It has often been said that medicine is an art based on conjecture and improved by murder. Valethamate bromide is a drug widely used all over India by obstetricians for facilitating cervical dilatation in the first stage of labor.[1] It is sold under many brand names such as Epidosin, Dilaton, Valosin, Valamate, Osdil etc. Even though this drug is available in India for more than a quarter of a century, it is still not listed in the Indian Pharmacopoeia. None of the well established textbooks of pharmacology from North America and the United Kingdom mention the drug, though one popular textbook from India makes a mention.[2] Reliable sources of information like the British National Formulary also do not list it. A Google Scholar search on the term produces ten hits and a PubMed search on the molecule throws up just seven articles with only two clinical trials published in peer reviewed indexed journals. This seems to be the total quantity of information from the web sources. Yet, it is listed in the Tamil Nadu Medical Services Corporation procurement list as the item with the drug code 460.[3]
This raises an interesting question. How and why is this drug so popular when there is so little information of its safety and efficacy? It is obvious that the drug is being used mainly due to three reasons. First, cervical ripening and dilatation is a natural feature of labor and using drugs to hasten the process may be beneficial in crowded labor rooms to facilitate the reduction in time spent in monitoring women in labor rather than any sound medical reason. Second, there are also no “new” drugs for this indication since evaluating drugs in pregnant women has its own share of problems and ethical concerns. Therefore, continuing to use something which was introduced when the criteria for using drugs were less stringent is easy. Third, the promotional pressures from the pharmaceutical companies are relentless. Obstetricians may be duped to believe that they are indeed using a drug which is listed in the Indian Pharmacopoeia (I.P.)
The carton of Epidosin lists three ingredients on it [Figure 1]. One is valethamate bromide and the other two are normal saline and water for injection. It is interesting to note that both saline and water have I.P. listed next to their names. What is missed by the undiscerning mind is that there is no I.P next to valethamate bromide. This is perhaps why many practicing obstetricians have failed to question the very basis for the use of this drug. Is it efficacious? There are no studies which prove it, and the meagre data shows that it is ineffective.[4,5] One study which mentions that valethamate is as effective as drotavarine[6] is an unblinded, underpowered trial which has used inappropriate statistical tests for data analysis, while another study comparing the same drugs says it is less efficacious than drotavarine but has more side effects.[7]
Figure 1.
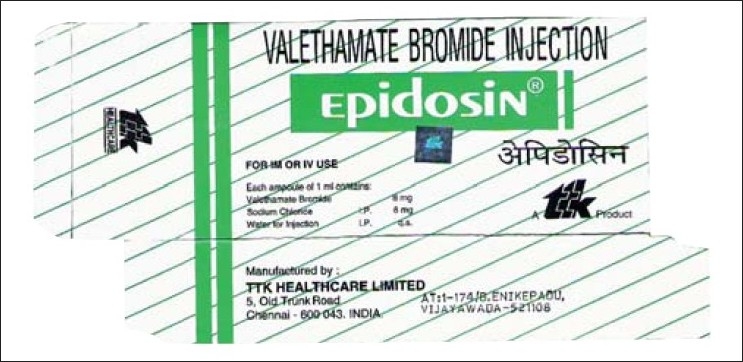
A photograph of the carton of Epidosin (valethamate bromide)
Is it safe? Not really, since two studies have recorded adverse events of moderate intensity[4 5] and one study noted mild events.[7] This brings us to the question, whether it should be used in pregnant women at all since both the efficacy and safety have not been adequately documented. The answer to this question can only be an emphatic “no”. The World Health Organization (WHO) has brought out guidelines outlining the criteria for the selection of drugs for any indication and valethamate bromide does not satisfy even one of them. It is hoped that obstetricians will stop using this drug in pregnant women until there is convincing evidence of its efficacy and safety and it is listed in the Indian Pharmacopoeia. Essential medicine lists and procurement lists should not list this drug which does not have the evidence to support its continued use in pregnant women.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Madhu C, Mahavarkar S, Bhave S. A randomised controlled study comparing drotaverine hydrochloride and valethamate bromide in the augmentation of labour. Arch Gynecol Obstet. 2009 doi: 10.1007/s00404-009-1188-8. DOI 101007/s00404-009-1188-8. [DOI] [PubMed] [Google Scholar]
- 2.Tripathi KD. 6th ed. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2008. Essentials of Medical Pharmacology. [Google Scholar]
- 3. [accessed on 13th November 2009]. http://www.tnmsc.com/tnmsc/new/html/services.php# .
- 4.Kuruvila S, Jasper P, Peedicayil A, Mathai M. A randomized controlled trial of valethamate bromide in acceleration of labor. Int J Gynaecol Obstet. 1992;38:93–96. doi: 10.1016/0020-7292(92)90042-h. [DOI] [PubMed] [Google Scholar]
- 5.Yilmaz B, Kart C, Kelekci S, Gokturk U, Sut N, Tarlan N, Mollamahmutoglu L. Meperidine versus valethamate bromide in shortening the duration of active labor. Int J Gynaecol Obstet. 2009 Nov;107:126–9. doi: 10.1016/j.ijgo.2009.06.021. Epub 2009 Aug 6. [DOI] [PubMed] [Google Scholar]
- 6.Ajmera SK, Shah PK, Shinde GA. Bombay Hospital Journal.Comparative Study of Drotaverine v/s Valethamate in Cervical Phase of Labour. [webpage on internet] [cited on: 2009 Nov 13]. Available at: http://bhj.org/journal/2006_4802_april/html/org_res_305-309.html .
- 7.Sharma JB, Pundir P, Kumar A, Murthy NS. Drotaverine hydrochloride vs.valethamate bromide in acceleration of labor. Int J Gynaecol Obstet. 2001;74:255–60. doi: 10.1016/s0020-7292(01)00448-9. [DOI] [PubMed] [Google Scholar]


