Abstract
Whereas cost-effective interventions exist for the control of micronutrient malnutrition (MN), in low-resource settings field-friendly tools to assess the effect of these interventions are underutilized or not readily available where they are most needed. Conventional approaches for MN measurement are expensive and require relatively sophisticated laboratory instrumentation, skilled technicians, good infrastructure, and reliable sources of clean water and electricity. Consequently, there is a need to develop and introduce innovative tools that are appropriate for MN assessment in low-resource settings. These diagnostics should be cost-effective, simple to perform, robust, accurate, and capable of being performed with basic laboratory equipment. Currently, such technologies either do not exist or have been applied to the assessment of a few micronutrients. In the Demographic and Health Surveys (DHS), a few such examples for which “biomarkers” of nutrition development have been assessed in low-resource settings using field-friendly approaches are hemoglobin (anemia), retinol-binding protein (vitamin A), and iron (transferrin receptor). In all of these examples, samples were collected mainly by nonmedical staff and analyses were conducted in the survey country by technicians from the local health or research facilities. This article provides information on how the DHS has been able to successfully adapt field-friendly techniques in challenging environments in population-based surveys for the assessment of micronutrient deficiencies. Special emphasis is placed on sample collection, processing, and testing in relation to the availability of local technology, resources, and capacity.
BIOMARKER COLLECTION IN THE DEMOGRAPHIC AND HEALTH SURVEYS
The Demographic and Health Survey (DHS) program has been at the forefront of incorporating biomarker testing in large-scale national household surveys. To date, the DHS has measured 16 different biomarkers in >80 developing countries in >200 surveys (Table 1). This has been possible because of the investment DHS puts into training and building in-country capacity and the availability of new or existing technologies and tests that can be adapted and applied to a survey.
TABLE 1.
Biomarker tests conducted under the Monitoring and Evaluation to Assess and Use Results (MEASURE) Demographic and Health Survey (DHS) project1
| Biomarker | Year test first done | No. of surveys including test | Population typically tested2 | Sampling method and equipment used |
| Anthropometric measurements (weight, height, age) | 1987 | 147 | Women aged 15–49 y; children aged 0 (6)–59 mo; men aged 15–59 y | Noninvasive, measuring board and scale |
| Hemoglobin (for anemia) | 1995 | 78 | Women aged 15–49 y; children aged 0–59 mo; men aged 15–59 y | Capillary blood, HemoCue portable analyzer3 |
| HIV | 2001 | 41 | Women aged 15–49 y; men aged 15–59 y | Capillary blood, DBSs |
| Blood pressure | 1998 | 8 | Women aged 15–49 y; men aged 15–59 y | Noninvasive, automatic cuff |
| Syphilis | 1996 | 6 | Women aged 15–49 y; men aged 15–59 y | Venous blood, RPR |
| Vitamin A | 1996 | 4 | Women aged 15–49 y; children aged 0 (6 or 12)–59 mo | Capillary blood, DBSs, HPLC, RBP-EIA |
| Malaria | 2006 | 3 | Women aged 15–49 y; children aged 0 (6)–59 mo | Capillary blood, RDT, thick/thin slides |
Other, less-common tests: hepatitis B, hepatitis C, herpes, measles, tetanus, chlamydia, diabetes, lipids, C-reactive protein, transferrin. DBSs, dried blood spots; RPR, rapid plasma reagin; RBP-EIA, retinol binding protein enzyme immunoassay; RDT, rapid diagnostic test.
Varies by country; the numbers in parentheses reflect the variation in ages in a typical DHS population (eg, men aged 15–54 or 15–64 y; children aged 0–59 or 6–59 mo).
HemoCue AB, Angelhom, Sweden.
Ideally, tests for measuring biomarkers in population-based surveys should be field-friendly, defined as follows:
1) Tests should be able to be applied directly in the field.
2) Tests should require minimal equipment.
3) Field staff should require minimal training to be able to perform the tests.
4) Samples used in the tests should be easy to collect, handle, and transport.
5) There should be a short turnaround time for results if the testing is done in a central laboratory.
To support field testing, the equipment or kits for the tests need to be rugged, portable, resilient to extreme temperatures and humidity, and relatively inexpensive. Furthermore, the tests should perform well under field conditions and show a high degree of accuracy, sensitivity, and specificity for the biomarkers of interest. Meeting all of these conditions is a challenge. However, the confluence of advances in research and technology, increasing donor interest, and the capacity of DHS to implement biomarker tests in challenging environments have made it possible for DHS to incorporate more biomarkers in survey operations.
Tests for biomarkers in population-based surveys are commonly performed by nonmedical staff, and therefore the tests have to be the least intrusive as possible and should preferably deliver results in a short period of time because testing is conducted in the household and more often than not includes young children aged <5 y.
Most tests require the collection of blood, which produces layers of complexities and considerations for survey operations. First, the intrusiveness of the method could reduce the response rate for the test and possibly for the overall interview as well. Expert training of the interviewer/tester in how to approach the potential respondents in a sensitive manner, coupled with proficiency in the application of the test, has been critical in maintaining high response rates. Here again, technology comes to the aid of measurement. For example, in the case of the measurement of hemoglobin for the determination of anemia, development of portable analyzers such as the HemoCue (HemoCue AB, Angelhom, Sweden) have made it possible to use a very small amount of blood from a finger prick to produce reliable results within a few minutes.
In the past, many tests required the collection of venous blood, making it necessary to have specially trained phlebotomists, which made the survey operation more expensive. However, the main challenge in collecting venous whole blood is the preservation of the integrity of the blood components before analysis in a central laboratory. The preservation of venous whole blood samples requires a “cold chain” in the field setting, which has to be maintained from the point of collection up to the storage and processing of samples at the central laboratory. Furthermore, a relatively large sample of blood is often collected. The collection of blood on filter paper cards as dried blood spots (DBSs) has facilitated the addition of biomarkers other than HIV in DHS.
The increased application of tests based on the collection of DBSs has revolutionized the capacity to obtain biomarkers in field settings. DBS samples have several advantages over venous blood (1, 2). The use of DBS samples has eliminated the need for a cold chain and refrigeration of specimens in the field, reducing considerably the complexity of storage in remote areas and transport to the laboratory. DBSs are obtained from the prick of a finger (or a heel in the case of very young children), thus reducing the need for syringes and needles and test tubes and racks and reducing the invasiveness of the procedure. DBS samples are collected in preprinted circles on a filter paper card, dried overnight, protected with glassine paper, sealed tightly in commercially available plastic bags with desiccants and humidity indicators, and then transported with relatively little effort to the central laboratory for testing. Many new tests, including those for HIV, micronutrients, infectious diseases, and tracking immunization coverage, are increasingly being validated for use with DBSs.
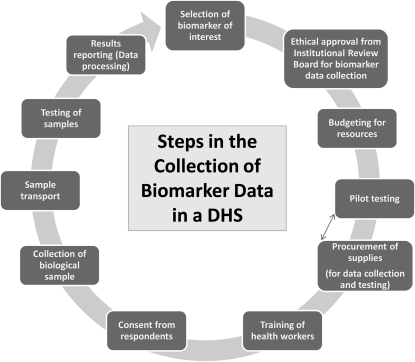
The availability of simpler tests, however, does not mean that conducting biomarker tests in the field is simple. Due consideration needs to be given to protection of human subjects (including protection of the persons collecting samples), informed consent procedures, formal approval of the survey protocol by one or more institutional review boards, procurement of all equipment and supplies and delivery in a timely manner (including attention to customs requirements and local rules and regulations), comprehensive training and field practice, preparation of detailed manuals for blood collectors and field supervisors, the logistics of getting needed supplies into the field, careful arrangements for the safe disposal of biohazardous waste, assessments of laboratory capabilities, and the preparation of laboratory protocols. The procedural steps for the collection of biomarkers in a typical DHS are outlined in Figure 1.
FIGURE 1.
Procedural steps for the collection of biomarker data in a typical Demographic and Health Survey (DHS).
This article focuses on the DHS experience in using field-friendly technologies to assess micronutrient deficiencies in Tanzania and Uganda.
OVERVIEW OF VITAMIN AND MINERAL DEFICIENCIES IN TANZANIA AND UGANDA
Vitamin and mineral deficiencies (VMDs) remain a significant public health problem affecting all segments of society in both industrialized and developing countries. In many developing countries, the problems are especially severe. The prevalence of vitamin A, iron, zinc, folate, and vitamin B-12 deficiencies are particularly high and are associated with significant adverse health consequences, including impaired immune function, slowed cognitive development, and an increased risk of severe infection and mortality. According to a 1998 report from the Tanzania Food and Nutrition Centre, the prevalence of VAD in lactating women was 69% and was 24% in children aged 6–59 mo (3). More recently, the Tanzania DHS (TDHS) found that the prevalence of anemia in pregnant women and children aged 6–59 mo was 48.4% and 71.8%, respectively (4). The picture of micronutrient deficiencies in Uganda is as dire as in Tanzania. The 2006 Uganda DHS (UDHS) report cited a prevalence of anemia of 49% in women aged 15–54 y and 73% in children aged 6–59 mo (5). The prevalence of VAD in these 2 groups was 19% and 20%, respectively. The last report on iron deficiency (ID) in Uganda on the basis of serum ferritin concentrations quoted a prevalence of 51.1% in pregnant women, 37.9% in nonpregnant women, and 45.4% in 9-mo-old infants (6). These data indicate that VMDs are a public health problem in Tanzania and Uganda. Consequently, Tanzania and Uganda, like other countries that aim to eliminate hunger and poverty and reduce preventable maternal and child mortality to meet a number of the Millennium Development Goals, have initiated many public health initiatives focused on the elimination and/or control of VMD. The planning and implementation of these initiatives can be better guided by collecting baseline data on VMD, as was carried out in the UDHS 2006 and the TDHS 2009.
APPLICATION OF FIELD-FRIENDLY TECHNIQUES TO ASSESS VAD AND ID IN THE UDHS AND TDHS
Subjects and methods
The UDHS was conducted in 2006, and the TDHS 2009 is currently collecting data (data collection is expected to end in mid-May). In the UDHS and the TDHS, concentrations of retinol-binding protein (RBP; a proxy for retinol) and transferrin receptor (sTfR) were measured to assess the prevalence of VAD and ID, respectively. We also measured C-reactive protein (CRP) to adjust the RBP data for the effect of inflammation/infection. DBS samples were collected from women and children, as described below, and transferred to a central laboratory for testing. All testing was carried out by local personnel using commercially obtained test kits. Hemoglobin was also measured in the household in women and children using a portable device (HemoCue) to assess the prevalence of anemia.
Sample size calculations in the DHS
In DHS surveys, sample size is determined for various indicators including fertility and mortality of a given population. Typically, all households or half of the sampled households are included for anemia testing. Sample size determinations are usually made for smaller regions and extended to the entire population. Depending on the country, a sample of 300–1500 households comprises a smaller region. There are various factors used in calculating the sample size for biomarkers to be included in the survey. One of the factors used is the “indicator value,” which is derived from the prevalence of a given condition. The indicator value for biomarkers of nutrition is almost >20% in most of the survey countries. The values for prevalence, total sample size, design effect (calculated for each variable and an average is used), and relative errors are inserted into the formula for calculation of the sample size required for biomarker testing. Typically, as the indicator value increases, the sample required for smaller regions will be low. In addition, budget plays an important role in determining sample size for a given survey, an undersize sample may not have the power to show the desired results; however, an oversized sample may be a waste of resources. Sample size is determined during the planning phases of the survey because it has direct bearing on the cost and procurement of supplies for biomarkers tested.
Sample collection
Obtaining consent
Before sample collection, verbal informed consent was obtained directly from respondents aged 18–49 y. For respondents aged 15–17 y, verbal informed consent was first obtained from the parent or adult responsible for the youth. If the parent or adult consented to the testing of the youth, assent was required from the youth to proceed with the blood collection. In cases in which the parent gave consent but the youth did not assent, no blood samples were collected. Verbal consent was obtained from parents or adults responsible for children aged 6–59 mo.
Blood collection
Although it is recognized that serum is the sample of choice for many biochemical assays, DHS typically collects blood samples as DBSs because of reasons mentioned previously. For the measurement of CRP, RBP, and sTfR in the UDHS and TDHS, we collected DBS samples for the analysis of these markers. The sample collection procedure has been described in detail elsewhere (7). Briefly, blood was collected directly from a finger prick by allowing blood drops to fall freely inside of preprinted circles on special filter paper cards (Whatman 903; GE Healthcare Bio-Sciences Corp, Piscataway, NJ), dried overnight, packaged, and sent to the laboratory for testing.
Specifically, either the third or fourth finger (ring finger; counting from the thumb) was cleaned with a cotton swab containing 70% alcohol, air-dried, and punctured with a sterile, retractable lancet (2.5 mm depth; Owen Mumford, Marietta, GA). The first blood drop was wiped away with a sterile gauze pad, and the circles on the filter paper card were filled with free-flowing blood (one drop of blood per circle) from the puncture. A Band-Aid (plaster; NDC Inc, La Vergne, TN) was used to cover the puncture site after the blood had stopped flowing.
The filter paper cards with the blood spots were then placed horizontally in a drying rack that was fixed to the inside of a plastic, rectangular box with a lid (drying box). Desiccants (Minipak; Multisorb Technologies, Buffalo, NY) were placed in the box to keep the cards dry and to hasten the drying process. The amount of humidity inside the box was monitored with humidity indicator cards (Humonitor; Multisorb Technologies). After the DBSs had dried completely overnight, the field technician, wearing gloves, removed the filter paper cards one by one from the drying box, placed a sheet of glassine paper over the blood spots, and packed the cards individually in a low, gas-permeable, plastic zip-lock bag containing 2–3 sachets of desiccant and a humidity indicator card. The zip-lock bags containing the filter paper cards were then placed in a larger plastic bag in a portable battery-operated refrigerator at 4°C until they were transferred from the field site to the central laboratory for testing.
To transfer the samples to the central laboratory, the large plastic bags holding the DBS samples were moved from the portable refrigerator to a box containing ice packs and transported to the laboratory and stored at −20°C until tested.
To keep track of survey samples, at the time of collection a unique, alpha-numeric barcode identification was pasted on the filter paper card, and the same barcode was pasted onto a sample tracking form (sample transmittal sheet); this form sent along with the samples to the laboratory. At the laboratory, the barcode ID on each sample was checked against the barcode ID on the tracking form, and discrepancies between the number of samples received and the number of barcodes on the tracking form were noted. Samples without barcodes were not tested because the barcode IDs are the only link between the households' and respondents' demographic data.
LABORATORY ANALYSIS OF DBS FOR CRP, RBP, AND sTfR
All DBS samples collected in the UDHS 2006 and TDHS 2009 were checked individually for stability (gauged by a humidity indicator card), adequacy of spots, blood clots, smudges, contamination with dirt or another substance, or incompletely filled circles. Once the samples were deemed adequate for testing, they were allowed to reach room temperature and were tested for the 3 biomarkers using commercially available test kits, with the exception of CRP testing in the UDHS, which was tested using an in-house assay (CSDE Biodemography Core; University of Washington, Seattle, WA). For the TDHS, we used the Bender MedSystems instant enzyme immunoassay (Bender MedSystems, Vienna, Austria); for RBP, we used the Scimedx RBP Assay (Scimedx Corporation, Denville, NJ); and sTfR was determined by using a commercial enzyme immunoassay (TF-94; Ramco Laboratories, Stafford, TX) (8, 9). Because these assays were not developed for the DBS, it was necessary to assess the elution profile of the analytes of interest from the filter paper matrix, a procedure we believe should always be done. We validated the DBS as a sample matrix for CRP, RBP, and sTfR. The procedure for validation of DBS for these markers has been discussed previously (7).
DISCUSSION
The successful inclusion of multiple biomarkers of nutrition in 2 DHSs (and anemia and vitamin A testing in 78 surveys) exemplifies how biomarker collection can be effectively introduced into large, population-based surveys. Drawing on its extensive experience collecting biomarkers on anemia and especially HIV, and in obtaining anthropometric measurements, the DHS was able to overcome the following logistical challenges: sample collection, sample transport to a central testing facility, supervision of staff, management of a large number of samples, management of large volumes of data, and measurements of 3 biomarkers as part of a nationally representative survey. The successful outcome of these 2 surveys—Tanzania and Uganda—showed that it is feasible to measure biomarkers in challenging environments, and where local technical capacity may be limited, if adequate training of staff and sustained supervision of the project is made a priority of stakeholders. Because the DHS is the leading source of demographic and health data, the assessment of VMD in the context of a DHS provides a very rich blend of demographic, health, and nutrition data that are invaluable not only to ministries of health but to academicians, researchers, program managers, and public health workers.
Despite the extensive training of staff and capacity building that go into each DHS, there are still numerous challenges within the survey country that have a direct bearing on the outcome of the testing. These challenges must not only be recognized before implementation of the survey but must be addressed head on if the survey is to be successful. For example, in most DHS countries, infrastructure is a serious hindrance to testing large numbers of samples. There is usually inadequate space for sample analysis, lack of refrigerators and freezers for storage of samples, an unstable supply of electricity, and a lack of facilities to produce deionized water. In many laboratories, the equipment needed to conduct testing is not available or, if it is, has not been serviced or is not working properly. Furthermore, there is the issue of recruiting enough full-time staff to conduct the testing (which can last up to 4 mo) or the staff available may not have the skills to conduct the chosen assays. Whenever feasible, the DHS works with the survey implementing organization in the country to rectify these problems before implementation of a survey. The DHS provides intensive training to the staff, procures equipment and supplies, and provides ongoing technical support and supervision for the laboratory analyses. Apart from the on-the-ground challenges, the DHS also is also cognizant of the utility of measuring the respective biomarkers as a proxy for a vitamin or mineral deficiency in the context of a national survey and of the appropriate selection of the sample type and test kits for analysis.
In DHS surveys, the prevalence of anemia is used as a crude measure of iron deficiency (ID). Although anemia is a crude measure of ID, it is an effective screening tool for anemia in population-based surveys and allows for on-the-spot reporting of results to respondents. However, anemia testing when coupled with accurate measures of VMD can provide insight into the underlying cause of the condition. Consequently, the DHS incorporated the measurement of sTfR as a marker of ID in the UDHS and TDHS, in conjunction with hemoglobin measurements. The major advantages of measuring sTfR are that infection or inflammatory processes do not significantly affect the assay (10), and its concentration does not vary with age, sex, or pregnancy status (11, 12). Currently, the only major limitation with the use of sTfR is that there is no internationally certified standard available, and each method or kit has its own cutoff. Because different methods for the measurement of sTfR correlate very well (13), it is relatively easy to get the same prevalence rates when the appropriate cutoff is used.
The World Health Organization recommends the measurement of serum retinol (vitamin A) for assessment of vitamin A status at the population level. HPLC is the preferred method for the determination of vitamin A because HPLC is considered to be reliable and highly accurate. However, HPLC requires skilled staff, the equipment is expensive, and the analytic procedure is long and cumbersome. For the assessment of VAD as a public health concern, RBP has been recommended as a surrogate marker of retinol (14) and has been used by the DHS and others to assess VAD at the population level (9, 15, 16). RBP lends itself best to DHS because blood can be collected as DBSs and the samples analyzed in a central laboratory by enzyme immunoassay. Even though it is feasible to measure retinol in DBSs, the analysis requires the use of HPLC (17). Furthermore, RBP in DBSs is relatively stable compared with retinol in DBSs and is less likely to be degraded in the filter paper matrix when collected under harsh environmental conditions that exist in tropical countries.
Despite overcoming some of the challenges of assessing VAD in field settings by measurement of RBP in DBSs, there are limitations to using RBP (and retinol) for the assessment of VAD. Specifically, it is known that the concentrations of RBP (15) and serum retinol (18, 19) decrease during the acute phase response to infection. The modulatory effect of infection/inflammation on retinol and RBP concentrations is problematic, particularly in developing countries, in which there is a high burden of infection. Consequently, there is a high likelihood that apparently healthy young children and women recruited to participate in vitamin A surveys harbor a subclinical infection. In the UDHS 2006, the prevalence of any infection/inflammation (>5 mg CRP/L) was 34.9% and 58% in women and children, respectively (unpublished data, Macro International, 2006). Thus, to improve estimates of vitamin A deficiency in surveys that use serum retinol and RBP, the simultaneous assessment of positive acute-phase proteins such as CRP, α1-acid glycoprotein, and α1-antichymotrypsin is recommended (18–23). Furthermore, by measuring CRP, the RBP data can be adjusted for infection status, rather than excluding respondents with a subclinical infection from the data analysis, which could potentiate sampling bias (24).
CONCLUSIONS
In population-based surveys conducted in developing countries, a major hindrance to effective assessment of VMD is the lack of appropriate and cost-effective tools that can effectively and accurately assess micronutrient deficiencies. This is critical for establishing the magnitude of the problem, identifying subpopulations at greatest risk, better designing and prioritizing interventions, and monitoring and tracking the progress that has been achieved through micronutrient intervention programs. The 2 examples cited above show that it is feasible to measure biomarkers of nutrition in a nationally representative, population-based survey in the face of the numerous challenges that exist in most developing countries. The successful collection of biomarker data in the UDHS and TDHS serves as a model to incorporate biomarkers of nutrition in other surveys. In the future, it would be ideal to have an assay that can measure all of the biomarkers collected in the UDHS and TDHS from a single sample, as this has the potential to reduce survey cost by reducing the time required to analyze samples, thereby producing results in a shorter time frame and decreasing the amount of supplies and reagents required. An assay that meets many of these requirements is under development at the Program for Appropriate Technology in Health (PATH), Seattle, WA, but it is not yet ready for field use.
Acknowledgments
The authors' responsibilities were as follows—DB: conducted the literature review; MTK: conceptualized the outline of the manuscript and created the DHS biomarker data collection model; JKS: contributed the section on sample size; DAG: drafted the manuscript; JKS and MTK: revised the manuscript; and DAG, JKS, and MTK: read and approved the final version of the manuscript. The authors declared that they had no conflicts of interest.
REFERENCES
- 1.Parker SP, Cubitt WD. The use of the dried blood spot sample in epidemiological studies. J Clin Pathol 1999;52:633–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDade TW, Williams S, Snodgrass JJ. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography 2007;44:899–925 [DOI] [PubMed] [Google Scholar]
- 3.Ballart A, Mugyabuso JKL, Ruhiye DRM, Ndossi GD, Basheke MM. The national vitamin A deficiency control program: preliminary report on the national vitamin A survey of 1997. Dar es Salaam, Tanzania: Tanzania Food and Nutrition Centre (TFNC Report No.1880) [Google Scholar]
- 4.National Bureau of Statistics, Tanzania and ORC Macro. Tanzania Demographic and Health Survey 2004-05. Dar es Salaam, Tanzania: National Bureau of Statistics and ORC Macro, 2005 [Google Scholar]
- 5.Uganda Bureau of Statistics, Macro International Inc UDHS 2006 Kampala. Calverton, MD: Uganda Bureau of Statistics and Macro International Inc, 2007 [Google Scholar]
- 6.Kiwanuka GN, Isharaza WK, Mahmoud S. Iron status of pregnant women at first antenatal booking in Mbarara University Teaching Hospital. Trop Doct 1999;29:228–30 [DOI] [PubMed] [Google Scholar]
- 7.Baingana RK, Matovu DK, Garrett D. Application of retinol-binding protein enzyme immunoassay to dried blood spots to assess vitamin A deficiency in a population-based survey: the Uganda Demographic and Health Survey 2006. Food Nutr Bull 2008;29:297–305 [DOI] [PubMed] [Google Scholar]
- 8.McDade TW, Shell-Duncan B. Whole blood collected on filter paper provides a minimally invasive method for assessing human transferrin receptor level. J Nutr 2002;132:3760–3 [DOI] [PubMed] [Google Scholar]
- 9.Shell-Duncan B, McDade T. Cultural and environmental barriers to adequate iron intake among northern Kenyan schoolchildren. Food Nutr Bull 2005;26:39–48 [DOI] [PubMed] [Google Scholar]
- 10.Ray A, Ndugwa C, Mmirot F, Ricks MO, Semba RD. Soluble transferrin receptor as an indicator of iron deficiency in HIV-infected infants. Ann Trop Paediatr 2007;27:11–6 [DOI] [PubMed] [Google Scholar]
- 11.Kohgo Y, Nishisato T, Kondo H, Tsushima N, Niitsu Y, Urushizaki I. Circulating transferrin receptor in human serum. Br J Haematol 1986;64:277–81 [DOI] [PubMed] [Google Scholar]
- 12.Carriaga MT, Skikne BS, Finley B, Cutler B, Cook JD. Serum transferrin receptor for the detection of iron deficiency in pregnancy. Am J Clin Nutr 1991;54:1077–81 [DOI] [PubMed] [Google Scholar]
- 13.Biesalski HK, Erhardt JG. Diagnosis of nutritional anemia–laboratory assessment of iron status :Kraemer K, Zimmermann MB. Nutritional anemia. Basel, Switzerland: Sight and Life Press, 2007:45–57 [Google Scholar]
- 14.de Pee S, Dary O. Biochemical indicators of vitamin A deficiency: serum retinol and serum retinol binding protein. J Nutr 2002;132:2895S–901S [DOI] [PubMed] [Google Scholar]
- 15.Baeten JM, Richardson BA, Bankson DD, et al. Use of serum retinol-binding protein for prediction of vitamin A deficiency: effects of HIV-1 infection, protein malnutrition, and the acute phase response. Am J Clin Nutr 2004;79:218–25 [DOI] [PubMed] [Google Scholar]
- 16.Gorstein JL, Dary O, Pongtorn, Shell-Duncan B, Quick T, Wasanwisut E. Feasibility of using retinol-binding protein from capillary blood specimens to estimate serum retinol concentrations and the prevalence of vitamin A deficiency in low-resource settings. Public Health Nutr 2008;11:513–20 [DOI] [PubMed] [Google Scholar]
- 17.Craft NE, Haitema T, Brindle LK, Yamini S, Humphrey JH, West KP., Jr Retinol analysis in dried blood spots by HPLC. J Nutr 2000;130:882–5 [DOI] [PubMed] [Google Scholar]
- 18.Filteau SM, Morris SS, Abbott RA, et al. Influence of morbidity on serum retinol of children in a community-based study in northern Ghana. Am J Clin Nutr 1993;58:192–7 [DOI] [PubMed] [Google Scholar]
- 19.Christian P, Schulze K, Stoltzfus RJ, West KP., Jr Hyporetinolemia, illness symptoms, and acute phase protein response in pregnant women with and without night blindness. Am J Clin Nutr 1998;67:1237–43 [DOI] [PubMed] [Google Scholar]
- 20.Paracha PI, Jamil A, Northrop-Clewes CA, Thurnham DI. Interpretation of vitamin A status in apparently healthy Pakistani children by using markers of subclinical infection. Am J Clin Nutr 2000;72:1164–9 [DOI] [PubMed] [Google Scholar]
- 21.Thurnham DI, McCabe GP, Northrop-Clewes CA, Nestel P. Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency: meta-analysis. Lancet 2003;362:2052–8 [DOI] [PubMed] [Google Scholar]
- 22.Thurnham DI, Mburu AS, Mwaniki DL, De Wagt A. Micronutrients in childhood and the influence of subclinical inflammation. Proc Nutr Soc 2005;64:502–9 [DOI] [PubMed] [Google Scholar]
- 23.Wieringa FT, Dijkhuizen MA, West CE, Northrop-Clewes CA. Muhilal. Estimation of the effect of the acute phase response on indicators of micronutrient status in Indonesian infants. J Nutr 2002;132:3061–6 [DOI] [PubMed] [Google Scholar]
- 24.Maqsood M, Dancheck B, Gamble MV, et al. Vitamin A deficiency and inflammatory markers among preschool children in the Republic of the Marshall Islands. Nutr J 2004;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]