Abstract
It is strongly established by numerous studies that oxidative stress-induced inflammation is one of the major causative agents in a variety of cancers. Various factors such as bacterial, viral, parasitic infections, chemical irritants, carcinogens are involved in the initiation of oxidative stress-mediated inflammation. Chronic and persistent inflammation promotes the formation of cancerous tumors. Recent investigations strongly suggest that aldose reductase [AR; AKR1B1], a member of aldo-keto reductase superfamily of proteins, is the mediator of inflammatory signals induced by growth factors, cytokines, chemokines, carcinogens etc. Further, AR reduced product(s) of lipid derived aldehydes and their metabolites such as glutathionyl 1,4-dihydroxynonanol (GS-DHN) have been shown to be involved in the activation of transcription factors such as NF-κB and AP-1 which transcribe the genes of inflammatory cytokines. The increased inflammatory cytokines and growth factors promote cell proliferation, a main feature involved in the tumorigenesis process. Inhibition of AR has been shown to prevent cancer cell growth in vitro and in vivo models. In this review, we have described the possible association between AR with oxidative stress- and inflammation- initiated carcinogenesis. A thorough understanding of the role of AR in the inflammation – associated cancers could lead to the use of AR inhibitors as novel chemotherapeutic agents against cancer.
Keywords: Aldose reductase, Oxidative stress, Inflammation, Cancer and NF-kB
INTRODUCTION
Constant exposure of cells in the body to oxygen, besides generating necessary to sustain life, generates highly reactive molecules known as free radicals or reactive oxygen species (ROS) [1–4]. These unstable free radicals or oxidants include hydroxyl radicals (OH−), singlet oxygen, superoxide anion (O2−) and hydrogen peroxide (H2O2) [1–4]. Under normal conditions antioxidant system of the cells minimizes the perturbations caused by ROS. However, when ROS exceeds the cellular antioxidants capacity, oxidative stress occurs. Therefore, oxidative stress is an outcome of continuous battle between inducers (pro-oxidants) and protective factors (anti-oxidants) of the cells of all aerobes [1–4]. The unstable ROS with high chemical reactivity cause lipid peroxidation and oxidation of proteins and nucleic acids which can increase the risk of mutagenesis and a number of other complications including inflammation [5–7]. Various reports suggest that intracellular ROS are generated by different mechanisms such as (a) mitochondrial respiratory chain reaction (b) membrane bound superoxide generating enzyme NADPH oxidase (c) phagocyte-derived oxidants by killing invading bacteria and parasites and (d) arachidonic metabolic reactions of Cox-2 [8–10]. The cytokines, chemokines – and growth factors– released under oxidative stress conditions could cause tissue damage and dysfunction leading to various inflammatory complications [11–14]. Thus, oxidative stress is thought to play an important causative role in the pathogenesis of numerous degenerative and chronic diseases such as diabetes, ageing, arthritis, Alzheimer’s and cancer.
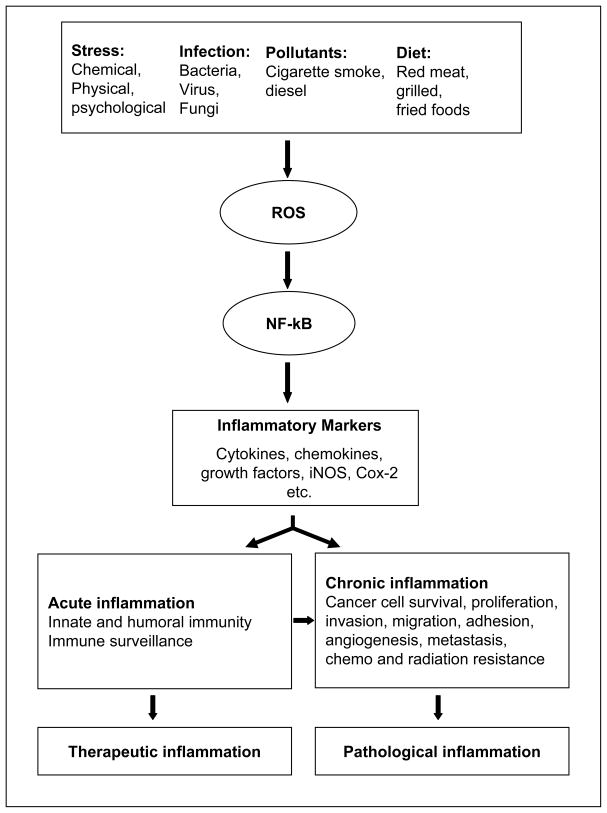
Inflammation is a host defense mechanism of the body to protect it from external or internal environmental stimuli [11–14]. The immune response of the body may be acute or chronic inflammatory response. Acute inflammation is pyrogenic, exists for short period of time and has therapeutic consequences [11–14], Fig. (1), whereas chronic inflammation lasts for a long period of time and can cause harmful diseases. For example, in acute inflammation particularly as a result of bacterial infections, neutrophils leave the vasculature and migrate toward the site of inflammation and cause phagocytic events by activating NADPH oxidase enzyme system to release large quantities of superoxide molecules and H2O2 radicals to kill the invading bacteria [15,16]. However, the bacterial debris which contains highly pro-inflammatory endotoxins is circulated in the blood and reaches various organs and cause damage. Further, bacterial endotoxins are recognized by circulating immune cells and trigger the gene induction of pro-inflammatory cytokines, chemokines and biosynthesis of nitric oxide (NO) and PGE2 and promote cell death or proliferation [15–17].
Fig. (1). Factors involved in oxidative stress/inflammation-induced carcinogenesis.
Various factors such as stress, infection, pollutants and diet cause generation of ROS, which are known to activate redox sensitive transcription factors such as NF-kB. Further, NF-kB transcribes various genes required for acute/chronic inflammation which cause inflammatory disorder such as cancer.
Chronic inflammation is mediated by mononuclear cells such as monocytes and macrophages [17]. These cells can be further stimulated to maintain inflammation through the action of an adaptive cascade involving lymphocytes, T cells, B cells and antibodies [12–14]. Chronic inflammation leads to increased production of tissue reactive oxygen and nitrogen intermediates [18]. Overwhelming levels of reactive oxygen and nitrogen species are known to be associated with oxidative stress and chronic inflammation [16–18]. These intermediates in turn alter the fate of the cells by altering signal transduction cascades leading to the activation of transcription factors such as NF-kB and AP-1 which mediate immediate cellular stress response [19]. Most of the studies indicate that NF-kB as a redox sensitive transcription factor. However, a few reports suggest that ROS do not mediate NF-kB activation directly [19–21]. For example, Hayakawa et al [21] have shown that the antioxidants such as N-acetyl-L-cysteine and pyrrolidine dithiocarbamate inhibit NF-kB activation independently of ROS.
It has been shown that chronic inflammation is associated with the progression of cancer through various sequential steps such as cellular transformation, promotion, survival, proliferation, invasion, angiogenesis and metastasis [20]. Although oxidative stress and inflammation are known to be major risk factors for most cancers, the mechanism of oxidative stress-induced carcinogenesis is not clearly known. Clinical and epidemiologic studies suggest a strong association between infectious agents and chronic inflammatory disorders and cancer. The failure of normal control mechanisms that limit immune response, leukocyte activation, leads to chronic inflammation and its consequences. The consequences of chronic inflammation are further augment permanently by metabolic alterations and malignant transformation as exemplified a strong association between chronic gastritis, hepatitis, and colitis and increased risk of primary carcinoma [23].
Recent studies indicate that polyol pathway enzyme aldose reductase (AR) catalyzes ROS-initiated lipid peroxidation generated lipid aldehydes and their glutathione (GSH) conjugates [24]. Further, AR has been shown to be oxidative responsive protein and inhibition of AR has shown to prevent cytokine- and growth factors induced inflammatory signals and proliferation of cancer cells in culture as well as nude mice xenograft models [25,26]. Since the main aim of this review is to understand the role of AR in cancer pathologies, we have searched the literature through Pubmed and Ovid by using keywords “aldose reductase” and “cancer” which resulted in more than 250 publications. After careful dissection of these abstracts, we found that there are very few publications actually related to AR and various forms of cancer. Further, we have only considered the papers on AR and not other aldo-keto reductases. In this review, we have mainly discussed the novel role of AR in the mediation of inflammatory carcinogenesis such as colon cancer and possible therapeutic intervention by AR inhibitors as anticancer drugs.
ALDOSE REDUCTASE
AR [EC1.1.1.21; AKR1B1] is a monomeric polyol pathway enzyme that belongs to aldo-keto reductase superfamily [27]. It reduces glucose to sorbitol in the presence of NADPH during hyperglycemic conditions with Km glucose in the mM range. The accumulated sorbitol is oxidized further to fructose in the presence of sorbitol dehydrogenase by utilizing NAD+ as a cofactor [27]. Under euglycemic conditions, only small percentage (>3%) of glucose enters the polyol pathway and most of glucose is phosphorylated to glucose-6-phosphate by hexokinase [27]. The reducing activity of AR against glucose was first describedin 1956 by Hers [28]. Later van Heyningen [29] reported that the activity of the AR dramatically increased during diabetic and galactosemic cataractogenesis which caused accumulation of AR -derived polyols such as sorbitol and galactitol in the ocular lens, thought to be the major cause of diabetic cataract. It was hypothesized that accumulation of sorbitol in the lens would cause osmotic swelling which would follow ionic imbalance and protein insolubilizationleading to diabetic cataractogenesis. A similar sequence of events was proposed to explain the biochemical mechanism of diabetic retinopathy, nephropathy, microangiopathy and neuropathy [30]. In hyperglycemic conditions there is a high demand for both NADPH and NAD cofactors by AR and sorbitol dehydrogenase because more than 30% of glucose is reduced to sorbitol by AR [31]. This pathway results in osmotic and oxidative stress due to accumulation of sorbitol and changes in the ratio of NADPH/NADP+. Since NADPH is used in many reductive metabolic pathways such as detoxification of ROS and peroxides, decrease in NADPH/NADP+ ratio could cause oxidative stress [27,31]. In mid 1980s we have demonstrated that accumulation of polyols could not be the main cause of diabetic cataractogenesis because antioxidants such as Trolox could prevent cataractogenesis in diabetic rats as good as or better than AR inhibitors, even though the levels of polyols were very high [32,33]. These results suggested that inhibition of AR is involved in other metabolic changes accompanied with hyperglycemia. Later, we and other investigators demonstrated that glucose is not the only inducer of AR activation and expression, rather AR is overexpressed under various conditions which induce oxidative stress and inflammation [34–38]. Indeed, this concept is further strengthened by our demonstration that AR efficiently catalyzes the reduction of one of the most abundant and toxic lipid aldehyde, 4-hydroxy-trans-2-nonenol (HNE) and its conjugate with GSH to 1, 4-dihydroxynonene (DHN) and GS-DHN, respectively, with Km in low micro molar range compared to Km glucose in mM range (50 – 100 mM) [24–27]. Interestingly, we have further shown that inhibition of AR prevents redox-signaling which cause cellular cytotoxicity by HNE and GS-HNE, but not by GS-DHN [24–26]. These studies suggested that the AR –catalyzed reduced form, GS-DHN, could be the main mediator of oxidative stress-induced cellular cytotoxicity. Further, our results in various cellular and animal models show that AR is an obligatory mediator of oxidative stress/inflammation induced by various triggers such as hyperglycemia, endotoxins, growth factors, cytokines and allergens [25,26, 39–41], which suggest possible use of AR inhibitors for the prevention of various inflammatory pathologies.
In addition to cellular studies, various rodent animal models have been used to examine the role of AR in various pathological conditions such as diabetes, cardiovascular, atherosclerosis, and cataract [30–45]. However, very limited studies have shown the involvement of AR in mediation of carcinogenic signals. Recently, by using nude mice xenograft models and azoxymethane (AOM) – induced aberrant crypt foci (ACF) formation in mice models, we have investigated the efficacy of AR inhibition on tumor/pre-neoplastic growth [25,42]. In AOM model, we have shown that administration of mice with AOM significantly elevated the levels of AR after 10 weeks of AOM treatment and inhibition of mice with AR inhibitor, sorbinil, prevented it. Further, we have also shown that increased inhibition of AR could also prevent the inflammatory diseases such as sepsis and asthma in mice models [30–40]. Further Ravindranath et al [43] have also shown that cecal ligation and puncture model of transgenic mice overexpressing human AR showed an enhanced inflammatory response including increased cytokines levels, neutrophil accumulation in the lung endothelial cells.
ALDOSE REDUCTASE INHIBITORS
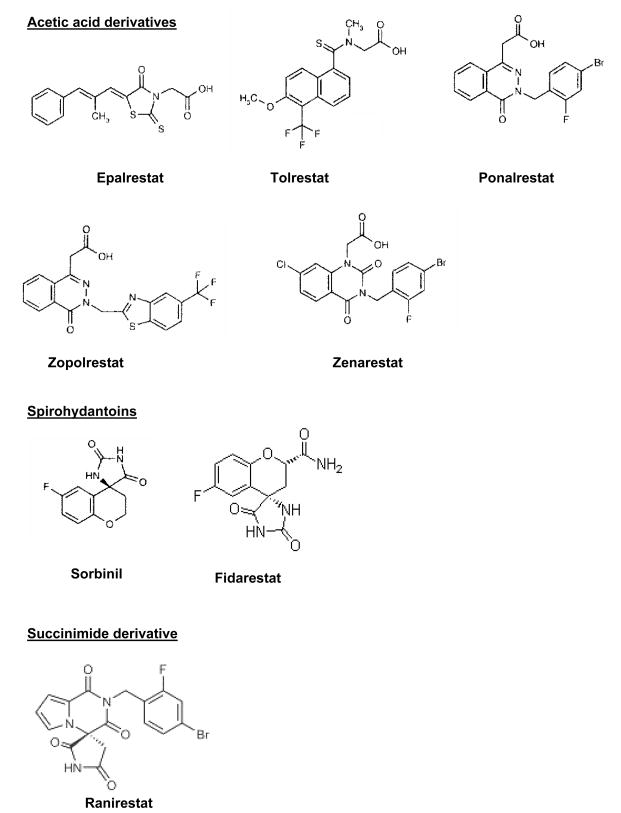
Various structurally different AR inhibitors have been developed to reduce accumulation of AR-catalyzed reduced polyol pathway products such as sorbitol and galactitol (in galactosemia) to prevent diabetic cataractogenesis, retinopathy and neuropathy [44,45]. Based on chemical structure, AR inhibitors can be classified in to three groups (Fig. (2). 1) Acetic acid derivatives (tolrestat, epalrestat, ponalrestat, zopolrestat, zenarestat), 2) spiro hydantoins (cyclic imides; sorbinil, fidarestat), 3) Succinimide class ranirestat (AS-3201) [44,45]. In animal studies, AR inhibitors have been shown to have promising effects in the prevention of cataract, retinal capillary basement membrane thickening and nerve conduction velocity deficits in diabetes [46,47]. However, to date none of the AR inhibitors such as fidarestat, zopolrestat, sorbinil were successful against FDA requirements in the market to reach humans in the USA against diabetic neuropathy as they lack the efficacy in reducing the associated symptoms [45]. The only AR inhibitor, epalrestat is in the market in Japan for the treatment human diabetic neuropathy. The newest AR inhibitor ranirestat is under phase 3 clinical trials in the USA, developed by Dainippon Pharmaceutical and Eisai for the treatment of diabetic complications such as neuropathy, cataract, retinopathy and nephropathy. Another most potent and highly specific AR inhibitor, fidarestat (IC50 9 nM) is developed by Sanwa Kagaku Kenkyyusho Co., Japan [45,48]. Fidarestat is water soluble and can rapidly distribute into the tissues and binds selectively to AR with high specificity and stays in circulation for longer periods without any major toxicity. Fidarestat was undergone phase III clinical trials for the treatment of diabetic neuropathy and found to be safe without any major irreversible toxicity [45,49]. However, fidarestat was failed at phase III clinical studies due to unsatisfactory prognosis in diabetic neuropathy patients.
Fig. (2). Structurally different inhibitors of AR.
The reasons for the failure of AR inhibitors in clinical trials may include 1) requirement of high dose of AR inhibitors compared to animals models 2) unavailability of highly specific inhibitors and 3) side effects such as hypersensitivity reactions as observed in older AR inhibitors with less specificity towards AR [44,45]. In view of this, the only way of minimizing the side effects and alleviate the inhibition of biologically important functions of AR includes targeted systemic delivery of AR inhibitors into the tissues by sequestering the drug into nanoparticles or development of highly specific inhibitors such as fidarestat. Our recent structural-activity studies suggest that AR has distinct GSH and aldehyde binding domains and selective modification of the enzyme active site prevents recognition and reduction of GSH conjugates without affecting aldehyde reduction [45,46]. These results suggest that AR mediates both the signaling and detoxification and designing the inhibitors which could selectively prevent cell injury without affecting antioxidant defense offers a new therapy for the treatment of oxidative-stress/inflammation -mediated cellular cytotoxicity.
ROLE OF AR IN CARCINOGENESIS
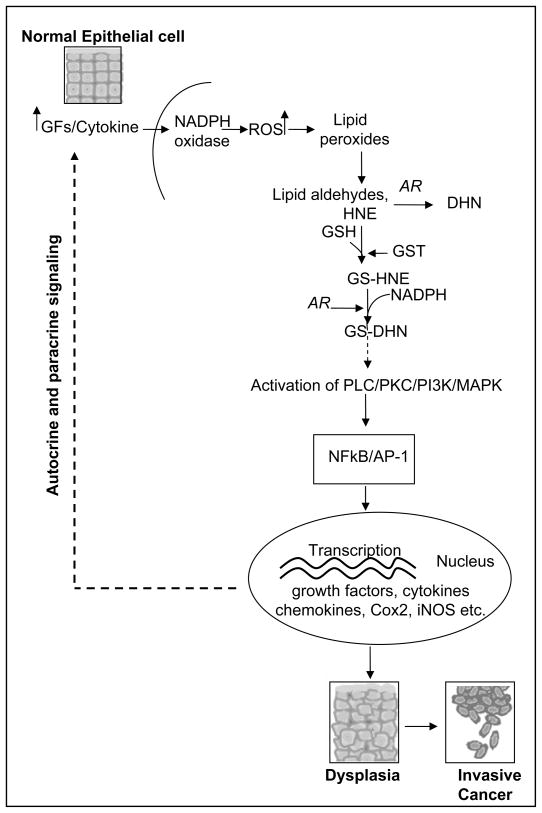
The abundance of AR in various organs and the low Km for lipid aldehydes and their glutathione conjugate and its role in inflammation suggest that this enzyme may be involved in diseases other than diabetes [27]. Various reports show that AR is overexpressed in several oxidative stress- and inflammation-related pathological conditions such as alcoholic liver cirrhosis, heart failure, myocardial ischemia, vascular inflammation, restenosis and cancer [34–38, 52,53]. For example, normal liver has little or no AR expression, whereas, the immunoreactivity of AR significantly increases in patients with alcoholic liver disease. The exact mechanism of AR induction in the liver is not known but may relate to oxidative stress-induced secretion of cytokines such as TNF-α which up-regulates the expression of AR during liver disease. Zeindl-Eberhart et al [52] also reported that AR is expressed in the liver during embryogenesis, is absent in the adult rat liver and is reexpressed and functionally active during liver carcinogenesis. Recently, Saraswat et al [54] demonstrated increased expression of AR in various cancerous tissues such as lung, breast, prostate, cervix, ovarian and colon. Further, they showed that the specific activity of AR was higher in tumor areas than non-tumor regions of tissues. Yoshitake et al [55] in cervical cancer demonstrated that an aldo-keto reductase family, AKR1B10, enzyme expression was associated with tumor recurrence after surgery and keratinization of squamous cell carcinoma in cervical cancer. Tanimoto et al [56] purified and characterized the AR from Engelbreth-Hom-Swarm tumor cells. However, the potential mechanism of involvement of AR in these cancers is unknown. It has been shown that various growth factors, cytokines and chemokines induce growth or apoptosis depending on the type of cells by generating ROS. ROS activate signaling pathways of PKC, PLC, DAG, PI3-K, which activate NF-kB and AP1 transcription factors known to transcribe inflammatory markers and cause cell growth as observed in cancer [24–27]. The major question is how ROS is involved in signaling? Although it has been known for a long time that ROS could cause lipid peroxidation and formation of lipid aldehydes, how they activate the signaling intermediates leading to NF-kB and AP1 activation is not clearly understood. A recent study demonstrates that alpha, beta unsaturated aldehydes promote human lung cancer cells (A549) growth in culture [57,58]. Further, our results show that GS-aldehydes are upstream to PLC, PKC, DAG, and PI3-K in the signaling pathways initiated by cytokines, chemokines and growth factors that cause activation of NF-kB and AP-1 [25–27], Fig. (3). Inhibition or ablation of AR significantly prevents the growth factors, cytokines, chemokines, and LPS-induced signals that activate the transcription factors and cause a marked increase in Cox-2 and prostaglandins along with other inflammatory markers [21–27, 40]. Based on our results, we hypothesize that AR inhibition could be an excellent method for treatment of inflammation – mediated neoplasia including colon, lung, breast and prostate cancers.
Fig. (3). AR mediation in oxidative stress – induced carcinogenesis.
Growth factors and cytokines generate ROS via NADPH oxidase. ROS cause peroxidation of membrane bound lipids and generate lipid aldehydes. AR efficiently catalyzes the reduction of one of the most abundant and toxic lipid aldehyde such as 4-hydroxy-trans-2-nonenol (HNE) and its conjugate with GSH to 1, 4-dihydroxynonene (DHN), and GS-DHN, respectively. Further, GS-DHN could be a major signaling molecule involved to activate redox-sensitive transcription factors such as NF-kB and AP1 via protein kinase cascade, PLC/PKC/PI3K/MAPK activation. The activated transcription factors translocate into the nucleus and transcribe various inflammatory genes which cause cell proliferation, dysplasia and cancer. The cytotoxic signals further augment the oxidative stress-induced carcinogenesis via autocrine and paracrine fashion.
A number of investigators have studied the efficacy of numerous chemopreventive agents against various cancers [59–61]. Most of them have antioxidant properties. The antioxidants obtained from extracts of tea, Black Cumin, Muscadine Grape Seed, the Native American Sacred herb “Tsi-Ahga” and Allicin-Release Product (ARP) from garlic have been used to study in cell and animal models to prevent carcinogenesis [60–62]. However, to date no suitable antioxidant is available with hydrophilic and hydrophobic properties that could be delivered to the cells. Most of these antioxidants are known to upregulate anti-oxidant enzymes through NRF2 and down regulate pro-inflammatory cytokines by inhibiting NF-κB [63–67]. AR expression has been shown to regulated by both NRF2 and NF-κB as AR promoter region has binding sites for their consensus sequences [67,68]. Even though there are few studies that show AR inhibitors by preventing the activation of NF-κB – dependent inflammatory signals could prevent inflammatory pathologies, no cohesive studies are available that show the interplay between NRF2 and AR that can affect the carcinogenesis. One of our interests is to examine how AR regulates NRF2 activity in cancer and immune cells and how AR regulated NRF2 modulates the tumor progression.
Role of AR in Colon Cancer
Worldwide colon cancer is the third most common cause of cancer and is the second leading cause of cancer deaths in the USA with over 1.2 million new cancer cases and 608,700 deaths estimated to have occurred in 2008 [69]. Familial and hereditary factors significantly contribute to colon carcinogenesis. The transition of normal epithelium to adenoma to carcinoma is associated with a variety of molecular and biochemical events such as genetic alterations, intestinal epithelial cell proliferation/differentiation and inflammation [70]. The major initiators of carcinogenesis include a) cells that suffered irreparable DNA damage due to increased free radicals which cause activation of specific nucleases and damage DNA, RNA, proteins and lipids, b) loss of extracellular stimulation which regulates cell growth and upregulation of growth factors and their receptors and c) autosomal dominant inheritance of cancer genes among the multiple family members [70,71]. In addition, risk factors of colon cancer include diet rich in fat, red meat, refined carbohydrates, and animal proteins along with low physical activity, overweight and obesity [71]. Furthermore, many chronic inflammatory diseases such as hepatitis, gastritis and ulcerative colitis are associated with an elevated risk of colon cancer [71,72]. However, it is not clear how cancer is initiated in the setting of chronic inflammation, but accumulating evidence strongly supports the association between inflammation and colon cancer. Furthermore, upregulation of cytokines such as TNF-α, IL-6, growth factors such as IGF-II, HGF, HGFR, EGFR, IGFR, VEGF, FGF and PDGF and their receptors have been observed in colon cancer cells obtained from colon cancer patients [25,26,73,74]. Exposure of cells to inflammatory cytokines and growth factors triggers upregulation of prostaglandins via Cox-2 and expand normal epithelial cells to dysplasia (precancer) and cancer [25,26]. Since redox-sensitive transcription factors such as NF-kB are known to transcribe the genes for cytokines and chemokines, the drugs and antioxidants that inhibit NF-kB are being used for intervention of colon cancer [25,75,76]. However, it is not clear how inflammation-associated increase in free radicals cause activation of NF-kB. It is plausible that during inflammation ROS and nitro compounds are produced by activated neutrophils and macrophages may act on cellular DNA during cell division to induce DNA mutations via NF-kB-mediated oncogenes. During chronic inflammation, many dividing cells become susceptible to DNA mutation [77]. For example, p53 mutations have been identified during chronic rheumatoid arthritis and inflammatory bowel disease [77]. Pro-inflammatory cytokines such as TNF-α plays a pivotal role in the pathogenesis of inflammatory bowel diseases like Crohn’s and ulcerative colitis which could cause increased risk of colon cancer [78].
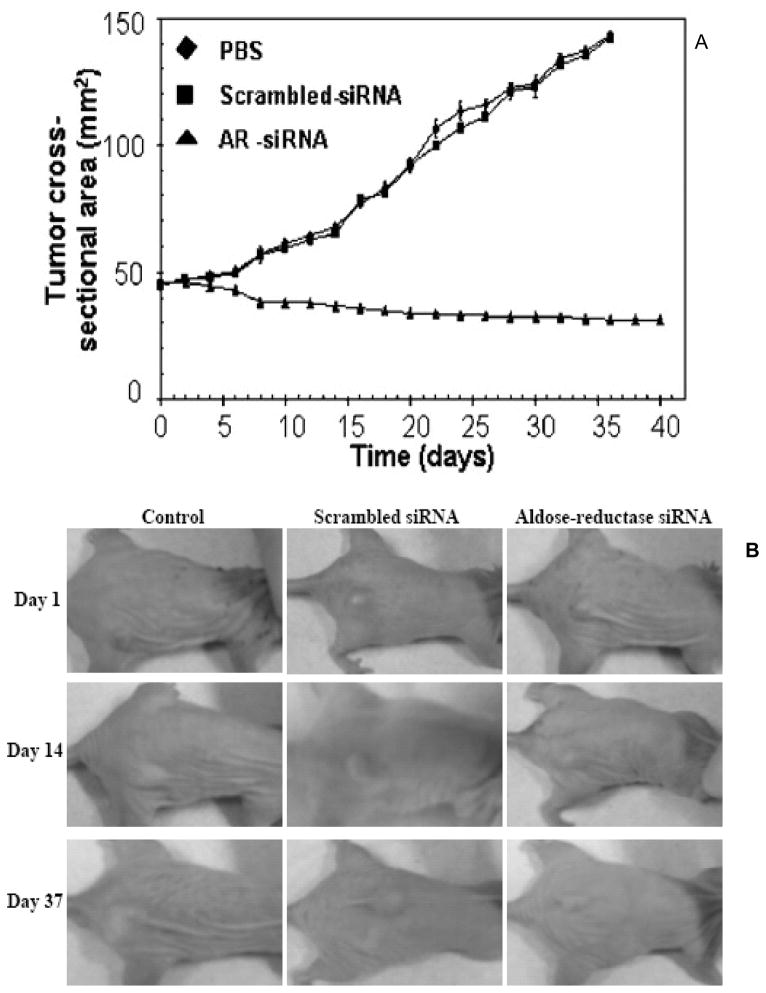
Our recent studies suggest that AR is a regulator of ROS signals induced by cytokines and growth factors (GF), leading to cell growth and differentiation in vascular cells [24–27]. Recently in human colon cancer cells, Caco-2, we have shown that inhibition of AR prevented the cytokine such TNF-α – or growth factors such as bFGF- or PDGF- induced proliferation, expression of Cox-2, activation of PKC, NF-kB and PGE2 production [25,26]. Most remarkably, in nude mice xenograft model, we have shown that in vivo administration of AR-small interfering RNA (siRNA) completely arrested tumor progression of SW480 human colon adenocarcinoma cells [25], Fig. (4). Further, pharmacological inhibition of AR with sorbinil prevents AOM-induced ACF formation in BALB/c mice. Similarly, AR gene knockout in mice significantly prevented AOM-induced ACF formation as well as AOM-induced expression of inflammatory markers, iNOS and Cox-2 and pre-neoplastic marker proteins, cyclin D1 and beta-catenin and activation of NF-kB in mice colons [42]. In addition, our results with cell cycle analysis suggest that inhibition of AR prevents growth factor -induced entry of cells to G1/S phase by accumulating at G2/M phase [25]. Growth factors promote tumor progression by upregulating important G1/S phase cell cycle proteins such as cyclin D1, cyclin E, cdks, PCNA and transcription factors including E2F-1 and c-myc through activation of AKT/PI3K [79]. Our recent studies indicate that AR inhibition prevents colon cancer cell growth by suppressing the E2F-1 mediated G1/S phase cell cycle transition [80]. There are only few studies that show how genetic modulation of AR could regulate carcinogenic signals in mouse models. We have shown that siRNA mediated knockdown of AR specifically in the tumor tissues prevents colon cancer growth in nude mice xenografts [25]. Further, we have also shown that AR deficient mice are resistant to the AOM – induced ACF formation and inflammatory protein expression [42]. More studies are required in mouse models to show how modulated levels of AR could contribute to carcinogenesis. Some of such studies are in progress in our laboratory. Overall the current findings indicate that AR is an excellent novel therapeutic target for prevention of colon cancer.
Fig. (4). AR mediates malignant growth in nude mice xenografts.
A). Effect of AR-siRNA on SW480 xenografts. An aliquot of 2 × 106 SW480 cells in 100 μL PBS was injected s.c. into one flank of each nu/nu nude mouse. When tumors reached a cross-sectional area of 45 mm2, animals were treated with PBS, scrambled siRNA, or AR-siRNA. At different days, tumors were measured in two dimensions using calipers. B). Photographs of animals taken at different days of tumor progression are shown. [Reproduced with permission from R Tammali et al.: Cancer Res. 66 (19): 9705 – 9713, 2006; Ref.: 25)
Role of AR in Hepatocarcinogenesis
Hepatocarcinogenesis refers to malignancy to the liver. In most of the cases liver becomes the secondary site for viral infection, cirrhosis or metastasis of the cancers from elsewhere of the body eg. Colon [81]. Overexpression of AR was first studied in the liver carcinogenesis among the various cancers. During embryonic development AR plays an important role in the liver to reduce carbohydrates. Increased expression of AR in the fetal liver up to 16th week of gestation and disappearance at later stages suggests that in normal adult liver AR is not required [82]. However, AR is reexpressed with functionally active enzyme in response to the loss or reduction of activity of various glycolytic enzymes and increased proliferative activity during hepatocarcinogenesis [83]. In addition, numerous studies support the overexpression of AR during liver carcinogenesis [84]. Takahashi et al [85] found that AR gene expression is induced in the livers of rats during development of hereditary hepatitis and hepatoma with aging. Further, increased expression of AR has been found in the cancerous lesions compared to uninvolved surrounding region of the liver. Scuric et al [83] found a significantly increased AR mRNA levels in the livers of hepatocellular cancer patients compared to normal liver. Together, these observations strongly suggest that AR is overexpressed during oxidative stress– induced hepatocarcinogenesis [86]. In this context, various reports show the use of antioxidants against inflammation/ROS -induced hepatocarcinogeneis. For example, the expression of AR in liver, formation of lipid peroxidation products such as malondialdehyde, nitric oxide and GST in N-nitrosodiethylamine – induced hepatocarcinogenesis were reduced by diallyl sulfide, an antioxidant, by lowering oxidative stress [86]. These results suggest that combating free radical mediated oxidative stress prevents liver carcinogenesis.
Role of AR in Cachexia syndrome
Cachexia syndrome is characterized by irreversible loss of body mass that can not be restored with nutritionally. The symptoms include loss of weight, adipose tissue, skeletal muscle atrophy, fatigue, weakness and significant loss of appetite [87–90]. Various pathological conditions such as cancer, AIDS, chronic obstructive pulmonary disease (COPD) and congestive heart failure (CHF) cause cachexia symptoms [87–89]. In cancer patients, cachexia symptoms were observed during end stage of cancer. The exact mechanism of development of cachexia is poorly understood. Recent reports suggest that altered tumor and/or host factors reduce muscle mass via decreasing protein synthesis and increasing protein degradation mechanisms [89,90]. During development of cachexia syndrome oxidative stress and inflammation have been shown to play a role by increasing the activation of ubiquitin-proteosome pathway, proteolysis-inducing factor, lipid mobilis factor and suppression of lipoprotein lipase activities. In addition it has been shown that pro-inflammatory cytokines such as TNF-α, IFN-γ, IL-1, IL-6 play significant role degradation of myofibrillar proteins in skeletal muscle during cachexia. There is considerable experimental evidence that TNF-α can induce lipid depletion in white adipose tissue by inhibiting lipoprotein lipase activity [91,92]. In 3T3-L1 adipocytes, inhibition of TNF-α selectively suppresses the lipoprotein lipase mRNA levels, which prevents storage of lipoproteins and increase of lipid flux in the circulation [92]. This mechanism has been linked with activation of MAPK, ERK, elevation of intracellular cAMP and NF-kB [92]. Similar to TNF-α, IL-1, IL-6, INF-g have been shown to decrease lipoprotein lipase activity [80,92,93]. Studies using murine colon-26 adenocarcinoma suggest that increased levels of IL-6, TNF-α, IFN-γ are correlated with the development of cachexia symptoms [93]. Watchorn et al [94] using primary cultures of human hepatocytes and the human cell HepG2 showed that proteolysis-inducing factor regulates production IL-6 and IL-8 via NF-kB and STAT3 [94]. Further, several reports showed that inhibition of NF-kB prevents cancer cachexia in various forms of cancer [95]. In murine myotubes, antioxidants such as curcumin and resveratrol completely attenuated the proteolysis-inducer factor -induced total protein degradation via inhibiting NF-kB [94,95]. Since pro-inflammatory cytokines, chemokines involved in cachexia syndrome are mediated via NF-kB, drugs which prevent NF-kB activation could offer better therapeutic option in the treatment of cancer cachexia. In this direction, our results show that inhibition of AR prevented the expression/secretion of inflammatory cytokines, chemokines via inhibiting NF-kB [39–41]. Kawamura et al [96, 97] first reported the role of AR in cancer is that inhibition of AR by ponalrestat significantly reduced the cachexia symptoms in mice induced by colon26 adenocarcinoma. Mice bearing subcutaneously injected colon26 adenocarcinoma cells developed cachexia symptoms with tumor growth [96]. Inhibition of AR by ponalrestat attenuated the cachexia symptoms such as reduction in the body mass, epididymal fat, gastrocnemius muscle, and carcass and prolonged the survival of mice. Inhibition of AR also prevented the LPS-induced production of IL-1 from monocytes in vitro and also LPS – induced release of IL-1 in the blood of mice. Further, in human melanoma cancer model inhibition of AR by ponalrestat prevented the cachexia syndrome in nude mice bearing human melanomas G361 and SEK1 cells by activating lipoprotein lipase activity in adipose tissue [97]. These results suggest that AR is involved in the development of cancer cachexia syndrome and inhibition of AR is therapeutic target to alleviate cancer cachexia. In addition, the recent advanced clinical data suggest that treatment of cancer patients with antioxidants is more promising in relieving the cachexia symptoms.
Role of AR in Chemotherapeutic Drug Resistance
Development of resistance to chemotherapeutic drugs is a major obstacle for the effective treatment of cancer [98–100]. Drug resistance affects the patients with various forms of cancers such as breast, ovarian, lung and colon [98–100]. A tumor usually contains distinct population of resistant and drug sensitive cells [101]. Most of the chemotherapeutic drugs which kill drug sensitive cells leave drug resistant cells [102]. Typically, this kind of chemotherapeutic therapy shrinks the tumor and appears to be successful but relapse of tumor and metastasis occurs by drug resistant cancer cells [102,103]. There are various possible mechanisms responsible for the development of drug resistance in the cancer cells. These include a) increased efflux of drugs b) enzymatic inactivation c) decreased permeability d) altered binding sites e) alternate metabolic pathways [101,103]. In this context various reports show that AR is involved in the development of resistance against various chemotherapeutic drugs such as daunorubicin, cisplatin etc [104,105]. The potential mechanism of AR involvement in drug resistance could be due to increased drug metabolism or increased efflux or decreased permeability of drugs from the cells [104]. Various studies in different cancer cells such as human stomach carcinoma cells, lung cancer and liver cancer, suggest that increased expression of AR contributes to carbonyl reduction and increased detoxification [105-107]. Lee et al [108] reported that overexpression of AR caused resistance to doxorubicin in liver cancer cells and inhibition of AR reversed the drug resistance. In addition, various reports show that increased expression of AR and intracellular accumulation of sorbitol may be one of the reasons to cause drug resistance in cancer cells [109]. Treatment of cancer cells with cisplatin in the presence of sorbitol reduced the cytotoxic effects through inhibition of Na+/K+ ATPase activity and thereby impairing the cellular uptake and intracellular accumulation of cisplatin [110,111]. These results clearly suggest AR involvement in the drug resistance. However, the mechanistic basis of AR activity and sorbitol accumulation is not clear, especially in understanding the AR-based drug resistance in the cancer cells. We speculate that there is an alternative signaling mechanism(s) involved with respect to AR since the oxidative stress/inflammation plays a major role in the development of drug resistance.
FUTURE PERSPECTIVES
Recent investigations show that AR is an obligatory mediator of oxidative stress– induced inflammatory patholologies such as diabetes, asthma, sepsis and cancer [112,113]. However, the role of AR in mediation of carcinogenic signals in not clear. Even though, number of studies demonstrate that AR is overexpressed in various forms of cancer, the investigations on how increased AR expression could contribute to tumor growth is not clear. Recent investigations suggest that the inhibition of AR could prevent cancer; however these studies are limited to mainly colon cancer. Therefore, extensive investigations are required to understand the efficacy of AR inhibitors in the prevention of cancers such as pancreatic, lung, prostate and breast. It is utmost important to understand the molecular events that link inflammation and cancer, and how AR regulates the inflammatory pathways that alter microenvironment of tumor. The precise mechanism(s) by which AR- catalyzed glutathione- lipid aldehydes-conjugates regulate the signaling pathways that can lead to cancer needs to be investigated. Further, more rigorous studies are required to demonstrate the significance of AR in cancer initiated by inflammation, environmental pollutants, xenobiotics, carcinogens and infections. Use of AR over-expressing transgenic animals and AR knockout animals for the cancer studies could fulfill the importance of this enzyme and will identify how genetically modulated levels of AR could contribute to cancer. Although, the current studies provided a promising role of AR in cancer progression and pharmacological inhibition or genetic ablation of this enzyme has been shown to prevent colon cancer, it is not known how AR inhibition prevents intrinsic and extrinsic signals that initiate and propagate carcinogenic signals that can cause metastatic spread of cancer. Comprehensive identification of AR regulated genetic variations that cause malignant growth needs to be determined. The role of AR in cancer invasion and angiogenesis needs to be explored. Such studies will lead to possible use of AR inhibitors as anti-cancer agents against various cancers. Further, combination studies with known chemotherapeutic drugs and AR inhibitors are required to expedite the use of AR inhibitors in clinical studies. Currently no AR inhibitor is under clinical evaluation in humans for the prevention of cancer. However, some of AR inhibitors have already gone through FDA’s Phase-iii clinical studies for diabetic complications and found to be safe for human use. Since AR inhibitors such as fidarestat have already gone through human studies without major irreversible toxicity, they can be developed easily as therapeutic drugs for treating various forms of cancer especially inflammation-mediated colon, lung, breast and prostate cancers relatively in a shorter time.
Acknowledgments
Supported by NIH grants CA129383 and DK36118 (to SKS), and GM71036 (to KVR).
Abbreviations
- AR
Aldose reductase
- AP1
Activator protein
- AOM
Azoxymethane
- Cox
Cyclooxygenase
- DHN
1,4-dihydroxynonene
- bFGF
Basic fibroblast growth factor
- GSH
Glutathione
- GS-HNE
Glutathionyl-4-hydroxynonenal
- GS-DHN
Glutathionyl-1,4-dihydroxynonene
- HGF
Hepatocyte growth factor
- HNE
4-hydroxy-trans-2-nonenal
- IFN
Interferon
- IGF
Insulin growth factor
- IL
Interleukin
- LPS
Lipopolysaccharide
- NF-kB
Nuclear factor kappa B
- PKC
Protein kinase C
- PLC
Phospho lipase C
- ROS
Reactive oxygen species
- siRNA
Small interfering RNA
- TNF
Tumor necrosis factor
Footnotes
Conflict of Interest: None
References
- 1.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki K. Anti-oxidants for therapeutic use: why are only a few drugs in clinical use? AdvDrug Deliv Rev. 2009;61:287–289. doi: 10.1016/j.addr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Chan EC, Jiang F, Peshavariya HM, Dusting GJ. Regulation of cell proliferation by NADPH oxidase-mediated signaling: potential roles in tissue repair, regenerative medicine and tissue engineering. Pharmacol Ther. 2009;122:97–108. doi: 10.1016/j.pharmthera.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Lau AT, Wang Y, Chiu JF. Reactive oxygen species: current knowledge and applications in cancer research and therapeutic. J Cell Biochem. 2008;104:657–667. doi: 10.1002/jcb.21655. [DOI] [PubMed] [Google Scholar]
- 5.Nair U, Bartsch H, Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: a review of published adduct types and levels in humans. Free Radic Biol Med. 2007;43:1109–1120. doi: 10.1016/j.freeradbiomed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol. 2008;153:6–20. doi: 10.1038/sj.bjp.0707395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartsch H, Nair J. Oxidative stress and lipid peroxidation-derived DNA-lesions in inflammation driven carcinogenesis. Cancer Detect Prev. 2004;28:385–391. doi: 10.1016/j.cdp.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Rao PV, Maddala R, John F, Zigler JS., Jr Expression of nonphagocytic NADPH oxidase system in the ocular lens. Mol Vis. 2004;10:112–121. [PubMed] [Google Scholar]
- 9.Vaquero EC, Edderkaoui M, Pandol SJ, Gukovsky I, Gukovskaya AS. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer ells. J Biol Chem. 2004;279:34643–34654. doi: 10.1074/jbc.M400078200. [DOI] [PubMed] [Google Scholar]
- 10.Tacchini L, Dansi P, Matteucci E, Desiderio MA. Hepatocyte growth factor signalling stimulates hypoxia inducible factor-1 (HIF-1) activity in HepG2 hepatoma cells. Carcinogenesis. 2001;22:1363–1371. doi: 10.1093/carcin/22.9.1363. [DOI] [PubMed] [Google Scholar]
- 11.Hoozemans JJ, Rozemuller AJ, Veerhuis R, Eikelenboom P. Immunological aspects of alzheimer's disease: therapeutic implications. BioDrugs. 2001;15:325–337. doi: 10.2165/00063030-200115050-00004. [DOI] [PubMed] [Google Scholar]
- 12.Márton IJ, Kiss C. Protective and destructive immune reactions in apical periodontitis. Oral Microbiol Immunol. 2000;15:139–150. doi: 10.1034/j.1399-302x.2000.150301.x. [DOI] [PubMed] [Google Scholar]
- 13.Khatami M. Inflammation, aging, and cancer: tumoricidal versus tumorigenesis of immunity: a common denominator mapping chronic diseases. Cell Biochem Biophys. 2009;55:55–79. doi: 10.1007/s12013-009-9059-2. [DOI] [PubMed] [Google Scholar]
- 14.Gauldie J. Inflammation and the aging process: devil or angel. Nutr Rev. 2007;65:S167–S169. doi: 10.1111/j.1753-4887.2007.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 15.Benjamim CF, Hogaboam CM, Kunkel SL. The chronic consequences of severe sepsis. J Leukoc Biol. 2004;75:408–412. doi: 10.1189/jlb.0503214. [DOI] [PubMed] [Google Scholar]
- 16.Andreasen AS, Krabbe KS, Krogh-Madsen R, Taudorf S, Pedersen BK, Møller K. Human endotoxemia as a model of systemic inflammation. Curr Med Chem. 2008;15:1697–1705. doi: 10.2174/092986708784872393. [DOI] [PubMed] [Google Scholar]
- 17.Sutcliffe AM, Clarke DL, Bradbury DA, Corbett LM, Patel JA, Knox AJ. Transcriptional regulation of monocyte chemotactic protein-1 release by endothelin-1 in human airway smooth muscle cells involves NF-kappaB and AP-1. Br J Pharmacol. 2009;157:436–450. doi: 10.1111/j.1476-5381.2009.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobliakov VA. Mechanisms of tumor promotion by reactive oxygen species. Biochemistry (Mosc) 2010;75:675–685. doi: 10.1134/s0006297910060015. [DOI] [PubMed] [Google Scholar]
- 19.Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solinas G, Marchesi F, Garlanda C, Mantovani A, Allavena P. Inflammation-mediated promotion of invasion and metastasis. Cancer Metastasis Rev. 2010;29:243–248. doi: 10.1007/s10555-010-9227-2. [DOI] [PubMed] [Google Scholar]
- 21.Hayakawa M, Miyashita H, Sakamoto I, Kitagawa M, Tanaka H, Yasuda H, Karin M, Kikugawa K. Evidence that reactive oxygen species do not mediate NF-kappaB activation. EMBO J. 2003;22:3356–3366. doi: 10.1093/emboj/cdg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:79–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Ramana KV, Bhatnagar A, Srivastava S, Yadav UC, Awasthi S, Awasthi YC, Srivastava SK. Mitogenic responses of vascular smooth muscle cells to lipid peroxidation-derived aldehyde 4-hydroxy-trans-2-nonenal (HNE): role of aldose reductase-catalyzed reduction of the HNE-glutathione conjugates in regulating cell growth. J Biol Chem. 2006;281:17652–17660. doi: 10.1074/jbc.M600270200. [DOI] [PubMed] [Google Scholar]
- 25.Tammali R, Ramana KV, Singhal SS, Awasthi S, Srivastava SK. Aldose reductase regulates growth factor-induced cyclooxygenase-2 expression and prostaglandin E2 production in human colon cancer cells. Cancer Res. 2006;66:9705–9713. doi: 10.1158/0008-5472.CAN-06-2105. [DOI] [PubMed] [Google Scholar]
- 26.Tammali R, Ramana KV, Srivastava SK. Aldose reductase regulates TNF-alpha-induced PGE2 production in human colon cancer cells. Cancer Lett. 2007;252:299–306. doi: 10.1016/j.canlet.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005;26:380–392. doi: 10.1210/er.2004-0028. [DOI] [PubMed] [Google Scholar]
- 28.Hers HG. The mechanism of the transformation of glucose in fructose in the seminal vesicles. Biochim Biophys Acta. 1956;22:202–203. doi: 10.1016/0006-3002(56)90247-5. [DOI] [PubMed] [Google Scholar]
- 29.van Heyningen R. Formation of polyols by the lens of the rat with ‘sugar’ cataract. Nature. 1959;468:194–195. [Google Scholar]
- 30.Varma SD, Mizuno A, Kinoshita JH. Diabetic cataracts and flavonoids. Science. 1977;195:205–206. doi: 10.1126/science.401544. [DOI] [PubMed] [Google Scholar]
- 31.Kinoshita JH, Kador P, Catiles M. Aldose reductase in diabetic cataracts. JAMA. 1981;246:257–261. [PubMed] [Google Scholar]
- 32.Srivastava SK, Petrash JM, Sadana IJ, Ansari NH, Partridge CA. Susceptibility of aldehyde and aldose reductases of human tissues to aldose reductase inhibitors. Curr Eye Res. 1982;2:407–410. doi: 10.3109/02713688209000786. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava SK, Ansari NH. Prevention of sugar-induced cataractogenesis in rats by butylated hydroxytoluene. Diabetes. 1988;37:1505–1508. doi: 10.2337/diab.37.11.1505. [DOI] [PubMed] [Google Scholar]
- 34.Ruef J, Liu SQ, Bode C, Tocchi M, Srivastava S, Runge MS, Bhatnagar A. Involvement of aldose reductase in vascular smooth muscle cell growth and lesion formation after arterial injury. Arterioscler Thromb Vasc Biol. 2000;20:1745–1752. doi: 10.1161/01.atv.20.7.1745. [DOI] [PubMed] [Google Scholar]
- 35.Donohue PJ, Alberts GF, Hampton BS, Winkles JA. A delayed-early gene activated by fibroblast growth factor-1 encodes a protein related to aldose reductase. J Biol Chem. 1994;269:8604–8609. [PubMed] [Google Scholar]
- 36.Tawata M, Ohtaka M, Hosaka Y, Onaya T. Aldose reductase mRNA expression and its activity are induced by glucose in fetal rat aortic smooth muscle (A10) cells. Life Sci. 1992;51:719–726. doi: 10.1016/0024-3205(92)90480-d. [DOI] [PubMed] [Google Scholar]
- 37.Seo HG, Nishinaka T, Yabe-Nishimura C. Nitric oxide up-regulates aldose reductase expression in rat vascular smooth muscle cells: a potential role for aldose reductase in vascular remodeling. Mol Pharmacol. 2000;57:709–717. doi: 10.1124/mol.57.4.709. [DOI] [PubMed] [Google Scholar]
- 38.Ramana KV, Chandra D, Srivastava S, Bhatnagar A, Aggarwal BB, Srivastava SK. Aldose reductase mediates mitogenic signaling in vascular smooth muscle cells. J Biol Chem. 2002;277:32063–32070. doi: 10.1074/jbc.M202126200. [DOI] [PubMed] [Google Scholar]
- 39.Yadav UC, Ramana KV, Aguilera-Aguirre L, Boldogh I, Boulares HA, Srivastava SK. Inhibition of aldose reductase prevents experimental allergic airway inflammation in mice. PLoS One. 2009;4:e6535. doi: 10.1371/journal.pone.0006535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J Biol Chem. 2006;281:33019–33029. doi: 10.1074/jbc.M603819200. [DOI] [PubMed] [Google Scholar]
- 41.Ramana KV, Reddy AB, Tammali R, Srivastava SK. Aldose reductase mediates endotoxin-induced production of nitric oxide and cytotoxicity in murine macrophages. Free Radic Biol Med. 2007;42:1290–1302. doi: 10.1016/j.freeradbiomed.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tammali R, Reddy AB, Ramana KV, Petrash JM, Srivastava SK. Aldose reductase deficiency in mice prevents azoxymethane-induced colonic preneoplastic aberrant crypt foci formation. Carcinogenesis. 2009;30:799–807. doi: 10.1093/carcin/bgn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ravindranath TM, Mong PY, Ananthakrishnan R, Li Q, Quadri N, Schmidt AM, Ramasamy R, Wang Q. Novel role for aldose reductase in mediating acute inflammatory responses in the lung. J Immunol. 2009;183:8128–8137. doi: 10.4049/jimmunol.0900720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jianghua Liu, Gebo Wen, Deliang Cao. Aldo-Keto Reductase Family 1 Member B1 Inhibitors: Old Drugs with New Perspectives. Recent Patents on Anti-Cancer Drug Discovery. 2009;4:246–253. doi: 10.2174/157489209789206931. [DOI] [PubMed] [Google Scholar]
- 45.Schemmel KE, Rosalyn S, Padiyara RS, D'Souza JJ. Aldose reductase inhibitors in the treatment of diabetic peripheral neuropathy: a review. J Diabetes Complications. 2010;24:354–360. doi: 10.1016/j.jdiacomp.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Cheung AK, Fung MK, Lo AC. Aldose reductase deficiency prevents diabetes-induced blood-retinal barrier breakdown, apoptosis and glial reactivation in the retina of db/db mice. Diabetes. 2005;54:3119–3125. doi: 10.2337/diabetes.54.11.3119. [DOI] [PubMed] [Google Scholar]
- 47.Yamaoka T, Nishimura C, Yamashita K. Acute onset of diabetic pathological changes in transgenic mice with human aldose reductase cDNA. Diabetologia. 1995;38:255–261. doi: 10.1007/BF00400627. [DOI] [PubMed] [Google Scholar]
- 48.Hotta N, Toyota T, Matsuoka K. Clinical efficacy of fidarestat, a novel aldose reductase inhibitor, for diabetic peripheral neuropathy. Diabetes Care. 2001;24:1776–1782. doi: 10.2337/diacare.24.10.1776. [DOI] [PubMed] [Google Scholar]
- 49.Asano T, Saito Y, Kawakami M. Erythrocytic sorbitol contents in diabetic patients correlate with blood aldose reductase protein contents and plasma glucose levels, and are normalized by the potent aldose reductase inhibitor fidarestat (SNK-860) Journal of Diabetes and its Complications. 2004;18:336–342. doi: 10.1016/j.diacomp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Singh R, White MA, Ramana KV, Petrash JM, Watowich SJ, Bhatnagar A, Srivastava SK. Structure of a glutathione conjugate bound to the active site of aldose reductase. Proteins. 2006;64:101–110. doi: 10.1002/prot.20988. [DOI] [PubMed] [Google Scholar]
- 51.Ramana KV, Dixit BL, Srivastava S, Balendiran GK, Srivastava SK, Bhatnagar A. Selective recognition of glutathiolated aldehydes by aldose reductase. Biochemistry. 2000;39:12172–12180. doi: 10.1021/bi000796e. [DOI] [PubMed] [Google Scholar]
- 52.Zeindl-Eberhart E, Jungblut PR, Otto A, Rabes HM. Identification of tumor- associated protein variants during rat hepatocarcinogenesis. Aldose reductase. J Biol Chem. 1994;269:14589–14594. [PubMed] [Google Scholar]
- 53.Lefrançois-Martinez AM, Bertherat J, Val P, Tournaire C, Gallo-Payet N, Hyndman D, Veyssière G, Bertagna X, Jean C, Martinez A. Decreased expression of cyclic adenosine monophosphate-regulated aldose reductase (AKR1B1) is associated with malignancy in human sporadic adrenocortical tumors. J Clin Endocrinol Metab. 2004;89:3010–3019. doi: 10.1210/jc.2003-031830. [DOI] [PubMed] [Google Scholar]
- 54.Saraswat M, Mrudula T, Kumar PU, Suneetha A, Rao TS, Srinivasulu M, Reddy B. Overexpression of aldose reductase in human cancer tissues. Med Sci Monit. 2006;12:CR525–529. [PubMed] [Google Scholar]
- 55.Yoshitake H, Takahashi M, Ishikawa H, Nojima M, Iwanari H, Watanabe A, Aburatani H, Yoshida K, Ishi K, Takamori K, Ogawa H, Hamakubo T, Kodama T, Araki Y. Aldo-keto reductase family 1, member B10 in uterine carcinomas: a potential risk factor of recurrence after surgical therapy in cervical cancer. Int J Gynecol Cancer. 2007;17:1300–1306. doi: 10.1111/j.1525-1438.2007.00932.x. [DOI] [PubMed] [Google Scholar]
- 56.Tanimoto T, Sato S, Kador PF. Purification and properties of aldose reductase and aldehyde reductase from EHS tumor cells. Biochem Pharmacol. 1990;39:445–453. doi: 10.1016/0006-2952(90)90049-q. [DOI] [PubMed] [Google Scholar]
- 57.Hung HS, Wu WJ, Cheng YW, Wu TC, Chang KL, Lee H. Association of cooking oil fumes exposure with lung cancer: involvement of inhibitor of apoptosis proteins in cell survival and proliferation in vitro. Mutat Res. 2007;628:107–716. doi: 10.1016/j.mrgentox.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 58.Horton ND, Mamiya BM, Kehrer JP. Relationships between cell density, glutathione and proliferation of A549 human lung adenocarcinoma cells treated with acrolein. Toxicology. 1997;122:111–122. doi: 10.1016/s0300-483x(97)00086-3. [DOI] [PubMed] [Google Scholar]
- 59.Yu H, Pan C, Zhao S, Wang Z, Zhang H, Wu W. Resveratrol inhibits tumor necrosis factor-alpha-mediated matrix metalloproteinase-9 expression and invasion of human hepatocellular carcinoma cells. Biomed Pharmacother. 2008;62:366–372. doi: 10.1016/j.biopha.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 60.Huang CS, Fan YE, Lin CY, Hu ML. Lycopene inhibits matrix metalloproteinase-9 expression and down-regulates the binding activity of nuclear factor-kappa B and stimulatory protein-1. J Nutr Biochem. 2007;18:449–456. doi: 10.1016/j.jnutbio.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 61.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N- acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- 63.Morin P, Jr, Ni Z, McMullen DC, Storey KB. Expression of Nrf2 and its downstream gene targets in hibernating 13-lined ground squirrels, Spermophilus tridecemlineatus. Mol Cell Biochem. 2008;312:121–129. doi: 10.1007/s11010-008-9727-3. [DOI] [PubMed] [Google Scholar]
- 64.Chew EH, Nagle AA, Zhang Y, Scarmagnani S, Palaniappan P, Bradshaw TD, Holmgren A, Westwell AD. Cinnamaldehydes inhibit thioredoxin reductase and induce Nrf2: potential candidates for cancer therapy and chemoprevention. Free Radic Biol Med. 2010;48:98–111. doi: 10.1016/j.freeradbiomed.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 65.Kang ES, Kim GH, Kim HJ, Woo IS, Ham SA, Jin H, Kim MY, Kim HJ, Lee JH, Chang KC, Seo HG, Hwang JY. Nrf2 regulates curcumin-induced aldose reductase expression indirectly via nuclear factor-kappaB. Pharmacol Res. 2008;58:15–21. doi: 10.1016/j.phrs.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 66.Liao BC, Hsieh CW, Liu YC, Tzeng TT, Sun YW, Wung BS. Cinnamaldehyde inhibits the tumor necrosis factor-alpha-induced expression of cell adhesion molecules in endothelial cells by suppressing NF-kappaB activation: effects upon IkappaB and Nrf2. Toxicol Appl Pharmacol. 2008;229:161–171. doi: 10.1016/j.taap.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 67.Kang ES, Woo IS, Kim HJ, Eun SY, Paek KS, Kim HJ, Chang KC, Lee JH, Lee HT, Kim JH, Nishinaka T, Yabe-Nishimura C, Seo HG. Up-regulation of aldose reductase expression mediated by phosphatidylinositol 3-kinase/Akt and Nrf2 is involved in the protective effect of curcumin against oxidative damage. Free Radic Biol Med. 2007;43:535–545. doi: 10.1016/j.freeradbiomed.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 68.Nishinaka T, Yabe-Nishimura C. Transcription factor Nrf2 regulates promoter activity of mouse aldose reductase (AKR1B3) gene. J Pharmacol Sci. 2005;97:43–51. doi: 10.1254/jphs.fp0040404. [DOI] [PubMed] [Google Scholar]
- 69.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 70.Kim Y. Nutritional epigenetics: impact of folate deficiency on DNA methylation and colon cancer susceptibility. J Nutr. 2005;135:2703–2709. doi: 10.1093/jn/135.11.2703. [DOI] [PubMed] [Google Scholar]
- 71.Weinstein IB. Nonmutagenic mechanisms in carcinogenesis: role of protein kinase C in signal transduction and growth control. Environ Health Perspect. 1991;93:175–179. doi: 10.1289/ehp.9193175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu ZX, Kiran RP, Bennett AE, Ni RZ, Shen B. Diagnosis and management of dysplasia and cancer of the ileal pouch in patients with underlying inflammatory bowel disease. Cancer. 2011 doi: 10.1002/cncr.25886. [DOI] [PubMed] [Google Scholar]
- 73.Di Popolo A, Memoli A, Apicella A. IGF-II/IGF-I receptor pathway up-regulates COX-2 mRNA expression and PGE2 synthesis in Caco-2 human colon carcinoma cells. Oncogene. 2000;19:5517–1524. doi: 10.1038/sj.onc.1203952. [DOI] [PubMed] [Google Scholar]
- 74.Nakao S, Ogata Y, Yamamoto Y, Furuyama S, Sugiya H. Platelet-derived growth factor-induced arachidonic acid release for enhancement of prostaglandin E(2) synthesis in human gingival fibroblasts pretreated with interleukin-1s. J Cell Biochem. 2004;92:579–590. doi: 10.1002/jcb.20086. [DOI] [PubMed] [Google Scholar]
- 75.Rao CV, Reddy BS. NSAIDs and chemoprevention. Curr Cancer Drug Targets. 2004;4:29–42. doi: 10.2174/1568009043481632. [DOI] [PubMed] [Google Scholar]
- 76.Swamy MV, Herzog CR, Rao CV. Inhibition of COX-2 in colon cancer cell lines by celecoxib increases the nuclear localization of active p53. Cancer Res. 2003;63:5239–5242. [PubMed] [Google Scholar]
- 77.Schetter AJ, Heegaard NH, Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hansen R, Thomson JM, El-Omar EM, Hold GL. The role of infection in the aetiology of inflammatory bowel disease. J Gastroenterol. 2010;45:266–276. doi: 10.1007/s00535-009-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pardee AB, Li CJ, Reddy GP. Regulation in S phase by E2F. Cell Cycle. 2004;3:1091–2004. [PubMed] [Google Scholar]
- 80.Ramana KV, Tammali R, Srivastava SK. Inhibition of aldose reductase prevents growth factor-induced G1-S phase transition through the AKT/phosphoinositide 3-kinase/E2F-1 pathway in human colon cancer cells. Mol Cancer Ther. 2010;9:813–824. doi: 10.1158/1535-7163.MCT-09-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gustot T, Durand F, Lebrec D, Vincent JL, Moreau R. Severe sepsis in cirrhosis. Hepatology. 2009;50:2022–2033. doi: 10.1002/hep.23264. [DOI] [PubMed] [Google Scholar]
- 82.Samanta BK, Chandra NC, Ghosh S, Mukherjee KL. Aldose metabolism in developing human fetal brain and liver. Experientia. 1984;40:1420–1422. doi: 10.1007/BF01951922. [DOI] [PubMed] [Google Scholar]
- 83.Scuric Z, Stain SC, Anderson WF, Hwang JJ. New member of aldose reductase family proteins overexpressed in human hepatocellular carcinoma. Hepatology. 1998;27:943–950. doi: 10.1002/hep.510270408. [DOI] [PubMed] [Google Scholar]
- 84.Takahashi M, Hoshi A, Fujii J, Miyoshi E, Kasahara T, Suzuki K, Aozasa K, Taniguchi N. Induction of aldose reductase gene expression in LEC rats during the development of the hereditary hepatitis and hepatoma. Jpn J Cancer Res. 1996;87:337–341. doi: 10.1111/j.1349-7006.1996.tb00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takahashi M, Fujii J, Miyoshi E, Hoshi A, Taniguchi N. Elevation of aldose reductase gene expression in rat primary hepatoma and hepatoma cell lines: implication in detoxification of cytotoxic aldehydes. Int J Cancer. 1995;62:749–754. doi: 10.1002/ijc.2910620617. [DOI] [PubMed] [Google Scholar]
- 86.Ibrahim SS, Nassar NN. Diallyl sulfide protects against N-nitrosodiethylamine-induced liver tumorigenesis: role of aldose reductase. World J Gastroenterol. 2008;40:6145–6153. doi: 10.3748/wjg.14.6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ockenga J, Valentini L. Review article: anorexia and cachexia in gastrointestinal cancer. Aliment Pharmacol Ther. 2005;22:583–594. doi: 10.1111/j.1365-2036.2005.02628.x. [DOI] [PubMed] [Google Scholar]
- 88.Saini A, Faulkner S, Al-Shanti N, Stewart C. Powerful signals for weak muscles. Ageing Res Rev. 2009;8:251–267. doi: 10.1016/j.arr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 89.Michael J, Tisdale Cancer cachexia. Langenbecks Arch Surg. 2004;389:299–305. doi: 10.1007/s00423-004-0486-7. [DOI] [PubMed] [Google Scholar]
- 90.Lorite MJ, Cariuk P, Tisdale MJ. Induction of muscle protein degradation by a tumour factor. Br J Cancer. 1997;76:1035–1040. doi: 10.1038/bjc.1997.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li YP, Reid MB. NF-kB mediates the protein loss induced by TNF-a in differentiated skeletal muscle myotubes. Am J Physiol. 2000;279:R1165–R1170. doi: 10.1152/ajpregu.2000.279.4.R1165. [DOI] [PubMed] [Google Scholar]
- 92.Berg M, Fraker DL, Alexander HR. Characterization of differentiation factor/leukaemia inhibitory factor effect on lipoprotein lipase activity and mRNA in 3T3 L1 adipocytes. Cytokine. 1994;6:425–432. doi: 10.1016/1043-4666(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 93.Fujita J, Tsujinaka T, Yano M. Anti-interleukin-6 receptor antibody prevents muscle atrophy in colon-26 adenocarcinoma-bearing mice with modulation of lysosomal and ATPubiquitin-dependent proteolytic pathways. Int J Cancer. 1996;68:637–643. doi: 10.1002/(SICI)1097-0215(19961127)68:5<637::AID-IJC14>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 94.Watchorn TM, Waddell ID, Dowidar N, Ross JA. Proteolysis-inducing factor regulates hepatic gene expression via the transcription factors NF-kB and STAT3. FASEB J. 2001;15:562–564. doi: 10.1096/fj.00-0534fje. [DOI] [PubMed] [Google Scholar]
- 95.Wyke SM, Russell ST, Tisdale MJ. Induction of proteasome expression in skeletal muscle is attenuated by inhibitors of NF-kappaB activation. Br J Cancer. 2004;91:1742–1750. doi: 10.1038/sj.bjc.6602165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kawamura I, Lacey E, Yamamoto N, Sakai F, Takeshita S, Inami M, Nishigaki F, Naoe Y, Tsujimoto S, Manda T, Shimomura K, Goto T. Ponalrestat, an aldose reductase inhibitor, inhibits cachexia syndrome induced by colon26 adenocarcinoma in mice. Anticancer Res. 1999;19:4105–4111. [PubMed] [Google Scholar]
- 97.Kawamura I, Lacey E, Inami M, Nishigaki F, Naoe Y, Tsujimoto S, Manda T, Goto T. Ponalrestat, an aldose reductase inhibitor, inhibits cachexia syndrome in nude mice bearing human melanomas G361 and SEKI. Anticancer Res. 1999;19:4091–4097. [PubMed] [Google Scholar]
- 98.Nadkar A, Pungaliya C, Drake K, Zajac E, Singhal SS, Awasthi S. Therapeutic resistance in lung cancer. Expert Opin Drug Metab Toxicol. 2006;2:753–777. doi: 10.1517/17425255.2.5.753. [DOI] [PubMed] [Google Scholar]
- 99.Baguley BC. Multidrug resistance in cancer. Methods Mol Biol. 2010;596:1–14. doi: 10.1007/978-1-60761-416-6_1. [DOI] [PubMed] [Google Scholar]
- 100.Liu FS. Mechanisms of chemotherapeutic drug resistance in cancer therapy--a quick review. Taiwan J Obstet Gynecol. 2009;48:239–244. doi: 10.1016/S1028-4559(09)60296-5. [DOI] [PubMed] [Google Scholar]
- 101.Giménez-Bonafé P, Tortosa A, Pérez-Tomás R. Overcoming drug resistance by enhancing apoptosis of tumor cells. Curr Cancer Drug Targets. 2009;9:320–340. doi: 10.2174/156800909788166600. [DOI] [PubMed] [Google Scholar]
- 102.Dean M. ABC transporters, drug resistance, and cancer stem cells. J Mammary Gland Biol Neoplasia. 2009;14:3–9. doi: 10.1007/s10911-009-9109-9. [DOI] [PubMed] [Google Scholar]
- 103.Hait WN, Hambley TW. Targeted cancer therapeutics. Cancer Res. 2009;69:1263–1267. doi: 10.1158/0008-5472.CAN-08-3836. [DOI] [PubMed] [Google Scholar]
- 104.Bando T, Fujimura M, Kasahara K, Shibata K, Shirasaki H, Heki U, Iwasa K, Ueda A, Tomikawa S, Matsuda T. Exposure to sorbitol induces resistance to cisplatin in human non-small-cell lung cancer cell lines. Anticancer Res. 1997;17:3345–3348. [PubMed] [Google Scholar]
- 105.Balendiran GK, Martin HJ, El-Hawari Y, Maser E. Cancer biomarker AKR1B10 and carbonyl metabolism. Chem Biol Interact. 2009;178:134–137. doi: 10.1016/j.cbi.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Verma M, Martin HJ, Haq W, O'Connor TR, Maser E, Balendiran GK. Inhibiting wild-type and C299S mutant AKR1B10; a homologue of aldose reductase upregulated in cancers. Eur J Pharmacol. 2008;584:213–221. doi: 10.1016/j.ejphar.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hasuike Y, Moriguchi R, Hata R, Miyagawa K, Kuragano T, Aizawa M, Yamamoto S, Yanase K, Izumi M, Tanimoto T, Nakanishi T. Role of aldose reductase in the peritoneal changes of patients undergoing peritoneal dialysis. Am J Nephrol. 2007;7:622–629. doi: 10.1159/000108358. [DOI] [PubMed] [Google Scholar]
- 108.Lee YS, Paek KS, Kang ES, Jang HS, Kim HJ, Kang YJ, Kim JH, Lee HT, Lee JH, Chang KC, Nishinaka T, Seo HG. Involvement of nuclear factor kappaB in up-regulation of aldose reductase gene expression by 12-O-tetradecanoylphorbol-13-acetate in HeLa cells. Int J Biochem Cell Biol. 2005;37:2297–2309. doi: 10.1016/j.biocel.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 109.Kamiya H, Nakamura J, Hamada Y, Nakashima E, Naruse K, Kato K, Yasuda Y, Hotta N. Polyol pathway and protein kinase C activity of rat Schwannoma cells. Diabetes Metab Res Rev. 2003;19:131–139. doi: 10.1002/dmrr.354. [DOI] [PubMed] [Google Scholar]
- 110.Lee EK, Regenold WT, Shapiro P. Inhibition of aldose reductase enhances HeLa cell sensitivity to chemotherapeutic drugs and involves activation of extracellular signal-regulated kinases. Anticancer Drugs. 2002;13:859–868. doi: 10.1097/00001813-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 111.Lee KW, Ko BC, Jiang Z, Cao D, Chung SS. Overexpression of aldose reductase in liver cancers may contribute to drug resistance. Anticancer Drugs. 2001;12:129–132. doi: 10.1097/00001813-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 112.Ramana KV, Srivastava SK. Aldose reductase: a novel therapeutic target for inflammatory pathologies. Int J Biochem Cell Biol. 2010;42:17–20. doi: 10.1016/j.biocel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Srivastava SK, Yadav UC, Reddy AB, Saxena A, Tammali R, Shoeb M, Ansari NH, Bhatnagar A, Petrash MJ, Srivastava S, Ramana KV. Aldose reductase inhibition suppresses oxidative stress-induced inflammatory disorders. Chem Biol Interact. 2011 doi: 10.1016/j.cbi.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]