Abstract
Background: Although long-chain omega-3 fatty acid (n−3 FA) consumption estimated via food-frequency questionnaires has been associated with a higher incidence of diabetes, limited prospective data on diabetes risk are available that use objective biomarkers of n−3 FAs.
Objective: We sought to examine the relation between plasma phospholipid n−3 FAs and incident diabetes.
Design: We prospectively analyzed data in 3088 older men and women (mean age: 75 y) from the Cardiovascular Health Study (1992–2007). Plasma phospholipid n−3 FAs were measured by using gas chromatography, and incident diabetes was ascertained by using information on hypoglycemic agents and serum glucose. We used Cox proportional hazards models to estimate multivariable-adjusted relative risks.
Results: During a median follow-up of 10.6 y, 204 new cases of diabetes occurred. In a multivariable model that controlled for age, sex, race, clinic site, body mass index, alcohol intake, smoking, physical activity, LDL cholesterol, and linoleic acid, relative risks (95% CIs) for diabetes were 1.0 (reference), 0.96 (0.65, 1.43), 1.03 (0.69, 1.54), and 0.64 (0.41, 1.01) across consecutive quartiles of phospholipid eicosapentaenoic acid and docosahexaenoic acid (P for trend = 0.05). Corresponding relative risks (95% CIs) for phospholipid α-linolenic acid (ALA) were 1.0 (reference), 0.93 (0.65, 1.34), 0.99 (0.68, 1.44), and 0.57 (0.36, 0.90) (P for trend = 0.03).
Conclusions: With the use of objective biomarkers, long-chain n−3 FAs and ALA were not associated with a higher incidence of diabetes. Individuals with the highest concentrations of both types of FAs had lower risk of diabetes.
See corresponding editorial on page 369.
INTRODUCTION
Type 2 diabetes is a highly prevalent disease with a lifetime risk ranging from 27% to 53% at birth in the United States (1). Modifiable lifestyle factors, including diet, have been recognized to play an important role (2–5) in the development of diabetes and its cardiovascular consequences. Among dietary components, long-chain n−3 fatty acids (FAs) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) and, to a lesser extent, the plant-derived n−3 FA α-linolenic acid (ALA) have been shown to confer some cardiac benefits (6–11), including beneficial effects of n−3 FAs on the incidence of cardiovascular diseases in older adults (12–15). Although a recent n−3 FA trial in postmyocardial infarction patients reported no association between n−3 FAs on cardiovascular events in general, a post hoc analysis revealed strong reduction in cardiovascular events in diabetic patients (16). In contrast, limited and inconsistent data have been reported on the effects of n−3 FAs on risk of diabetes. Several prospective cohorts have reported significant, positive associations between estimated dietary n−3 FA consumption and incidence of diabetes (17–19), whereas others have shown no significant associations (20–23). Some of these studies have relied on dietary questionnaires (17–19) to estimate the consumption of n−3 FAs, which may be prone to a measurement error of n−3 FAs consumed. In contrast to estimates from dietary questionnaires, the measurement of circulating fatty acids provides an objective measure of exposure and also allows for the assessment of individual n−3 FAs such as ALA, EPA, and DHA. Two previous studies that used objective biomarker measurements showed no significant association between plasma concentrations of marine n−3 FAs and incident diabetes (20, 21). Because the 2 studies (20, 21) were limited to mostly younger adults and had relatively short follow-ups, it is unclear whether n−3 FAs influenced risk of diabetes in older adults.
Because of the inconsistency of reports that focused on the association between n−3 FAs and incident diabetes and the importance of understanding this relation, we prospectively investigated the association between plasma phospholipid n−3 FA concentrations, which are objective biomarkers of exposure, and new-onset diabetes in 3088 older US adults. We hypothesized that the long-chain n−3 FAs EPA+DHA would be associated with higher risk of diabetes, whereas the plant-derived n−3 FA ALA would not be associated with higher risk of diabetes.
SUBJECTS AND METHODS
Study population
The Cardiovascular Health Study (CHS) is a prospective, population-based cohort study of cardiovascular disease in older adults. In 1989–1990, 5201 men and women aged ≥65 y were recruited from a random sample of Medicare-eligible residents in the following 4 US communities: Forsyth County, NC; Sacramento County, CA; Washington County, MD; and Allegheny County, PA. A supplemental cohort of 687 predominantly African American men and women was recruited in 1992–1993 from 3 of the same communities (excepting Washington County) by using the same sampling and recruitment methods. The institutional review board of each center approved the study, and all participants gave informed written consent to participate in the study. Details of the study design, sampling, and recruitment have been published (24).
Measurements of n−3 FAs in plasma specimens taken at the 1992–1993 examination (the baseline for the current analysis) were available for 3941 participants. We excluded from this analysis participants who had prevalent diabetes (n = 594) or for whom the prevalent diabetes status could not be determined (n = 64). We also excluded participants who had no diabetes ascertainment beyond baseline (n = 94) or were missing covariate data (n = 100). One participant was excluded because of an EPA value outside 10 SDs. The final analysis sample included 3088 participants.
Assessment of nminus3 and nminus6 FAs
Plasma samples collected at the 1992–1993 examination were stored at −70°C until analyzed at the Fred Hutchinson Cancer Research Center (Seattle, WA). Total lipids were extracted from plasma by using the method of Folch et al (25). Phospholipids were separated from neutral lipids by one-dimensional thin-layer chromatography with 250-m Silica Gel G plates (Analtech Inc, Newark, DE) and a 67.5:15:0.75 hexane:ether:acetic acid development solvent with 0.005% butylated hydroxytoluene. Phospholipid fractions were directly transesterified to prepare fatty acid methyl esters by using the method of Lepage and Roy (26). We separated fatty acid methyl esters of each individual fatty acid by using gas chromatography as previously described (14). n−3 FAs were expressed as percentages of total fatty acids by weight. Interassay CVs were 1.6%, 2.1%, 3.1%, and 0.6% for DHA, EPA, ALA, and linoleic acid, respectively.
Ascertainment of type 2 diabetes in the CHS
In this cohort, we assessed medication use at baseline and annually by using a medication inventory (27) through 2007. In addition, fasting glucose was measured during examinations in years 1992–1993, 1996–1997, and 2005–2006. Incident diabetes was ascertained if any of the following conditions were present: 1) the new use of insulin or oral hypoglycemic agents, 2) a fasting glucose concentration ≥7 mmol/L (126 mg/dL), or 3) a nonfasting glucose concentration ≥11.1 mmol/L (200 mg/dL). A detailed description of the diabetes definition in the CHS has been published elsewhere (2).
Other variables
Comprehensive information on health-related factors was collected at baseline and annually thereafter in a standardized fashion. Age, sex, race, years of education, physical activity, smoking status, alcohol consumption, and dietary habits were based on self-reports. The leisure-time activity (kcal/wk) was assessed by using a modified Minnesota Leisure-Time Activities questionnaire (28). The usual diet was assessed by using a picture-sort food-frequency questionnaire (29) in 1989–1990 and a food-frequency questionnaire (1995–1996 examination). Weight, height, and waist circumference were measured by using standardized protocols. Body mass index (BMI; calculated as weight in kilograms divided by height in meters squared. Glucose, insulin, and lipids were measured in fasting blood specimens (30). A homeostasis model assessment of insulin resistance was calculated as follows:
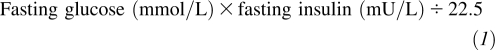 |
Statistical analysis
We categorized participants by quartiles of EPA, DHA, EPA+DHA, and ALA, and used Cox proportional hazards regression to estimate the relative risk (RR) of incident diabetes associated with these categories by using lowest quartiles as reference groups. The time at risk was calculated as the interval in days from the date of the 1992–1993 examination to the earliest of the date of the follow-up contact at which diabetes was ascertained, date of the last informative contact, or date of the 2007–2008 contact.
In preliminary analyses, models were fit in men and women separately to evaluate the potential for effect modification by sex. For each analyte, there was no significant sex-by-analyte interaction, and hazard ratio estimates were in the same direction and of a similar magnitude in the separate models; therefore, men and women were pooled for all subsequent analyses. Multivariable models were adjusted for age, race (black or non-black), sex, clinic site, BMI, alcohol consumption (0, <7–13, and ≥14 drinks/wk), physical activity (categories were based on quintiles), current smoking, linoleic acid, and LDL cholesterol. Tests for trends across quartiles were performed by fitting a continuous variable with assigned values equal to the median amount of the analyte within each category.
In secondary analyses performed in participants enrolled in 1989–1990 and included in the analyses of fatty acids and diabetes (n = 2831), we calculated the RR of incident diabetes associated with estimated dietary EPA, DHA, ALA, and fish consumption calculated by using the cumulative average of the 2 food-frequency questionnaires. Total fish consumption was calculated by summing the intake of tuna fish and other broiled or baked fish, as previously described (31). Last, we conducted a sensitivity analysis by excluding people who reported the use of fish-oil supplements (n = 117). We evaluated the validity of the proportional hazards assumption by using Schoenfeld residuals and showed no meaningful departures. Statistical analysis was performed with Stata software 10.1 (StataCorp, College Station, TX).
RESULTS
Of the 3088 participants, 38.9% of subjects were men and 10.8% of subjects were African-Americans. The mean age was 75.6 ± 5.5 y (range: 65–98 y) for men and 74.7 ±5.0 y (range: 65–98 y) for women. Baseline characteristics according to plasma phospholipid marine n−3 FAs and ALA are shown in Table 1. Higher concentrations of EPA+DHA were associated with female sex, lower levels of physical activity, a higher educational attainment, a lower prevalence of smoking, lower concentrations of triglycerides, and higher prevalences of moderate drinking, fish consumption, and use of fish oil supplements. Higher concentrations of ALA were related to female sex, education, nonsmoking, lower BMI and waist circumference, lower triglycerides, and higher fish consumption.
TABLE 1.
Characteristics of 3088 participants at the 1992–1993 examination according to quartiles of plasma n−3 fatty acids1
| EPA+DHA |
ALA |
|||||||
| Quartile 1, low (n = 772) | Quartile 2 (n = 772) | Quartile 3 (n = 772) | Quartile 4, high (n = 772) | Quartile 1, low (n = 785) | Quartile 2 (n = 778) | Quartile 3 (n = 753) | Quartile 4, high (n = 772) | |
| Range (% of total plasma fatty acids) | ≤2.82 | >2.82–3.42 | >3.42–4.22 | >4.22 | ≤0.11 | >0.11–0.14 | >0.14–0.18 | >0.18 |
| Median (% of total plasma fatty acids) | 2.47 | 3.13 | 3.74 | 5.02 | 0.10 | 0.13 | 0.16 | 0.21 |
| Characteristic | ||||||||
| Age (y) | 74.9 ± 5.22 | 75.1 ± 5.3 | 75.0 ± 5.2 | 75.1 ± 5.33 | 74.9 ± 5.0 | 74.9 ± 5.3 | 75.1 ± 5.3 | 75.2 ± 5.33 |
| Men (%) | 44.0 | 38.6 | 35.4 | 37.7 | 46.6 | 39.9 | 37.1 | 32.0 |
| Black (%) | 3.4 | 7.6 | 14.4 | 17.9 | 13.1 | 11.3 | 12.0 | 6.9 |
| High school education or less (%) | 61.5 | 57.7 | 50.1 | 43.8 | 57.7 | 55.4 | 50.7 | 49.2 |
| Current smoker (%) | 13.6 | 9.7 | 7.8 | 6.2 | 10.6 | 12.3 | 6.5 | 7.8 |
| Alcoholic drinks/wk (%) | ||||||||
| None | 57.2 | 55.7 | 51.4 | 45.5 | 53.5 | 54.8 | 54.2 | 47.3 |
| <7–13 | 36.3 | 36.9 | 41.6 | 48.3 | 41.5 | 39.4 | 38.8 | 43.4 |
| ≥14 | 6.5 | 7.4 | 7.0 | 6.2 | 5.0 | 5.8 | 7.0 | 9.3 |
| Prevalent coronary disease (%) | 20.2 | 20.1 | 20.3 | 21.13 | 20.0 | 23.8 | 20.1 | 17.93 |
| Use of fish-oil supplements (%) | 1.3 | 1.9 | 5.2 | 6.7 | 3.4 | 3.7 | 4.7 | 3.4 |
| BMI (kg/m2) | 26.0 ± 4.4 | 26.5 ± 4.4 | 26.6 ± 4.7 | 26.3 ± 4.53 | 27.3 ± 4.8 | 26.5 ± 4.4 | 26.2 ± 4.5 | 25.4 ± 4.13 |
| Waist circumference (cm) | 95.7 ± 12.9 | 96.7 ± 12.5 | 96.8 ± 12.8 | 95.0 ± 13.03 | 99.0 ± 13.0 | 96.3 ± 12.3 | 95.6 ± 12.8 | 93.3 ± 12.4 |
| Physical activity (kcal/wk) | 960 (338, 2154)4 | 971 (368, 2010) | 836 (270, 1913) | 856 (305, 1905)3 | 915 (341, 2171) | 881 (270, 1890) | 840 (270, 1980) | 1016 (374, 2004)3 |
| Triglyceride (mg/dL) | 122 (93, 172) | 126 (91, 176) | 122 (91, 169) | 112 (81, 158) | 127 (94, 168) | 119 (91, 170) | 120 (89, 167) | 121 (83, 173)3 |
| LDL cholesterol (mg/dL) | 121.7 ± 34.2 | 119.6 ± 33.1 | 121.8 ± 33.1 | 120.3 ± 31.93 | 123.4 ± 32.5 | 122.2 ± 34.8 | 121.7 ± 31.6 | 116.0 ± 32.9 |
| Glucose (mg/dL) | 97.2 ± 9.9 | 97.4 ± 9.8 | 97.3 ± 9.6 | 97.0 ± 10.03 | 98.7 ± 10.1 | 97.6 ± 9.7 | 96.6 ± 9.3 | 96.0 ± 9.9 |
| Insulin (IU/mL) | 10.4 ± 6.8 | 11.6 ± 7.0 | 12.1 ± 16.1 | 10.4 ± 5.53 | 12.2 ± 8.2 | 11.7 ± 16.0 | 10.6 ± 5.9 | 9.9 ± 5.0 |
| HOMA-IR | 2.1 (1.5, 3.0) | 2.4 (1.6, 3.4) | 2.2 (1.6, 3.3) | 2.2 (1.6, 3.1)3 | 2.4 (1.7, 3.6) | 2.3 (1.6, 3.2) | 2.1 (1.6, 3.1) | 2.0 (1.4, 2.9) |
| Dietary EPA+DHA (g/d)5 | 0.17 (0.10, 0.30) | 0.25 (0.16, 0.46) | 0.36 (0.22, 0.57) | 0.56 (0.33, 0.76) | 0.27 (0.15, 0.53) | 0.29 (0.16, 0.55) | 0.29 (0.17, 0.55) | 0.35 (0.19, 0.60) |
| Dietary ALA (g/d)5 | 1.35 (1.11, 1.64) | 1.33 (1.10, 1.61) | 1.37 (1.13, 1.62) | 1.35 (1.09, 1.61)3 | 1.27 (1.06, 1.52) | 1.33 (1.08, 1.62) | 1.38 (1.13, 1.65) | 1.41 (1.16, 1.66) |
| Dietary linoleic acid (g/d)5 | 11.7 (9.5, 14.3) | 11.6 (9.6, 14.3) | 11.5 (9.5, 13.9) | 11.4 (8.9, 13.6) | 11.2 (9.3, 13.7) | 11.5 (9.3, 13.9) | 11.8 (9.4, 14.1) | 11.8 (9.7, 14.0) |
| Fish servings/wk5 | 0.8 (0.5, 1.5) | 1.2 (0.7, 1.9) | 1.6 (0.9, 2.3) | 2.0 (1.5, 3.0) | 1.3 (0.7, 2.0) | 1.3 (0.7, 2.0) | 1.5 (0.8, 2.2) | 1.5 (0.8, 2.3) |
EPA+DHA, eicosapentaenoic acid and docosahexaenoic acid; ALA, α-linolenic acid; HOMA-IR, homeostatic model assessment of insulin resistance. Linear and logistic regression was used to assess linear trends of characteristics across quartiles of fatty acids and were significant (P < 0.05) unless otherwise noted.
Mean ± SD (all such values).
Linear test for trend, P > 0.05.
Median; interquartile range in parentheses (all such values).
Cumulative average of energy-corrected values from food-frequency questionnaires administered in 1989–1990 and 1995–1996 in participants enrolled in 1989–1990 only (n = 2831).
During an average follow up of 9.6 y, 204 incident cases of diabetes occurred. Crude incidence rates of diabetes were 68.4, 74.7, 79.9, and 52.0 cases per 10,000 person-years from the lowest to highest quartiles of EPA+DHA. Corresponding values for ALA were 87.1, 78.1, 72.6, and 37.7 cases per 10,000 person-years, respectively. In a multivariable adjusted model, the concentration of plasma phospholipid EPA+DHA was not associated with an increased risk of diabetes (Table 2). Individuals with the highest concentrations of EPA+DHA had lower risk of diabetes [RR (95% CI): 0.64 (0.41, 1.01); Table 2]. When analyzed separately, neither DHA nor EPA was associated with an increased risk of diabetes [fully adjusted RR (95% CI) for comparison of highest to lowest quartiles of EPA: 1.17 (0.71, 1.92) and DHA: 0.70 (0.44, 1.11)]. The exclusion of participants who were taking fish oil supplements did not materially alter the association between plasma phospholipid EPA+DHA and incident diabetes [RR: 1.0 (reference), 0.95 (0.64, 1.42), 1.05 (0.70, 1.57), and 0.66 (0.41, 1.05) from the lowest to highest quartiles of EPA+DHA; P for trend = 0.07]. A similar association was observed between plasma phospholipid ALA with a 43% lower risk of diabetes when the highest to lowest quartiles of ALA were compared (95% CI: 10%, 64%; Table 2). The exclusion of people who were using fish-oil supplements did not alter these findings [adjusted RR (95% CI): 1.0 (reference), 0.94 (0.65, 1.36), 0.96 (0.66, 1.40), and 0.57 (0.36, 0.90) (P for trend = 0.03) across consecutive quartiles of ALA].
TABLE 2.
Relative risk of incident diabetes according to quartiles of plasma n−3 fatty acids1
| EPA+DHA |
ALA |
|||||||||
| Quartile 1 (n = 772) | Quartile 2 (n = 772) | Quartile 3 (n = 772) | Quartile 4 (n = 772) | P for trend | Quartile 1 (n = 785) | Quartile 2 (n = 778) | Quartile 3 (n = 753) | Quartile 4 (n = 772) | P for trend | |
| Range (% of total plasma fatty acids) | ≤2.82 | >2.82–3.42 | >3.42–4.22 | >4.22 | — | ≤0.11 | >0.11–0.14 | >0.14–0.18 | >0.18 | — |
| No. of cases of incident diabetes | 50 | 55 | 59 | 40 | — | 65 | 57 | 53 | 29 | — |
| Person-years of follow-up | 7310 | 7366 | 7384 | 7688 | — | 7459 | 7301 | 7300 | 7688 | — |
| Model2 | ||||||||||
| 1 | 1.00 (reference) | 1.05 (0.71, 1.54) | 1.11 (0.76, 1.63) | 0.72 (0.47, 1.11) | 0.12 | 1.00 (reference) | 0.91 (0.64, 1.30) | 0.83 (0.58, 1.20) | 0.46 (0.29, 0.71) | <0.001 |
| 2 | 1.00 (reference) | 1.00 (0.68, 1.48) | 1.13 (0.77, 1.66) | 0.73 (0.47, 1.13) | 0.17 | 1.00 (reference) | 0.95 (0.66, 1.36) | 0.91 (0.63, 1.32) | 0.56 (0.36, 0.88) | 0.02 |
| 3 | 1.00 (reference) | 0.96 (0.65, 1.43) | 1.03 (0.69, 1.54) | 0.64 (0.41, 1.01) | 0.05 | 1.00 (reference) | 0.93 (0.65, 1.34) | 0.99 (0.68, 1.44) | 0.57 (0.36, 0.90) | 0.03 |
EPA+DHA, eicosapentaenoic acid and docosahexaenoic acid; ALA, α-linolenic acid.
Values are relative risks; 95% CIs in parentheses (calculated by using Cox proportional hazards). Model 1 adjusted for age, race (black or nonblack), sex, and clinic site. Model 2 adjusted for age, race (black or nonblack), sex, clinic site, BMI, alcohol consumption, physical activity, and current smoking. Model 3 adjusted for age, race (black or nonblack), sex, clinic site, BMI, alcohol consumption, physical activity, current smoking, plasma phospholipid linoleic acid, and LDL cholesterol.
In a secondary analysis, self-reported fish intake was not associated with an increased risk of diabetes (Table 3). We showed no association between dietary EPA+DHA and incident diabetes (Table 4). For dietary ALA, only the highest quartile was associated with a nonsignificant 50% lower risk of diabetes compared with that of the first quartile (Table 4).
TABLE 3.
Relative risk of incident diabetes according to consumption of fish for participants enrolled in 1989–1990 (n = 2831)
| Frequency of fish consumption1 |
||||||
| <1/mo (n = 168) | 1–3/mo (n = 768) | 1–2/wk (n = 1500) | 3–4/wk (n = 308) | ≥5/wk (n = 87) | P for trend | |
| No. of cases of incident diabetes | 9 | 52 | 94 | 17 | 5 | — |
| Person-years of follow-up | 1434 | 6763 | 15235 | 2936 | 839 | — |
| Model2 | ||||||
| 1 | 1.00 (reference) | 1.27 (0.63, 2.59) | 1.05 (0.53, 2.08) | 1.05 (0.46, 2.37) | 1.08 (0.36, 3.26) | 0.59 |
| 2 | 1.00 (reference) | 1.23 (0.60, 2.53) | 0.98 (0.49, 1.96) | 0.90 (0.39, 2.07) | 1.03 (0.34, 3.19) | 0.46 |
| 3 | 1.00 (reference) | 1.21 (0.59, 2.48) | 1.00 (0.50, 2.01) | 0.93 (0.40, 2.14) | 1.07 (0.35, 3.30) | 0.59 |
Cumulative average from food-frequency questionnaires administered in 1989–1990 and 1995–1996.
Values are relative risks; 95% CIs in parentheses (calculated by using Cox proportional hazards). Model 1 adjusted for age, race (black or nonblack), sex, and clinic site. Model 2 adjusted for age, race (black or nonblack), sex, clinic site, BMI, alcohol consumption, physical activity, current smoking, and total energy intake. Model 3 adjusted for age, race (black or nonblack), sex, clinic site, BMI, alcohol consumption, physical activity, current smoking, total energy intake, and LDL cholesterol.
TABLE 4.
Relative risk of incident diabetes according to dietary eicosapentaenoic acid and docosahexaenoic acid (EPA+DHA) and α-linolenic acid (ALA) as estimated from food-frequency questionnaires for participants enrolled during 1989–1990 (n = 2826)1
| EPA+DHA |
ALA |
|||||||||
| Quartile 1 (n = 707) | Quartile 2 (n = 706) | Quartile 3 (n = 707) | Quartile 4 (n = 706) | P for trend | Quartile 1 (n = 707) | Quartile 2 (n = 706) | Quartile 3 (n = 707) | Quartile 4 (n = 706) | P for trend | |
| Range2 | ≤0.17 | >0.17–0.30 | >0.30–0.56 | >0.56 | — | ≤0.11 | >0.11–0.14 | >0.14–0.18 | >0.18 | — |
| No. of cases of incident diabetes | 46 | 51 | 36 | 44 | — | 45 | 46 | 55 | 31 | — |
| Person-years of follow-up | 6566 | 6615 | 7083 | 6904 | — | 6605 | 7045 | 7085 | 6434 | — |
| Model3 | ||||||||||
| 1 | 1.00 (reference) | 1.11 (0.75, 1.66) | 0.75 (0.48, 1.17) | 0.97 (0.64, 1.49) | 0.57 | 1.00 (reference) | 0.92 (0.61, 1.39) | 1.10 (0.74, 1.63) | 0.70 (0.44, 1.11) | 0.22 |
| 2 | 1.00 (reference) | 1.07 (0.72, 1.60) | 0.75 (0.48, 1.16) | 0.99 (0.64, 1.52) | 0.67 | 1.00 (reference) | 0.88 (0.58, 1.34) | 1.13 (0.75, 1.69) | 0.70 (0.44, 1.11) | 0.26 |
| 3 | 1.00 (reference) | 1.11 (0.74, 1.66) | 0.78 (0.50, 1.22) | 1.04 (0.67, 1.60) | 0.81 | 1.00 (reference) | 0.82 (0.51, 1.33) | 0.99 (0.56, 1.77) | 0.50 (0.24, 1.05) | 0.09 |
Values are cumulative averages of energy-corrected values from food-frequency questionnaires administered in 1989–1990 and in 1995–1996.
Values are shown in mg/d for EPA+DHA and in g/d for ALA.
Values are relative risks; 95% CIs in parentheses (calculated by using Cox proportional hazards). Model 1 adjusted for age, race (black or nonblack), sex, and clinic site. Model 2 adjusted for age, race (black or nonblack), sex, clinic site, BMI, alcohol consumption, physical activity, and current smoking. Model 3 adjusted for age, race (black or nonblack), sex, clinic site, BMI, alcohol consumption, physical activity, current smoking, LDL cholesterol, and linoleic acid.
DISCUSSION
In this large prospective study of older adults, plasma phospholipid EPA+DHA and ALA were not associated with an increased risk of diabetes. Individuals with the highest concentrations of EPA+DHA or ALA had lower risk of diabetes. The exclusion of the use of fish-oil supplements did not materially alter these associations. In contrast, dietary EPA+DHA and fatty fish consumption were not associated with diabetes, whereas higher concentrations of dietary ALA were suggestive of lower risk of diabetes.
EPA+DHA and risk of diabetes
The lack of an increased risk of diabetes with phospholipid marine n−3 FAs (EPA+DHA) in the current study was consistent with reports from the Atherosclerosis Risk in Communities study (20). In addition, Vessby et al (22) did not find a significant association between serum n−3 FAs (cholesteryl esters) and incident diabetes in men after 10 y of follow-up. In the Melbourne Collaborative Study (23) of nearly 4000 men and women, plasma phospholipids EPA+DHA were not associated with incident diabetes. In a case-control study (32) of 383 subjects, plasma phospholipids EPA+DHA were not associated with the odds of diabetes, which were findings that were consistent with our report.
Among studies that used food-frequency questionnaires to estimate marine n−3 FAs, findings on the association between EPA+DHA and diabetes risk have been inconsistent. The Iowa Women's Study reported no association between dietary long-chain n−3 FAs and diabetes (17). In addition, Hodge et al (23) reported no relation between dietary long-chain n−3 FAs and risk of diabetes. Those data were consistent with our findings of no association between dietary EPA+DHA and incident diabetes. In contrast, a pooled analysis of 3 large cohorts reported a 24% increased risk of diabetes in the highest quintile of dietary long-chain n−3 FAs compared with the lowest quintile (18). In addition, data from the Women's Health Study showed a positive and graded association between dietary long-chain n−3 FAs and incident diabetes [RR (95% CI): 1.44 (1.25, 1.65) for comparison of highest compared with lowest quintiles] (19).
Our findings of no increased risk of diabetes with dietary or plasma phospholipid EPA+DHA suggested that marine n−3 FAs may not play a major role in the development of diabetes in older adults. The observed borderline significant lower risk of diabetes in the highest quartile of EPA+DHA suggested that long-chain n−3 FAs may even exert beneficial effects on diabetes risk. Plasma phospholipid n−3 FAs may not reflect the long-term intake of n−3 FAs. Hence, it is possible that a single measure of plasma phospholipid n−3 FAs might not capture the long-term intake of dietary n−3 FAs. In addition, plasma n−3 FAs concentrations may reflect metabolic processes in addition to dietary intakes such as the exchange between plasma and red blood cell membranes, which make it difficult to correlate dietary intake (measured via biomarkers) to diabetes risk. Alternatively, we could not exclude unmeasured or residual confounding as a partial explanation of the findings.
ALA and incident diabetes
Our observation of lower risk of diabetes with higher plasma phospholipid ALA was consistent with findings from the ARIC study (20) in which there was ≈30% lower risk of diabetes in the highest quintile of plasma phospholipid ALA compared with the lowest group (P for linear trend = 0.026). In a European case-control study (32), adjusted odds ratios (95% CIs) for diabetes were 1.0, 0.88 (0.50, 1.54) and 0.81 (0.46, 1.43) from the first to the third tertiles of plasma phospholipid ALA. The association between plasma phospholipid ALA and incident diabetes was consistent with the observed borderline significant association between dietary ALA and incident diabetes. In contrast, other investigators showed no association between phospholipid (23), total plasma (21), or dietary (18, 19) ALA and incident diabetes. Although these studies did not provide ALA concentrations for individual exposure categories for comparison, plasma phospholipid ALA in the studies (0.13–0.20% for diabetes cases and 0.15−0.22% for noncases) were comparable with values obtained in the current study (mean: 0.14% for diabetes cases and 0.15% for noncases).
nminus3 Fatty acids and diabetes-related traits
Limited and inconsistent data are available on potential mechanisms by which n−3 FAs may influence risk of diabetes. A 6-wk intervention with 4 g EPA or DHA led to a 1.40 mmol/L (P = 0.002) or 0.98 mmol/L (P = 0.002) increase in the fasting glucose concentration, respectively, compared with that of olive oil in patients with diabetes (33); in contrast, neither EPA nor DHA had any effect on fasting insulin, insulin sensitivity, C-peptide, or glycated hemoglobin (33). In a meta-analysis of 26 trials, each gram per day of DHA was associated with a 0.74-mmol/L (95% CI: 0.16, 1.32) increase in the fasting glucose concentration (34); in addition, there was a 0.38% (95% CI: 0.00%, 0.76%) increase in glycated hemoglobin for each gram per day increase in EPA; the corresponding increase for DHA was 0.6% (95% CI: 0.06%, 1.15%) (34). Other trials have reported increased fasting glucose or insulin with EPA or DHA compared with placebo (35, 36). However, other investigators reported no effects of fish oil or EPA+DHA on fasting glucose, glycated hemoglobin, or insulin sensitivity (37–39). There are limited trial data on the effects of ALA on glucose metabolism. In 2 small clinical trials, the intervention with ALA was associated with a reduced plasma glucose concentrations [pooled effect size: −0.20 mmol/L (95% CI: −0.30, −0.10 mmol/L)] (40). This benefit was consistent with the observed lower risk of diabetes in the higher category of plasma phospholipid or dietary ALA in our study. Additional studies that examine biologic mechanisms by which n−3 FAs might affect glucose metabolism are warranted.
Limitations and strengths
We had only one measurement of plasma phospholipids in this cohort. Hence, we were unable to account for the change in plasma n−3 FAs that resulted from the change in n−3 FA consumption over time. Plasma concentrations of n−3 FAs only reflect dietary intake across several weeks, whereas the adipocyte content of n−3 FAs reflects intakes over ≥3 mo. Furthermore, phospholipid n−3 FAs measured in this study reflected the dietary intake and the metabolism of fatty acids, including the exchange between red blood cell membranes and plasma. As an observational study, we could not exclude residual or unmeasured confounding as an alternative explanation of the observed associations. Our sample consisted of mostly whites and older adults, which thereby limits the generalizability of our findings. We had only 204 cases of incident diabetes in our study; hence, our statistical power to detect a modest effect of EPA+DHA on diabetes was limited. Strengths of the current study included the large sample size, availability of data on men and women, the use of biomarkers to measure n−3 FAs (more reproducible than a food questionnaire), and the long-term and nearly complete follow-up.
Conclusions
In conclusion, higher concentrations of plasma phospholipid ALA and EPA+DHA were associated with lower risk of diabetes in older adults. The absence of increased diabetes risk with higher concentrations of plasma phospholipid or dietary EPA+DHA did not support the hypothesis that long-chain n−3 FA consumption increases the risk of diabetes, which suggested that relations observed in previous studies may have been due to confounding or to other ingredients contained in fish.
Acknowledgments
We are indebted to the participants and staff of the CHS. A full list of participating CHS investigators and institutions is available at http://www.chs-nhlbi.org.
The authors’ responsibilities were as follows—LD: study design and drafting the manuscript; MLB: statistical analysis; IBK and XS: measurement of fatty acids; LD, JHI, KJM, DSS, and DM: obtained funding; DSS: data collection; and all authors: critical review of the manuscript. KJM is the principal investigator in an ongoing study funded by Harvard Medical School for which Beth Israel Deaconess Medical Center received a donation of DHA and placebo capsules from Martek Corp. Martek Corp provided no other resources or funds and had no role in the conduct or analysis of that study. Also, Martek Corp had no role in the current manuscript. DM received research grants from GlaxoSmithKline, Sigma Tau, Pronova, and the National Institutes of Health for an investigator-initiated, not-for-profit clinical trial of fish oil; travel reimbursement, honoraria, or consulting fees from Aramark, Unilever, SPRIM, Norwegian Seafood Export Council, and Nutrition Impact for topics related to diet and cardiovascular health; and royalties from UpToDate for an online chapter on fish oil. LD, MLB, RNL, IBK, XS, JHI, and DSS had no conflicts of interest.
REFERENCES
- 1.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA 2003;290:1884–90 [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Kamineni A, Carnethon M, Djousse L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med 2009;169:798–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001;345:790–7 [DOI] [PubMed] [Google Scholar]
- 4.Djoussé L, Hunt CS, Tang W, Eckfeldt JH, Province MA, Ellison CR. Dietary linolenic acid and fasting glucose and insulin: the National Heart, Lung, and Blood Institute Family Heart Study. Obesity (Silver Spring) 2006;14:295–300 [DOI] [PubMed] [Google Scholar]
- 5.Kochar J, Djousse L, Gaziano JM. Breakfast cereals and risk of type-2 diabetes in the Physicians’ Health Study I. Obesity (Silver Spring) 2007;15:3039–44 [DOI] [PubMed] [Google Scholar]
- 6.Djousse L, Arnett DK, Carr JJ, et al. Dietary linolenic acid is inversely associated with calcified atherosclerotic plaque in the coronary arteries: the NHLBI Family Heart Study. Circulation 2005;111:2921–6 [DOI] [PubMed] [Google Scholar]
- 7.Marchioli R, Barzi F, Bomba E, et al. Early protection against sudden death by n−3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation 2002;105:1897–903 [DOI] [PubMed] [Google Scholar]
- 8.de Lorgeril M, Renaud S, Mamelle N, et al. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 1994;343:1454–9 [DOI] [PubMed] [Google Scholar]
- 9.Albert CM, Campos H, Stampfer MJ, et al. Blood levels of long-chain n−3 fatty acids and the risk of sudden death. N Engl J Med 2002;346:1113–8 [DOI] [PubMed] [Google Scholar]
- 10.Jenkins DJ, Josse AR, Dorian P, et al. Heterogeneity in randomized controlled trials of long chain (fish) omega-3 fatty acids in restenosis, secondary prevention and ventricular arrhythmias. J Am Coll Nutr 2008;27:367–78 [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007;369:1090–8 [DOI] [PubMed] [Google Scholar]
- 12.Mozaffarian D, Psaty BM, Rimm EB, et al. Fish intake and risk of incident atrial fibrillation. Circulation 2004;110:368–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mozaffarian D, Longstreth WT, Jr, Lemaitre RN, et al. Fish consumption and stroke risk in elderly individuals: the cardiovascular health study. Arch Intern Med 2005;165:200–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. n−3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study. Am J Clin Nutr 2003;77:319–25 [DOI] [PubMed] [Google Scholar]
- 15.Mozaffarian D, Bryson CL, Lemaitre RN, Burke GL, Siscovick DS. Fish intake and risk of incident heart failure. J Am Coll Cardiol 2005;45:2015–21 [DOI] [PubMed] [Google Scholar]
- 16.Kromhout D, Giltay EJ, Geleijnse JM. n−3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med 2010;363:2015–26 [DOI] [PubMed] [Google Scholar]
- 17.Meyer KA, Kushi LH, Jacobs DR, Jr, Folsom AR. Dietary fat and incidence of type 2 diabetes in older Iowa women. Diabetes Care 2001;24:1528–35 [DOI] [PubMed] [Google Scholar]
- 18.Kaushik M, Mozaffarian D, Spiegelman D, Manson JE, Willett WC, Hu FB. Long-chain omega-3 fatty acids, fish intake, and the risk of type 2 diabetes mellitus. Am J Clin Nutr 2009;90:613–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Djousse L, Gaziano JM, Buring JE, Lee IM. Dietary omega-3 fatty acids and fish consumption and risk of type 2 diabetes. Am J Clin Nutr 2011;93:143–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Folsom AR, Zheng ZJ, Pankow JS, Eckfeldt JH. Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2003;78:91–8 [DOI] [PubMed] [Google Scholar]
- 21.Laaksonen DE, Lakka TA, Lakka HM, et al. Serum fatty acid composition predicts development of impaired fasting glycaemia and diabetes in middle-aged men. Diabet Med 2002;19:456–64 [DOI] [PubMed] [Google Scholar]
- 22.Vessby B, Aro A, Skarfors E, Berglund L, Salminen I, Lithell H. The risk to develop NIDDM is related to the fatty acid composition of the serum cholesterol esters. Diabetes 1994;43:1353–7 [DOI] [PubMed] [Google Scholar]
- 23.Hodge AM, English DR, O'Dea K, et al. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr 2007;86:189–97 [DOI] [PubMed] [Google Scholar]
- 24.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–76 [DOI] [PubMed] [Google Scholar]
- 25.Folch J. Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509 [PubMed] [Google Scholar]
- 26.Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 1986;27:114–20 [PubMed] [Google Scholar]
- 27.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol 1992;45:683–92 [DOI] [PubMed] [Google Scholar]
- 28.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 1978;31:741–55 [DOI] [PubMed] [Google Scholar]
- 29.Kumanyika SK, Tell GS, Shemanski L, Martel J, Chinchilli VM. Dietary assessment using a picture-sort approach. Am J Clin Nutr 1997;65:1123S–9S [DOI] [PubMed] [Google Scholar]
- 30.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem 1995;41:264–70 [PubMed] [Google Scholar]
- 31.Mozaffarian D, Gottdiener JS, Siscovick DS. Intake of tuna or other broiled or baked fish versus fried fish and cardiac structure, function, and hemodynamics. Am J Cardiol 2006;97:216–22 [DOI] [PubMed] [Google Scholar]
- 32.Patel PS, Sharp SJ, Jansen E, et al. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Am J Clin Nutr 2010;92:1214–22 [DOI] [PubMed] [Google Scholar]
- 33.Woodman RJ, Mori TA, Burke V, Puddey IB, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am J Clin Nutr 2002;76:1007–15 [DOI] [PubMed] [Google Scholar]
- 34.Friedberg CE, Janssen MJ, Heine RJ, Grobbee DE. Fish oil and glycemic control in diabetes. A meta-analysis. Diabetes Care 1998;21:494–500 [DOI] [PubMed] [Google Scholar]
- 35.Mori TA, Burke V, Puddey IB, et al. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr 2000;71:1085–94 [DOI] [PubMed] [Google Scholar]
- 36.Mostad IL, Bjerve KS, Bjorgaas MR, Lydersen S, Grill V. Effects of n−3 fatty acids in subjects with type 2 diabetes: reduction of insulin sensitivity and time-dependent alteration from carbohydrate to fat oxidation. Am J Clin Nutr 2006;84:540–50 [DOI] [PubMed] [Google Scholar]
- 37.Montori VM, Farmer A, Wollan PC, Dinneen SF. Fish oil supplementation in type 2 diabetes: a quantitative systematic review. Diabetes Care 2000;23:1407–15 [DOI] [PubMed] [Google Scholar]
- 38.Kabir M, Skurnik G, Naour N, et al. Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr 2007;86:1670–9 [DOI] [PubMed] [Google Scholar]
- 39.Griffin MD, Sanders TA, Davies IG, et al. Effects of altering the ratio of dietary n−6 to n−3 fatty acids on insulin sensitivity, lipoprotein size, and postprandial lipemia in men and postmenopausal women aged 45-70 y: the OPTILIP Study. Am J Clin Nutr 2006;84:1290–8 [DOI] [PubMed] [Google Scholar]
- 40.Wendland E, Farmer A, Glasziou P, Neil A. Effect of alpha linolenic acid on cardiovascular risk markers: a systematic review. Heart 2006;92:166–9 [DOI] [PMC free article] [PubMed] [Google Scholar]


