presented in Table 1. Participants were on average 20 y of age at enlistment and 41 y of age at follow-up; 95% were white. In young adulthood, participants had an average BMI of 22, which is in the normal-weight range. Fourteen percent of participants were in the overweight range (BMI ≥25 and <30), 1.4% classified as obese (BMI ≥ 30 and <40) and 0% classified as morbidly obese (BMI ≥ 40). In middle age, the average BMI was 26, which is in the overweight range. Forty-four percent of participants would be classified as overweight, 11.6% as obese, and 0.4% as morbidly obese. The increase in BMI was 3.99 units, or 13.23 kg (29.11 lb), on average across the 21-y follow-up. Seven percent maintained or lost weight, whereas 93% gained weight.
TABLE 1.
Demographic and descriptive statistics for the Vietnam-Era Twin Registry sample
| Monozygotic twins(n = 3932; 1966 pairs) | Dizygotic twins(n = 3058; 1529 pairs) | P | |
| Age (y) | |||
| Young adult | 20.01 ± 1.631 | 19.99 ± 1.58 | 0.532 |
| Middle age | 40.66 ± 3.11 | 40.61 ± 2.75 | 0.49 |
| BMI (kg/m2) | |||
| Young adult | 22.01 ± 2.93 | 22.09 ± 2.94 | 0.57 |
| Middle age | 25.99 ± 3.73 | 26.10 ± 3.70 | 0.82 |
| Race [n (%)] | 0.553 | ||
| White | 3682 (95) | 2862 (95) | |
| African American | 169 (4) | 131 (4) | |
| Hispanic | 0 (0) | 2 (0.1) | |
| Other | 14 (0.1) | 4 (0.1) | |
| Social contact [n (%)] | <0.001 | ||
| Almost daily | 958 (24) | 306 (10) | |
| Weekly | 1112 (28) | 654 (21) | |
| Monthly | 1120 (28) | 940 (31) | |
| Yearly | 674 (17) | 1056 (35) | |
| Less than yearly | 68 (2) | 102 (3) |
Mean ± SD (all such values).
t test.
Chi-square test.
Monozygotic twin pairs reported significantly more social contact than did dizygotic twin pairs (P < 0.001). Slightly more than one-half (52%) of monozygotic twin pairs reported daily or weekly contact, whereas 31% of dizygotic twins reported that frequency of contact.
Regression models
In linear regression models, both twin-pair zygosity (β = 0.099, P < 0.001) and frequency of social contact (β = 0.041, P = 0.02) significantly predicted twin-pair differences in BMI change over the 21-y follow-up. No significant interaction between zygosity and frequency of social contact was observed (P = 0.38).
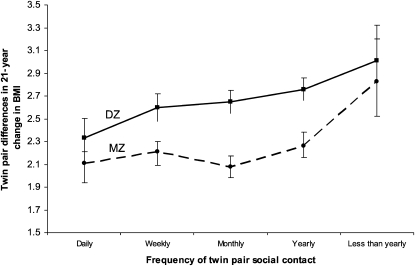
The association of zygosity and frequency of social contact with twin-pair similarities in BMI change over the 21-y follow-up is depicted in Figure 2; higher scores reflected greater within-pair differences (ie, less similarity). Monozygotic twin pairs were more similar in their change in BMI than were dizygotic twin pairs. Independently, however, twin pairs reporting greater social contact were also more similar in their change in BMI than were twin pairs reporting less social contact.
FIGURE 2.
Twin-pair differences in BMI change over 21 y of follow-up by frequency of social contact and zygosity. The analyses were conducted by using linear regression [n = 3495 twin pairs; 1966 monozygotic (MZ) and 1529 dizygotic (DZ)]. Twin-pair zygosity (β = 0.099, P < 0.001) and frequency of social contact (β = 0.041, P = 0.02) significantly predicted twin-pair differences in BMI changes over the 21 y of follow-up. No significant interaction between zygosity and frequency of social contact was observed (P = 0.38).
Similar results were found for change in weight. Monozygotic twin pairs were more similar in weight change than were dizygotic twin pairs (P < 0.001); however, again, a greater frequency of social contact between twins independently predicted more similarity in weight change over time (P = 0.008). No significant interaction of zygosity and frequency of social contact was observed (P = 0.43).
When examined individually, both phone contact and in-person contact significantly predicted twin-pair similarity in weight (P < 0.02) and BMI change (P < 0.04), independent of zygosity.
Twin structural equation modeling
Results of the twin structural equation modeling for BMI change from young adulthood to middle age are presented in Table 2. Model 1 for BMI change depicts the full model in which frequency of social contact, additive genetic effects, shared environment, and nonshared environment are simultaneously estimated. Twin-pair social contact accounted for 16% of the variance, in addition to significant additive genetic (42%) and nonshared environmental effects (41%). No significant contribution of additional shared environmental effects was observed (1%).
TABLE 2.
Contribution of twin-pair social contact to the heritability of change in BMI and weight from young adulthood to middle age (n = 3495 twin pairs; 1966 monozygotic and 1529 dizygotic)1
| a2 | c2 | e2 | Social contact | −2LL | df | Δχ2(1) | P | |
| BMI change (kg/m2) | ||||||||
| Model 1 | 0.42 (0.31, 0.50)2 | 0.01 (0.00, 0.11) | 0.41 (0.36, 0.45) | 0.16 (0.07, 0.25) | 18,616.059 | 6859 | ||
| Model 2 | 0.49 (0.38, 0.56) | 0.04 (0.00, 0.14) | 0.47 (0.44, 0.50) | X | 18,627.433 | 6860 | 11.37 | <0.001 |
| Weight change (kg) | ||||||||
| Model 1 | 0.43 (0.31, 0.51) | 0.02 (0.00, 0.12) | 0.40 (0.36, 0.45) | 0.15 (0.06, 0.24) | 18,675.91 | 6959 | ||
| Model 2 | 0.50 (0.40, 0.57) | 0.04 (0.00, 0.14) | 0.46 (0.43, 0.49) | X | 18,686.42 | 6960 | 10.51 | <0.001 |
All analyses were conducted by using twin structural equation modeling. a2, additive genetic variance; c2, shared environmental variance; e2, nonshared environmental variance; −2LL, –2 log-likelihood; X, a parameter that was fixed to zero in the model for model comparison; Δχ2(1), change in chi-square with 1 df.
Parameter estimate; 95% CI in parentheses (all such values).
In model 2, the twin social contact parameter was fixed to zero to determine whether a significant decrement in model fit relative to model 1 occurred. The change in model fit was highly significant (P < 0.001), which indicated that twin-pair social contact contributed significantly to variation in change in BMI from young adulthood to middle age.
Similar results were obtained for weight change. In the full model (weight change, model 1), social contact, additive genetic, and nonshared environmental effects each contributed significantly to the variance in weight change. When the effects of social contact were fixed to zero (weight change, model 2), a significant decrement in model fit was observed (P < 0.001), which indicated that social contact contributed significantly to weight change. Social contact accounted for 15% of the variation in weight change.
Homophily
If people of similar physical characteristics tend to associate with one another (homophily), then, in this case, twins’ similarity in weight ought to predict the amount of social contact over time. Therefore, we examined whether within-pair differences in BMI and weight in young adulthood predicted the frequency of social contact in middle age using pairwise linear regression models. No significant associations were seen (P > 0.13).
DISCUSSION
The results of this study document that twin pairs who contacted each other more frequently were more similar in weight and BMI change from young adulthood to middle age. In twin modeling, the frequency of social contact between twins accounted for 15% to 16% of the variability in BMI and weight change. In addition, BMI and weight gain were significantly heritable, with no contribution of shared environmental factors. Thus, our results indicate that social contact among twins predicted similarity in weight and BMI change in twin pairs above and beyond the well-established genetic influences and beyond the effects of shared environment, including contextual factors that contribute to twin pair similarity.
The vast majority of participants in this study experienced weight and BMI gain from young adulthood to middle age. Young adulthood is increasingly recognized as a critical time to study factors contributing to weight gain, because young adults experience the greatest rate of weight gain, averaging 0.5–1.0 kg (1–2 lb) per year (27, 28). The average weight changes in this cohort of men mirrored those found in the literature, with an average weight gain of 13.23 kg (29.11 lb), an increase in BMI of 3.99 over 21 y of follow-up, or an average weight gain of 0.6 kg (1.4 lb) per year. The extent to which social contact contributes to weight change in other age groups or in different age cohorts or to weight loss remain interesting questions.
Our study addressed controversies raised in the literature. The conclusion that social networks contribute to weight gain in the original Christakis and Fowler (3) article had been criticized for lack of sufficient control for other contextual factors that might contribute to similarities in weight gain and the potential for individuals to select friends of similar weight (homophily) (6). In twin models, variance attributable to environmental factors that contribute to twin-pair similarity is routinely estimated. We found no evidence that other shared environmental factors contributed significantly to change in BMI or weight. With regard to homophily, we found no significant association between similarity in BMI in young adulthood and later social contact in this sample, but note that other forms of homophily that we did not assess in this study may have been relevant to weight change. Thus, our results suggest that the effects of social contact on weight change are not attributable to homophily based on similarity in weight or to shared environmental effects.
The results also raise important issues related to a key assumption in estimating genetic effects based on twin models, namely that monozygotic and dizygotic twins are equally correlated for environmental factors of relevance to the trait of interest, ie, the equal environments assumption (25, 26). To the extent that monozygotic twins share more environmental similarity than dizygotic twins, it is possible that the increased similarity among monozygotic, relative to dizygotic twins, reflects, in part, environmental effects and therefore may bias parameter estimates, including an upward bias of heritability. Consistent with the literature (29–31), we found greater social contact among monozygotic than among dizygotic twin pairs, which suggests that monozygotic twins share more similar environments than do dizygotic twins. In addition, largely in contrast with the literature, which has focused primarily on psychiatric outcomes (25, 26), we found a significant effect of this differential contact on our outcome of interest—twin similarity for weight change. Interestingly, our results are consistent with the one twin study that assessed the effect of twin-pair social contact on similarity in BMI, albeit cross-sectionally (10).
Social contact accounted for a difference in heritability of 0.07 in BMI change (Table 2, model 1 compared with model 2). This suggests that, although a lack of accounting for twin-pair social contact does result in a statistically significant violation of the equal environments assumption, the magnitude of this effect is not large. The models produce parameter estimates within the range of prior research regardless of whether frequency of social contact between twins is taken into account. Nonetheless, given the rarity of this type of finding in the literature, replication studies are warranted to more fully characterize the magnitude of this effect and its importance to twin and genetic studies of weight and weight change. Furthermore, it is plausible that some of the change in heritability across models may reflect gene-environment correlation or interaction, which suggests that it may be fruitful to further investigate social contact as a predictor in models of gene-environment correlation or interaction in weight change.
Social contacts are thought to affect weight status via social norms and social modeling (3). Laboratory and quasi-experimental studies have shown that social norms and modeling strongly affect eating and activity behaviors. For example, Pliner and Mann (32) randomly assigned participants to 1 of 3 conditions—no eating norm, inhibited eating norm (“previous participants have not eaten much”), or augmented eating norm (“previous participants have eaten a lot”)—and showed that participants in the augmented norm condition ate significantly more than participants in the inhibited and no-norm conditions. Similarly, confederates who model stair use have been shown to prompt stair use in participants (33). Moreover, Leahey et al (8) showed that having more social contacts trying to lose weight was associated with a greater intention to lose weight. Furthermore, social norms for weight control (ie, beliefs about what is socially acceptable) fully mediated this effect. Taken together, these findings suggest that social norms and social modeling are strong candidate mechanisms for the effects of twin-pair contact on weight gain.
The strengths of our research include the 21-y longitudinal design in twins and similar weight gains as reported in prior studies of young adults. The limitations pertain to the generalizability of our results, because our cohort was entirely composed of male twins of a limited age range, who are predominantly of white descent, and data collection occurred from 1968 to 1990. In addition, we did not have a measure of social contact in young adulthood, our measure of social contact covered only 5 y before the assessment, and we did not include other potential sources of violation of the equal environments assumption (ie, differential treatment by parents) or gene-environment correlation or interaction. We also were not able to characterize the genetic and environmental underpinnings of frequency of social contact. Therefore, how social contact influences weight change, either through genetic, shared environment, or complex gene-environment correlation or gene-environment interaction is unclear. Finally, the military had weight restrictions at entry; thus, our sample may have been biased toward including individuals of normal weight at enlistment.
In summary, this article extends the growing literature on social contact as a predictor of weight gain. Frequency of twin-pair social contact significantly predicted similarity in weight gain in twin pairs above and beyond genetic and other shared environmental influences on weight gain.
Acknowledgments
Numerous organizations provided invaluable assistance in the conduct of this study, including the Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. Most importantly, we gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families, without whose contribution this research would not have been possible.
The authors’ responsibilities were as follows—JMM, KJ, and TML: designed the research; CEF, MJL, and WSK: conducted the research; JMM, KJ, and HX: analyzed the data or performed the statistical analysis; JMM, CEF, KJ, TML, HX, RRW, ML, and WSK: wrote the manuscript; and JMM: had primary responsibility for the final content. All authors declared financial support received for this work and did not report any financial involvement or affiliation with any organization whose financial interests may be affected by material in this manuscript or which might potentially bias it.
REFERENCES
- 1.NHLBI Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. Obes Res 1998;6:51S–209S [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010;303:235–41 [DOI] [PubMed] [Google Scholar]
- 3.Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med 2007;357:370–9 [DOI] [PubMed] [Google Scholar]
- 4.Fowler JH, Christakis NA. Estimating peer effects on health in social networks: a response to Cohen-Cole and Fletcher; and Trogdon, Nonnemaker, and Pais. J Health Econ 2008;27:1400–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenquist JN, Murabito J, Fowler JH, Christakis NA. The spread of alcohol consumption behavior in a large social network. Ann Intern Med 2010;152:426–33, W141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen-Cole E, Fletcher JM. Is obesity contagious? Social networks vs. environmental factors in the obesity epidemic. J Health Econ 2008;27:1382–7 [DOI] [PubMed] [Google Scholar]
- 7.Halliday TJ, Kwak S. Weight gain in adolescents and their peers. Econ Hum Biol 2009;7:181–90 [DOI] [PubMed] [Google Scholar]
- 8.Leahey TM, Gokee LaRose J, Wing RR. Social influences are associated with BMI and weight loss intentions in young adults. Obesity (Silver Spring) (Epub ahead of print 16 December 2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trogdon JG, Nonnemaker J, Pais J. Peer effects in adolescent overweight. J Health Econ 2008;27:1388–99 [DOI] [PubMed] [Google Scholar]
- 10.Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet 1997;27:325–51 [DOI] [PubMed] [Google Scholar]
- 11.Franz CE, Grant MD, Jacobson KC, et al. Genetics of body mass stability and risk for chronic disease: a 28-year longitudinal study. Twin Res Hum Genet 2007;10:537–45 [DOI] [PubMed] [Google Scholar]
- 12.Jacobson KC, Rowe DC. Genetic and shared environmental influences on adolescent BMI: interactions with race and sex. Behav Genet 1998;28:265–78 [DOI] [PubMed] [Google Scholar]
- 13.Romeis JC, Grant JD, Knopik VS, Pedersen NL, Heath AC. The genetics of middle-age spread in middle-class males. Twin Res 2004;7:596–602 [DOI] [PubMed] [Google Scholar]
- 14.Fabsitz RR, Sholinsky P, Carmelli D. Genetic influences on adult weight gain and maximum body mass index in male twins. Am J Epidemiol 1994;140:711–20 [DOI] [PubMed] [Google Scholar]
- 15.Stunkard AJ, Foch TT, Hrubec Z. A twin study of human obesity. JAMA 1986;256:51–4 [PubMed] [Google Scholar]
- 16.Eisen S, True W, Goldberg J, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: method of construction. Acta Genet Med Gemellol (Roma) 1987;36:61–6 [DOI] [PubMed] [Google Scholar]
- 17.Goldberg J, True W, Eisen S, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: ascertainment bias. Acta Genet Med Gemellol (Roma) 1987;36:67–78 [DOI] [PubMed] [Google Scholar]
- 18.Henderson WG, Eisen S, Goldberg J, True WR, Barnes JE, Vitek ME. The Vietnam Era Twin Registry: a resource for medical research. Public Health Rep 1990;105:368–73 [PMC free article] [PubMed] [Google Scholar]
- 19.Newton-Cheh C, Johnson T, Gateva V, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet 2009;41:666–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kremen WS, Thompson-Brenner H, Leung YM, et al. Genes, environment, and time: the Vietnam Era Twin Study of Aging (VETSA). Twin Res Hum Genet 2006;9:1009–22 [DOI] [PubMed] [Google Scholar]
- 21.Villanueva EV. The validity of self-reported weight in US adults: a population based cross-sectional study. BMC Public Health 2001;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neale M, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht, Germany: Kluwer Academic Publishers, 1992 [Google Scholar]
- 23.Mazzeo SE, Mitchell KS, Bulik CM, Aggen SH, Kendler KS, Neale MC. A twin study of specific bulimia nervosa symptoms. Psychol Med 2010;40:1203–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neale M, Boker S, Xie G, Maes H. Statistical modeling. 6th ed. Richmond, VA: Department of Psychiatry, 2002 [Google Scholar]
- 25.Xian H, Scherrer JF, Eisen SA, et al. Self-reported zygosity and the equal-environments assumption for psychiatric disorders in the Vietnam Era Twin Registry. Behav Genet 2000;30:303–10 [DOI] [PubMed] [Google Scholar]
- 26.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A test of the equal-environment assumption in twin studies of psychiatric illness. Behav Genet 1993;23:21–7 [DOI] [PubMed] [Google Scholar]
- 27.Lewis CE, Jacobs DR, Jr, McCreath H, et al. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol 2000;151:1172–81 [DOI] [PubMed] [Google Scholar]
- 28.Truesdale KP, Stevens J, Lewis CE, Schreiner PJ, Loria CM, Cai J. Changes in risk factors for cardiovascular disease by baseline weight status in young adults who maintain or gain weight over 15 years: the CARDIA study. Int J Obes (Lond) 2006;30:1397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kendler KS, Heath A, Martin NG, Eaves LJ. Symptoms of anxiety and depression in a volunteer twin population. The etiologic role of genetic and environmental factors. Arch Gen Psychiatry 1986;43:213–21 [DOI] [PubMed] [Google Scholar]
- 30.Rose RJ, Kaprio J, Williams CJ, Viken R, Obremski K. Social contact and sibling similarity: facts, issues, and red herrings. Behav Genet 1990;20:763–78 [DOI] [PubMed] [Google Scholar]
- 31.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A population-based twin study of major depression in women. The impact of varying definitions of illness. Arch Gen Psychiatry 1992;49:257–66 [DOI] [PubMed] [Google Scholar]
- 32.Pliner P, Mann N. Influence of social norms and palatability on amount consumed and food choice. Appetite 2004;42:227–37 [DOI] [PubMed] [Google Scholar]
- 33.Adams MA, Hovell MF, Irvin V, Sallis JF, Coleman KJ, Liles S. Promoting stair use by modeling: an experimental application of the Behavioral Ecological Model. Am J Health Promot 2006;21:101–9 [DOI] [PubMed] [Google Scholar]