Abstract
The ability to develop evidence-based clinical guidance and effective programs and policies to achieve global health promotion and disease prevention goals depends on the availability of valid and reliable data. With specific regard to the role of food and nutrition in achieving those goals, relevant data are developed with the use of biomarkers that reflect nutrient exposure, status, and functional effect. A need exists to promote the discovery, development, and use of biomarkers across a range of applications. In addition, a process is needed to harmonize the global health community's decision making about what biomarkers are best suited for a given use under specific conditions and settings. To address these needs, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services, organized a conference entitled “Biomarkers of Nutrition for Development: Building a Consensus,” which was hosted by the International Atomic Energy Agency. Partners included key multilateral, US agencies and public and private organizations. The assembly endorsed the utility of this initiative and the need for the BOND (Biomarkers of Nutrition for Development) project to continue. A consensus was reached on the requirement to develop a process to inform the community about the relative strengths or weaknesses and specific applications of various biomarkers under defined conditions. The articles in this supplement summarize the deliberations of the 4 working groups: research, clinical, policy, and programmatic. Also described are content presentations on the harmonization processes, the evidence base for biomarkers for 5 case-study micronutrients, and new frontiers in science and technology.
INTRODUCTION
The global health community has increasingly recognized the integral role of food and nutrition in health maintenance and disease prevention. It is estimated that, globally, maternal and child undernutrition results in 3.5 million deaths per year and accounts for 35% of the disease burden in children <5 y of age (1). Within that burden of undernutrition is the “hidden hunger” of single and multiple micronutrient insufficiencies affecting ≈2 billion individuals in both developed and developing countries (2). These micronutrient deficiencies occur in both overweight and underweight individuals (3). Coincidentally, overweight and obesity are becoming more prevalent, with an estimated one billion adults and 22 million children classified as overweight (4). Thus, the “dual burden” of over- and undernutrition presents a major challenge (5).The ability to assess the role of nutrition in disease prevention and health promotion is predicated on the availability of accurate and reliable biomarkers that reflect nutrient exposure, status, and effect. The recent Lancet series on maternal and child undernutrition highlighted the need for the “development of methods to assess nutritional status and its determinants” as a critical area of research (1).
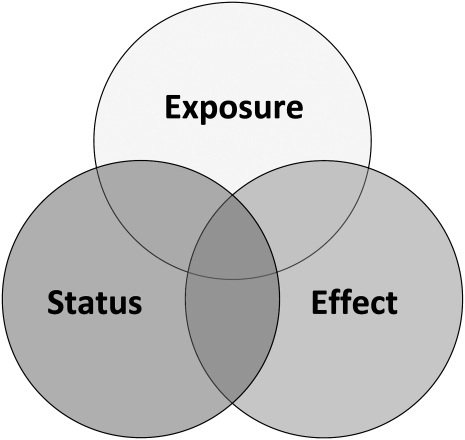
Biomarkers are essential components for clinical and community assessment, yet confusion remains surrounding their use and application. In the context of food and nutrition, 2 questions stand: “What is a nutritional biomarker?” and “What does it measure?” One definition of a biomarker is “a distinctive biological or biologically derived indicator (as a biochemical metabolite in the body) of a process, event, or condition (as in aging, disease, or exposure to a toxic substance)” (6). Others view biomarkers more as measurable molecules or “characteristics” that are the responses to disease or interventions. In the context of nutrition, biomarkers are often categorized as indicators of exposure, status, and function or effect (Figure 1).
FIGURE 1.
The class of nutritional biomarkers and their overlap. Exposure—definition would be contingent on the goal of the evaluation: clinical (what is the baseline information to distinguish between dietary insufficiency compared with a physiologic response to a clinical condition or intervention?); program (at a program level what is the amount of intake of the target population and does it indicate a need, risk, or favorable response to an intervention?). Status—where does the individual or group stand relative to an accepted standard: clinical (a condition that is subject to change); clinical/population (the relative position or standing of somebody or something in a society or other group). Function/effect—direct: did it get from mouth to cell and is it being used (eg, enzyme stimulation assays to evaluate incorporation of cofactors into dependent enzymes, such as vitamin B-6/pyridoxine into erythrocyte glutamic pyruvic transaminase or thiamine into transketolase)? Does the function matter beyond a reflection of nutrient status? Indirect: is the nutrient-dependent system functioning better or worse? Systemic effect of nutrient problem (eg, growth and zinc, vision and vitamin A).
What might be a useful index of nutrient exposure may not necessarily reflect nutrient status, which, in turn, may not necessarily reflect the effect or function of that nutrient (Figure 1). The choice of a biomarker is therefore contingent on issues related to interpretation, implementation, the context of use, and capacity/resource needs. To some, clinicians for example, the goal is to find biomarkers that reflect all 3 components: exposure, status, and effect of a nutrient. These components might be less useful to an agency seeking to estimate populations at risk of a particular nutrient deficiency.
There are 4 main user communities for nutritional biomarkers: 1) research (including basic research into the role of nutrition in biological systems and clinical and operations research), 2) clinical care, 3) programs (surveillance to identify populations at risk, monitoring, and evaluation of public health programs), and 4) policy (evaluation of the evidence base to make national or global policy about diet and health, funding agencies making decisions about priorities in food and nutrition). Each use has its own specific user needs, as well as overlapping needs.
In terms of the current state of science, systematic reviews have been used to evaluate biomarkers for a range of micronutrients, and the results of those reviews were recently discussed in a report from the “Biomarkers of Micronutrient Status: EURRECA Workshop” (7). These results emphasized the lack of clarity on the definition of biomarkers and their application and purpose. The proceedings also emphasized an urgent need for a focused research agenda, especially from a physiologic development perspective, and irrespective of the specific micronutrient. Even for nutrients for which there is a significant body of evidence, many knowledge gaps exist that have prevented the implementation of recommendations, even for the best-validated biomarkers.
BIOMARKERS OF NUTRITION FOR DEVELOPMENT: PROJECT DESCRIPTION
The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) conceptualized an initiative entitled “Biomarker of Nutrition for Development (BOND)” in 2009 (http://www.nichd.nih.gov/global_nutrition/programs/bond/). The goal of BOND is to address 2 core issues: 1) the continuing need to identify, develop, and implement valid and reliable biomarkers and 2) the need to harmonize the global health and nutrition communities' decision-making process for determining which biomarkers are most useful under defined conditions and settings. BOND is intended to provide guidance, both for the selection and interpretation of biomarkers and to support a research agenda aimed at discovery, development, and use of biomarkers to be incorporated into the community's research, clinical, program, and policy-making activities.
The initial activity of the project was a workshop “Biomarkers of Nutrition for Development: Building a Consensus,” organized by the NICHD and hosted by the International Atomic Energy Agency of the United Nations System (IAEA) in February 2010. Seventy-eight participants attended who represented the breadth of the global food and nutrition enterprise; multilateral, unilateral, and nongovernmental organizations; foundations; and the private sector (see Appendix A).
The working groups (WGs) were asked to develop an approach to be used in the evaluation of currently available biomarkers for a set of 5 nutrient case studies (iron, zinc, vitamin A, folate, vitamin B-12). For the purpose of creating a structure that would include considerations applicable to each nutrient, each WG identified key questions and considerations for biomarker selection from their user perspective. The 5 case-study nutrients were selected by a steering committee and were based on the perceived public health importance and unique challenges related to each nutrient. This was a didactic exercise conducted to explore how best to proceed with developing a resource useful for the global food/nutrition community. It was not an attempt to make recommendations about specific biomarkers.
The workshop facilitated deliberations of the WGs by presenting expert reviews on each case nutrient within group discussion sessions (Workshop Agenda; Appendix B). This supported the development of agreement regarding the general principles of a biomarker definition and the formation of a process to identify the relative strengths or weaknesses of various biomarkers for specific applications.
The following is a synopsis of the reports prepared by the WGs followed by a series of reviews based on the presentations made. The full reports of the WGs are available on request.
WORKING GROUP OVERVIEW
The organizers recognized that the 4 WG categories represent a continuum of activity across the nutrition enterprise. Consequently, the WGs were asked not only to represent their particular concepts and principles but also to identify cross-cutting and intersecting interests. For example, clinicians are often involved in research and program evaluation, policy makers frequently identify areas of research need, and so on. The BOND workshop was designed with these linkages in mind, allowing for substantial interfacing of the WGs to exploit common areas of interest. This helped to focus discussion on the particular needs of each user group as well as on the themes common to all users. The following sections represent the composite work of WG reports.
OVERVIEW OF BIOMARKER RESEARCH AGENDA
BOND participants emphasized the need to identify and validate nutritional biomarkers to assess nutrient exposure, status, and functional effect at both the individual and population levels. They emphasized that, whereas sensitive and reliable biomarkers are required for use with subjects of all ages, the greatest needs for such tools are for applications involving pregnant women, preterm/low-birth-weight infants, term infants, and children.
There was agreement on the overarching research goal of biomarker needs assessment, beginning with all dietary essential nutrients. Features of such an assessment are included in Table 1. A number of “cross-cutting” issues were identified that affect biomarker discovery, development, and implementation irrespective of use. Many of these are listed in Table 1, and they are covered in individual report summaries below.
TABLE 1.
Elements of biomarker research agenda
| • Elucidation of factors affecting nutrient homeostasis, including the relation between circulating nutrient concentration and specific tissues (eg, gastrointestinal, liver) |
| • Evaluation of the relative response (eg, change in concentration or activity) of biomarkers of specific nutrients in individuals with presumed normal, depleted, or replete status |
| • Assessment of the utility of biomarkers for uses at both the individual and population levels |
| • Elucidation of nutrient-nutrient and genome-nutrient (including polymorphisms and epigenetics) interactions |
| • Identification and development of reliable, standard assays and reference materials |
| • Identification of informative combinations of biomarkers to provide more sensitivity and specificity for assessing nutrient status/effect |
| • Improved biochemical indexes of inflammation and infection |
| • Development of multiplex assays (multiple assay platforms) for clinical and program use |
| • Development of methods to distinguish primary nutritional problems from secondary effects due to disease |
| • Elucidation of exposure thresholds of both deficiency and toxicity for clinical diagnosis and/or population-based use with an emphasis on distinctions by life cycle, particularly for infants and pregnant women |
| • Conduct dose-response studies to establish dietary references and biomarker cutoffs to determine the heterogeneity of responses in different age groups and different settings |
| • Mining of available data sets on children and young women of child-bearing age to establish the relation of transient changes in nutrient status/exposure to long-term health promotion and disease risk |
| • Implementation of research to identify social/cultural factors that may influence the ability to collect sample (eg, resistance to venous blood collection) |
BOND participants were requested to address certain procedural issues that influence not only the discovery and development of biomarkers but also the codification of a biomarker as an acceptable standard by the community. Several considerations were recognized that affect interpretation and conclusions derived from data (eg, subjects with well-defined controlled intake compared with data from free-living populations in whom intake was uncontrolled).
Participants recognized that systematic reviews have been valuable for evaluating the validity of particular biomarkers. However, this approach is limited by the fact that the evidence base may not allow a priori questions related to the informative value of a specific biomarker. This situation is exacerbated by the general lack of randomized trials designed to test biomarkers.
RECOMMENDATION FOR CONTINUED EFFORT
There was an endorsement on the utility and need for the BOND initiative, and a request that the NICHD continue to facilitate that goal. Several follow-up activities were proposed and endorsed:
Publication of the proceedings of the meeting in a peer-reviewed journal
Dissemination of the proceedings and BOND-related activities via participation in relevant meetings of the nutrition community (eg, Experimental Biology)
Development of resources to include 1) a BOND website linked with other ongoing programs involved in biomarker-related work [eg, EURopean micronutrient RECommendations Aligned (EURRECA), World Health Organization (WHO)] and 2) virtual resource for organizing biomarker information according to user needs, starting with the case study nutrients (iron, zinc, vitamin A, folate, vitamin B-12).
Development of a targeted research agenda based on the review and deliberations of expert panels and the continued involvement of the BOND Steering Committee in the development of these resources
WORKING GROUP REPORTS
The following are summaries derived from the 4 WG reports and discussions.
Research WG report
Chair: Gerald Combs Jr, US Department of Agriculture, Washington, DC Rapporteur: Samir Samman, University of Sydney, Sydney, Australia
The Research WG had a unique role to play in this process because their work reflected not only the needs of those actively engaged in food/nutrition-related research but also those most actively engaged in the discovery and development of biomarkers. Their report reflected aspects of both.
Currently, nutrition research is limited by the lack of a well-developed approach to experimental design that includes the effective use of nutritional biomarkers. Although a wide variety of biomarkers are available for use, in many cases a lack of international consensus exists regarding the appropriateness of those uses.
The ability to move toward such a consensus calls for the identification of the following:
The most useful biomarkers (those with the greatest inferential value for assessing nutrient status, including utility in distinguishing among deficiency, adequacy, and toxicity as well as assessing aspects of physiologic function and/or health)
Useful combinations of biomarkers where relevant
Limitations of biomarkers (including interindividual variation that limits applicability for use with individuals and/or groups and the effects of many of the cross-cutting issues itemized in Table 2—eg, age, genotype, and inflammation/infection)
Reference values (identification of cutoffs, where appropriate, for the identification of risks of nutrient deficiency and toxicity)
TABLE 2.
Cross-cutting issues in biomarker selection
| Biological |
| • Developmental stage |
| • Physiology (eg, growth, pregnancy) |
| • Inflammation and/or infection (chronic vs acute) |
| • Disease-specific considerations (including relevant genetic polymorphisms) |
| • Systems biology: integrity of gastrointestinal tract, hepatic, or other related systems |
| • Routes of exposure (eg, affected by dietary intake, short- vs long-term exposure, food-based vs supplementation, intravenous) |
| • Pharmacology (drug/nutrient interactions) |
| • Sample size (amounts of biological tissue required) |
| Assay-specific issues |
| • Cost |
| • Technical and human capacity |
| • Environmental conditions of use and storage |
| • Sampling burden (field to laboratory) |
| • Analysis burden (eg, laboratory-specific needs) |
| • Amenable to multiplexing (eg, multiple assay platforms) |
| • Standardized and validated assay |
| • Recognized/accepted standard reference materials |
The Research WG recognized that, in principle, the various applications for biomarkers described in Table 3 constitute a de facto process of “translational science” whereby discoveries yield new knowledge, which then informs practice. In practice, the translational use of nutritional biomarkers must start with the identification and validation of such biomarkers. The actual discovery or identification of new biomarkers may involve studies in vitro, in cells, in in vivo animal models, and in human subjects. Often such research can be nested within other nutritional studies.
TABLE 3.
Research applications for biomarkers
| Application | Elements |
| Molecular biology (use of cultured cells) | Need biomarkers to test specific hypotheses that can provide information about the contents of and metabolic responses to nutrients. |
| Animal-based research [model systems (rodent/nonhuman primate) and livestock/domestic] | Requires biomarkers capable of providing information about the nutrient contents of foods/nutrient intervention, baseline tissue concentrations, and metabolic/physiologic responses of tissues to nutrients under defined experimental (eg, dietary, physiologic, genetic, pharmacologic, and/or physical) conditions. |
| Human studies (metabolic studies, epidemiology, clinical trials, community-based interventional studies, and program evaluation) | Studies to elucidate the metabolism of specific nutrients, determine quantitative dietary nutrient requirements, evaluate factors that may affect those requirements, evaluate the efficacy and sustainability of interventions and programs, and identify nutrient-health relations. |
| Clinical trials including community-based intervention trials test specific hypotheses and demand biomarkers to assess nutritional status and metabolic/physiologic responses to interventions across the life cycle. | |
| Program evaluation requires biomarkers to assess nutritional status and physiologic responses to program participation. In this respect, programs are much like uncontrolled interventions, calling for the use of baseline (preparticipation) evaluation as the basis of comparison. |
In addition to the needs identified above, the Research WG recognized that considerations of the wide array of research needs raises points relevant to specific applications of prospective nutritional biomarkers, each of which must be addressed in the context of specific experimental needs or conditions of use. Many of these represent common themes emphasized by all groups and include the following: informative value regarding nutritional status, reflection of body stores, reflection of utilization and function of the nutrient or nutrients, responsiveness to changes in intake and/or status, degree of specificity, use of multiple biomarkers for specific nutrients, availability of consensus reference values, practical needs for sample collection and storage, quality/assay standardization metrics, technical feasibility, and cost.
Clinical WG report
Chair: Bernard Brabin, Liverpool School of Tropical Medicine, United Kingdom
Rapporteur: Frank Greer, University of Wisconsin-Madison, Madison, WI
The Clinical WG definition (Table 4) reflects the reality that clinicians are asked to assess the nutritional status of people who are healthy or sick or who have subclinical illness. Disease itself has a major effect on nutrient metabolism, but the disease is not the only factor the clinician has to consider in assessing the nutritional status of an individual patient. Many of those other considerations are outlined above as cross-cutting issues and include life stage, medications, metabolic abnormalities, sex, and genetic variation. Each must be taken into consideration to put the results of biomarker tests into a relevant context for clinical care. A biomarker may also have utility in predicting future risk of disease or long-term functional outcomes if abnormal values persist. Relevant clinical factors and underlying biological conditions are summarized in Table 5.
TABLE 4.
Clinical definition of a biomarker1
| A biomarker is a biological characteristic that can be objectively measured and that serves as an indicator of normal biological processes, pathogenic processes, or responses to therapeutic interventions. Biomarkers can be broadly characterized into 3 groups: those that measure physical or genetic traits (anthropometric indexes, metabolic gene polymorphisms), those that measure chemical or biochemical agents in the biological system (plasma retinol, iron, zinc), and those that assess a measureable physiologic function (test of night vision, cognitive assessment) or future clinical risk. |
From reference 8.
TABLE 5.
Uses of biomarkers in clinical settings
| Condition | Elements |
| Apparently “healthy” patients | Biomarkers should assess |
| • Reserves | |
| • Pool size | |
| • Tissue amounts of the nutrient | |
| The biomarker should also be useful in determining the response to clinical treatment of the deficiency or disease state. | |
| “Sick” patients | The ideal biomarker should assess |
| • The patient's status for a specific clinical problem | |
| • Reflect a current state of deficiency or clinical disease | |
| The utility of a biomarker also relates to the acuteness or severity of a condition and the response needed. |
In addition to the cross-cutting issue and items outlined previously, the Clinical WG highlighted the importance of genetics (including gene polymorphisms) as an area of particular relevance to the determination of the clinical usefulness of biomarkers. The WG cited several examples including the effect of genetic hemoglobinopathies on iron metabolism and assessment (9). Other examples included the polymorphisms affecting vitamin A, vitamin B-12, and folate. Our knowledge of how genetic variability influences the clinical interpretation of biomarkers in individuals is currently limited and will greatly increase as research continues in this major new field of investigation.
As outlined in their definition, measurement of biomarkers relies on the collection of biological fluid or tissue biopsy material with the application of standardized biochemical procedures. A number of problems around the setting (collection and processing site) include timing of the collection, relation to meals, patient discomfort from the procedure, risk of infection from the collection, and subject compliance in general. Often there is a need for sophisticated equipment, reagents, and instrumentation (eg, refrigerators, freezers, centrifuges, spectrometers, chromatography equipment). In short, the collection of even the gold-standard biomarker for a nutrient may not be feasible or perhaps desirable in many settings.
There was a clear consensus from the WGs that appropriate biomarkers were not yet available for assessing the status of many nutrients. In some cases, newer approaches may be needed to identify potential biomarkers; examples of such new technologies include gene expression arrays to detect genes coding for particular proteins—eg, specific nutrient transport proteins or storage proteins proteomic techniques to directly identify target protein biomarkers. As methods are streamlined and simplified, these new biomarkers could then be used in studies to assess their usefulness in clinical or field conditions, particularly in low-resource settings.
The Clinical WG endorsed the need for the application and utility of biomarkers in the context of large, multicenter trials with long-term follow-up to provide adequate databases. Cohort studies in children and adolescent girls (young women of child-bearing age) followed through adulthood are also needed to establish the relation of biomarkers to transient changes as well as long-term risks and outcomes. An example of this is the National Children's Study currently underway in the United States. In addition, prospective dose-response studies evaluating different nutrient intakes on biomarker responses in multicentered trials are required to determine the heterogeneity of responses in different age and racial groups living under different environmental conditions.
Program WG report
Chair: Emorn Wasantwisut, Mahidol University, Thailand
Rapporteur: Lynnette Neufeld, The Micronutrient Initiative, Ottawa, Canada
In most countries, specific programs are in place to increase the intake of micronutrients from food and supplementary sources (eg, food fortification and promotion of dietary diversity). Many such programs are intended to prevent and treat specific micronutrient deficiencies and their functional consequences. In the context of programs, biomarkers are needed to determine the need for programs in populations (both exposure and status) and to assess changes in response to program interventions and/or conditions in those populations over time.
Programs should be designed and implemented on the basis of an identified need at the community (local and national) level to address a high prevalence of a nutrient-specific functional problem (eg, a high prevalence of night-blindness) or on evidence of limited nutrient exposure. In the context of program evaluation, biomarkers should ideally be included that are in the causal pathway between exposure, the program interventions, and functional outcomes.
In addition to status and function, program managers require information related to the delivery, acceptance, and utilization of interventions, which cannot be provided from biomarkers. The identification of specific and appropriate objectives for the use of biomarkers and the recognition of the strengths and limitations for their use in programs are vital. Most countries where micronutrient deficiencies remain public health problems require capacity strengthening in the planning stage to ensure that the objectives and opportunities for the use of biomarkers are appropriately identified. Program managers require specific information related to the use of biomarkers (eg, sample size, timing and frequency of measurements) to meet specific program objectives, and capacity to provide this support is often limited. Capacity development for in-country collection, processing, and analysis of samples in standardized laboratories is also required.
Currently available biomarkers have a number of important limitations for use in the programmatic context, particularly in resource-constrained settings with high disease burden. For the program manager, although biomarkers provide estimates of deficiency or excess, in isolation (ie, without some other reflection of function or effect) they may not in and of themselves reflect optimal health or even serve as an accurate reflection of nutrient status. Programs that use low doses of nutrients to prevent and ameliorate nutrient insufficiency at a population level are appropriate in many settings, and biomarkers are often used effectively to measure the success of such efforts. However, under certain circumstances, interventions and programs could be strengthened by the ability to target the individual and specific health outcomes rather than nutrient status alone. A well-known example is the current WHO recommendation to provide iron supplements only to anemic individuals with risk of iron deficiency in malaria endemic areas (10). In the absence of markers reflecting the larger health context (eg, inflammation associated with malaria), the interpretation of the iron biomarker alone is compromised. In addition, in a program context, better information about the effect of such genetic conditions such as thalassemia on specific biomarkers and implications for cutoffs, use, and interpretation is urgent. In the future, programs would be greatly improved with the inclusion of biomarkers that could simultaneously reflect the status of multiple micronutrients, going beyond the usual selected few (ie, vitamin A, iron, zinc, iodine, folate) and in line with assessment of status associated with optimal health.
The Program WG recommended the development of a guide that would assist program managers in the selection, use, and appropriate interpretation of biomarkers to improve programs.
Policy WG report
Chair: Mary L'Abbé, University of Toronto, Toronto, Canada
Rapporteur: Jonathan Gorstein, University of Washington, Seattle, WA
Malnutrition (under-/overnutrition involving macro-/micronutrients) underlies significant public health problems in many parts of the world. From the policy perspective, robust evidence on the magnitude of these nutritional problems is required to establish priorities for investment and track the implementation and effectiveness of intervention programs. Furthermore, a need exists to assess toxicity and nutrient excess, as this is also critical for policy makers, especially when interventions are being planned or evaluated. A definition of a biomarker and uses relevant to policymakers are found in Table 6.
TABLE 6.
Definition of biomarker for policy use
| Definition: an indicator that provides evidence on the magnitude and distribution of individual nutrient deficiencies/excesses, which has been subjected to scientific review and for which there is international consensus. |
| A biomarker should |
| • Have well-defined criteria for its application both for individuals and for population groups |
| • Have standardized methodologies |
| • Have evidence-based cutoffs to distinguish between “normal” status and varying degrees of deficiency or excess |
| • Be responsive to interventions that aim to improve status and prevent deficiency of a particular nutrient |
| • Serve to guide resource allocation decisions as to whether investments are effective |
Criteria for considering the utility of a particular biomarker within a policy context are listed in Table 7. Consequent to resource availability and priority, in most cases policy makers or national governments confine the choice of biomarkers to relatively few—usually only 1 or 2 biomarkers are used to describe the status of the population. Thus, the biomarkers selected must be the most relevant to support achievement of the user's goals and objectives. The Policy WG reiterated many of the core characteristics required for a biomarker outlined by the other WGs, including utility for assessing exposure or intake of a nutrient, status of the population, and whether the biomarker reflects short- or long-term status/exposure or a particular biochemical, physiologic, neurological or behavioral function. The Policy WG emphasized the need for biomarkers that have been validated and endorsed by the international community to ensure a level of confidence in the data.
TABLE 7.
Criteria for biomarker use in policy context
| • Generally regarded by the global community as the best biomarker for a given use under defined conditions |
| • Standardized cutoff established by accepted authoritative body (eg, World Health Organization) |
| • Relevant at the population level rather than the individual level |
| • Generally should be the best 1 or 2 markers for a given nutrient for use at population level |
| • Should distinguish between short- and long-term status/exposure |
| • To the extent possible, should distinguish between effect of nutrient exposure and a nutrient/disease-related outcome |
| • Should reflect a biologically relevant response to an intervention |
| • Cutoffs for biomarker must be relevant and interpretable within the context of the country, region, or population of interest |
Ultimately, the collection of biomarker data for policy applications will have to balance different factors while still ensuring that the data are meaningful to estimate the magnitude and distribution of nutrient status and deficiency, and track effects of interventions. Nutritional assessments done to formulate policy are usually based on surveys of populations rather than individual/patient-based data. Such monitoring is usually done at the country or regional level, and the general policy uses include the following:
Identification of nutritional problems within a population or population subgroup
Assessment of the efficacy of policies introduced to reduce the identified problem
Monitoring of other potential effects
The WG emphasized that the selection of a biomarker, irrespective of use, is often constrained by the setting both in terms of the environment (eg, sanitation, temperature) and technical capacity (eg, trained technicians, requisite equipment, sample collection procedures, storage needs and facilities). There is a need to consider costs involved for all aspects, from specimen collection and specimen transport to laboratory analysis. Finally, it was recognized that there are often limitations to the ability to collect specimens due to social/cultural constraints (eg, a lack of community acceptance).
The Policy WG endorsed the development and use of diagnostic platforms that enable direct analysis and data storage at the field/community level because they can potentially provide immediate results and feedback that can be used for individual or population screening and decision making. The need was recognized for potential compromise between the rapid response required and availability and precision of results.
EVALUATION OF BIOMARKERS AND RESEARCH NEEDS BY CASE-STUDY NUTRIENTS
In addition to outlining conceptual issues, each WG was also asked to apply those concepts to an evaluation of currently available biomarkers for 5 case-study nutrients (vitamin A, iron, zinc, folate, vitamin B-12). A summary of their assessments as an exploration of how currently available biomarkers might be viewed in the context of specific user needs is shown in Table 8. This includes their considerations of research needs for those nutrients.
TABLE 8.
Application of working group principles to case-study biomarkers1
| Assay | Overview: from user group perspective | Utility for assessing exposure, status, and function | Additional considerations/confounders |
| Vitamin A | |||
| Serum retinol | General: widely used and characterized. Requires well-trained technicians and equipment support and maintenance. | Exposure: reflects long-term intake; not sensitive to acute changes in vitamin A intake. | Determinants/confounders: age, infection/inflammation, protein-energy malnutrition, zinc/iron deficiency. |
| Program: useful for population and individual levels; however, no value for assessing exposure and is confounded by presence of inflammation/infection. | Status: indicator of severe and moderate deficiency, few examples for evaluating toxicity and not for mild deficiency; does not always respond to intervention strategies. | Additional markers: acute phase protein, eg, AGP/CRP; for excess may combine with free retinyl esters and retionoic acid. | |
| Clinical: relative strength for assessing deficiency and toxicity in individuals and population; relative weakness for assessing intake/exposure; large variations in individual responses to doses of retinol | Function: no direct evidence of cutoff value where morbidity/mortality effects begin to occur; low concentrations likely to be associated with adverse ocular function but not validated; concentrations at 10 μg/dL were originally selected because of its association with xerophthalmia. | ||
| Policy: generally useful except in areas of high disease prevalence. Internationally recognized cutoffs; pregnancy cutoffs need review, may not be useful in infants aged <6 mo. | |||
| Zinc | |||
| Serum zinc | General: issues with contamination, specimen handling. | Exposure: corroboration of dietary insufficient when assessed in combination with intake data; responsive to zinc interventions. | Determinants/confounders: age, sex, pregnancy, infection/inflammation, muscle turnover during rapid growth, chronic disease, fasting/starvation, diurnal/circadian. |
| Program: combination of stunting/dietary zinc/serum zinc useful to target programs; serum zinc useful for program monitoring/evaluation. | |||
| Clinical: low sensitivity at individual level; limited utility in discriminating between states. | Status: better indicator of long-term status; responds slowly to marginal zinc intakes and the degree of change is small and inconsistent; thus limited for detection of mild depletion. | Additional markers: stunting prevalence, dietary intake. | |
| Policy: internationally recognized cutoffs. | |||
| Function: growth is the primary functional outcome most often associated with serum zinc: if long-term, may reflect risk of impaired function in populations; at individual level has not been found to be consistently predictive of functional outcomes. | |||
| Iron | |||
| Serum ferritin | General: standardized assay with accepted cutoffs available. | Exposure: indicates response to increased consumption in absence of confounders; not a measure of severe deficiency. | Determinants/confounders: age, sex, pregnancy, lactation, infection/ inflammation, polymorphisms, malignancy, hyperthyroidism, liver disease, alcohol. |
| Program: useful for survey data in absence of inflammation. | Status: same as intake; better for long- rather than short-term deficiency. | Additional markers: acute phase protein assay, eg, AGP/CRP. | |
| Clinical: utility affected by its role as an acute phase reactant; not an issue in otherwise healthy individuals. | Function: has been associated with delayed cognitive/motor development and abnormal emotional maturation. | ||
| Policy: for deficiency cutoffs accepted when inflammation is not a factor; cutoffs not established for excess. | |||
| Serum ferritin/soluble transferrin receptor | General: relatively expensive test kits available. | Exposure: quantitative assay for the complete spectrum of iron status; no data on iron overload; highly responsive to changes in intake. | Determinants/confounders: age, sex, pregnancy, lactation, infection/inflammation (less than serum ferritin), polymorphisms. |
| Program: viewed as having great utility for intervention studies, although inadequate data on effect of inflammation. | Status: same as intake. | Additional markers: acute phase protein, eg, AGP/CRP. | |
| Clinical: for individuals serum ferritin is sufficient unless in the presence of inflammation. | Function: insufficient data. | ||
| Policy: cutoffs available but further validation and consensus needed. | |||
| Hepcidin | General: disparate methodologies and technically complex cutoffs not widely available. Because it reflects an important aspect of iron homeostasis, it offers great future potential. | Exposure: insufficient data. | Determinants/confounders: infection/inflammation. |
| Status: better for short- rather than long-term deficiency; poor responsiveness to change. | |||
| User: because of the current limitations it is not useful for clinical, policy, programs. | Function: although it is presumptively a reflection of iron homeostasis, there are insufficient data with regard to its ability to reflect any other aspect of iron function or effect. | ||
| Vitamin B-12 | |||
| Methylmalonic acid | General: well validated but expensive and requires special instrumentation | Exposure: very sensitive and moderately specific; reverts to normal within 7–20 d of repletion. | Determinants/confounders: renal impairment, bacterial overgrowth, age (older adults exposure and status do not always correlate). |
| Program: useful at the population level although not widely used. | Status: good for short- and long-term status; less likely to reflect high intakes because absorption is inversely related to intake. | Additional markers: improved predictive value when used in conjunction with serum vitamin B-12 or holoTC. | |
| Clinical: useful for individuals. | |||
| Policy: generally agreed on cutoffs but no international consensus; cutoffs related to functional changes not clear, especially for tissue functions. | Function: good and specific indicator of the adequacy of vitamin B-12 status to support coenzyme function. | ||
| Folate | |||
| Red blood cell folate | General: assay standardized but in need of regional reference laboratories. | Exposure: not reflective of current intake; frequently low concentrations due to vitamin B-12 deficiency | Determinants/confounders: age, pregnancy, alcohol consumption, smoking, drug interactions (antifolates), thalassemia, SNPs. |
| Policy: generally agreed on cutoffs but no international consensus. | Status: reflects long-term status; compromised sensitivity (normal concentrations in folate deficiency) and specificity (low concentrations in vitamin B-12 deficiency). | Additional markers: serum folate useful for intake/short-term status, affected by vitamin B-12 deficiency. | |
| Clinical: useful for individual assessment. | |||
| Program: useful to detect prevalence of deficiency and for monitoring/evaluation. | Function: probably not (bioactive form at the tissue level will affect function) but associated with neural tube defects risk and increased tHcy. | ||
SNPs, single nucleotide polymorphisms; AGP/CRP, α1-acid glycoprotein/C-reactive protein; tHcy, total homocysteine; holoTC, holotranscobalamin.
This table (Table 8) is not intended to be an exhaustive evaluation of all currently available biomarkers for each of the case study nutrients. Moreover, it is not to be interpreted as a set of recommendations for biomarkers endorsed by the BOND participants. It is a reflection of relative strengths and weaknesses identified during the WG deliberations that can inform future efforts to harmonize biomarker selection as well as efforts to improve and expand the biomarker toolkit.
RESEARCH NEEDS BY NUTRIENT
Research needs identified by the WGs for each of the case nutrients are summarized in Table 9. This list is not exhaustive but indicates user needs. It is hoped that this listing will form the basis for a targeted research agenda to advance the discovery and identification, development, and use of biomarkers across the range of users represented by the BOND initiative.
TABLE 9.
Research needs by nutrients
| Nutrient |
| Vitamin A |
| • Evaluate the relative merits of currently used biomarkers of vitamin A (eg, retinol binding protein compared with retinol at different stages of vitamin A status) in terms of cost-effectiveness, feasibility, and diagnostic performance |
| • Identify biomarkers that will be most appropriate to validate dietary estimates of vitamin A intake to monitor dietary interventions and food-based strategies |
| • Identify biomarkers that are sensitive to acute changes in vitamin A intake |
| • Identify biomarkers that can classify subclinical vitamin A deficiency (eg, the meaning of the serum retinol range between 0.70 μmol/L and 1.05 μmol/L is unclear, and methods are needed to assess the link between mild ranges of vitamin A deficiency and health consequences) |
| • Test the diagnostic performance of retinyl esters (including vitamin A toxicity) against robust gold-standard measures of hepatic stores (isotope dilution methods) |
| • Validation of new methods for quantifying dark adaptation |
| • Standardize approaches used to interpret biomarker distributions in the presence of inflammation and infection |
| • Adapt current or new vitamin A biomarkers to increase feasibility in the field (eg, methods that do not require cold storage such as dried blood spot assay) |
| • Develop guidelines for the use of modified relative dose response as a sensitive indicator for program evaluation (subsample where status measures are used) |
| Iron |
| • Identify additional iron biomarkers, eg, hepcidin, nontransferrin bond iron (NTBI) and their utility under defined conditions of use |
| • Explore the potential of new noninvasive technologies to assess iron status and effect such as MRI (magnetic resonance imaging) assessment of tissue-specific iron, noninvasive liver iron quantification by SQUID (superconducting quantum interference device) bio-susceptometry, zinc-protoporphyrin determination by direct measurement of fluorescence of mucosal/epithelial tissue |
| • Improve technology to ensure that rigorously standardized specific iron biomarkers are available |
| • Iron measurements across the life cycle; with specific emphasis on women of reproductive age and during pregnancy, infants and children aged <5 y, and the elderly |
| • Special emphasis on outcomes most relevant to resource-limited settings including neonatal and infant mortality, iron status of infants during the first 6 mo of life, and infant cognitive, motor, and behavioral development |
| • Biomarkers of iron exposure are needed, particularly during pregnancy (storage markers of iron are less useful because maternal iron stores are frequently low or depleted during the third trimester) |
| • Determine the association of iron status in infants at birth and again at age 6 mo in relation to maternal iron status including an evaluation of the effect of different exposure scenarios (maternal supplements vs food-based interventions) during pregnancy on infant outcomes |
| • Assess functional deficits in relation to body iron stores particularly in infants and young children. Current methodologies might include immunologic responsiveness, molecular assessment of enzyme activity, use of visual and/or auditory evoked potentials that can be measured beginning shortly after birth |
| • Identify measures of iron excess |
| • Examine the effect of inflammation, including infections and neoplastic disorders on iron biomarkers; this would include further exploring the use of acute phase proteins, eg, C-reactive protein (CRP) and α1-acid glycoprotein (AGP) to evaluate serum ferritin cutoffs |
| • Development of multiplex assays to measure iron and inflammation (eg, ferritin, short tandem repeat, CRP, AGP) that are appropriate for field use |
| • Evaluate the utility of iron biomarkers in populations with high prevalence of α-thalassemia and relevant genetic polymorphisms |
| • Reassess the utility of hemoglobin for establishing the severity of nutritional iron deficiency in population surveys |
| • Establish international standards and cutoffs for primary iron biomarkers (hemoglobin, ferritin, serum transferrin receptor) that are life course/sex specific |
| • Establish processes to harmonize international standards for biomarkers to improve the assay calibration and utility (eg, specific reference was made to the serum transferrin receptor/ferritin ratio to assess body iron status in healthy and unhealthy populations for program evaluation as well as for individuals) |
| Folate/vitamin B-12 |
| • Build a greater understanding of single-carbon metabolism and identify biomarkers that reflect the interactions between components of single-carbon metabolism (eg, folate, vitamin B-12, choline) |
| • Discover and/or validate biomarkers that can measure potential adverse effects of overexposure to folate |
| • Identify folate biomarkers for large-scale/population-based screening purposes |
| • Validation of percentage bioavailability correction needed with high intakes of vitamin B-12, to reflect actual percentage absorbed; determine intakes needed to normalize vitamin B-12 biomarkers in healthy populations using correct bioavailability values |
| • Evaluate the relative utility of transcobalamin II (holoTC) compared with serum vitamin B-12 for various purposes |
| • Identify biomarkers of short-term, inadequate vitamin B-12 status |
| • Explore linkages of folate biomarkers to function (eg, neural tube defects) and determine cutoffs indicating increased risk of tissue dysfunction |
| • Develop clear recommendations on when to measure red blood cell folate vs serum folate |
| • Improve understanding of the functional effect of folate status, particularly with regard to its effect on DNA methylation or damage and the relation to increased risk of chronic disease |
| • Risks associated with high serum or red blood cell folate and unmetabolized, free folic acid, especially where vitamin B-12 depletion and deficiency are prevalent |
| • Develop cutoffs for serum, red blood cell, or free folic acid that reflect increased risk of possible adverse effects, especially in the presence of vitamin B-12 deficiency |
| • Identify biomarkers of folate status that will be most useful in the evaluation of health effects of folate intervention programs in healthy and high disease-prevalent (eg, malaria) settings |
| Zinc |
| • Identify biomarkers of zinc function across the life course and identify biomarkers that reflect short-term changes in zinc function with marginal intakes |
| • Improve understanding of factors affecting zinc homeostasis including those signals for zinc mobilization into and out of endogenous zinc pools, factors affecting conservation of endogenous zinc secretion, and signals affecting appetite in zinc deficiency |
| • Identify biomarkers that show cellular response to acute, short-term zinc depletion or supplementation |
| • Evaluate the utility, sensitivity, and specificity of multiple related functional endpoints, eg, oxidative stress, cellular transcription factors for assessing zinc status, and response to exposure |
| • Assess effect of chronic or acute infection on zinc status in the context of various exposure scenarios (eg, short- vs long-term dietary insufficiency) |
| • Support efforts to discover, identify, and develop new biomarkers of zinc exposure, status, and function; a need exists for further development of accurate food composition tables (that include zinc content of indigenous foods and phytate content) to assess dietary zinc |
| • Develop an algorithm to assess zinc bioavailability from various whole diets |
| • Establish the viability of currently available or new biomarkers of zinc to assess functional effect of zinc status, further evaluate their use to assess growth, immune status, fat-free mass, morbidity, dermatitis |
| • Determine which zinc-dependent functions are prioritized and sustained and which are sacrificed in deficiency |
| • Use new technologies including “-omics” (eg, metabolomics) to identify metabolites that might be most responsive to changes in zinc status/exposure |
| • Explore the value of algorithms that might include combinations of non–zinc-specific tests to provide adequate reflection of functional effect of zinc [eg, low serum plasma zinc plus low insulin-like growth factor or serum plasma zinc plus immune marker (eg, CRP)] |
| • Develop international standards for poor dietary zinc bioavailability, eg, phytate/zinc ratios, estimates of percentage absorbed zinc |
| • Develop valid and field-friendly methods to assess zinc status that require small samples, that do not require stringent storage conditions, and that can be run at local laboratories/clinics |
| • Determine whether cord blood for serum plasma zinc is a useful measure of zinc stores at birth |
| • Determine whether urinary zinc is a useful measure of zinc and develop accepted cutoffs |
Acknowledgments
We acknowledge the following WG rapporteurs: Jonathan Gorstein, Frank Greer, Lynnette Neufeld, and Samir Samman, as well as the “Biomarkers of Nutrition for Development: Building a Consensus” meeting participants. DJR acknowledges the coauthors of this Executive Summary (SN, BB, GC, ML, and EW, who served as the Chairs of the User Group panels; ID-H served as Chair of the Workshop Steering Committee as well as overall meeting Chair) for their efforts in developing the individual user group content of this report and for their support in the preparation and review of this manuscript.
The authors' responsibilities were as follows—GC: drafted the Research Working Group (WG) report; BB: drafted the Clinical WG report; EW: drafted the Program WG report; MRL: drafted the Policy WG report; DJR and SN: wrote the manuscript; ID-H: reviewed the manuscript; and DJR: had primary responsibility for final content. The nongovernmental contributors to this meeting included the Bill and Melinda Gates Foundation (BMGF) obtained through a competitive conference grant application process. Once awarded, the BMGF was informed of the progress of the conference and received a final report as the required “deliverable.” Additional support came in the form of a restricted gift to the NICHD gift fund from PepsiCo to support this workshop and did not reflect any obligation on the part of the NICHD to PepsiCo for the planning or outcome of this meeting. The authors declared no conflicts of interest.
APPENDIX A
Program participants
Lindsay Allen, Western Human Nutrition Research Center, US Department of Agriculture, and Department of Nutrition, University of California, Davis, 430 West Health Sciences Drive, University of California, Davis, Davis, CA 95616. E-mail: lindsay.allen@ars.usda.gov.
Stephanie Atkinson, Department of Pediatrics, McMaster University, 1200 Main Street West, HSC-3V42, Hamilton, ON, L8N 3Z5 Canada. E-mail: satkins@mcmaster.ca.
Sunethra Atukorala, Department of Biochemistry and Molecular Biology, Faculty of Medicine, University of Colombo, Kynsey Road, PO Box 271, Colombo 8, Sri Lanka. E-mail: sunethra@eol.lk.
Lisa Bero, Medicines, Access and Rational Use, Essential Medicines and Pharmaceutical Policies, World Health Organization, 20 Avenue Appia, CH1211 Geneva 27, Switzerland. E-mail: berol@who.int.
HK Biesalski, Department of Biological Chemistry and Nutrition, University of Hohenheim, Garbenstrasse 30, D 70593 Stuttgart, Germany. E-mail: biesal@uni-hohenheim.de.
Martin Bloem, Nutrition and HIV/AIDS Policy, United Nations World Food Programme, Via Cesare Giulio Viola, 68/70, Parco de Medici, 00148 Rome, Italy. E-mail: martin.bloem@wfp.org.
Erick Boy-Gallego, HarvestPlus, 180 Elgin Street, Suite 1000, Ottawa, ON, K2P 2K3 Canada. E-mail: e.boy@cgiar.org.
Bernard Brabin, Tropical Pediatrics, Liverpool School of Tropical Medicine, Pembroke Place, Liverpool L3 5QA, United Kingdom. E-mail: b.j.brabin@liv.ac.uk.
Gary Brittenham, Columbia University, College of Physicians and Surgeons, Children's Hospital of New York, Harkness Pavillion, Room CHN 10-08, 3959 Broadway, New York, NY 10032. E-mail: gmb31@columbia.edu.
Kenneth Brown, Helen Keller International, University of California, Davis, PO Box 29, 898, Dakar-Yoff, Senegal. E-mail: kbrown@hki.org.
Paul Coates, Office of Dietary Supplements, National Institutes of Health, US Department of Health and Human Services, Suite 3B01, 6100 Executive Boulevard, Bethesda, MD 20892-7517. E-mail: coatesp@mail.nih.gov.
Gerald Combs Jr, Grand Forks Human Nutrition Research Center, US Department of Agriculture, 2420 2nd Avenue North, Grand Forks, ND 58203. E-mail: gcombs@gfhnrc.ars.usda.gov.
Ian Darnton-Hill, Boden Institute of Obesity, Nutrition, and Exercise, The University of Sydney, Australia; Friedman School of Nutrition Science and Policy, Tufts University, C/O 71 Broadway, Apartment 2E, New York, NY 10006. E-mail: iandarntonhill@aol.com.
Omar Dary, A2Z/USAID Micronutrient and Child Blindness Program, Academy for Educational Development (AED), 1825 Connecticut Avenue, Suite 800, Washington, DC 20009-5721. E-mail: odary@aed.org.
Lena Davidsson, Nutritional and Health Related Environmental Studies Section, Division of Human Health, International Atomic Energy Agency, PO Box 100, A-1400 Vienna, Austria. E-mail: l.davidsson@iaea.org.
Cindy Davis, Nutritional Science Research Group, National Cancer Institute, National Institutes of Health, US Department of Health and Human Services, EPN 3159, 6130 Executive Boulevard, Rockville, MD 20892. E-mail: davisci@mail.nih.gov.
Saskia de Pee, Policy, Strategy, and Programme Support Division, United Nations World Food Programme, Via Cesare Giulio Viola, 68/70, Parco de Medici, 00148 Rome, Italy. E-mail: depee.saskia@gmail.com.
Ali Dhansay, Medical Research Council, PO Box 19070, 7505 Tygerberg, South Africa. E-mail: ali.dhansay@mrc.ac.za.
Juergen Erhardt, SEAMEO-TROPMED, Regional Center for Community Nutrition, University of Indonesia, Salemba Raya 6, Jakarta 10430, Indonesia. E-mail: juergen.erhardt@nutrisurvey.net.
Susan Fairweather-Tait, Mineral Metabolism, University of East Anglia, Norwich, NR4 7TJ United Kingdom. E-mail: s.fairweather-tait@uea.ac.uk.
Rafael Flores-Ayala, International Micronutrient Malnutrition Prevention & Control (IMMPaCt) Program, Nutrition Branch, Centers for Disease Control and Prevention, US Department of Health and Human Services, 4470 Buford Highway, NE MS K- 25, Atlanta, GA 300341-3724. E-mail: rnf2@cdc.gov.
Dean Garrett, Program for Appropriate Technology in Health, MEASURE Demographic Health Surveys (DHS) Project, 1455 NW Leary Way, Seattle, WA 98107. E-mail: dgarrett@path.org.
Jonathan Gorstein, Global Health, University of Washington, H-688 Health Sciences Building, Box 357660, International Training and Education Center on HIV (I-TECH), Seattle, WA 98195. E-mail: gorstein@u.washington.edu.
Ralph Green, Departments of Pathology and Laboratory Medicine and Internal Medicine, University of California, Davis, Medical Center, 2315 Stockton Boulevard, Sacramento, CA 95817. E-mail: ralph.green@ucdmc.ucdavis.edu.
Frank Greer, Department of Pediatrics, University of Wisconsin School of Medicine and Public Health, Meritor Hospital, Perinatal Center, 202 South Park Street, Madison, WI 53715. E-mail: frgreer@pediatrics.wisc.edu.
Yiwu He, Bill and Melinda Gates Foundation, PO Box 23350, Seattle, WA 98102. E-mail: yiwu.he@gatesfoundation.org.
Douglas Heimburger, Education and Training, Vanderbilt Institute for Global Health, 2525 West End Boulevard Nashville, TN 37203. E-mail: douglas.heimburger@vanderbilt.edu.
Richard Hurrell, Laboratory for Human Nutrition, Institute of Food Science and Nutrition, Swiss Federal Institute of Technology, LFV D 20, Schmelzbergstrasse 7, 8092 Zurich, Switzerland. E-mail: richard.hurrell@ilw.agrl.ethz.ch.
Kazi Jamil, Clinical Sciences Division, International Centre for Diarrhoeal Disease Research, Bangladesh, Mohakhali, Dhaka-1212, Bangladesh. E-mail: jamil@icddrb.org.
Emmanuel Kafwembe, Tropical Diseases Research Centre, PO Box 71769, Ndola, Zambia. E-mail: ekafwembe1951@yahoo.com.
Janet King, University of California, Berkeley and Davis, and Children's Hospital Oakland Research Institute, 5700 Martin Luther King Jr Way, Oakland, CA 94609. E-mail: jking@chori.org.
Klaus Kraemer, Sight and Life, PO Box 2116, CH-4002 Basel, Switzerland. E-mail: klaus.kraemer@sightandlife.org.
Nancy Krebs, Department of Pediatrics, Center for Human Nutrition, University of Colorado Denver, Research Complex 2, Room 5025, 12700 East 19th Avenue, C225, Aurora, CO 80045. E-mail: nancy.krebs@uchsc.edu.
Bruce Kristal, Department of Surgery, Harvard Medical School, and Department of Neurosurgery, Brigham and Women's Hospital, 221 Longwood Avenue, Boston, MA 02115. E-mail: bkristal@partners.org.
Regina Kulier, Guidelines Review Committee Secretariat, Information, Evidence, and Research, Department of Research, Policy, and Cooperation, World Health Organization, 20, Avenue Appia, CH1211 Geneva 27, Switzerland. E-mail: kulierr@who.int.
Roland Kupka, Regional Office for West and Central Africa, United Nations Children's Fund, BP 29720, Dakar, Senegal. E-mail: rkupka@unicef.org.
Mary L'Abbé, Department of Nutrition Sciences, Faculty of Medicine, University of Toronto, FitzGerald Building, 150 College Street, Toronto, ON, M5S 3E2 Canada. E-mail: mary.labbe@utoronto.ca.
Carol Levin, Technology Solutions, PATH, 2201 Westlake Avenue, Seattle, WA 98107. E-mail: clevin@path.org.
Sean Lynch, Eastern Virginia Medical School, 151 Breezy Point Drive, Grafton, VA 23692. E-mail: srlynch@visi.net.
Barbara MacDonald, Performance Measurement and Research, Global Alliance for Improved Nutrition, PO Box 55, 1211 Geneva 20, Switzerland. E-mail: bmacdonald@gainhealth.org.
Yvonne Maddox, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services, 31 Center Drive, Room 2A03, Bethesda, MD 20892. E-mail: maddoxy@exchange.nih.gov.
Elizabeth Madraa, Food and Nutrition Division, Ministry of Health, Uganda, Plot 6, Lourdel Road, Nakasero, Uganda. E-mail: emadraa@yahoo.com.
Helene McNulty, Biomedical Sciences Research Institute, School of Biomedical Sciences, University of Ulster, Coleraine Campus, Room W2047, Cromore Road, Coleraine BT52 1SA, Ireland. E-mail: h.mcnulty@ulster.ac.uk.
Mark Miller, Division of International Epidemiology and Population Studies, Fogarty International Center, National Institutes of Health, US Department of Health and Human Services, 31 Center Drive–MSC 2220, Bethesda, MD 20892-2220. E-mail: millemar@mail.nih.gov.
John Milner, Nutritional Science Research Group, National Cancer Institute, National Institutes of Health, US Department of Health and Human Services, 6130 Executive Boulevard–MSC 7328, Rockville, MD 20982. E-mail: john.milner@nih.hhs.gov.
K Madhavan Nair, Micronutrient Research Group, Department of Biophysics, National Institute of Nutrition, India, and Indian Council of Medical Research, Jamai-Osmania, Hyderabad 500 604, Andhra Pradesh, India. E-mail: nairthayil@hotmail.com.
Sorrel Namasté, Endocrinology, Nutrition, and Growth Branch, Center for Research for Mothers and Children, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services, 6100 Executive Boulevard, Suite 4B09A, Rockville, MD 20852. E-mail: namastes@mail.nih.gov.
Lynnette Neufeld, The Micronutrient Initiative, 180 Elgin Street, Suite 1000, Ottawa, ON, K2P 2K3 Canada. E-mail: lneufeld@micronutrient.org.
Christina Northrop-Clewes, Performance Measurement and Research, Global Alliance for Improved Nutrition, Rue de Vermont 37-39, PO Box 55, 1211 Geneva 20, Switzerland. E-mail: cclewes@gainhealth.org.
Jean-Bosco Ouédraogo, Institut de recherché santé det société (IRSS), 399, Avenue de la Liberte, 01 BP 545 Bobo Dioulasso, Burkina Faso. E-mail: jbouedraogo.irss@fasonet.bf.
Juan Pablo Pena-Rosas, Micronutrients Unit, Department of Nutrition for Health and Development, World Health Organization, 20, Avenue Appia, CH-1211 Geneva 27, Switzerland. E-mail: penarosasj@who.int.
Mary Penny, Instituto de Investigación Nutricional, Av La Molina 1885, Lima 12, Peru. E-mail: mpenny@iin.sld.pe.
Christine Pfeiffer, Nutritional Biomarkers Branch, Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention, US Department of Health and Human Services, 4770 Buford Highway NE, MSF-55, Atlanta, GA 30341. E-mail: cpfeiffer@cdc.gov.
Kamija Phiri, Department of Haematology, University of Malawi College of Medicine, P/Bag 360, Blantyre 3, Malawi. E-mail: kamijaphiri@gmail.com.
Francesca Joseline Marhone Pierre, Haiti State University, Ministry of Health and Population of Haiti, and Department of Biology and Nutrition, Catholic University of Notre Dame, 6, Rue Sapotille, PO Box 1522, Port-au-Prince, Haiti. E-mail: franjomapi@hotmail.com.
Mark Pirner, Clinical and Scientific Development Strategy, PepsiCo, 700 Anderson Hill Road, Purchase, NY 10577. E-mail: mark.pirner@pepsico.com.
Daniel Raiten, Endocrinology, Nutrition, and Growth Branch, Center for Research for Mothers and Children, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services, 6100 Executive Boulevard, Suite 4B11, Rockville, MD 20852. E-mail: raitend@mail.nih.gov.
Fabian Rohner, Performance Measurement and Research, Global Alliance for Improved Nutrition, PO Box 55, 1211 Geneva 20, Switzerland. E-mail: frohner@gainhealth.org.
Irwin Rosenberg, The Jean Mayer US Department of Agriculture Human Nutrition Research Center on Aging, Tufts University, 711 Washington Street, Boston, MA 02111. E-mail: irwin.rosenberg@tufts.edu.
Rob Russell, Tufts University, and Office of Dietary Supplements, National Institutes of Health, US Department of Health and Human Services, 216 Pleasant Street, Arlington, MA 02476. E-mail: rob.russell@tufts.edu.
Samir Samman, School of Molecular and Microbial Biosciences, G08, University of Sydney, Sydney NSW 2006, Australia. E-mail: s.samman@usyd.edu.au.
Jacob Selhub, Vitamin Metabolism Laboratory, The Jean Mayer US Department of Agriculture Human Nutrition Research Center on Aging, Tufts University, 711 Washington Street, Boston, MA 02111. E-mail: jacob.selhub@tufts.edu.
Noel Solomons, Center for Studies of Sensory Impairment, Aging and Metabolism, 17a Avendia Marisca No. 16-89, Zona 11, Guatemala City 01011, Guatemala. E-mail: cessiam@guate.net.gt.
Pamela Starke-Reed, Division of Nutrition Research Coordination, National Institutes of Health, US Department of Health and Human Services, 6707 Democracy Boulevard–MSC 5462, Bethesda, MD 20892. E-mail: pamela.starke-reed@nih.hhs.gov.
Patrick Stover, Division of Nutritional Sciences, Cornell University, 127 Savage Hall, Ithaca, NY 14853. E-mail: pjs13@cornell.edu.
Christine Swanson, Office of Dietary Supplements, National Institutes of Health, US Department of Health and Human Services, 6100 Executive Boulevard, Room 3B01, Bethesda, MD 20892-7517. E-mail: swansonc@od.nih.gov.
Sherry Tanumihardjo, University of Wisconsin, National Science Building, 1415 Linden Drive, Madison, WI 53706. E-mail: sherry@nutrisci.wisc.edu.
David Thurnham, University of Ulster, Center for Molecular Biosciences, Cromore Road, Coleraine BT52 1SA, Ireland. E-mail: di.thurnham@ulster.ac.uk.
Ricardo Uauy, Nutrition and Public Health Research Unit, London School of Hygiene and Tropical Medicine, Room 182, Kepple Street, London WC1E HT, United Kingdom. E-mail: ricardo.uauy@lshtm.ac.uk.
Emorn Wasantwisut, Mahidol University, Salaya, Phutthamonthon, Nakhon Pathom 73170, Thailand. E-mail: numdk@mahidol.ac.th.
Keith West Jr, Program and Center for Human Nutrition, Department of International Health, Johns Hopkins Bloomberg School of Public Health, 615 North Wolfe Street W2041, Baltimore, MD 21205. E-mail: kwest@jhsph.edu.
Sedigheh Yamini, Nutrition Science Evaluation, Office of Nutrition, Labeling, and Dietary Supplements, Center for Food Safety and Applied Nutrition, Food and Drug Administration, US Department of Health and Human Services, 5100 Paint Branch Parkway, HFS 830, College Park, MD 20740. E-mail: essie.yamini@fda.hhs.gov.
Xiaoguang Yang, National Institute for Nutrition and Food Safety, China Centre for Disease Control and Prevention, and Key Lab of Trace Element Nutrition, Ministry of Health of China, 29 Nan Wei Road, Beijing, Xuanwu District 100050, China. E-mail: xgyangcdc@vip.sina.com.
Michael Zimmermann, Micronutrients and International Health, Laboratory of Human Nutrition, Institute for Food Science and Nutrition, Swiss Federal Institute of Technology (ETH), Schmelzberstrasse 7, LFV E19, ETH Zentrum, CH-8092 Zurich, Switzerland. E-mail: michael.zimmermann@ilw.agrl.ethz.ch.
APPENDIX B
“Biomarkers of Nutrition for Development: Building a Consensus”
Hosted by the International Atomic Energy Agency
Organized in collaboration with the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services
February 8–10, 2010
Venue: International Atomic Energy Agency Headquarters
Vienna, Austria
Board Room A (M Building)
AGENDA
Monday, February 8
9:00–9:45 Session I: Welcoming Remarks and Introductions
Chair: Lena Davidsson, International Atomic Energy Agency
9:00–9:10 Welcome from the International Atomic Energy Agency
Werner Burkart, International Atomic Energy Agency
9:10–9:20 Welcome from the National Institutes of Health
Yvonne Maddox, National Institutes of Health, US Department of Health and Human Services
9:20–9:30 Welcome from the Bill and Melinda Gates Foundation
Yiwu He, Bill and Melinda Gates Foundation
9:30–9:40 BOND Overview
Daniel Raiten, National Institutes of Health, US Department of Health and Human Services
9:40–9:50 Meeting Goals and Agenda
Ian Darnton-Hill, The University of Sydney & Tufts University
9:50–12:15 Session II: Reports from User Working Groups
Chair: Rob Russell, Tufts University & National Institutes of Health, US Department of Health and Human Services
Session objectives: obtain user group views on how best to define biomarkers of:
-Exposure
-Status (Distinction between exposure vs assimilation/effect?)
-Functional Effect (A reflection of biological systems performance or a health/disease relationship?)
9:50–10:00 Introduction
Rob Russell, Tufts University & National Institutes of Health, US Department of Health and Human Services
10:00–10:20 Report from Research Working Group
Gerald Combs, US Department of Agriculture
10:20–10:45 Break
10:45–11:05 Report from Clinical Working Group
Bernard Brabin, Liverpool School of Tropical Medicine
11:05–11:25 Report from Policy Working Group
Mary L'Abbé, University of Toronto
11:25–11:45 Report from Program Working Group
Emorn Wasantwisut, Mahidol University
11:45–12:15 Panel Discussion
Working Group Chairs
12:15–13:15 Lunch
13:15–15:10 Session III: Defining the Process
Chair: Ricardo Uauy, London School of Hygiene and Tropical Medicine
Session objectives: review examples of harmonization processes and assess relative strengths with regard to meeting user group needs.
13:15–13:25 Introduction: User group needs and potential process for harmonization and decision making
Ricardo Uauy, London School of Hygiene and Tropical Medicine
13:25–13:40 European Micronutrient Recommendations Aligned (EURRECA) Process
Susan Fairwather-Tait, University of East Anglia
13:40–13:55 Dietary Reference Intake (DRI) Process
Stephanie Atkinson, McMaster University
13:55–14:10 World Health Organization Evidence-based Guideline Development Process
Regina Kulier, World Health Organization
14:10–15:10 Panel Discussion
15:10–15:40 Break
15:40–17:15 Session IV: Workshop on Defining the Process
Chair: Ian Darnton-Hill, TheUniversity of Sydney & Tufts University
15:40–15:50 Introduction: Charge to the breakout groups
Ian Darnton-Hill, The University of Sydney & Tufts University
15:50–17:15 Concurrent group discussions
Group 1: Research
Group 2: Clinical
Group 3: Policy
Group 4: Program
17:30–18:30 Reception hosted by the International Atomic Energy Agency (IAEA)
Tuesday, February 9
8:30–9:30 Session V: Workshop Presentations on Defining the Process
Chair: Ian Darnton-Hill,The University of Sydney & Tufts University
8:30–8:40 Group 1: Research
8:40–8:50 Group 2: Clinical
8:50–9:00 Group 3: Policy
9:00–9:10 Group 4: Program
9:10–9:30 Discussion
9:30–13:20 Session VI: Case Studies
Chair: Daniel Raiten, National Institutes of Health, US Department of Health and Human Services
Session objectives: focus on the evidence base to support recommendations for biomarkers of exposure, status, and function/effect.
9:30–9:40 Introduction: Goals and justification for case studies
Daniel Raiten, National Institutes of Health, US Department of Health and Human Services
9:40–11:40 Case studies: Vitamins
Chair: Roland Kupka, United Nations Children's Fund
9:40–10:00 Vitamin A
Sherry Tanumihardjo, University of Wisconsin
10:00–10:20 Folic acid/Vitamin B-12
Ralph Green, University of California, Davis
10:20–10:40 Break
10:40–11:40 Panel discussion
11:40–13:20 Case studies: Minerals
Chair: Rafael Flores-Ayala, Centers for Disease Control and Prevention, US Department of Health and Human Services
11:40–12:00 Iron
Sean Lynch, Eastern Virginia Medical School
12:00–12:20 Zinc
Janet King, Children's Hospital Oakland Research Institute
12:20–13:20 Panel discussion
13:20–14:30 Lunch
14:30–17:30 Session VII: Workshop on Case Studies
Chair: Ian Darnton-Hill, The University of Sydney & Tufts University
Session objectives: identify key questions to be addressed for each of the case study nutrients, evaluate the strength of evidence to make recommendations for each user group, and identify research gaps.
14:30–14:40 Introduction: Charge to the breakout groups
Ian Darnton-Hill, The University of Sydney & Tufts University
14:40–17:30 Concurrent group discussions
Group 1: Research
Group 2: Clinical
Group 3: Policy
Group 4: Program
Wednesday, February 10
8:30–9:30 Session VIII: Workshop Presentations on Case Studies
Chair: Ian Darnton-Hill, The University of Sydney & Tufts University
8:30–8:40 Group 1: Research
8:40–8:50 Group 2: Clinical
8:50–9:00 Group 3: Policy
9:00–9:10 Group 4: Program
9:10–9:30 Discussion
9:30–10:45 Session IX: New Frontiers in Science and Technology
Chair: John Milner, National Institutes of Health, US Department of Health and Human Services
Session objectives: based on context, what are best new candidates?; role of “-omics” (metabolomics, proteomics, genomics, nutrigenomics):
-Capacity/resource/training needs
-Specific issues regarding settings: primary care vs referral vs academic
9:30–9:40 Introduction
John Milner, National Institutes of Health, US Department of Health and Human Services
9:40–9:55 Field Friendly Techniques
Dean Garrett, PATH and MEASURE Demographic Health Surveys (DHS) Project
9:55–10:10 “-omics”
Mark Pirner, PepsiCo
10:10–10:25 Nuclear Techniques
Lena Davidsson, International Atomic Energy Agency
10:25–10:45 Discussion
10:45–11:00 Break
11:00–12:15 Session X: Next Steps
Chairs: Ian Darnton-Hill, The University of Sydney & Tufts University
Daniel Raiten, National Institutes of Health, US Department of Health and Human Services
-Open meeting adjourned-
13:15–17:00 Steering Committee Consortium
Session objectives: discuss next steps and responsibilities, which will include:
-Do we have an agreement on a process?
-Can we delegate responsibilities?
-How can we best proceed?
REFERENCES
- 1.Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371:243–60 [DOI] [PubMed] [Google Scholar]
- 2.Ramakrishnan U. Prevalence of micronutrient malnutrition worldwide. Nutr Rev 2002;60:S46–52 [DOI] [PubMed] [Google Scholar]
- 3.Garcia OP, Long KZ, Rosado JL. Impact of micronutrient deficiencies on obesity. Nutr Rev 2009;67:559–72 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization Global strategy on diet, physical activity and health. Geneva, Switzerland: World Health Organization, 2004 [Google Scholar]
- 5.Jehn M, Brewis A. Paradoxical malnutrition in mother-child pairs: untangling the phenomenon of over- and under-nutrition in underdeveloped economies. Econ Hum Biol 2009;7:28–35 [DOI] [PubMed] [Google Scholar]
- 6.Merriam-Webster Online Dictionary. Definition of “biomarker.” Available from: http://www.merriam-webster.com/dictionary/biomarker (cited 2010)
- 7.Hooper L, Ashton K, Harvey LJ, Decsi T, Fairweather-Tait SJ. Assessing potential biomarkers of micronutrient status by using a systematic review methodology: methods. Am J Clin Nutr 2009;89:1953S–9S [DOI] [PubMed] [Google Scholar]
- 8.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89–95 [DOI] [PubMed] [Google Scholar]
- 9.Demir A, Yarali N, Fisgin T, Duru F, Kara A. Serum transferrin receptor levels in beta-thalassemia trait. J Trop Pediatr 2004;50:369–71 [DOI] [PubMed] [Google Scholar]
- 10.Fontaine O. Conclusions and recommendations of the WHO Consultation on prevention and control of iron deficiency in infants and young children in malaria-endemic areas. Food Nutr Bull 2007;28:7. [DOI] [PubMed] [Google Scholar]