Abstract
The detection of intracellular microbial DNA is critical to an appropriate innate immune response, however current knowledge on how such DNA is sensed is limited. Here we identify IFI16, a PYHIN protein, as an intracellular DNA sensor mediating interferon-β (IFNβ)-induction. IFI16 directly associated with IFNβ-inducing viral DNA motifs. STING, a critical mediator of IFNβ responses to DNA, was recruited to IFI16 after DNA stimulation. Reduction of expression of IFI16, or its murine ortholog p204, by RNA interference inhibited DNA- and herpes simplex virus (HSV)-1-induced gene induction and IRF3 and NFκB activation. IFI16/p204 is the first PYHIN protein shown to be involved in IFNβ induction, and thus together with AIM2, a PYHIN protein that senses DNA for caspase 1 activation, is part of a new family of innate DNA sensors which we term AIM2-like receptors (ALRs).
INTRODUCTION
An effective immune response to viruses is dependent on the induction of host cytokines and type I interferons such as interferon-β (IFNβ)1. This largely occurs in response to signalling by intracellular pattern recognition receptors that detect viral nucleic acids, such as viral RNA and DNA, viral replicative intermediates and viral transcription products2.
The innate immune response to viral RNA has been well-characterised, in that the endosomal Toll-like receptors (TLRs) and cytoplasmic RIG-I-like receptors (RLRs) sense viral RNA leading to IFNβ induction via activation of downstream signalling pathways2. Thus TLR3 senses double-stranded RNA (dsRNA) leading to the recruitment of the TLR adaptor protein TRIF, which then triggers the activation of TANK-binding kinase 1(TBK1) and IKKβ, kinases that phosphorylate and activate IRF3 and NF-κB respectively3, 4. Thereafter, IRF3 and NF-κB mediate an anti-viral gene induction program that includes production of IFNβ. TLR7 and TLR8 both bind viral single-stranded RNA (ssRNA) leading to recruitment of MyD88, and like TLR3 activate IKKβ and NF-κB, while in contrast to TLR3 they activate IKKα leading to IRF7 activation and induction of the type I interferon IFN-α2. Cytosolic RNA is detected by the RLRs RIG-I and Mda-5, which via the adaptor protein IPS-1 (also known as MAVS, Cardif or VISA) turn on a signalling pathway similar to TLR3, in that IFNβ induction occurs via TBK1-mediated activation of IRF32.
In contrast to the situation for viral RNA described above, the current knowledge of intracellular DNA sensors that mediate IFNα, IFNβ and cytokine induction in response to DNA viruses, such as herpes viruses and poxviruses, is much more limited. TLR9 can detect some viral DNAs in endosomes in plasmacytoid dendritic cells (DCs), leading to IFNα induction via MyD88 and IKKα 5. However it has been known for some time that exogenous dsDNA introduced into the cytoplasm, such as would be the case during infection by a DNA virus, leads to a potent innate immune response in multiple cell types, the hallmark of which is the induction of IFNβ (reviewed in 6). An innate immune response has been observed in response to bacterial and viral DNA, self DNA from apoptotic cells, and synthetic oligonucleotides such as poly(dA-dT) and a ds 45mer oligonucleotide termed IFN-stimulatory DNA (ISD). In all these cases, the induction of IFNb relies on signalling though TBK1 and IRF37,8. Apart from TBK1 and IRF3, a further critical downstream component of the response to intracellular DNA has recently been identified as STING (also called MITA and ERIS)9-11. STING was shown to be required for IFNβ induction by poly(dA-dT), ISD and herpes simplex virus-1 (HSV-1) in mouse embryonic fibroblasts (MEFs) and monocytes, and for the immune response to HSV-1 in vivo12. STING acted ‘upstream’ of TBK1, and in the presence of intracellular DNA, relocalised from the endoplasmic recticulum to to cytoplasmic foci containing TBK112, 13. However, although cytoplasmic IFNβ responses to intracellular DNA have been shown to involve STING, TBK1 and IRF3, the identity of the upstream DNA sensors that would engage with the STING-TBK1-IRF3 pathway has remained elusive.
Two such intracellular DNA sensor candidates leading to IFNβ induction have recently been identified, namely DAI (DNA-dependent activator of IRFs) and RNA polymerase III (Pol III). DAI was discovered based on the ability of poly(dA-dT) to induce IFNβ when transfected into cells14. However, the role of DAI has been shown to be very cell-type specific, and DAI-deficient murine embryonic fibroblasts (MEFs) and monocytes responded normally to poly(dA-dT)15, 16. Pol III was also shown to respond to transfected poly(dA-dT), which it transcribed into an RNA ligand for RIG-I, leading to DNA-mediated IFNβ induction via the RIG-I pathway17, 18. However, the ability of many exogenous DNAs, especially non-AT rich DNA, to induce IFNβ in multiple cell types is not accounted for by DAI and Pol III, and their relevance in pathogen detection is still unclear. Clearly then, at least one further intracellular sensor for viral DNA leading to IFNβ induction remains to be identified6.
To search for novel cytoplasmic DNA sensors, we used an IFNβ-inducing vaccinia virus (VACV) DNA motif to affinity purify DNA-binding proteins from cytosolic extracts of human monocytes. This VACV motif, when transfected into cells induced IFNβ in a TLR- DAI- and Pol III-independent, but STING- TBK1- and IRF3-dependent manner. Among the proteins identified that interacted with this DNA was IFI16, a member of the PYHIN protein family that contains a pyrin domain and two DNA binding HIN domains. IFI16 was shown to directly bind to the IFNβ–stimulating viral DNA, and to recruit STING upon stimulation of cells with transfected DNA. Small interfering RNA (siRNA) targeting IFI16, or its murine ortholog p204, inhibited DNA-, but not RNA-induced IRF3 and NF-κB activation, and IFNβ induction. Importantly, responses to a DNA virus, but not to an RNA virus, were also dependent on p204, in that HSV-1-, but not Sendai virus-stimulated transcription factor activation and gene induction were strongly impaired by p204 siRNA. Interestingly, AIM2, another PYHIN protein, has previously been shown to be a sensor for cytosolic DNA for the signalling pathway that activates caspase 1, leading to interleukin-1β release19-22. Thus we propose that the PHYIN proteins represent a new family of innate DNA sensors termed AIM2-like receptors (ALRs).
RESULTS
IFNβ induction in monocytes by viral DNA sequences
In order to investigate the cellular response to exogenous DNA we first compared the ability of different types of exogenous DNA to stimulate cytosolic DNA sensing pathways in human cells. Cells were transfected with poly(dA-dT), viral DNA from VACV, mammalian DNA or bacterial DNA, and induction of the IFNβ promoter was measured. In HEK293 cells, which have previously been shown to have a functional Pol III DNA sensing pathway17, 18, only poly(dA-dT) stimulated the IFNβ promoter (Fig. 1a). In contrast, the situation was quite different in human monocytic THP-1 cells, since in those cells all the dsDNAs that were tested induced IFNβ mRNA when transfected into cells (Fig. 1b). This suggested the existence of one or more DNA sensing pathways for non-AT rich dsDNA in THP-1 cells, that were not represented in HEK293 cells. In order to select a defined IFNβ–inducing viral dsDNA to screen for novel host DNA sensors in THP-1 cells, we noted a 70 b.p. long sequence that is strongly conserved in different poxviral genomes such as VACV in the inverted terminal repeat region, and often repeated multiple times23 (Supplementary Fig. 1). When transfected into cells, this dsDNA 70 b.p. motif (hereafter referred to as VACV 70mer) strongly induced IFNβ in THP-1 cells (Fig. 1c and Supplementary Fig. 2a) and in MEFs (Fig. 1d), but was only stimulatory when ds and not ss (Fig. 1c, d). Furthermore, VACV 70mer also induced IFNβ in human peripheral blood mononuclear cells, immortalized murine bone marrow-derived macrophages (BMDMs) and in murine bone marrow-derived DCs (Supplementary Fig. 2), but not in HEK293 cells (Fig. 1e).
Figure 1.
Induction of IFNβ by a VACV DNA motif. a, HEK293T cells were transfected with nucleic acids (0.5 and 5 μg/ml) for 16 h and IFNβ promoter activity was measured by reporter gene assay. b, PMA-treated THP-1 cells were transfected with 1 μg/ml poly(dA-dT) or with 200 ng/ml DNA isolated from VACV, calf thymus or Listeria for 6 h and IFNβ mRNA was measured. c, d THP1 (c) or immortalized MEF (d) cells were transfected with 1 μg/ml (c) or 5 μg/ml (d) nucleic acids for 6 h and IFNβ mRNA was measured. e, HEK293T cells were transfected with 5 μg/ml nucleic acids for 16 h, and IFNβ promoter activity was measured. f, g, THP-1 (f) or RAW264.7 (g) cells were transfected with different lengths (b.p.) of 1 μg/ml VACV 70mer-derived DNA, GC-rich 70mer (70(GC)) or interferon stimulatory DNA (ISD) for 6 h and IFNβ mRNA was measured. Error bars indicate s.d.
We next assessed whether the particular sequence of the VACV 70mer, and/or its length were critical to the IFNβ response, and found that the response to VACV 70mer was independent of AT content, but strongly dependent on length, since in either human or murine monocytic cells changing the AT content from 67% to 10% did not affect the response, whereas even a reduction of 10 b.p. in length impaired IFNβ induction (Fig. 1f, g). Consistent with the cytosolic IFNβ response being length-dependent but sequence-independent, the previously described 45 b.p. ISD7 gave a comparable IFNβ response to a 50 b.p. oligonucleotide derived from the VACV 70mer (Fig. 1f, g). Furthermore, an unrelated dsDNA 60mer oligonucleotide derived from the HSV-1 genome (herein referred to as HSV 60mer) also induced IFNβ (Supplementary Fig. 3a). A number of different oligonucleotides derived from the HSV-1 genome were screened for IFNβ-inducing capacity (data not shown) and the HSV 60mer was selected as having the highest (see Methods for sequence).
IFNβ induction via a novel cytoplasmic DNA sensor requiring TBK1 and IRF3
We next investigated the role of known DNA sensors in the IFNβ response to the VACV 70mer and HSV 60mer. The response to VACV 70mer in BMDMs lacking the TLR signalling adaptors MyD88 or TRIF was normal (Fig. 2a, c) while TLR-induced IFNβ expression was strongly inhibited (Fig. 2b, d). Analogously, the response to the HSV 60mer was unimpaired in macrophages lacking both MyD88 and TRIF (Supplementary Fig. 3a). BMDMs lacking DAI also responded normally to VACV 70mer (Fig. 2e), and to poly(dA-dT) (Fig. 2f). The IFNβ response to poly(dA-dT) is known to involve transcription of the DNA into RNA RIG-I ligands17, 18. Consistent with this, when RNA was extracted from poly(dA-dT)-treated murine macrophages, MEF or human THP-1 cells, and transfected into HEK293 cells, this RNA induced the IFNβ promoter (Fig. 2g and Supplementary Fig. 4a, b). In contrast, there was no IFNβ promoter induction in HEK293 cells transfected with RNA extracted from cells stimulated with VACV 70mer (Fig. 2g and Supplementary Fig. 4a, b), indicating that, unlike poly(dA-dT), the VACV 70mer is not transcribed into immune-stimulatory RNA . The HSV-1 60mer also induced IFNβ independently of Pol III, since IFNβ induction was not prevented by the Pol III inhibitor ML60812 (Supplementary Fig. 3b). Consistent with this, there was no response to the HSV 60mer in HEK293 cells where the Pol III pathway is operational (Supplementary Fig. 3c).
Figure 2.
Induction of IFNβ by a VACV DNA motif is independent of known DNA sensing pathways. a-f, h-j, Immortalised BMDMs or MEFs derived from mice lacking signalling components as indicated were transfected with 5 μg/ml VACV 70mer (a, c, e, h-j) or poly(dA-dT) (f) or stimulated with LPS (b) or poly(I:C) (d) for 6 h and IFNβ mRNA measured. Values are expressed as % of stimulation observed in cells from wild type mice. Data is from three independent experiments performed in triplicate and error bars represent s.e.m. g, Immortalised BMDMs were transfected with nucleic acid for 6h and IFNβ mRNA measured (left panel). 100 ng of the RNA extracted from BMDMs was transfected into HEK293T cells for 16 h, and IFNβ promoter activity measured (right panel). Data from one experiment of three is shown where error bars represent s.d.
All cytosolic DNA sensing pathways inducing IFNβ described thus far, including those where no receptor has been identified such as for the ISD, are known to do so via activation of the transcription factor IRF3 by a complex containing TBK16. Consistent with this, the VACV 70mer response in MEFs was abolished in the absence of IRF3 (Fig. 2h), while in either MEFs or BMDMs lacking TBK1, the VACV 70mer response was strongly impaired (Fig. 2i, j). Hence IFNβ induction by the VACV 70mer is TLR-, DAI- and Pol III-independent, yet TBK1- and IRF3-dependent.
Identification of IFI16 as a candidate DNA sensor for viral DNA
Since the IFNβ response to VACV 70mer was not mediated by any of the known DNA-sensing pathways, we designed a screen to isolate novel cytoplasmic DNA sensors. Biotinylated ss or ds VACV 70mer was coupled to streptavidin beads in order to affinity purify DNA-binding proteins from cytosolic extracts of THP-1 cells. Proteins interacting with VACV 70mer were identified by mass spectrometry (see Methods for further details). Among the proteins identified was IFI16, a member of the PYHIN protein family that contains a pyrin domain and two DNA binding HIN domains (Fig. 3a). Interestingly, AIM2, another PYHIN protein, has been shown to be a receptor for cytosolic DNA that regulates caspase 1 activation, leading to interleukin-1β release19-22. However, no PYHIN protein has yet been implicated in mediating an IFNβ response to DNA.
Figure 3.
IFI16 binds to immune stimulatory viral DNA. a, Schematic representation of human PYHINs. Aa, amino acids. b, Cytoplasmic extracts from PMA-treated THP-1 cells were incubated with biotinylated ss or dsVACV 70mer immobilised on streptavidin beads. Precipitated proteins were immunoblotted with an IFI16-specific antibody as indicated. c, Left panel: THP-1 cells were transfected with 1 μg/ml dsVACV 70mer or poly(I:C) in the absence or presence of 1 μg/ml ssVACV 70mer and IFNβ mRNA was measured after 6 h. Right panel: HEK293T cells were transfected with 50 ng/ml poly(dA-dT) alone, or together with 50 ng/ml ss or dsVACV 70mer and IFNβ promoter activity measured after 16 h. Data from one experiment of three is shown where error bars represent s.d. d, PMA/IFN α-treated THP-1 cells grown on coverslips were transfected with 2.5 μg/ml VACV 70mer or poly(I:C) for 1 h. Cells were fixed and stained with anti-IFI16 antibody (red). DNA and poly(I:C) were visualised with DAPI (blue). e, FITC-labelled HSV-1 60mer was transfected into PMA-treated THP-1 cells for 3 h. Cells were fixed and stained for IFI16 (red). DAPI-stained DNA is shown in blue (upper panel) and the HSV 60mer is shown in green (lower panel). Scale bar: 10 μm. f, AlphaScreen assessment of IFI16 HIN domains binding dsVACV 70mer. Left panel, 30 nM DNA with increasing concentration of HIN domains or GB1 expression tag. Right panel, 30 nM protein domain with increasing concentrations of biotin-70mer.
In THP-1 cells cytosolic IFI16 was detectable by immunoblot in a complex with immobilised ss or dsVACV 70mer (Fig. 3b), consistent with the fact that its HIN domains were previously shown to bind both ssDNA and dsDNA24. Given that the ssVACV 70mer was unable to induce IFNβ (Fig. 1c), we reasoned that it might bind IFI16 but fail to induce signalling to IFNβ, and consistent with this hypothesis, The ssVACV 70mer acted as an antagonist of dsVACV 70mer-induced IFNβ when co-transfected into THP-1 cells, while not affecting the response to poly(I:C) (Fig. 3c). Furthermore, poly(dA-dT)-induced IFNβ in HEK293 cells was also unaffected by transfection of the ssVACV 70mer (Fig. 3c). It was previously shown that IFI16 was mainly localized to the nucleus when overexpressed in HEK293 cells19, which was confirmed in this study (data not shown). However in THP-1 cells, endogenous IFI16 was isolated from the cytoplasm in association with VACV 70mer (Fig. 3b), and while predominantly nuclear, was clearly visible in the cytoplasm by immunofluorescence (Fig. 3d). Further, mitotracker co-staining indicated that IFI16 might partially localize to mitochondria (Supplementary Fig. 5a). Compellingly, when cells were stained with DAPI to detect nucleic acid, VACV 70mer, but not poly(I:C), was found to co-localize with endogenous IFI16 in the cytoplasm (Fig 3d). Furthermore, FITC-labelled HSV 60mer also co-localised with endogenous cytoplasmic IFI16 (Fig. 3e). In order to determine whether IFI16 was capable of directly binding the viral DNAs in vitro, the HIN domains of IFI16 were expressed in E.coli and purified (see Supplementary methods and Supplementary Fig. 6). An AlphaScreen was then used to determine in vitro binding between viral DNA and purified IFI16 HIN domains. Biotinylated dsVACV 70mer was found to bind cooperatively to HINb, and to the region of IFI16 containing both HINa and HINb (HINab) with higher affinity than to HINb alone, but not to bind HINa alone (Fig. 3f). Similar results were obtained for the HSV 60mer (Supplementary Fig. 3d). The co-localization of endogenous cytoplasmic IFI16 with IFNβ inducing viral DNA, and its direct and cooperative binding to this DNA in vitro suggested that IFI16 might be a novel DNA sensor mediating IFNβ induction.
STING is recruited to IFI16 upon DNA stimulation
As well as directly binding IFNβ-stimulating DNA, IFI16 in cytosolic lysates recruited downstream signalling molecules known to be involved in IFNβ induction: like IFI16 both cytosolic TBK1 and DDX3 (which has a role in IRF3 activation by TBK125), were enriched in immobilised dsVACV 70mer complexes, compared to ssVACV 70mer complexes (Fig. 4a). STING is a recently identified signalling protein known to be required for TBK1-dependent IFNβ responses to viruses and DNA12, 13. Interestingly, transfection of VACV 70mer into cells caused a DNA-dependent association between endogenous STING and IFI16 in THP-1 cells (Fig. 4b). Furthermore, IFI16 from DNA-stimulated THP1 cells associated with immobilized Myc-tagged human STING and to a lesser extent mouse STING (Fig. 4c). STING was not only recruited to IFI16 after viral DNA stimulation, but was also required for IFNβ induction by VACV 70mer or HSV 60mer since in BMDMs lacking STING, IFNβ secretion from cells transfected with the viral DNAs was completely inhibited (Fig. 4d, e). In contrast to the role of STING in the 70mer-IFI16 pathway to IFNβ, there was no role for ASC (apoptosis-associated speck like protein containing a CARD domain), the AIM2 adaptor that mediates caspase 1 activation by viruses and poly(dA-dT)19, since in BMDMs lacking ASC both VACV 70mer- and HSV 60mer-induced IFNβ expression was unimpaired, as was the poly(dA-dT) response (Fig. 4f). Thus STING and ASC likely have distinct roles in regulating PYHIN-mediated IFNβ induction and caspase 1 activation respectively.
Figure 4.
Role for STING in IFI16-mediated IFNβ induction. a, Cytoplasmic extracts from PMA-treated THP-1 cells were incubated with biotinylated ss or dsVACV 70mer immobilised on streptavidin beads. Precipitated proteins were immunoblotted as indicated. b, PMA/IFNα pre-treated THP1 cells were transfected with 1 μg/ml VACV 70mer for 4 h. Resulting lysates were incubated with anti-STING antibody and immuno-precipitated proteins were immunoblotted as indicated. c, Myc-tagged human (hu) or mouse (mur) STING were overexpressed in HEK293 cells, immobilized on sepharose beads, and incubated with lysates from DNA-treated THP1 cells. Co-immunoprecipitated IFI16 was detected by immunoblotting. Ab, antibody heavy chain. d, e, BMDMs lacking STING were transfected with either VACV 70mer , HSV 60mer or infected with HSV-1 or Sendai virus for 18 h as indicated and IFNβ protein release was measured by ELISA. f, Immortalised BMDMs from ASC-null mice were transfected with DNA as indicated for 18 h and IFNβ protein secretion was measured by ELISA. Data from one experiment of at least 2 (performed in triplicate) is shown where error bars represent s.d. (d-f).
Gene induction and signalling by viral DNA requires IFI16/p204
Further evidence for a role for IFI16 in mediating an IFNβ response to DNA came from the observation that there was a correlation between expression of IFI16 and responsiveness of cells to VACV 70mer. Thus, PMA-treated THP-1 cells displayed enhanced IFI16 expression and increased VACV 70mer-induced IFNβ expression compared to untreated cells (Fig. 5a), while in HEK293 cells which failed to respond to VACV 70mer (Fig. 1e), IFI16 expression was undetectable (Fig. 5a and Supplementary Fig. 5b). In THP-1 cells, IFI16 expression was inducible by both IFNα and VACV 70mer (Supplementary Fig. 5b, c), consistent with an antiviral role for IFI16. To clarify the role of IFI16 in mediating IFNβ induction by the VACV 70mer we used transient transfection of short interfering RNA oligonucleotides (siRNAs) to knock down IFI16 in THP-1s, which led to reduced expression of IFI16 protein (Fig. 5b). Importantly, this led to an inhibition of IFNβ induction in response to VACV 70mer (Fig. 5c). The IFNβ response to HSV 60mer was also inhibited in human cells by siRNA targeting IFI16 (Supplementary Fig. 7a).
Figure 5.
IFI16/p204 is required for DNA-mediated gene induction. a, Left panel, IFI16 mRNA was measured in THP-1, PMA-treated THP-1, or HEK293 cells. Right panel, THP-1 or PMA-treated THP-1 cells were transfected with VACV 70mer for 6 h and IFNβ mRNA measured. b, c, siRNA-treated THP-1 cells were transfected with 1 μg/ml VACV 70mer for 6 h, before detection of IFI16 protein expression by immunoblotting (b) or measurement of IFNβ mRNA (c). d, Domain organization of human IFI16 compared to murine members of the PYHIN family, drawn to scale. Boxes represent conserved domains. aa, amino acids. Of the murine proteins, p204 (*) is most similar to human IFI16, in terms of domain structure and aa identity. e, f, siRNA-treated RAW264.7 cells were transfected with 1 μg/ml VACV 70mer for 6 h and p204 protein expression was analysed by immunoblotting (e) or p204, IFNβ, CCL5 and TNF mRNA was measured (f). g, siRNA-treated MEF cells were transfected with 1 μg/ml VACV 70mer for 6 h and p204, IFNβ, CCL5 and TNF mRNA was measured. h, siRNA-treated RAW264.7 cells were treated with HSV 60mer DNA for 6 h before measurement of IFNβ mRNA. i, BMDMs were electroporated with siRNA prior to transfection with oligomers for 18 h, and IFNβ protein release was measured. Data from one experiment of 2-3 (performed in triplicate) is shown (a, c, f-i) where error bars represent s.d. *P < 0.05 compared with control siRNA.
We next considered whether IFI16 might also have a role in DNA sensing in murine cells. Upon examining the murine PYHIN family, only one member was found to display the same domain structure as IFI16, namely p204, in that this PYHIN protein also contained one pyrin and two HIN domains (Fig. 5d). Also, a BLAST search with human IFI16 revealed p204 as the most similar murine PYHIN protein (37% amino acid identity). Expression of p204 was detected in murine RAW264.7 cells by immunoblot, and this increased upon transfection with VACV 70mer (Fig. 5e). Treatment of these cells with siRNA targeting p204 led to reduced p204 protein (Fig. 5e) and mRNA (Fig. 5f) levels. This led to impaired VACV 70mer-mediated gene induction of IFNβ, CCL5 and TNF (Fig. 5f). The requirement for p204 for transfected DNA-mediated gene induction was not restricted to RAW264.7 cells, since in MEFs, p204 siRNA strongly reduced p204 mRNA (Fig. 5g) and also led to potent inhibition of VACV 70mer-mediated IFNβ, CCL5 and TNF induction (Fig. 5g). Suppression of p204 expression by siRNA in RAW264.7 cells also inhibited the IFNβ response to the HSV 60mer (Fig. 5h). Furthermore, in BMDMs, p204 siRNA inhibited both VACV 70mer- and HSV 60mer-stimulated IFNβ protein secretion from cells (Fig. 5i). Hence the IFI16/p204 DNA sensing pathway is not restricted to one DNA sequence nor to one cell type, and is operational both in human and murine cells.
A role for p204 as a sensor for DNA predicts that inhibition of p204 expression should affect DNA-mediated gene induction due to an effect upstream of transcription factor activation, and therefore we next examined the effect of p204 siRNA on VACV 70mer-induced NF-κB and IRF3 activation. To measure transcription factor activation, the translocation of endogenous p65 (an NF-κB subunit) and IRF3 from the cytosol to the nucleus was monitored by confocal microscopy upon DNA transfection of RAW264.7 cells, in the presence of either control or p204-targetting siRNA. Transfection of cells with VACV 70mer or poly(I:C) for 6 h led to an accumulation of both p65 and IRF3 in the nucleus in the presence of the control siRNA (Fig. 6a, b). Compellingly, both p65 and IRF3 translocation was prevented in the presence of p204 siRNA in cells transfected with DNA but not with RNA (Fig. 6a, b). This confirmed that p204 has a role in gene induction upstream of transcription factor activation. Furthermore, it showed a role for p204 upstream of both the NF-κB and IRF3 pathways for DNA and not RNA, consistent with a role in direct DNA sensing.
Figure 6.
p204 is required for VACV 70mer DNA-stimulated transcription factor activation. a, siRNA-treated RAW264.7 cells grown on glass coverslips were mock-transfected or transfected with 2.5 μg/ml VACV 70mer or poly(I:C) for 6 h, fixed and stained for NF-κB p65 (red) and IRF3 (green). Nuclei were visualised by DAPI (blue). b, Cells treated as in (a) were qualitatively examined to assess staining of either p65 (left panel) or IRF3 (right panel) in the nucleus. Cells showing nuclear staining were counted and expressed as a percentage of total number of cells. At least 200 cells were counted per sample. Shown is a representative of four independent experiments.
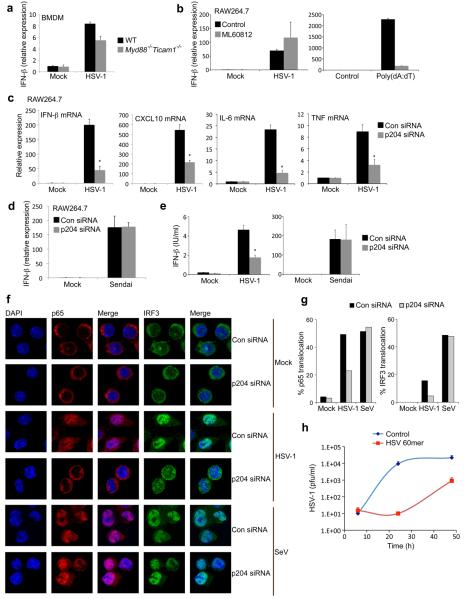
IFI16/p204 is required for gene induction and signaling responses to HSV-1
Current knowledge of IFNβ-inducing DNA sensors that would operate during viral infection by DNA viruses such as HSV-1 is very limited26. We therefore examined the role of various DNA sensing pathways in mediating gene induction and signalling responses to HSV-1 in monocytes. The IFNβ response to live HSV-1 in BMDMs was largely TLR-independent, since in cells lacking both MyD88 and TRIF, only marginal inhibition of IFNβ mRNA induction was observed (Fig. 7a). The IFNβ response to HSV-1 in monocytes was also Pol III-independent, since in contrast to a previous study17, we found no impairment of IFNβ induction by HSV-1 in the presence of the Pol III inhibitor, although as expected the response to poly(dA-dT) was inhibited (Fig. 7b). Furthermore, consistent with a lack of a role for Pol III in sensing HSV-1, no IFNβ induction was detectable in HSV-1-infected HEK293 cells, cells in which the Pol III DNA sensing pathway is operational (Supplementary Fig. 3c). The IFNβ response to HSV-1 was STING-dependent (Fig. 4e) as previously shown12.
Figure 7.
IFI16/p204 is required for the innate immune response to HSV-1. a, BMDMs were infected with HSV-1 (MOI=10) for 6 h and IFNβ mRNA was measured. b, RAW264.7 cells were pre-treated with ML60812 for 2 h prior to infection with HSV-1 (MOI=10), or transfection with poly(dA-dT) for 6 h. c, d, siRNA-treated RAW264.7 cells were infected with HSV-1 or Sendai virus for 6 h and IFNβ (c, d), CXCL10 (c), IL-6 (c) and TNF (c) mRNA was measured. e, siRNA-treated RAW264.7 cells were infected with HSV1 (MOI 10) or Sendai virus for 20 h and IFNβ protein expression determined by ELISA. f, siRNA-treated RAW264.7 cells grown on glass coverslips were mock-infected or infected with HSV-1 or Sendai virus for 6 h, fixed and stained for NF-κB p65 (red) and IRF3 (green). Nuclei were visualised by DAPI (blue). g, Cells treated as in (f) were qualitatively examined to assess staining of either p65 (left panel) or IRF3 (right panel) in the nucleus. Cells showing nuclear staining were counted and expressed as a percentage of total number of cells. At least 200 cells were counted per sample. Shown is a representative of three independent experiments. h, RAW264.7 cells transfected with HSV 60mer (2 μg/ml) were infected with HSV-1 (MOI 1) and culture supernatants were harvested at the indicated time points post infection for viral quantification by plaque assay. Data from one experiment of 2-3 (performed in triplicate) is shown (a-e, h) where error bars represent s.d.*P < 0.001 compared with control siRNA.
In contrast to the minor role for TLRs, and the absence of a role for Pol III in IFNβ induction by HSV-1 in monocytes, treatment of cells with p204 siRNA led to a dramatic inhibition of HSV-1-induced IFNβ mRNA (Fig. 7c) and protein (Fig. 7e), while the IFNβ response to an RNA virus (Sendai) was unaffected (Fig. 7d, e). This confirmed that knockdown of p204 expression did not lead to a global reduction in gene induction. The IFNβ response to HSV-1 was also inhibited in human cells by siRNA targeting IFI16 (Supplementary Fig. 7a). Induction of the IRF3-dependent gene CXCL10, and the NF-κB-dependent genes IL-6 and TNF by HSV-1 infection of RAW264.7 cells were also impaired by p204 siRNA (Fig. 7c). Consistent with this, and similar to the case for the viral DNA oligonucleotides (Fig. 6a), p204 was required for HSV-1-induced translocation of NF-κB (p65) and IRF3 from the cytosol to the nucleus (Fig. 7f, g). In contrast, Sendai virus-stimulated transcription factor translocation was unaffected by p204 siRNA (Fig. 7f, g). Further, triggering the p204 pathway induced anti-HSV-1 activity in cells, since pre-treatment of cells with the HSV 60mer potently suppressed HSV-1 replication (Fig. 7h).
HSV-1 replicates in the nucleus. Therefore IFI16/p204 could in principle detect HSV-1 DNA in that compartment, and certainly the nucleus contains high levels of IFI16 (Fig. 3d). However, using a specific labelled oligonucleotide that is complementary to HSV-1 DNA, we demonstrate for the first time that HSV-1 DNA is detectable in the cytoplasm mislocated from the viral capsid (as stained by an antibody to the viral protein VP5) in RAW264.7 macrophages (Supplementary Fig. 8), BMDMs, and THP-1 cells (not shown). Thus IFI16/p204 might detect the presence of HSV-1 DNA either in the cytoplasm and/or the nucleus.
Together, these results demonstrate that human IFI16 and murine p204 are PYHIN proteins that act as sensors for exogenous DNA, but not RNA, directly detecting the presence of viral DNA leading to transcription factor activation and gene induction via a STING-dependent pathway.
DISCUSSION
Currently there is much interest in defining the mechanisms whereby the innate immune response detects exogenous DNA, since this detection process is critical in order to understand how cells respond to DNA viruses, and to immune-stimulatory bacterial and self-DNA6. Although IFI16 has previously been shown to have a role in regulating cell proliferation and differentiation27, our data here identify IFI16/p204 as a critical sensor for exogenous dsDNA, especially with regard to IRF3- and NF-κB-dependent gene induction, and as essential for the IFNβ response to live HSV-1. Until now, the sensor for intracellular non-AT-rich dsDNA in MEFs and some monocyte-derived cells was undefined6, although IRF3, TBK-1 and STING were known to be required7, 8, 12. Since the IFI16/p204 pathway to IFNβ induction via STING, TBK-1 and IRF3 identified here operates in both MEFs and macrophages it may account for these responses.
IFI16 is the first example of a pyrin domain-containing protein sensing DNA to mediate IFNβ induction. We showed that the HIN domains of IFI16 bound the VACV 70mer and the HSV 60mer, but it remains to be determined which features of the dsDNA are critical for this interaction, and how dsDNA stimulates IFI16 to recruit STING. It is possible that DNA of a certain length in the cytosol causes oligomerisation of IFI16 leading to signalling, consistent with our observation that the IFNβ response was strongly length-dependent.
Previously pyrin domains have been shown to mediate inflammasome activation. For example, AIM2 is another PYHIN family member which does sense DNA and poxviruses, but this leads to activation of caspase 1, via the adaptor ASC, and not IFNβ induction19. In contrast, here IFI16 recruited STING, and required STING but not ASC, for DNA-mediated IFNβ induction. Further, previously it has been shown that IFI16 and ASC do not interact19. Interestingly, within the PYHINfamily, the pyrin domain of AIM2 is most like inflammasome-related pyrin domains, while the pyrin domains of the rest of the family are distinct from AIM2, and more like IFI1628. It remains to be determined whether other PYHIN family members may also be capable of regulating STING-dependent pathways that may be independent of ASC.
Given that IFI16 senses DNA to induce IFNβ, and AIM2 senses DNA to activate caspase 1, we propose that the PYHIN proteins represent a new family of innate DNA sensors termed AIM2-like receptors (ALRs). Thus while exogenous RNA is sensed by both endosomal TLRs (TLR3, 7, 9) and intracellular RLRs2, so exogenous DNA is sensed by both endosomal TLR9 and intracellular ALRs. Similar to the RLR family, the ALR family contains sensors for IFNβ induction (IFI16 compared to RIG-I and Mda5), inflammasome activators (AIM2 compared to RIG-I29) and negative regulators (p20221 compared to LGP230). Further, like the RLR family, the ALR family is subject to viral targeting: the poxviral protein M013 contains a pyrin domain and binds to ASC31, which would inhibit the AIM2 response, while interestingly it has also been recently shown to inhibit poxviral-stimulated induction of NF-κB-dependent genes32. Also, the human cytomegalovirus protein pUL83, a known antagonist of IFN-inducible genes, has recently been shown to interact with IFI1633. If IFI16 also has a role in sensing that DNA virus that could explain the ability of pUL83 to potently inhibit the IFN response.
It is possible that IFI16 also has a role in sensing bacterial DNA during infection with intracellular bacteria. Type I IFN induction in response to Listeria is independent of TLRs and RNA sensing and requires IRF334 and STING12, while IFNβ induction in response to Chlamydia infection also involves a TLR- and RLR-independent but STING-dependent pathway35. Finally, DNA detection in the cytoplasm leading to IFNβ induction is thought to be one trigger for autoimmune conditions such as systemic lupus erythematosus (SLE), and it is interesting to note that anti-IFI16 antibodies are present, and IFI16 expression levels are elevated, in patients with SLE36-38. Thus detection of DNA by IFI16/p204 may have a role not only in anti-viral innate immune responses but also in responses to bacterial pathogens, and in autoimmunity.
METHODS
Mice, cells and viruses
MyD88-/- and TRIF-/- mice were obtained from S. Akira, and were crossed to generate MyD88-/-/TRIF-/- mice. DAI-/-, IRF3-/-, TNFR1-/- and TBK1-/-/TNFR1-/- mice were from S. Akira, T. Taniguchi, M. Kelliher and T. Mak respectively. STING -/- femurs were from G. Barber. Immortalized macrophage cell lines were generated from mouse femurs using J2 recombinant retrovirus carrying v-myc and v-raf/mil oncogenes39 as described40. Immortalized MEFs were generated from IRF3-/- and TBK1-/- mice. THP-1 cells were grown in RPMI containing 10% FBS, and were treated with 50 or 100 nM PMA and 1000U/ml IFNα for at least 16h where indicated. All other cells were grown in DMEM containing 10% FBS. For viral infection, HSV-1 strain KOS, and Sendai virus strain Cantell (a gift from I. Julkunen) were used.
Antibodies
p204 antibody41 was a gift from C. Liu. STING antibody9 was a gift from G.Barber. Other antibodies were from the following sources: IFI16 and p65 (Santa Cruz), β-actin (Sigma), TBK1 (Cell Signalling), DDX3 (Bethel Laboratories), mouse IRF3 (Zymed), VP5 (Virusys), myc (Sigma).
Nucleic acids and transfection
HEK293 cells were transfected using 4μl/ml GeneJuice (Merck). All other cells were transfected using 1μl/ml Lipofectamine2000 (Invitrogen). Poly(I:C), calf thymus DNA and poly(dA-dT) were from Sigma, and herring sperm DNA from Promega. Listeria monocytogenes DNA was a gift from S. Corr. VACV DNA was purified from Western Reserve strain virus core particles by phenol-chloroform extraction. Oligonucleotides were synthesised by MWG Biotech, sequences are as follows: VACV 70mer, 5′-CCATCAGAAAGAGGTTTAATATTTTTGTGAGACCATCGAAGAGAGAAAGA GATAAAACTTTTTTACGACT-3′; GC-rich 70mer, 5′-CCGCCAGCCCGCGGGCTGGCGCCCCCACTCGGGCCGTCGGGGCCGCGCCT CCCCCGCGAGGCCGCCGGCG-3′; ISD, 5′-TACAGATCTACTAGTGATCTATGACTGATCTGTACATGATCTACA-3′; HSV-1 60mer, 5′-TAAGACACGATGCGATAAAATCTGTTTGTAAAATTTATTAAGGGTACAAA TTGCCCTAGC-3′. The VACV 30mer, 50mer and 60mer sequences are identical to the 5′ portion of the VACV 70mer, but terminating at position 30, 50 and 60, respectively.
AlphaScreen
The AlphaScreen was set up as an association assay. Purified recombinant His-tagged IFI16 HIN domains were incubated at the indicated concentrations with biotinylated DNA and binding was measured as previously described19.
RNA analysis
Human IFNβ mRNA was quantified by real-time PCR using the TaqMan gene expression assay Hs00277188_s1 and β-actin endogenous control VIC/MGB probe (Applied Biosystems). Other mRNAs were quantified using SYBR Green with the following primers: F-IFNβ(mouse), 5′-ATGGTGGTCCGAGCAGAGAT-3′; R-IFNβ(mouse), 5′-CCACCACTCATTCTGAGG-3′; F-CCL5(mouse), 5′-CTCACCATATGGCTC GGACA-3′; R-CCL5(mouse), 5′-ACAAACACGACTGCAAGATTG G-3′; F-TNF(mouse), 5′-TCCCCAAAGGGATGAGAAGTT-3′; R-TNF(mouse), 5′-GTTTGCTACGACGTG GGCTAC-3′; F-β-actin(mouse), 5′-TCCAGCCTTCCTTCTTGG GT-3′; R-β-actin(mouse), 5′-GCACTGTGTTGGCATAGAGGT-3′; F-p204, 5′-TGGTCCCAAACAAGTGATGGTGC-3′; R-p204, 5′-TCAGTTTCAGTAGCCACGGTAGCA-3′; F-IFI16, 5′-CCGTTCATGACCAGCATAGG-3′; R-IFI16, 5′-TCAGTCTTGGTTTCAACGTGGT-3′. In Fig. 5h and 7, the following real-time PCR primers were used: F-TNFα, 5′-ATCGGCTGGCACCACTAGTT-3′; R-TNFα, 5′-GTAGCCCACGTCGTAGCAAAC-3′; F-IL6, 5′-AGAATTGCCATTGCACA-3′; R-IL6, 5′-CTCCCAACAGACCTGTCTATA-3′; β–actin-F, 5′-TAGCACCATGAAGATCAAGAT-3′; β–actin-R, 5′-CCGATCCACACAGAGTACTT-3′; IFNβ-F, 5′-CACGCTGCGTTCCTGCTGTG-3′, IFNβ-R, 5′-AGTCCGCCCTGTAGGTGA GGTT-3′; CXCL10-F, 5′-CGATGACGGGCCAGTGAGAATG-3′; CXCL10-R, 5′-TCAACACGTGGGCAGGATAGGCT-3′. mRNA expression levels were normalized to β-actin mRNA levels.
ELISA
Cell culture supernatants were assayed for IFNβ by a custom ELISA, as previously described42.
Luciferase assays
IFNβ promoter activation in HEK293 cells was measured as previously described25.
Oligonucleotide pull-down and mass spectrometry
Cytoplasmic extracts were generated by disrupting PMA-treated THP1 cells in extraction buffer (10mM HEPES, pH7.9, 1.5mM MgCl2, 10mM KCl, 0.5mM EDTA, 0.5mM DTT, protease inhibitors) and pelleting nuclei by centifugation at 1000 g for 10min. The superntant was further centrifuged at 15,700 g for 10min and pre-cleared with resin. 5 mg of total cytoplasmic protein was used for each nucleic acid affinity purification, together with 2 nmoles of ds or ss 5′ biotinylated VACV 70mer pre-coupled to 100μl of Streptavidin UltraLink® Resin (Pierce; 50 % slurry). Precipitated DNA-protein complexes were washed extensively in NP40-containing lysis buffer (0.5 % NP40, 100mM NaCl, 5% glycerol, 0.5mM EDTA and 50mM HEPES pH7.5, 1mM NaVO4, protease inhibitors) and in 5 mM HEPES pH 7.5. Proteins were eluted by boiling in 1% SDS, resolved by SDS-PAGE, subjected to trypsin digestion and analyzed by liquid chromatography mass spectrometry.
Co-immunoprecipitation
PMA- and IFNα-treated THP1s were either mock-transfected or transfected with 1μg/ml VACV 70mer DNA for 4h. Cells were lysed in NP40-containing lysis buffer (0.5 % NP40, 100mM NaCl, 10% glycerol, 1mM EDTA and 50mM Hepes pH7.5, 1mM NaVO4, protease inhibitors), pre-cleared and immunoprecipitated with anti-STING antibody. For co-immunoprecipitation with myc-STING, coding regions of human and mouse STING were amplified by PCR from full-length I.M.A.G.E. cDNA clones (IRATp970D0274D and IRAVp968F0688D, obtained from Imagenes) and cloned into the vector pCMV-myc (Clontech). STING constructs and empty vector were transfected into HEK293T cells using calcium phosphate. 24h after transfection, cells were lysed in NP40-containing lysis buffer, and immunoprecipitated using immobilized anti-myc antibody. Immunoprecipitates were washed in lysis buffer, and then incubated with pre-cleared lysates from PMA- and IFNα-treated THP1 cells stimulated with 1μg/ml 70mer. Immunoprecipitated proteins were washed in lysis buffer, eluted, resolved by SDS-PAGE and detected by Western blotting.
RNA interference
siRNAs were chemically synthesised by Invitrogen (Stealth RNAi siRNAs) or by QIAGEN. Sequences were as follows: IFI16 siRNA, 5′-GGUGCUGAACGCAACAGAAUCAUUU-3′ (Invitrogen); p204 siRNA 1, 5′-UUAGUUUACUGCCUGGUUCACACCU-3′ (Invitrogen); p204 siRNA 2, 5′-CGGAGAGGAAUAAAUUCAUTT-3′ (QIAGEN). p204 siRNA 2 was used in Fig. 5h, 5i and 7, together with the negative control siRNA, 5′-UUCUCCGAACGUGUCA CGUTT-3′ (QIAGEN). In all other experiments, the Stealth RNAi negative control siRNA cat. no 12935-300 (Invitrogen) was used. 1× 105 cells/well were seeded in 12-well plates and transfected with 12.5 pmol/ml (or 6.25 pmol/ml for THP-1 cells) siRNA using 1μl/ml lipofectamine. RAW264.7 and THP1 cells were treated twice with siRNA on consecutive days, and grown for a further 48 h before stimulation. MEFs were treated once with siRNAs, and stimulated 48 h later.
HSV replication assay
RAW 264.7 cells were transfected with the HSV 60mer (2μg/ml) 24h prior to infected with HSV-1 (MOI 1). Supernatants were harvested 6, 24, and 48h post infection, and virus was quantitated using standard plaque assay on Vero cells26.
Confocal microscopy
Cells grown on glass coverslips were fixed in 4% paraformaldehyde, and permeabilized in 0.5% Triton X-100. Coverslips were pre-incubated in 5% BSA/0.05% Tween20/PBS or in 1%FCS/PBS, and stained for 1-3h with primary antibodies (1:100) at 25°C, and for 1h with Alexa488- and Alexa647-labelled secondary antibodies (1:500). Coverslips were mounted in MOWIOL 4-88 (Calbiochem) containing 1μg/ml DAPI. Images were taken on an Olympus FV1000 scanning confocal microscope.
Statistics
Statistical significance was determined by Student’s t-test.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank C.Liu for the anti-p204 antibody, S. Akira, T. Taniguchi, M. Kelliher , T. Mak and G.Barber for mice or femurs, D.Golenbock for immortalized BMDMs lacking MyD88 or TRIF and I. Julkunen for Sendai virus. This work was funded by Science Foundation Ireland grant 07/IN1/B934 (L.U., M.B., A.G.B.), the Irish Health Research Board (S.K.), the Danish Medical Research Council grant 09-072636 (S.R.P.), the Lundbeck Foundation grants R17-A1528 (S.R.P.) and R34-A3855 (K.A.H.), and by NIH grants AI083713 and AI067497 (to K.A.F). S.B.J. was supported by a PhD scholarship from the Faculty of Health Sciences, Aarhus. T.S.X. is supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH, USA.
REFERENCES
- 1.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 2.Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-[kappa]B by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 4.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 5.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hornung V, Latz E. Intracellular DNA recognition. Nat Rev Immunol. 2010;10:123–130. doi: 10.1038/nri2690. [DOI] [PubMed] [Google Scholar]
- 7.Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Ishii KJ, et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7:40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong B, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Sun W, et al. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc Natl Acad Sci U S A. 2009;106:8653–8658. doi: 10.1073/pnas.0900850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNAmediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saitoh T, et al. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0911267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takaoka A, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007 doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 15.Lippmann J, et al. IFNbeta responses induced by intracellular bacteria or cytosolic DNA in different human cells do not require ZBP1 (DLM-1/DAI) Cell Microbiol. 2008;10:2579–2588. doi: 10.1111/j.1462-5822.2008.01232.x. [DOI] [PubMed] [Google Scholar]
- 16.Ishii KJ, et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- 17.Chiu YH, Macmillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138:576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ablasser A, et al. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat Immunol. 2009;10:1065–1072. doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts TL, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 22.Burckstummer T, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 23.Massung RF, Knight JC, Esposito JJ. Topography of variola smallpox virus inverted terminal repeats. Virology. 1995;211:350–355. doi: 10.1006/viro.1995.1416. [DOI] [PubMed] [Google Scholar]
- 24.Yan H, et al. RPA nucleic acid-binding properties of IFI16-HIN200. Biochim Biophys Acta. 2008;1784:1087–1097. doi: 10.1016/j.bbapap.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Schroder M, Baran M, Bowie AG. Viral targeting of DEAD box protein 3 reveals its role in TBK1/IKKepsilon-mediated IRF activation. EMBO J. 2008;27:2147–2157. doi: 10.1038/emboj.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasmussen SB, et al. Type I interferon production during herpes simplex virus infection is controlled by cell-type-specific viral recognition through Toll-like receptor 9, the mitochondrial antiviral signaling protein pathway, and novel recognition systems. J Virol. 2007;81:13315–13324. doi: 10.1128/JVI.01167-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luan Y, Lengyel P, Liu CJ. p204, a p200 family protein, as a multifunctional regulator of cell proliferation and differentiation. Cytokine Growth Factor Rev. 2008;19:357–369. doi: 10.1016/j.cytogfr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu T, Rojas A, Ye Y, Godzik A. Homology modeling provides insights into the binding mode of the PAAD/DAPIN/pyrin domain, a fourth member of the CARD/DD/DED domain family. Protein Sci. 2003;12:1872–1881. doi: 10.1110/ps.0359603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poeck H, et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1beta production. Nat Immunol. 2009 doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- 30.Rothenfusser S, et al. The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J Immunol. 2005;175:5260–5268. doi: 10.4049/jimmunol.175.8.5260. [DOI] [PubMed] [Google Scholar]
- 31.Johnston JB, et al. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity. 2005;23:587–598. doi: 10.1016/j.immuni.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Rahman MM, Mohamed MR, Kim M, Smallwood S, McFadden G. Co-Regulation of NF-kappaB and Inflammasome-Mediated Inflammatory Responses by Myxoma Virus Pyrin Domain-Containing Protein M013. PLoS Pathog. 2009;5:e1000635. doi: 10.1371/journal.ppat.1000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cristea IM, et al. Human cytomegalovirus pUL83 stimulates activity of the viral immediate-early promoter through its interaction with the cellular IFI16 protein. J Virol. 2010;84:7803–7814. doi: 10.1128/JVI.00139-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monroe KM, McWhirter SM, Vance RE. Induction of type I interferons by bacteria. Cell Microbiol. 2010;12:881–890. doi: 10.1111/j.1462-5822.2010.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prantner D, Darville T, Nagarajan UM. STING Is Critical for Induction of IFN-{beta} during Chlamydia muridarum Infection. J Immunol. doi: 10.4049/jimmunol.0903704. jimmunol.0903704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mondini M, et al. Role of the interferon-inducible gene IFI16 in the etiopathogenesis of systemic autoimmune disorders. Ann N Y Acad Sci. 2007;1110:47–56. doi: 10.1196/annals.1423.006. [DOI] [PubMed] [Google Scholar]
- 37.Choubey D, Panchanathan R. Interferon-inducible Ifi200-family genes in systemic lupus erythematosus. Immunology Letters. 2008;119:32–41. doi: 10.1016/j.imlet.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimkong I, Avihingsanon Y, Hirankarn N. Expression profile of HIN200 in leukocytes and renal biopsy of SLE patients by real-time RTPCR. Lupus. 2009;18:1066–1072. doi: 10.1177/0961203309106699. [DOI] [PubMed] [Google Scholar]
- 39.Roberson SM, Walker WS. Immortalization of cloned mouse splenic macrophages with a retrovirus containing the v-raf/mil and v-myc oncogenes. Cell Immunol. 1988;116:341–351. doi: 10.1016/0008-8749(88)90236-5. [DOI] [PubMed] [Google Scholar]
- 40.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu CJ, Wang H, Lengyel P. The interferon-inducible nucleolar p204 protein binds the ribosomal RNA-specific UBF1 transcription factor and inhibits ribosomal RNA transcription. EMBO J. 1999;18:2845–2854. doi: 10.1093/emboj/18.10.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts ZJ, et al. The chemotherapeutic agent DMXAA potently and specifically activates the TBK1-IRF-3 signaling axis. J Exp Med. 2007;204:1559–1569. doi: 10.1084/jem.20061845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.