Abstract
Parkinsonian rigidity is characterized by an increased resistance of a joint to externally imposed motion that remains uniform with changing joint angle. Two candidate mechanisms are proposed for the uniformity of rigidity, involving neural-mediated excitation of shortening muscles, i.e., shortening reaction (SR), or inhibition of stretched muscles, i.e., stretch-induced inhibition (SII). To date, no study has addressed the roles of these two phenomena in rigidity. The purpose of this study was to differentiate these two phenomena, and to quantify the potential contribution of each to wrist joint moment in 17 patients with parkinsonian rigidity, in both Off- and On-medication states. Joint position, torque, and EMGs of selected muscles were collected during externally imposed flexion and extension motions. Moments of shortened and stretched muscles were estimated using a biomechanical model. Slopes of the estimated torque–angle curve were calculated for shortened and stretched muscles, separately. A mixed model ANOVA was performed to compare the contribution between the two mechanisms. During flexion, slopes were significantly (P = 0.003) smaller for SR than for SII, whereas during extension, slopes for SII were significantly (P = 0.003) smaller. Results showed that both SR and SII contributed to rigidity. Which mechanism predominates appeared to be associated with the direction of movement. The findings provide new insights into the biomechanical underpinnings of this common symptom in Parkinson’s disease.
Keywords: Rigidity, Biomechanical modeling, Shortening reaction, Stretch-induced inhibition, Parkinson’s disease
Introduction
Rigidity is one of the clinical hallmarks in Parkinson’s disease (PD) and it responds well to dopaminergic medication, thereby forming an integral part of clinical diagnosis and therapeutic evaluation in PD. Rigidity is defined as an increased resistance to passive movement of a limb and is felt as a constant resistance persisting throughout its range of passive motion (Fung and Thompson 2002). In particular, parkinsonian rigidity is widely described as providing a “lead-pipe” type of resistance, a unique characteristic distinguishing the increased muscle tone in rigidity from that associated with spasticity and from other forms of abnormal muscle tone. An elucidation of the underlying mechanisms bears scientific merit and clinical significance.
There are two basic elements in parkinsonian rigidity: (1) hypertonia, which is an increased resistance of the joint to passive movement; and (2) uniformity of the resistance or “lead-pipe” resistance, in which the mechanical resistance stays relatively constant across the range of joint motion. Passive movement in this context refers to the absence of voluntary muscle activation during externally imposed joint motion.
In spite of previous efforts to characterize the mechanisms of rigidity, the physiological and biomechanical mechanisms are still poorly understood. The main established finding in this arena is an exaggeration of the long-latency stretch reflex (Lee and Tatton 1975; Rothwell et al. 1983), which is potentially responsible for the hypertonia of rigidity. The latency for this component is approximately 60 ms for the forearm muscles (Lee and Tatton 1982). However, the increased long-latency stretch reflex cannot account for the uniformity of the imposed resistance with changing joint angle. Elicitation of the long-latency stretch reflex depends on both stretch velocity and stretch duration, specifically the interaction of the two factors. A low velocity with sufficient stretch duration can also evoke long-latency EMG components (Lee and Tatton 1982). Additionally, no prior study has addressed how the underlying muscle activation accounts for the uniformity of rigidity.
Biomechanically, rigidity can be quantified by the amount of torque required to change joint position during externally imposed movement, and it is characterized by a constant resistance displayed as a flat “torque–angle” relationship during the stretch, thus promoting a perception of constant resistance (Xia and Rymer 2004). It is well known, however, that when an active muscle is stretched, the muscle force output generally increases in proportion with the increase of muscle length, generating mechanical properties analogous to those of a spring, hence the “spring-like” property (Gordon et al. 1966; Matthews 1959; Rack and Westbury 1969). This principle also applies to the passive condition in PD, because patients with parkinsonian rigidity are unable to remain completely relaxed, exhibiting increased background EMG activity (Burke et al. 1977; Marsden 1982; Wallin et al. 1973).
Given the spring-like property, the torque–angle relationship in healthy subjects, may display a relatively steep curve, or a constant or non-linear shape due to the interaction of numerous muscles acting together or in opposition across a given joint, and to the interaction of the individual muscle force and its changing moment arm as a function of joint position. The patterns of torque–angle curve are dependent on the joint of interest, the operating range of motion and the direction of the movement (Buchanan et al. 1998; Delp et al. 1996; Knutson et al. 2000). In addition, this spring-like behavior can be modified by changes in the neural excitation signals, potentially generating a flat torque–angle relationship equivalent to the constant resistance perceived in parkinsonian rigidity. Most importantly for our current study, a constant torque–angle curve could result from either a shortening reaction (SR) or a stretch-induced inhibition (SII) or a combination of the two. The potential origins of both phenomena are described as follows.
There have been longstanding reports of substantial muscle activation in shortening muscles during passive movements. Over a century ago, Westphal (1880) observed muscular contraction in the passively shortened skeletal muscles. Later, Sherrington (1909) described analogous findings in both the spinal dog and the decerebrate cat under the name “shortening reaction,” a terminology that has been used since. Sherrington (1909) also reported a “lengthening reaction” in the spinal animals. When an examiner externally bent or flexed the knee against a tonic extensor contraction, the examiner felt the opposition offered by the extensor gave away almost abruptly at a certain pressure, and the knee could then be flexed without opposition.
The clasp-knife reflex associated with spasticity in humans and animals is characterized by an abrupt decline in muscle force that occurs when a spastic limb is moved beyond a certain angle (Burke et al. 1970, 1972; Rymer et al. 1979). There is potential common ground between the lengthening reaction in animals and the clasp-knife phenomenon in human spasticity, in that the essential feature of both phenomena is the sudden release of the resistance due to the continuous stretch of the elongated muscle, referred to as “stretch-induced inhibition”. However, none of the previous studies on parkinsonian rigidity has explored a potential association of rigidity with lengthening reaction or stretch-induced inhibition. The exploration of this phenomenon is of potential clinical significance, as the differentiation between rigidity and spasticity is not straightforward in a clinical scenario (Fung and Thompson 2002).
In parkinsonian rigidity, the torque produced by the stretched muscles is potentially increased due to the exaggerated long-latency stretch reflexes (Lee and Tatton 1975; Rothwell et al. 1983) and to enhanced tonic muscle responses (Dietrichson 1971) in cases of sufficiently high stretching velocity and sufficiently long displacement duration. There are two ways in which a joint could generate relatively constant torque with the changing joint position. First, if there is an inappropriate activation of SR (e.g., during extension movement), the increasing force generated by the stretched flexor could be offset by increasing activation of the shortening extensor. This muscle interaction could lead to a flat torque–angle relationship, and provide the perception of constant rigidity. Another possibility is that a gradual reduction in activation of stretched muscle at an elongated muscle length counteracts the otherwise gradual increase in muscle force (i.e., spring-like or elastic-like muscle force) as the muscle length of the stretched flexors is elongated throughout the stretch. Due to this counteracting effect, the net torque could remain relatively constant throughout the range of joint motion.
Potentially, both SR and SII have counteracting effects within a specified range of joint movement, generating the promotion of constant rigidity (uniformity) as defined in parkinsonian rigidity. During passive movements, the two mechanisms are potentially generating counteracting effects on the net torque resistance simultaneously, because one group of muscles is shortened whereas the other group of muscles is stretched. A dissociation of the two mechanisms is not readily available and technically challenging. Therefore, the objective of the present study was to differentiate these two physiological mechanisms and to quantify the contribution of each mechanism to PD rigidity, by employing a biomechanical model of the upper extremity including the wrist joint.
Methods
Subjects
Seventeen subjects with idiopathic PD participated in the current study. Subjects’ ages ranged from 46 to 74 years (62.0 ± 8.9 years). Table 1 summarizes participants’ demographic characteristics and clinical information. The unified Parkinson’s disease rating scale (UPDRS; Fahn and Elton 1987) was used to assess each subject’s clinical degree of rigidity at the wrist joint. Informed consent was obtained prior to subjects’ participation in the study. The experimental protocol was approved by the Institutional Review Board of Creighton University, Omaha, Nebraska, USA and was conducted in accord with the Declaration of Helsinki.
Table 1.
Patients’ clinical information
| Patient | Age (years) | Disease duration (years) | Sex | Arm tested | Rigidity (UPDRS)a |
Medication | |
|---|---|---|---|---|---|---|---|
| Off | On | ||||||
| #1 | 72 | 3.5 | F | R | 2 | 1 | R 3.0 mg (4×); C/L 25/100 (1×) |
| #2 | 62 | 5.5 | F | L | 3 | 2 | R 1.0 mg (3×); C/L 25/100 (3×); S 5.0 mg (2×) |
| #3 | 74 | 2 | F | L | 2 | 1 | C/L 25/100 (3×) |
| #4 | 62 | 11 | F | R | 3 | 1 | Pra 1.5 (3×); C/L 50/200 (4×); CLE 100 (2×) |
| #5 | 56 | 6.5 | M | L | 2 | 0 | E 200 (3×); R 1 mg (3×); S 1.0 mg (1×) |
| #6 | 71 | 5 | F | R | 2 | 1 | C/L 25/100 (3×) |
| #7 | 55 | 10 | M | L | 3 | 1 | R 1.0 mg (40×); C/L 25/250 (4×) |
| #8 | 65 | 3 | M | L | 2 | 1 | C/L 25/100 (3×) |
| #9 | 69 | 3 | F | R | 2 | 2 | Pra 1.5 (3×) |
| #10 | 48 | 1.5 | F | L | 2 | 1 | C/L 25/100 (3×); Pra 1.5 mg |
| #11 | 73 | 0.5 | M | L | 3 | 2 | C/L 25/100 (3×) |
| #12 | 56 | 4.5 | F | R | 2 | 1 | R 3 mg (3×); S 1.0 mg; C/L 25/100 (3×) |
| #13 | 57 | 13 | M | L | 3 | 3 | E 200 mg (3×); Am 200 (3×); C/L 25/100 (3×) |
| #14 | 46 | 1 | F | L | 3 | 1 | R 1.0 mg (3×); C/L 25/100 |
| #15 | 63 | 7 | F | R | 2 | 1 | S 1.0 mg; Pra 1.5 mg (3×) |
| #16 | 59 | 8 | M | R | 2 | 1 | R 1.0 mg (3×); C/L 25/100 (3×) |
| #17 | 71 | 9 | F | L | 2 | 1 | Pra 1.0 mg (3×); C/L 25/100 (4×); CLE 100 mg (4×); |
Am amantadine, E entacopone, R ropinirole, S selegiline, Pra pramipexole, C/L carbidopa/levodopa, CLE carbidopa/levodopa/entacapone
UPDRS (unified Parkinson’s disease rating scale). Rigidity: 0, absent; 1, slight; 2, mild to moderate; 3, marked; 4, severe
Inclusion and exclusion criteria
Verbal medical history and the Motor Section (Part III) of the UPDRS were used to screen all patients for inclusion in the current study. The inclusion criteria were: (1) age range from 30 to 75 years old, (2) currently treated by dopaminergic medication, and (3) the presence of rigidity as assessed by the UPDRS scale (≥2, mild to moderate, or marked) in one or both arms and minimal tremor (≤1, slight and infrequently present) in the tested arm when dopaminergic medication was withdrawn. The exclusion criteria were as follows: (1) insufficient range of motion in flexion or extension (<30°); and/or (2) cognitive impairments that may have prevented the subject from giving informed consent, understanding instructions, or providing adequate feedback.
Experimental procedure
A detailed experimental procedure has been described previously (Xia et al. 2006). In brief, each subject was initially evaluated with the Motor Section (Part III) of the UPDRS. Each subject was then seated in a height-adjustable chair with the arm exhibiting the greater amount of rigidity placed in the apparatus via a manipulandum. The center of rotation of the subject’s wrist was aligned with the center of rotation of the apparatus, while the forearm was held in neutral pronation and supination and stabilized with a vacuum bag splint. The motion of the wrist was restricted to flexion and extension in the horizontal plane.
Subjects were instructed to relax as much as they were able to while the servomotor induced a complete cycle of wrist flexion and extension motions through a 60° range of motion in each trial, beginning at an extended position of 30°, moving to a flexed position of 30° (flexion motion), holding for one second, and then returning to the original position (extension motion). Flexion and extension movements were conducted at 50°/s. This velocity was chosen for two reasons: (1) parkinsonian rigidity is the resistance to a continued sustained stretch, especially to slow rates of stretch (Marsden 1990), and (2) The slow velocity better approximated the application of the selected model which was based on isometric conditions measured at multiple positions of the wrist joint (Delp et al. 1996; Holzbaur et al. 2005). All trials were followed by a short period of rest from 30 to 60 s to minimize fatigue and to avoid possible motor adaptation.
Subjects were first tested in the “Off-medication” state, requiring an overnight withdrawal of dopaminergic medication for at least 12 h, when most of the beneficial effects of treatment was eliminated (Defer et al. 1999). After the Off-medication tests were completed, subjects resumed their regular dose of medication in the laboratory. Approximately 1 h later, when medication started taking effects, which were confirmed and validated by the subjects, each subject was re-examined with the Motor Section of the UPDRS. Then, the testing protocol was repeated in the state of defined “On-medication” (Brown and Marsden 1999; Robichaud et al. 2002).
EMG and kinetic recordings
Using active surface differential electrodes, which measured 41 by 20 mm and had an inter-electrode distance of 10 mm (Delsys Inc., Boston, USA), surface electromyograms (EMGs) were recorded over the bellies of wrist and finger flexor muscles: [flexor carpi radialis (FCR), flexor carpi ulnaris (FCU), and flexor digitorum superficialis (FDS)], and wrist and finger extensor muscles: [extensor carpi radialis longus and brevis (ECRLB), extensor carpi unlaris (ECU), and extensor digitorum communis (EDC)]. Electrode placements followed the recommendations of Perotto (1994) and were confirmed with manual muscle testing. EMG signals were amplified (×10 k) and bandpass filtered (20–450 Hz) before being sampled at 1,000 Hz per EMG channel.
Angular position of the wrist joint was measured using an emulated encoder output from the servomotor controller (SC904 series, Pacific Scientific, USA), while velocity was calculated by differentiating the positional signal. The joint torque was measured with a strain gauge torque transducer (TRT-200, Transducer Techniques, USA). The position signal was sampled at 100 Hz and the torque signal was sampled at 1,000 Hz. Data capture was controlled by LabView codes (National Instruments, Texas, USA).
Application of a biomechanical model in differentiation between shortening reaction and stretch-induced inhibition
An upper extremity model was used to estimate muscle force, moment arm, and moment generated by each individual muscle across the wrist joint (Holzbaur et al. 2005). In brief, this model includes 15 degrees of freedom representing the shoulder, elbow, wrist, thumb and index finger, and 50 muscle compartments crossing these joints. The model estimates the muscle–tendon length and moment arm of each muscle over a wide range of motion and estimates maximum active force and passive force that each muscle produces at a given joint position. The characteristics of the wrist joint in the model were built on an earlier model on the wrist alone (Gonzalez et al. 1997). The modeling capacity of the wrist joint covers wrist flexion/extension ranging from 70° of extension to 70° of flexion, including the contributions of wrist muscles and extrinsic finger muscles (Gonzalez et al. 1997; Holzbaur et al. 2005).
We calculated the moment (torque) generated by each of the muscles, including both shortening muscles and stretched muscles. The moment that each individual muscle generated was computed as a product of its moment arm multiplied by the force it generated at a given joint position:
m moment by muscle i; ma moment arm for muscle i; f muscle force generated by muscle i; i major muscles contributing to wrist flexion or extension moment, including dedicated wrist muscles and finger extrinsic muscles.
Estimation of muscle moment
The model was implemented through Software for Interactive Musculoskeletal Modeling (SIMM version 4.0; MusculoGraphics, Inc., Santa Rosa, CA, USA). Experimental data, including EMGs and joint angular position, were fed into the model as input signals. Muscle force generated by each individual muscle was estimated as a function of joint position resulting from SIMM as output signals. The moment arm profile for involved groups of muscle was obtained by the model via SIMM (Holzbaur et al. 2005). Moments generated by stretched muscles were summed together and moments by shortening muscles summed together.
Using custom software (SIMM Convert 2.1, Nvision Solutions, Ltd., Raleigh, NC, USA), surface EMG and position data were converted for import into SIMM 4.0, including expanding the EMG array from six collected channels to 13 channels required for implementation of the selected biomechanical model (Holzbaur et al. 2005). Specifically, the SIMM Convert software replicated the FDS EMG signal that was collected to fill the arrays of the flexors for digit 5 (FDSL), digit 4 (FDSR), digit 3 (FDSM), and digit 2(FDSI). Further, the EDC EMG signal that was collected was replicated to fill the arrays of the extensors for digit 5 (EDCL), digit 4 (EDCR), digit 3 (EDCM), and digit 2 (EDCI). The ECR EMG signals were used to fill two arrays including the ECR longus (ECRL) and ECR brevis (ECRB). Activation arrays for each of these muscles were required to implement the biomechanical model. The SIMM Convert software did not normalize or modify the values of the EMG signals, because the normalization of small EMG signals under passive movement conditions to a maximum voluntary isometric contraction would have resulted in extremely low level of muscle activation. Therefore, the model of rigidity would have been dominated by the visco-elastic properties of the muscle–tendon unit, minimizing the contributions of neural reflexes to parkinsonian rigidity.
The SIMM software, using the biomechanical model by Holzbaur et al. (2005), estimated moment arms for the flexors and extensors using the partial velocity method (Delp and Loan 1995). For each muscle, SIMM calculated active muscle force using an assumed fiber length based on joint position and surface EMG to scale the length–tension relationship. SIMM estimated the passive force generation based on fiber length (joint position). The active and passive forces were then summed and multiplied by the moment arm to obtain the total estimated moment for each muscle. Muscle moments were imported into custom software (MatLab 2009a, The MathWorks, Natick, MA, USA) in which onset and termination of the flexion and extension movements were determined manually from the position signal. Estimated muscle moments were grouped by function (finger and wrist flexors vs. finger and wrist extensors) while the estimated net moment was defined as the sum of all estimated muscle moments.
To quantify individual contributions of shortened and stretched muscle groups to the uniformity of rigidity, the slopes of the torque–angle curve were calculated for shortened muscles and stretched muscles, separately, during the flexion and extension movements in both the Off-medication and On-medication states.
Statistical analyses
The calculated slope data were analyzed using a mixed model of analysis of variation (ANOVA). The linear mixed model included three fixed effects, medication state (Off-medication vs. On-medication), direction of movement (flexion vs. extension), and muscle group (shortening muscles vs. lengthening muscles), as well as the two-way and three-way interactions of the fixed effects. The mixed model also incorporated the subject as a random effect. Therefore, the multiple observations from a single subject are not considered to be independent of each other as in an ordinary ANOVA. The model first examined the two-way and three-way interaction effects. Provided that there was no evidence showing significant interaction effects, the comparison between two levels of each fixed effect was carried out using T-tests and confidence intervals. For all statistical tests, differences were considered significant when P < 0.05. The statistical analysis was performed using the mixed procedure in SAS 9.2 (SAS Institute Inc., Cary NC, USA).
Results
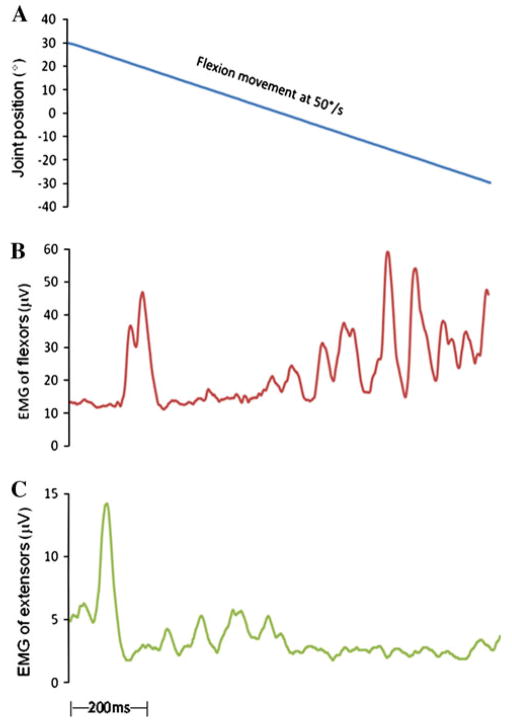
Figure 1 illustrates representative examples of shortening reaction and stretch-induced inhibition recorded during passive flexion movement (Fig. 1a) from a patient in the Off-medication state. In this example, the shortening reaction was associated with activation in wrist flexor muscles (Fig. 1b). During the flexion movement, there was a large initial stretch reflex in the wrist extensor muscles. The initial stretch reflex was followed by a period of sustained activity and curtailed by an evident decline when the progressive movement approached at almost the neutral position and the muscle length of the extensors was elongated, demonstrating the stretch-induced inhibition (Fig. 1c).
Fig. 1.
Kinematic and EMG recordings during passive flexion movement obtained from a patient in the Off-medication state. a Wrist joint position during the passive flexion movement; the subject’s wrist joint was externally rotated from 30° flexion to 30° extension at 50°/s. b SR was recorded in shortened flexors in the Off state in a PD subject. There was an increased EMG activation in passively shortened muscles. c SII was observed in the stretched extensor muscles during the same movement. There was an EMG reduction, when the stretch exceeded the neutral position and the muscle length was elongated
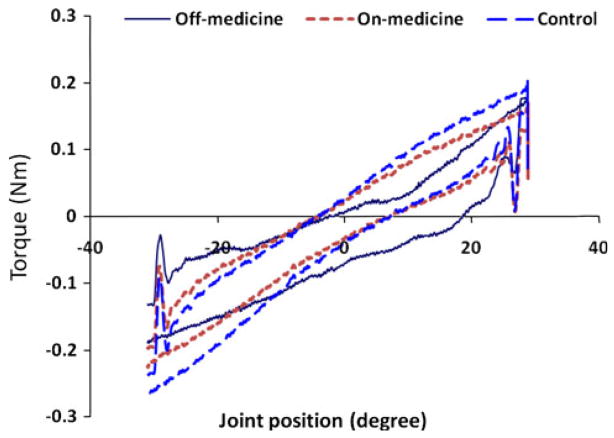
The net torque–angle curves obtained from the experimental recordings are illustrated for the same patient with PD in both the Off- and On-medication and are compared with the net torque–angle plot for an age-matched control subject (Fig. 2). Extension movement is shown as the upper traces and the flexion as the lower ones. The torque–angle slopes in the Off-medication were the smallest among all three measures for both movements, which were 3.9 and 4.1 in the PD Off state, 5.3 and 5.4 in the PD On state, and 6.4 and 6.5 milliNm/deg in the control subject for the extension and flexion movements, respectively.
Fig. 2.
Comparison of the net torque–angle patterns made between a subject with PD in the Off-medication (solid line) and On-medication (dotted line) states and an age-matched control subject (dashed line) obtained from the recordings during the experiment. The torque–angle slopes were 3.9 and 4.1 milliNm/deg associated with extension movement and flexion movement for the PD subject in the Off-medication and 5.3 and 5.4 milliNm/deg in the On-medication. The slopes were 6.4 and 6.5 milliNm/deg for the two respective movements in the control subject. Upper curves extension movement; Lower curves flexion movement
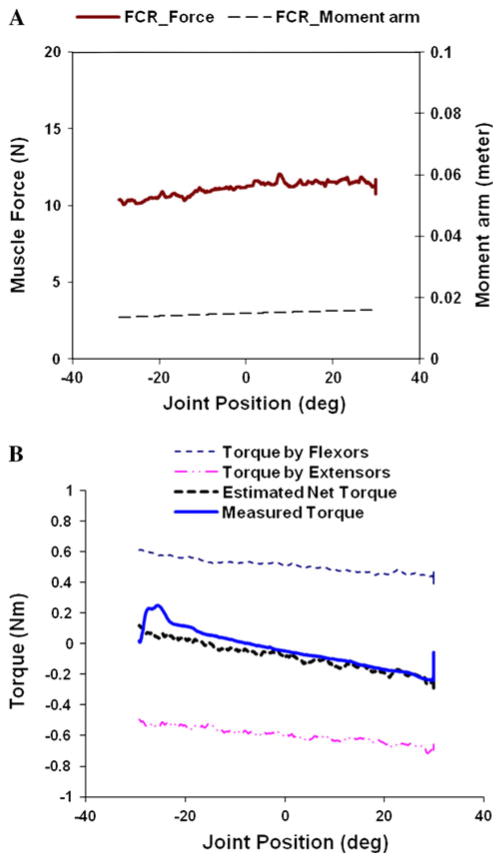
Figure 3 compares the estimated torque by the model with the experimentally measured torque from a subject in the Off-Medication state. Figure 3a shows the plots of the muscle force and moment arm of the FCR as a function of joint position while the subject’s wrist joint was extended from −30° to +30°. Moments generated by stretched muscles (i.e., wrist and finger flexors in this case) were summed together and moments by shortening muscles summed together (refer to “Torque by flexors” and “Torque by extensors” of Fig. 3b). Stretch-induced inhibition was manifested in the line of Torque by flexors, showing that the force generated by the stretched muscles was progressively reducing as the stretch continued. On the other hand, the muscle force generated by the shortening muscles increased gradually throughout the extension movement, due largely to the shortening reaction. Summation of the two lines is plotted as the line of “Estimated net torque,” which is closely matched with experimentally measured torque shown as the line “Measured torque.” The estimated and the measured torque traces were independently matched as the measured torque did not play any role in the calculation of the estimated force by individual muscles.
Fig. 3.
A representative example of the estimated flexor and extensor torques, estimated net and the measured torque in a PD patient under Off-medication condition. a Muscle force (thicker line) generated by FCR and moment arm (dashed line) for FCR are plotted as a function of wrist joint position during externally induced extension movement (50°/s) from 30° flexed position (minus sign) to 30° extended position (plus sign). b Torque–angle plot illustrating “stretch-induced inhibition” and “shortening reaction”, and net torque plots estimated by the model and measured during the experiment
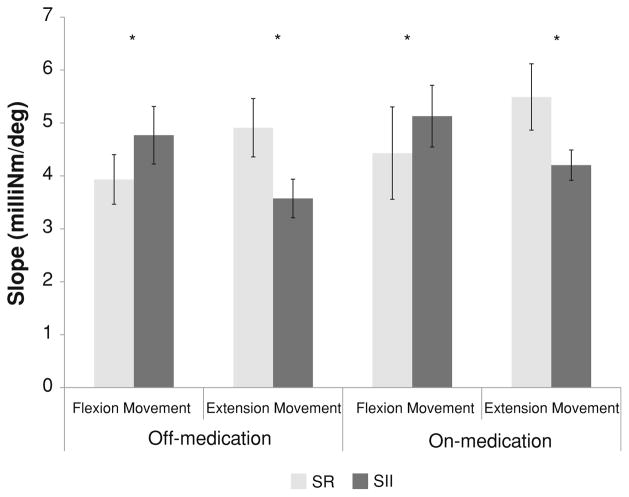
The slopes of the estimated torque–angle plots were calculated for the flexor and extensor compartments in all subjects (Fig. 4). Torque–angle slopes were significantly different between the shortening muscles and lengthening muscles [t(83) = 3.02; P = 0.003]. Dopaminergic medication resulted in consistent increases in slopes for both shortened and stretched muscles in the directions of flexion and extension movement. However, the effect did not generate a significant difference on estimated torque–angle slopes associated with either muscle group [t(83) = 1.09; P = 0.279]. Torque–angle slopes were not significantly different between the flexion and extension movements [t(83) = 0.06; P = 0.952]. There were no significant interactions of medication by movement [F(1,83) = 0.06; P = 0.800], movement by muscle [F(1,83) = 0.630; P = 0.431], medication by muscle [F(1,83) = 0.02; P = 0.894], or medication by movement by muscle [F(1,83) = 0.00; P = 0.949].
Fig. 4.
Averaged slopes of the estimated torque–angle plots, obtained from all 17 subjects with PD, for shortened muscle groups (SR gray bars) and stretched muscle groups (SII black bars) during passive flexion and extension movements when subjects were tested in Off-medication and On-medication states. Error bars standard error means. *Statistically significant difference between SR and SII. *P < 0.005
Discussion
The purpose of this study was to dissociate the two physiological mechanisms responsible for the lead-pipe characteristics of parkinsonian rigidity and to quantify the level of contribution made by each mechanism. The upper extremity model implemented via the SIMM environment allows us to differentiate the two mechanisms.
We have proposed that SR and SII are potential mechanisms responsible for the uniform characteristics of parkinsonian rigidity. The rationale for this proposition arises from the spring-like property of the muscle. Unimpaired individuals are able to relax during passive movement and the wrist muscles remain relatively slack in the central range. This is supported by the finding of Delp et al. (1996) who reported that passive moments of the wrist joint in the central 150° range of motion produced torque near 0 Nm. In contrast, patients with parkinsonian rigidity manifest a constant background muscle activity and muscle tension due to afferent discharge (Burke et al. 1977; Marsden 1982; Wallin et al. 1973).
Previous studies have illustrated that the net torque–angle curve of the wrist exhibits an elevated initial torque level and remained relatively constant throughout the range of motion in the Off-medication states (Xia and Rymer 2004; Xia et al. 2006). These previous studies also reported that with the administration of dopaminergic medication, the net torque–angle curve became steeper. Medication diminished the effects of SR and SII on the torque–angle pattern of the wrist joint.
A number of previous studies have reported a presence of SR in PD (Angel and Lewitt 1978; Berardelli and Hallett 1984; Diener et al. 1987; Xia and Rymer 2004). The core finding reported in these earlier studies was that SR was enhanced in PD. However, none of those studies elucidated the role SR may play in parkinsonian rigidity and how SR may contribute to the biomechanical characteristics associated with parkinsonian rigidity. Until recently, SR has been suggested to account for the uniform or lead-pipe resistance which uniquely defines parkinsonian rigidity (Xia and Rymer 2004). Although SII is a well-known phenomenon associated with spasticity in humans and animals (Burke et al. 1970; Burke et al. 1972; Rymer et al. 1979), it has never been examined in PD.
The present study examined both SR and SII as candidate mechanisms responsible for the uniform resistance in rigidity. The results obtained by an application of the upper extremity model (Holzbaur et al. 2005) have shown that, during the passive flexion movement, SR plays a predominant role in the genesis of uniformity of rigidity, whereas SII is a primary contributor to the uniformity of rigidity during the passive extension movement. Results obtained from this study also suggested that SR was more prominent in the flexors compared to the extensors as the estimated slope was smaller in flexion movement than in the extension movement (see Fig. 4). A majority of the previous reports on SR associated with PD examined exclusively the flexor muscles in the leg. SR was demonstrated in tibialis anterior during passive dorsiflexion of the ankle (Angel and Lewitt 1978; Berardelli and Hallett 1984; Diener et al. 1987). Andrews et al. (1972) compared the SR evoked in biceps with that elicited in triceps surae in parkinsonian rigidity. They found that SR is relatively more identifiable in the elbow flexors (biceps muscle). Our findings are consistent with those reported in the literature in that flexor muscles exhibit SR more than extensor muscles including multiple joints being examined.
In contrast, the clasp-knife phenomenon, equivalent to SII in the present study, has only been investigated in upper motor neuron syndromes, such as spasticity in human subjects and animal models (Burke et al. 1970, 1971, 1972; Rymer et al. 1979). Burke et al. (1970, 1971) examined the clasp-knife phenomenon in quadriceps and hamstrings in human spasticity. Stretch reflexes were recorded in both quadriceps and hamstrings of the patients. Clasp-knife phenomenon was observed in quadriceps but was absent in the spastic hamstrings. The length-dependent inhibition of the stretch reflex underlies the clasp-knife phenomenon. There is a similarity of the human spasticity to animal preparation in that both displayed the sudden release of the resistance due to the continuous stretch of the elongated muscle. Clasp-knife responses were consistently observed in the quadriceps and soleus muscles in spinal cats (Burke et al. 1972; Rymer et al. 1979). Concurrent muscle afferent recordings suggested that clasp-knife reflex may be mediated by the central inhibitory effects of groups III and IV (and possibly non-spindle group II muscle afferents; Rymer et al. 1979).
Current investigation of parkinsonian rigidity has suggested that the clasp-knife reflex is a contributor to rigidity in PD. It is present in both flexor and extensor muscle groups of the wrist joint, as the estimated torque–angle slopes showed the contribution of SII to the uniformity of rigidity during both flexion and extension movements. The underlying mechanism responsible for rigidity is yet to be investigated and better explored in animal models, which obviously is beyond the scope of the present study.
In an earlier pilot study by the authors, only the SR was examined (Xia and Rymer 2004). In the present study, both physiological mechanisms were explored in patients with parkinsonian rigidity. Through the application of the upper extremity model via the SIMM program, we have dissociated the two mechanisms and quantified the contribution by each. Current findings obtained through the modeling approach show that both SR and SII contribute significantly to the uniformity of PD rigidity in both the flexion and extension movements. The smaller slope associated with SR during flexion movement suggested that SR played a primary role compared to SII, whereas the two changed their role during the extension movement (Fig. 4).
With dopaminergic therapy, the torque–angle curves became consistently steeper as compared to those in the Off-medication state, although the changes did not reach a statistically significant level (Fig. 4). The effect of medication was manifested in all the torque–angle slopes, as the estimated slopes were consistently greater in the On-medication for both shortening and lengthening muscle groups during flexion and extension movements, compared to those in the Off-medication condition. The slope connected with SR continued to be less than that associated with SII for the flexion movement, whereas the relationship between the SR and SII reversed for the slope data during extension movement. This consistent pattern confirmed the contribution of each mechanism to lead-pipe characteristics of PD rigidity. Both SR and SII appear to respond to medication treatment. On the other hand, the change in torque–angle slope due to medication is not so dramatic for the dissociated muscle groups, i.e., either the shortening or lengthening muscle group.
There are a few potential explanations for the observed medication effect. First, the net torque (or moment) at a given joint position is a summation of the moments generated by all the flexors and the moments by all the extensors across the wrist joint. For computational purposes, the moment arm for the flexor and extensor muscles has the opposite signs, i.e., a plus sign (+) for the flexors and a minus sign (−) for the extensors when the wrist of the right side was tested, or vice versa for the left side. The torque–angle plot can be fit by a linear regression for each dissociated muscle group. The slope of the net torque–angle is equal to the summed slope of flexor and extensor groups. Thus, the net joint torque–angle curve is always steeper than the torque–angle plot associated with either flexor or extensor muscle groups.
Second, surface electrodes were employed to monitor forearm muscle EMG activity. Surface EMG has inherent limitations including cross-talk and lack of specificity, and these limitations may be more prevalent in the small musculature of the forearm and deeper muscles such as flexor digitorum profundus (FDP). However, monitoring six key muscles that contribute considerable torque at the wrist would provide the best balance of capturing a representative sample of motor units from the key muscles of interest and non-invasive way of monitoring muscle activation while encouraging subject compliance and relaxation during the testing.
Another explanation lies in the limitations of the biomechanical model applied in this study. The model of the upper extremity (Holzbaur et al. 2005) is an integral model representing multiple joints. The model is useful for a broad range of studies, but may not be well suited to all studies of upper extremity biomechanics. The model was developed based on the data obtained from studies of normal cadaveric specimens (Murray et al. 2002). The model could not account for changes in mechanical properties of muscle and tissue that have been shown resulting from pathological biomechanics such as PD (Dietz et al. 1981; Watts et al. 1986; Xia et al. 2010). Therefore, a certain amount of discrepancy is expected from the application of the model to rigidity in patients with PD.
Conclusions
The findings of this study are useful in elucidating the underlying mechanisms responsible for the lead-pipe characteristics defining parkinsonian rigidity. The knowledge gained from this study provides new insights into the biomechanical and physiological underpinnings of this common symptom in patients with PD. The use of a biomechanical model may offer a means of assessing the efficacy of rehabilitation programs and therapeutic interventions.
Acknowledgments
This study was supported in part by the National Institutes of Health under grant R15-HD061022, in part under grant Nebraska Tobacco Settlement Biomedical Research Development Fund, and in part under Faculty Development Fund of School of Pharmacy and Health Professions, Creighton University, USA. Authors wish to thank all the subjects for their participation in the study.
Contributor Information
Ruiping Xia, Email: ruipingxia@creighton.edu, Rehabilitation Science Research Laboratory, Department of Physical Therapy, Creighton University, 2500 California Plaza, Omaha, NE 68178, USA.
Douglas Powell, Rehabilitation Science Research Laboratory, Department of Physical Therapy, Creighton University, 2500 California Plaza, Omaha, NE 68178, USA.
W. Zev Rymer, Sensory Motor Performance Program, Rehabilitation Institute of Chicago, Chicago, IL, USA.
Nicholas Hanson, Rehabilitation Science Research Laboratory, Department of Physical Therapy, Creighton University, 2500 California Plaza, Omaha, NE 68178, USA.
Xiang Fang, Division of Biostatistics, Office of Research and Compliance, Creighton University, Omaha, NE, USA.
A. Joseph Threlkeld, Rehabilitation Science Research Laboratory, Department of Physical Therapy, Creighton University, 2500 California Plaza, Omaha, NE 68178, USA.
References
- Andrews CJ, Burke D, Lance JW. The response to muscle stretch and shortening in parkinsonian rigidity. Brain. 1972;95:795–812. doi: 10.1093/brain/95.4.795. [DOI] [PubMed] [Google Scholar]
- Angel RW, Lewitt PA. Unloading and shortening reactions in parkinson’s disease. J Neurol Neurosurg Psychiatry. 1978;41:919–923. doi: 10.1136/jnnp.41.10.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A, Hallett M. Shortening reaction of human tibialis anterior. Neurology. 1984;34:242–245. doi: 10.1212/wnl.34.2.242. [DOI] [PubMed] [Google Scholar]
- Brown P, Marsden CD. Bradykinesia and impairment of EEG desynchronization in Parkinson’s disease. Mov Disord. 1999;14:423–429. doi: 10.1002/1531-8257(199905)14:3<423::aid-mds1006>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Buchanan TS, Delp SL, Solbeck JA. Muscular resistance to varus and valgus loads at the elbow. J Biomech Eng. 1998;120:634–639. doi: 10.1115/1.2834755. [DOI] [PubMed] [Google Scholar]
- Burke D, Gillies JD, Lance JW. The quadriceps stretch reflex in human spasticity. J Neurol Neurosurg Psychiatry. 1970;33:216–223. doi: 10.1136/jnnp.33.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gillies JD, Lance JW. Hamstrings stretch reflex in human spasticity. J Neurol Neurosurg Psychiatry. 1971;34:231–235. doi: 10.1136/jnnp.34.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Knowles L, Andrews C, Ashby P. Spasticity, decerebrate rigidity and the clasp-knife phenomenon: an experimental study in the cat. Brain. 1972;95:31–48. doi: 10.1093/brain/95.1.31. [DOI] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Wallin BG. Reflex mechanisms in Parkinsonian rigidity. Scand J Rehabil Med. 1977;9:15–23. [PubMed] [Google Scholar]
- Defer GL, Widner H, Marie RM, Remy P, Levivier M. Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD) Mov Disord. 1999;14:572–584. doi: 10.1002/1531-8257(199907)14:4<572::aid-mds1005>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Delp SL, Loan JP. A graphics-based software system to develop and analyze models of musculoskeletal structures. Comput Biol Med. 1995;25:21–34. doi: 10.1016/0010-4825(95)98882-e. [DOI] [PubMed] [Google Scholar]
- Delp SL, Grierson AE, Buchanan TS. Maximum isometric moments generated by the wrist muscles in flexion–extension and radial-ulnar deviation. J Biomech. 1996;29:1371–1375. doi: 10.1016/0021-9290(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Diener C, Scholz E, Guschlbauer B, Dichgans J. Increased shortening reaction in Parkinson’s disease reflects a difficulty in modulating long loop reflexes. Mov Disord. 1987;2:31–36. doi: 10.1002/mds.870020104. [DOI] [PubMed] [Google Scholar]
- Dietrichson P. Tonic ankle reflex in parkinsonian rigidity and in spasticity. The role of the fusimotor system. Acta Neurol Scand. 1971;47:163–182. doi: 10.1111/j.1600-0404.1971.tb07474.x. [DOI] [PubMed] [Google Scholar]
- Dietz V, Quintern J, Berger W. Electrophysiological studies of gait in spasticity and rigidity. Evidence that altered mechanical properties of muscle contribute to hypertonia. Brain. 1981;104:431–449. doi: 10.1093/brain/104.3.431. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL. Members of the UPDRS development committee. Unified Parkinson’s disease rating scale. In: Fahn S, et al., editors. Recent developments in Parkinson’s disease. Macmillian Healthcare; Florham, New Jersey: 1987. pp. 153–164. [Google Scholar]
- Fung VS, Thompson PD. Rigidity & spasticity. In: Jankovic JJ, Tolosa E, editors. Parkinson’s disease and movement disorders. 4. Lippencott Williams & Wilkens; Philadelphia: 2002. pp. 473–482. [Google Scholar]
- Gonzalez RV, Buchanan TS, Delp SL. How muscle architecture and moment arms affect wrist flexion–extension moments. J Biomech. 1997;30:705–712. doi: 10.1016/s0021-9290(97)00015-8. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966;184:170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbaur KR, Murray WM, Delp SL. A model of the upper extremity for simulating musculoskeletal surgery and analyzing neuromuscular control. Ann Biomed Eng. 2005;33:829–840. doi: 10.1007/s10439-005-3320-7. [DOI] [PubMed] [Google Scholar]
- Knutson JS, Kilgore KL, Mansour JM, Crago PE. Intrinsic and extrinsic contributions to the passive moment at the metacarpophalangeal joint. J Biomech. 2000;33:1675–1681. doi: 10.1016/s0021-9290(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Lee RG, Tatton WG. Motor responses to sudden limb displacements in primates with specific cns lesions and in human patients with motor system disorders. Can J Neurol Sci. 1975;2:285–293. doi: 10.1017/s0317167100020382. [DOI] [PubMed] [Google Scholar]
- Lee RG, Tatton WG. Long latency reflexes to imposed displacements of the human wrist: dependence on duration of movement. Exp Brain Res. 1982;45:207–216. doi: 10.1007/BF00235780. [DOI] [PubMed] [Google Scholar]
- Marsden CD. The mysterious motor function of the basal ganglia: the Robert Wartenberg Lecture. Neurology. 1982;32:514–539. doi: 10.1212/wnl.32.5.514. [DOI] [PubMed] [Google Scholar]
- Marsden CD. Neurophysiology. In: Stern G, editor. Parkinson’s disease. Johns Hopkins University Press; Baltimore: 1990. pp. 57–98. [Google Scholar]
- Matthews PB. The dependence of tension upon extension in the stretch reflex of the soleus muscle of the decerebrate cat. J Physiol. 1959;147:521–546. doi: 10.1113/jphysiol.1959.sp006260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray WM, Buchanan TS, Delp SL. Scaling of peak moment arms of elbow muscles with upper extremity bone dimensions. J Biomech. 2002;35:19–26. doi: 10.1016/s0021-9290(01)00173-7. [DOI] [PubMed] [Google Scholar]
- Perotto AO. Anatomical guide for the electromyographer: the limb and trunk. Springfield; Illinois, USA: 1994. [Google Scholar]
- Rack PM, Westbury DR. The effects of length and stimulus rate on tension in the isometric cat soleus muscle. J Physiol. 1969;204:443–460. doi: 10.1113/jphysiol.1969.sp008923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaud JA, Pfann KD, Comella CL, Corcos DM. Effect of medication on EMG patterns in individuals with Parkinson’s disease. Mov Disord. 2002;17:950–960. doi: 10.1002/mds.10218. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Obeso JA, Traub MM, Marsden CD. The behaviour of the long-latency stretch reflex in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1983;46:35–44. doi: 10.1136/jnnp.46.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymer WZ, Houk JC, Crago PE. Mechanisms of the clasp-knife reflex studied in an animal model. Exp Brain Res. 1979;37:93–113. doi: 10.1007/BF01474257. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. On plastic tonus and proprioceptive reflexes. Quart J Exp Physiol. 1909;2:109–156. [Google Scholar]
- Wallin BG, Hongell A, Hagbarth KE. Recordings from muscle afferents in Parkinsonian rigidity. In: Desmedt JE, editor. New development in electromyography and clinical neurophysiology. Vol. 3. Karger, Basel: 1973. pp. 263–272. [Google Scholar]
- Watts RL, Wiegner AW, Young RR. Elastic properties of muscles measured at the elbow in man: II. Patients with parkinsonian rigidity. J Neurol Neurosurg Psychiatry. 1986;49:1177–1181. doi: 10.1136/jnnp.49.10.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal C. Uber eine art paradoxer muskel-contraction. Arch Psychiat und Nervenkr. 1880;10:243–248. [Google Scholar]
- Xia R, Rymer WZ. The role of shortening reaction in mediating rigidity in parkinson’s disease. Exp Brain Res. 2004;156:524–528. doi: 10.1007/s00221-004-1919-9. [DOI] [PubMed] [Google Scholar]
- Xia R, Markopoulou K, Puumala SE, Rymer WZ. A comparison of the effects of imposed extension and flexion movements on parkinsonian rigidity. Clin Neurophysiol. 2006;117:2302–2307. doi: 10.1016/j.clinph.2006.06.176. [DOI] [PubMed] [Google Scholar]
- Xia R, Radovic M, Mao ZH, Threlkeld AJ. System identification and modeling approach to characterizing rigidity in Parkinson’s disease: neural and non-neural contributions. Proceedings of the 4th international conference on bioinformatics and biomedical engineering (iCBBE 2010), Paper No. 40046; 2010. p. 4. [DOI] [Google Scholar]