Abstract
Background: Various definitions, criteria, tests, and cutoffs have been used to define vitamin B-12 status; however, a need exists for the systematic study of vitamin B-12 status in the United States because of concerns about high folic acid intakes and the potential for associated adverse effects.
Objective: The objective was to determine the effect of different cutoff choices on outcomes and of the different degrees of serum vitamin B-12 status, definable by the concurrent use of a functional and circulating marker as the first steps to developing a data-based consensus on the biochemical diagnosis of vitamin B-12 deficiency.
Design: Data from NHANES, a nationally representative cross-sectional survey, were examined for adults aged >19 y (mean ± SD age: 45 ± 1 y) from 1999 to 2004 (n = 12,612).
Results: Commonly used cutoffs had a greater effect on prevalence estimates of low vitamin B-12 status with the use of vitamin B-12 than with the use of methylmalonic acid (MMA; 3–26% and 2–6%, respectively). A cutoff of >148 pmol/L for vitamin B-12 and of ≤210 nmol/L for MMA resulted in significant misclassifications. Approximately 1% of adults had a clear vitamin B-12 deficiency (low vitamin B-12 and elevated MMA); 92% of adults had adequate vitamin B-12 status. A high percentage of younger women characterized the group with low vitamin B-12 and normal MMA (2% of adults) and may have falsely reflected low vitamin B-12. Adults with elevated MMA (5%) only were demographically similar (ie, by age and race) to the deficient group and may have included some individuals with early vitamin B-12 deficiency.
Conclusions: These analyses indicate the challenges of assessing vitamin B-12 status when uncertainties exist about the appropriate cutoffs. Future studies should determine definable endpoints to achieve this goal.
INTRODUCTION
Vitamin B-12 is essential for DNA synthesis to maintain normal hematologic and neurologic functions. A marked interest in monitoring the vitamin B-12 status of the US population was triggered by the 1996 implementation of the national folic acid fortification of cereal grains because of concerns that an improved folate status could adversely affect persons at risk of vitamin B-12 insufficiency, particularly the elderly, for whom a relatively high prevalence has been reported (1, 2). NHANES has measured 3 biomarkers of vitamin B-12 status over the past 3 decades: serum vitamin B-12, methylmalonic acid (MMA), and total homocysteine (tHcy) (3). Serum vitamin B-12 directly reflects the range of circulating vitamin B-12 concentrations in individuals, although not necessarily tissue concentrations. MMA accumulates in the absence of adequate vitamin B-12 and is therefore a functional indicator of vitamin B-12 status (2, 4). Like MMA, tHcy accumulates in the absence of vitamin B-12. However, tHcy is less specific for vitamin B-12 deficiency than is MMA because it also accumulates in the absence of other nutrients, such as folate.
The primary challenge with using all biomarkers of vitamin B-12 status is the lack of scientific consensus regarding cutoffs to determine low status. This limitation is particularly critical for identifying individuals with subclinical vitamin B-12 deficiency who lack clinically relevant endpoints and often depend on other biomarkers for comparison and validation (3, 5). For serum vitamin B-12 and MMA, commonly used cutoffs often come from statistical distributions of different populations. Thus, the reference intervals may be both population and method dependent, and researchers have published many different cutoffs in the literature.
Another complication is that each biomarker is subject to sensitivity and specificity limitations. These limitations are particularly challenging for accurately identifying subclinical vitamin B-12 deficiency, which is more common than the more severe clinical deficiency (6). To minimize the effect of these vitamin B-12 biomarker limitations, experts recommend concurrently examining a functional biomarker and a biomarker that directly measures blood vitamin content when assessing vitamin B-12 status (7). However, to our knowledge, NHANES data have not been analyzed previously to evaluate the combined use of serum vitamin B-12 and MMA for assessing vitamin B-12 status in the US population. We examined NHANES 1999–2004 data on the combined application of several commonly used cutoffs for serum vitamin B-12 and MMA to create metabolic profiles to assess the prevalence of low vitamin B-12 status in the US population.
SUBJECTS AND METHODS
Subjects
NHANES is a nationally representative, cross-sectional survey of the noninstitutionalized US population. The survey uses a complex, stratified, multistage probability cluster sampling design. The National Center for Health Statistics of the Centers for Disease Control and Prevention administers NHANES. NHANES personnel first interview survey participants in their homes. During this household interview, interviewers collect information on demographic characteristics, dietary supplement use, and some health-related issues. Participants undergo a physical examination and blood collection in a mobile examination center ≈1–2 wk after the household interview. They also complete a 24-h dietary recall. Survey personnel obtain written informed consent from all participants or proxies, and the Research Ethics Review Board of the National Center for Health Statistics approved the survey protocol. Interview and examination response rates for each survey year are publically available (8).
For this study, we combined data from NHANES 1999–2000, 2001–2002, and 2003–2004 (n = 31,126). We excluded data on anyone younger than 19 y (n = 14,942), those with interview data only (ie, lacking mobile examination center data, n = 1160), lactating (n = 145) and pregnant (n = 801) women, and those with missing MMA data (n = 918). We also excluded data for individuals with high creatinine concentrations (>120 μmol/L for men, >110 μmol/L for women; n = 548) because impaired kidney function increases MMA and tHcy independent of vitamin B-12 status (9). Thus, the final analytic sample consisted of 12,612 adult participants.
Vitamin B-12 exposure
Dietary supplement information was collected via the NHANES Dietary Supplement Questionnaire to determine a sample person's use of vitamins, minerals, herbs, and other dietary supplements over the past 30 d. Detailed information about name and type, consumption frequency, duration of use, and amount taken was collected for each reported dietary supplement. We calculated the average daily intake of vitamin B-12 from all dietary supplements using the number of days of reported consumption of the supplement, the reported amount that respondents took per day, and the serving size unit from the product label, as we described previously (10).
Data on one 24-h dietary recall are available for each participant for 1999–2002 and for two 24-h dietary recalls for 2003–2004. We used the average vitamin B-12 intakes from the two 24-h dietary recalls in 2003–2004 instead of trying to adjust the data for within-person variability to use these data in conjunction with the 1999–2002 data. Because of limitations in the food-composition databases, estimates of vitamin B-12 intake in 1999–2004 do not account for bioavailability differences between naturally occurring and added vitamin B-12 sources. Added vitamin B-12 is more bioavailable and is present in fortified foods and dietary supplements (11). We combined data on the average daily intake of vitamin B-12 from supplemental sources with the daily estimates of vitamin B-12 intake from foods to reflect total vitamin B-12 exposure.
Cognitive test scores
We used a single measure of cognitive function, the Digit Symbol-Coding (DSC) subtest of the Wechsler Adult Intelligence Scale III (The Psychological Corporation) (12); this instrument, whereas sensitive, is not comprehensive or indicative of all domains of cognitive function. The DSC score is the total number of symbols that participants draw correctly in 120 s. To obtain a high score on the test, participants must have an adequate speed of response, attention, and memory (13). We used < 20th percentile (scores < 34 on the DSC) to indicate cognitive impairment as previously used by Morris et al (14) in NHANES. DSC scores were only available for those aged ≥60 y in NHANES 1999–2002 (n = 2393).
Medications
We used data from the NHANES prescription drug questionnaire to evaluate the use of prescription medications that may adversely affect vitamin B-12 status. Within users of NHANES Class 87 drugs, we identified participants who reported the use of proton-pump inhibitors and H2-receptor antagonists (15). No data were collected on the length of time that participants took these medications.
Biochemical methods
NHANES 1999–2004 measured serum folate, red blood cell (RBC) folate, and serum vitamin B-12 using the Quantaphase II radioassay (Bio-Rad, Hercules, CA). NHANES measured plasma tHcy using a fluorescence polarization immunoassay reagent kit (Abbott Laboratories, Abbott Park, IL) (16). NHANES assayed plasma MMA by gas chromatography–mass spectrometry after cyclohexanol derivation of the extracted MMA. NHANES 1999–2000 measured serum creatinine by the Jaffe method using the Hitachi 917 multichannel analyzer, and NHANES 2001–2004 used the Jaffe rate method (kinetic alkaline picrate) with the Beckman Synchron LX20 modular chemistry analyzer. We adjusted serum creatinine data for NHANES 1999–2000 using the following equation: 0.147 + 1.013 × uncorrected serum creatinine (mg/dL) as the analytic note recommends (17). Complete details and documentation for each of these methods are publically available on the NHANES website (18–20).
We applied several cutoff values to the biomarker data to determine sufficiency and illustrate changes in status (ie, categories across status). We reviewed serum vitamin B-12 concentrations to determine the sufficiency using cutoff values of <148 pmol/L (21–24), 200 pmol/L (25–27), and 258 pmol/L (7, 28, 29). We also used vitamin B-12 status based on the cutoffs that Stabler et al (30) originally proposed and that we modified for this analysis. The original criteria had 3 concentrations of vitamin B-12 status: <148 pmol/L (<200 pg/mL), 148–222 pmol/L (200–300 pg/mL), and >223 pmol/L (>300 pg/mL). For this analysis, we added an additional cutoff of >296 pmol/L (>400 pg/mL) to the original Stabler et al criteria (30). We classified elevated MMA using 2 concentrations: >376 nmol/L (31–35) and >271 nmol/L (24, 28, 32, 36–39). We also examined 4 categories of MMA based on the above cutoffs (>376 and >271 nmol/L) and >210 nmol/L (14, 23, 40, 41). We defined elevated tHcy as a concentration >13 μmol/L (4, 16). High serum folate was defined as a concentration >59 nmol/L.
Metabolic profiles
We defined 4 metabolic profiles based on combinations of “normal” and “abnormal” vitamin B-12 and MMA concentrations, applying the most frequently used cutoffs for vitamin B-12 (<148 pmol/L) and MMA (>271 nmol/L). The use of MMA > 210 nmol/L may falsely elevate prevalence estimates for deficiency in the elderly because the cutoff was developed in a younger reference population, whereas the use of >376 nmol/L may underestimate prevalence because it was based on 3 SD limits and/or patient samples with known low vitamin B-12 concentrations (1). Profile 1 consisted of a low serum vitamin B-12 concentration together with an elevated MMA concentration, profile 2 consisted of a low serum vitamin B-12 concentration without an elevated MMA concentration, profile 3 consisted of an elevated MMA concentration without a low vitamin B-12 concentration, and profile 4 consisted of normal vitamin B-12 and MMA concentrations.
Statistical analysis
We performed all statistical analyses using SAS (version 9; SAS Institute Inc, Cary, NC) and SAS-callable SUDAAN (version 9; Research Triangle Institute, Research Triangle Park, NC) software. We used sample weights with SUDAAN to represent the US population, account for differential nonresponse and noncoverage, and adjust for the planned oversampling of some groups. We estimated SEs for all statistics of interest by Taylor series linearization.
We estimated descriptive statistics (means, medians, and percentages/prevalences) for all variables using proc crosstab, proc descript, and/or proc regress in SAS-callable SUDAAN. We adjusted for the covariates of sex, age, and race-ethnicity. We also controlled all group comparisons of biochemical measures for length of food fasting before blood collection, session of sample collection, and serum creatinine concentration.
We compared least-squares means by examining the linear trend across the 4 vitamin B-12 and MMA status groups. We examined differences between the metabolic profiles using the effects statement (proc regress). We log-transformed nonnormal variables before statistical analysis and established significance at P < 0.05.
RESULTS
Most individuals in our sample were non-Hispanic white (72%), and about one-half (51%) were women. The mean age of the sample was 45 ± 1 y, and ≈10% of the participants were aged >60 y (data not shown). About 25% of the sample reported the use of a dietary supplement containing vitamin B-12.
The prevalence of low vitamin B-12 status ranged from 3% to 26%, depending on the serum vitamin B-12 cutoff (Table 1). When we used <148 pmol/L as the cutoff, the prevalence of low vitamin B-12 status was 2.9% and increased significantly with age, was higher in women than in men, and did not differ by race-ethnicity. When we used <200 pmol/L as the cutoff, the prevalence of low vitamin B-12 concentrations was ≈10.6% and remained higher in those aged ≥60 y than in those aged 19–39 y but did not differ from the prevalence in those aged 40–59 y. Non-Hispanic whites had a higher prevalence than did non-Hispanic blacks or Mexican Americans, and women were more likely to have a low vitamin B-12 status than were men. When <258 pmol/L was used as the cutoff, 25.7% were classified as having low vitamin B-12 status; however, the prevalence differed significantly between all 3 racial-ethnic groups and no longer differed by age. The higher prevalence of low vitamin B-12 in women remained constant across all the vitamin B-12 cutoffs.
TABLE 1.
Prevalence of low serum vitamin B-12 and high methylmalonic acid (MMA) concentrations by several cutoffs and elevated total homocysteine (tHcy) in the US adult population (≥19 y of age) by age, race-ethnicity, and sex (1999–2004)1
| No. of subjects | Serum vitamin B-12 <148 pmol/L | Serum vitamin B-12 <200 pmol/L | Serum vitamin B-12<258 pmol/L | MMA >376 nmol/L | MMA >271 nmol/L | tHcy >13 μmol/L | |
| % | % | % | % | % | % | ||
| Total sample | 12,612 | 2.9 ± 0.22 | 10.6 ± 0.4 | 25.7 ± 0.6 | 2.3 ± 0.2 | 5.8 ± 0.3 | 6.1 ± 0.3 |
| Age | |||||||
| 19–39 y | 4538 | 2.0 ± 0.2a | 9.9 ± 0.4a | 26.1 ± 0.7a | 1.2 ± 0.2a | 3.9 ± 0.4a | 2.6 ± 0.4a |
| 40–59 y | 5793 | 3.1 ± 0.3b | 10.9 ± 0.6a,b | 25.3 ± 0.8a | 2.0 ± 0.3b | 5.2 ± 0.4b | 6.6 ± 0.6b |
| ≥60 y | 2281 | 5.0 ± 0.7c | 11.4 ± 1.0b | 25.8 ± 0.9a | 7.7 ± 0.7c | 15.9 ± 1.0c | 18.1 ± 1.0c |
| Race-ethnicity | |||||||
| Non-Hispanic white | 6202 | 2.9 ± 0.2a | 11.1 ± 0.5a | 27.2 ± 0.7a | 2.2 ± 0.2a | 6.0 ± 0.4a | 6.1 ± 0.4a |
| Non-Hispanic black | 2422 | 2.8 ± 0.4a | 7.0 ± 0.6b | 16.8 ± 0.9b | 1.1 ± 0.2b | 2.4 ± 0.3b | 7.3 ± 0.5a |
| Mexican American | 2964 | 2.4 ± 0.3a | 7.0 ± 0.6b | 20.6 ± 1.6c | 2.6 ± 0.3a | 4.9 ± 0.9a | 4.7 ± 0.3b |
| Sex | |||||||
| Men | 6321 | 2.4 ± 0.2a | 9.1 ± 0.5a | 23.6 ± 0.6a | 2.5 ± 0.3a | 5.9 ± 0.4a | 7.4 ± 0.5a |
| Women | 6291 | 3.3 ± 0.3b | 12.0 ± 0.5b | 27.7 ± 0.8b | 2.2 ± 0.2a | 5.7 ± 0.3a | 4.9 ± 0.4b |
We combined the data for adults aged ≥19 y (n = 16,184) from NHANES 1999–2000, 2001–2002, and 2003–2004. We excluded data from respondents with interview data only (ie, no mobile examination center data, RIDSTATR = 1; n = 1160), high creatinine concentrations (>120 μmol/L for men, >110 μmol/L for women; n = 548), and missing MMA data (n = 918). We also excluded data for lactating (n = 145) and pregnant (n = 801) women. The analytic sample included data for 12,612 respondents. NHANES assessed serum vitamin B-12 by using the Quantaphase II radioassay from Bio-Rad (Hercules, CA), plasma MMA by using gas chromatography–mass spectrometry, and plasma homocysteine with a fluorescence polarization immunoassay reagent kit from Abbott Laboratories (Abbott Park, IL). The analysis was controlled for age, sex, race-ethnicity, serum creatinine, session of blood collection, and hours of fasting before blood collection. Values within age, race-ethnicity, and sex groups for a given status category with different superscript letters are significantly different, P ≤ 0.05. Nonnormal variables were log transformed before statistical analysis.
Least-squares mean ± SE (all such values).
The prevalence of elevated MMA was 2.3% at a cutoff of 376 nmol/L and 5.8% at a cutoff of 271 nmol/L (Table 1). Regardless of the cutoff we used, the prevalence of elevated MMA significantly increased with age, was lower in non-Hispanic blacks than in the other 2 race-ethnicity groups, and did not differ by sex. About 6% of the US adult population had elevated tHcy concentrations. The prevalence of high tHcy concentrations increased with age, was lower in Mexican Americans than in the other 2 race-ethnicity groups, and was lower in women than in men.
Vitamin B-12 status groups (modified Stabler approach)
Among the <3% of the US adult population with vitamin B-12 concentrations <148 pmol/L, only 22–44% had elevated MMA concentrations (depending on the cutoff), and only 32% had elevated tHcy concentrations (Table 2). Another 13% of US adults had serum vitamin B-12 concentrations of 148 to 222 pmol/L, and an additional 22% had vitamin B-12 concentrations of 223 to 296 pmol/L. Most US adults (62%) had serum vitamin B-12 concentrations >296 pmol/L. Of this group, only a small percentage (<10%) had elevated MMA or tHcy concentrations. Each successive cutoff rise showed a significant inverse linear trend across the vitamin B-12 groups with regard to MMA and tHcy; the prevalence of elevated MMA (regardless of the cutoff used) and tHcy decreased with increasing vitamin B-12 cutoff. RBC and serum folate concentrations increased with increasing vitamin B-12 status. We observed a trend toward increasing prevalence of high serum folate concentrations with increasing vitamin B-12 concentrations.
TABLE 2.
Categories of increasing serum vitamin B-12 concentrations and descriptions of demographic, biochemical, dietary, medication, and cognitive variables in the US population, 1999–20041
| Serum vitamin B-12 | ||||
| <148 pmol/L(<200 pg/mL) | 148–222 pmol/L(200–300 pg/mL) | 223–296 pmol/L(301–400 pg/mL) | >296 pmol/L(>400 pg/mL) | |
| No. of subjects | 390 | 1424 | 2698 | 8100 |
| Subjects per group (%) | 3 | 13 | 22 | 62 |
| Demographic characteristics2 | ||||
| Age (y) | 51 ± 13 | 44 ± 1 | 44 ± 1 | 46 ± 14 |
| >60 y (%) | 32 ± 2 | 18 ± 1 | 18 ± 1 | 22 ± 14 |
| Male sex (%) | 41 ± 2 | 44 ± 2 | 49 ± 1 | 50 ± 14 |
| Non-Hispanic white (%) | 72 ± 4 | 79 ± 2 | 74 ± 2 | 70 ± 2 |
| Non-Hispanic black (%) | 10 ± 2 | 5 ± 1 | 8 ± 1 | 12 ± 1 |
| Mexican American (%) | 6 ± 1 | 5 ± 1 | 7 ± 1 | 8 ± 1 |
| MMA (nmol/L)25 | 369 ± 311 | 187 ± 4 | 155 ± 2 | 134 ± 14 |
| High, >376 nmol/L (%) | 22 ± 2.4 | 6 ± 0.7 | 2 ± 0.3 | 1 ± 0.14 |
| High, >271 nmol/L (%) | 32 ± 3.0 | 14 ± 1.1 | 6 ± 0.5 | 3 ± 0.34 |
| High, >210 nmol/L (%) | 44 ± 3.3 | 26 ± 1.5 | 14 ± 0.7 | 8 ± 0.54 |
| Total homocysteine (μmol/L)25 | 12.6 ± 0.5 | 10.0 ± 0.2 | 8.9 ± 0.08 | 7.9 ± 0.044 |
| High, >13 μmol/L (%) | 32 ± 2.3 | 14 ± 1.1 | 7 ± 0.6 | 3 ± 0.34 |
| Folate status25 | ||||
| Red blood cell folate (nmol/L) | 559 ± 19 | 567 ± 8 | 612 ± 7 | 712 ± 74 |
| Serum folate (nmol/L) | 25.3 ± 1.1 | 25.2 ± 0.6 | 27.4 ± 0.4 | 36.0 ± 0.64 |
| High, >59 nmol/L (%) | —6 | 2.9 ± 0.6 | 4.9 ± 0.4 | 12.6 ± 0.74 |
| Diet and supplements | ||||
| User of vitamin B-12 supplements (%) | 16 ± 3 | 14 ± 1 | 18 ± 1 | 30 ± 14 |
| Vitamin B-12 (μg/d)7 | 24 ± 6 | 20 ± 3 | 37 ± 9 | 50 ± 44 |
| Vitamin B-12, diet (μg/d)2 | 4.3 ± 0.2 | 4.2 ± 0.2 | 5.1 ± 0.1 | 5.6 ± 0.14 |
| Total vitamin B-12 (μg/d)2 | 6.4 ± 1.3 | 6.5 ± 0.5 | 11.9 ± 1.7 | 20.7 ± 1.34 |
| Prescription medication use2 | ||||
| Proton pump inhibitors | 4.6 ± 1.4 | 5.9 ± 0.8 | 6.4 ± 0.6 | 5.8 ± 0.4 |
| H2-Receptor antagonists | 2.7 ± 1.0 | 2.2 ± 0.4 | 2.4 ± 0.4 | 1.8 ± 0.2 |
| Either of the above medications | 7.3 ± 1.7 | 8.1 ± 0.8 | 8.7 ± 0.7 | 7.67 ± 0.4 |
| Cognitive scores8 | 42 ± 2 | 47 ± 1 | 47 ± 1 | 48 ± 14 |
| DSC <34 points (%) | 33 ± 5 | 26 ± 3 | 25 ± 3 | 23 ± 14 |
| Age >60 y, 1999–2002 (n) | 113 | 285 | 455 | 1540 |
We combined the data for adults aged ≥19 y (n = 16,184) from NHANES 1999–2000, 2001–2002, and 2003–2004. We excluded data from respondents with interview data only (ie, no mobile examination center data, RIDSTATR = 1; n = 1160), high creatinine concentrations (>120 μmol/L for men, >110 μmol/L for women; n = 548), and missing methylmalonic acid (MMA) data (n = 918). We also excluded data for lactating (n = 145) and pregnant (n = 801) women. The analytic sample included data for 12,612 respondents. NHANES assessed serum vitamin B-12 by using the Quantaphase II radioassay from Bio-Rad (Hercules, CA), plasma MMA by using gas chromatography–mass spectrometry, and plasma homocysteine with a fluorescence polarization immunoassay reagent kit from Abbott Laboratories (Abbott Park, IL). DSC, Digit Symbol-Coding.
This analysis was controlled for age, sex, and race-ethnicity.
Least-squares mean ± SE (all such values).
Significant linear trend across vitamin B-12 groups. We log-transformed nonnormal variables before statistical analysis, P ≤ 0.05.
This analysis controlled for serum creatinine concentration, session of blood collection, and hours of fasting before blood collection.
The relative SE was >40%, which is not reliable.
Estimates are for users of dietary supplements only; total is for users and nonusers of supplements combined.
Based on the DSC subtest of the Wechsler Adult Intelligence Scale III (The Psychological Corporation, San Antonio, TX). Cognitive function scores were available for those aged ≥60 y in NHANES 1999–2002 (n = 2393).
The use of vitamin B-12–containing dietary supplements and the amount of vitamin B-12 that users consumed was highest in respondents with serum vitamin B-12 concentrations >296 pmol/L. Vitamin B-12 intake from the diet and total vitamin B-12 intake (from diet + supplements in users and nonusers combined) increased with serum vitamin B-12 status. The use of proton pump inhibitors and H2-receptor antagonists had no relation with vitamin B-12 status. Cognitive function scores increased, and the prevalence of impairment decreased across increasing serum vitamin B-12 concentrations for adults aged ≥60 y. We found no significant differences in mean corpuscular volume (MCV) or in the prevalence of macrocytosis across vitamin B-12 groupings (data not shown).
MMA status groups
We examined the data for 4 groups defined by their MMA concentrations (Table 3). Very few people had MMA concentrations >376 nmol/L (2.3%), but 27–66% of this group had a low serum vitamin B-12 concentration (depending on the vitamin B-12 cutoff); another 3% had MMA concentrations of 272 to 376 nmol/L, and yet another 8% had concentrations of 210 to 271 nmol/L. Most (≈86%) had MMA concentrations <210 nmol/L; of this group, ≈2% had low vitamin B-12 concentrations (<148 pmol/L).
TABLE 3.
Categories of decreasing plasma methylmalonic acid (MMA) concentrations and descriptions of demographic, biochemical, dietary, medication, and cognitive variables in the US population, 1999–20041
| Plasma MMA | ||||
| >376 nmol/L | 272–376 nmol/L | 210–271 nmol/L | <210 nmol/L | |
| No. of subjects | 375 | 451 | 1042 | 10,744 |
| Subjects per group (%) | 2.3 | 3.4 | 8.1 | 86.2 |
| Demographic characteristic2 | ||||
| Age (y) | 57 ± 13 | 52 ± 1 | 51 ± 1 | 44 ± 14 |
| >60 y (%) | 47 ± 4 | 38 ± 3 | 34 ± 2 | 18 ± 14 |
| Male sex (%) | 53 ± 4 | 50 ± 3 | 52 ± 2 | 49 ± 1 |
| Non-Hispanic white (%) | 69 ± 4 | 80 ± 3 | 78 ± 2 | 71 ± 2 |
| Non-Hispanic black (%) | 4 ± 1 | 4 ± 1 | 6 ± 1 | 11 ± 14 |
| Mexican American (%) | 8 ± 2 | 4 ± 1 | 5 ± 1 | 7 ± 1 |
| Serum vitamin B-12 (pmol/L)25 | 224 ± 11 | 294 ± 11 | 339 ± 21 | 406 ± 94 |
| <148 pmol/L (%)25 | 27 ± 3 | 9 ± 2 | 5 ± 1 | 2 ± 0.14 |
| <200 pmol/L (%)25 | 49 ± 3 | 33 ± 3 | 19 ± 2 | 8 ± 0.44 |
| <258 pmol/L (%)25 | 66 ± 3 | 50 ± 3 | 40 ± 2 | 19 ± 14 |
| Total homocysteine (μmol/L)25 | 13.4 ± 1 | 10.5 ± 0.2 | 9.4 ± 0.1 | 8.3 ± 0.54 |
| High, >13 μmol/L (%) | 40 ± 4 | 18 ± 2 | 10 ± 1 | 4 ± 0.34 |
| Folate status25 | ||||
| Red blood cell folate (nmol/L) | 550 ± 18 | 600 ± 21 | 653 ± 11 | 674 ± 74 |
| Serum folate (nmol/L) | 28.6 ± 1.2 | 28.4 ± 1.2 | 29.8 ± 0.8 | 32.9 ± 0.54 |
| High folate, >59 nmol/L (%) | —6 | 8.3 ± 1.5 | 7.3 ± 1.1 | 9.7 ± 0.54 |
| Diet and supplements | ||||
| User of vitamin B-12 supplements (%) | 13 ± 3 | 18 ± 3 | 21 ± 2 | 26 ± 14 |
| Vitamin B-12 (μg/d)7 | 29 ± 5 | 40 ± 9 | 43 ± 9 | 45 ± 44 |
| Vitamin B-12, diet (μg/d) | 5.0 ± 0.7 | 4.7 ± 0.2 | 4.6 ± 0.2 | 5.3 ± 0.14 |
| Total vitamin B-12 (μg/d) | 5.6 ± 1.8 | 9.6 ± 1.6 | 12.2 ± 2.2 | 17.5 ± 1.24 |
| Prescription medication use (%)2 | ||||
| Proton pump inhibitors | 3.3 ± 1.2 | 4.8 ± 1.2 | 7.9 ± 1.0 | 5.8 ± 0.3 |
| H2-Receptor antagonists | —6 | 3.5 ± 1.1 | 2.4 ± 0.6 | 1.9 ± 0.2 |
| Either of the above medications | 4.8 ± 1.6 | 8.3 ± 1.5 | 10.3 ± 1.2 | 7.7 ± 0.3 |
| Cognitive scores38 | 41 ± 2 | 42 ± 1 | 47 ± 1 | 48 ± 14 |
| DSC <34 points (%) | 41 ± 6 | 34 ± 4 | 28 ± 4 | 22 ± 14 |
| Age >60 y, 1999–2002 (n) | 135 | 120 | 306 | 1832 |
We combined the data for adults aged ≥19 y (n = 16,184) from NHANES 1999–2000, 2001–2002, and 2003–2004. We excluded data from respondents with interview data only (ie, no mobile examination center data, RIDSTATR = 1; n = 1160), high creatinine concentrations (>120 μmol/L for men, >110 μmol/L for women; n = 548), and missing MMA data (n = 918). We also excluded data for lactating (n = 145) and pregnant (n = 801) women. The analytic sample included data for 12,612 respondents. NHANES assessed serum vitamin B-12 by using the Quantaphase II radioassay from Bio-Rad (Hercules, CA), plasma MMA by using gas chromatography–mass spectrometry, and plasma homocysteine with a fluorescence polarization immunoassay reagent kit from Abbott Laboratories (Abbott Park, IL). DSC, Digit Symbol-Coding.
This analysis was controlled for age, sex, and race-ethnicity.
Least-squares mean ± SE (all such values).
Significant linear trend across MMA groups. We log-transformed nonnormal variables before statistical analysis, P ≤ 0.05.
This analysis controlled for serum creatinine concentration, session of blood collection, and hours of fasting before blood collection.
The relative SE was >40%, which is not reliable.
Estimates are for users of dietary supplements only; total is for users and nonusers of supplements combined.
Based on the DSC subtest of the Wechsler Adult Intelligence Scale III (The Psychological Corporation, San Antonio, TX). Cognitive function scores were available for those aged ≥60 y in NHANES 1999–2002 (n = 2393).
As MMA increased, so did the mean age of the groups and the prevalence of those aged >60 y. The percentage of the group that was of non-Hispanic black ethnicity decreased as MMA concentrations increased. The prevalence of low vitamin B-12 status increased across increasing MMA concentration categories with all cutoffs. Similarly, tHcy and the percentage of US adults with elevated tHcy concentrations increased with increasing MMA concentration categories. We found no differences in MCV concentrations or in the prevalence of macrocytosis (data not shown). Both serum and RBC folate concentrations decreased across increasing MMA categories. We observed a trend toward increasing prevalence in those with high serum folate with decreasing MMA concentrations.
Use of dietary supplements containing vitamin B-12 and vitamin B-12 intake from supplements increased with decreasing MMA concentration categories. Dietary vitamin B-12 intakes had no relation with MMA concentrations, but total vitamin B-12 dietary exposure had a significant inverse relation with MMA concentrations. The use of proton pump inhibitor or H2-receptor antagonist medications had no relation with MMA concentration categories. Cognitive function scores decreased and the prevalence of impairment increased with increasing MMA categories. About 40% of those with the highest MMA concentrations (>376 nmol/L) had cognitive impairment.
Metabolic profiles based on MMA and vitamin B-12 biomarkers together
About 1% of the adult US adult population had low serum vitamin B-12 and elevated MMA concentrations (profile 1; Table 4); in addition to the very high MMA concentration (mean ± SE: 813 ± 93 nmol/L), tHcy was also quite elevated (mean: 18 μmol/L); 65% had concentrations >13 μmol. This profile thus reflected vitamin B-12 deficiency, often of more than a mild degree. Profile 2 (low vitamin B-12 with normal MMA concentrations, 2%) and profile 3 (normal vitamin B-12 with elevated MMA concentrations, 5%) were slightly more common. Profile 4, making up 92% of the study population, had both normal vitamin B-12 and MMA, which suggests that this profile reflected normal vitamin B-12 status.
TABLE 4.
Profiles of serum vitamin B-12 and plasma methylmalonic acid (MMA) by various covariables in the United States, 1999–20041
| Profile 1:vitamin B-12 <148 pmol/L,MMA >271 nmol/L | Profile 2:vitamin B-12 <148 pmol/L,MMA ≤271 nmol/L | Profile 3:vitamin B-12 ≥148 pmol/L,MMA >271 nmol/L | Profile 4:vitamin B-12 ≥148 pmol/L,MMA ≤271 nmol/L | |
| No. of subjects | 143 | 247 | 683 | 11,539 |
| Subjects per group (%) | 1 | 2 | 5 | 92 |
| Demographic characteristic2 | ||||
| Age (y) | 58 ± 1a,c,33 | 47 ± 1b | 53 ± 1c | 45 ± 1d |
| >60 y (%) | 51 ± 6a | 23 ± 2b | 40 ± 2a | 19 ± 1b |
| Male sex (%) | 51 ± 5a | 36 ± 3b | 51 ± 2a | 49 ± 1a |
| Non-Hispanic white (%) | 78 ± 1a | 71 ± 2a | 77 ± 1a | 72 ± 1a |
| Non-Hispanic black (%) | 3 ± 1a | 13 ± 2b | 4 ± 1a | 11 ± 1b |
| Mexican American (%) | 5 ± 1.5a | 5 ± 1a | 5 ± 1a | 7 ± 1a |
| Serum vitamin B-12 (nmol/L)24 | 101 ± 10a | 130 ± 5b | 296 ± 9c | 404 ± 9d |
| MMA (nmol/L)24 | 813 ± 93a | 159 ± 4b | 381 ± 8c | 132 ± 1d |
| Total homocysteine (μmol/L)24 | 18.0 ± 1.3a | 10.4 ± 0.4b | 11.0 ± 0.3b | 8.3 ± 0.1c |
| High, >13 μmol/L (%)4 | 65 ± 4a | 17 ± 3b | 22 ± 2b | 4 ± 0.3c |
| Folate status24 | ||||
| Red blood cell folate (nmol/L) | 476 ± 33a | 597 ± 21b | 601 ± 16b | 673 ± 7c |
| Serum folate (nmol/L) | 26 ± 1.9a,b | 25 ± 1.3a | 29 ± 1.0b | 33 ± 0.5c |
| High, >59 nmol/L (%) | —5 | 4.3 ± 1.7a | 7.3 ± 1.1b | 9.6 ± 0.5b |
| Diet and supplements | ||||
| User of vitamin B-12 supplements (%) | 16 ± 4a | 16 ± 3a | 16 ± 2a | 26 ± 1b |
| Vitamin B-12 (μg/d)6 | 13 ± 5a | 23 ± 7a | 34 ± 7b | 46 ± 4c |
| Vitamin B-12, diet (μg/d)2 | 4.5 ± 0.5a,b | 4.2 ± 0.3a | 4.9 ± 0.4a,b | 5.3 ± 0.1b |
| Total vitamin B-12 (μg/d)2 | 3.2 ± 1.3a | 7.9 ± 1.7b | 9.2 ± 1.2b | 17.2 ± 1.0c |
| Prescription medication use (%)2 | ||||
| Proton pump inhibitors | —5 | 5.2 ± 1.8a,b | 4.4 ± 1.0b | 6.0 ± 0.3a |
| H2-receptor antagonists | —5 | 3.3 ± 1.3a | 2.9 ± 0.9a | 1.9 ± 0.2a |
| Either of the above medications | —5 | 8.5 ± 2.9a | 7.3 ± 1.1a | 8.0 ± 0.4a |
| Cognitive scores27 | 38 ± 2a | 44 ± 2a,b | 43 ± 1a | 48 ± 1b |
| DSC <34 points (%) | 40 ± 7a | 28 ± 4a,b | 36 ± 5a | 22 ± 2b |
| Age >60 y, 1999–2002 (n) | 55 | 58 | 200 | 2080 |
We combined the data for adults aged ≥19 y (n = 16,184) from NHANES 1999–2000, 2001–2002, and 2003–2004. We excluded data from respondents with interview data only (ie, no mobile examination center data, RIDSTATR = 1; n = 1160), high creatinine concentrations (>120 μmol/L for men, >110 μmol/L for women; n = 548), and missing MMA data (n = 918). We also excluded data for lactating (n = 145) and pregnant (n = 801) women. The analytic sample included data for 12,612 respondents. NHANES assessed serum vitamin B-12 by using the Quantaphase II radioassay from Bio-Rad (Hercules, CA), plasma MMA by using gas chromatography–mass spectrometry, and plasma homocysteine with a fluorescence polarization immunoassay reagent kit from Abbott Laboratories (Abbott Park, IL). Differences between the metabolic profiles were examined by using the effects statement (proc regress). Means across profiles with different superscript letters are significantly different, P ≤ 0.05. Nonnormal variables were log transformed before statistical analysis. DSC, Digit Symbol-Coding.
This analysis was controlled for age, sex, and race-ethnicity.
Least-squares mean ± SE (all such values).
This analysis was controlled for serum creatinine, session of blood collection, and hours of fasting before blood collection.
The relative SE was >40%, which is not reliable.
Estimates are for users of dietary supplements only; total is for users and nonusers of supplements combined.
Based on the DSC subtest of the Wechsler Adult Intelligence Scale III (The Psychological Corporation, San Antonio, TX). Cognitive function scores were available for those aged ≥60 y in NHANES 1999–2002 (n = 2393).
Falling between the 2 poles, profile 2 (low vitamin B-12 with normal MMA) and profile 3 (normal vitamin B-12 with elevated MMA) accounted for 2% and 5% of the population, respectively. Demographically, profile 3 resembled the vitamin B-12–deficient profile 1 in mean age, proportion >60 y of age, sex distribution, and ethnic distribution, including a low proportion of non-Hispanic blacks. Profile 2, on the other hand, resembled the vitamin B-12–sufficient profile 4 in having the fewest subjects >60 y of age and in ethnic distribution; profile 2 also had the lowest proportion of men in the study.
Serum vitamin B-12 and MMA differed significantly, in part, as expected from selection, but also unexpectedly because even the “normal” values in profiles 2 and 3 differed from the normal values in profile 4 and the “abnormal” values differed from profile 1 (Table 4). Profiles 2 and 3 had similar concentrations of tHcy, with a mean within the reference interval, but differed significantly from both profiles 1 and 4. The same pattern was evident for RBC folate concentrations. Serum folate concentrations were highest in profile 4, whereas profile 1 did not differ from profiles 2 and 3; the percentage of high serum folate was lower in profile 2 than in profiles 3 or 4, however.
Subjects in profile 4 reported using dietary supplements containing vitamin B-12 more than did subjects in the other profiles and had the highest intake of vitamin B-12 from supplements (Table 4). Dietary intakes of vitamin B 12 did not differ greatly across profiles, but total intakes (diet plus supplements) were highest in profile 4. Proton pump inhibitor use was greater in profile 4 than in profile 3, but was not different from profile 2; no differences were observed for the reported use of H2-receptor antagonists. Mean cognitive scores were higher and the prevalence of low scores was lower in profile 4 than in profile 1, but did not differ from profile 2.
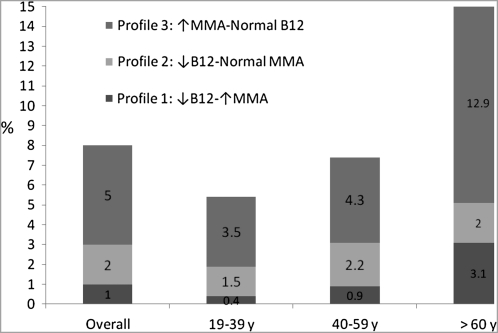
The prevalence of metabolic profiles 1–3 increased with age (Figure 1). About 21% of elderly adults (>60 y) had at least one abnormal functional or circulating biomarker of vitamin B-12 status. An elevated MMA concentration alone was more common than either a low serum vitamin B-12 concentration or the combination of a low serum vitamin B-12 and an elevated MMA concentration.
FIGURE 1.
The prevalence of different metabolic profiles of biomarkers of vitamin B-12 (B12) status. Shaded bars show least-squares means for respondents with low vitamin B-12 (<148 pmol/L) and normal methylmalonic acid (MMA; <271 nmol/L) concentrations, elevated MMA (>271 nmol/L) and normal vitamin B-12 (>148 pmol/L) concentrations, and low vitamin B-12 (<148 pmol/L) and elevated MMA (>271 nmol/L) concentrations for the US population and by age groups (NHANES, 1999–2004). We combined the data for adults aged ≥19 y (n = 16,184) from NHANES 1999–2000, 2001–2002, and 2003–2004. We excluded data from respondents with interview data only (ie, no mobile examination center data, RIDSTATR = 1; n = 1160), high creatinine concentrations (>120 μmol/L for men, >110 μmol/L for women; n = 548), and missing MMA data (n = 918). We also excluded data for lactating (n = 145) and pregnant (n = 801) women. The analytic sample included data for 12,612 respondents. NHANES assessed serum vitamin B-12 by using the Quantaphase II radioassay from Bio-Rad (Hercules, CA), plasma MMA by using gas chromatography–mass spectrometry, and plasma homocysteine with a fluorescence polarization immunoassay reagent kit from Abbott Laboratories (Abbott Park, IL). Our analysis controlled for age, sex, race-ethnicity, serum creatinine concentration, session of blood collection, and hours of fasting before blood collection.
DISCUSSION
The nationally representative data in this article confirm most of the broader, previously reported demographic findings relevant to vitamin B-12 status (ie, relations with age, race, and sex). We extend that existing information by exploring in greater depth the influence of cutoffs on population outcomes and characteristics and by analyzing the interactions between concurrent vitamin B-12 and MMA changes more closely because of the diagnostic limitations of every biomarker when it stands alone.
Four metabolic profiles have emerged from an examination of the concurrent use of serum vitamin B-12 and MMA concentrations to assess vitamin B-12 status in NHANES (Table 4, Figure 1). Profile 1 had a high likelihood of vitamin B-12 deficiency; although the prevalence was low (1%, one-half of whom were >60 y of age), it represented a large absolute number of Americans (n = 1,694,748). Moreover, the high mean MMA (813 nmol/L) and tHcy (18 μmol/L) concentrations suggest that many of the subjects had more advanced vitamin B-12 deficiency and were at a considerable health risk if undiagnosed and untreated. Indeed, the prevalence resembled that of pernicious anemia in the elderly (42). Profiles 2 and 3, each with one normal and one abnormal biomarker, were more problematic but also presented a more important challenge to resolve because their respective 2% and 5% prevalences far exceeded the 1% prevalence of profile 1. Profile 4 included 92% of the population and probably represented vitamin B-12 adequacy because vitamin B-12 and MMA (and tHcy) are all within usual reference ranges. Profiles 2 and 3 were similar in their intermediacy between the healthy profile 4 and the deficient profile 1, but they may reflect different underlying metabolic processes and types of affected persons. Profile 2, with isolated vitamin B-12 abnormality, may have mainly represented falsely low vitamin B-12 concentrations; the mean MMA (and tHcy) concentrations were slightly higher than those in the presumably nondeficient profile 4, but they were nevertheless far below the most liberal cutoff for abnormality. Profile 2 also resembled the “normal” profile 4, demographically, much more closely than it resembled profile 1, for which the age was higher and the frequency of non-Hispanic blacks was lower. As summarized elsewhere, ≥22% of individuals with vitamin B-12 concentrations <148 pmol/L in surveys had no deficiency, as measured by other metabolic tests, and thus were likely to have low vitamin B-12 concentrations secondary to another cause (5, 7). Profile 2 may include individuals with an often genetic TC I deficiency, the frequency of which may be higher than that of pernicious anemia, and in whom serum vitamin B-12 is low but MMA and tHcy are not abnormal (39) or individuals who lack the common fucosyltransferase polymorphism associated with higher vitamin B-12 concentrations (43). Given the high proportion of younger women in profile 2, many of the low vitamin B-12 concentrations may have been affected by hormonal factors unrelated to functional vitamin B-12 status. For example, in women aged 19–39 y, oral contraceptive users had significantly lower mean serum vitamin B-12 concentrations than did nonusers (290 compared with 412 pmol/L; P < 0.001; data not shown). Additional heterogeneity must also be considered; 17% of profile 2 subjects had elevated tHcy, and some may have had mild vitamin B-12 deficiency, despite having normal MMA concentrations.
Profile 3 was characterized by a high MMA concentration alone; serum vitamin B-12 concentrations were within the defined normal range. This group may have reflected the superior sensitivity of MMA to vitamin B-12 in mild, purely biochemical vitamin B-12 deficiency (44), which may predominate in profile 3, as also suggested by the demographic similarities of profile 3 to profile 1; the significantly lower MMA elevation than in profile 1 suggests that the vitamin B-12 deficiency, if present, was milder. However, others in profile 3 may also have represented falsely elevated MMA concentrations for reasons not related to vitamin B-12 status (5). Our results indicate that only 22% of profile 3 had an elevated tHcy concentration. It is also notable that, within profile 3, those with elevated tHcy were older (63 compared with 50 y), were more likely to be male (57% compared with 47%), were more likely to be non-Hispanic black (6% compared with 3%), and had a lower mean vitamin B-12 concentration (249 compared with 309 pmol/L) compared with those without elevated tHcy (differences were statistically significant, data not shown). Taken together, these findings suggest that individuals with vitamin B-12 insufficiency may represent only a minor fraction of those with metabolic profile 3. Vogiatzoglou et al (9) found that identified determinants (age, creatinine, and serum vitamin B-12) accounted for <17% of the variation in plasma MMA.
MMA concentrations are highly dependent on kidney function and serum creatinine (9), even within the normal range (45). We excluded those with high serum creatinine concentrations, which are indicative of serious renal impairment, but also recognized that some residual effect on MMA concentrations might occur in persons with milder renal impairment. For this reason, we also statistically controlled every analysis for serum creatinine concentrations.
Misclassification of vitamin B-12 status
The consequences of extending the cutoff for serum vitamin B-12 beyond 148 pmol/L are clearly shown in Table 2. Only 6–26% (depending on demographic subset) of the added cohort with serum vitamin B-12 concentrations of 148 to 222 pmol/L had abnormal MMA concentrations and only 14% had elevated tHcy concentrations, and the loss of diagnostic specificity was even greater for the added cohort with vitamin B-12 concentrations of 223 to 296 pmol/L (2–14% abnormal MMA and 7% abnormal tHcy). Approximately 80% of the subjects in the 2 extended vitamin B-12 interval categories did not have an elevated MMA concentration (based on >271 nmol), and 79% did not have an elevated tHcy concentration. The findings suggest an unacceptable degree of false-positive misclassification of vitamin B-12 insufficiency when a vitamin B-12 cutoff > 148 pmol/L is used.
Cutoff challenges
The use of biomarker cutoffs of vitamin B-12 to estimate the prevalence of inadequate nutrient status is challenging. As our data show (Table 1), different cutoffs result in very different prevalence estimates (3–26% for low serum vitamin B-12 and 2.3–5.8% for elevated MMA); the prevalence estimates change to a greater degree for serum vitamin B-12 than for plasma MMA. Moving the vitamin B-12 cutoff up in 25–30% increments from 148 to 200 and then to 258 pmol/L increases the rates of subjects identified as low vitamin B-12 from 2.9% to 10.6% and then to 25.7%, respectively (Table 1). Moving MMA cutoffs down by a similar percentage from 376 to 271 nmol/L had smaller effect on rates of subjects identified as “high MMA” (from 2.3% to 5.8%; Table 1). Only when the MMA cutoff was set at 210 nmol/L did the rate of abnormality reach 13.8% (Table 3). The MMA cutoff shift also did not shift the demographic distribution as much as did changes in vitamin B-12 cutoff.
Cognitive function
In older adults, the prevalence of cognitive impairment was higher with lower vitamin B-12 status and elevated MMA concentrations. We saw a significant linear trend with both raw scores on cognitive function and the prevalence of cognitive impairment across vitamin B-12 and MMA status (Tables 2 and 3). About 40% of the highest MMA group (>376 nmol/L) had cognitive impairment (Table 3). The 2 metabolic profiles with elevated MMA concentrations had lower cognitive scores and a higher prevalence of impairment than did the profiles without elevated MMA concentrations (Table 4). On this basis, it is tempting to conclude that MMA may be a better biomarker for cognitive function than serum vitamin B-12 in older adults. However, these data must be interpreted with caution. First, NHANES data are cross-sectional in nature, so we cannot infer a cause-and-effect relation between MMA concentrations and cognitive function. Second, important confounders may be attributed to the relations; individuals in the profiles with poorer cognitive function were also older. Third, cognitive status is complex and difficult to measure; it is related to educational and socioeconomic status—neither of which were examined here. Thus, more data are needed before we can accurately interpret the relation between MMA and cognitive function in older adults. In NHANES 2013–2014, a more complete battery of cognitive function tests will be available.
Study limitations
The ideal approach for defining appropriate cutoffs for NHANES and other applications would be to conduct clinical trials in which a quantitative relation between biomarker concentrations and the appearance of clinical outcomes of presumed vitamin B-12 inadequacy is established and in which the measurement of the biomarkers is traceable to a higher order reference method (46). Unfortunately, this type of information cannot be obtained from a cross-sectional study such as NHANES, which cannot establish causality between biomarker concentrations and outcomes. However, our findings may prove useful for informing the design of interventional population-based trials designed to assess the effects of vitamin B-12 repletion through supplementation.
Currently, no consensus exists on the appropriate cutoffs for assessing serum vitamin B-12 and MMA status, particularly for population-based monitoring purposes. The approach and criteria used, the study population, and the measurement procedure can all affect the relevance of published cutoffs. The most appropriate cutoffs for assessing vitamin B-12 status when using NHANES data remain elusive, which has resulted in considerable uncertainty about the vitamin B-12 status of the US population. Future studies should seek to determine a definable clinical endpoint to achieve this goal. Until this is established, we cannot accurately estimate the public health burden of vitamin B-12 deficiency in the United States.
Acknowledgments
The authors' responsibilities were as follows—All authors contributed to the concept development and manuscript preparation and read and approved the final version of this manuscript. RC has submitted a patent application for work related to transcobalamin I deficiency and its genetic aspects. RG is a consultant for Emisphere and served on the scientific advisory board of Par Pharmaceutical Inc during the past year. None of the other authors had a personal or financial conflict of interest.
REFERENCES
- 1.Lindenbaum J, Rosenberg IH, Wilson PW, Stabler SP, Allen RH. Prevalence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr 1994;60:2–11 [DOI] [PubMed] [Google Scholar]
- 2.Pennypacker LC, Allen RH, Kelly JP, et al. High prevalence of cobalamin deficiency in elderly outpatients. J Am Geriatr Soc 1992;40:1197–204 [PubMed] [Google Scholar]
- 3.Yetley EA, Pfeiffer CP, Phinney KW, et al. Biomarkers of vitamin B-12 status in NHANES: a roundtable summary. Am J Clin Nutr (Epub ahead of print 25 May 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green R, Miller JW. Vitamin B12 deficiency is the dominant nutritional cause of hyperhomocysteinemia in a folic acid-fortified population. Clin Chem Lab Med 2005;43:1048–51 [DOI] [PubMed] [Google Scholar]
- 5.Carmel R. Biomarkers of cobalamin (vitamin B-12) status in the epidemiologic setting: a critical overview of context, applications, and performance characteristics of cobalamin, methylmalonic acid, and holotranscobalamin II. Am J Clin Nutr (Epub ahead of print 25 May 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmel R, Sarrai M. Diagnosis and management of clinical and subclinical cobalamin deficiency: advances and controversies. Curr Hematol Rep 2006;5:23–33 [DOI] [PubMed] [Google Scholar]
- 7.Carmel R, Green R, Rosenblatt DS, Watkins D. Update on cobalamin, folate, and homocysteine. Hematology Am Soc Hematol Educ Program 2003;62–81 [DOI] [PubMed] [Google Scholar]
- 8.Response rates & CPS population totals, National Health and Nutrition Examination Survey National Center for Health Statistics. Available from: http://www.cdc.gov/nchs/nhanes/response_rates_cps.htm (cited 19 March 2011) [Google Scholar]
- 9.Vogiatzoglou A, Oulhaj A, Smith AD, et al. Determinants of plasma methylmalonic acid in a large population: implications for assessment of vitamin B12 status. Clin Chem 2009;55:2198–206 [DOI] [PubMed] [Google Scholar]
- 10.Bailey RL, Dodd KW, Gahche JJ, et al. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003-2006. Am J Clin Nutr 2010;91:231–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Food and Nutrition Board Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press, 1998 [PubMed] [Google Scholar]
- 12.Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio, TX: Harcourt Brace and Company, 1997 [Google Scholar]
- 13. National Center for Health Statistics National Health and Nutrition Examination Survey. Household Interview Questionnaire. 2004. Available from: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm (cited 19 March 2011) [Google Scholar]
- 14.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors. Am J Clin Nutr 2010;91:1733–44 [DOI] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics Datasets and related documentation. Available from: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm (cited 19 March 2011)
- 16.Pfeiffer CM, Osterloh JD, Kennedy-Stephenson J, et al. Trends in circulating concentrations of total homocysteine among US adolescents and adults: findings from the 1991-1994 and 1999-2004 National Health and Nutrition Examination Surveys. Clin Chem 2008;54:801–13 [DOI] [PubMed] [Google Scholar]
- 17.Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988-1994, 1999-2004. Am J Kidney Dis 2007;50:918–26 [DOI] [PubMed] [Google Scholar]
- 18.National Center for Health Statistics National Health and Nutrition Examination Survey. Laboratory methods, 1999-2000. Available from: http://www.cdc.gov/nchs/nhanes/lab99_00.htm (cited 19 March 2011)
- 19.National Center for Health Statistics National Health and Nutrition Examination Survey. Laboratory methods, 2001-2002. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2001-2002/lab01_02.htm (cited 19 March 2011)
- 20.National Center for Health Statistics National Health and Nutrition Examination Survey. Laboratory methods, 2003-2004. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/lab03_04.htm (cited 19 March 2011)
- 21.Selhub J, Morris MS, Jacques PF. In vitamin B12 deficiency, higher serum folate is associated with increased total homocysteine and methylmalonic acid concentrations. Proc Natl Acad Sci USA 2007;104:19995–20000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Elevated serum methylmalonic acid concentrations are common among elderly Americans. J Nutr 2002;132:2799–803 [DOI] [PubMed] [Google Scholar]
- 23.Pfeiffer CM, Caudill SP, Gunter EW, Osterloh J, Sampson EJ. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999-2000. Am J Clin Nutr 2005;82:442–50 [DOI] [PubMed] [Google Scholar]
- 24.Vanderjagt DJ, Ujah IA, Patel A, et al. Subclinical vitamin B12 deficiency in pregnant women attending an antenatal clinic in Nigeria. J Obstet Gynaecol 2009;29:288–95 [DOI] [PubMed] [Google Scholar]
- 25.Wahlin A, Hill RD, Winblad B, Backman L. Effects of serum vitamin B12 and folate status on episodic memory performance in very old age: a population-based study. Psychol Aging 1996;11:487–96 [DOI] [PubMed] [Google Scholar]
- 26.Clarke R, Grimley Evans J, Schneede J, et al. Vitamin B12 and folate deficiency in later life. Age Ageing 2004;33:34–41 [DOI] [PubMed] [Google Scholar]
- 27.Hvas AM, Nexo E. Holotranscobalamin as a predictor of vitamin B12 status. Clin Chem Lab Med 2003;41:1489–92 [DOI] [PubMed] [Google Scholar]
- 28.Johnson MA, Hausman DB, Davey A, Poon LW, Allen RH, Stabler SP. Vitamin B12 deficiency in African American and white octogenarians and centenarians in Georgia. J Nutr Health Aging 2010;14:339–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson J, Iemolo F, Stabler SP, Allen RH, Spence JD. Vitamin B12, homocysteine and carotid plaque in the era of folic acid fortification of enriched cereal grain products. CMAJ 2005;172:1569–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stabler SP. Screening the older population for cobalamin (vitamin B12) deficiency. J Am Geriatr Soc 1995;43:1290–7 [DOI] [PubMed] [Google Scholar]
- 31.Savage DG, Lindenbaum J, Stabler SP, Allen RH. Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am J Med 1994;96:239–46 [DOI] [PubMed] [Google Scholar]
- 32.Allen RH, Stabler SP, Savage DG, Lindenbaum J. Diagnosis of cobalamin deficiency I: usefulness of serum methylmalonic acid and total homocysteine concentrations. Am J Hematol 1990;34:90–8 [DOI] [PubMed] [Google Scholar]
- 33.Lindenbaum J, Savage DG, Stabler SP, Allen RH. Diagnosis of cobalamin deficiency: II. Relative sensitivities of serum cobalamin, methylmalonic acid, and total homocysteine concentrations. Am J Hematol 1990;34:99–107 [DOI] [PubMed] [Google Scholar]
- 34.Stabler SP, Allen RH, Savage DG, Lindenbaum J. Clinical spectrum and diagnosis of cobalamin deficiency. Blood 1990;76:871–81 [PubMed] [Google Scholar]
- 35.Subar AF, Block G. Use of vitamin and mineral supplements: demographics and amounts of nutrients consumed. The 1987 Health Interview Survey. Am J Epidemiol 1990;132:1091–101 [DOI] [PubMed] [Google Scholar]
- 36.Rajan S, Wallace JI, Beresford SA, Brodkin KI, Allen RA, Stabler SP. Screening for cobalamin deficiency in geriatric outpatients: prevalence and influence of synthetic cobalamin intake. J Am Geriatr Soc 2002;50:624–30 [DOI] [PubMed] [Google Scholar]
- 37.Stabler SP, Allen RH, Fried LP, et al. Racial differences in prevalence of cobalamin and folate deficiencies in disabled elderly women. Am J Clin Nutr 1999;70:911–9 [DOI] [PubMed] [Google Scholar]
- 38.Kuzminski AM, Del Giacco EJ, Allen RH, Stabler SP, Lindenbaum J. Effective treatment of cobalamin deficiency with oral cobalamin. Blood 1998;92:1191–8 [PubMed] [Google Scholar]
- 39.Park S, Johnson MA, Shea-Miller K, De Chicchis AR, Allen RH, Stabler SP. Age-related hearing loss, methylmalonic acid, and vitamin B12 status in older adults. J Nutr Elder 2006;25:105–20 [DOI] [PubMed] [Google Scholar]
- 40.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr 2007;85:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selhub J, Morris MS, Jacques PF, Rosenberg IH. Folate-vitamin B-12 interaction in relation to cognitive impairment, anemia, and biochemical indicators of vitamin B-12 deficiency. Am J Clin Nutr 2009;89:702S–6S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carmel R. Prevalence of undiagnosed pernicious anemia in the elderly. Arch Intern Med 1996;156:1097–100 [PubMed] [Google Scholar]
- 43.Hazra A, Kraft P, Selhub J, et al. Common variants of FUT2 are associated with plasma vitamin B12 levels. Nat Genet 2008;40:1160–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carmel R, Green R, Jacobsen DW, Rasmussen K, Florea M, Azen C. Serum cobalamin, homocysteine, and methylmalonic acid concentrations in a multiethnic elderly population: ethnic and sex differences in cobalamin and metabolite abnormalities. Am J Clin Nutr 1999;70:904–10 [DOI] [PubMed] [Google Scholar]
- 45.Hvas AM, Juul S, Gerdes LU, Nexo E. The marker of cobalamin deficiency, plasma methylmalonic acid, correlates to plasma creatinine. J Intern Med 2000;247:507–12 [DOI] [PubMed] [Google Scholar]
- 46.Vesper HW, Thienpont LM. Traceability in laboratory medicine. Clin Chem 2009;55:1067–75 [DOI] [PubMed] [Google Scholar]