Abstract
We recently demonstrated that liposome-supported plasmon resonant gold nanoshells are degradable into components of a size compatible with renal clearance, potentially enabling their use as multifunctional agents in applications in nanomedicine, including imaging, diagnostics, therapy, and drug delivery (Troutman et al., Adv. Mater. 2008, 20, 2604–2608). When illuminated with laser light at the wavelength matching their plasmon resonance band, gold-coated liposomes rapidly release their encapsulated substances, which can include therapeutic and diagnostic agents. The present research demonstrates that release of encapsulated agents from gold-coated liposomes can be spectrally controlled by varying the location of the plasmon resonance band; this spectral tuning is accomplished by varying the concentration of gold deposited on the surface of liposomes. Furthermore, the amount of laser energy required for release is qualitatively explained using the concept of thermal confinement (Jacques, Appl. Opt. 1993, 32(3), 2447–2454). Overlapping thermal confinement zones can be avoided by minimizing the laser pulse width, resulting in lower energy requirements for liposomal content release and less global heating of the sample. Control of heating is especially important in drug delivery applications, where it enables spatial and spectral control of delivery and prevents thermal damage to tissue.
Keywords: optical imaging, contrast agents, drug delivery, plasmon resonance, controlled release, self assembly, liposomes, nanomedicine
1. INTRODUCTION
The controlled release of therapeutic and diagnostic agents may enable many medical interventions contemplated for the treatment of diseases. Liposomes of certain compositions have been shown to be capable of thermally controlled release of encapsulated agents1; this rapid thermal release occurs at characteristic temperatures, corresponding to phase transitions. However, in clinical applications, the local temperature modulation necessary for the spatial and temporal control of release is often difficult to achieve. In comparison, light-controlled content release may allow for precise, on-demand content delivery within individual cells in vitro or, when used in conjunction with catheter or endoscopic light delivery, may enable precise medicinal intervention in vivo.
We recently introduced degradable plasmon resonant nanoshells created through the deposition of gold onto the surface of liposomes2. Rather than a continuous metallic shell, as previously demonstrated by others3,4,5, this composite nanostructure is comprised of a shell-shaped array of discrete gold clusters supported by a spherical, metastable liposome core. This material uniquely combines the spectrally tunable optical properties of plasmon resonant coating with the biodegradability and ability to encapsulate and release content afforded by the liposome template.
We demonstrated that these plasmon resonant nanoshells are capable of light-induced content release6,7. We hypothesize that this light-controlled release from thermosensitive liposomes is permitted by a photothermal conversion process, in which laser light absorbed by the plasmon resonant coating results in an increase in temperature. When the local temperature reaches a phase transition of the liposomes, encapsulated content is released.
An important aspect of the laser-induced release mechanism is the extent to which the thermal energy propagates in the tissue. Excessive tissue heating can result in unwanted protein denaturation, cell death, and an inability to achieve spectrally selective content release from liposomes. Analysis of thermal diffusion in the tissue can be used to approximate the diameter of the spherical volume affected by these thermal changes:
| (1) |
where d is the thermal confinement diameter, tp is the laser pulse width, and κ is the thermal diffusivity of the medium8. Here, we demonstrate that, by using shorter laser pulse widths, the amount of energy required for nanocapsule content release can indeed be reduced. Therefore, short laser pulses can confine thermal energy to a volume within or near that of the nanoshells and maximize the efficiency of release.
2. MATERIALS AND METHODS
2.1 Liposome preparation
Liposomes were prepared from synthetic lipids using a lipid composition similar to one previously demonstrated to exhibit temperature-sensitive controlled release1; the logic supporting this composition is that the instability that occurs during the gel to liquid-crystalline phase transition of lipids sufficiently perturbs the liposome membrane to induce the leakage of contents. The membrane was composed of dipalmitoylphosphatidylcholine (DPPC), monopalmitoylphosphatidylcholine (MPPC), and dipalmitoylphosphatidylethanolamine-[N-methoxy(polyethylene glycol)-2000] (DPPE-PEG2000, all lipids from Avanti Polar Lipids; Alabaster, AL) in a 90:10:4 molar ratio. MPPC was included to facilitate membrane leakage at the phase transition temperature and DPPE-PEG2000 was included to improve colloidal stability.
The proper proportions of dry lipids were dispersed in chloroform and dried by convection with N2; this process was followed by overnight evaporation under vacuum. Dry lipids were then dispersed in phosphate buffered saline (PBS) containing 5 mM fluorescein to achieve a 60 mM lipid concentration. Liposomes were prepared by the standard freeze-thaw cycle method, which was followed by extrusion through 100 nm polycarbonate membranes, as detailed in previous publications9. Following extrusion, the liposome preparation was dialyzed at 4 °C against PBS to remove excess fluorescein. As determined by quasi-elastic light scattering, the average liposome diameter was 136 nm, intensity weighted (Malvern Zetasizer). All liposome preparations were stored at 4 °C to minimize content leakage.
2.2 Reduction of gold
The process for the reduction of gold to the surface of liposomes was similar to the technique previously reported2. To summarize, aqueous solutions of gold chloride and of ascorbic acid were prepared at concentrations of 100 mM and 500 mM, respectively. These solutions were added to a 1 mL quantity of the liposome preparation diluted to 20 mM lipids with PBS. For resonance wavelengths matched to a 690 nm laser diode, 24 μL of the gold chloride solution was added and gently swirled until uniformly distributed; this was followed by the addition of 36 μL of the ascorbic acid solution and gentle swirling until color, a feature characteristic of the presence of plasmon resonance, developed. Following reduction, liposomes were subjected to a single stage dialysis against PBS at 4 °C.
2.3 Extinction spectra
Extinction spectra were taken with a Cary 5 spectrophotometer in double beam mode against liposome samples prepared at the same time and in the same manner as those used for the release tests. Samples were diluted to 3 mM lipids to match spectrophotometric range of the instrument.
2.4 Light-induced content release
Content release from plasmon resonant liposomes was then tested by applying a light beam generated by a 690 nm laser diode (RPMC Lasers; O’Fallon, MO) to a liposome sample. The laser diode was driven at 1 W power by a constant current pulsed source (ILX Lightwave; Bozeman, MT), operating at 10% duty cycle and with frequencies of 1, 20, 100, and 500 kHz; therefore, the tested pulse widths were 100, 5, 1, and 0.2 μs. A 30 μL droplet of liposome solution having a 10 mM lipid concentration was retained in a semi-micro cuvette (1.5–3 mL volume; BrandTech; Essex, CT). Light from a laser diode was focused onto the volume of the liposome droplet using a 4.51 mm focal length aspheric lens (Thorlabs; Newton, NJ). Samples were exposed to pulses of laser light for varying durations of time in increments of 1 min, for up to 8 minutes.
Fluorescence measurements were recorded immediately after illumination. The 30 μL droplet of the irradiated solution was diluted with 1970 μL of PBS to obtain a 0.3 mM lipid concentration for measurement. Fluorescence emission spectra were collected over the range of 200 to 800 nm using a diode array spectrometer (Ocean Optics; Dunedin, FL). The excitation source was a 470 nm LED and emission measurements were taken with SpectraSuite Software using a 500 ms integration time. The maximum value of the emission spectrum for each illumination event was recorded and the percent release was determined using equation 2:
| (2) |
where I is the maximum intensity of fluorescence emission (generally near 515 nm) for an individually measured sample, I0 is the maximum fluorescence for an untreated or minimum temperature sample, and IT is the fluorescence maximum in the event of complete release, which was determined by replacing 10% of the PBS diluting solution with an aqueous 10% Triton X-100 solution.
3. RESULTS AND DISCUSSION
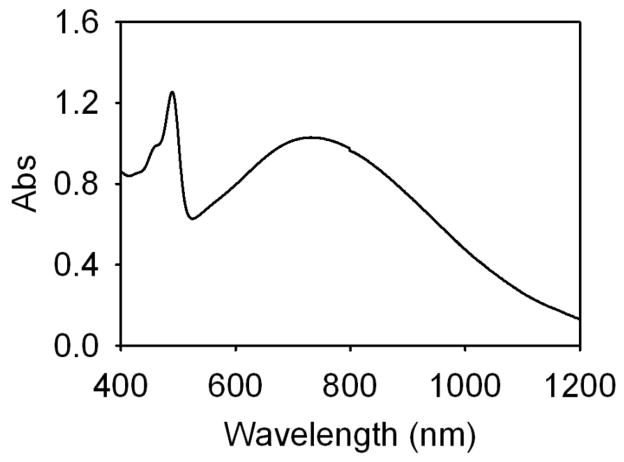
Figure 1 confirms the presence of plasmon resonance in the gold-coated liposome sample. The peak of the extinction spectra around 690 nm is the result of the reduced gold on the liposome surface. As previously described2, the spectral position of the plasmon resonance maxima is dependent on the quantity of gold reduced. Like other shell-type structures, these nanoparticles have broad resonance peaks, especially in the infrared. The presence of encapsulated fluorescein is evident in the extinction spectra by the narrow peak at 485 nm.
Fig. 1.
Extinction spectra of gold-coated liposomes. Liposomes were loaded with fluorescein.
As shown in Figure 2, about twice as much exposure time was needed to achieve 80% of total release using a laser pulse width of 100 μs as compared to the 5, 1, and 0.2 μs pulse widths. As the laser diode was pulsed using a constant current source at a 10% duty cycle, the illumination time is directly proportional to the amount of energy delivered; therefore, the 100 μs pulse width required about twice as much energy as the other tested pulse widths to achieve 80% of total content release.
Fig. 2.
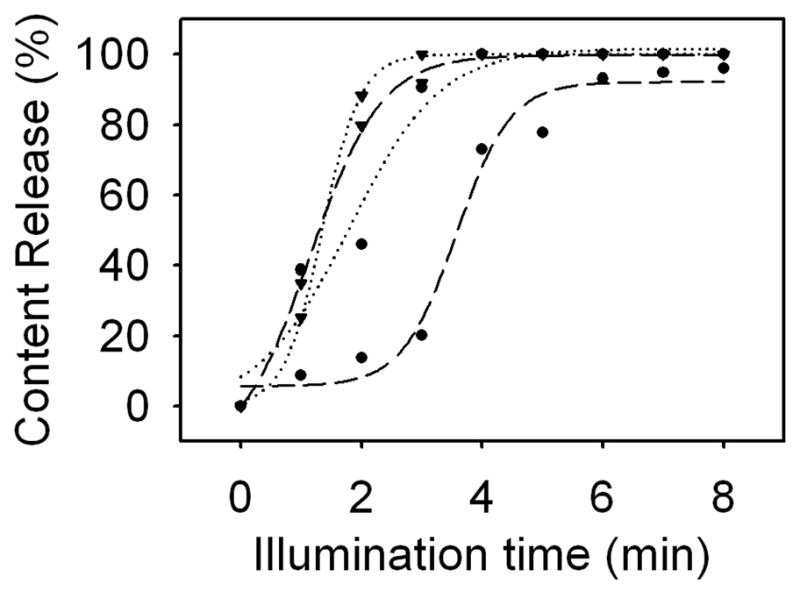
Light-mediated release from gold-coated liposomes resonant at 690 nm using 100 μs (circle, long dash), 5 μs (circle, dotted), 1 μs (inverted triangle, long dash), and 0.2 μs (inverted triangle, dotted) laser diode pulse widths. Shorter pulse widths generally elicit more rapid content release from gold-coated liposomes.
According to equation 1 and simple geometric calculations, at the concentration of gold-coated liposomes tested here, pulse widths above approximately 16 μs will result in global heating, i.e., thermal energy diffusing throughout the entire volume of the sample, and the liposomes will be in thermal equilibrium with the surrounding media. For liposomes having an average diameter of 136 nm, thermal changes should be entirely confined within the volume of the liposome at pulse width below 0.14 μs.
It appears that the 100 μs laser pulse width resulted in global heating, and subsequently less efficient content release. At pulse widths below those causing global heating, shorter pulses generally led to faster content release, as less energy is diffused into the suspension medium and subsequently producing unnecessary heating. This overall trend is apparent in Figure 2, but the energies required for full release using 5, 1, and 0.2 μs pulse widths are so similar that a qualitative analysis will require further experimentation. For in vitro and in vivo applications, confining thermal changes to the volume of the liposome is desirable for reducing the overall energy required for content release and avoiding unwanted heating and protein denaturation in surrounding cells and tissue that can lead to cell death. Furthermore, thermal confinement may allow for spectrally selective release, in which different wavelengths of light are used to release content from liposomes exhibiting corresponding plasmon resonance bands.
4. CONCLUSION
Using shorter laser pulse widths, we were able to elicit more efficient content release from gold-coated liposomes; this finding is in agreement with the model of thermal confinement. Confining the thermal changes necessary for content release from liposomes to the volume of the liposome decreases the effects of light-mediated release on tissue and may allow for spectrally selective release, thus presenting the possibility of using gold-coated liposomes in applications in nanomedicine, including diagnostic and therapeutic interventions.
Acknowledgments
This work was supported by grant CA120350 from the National Institutes of Health and grant CBET 0853921 from the National Science Foundation.
References
- 1.Needham D, Anyarambhatla G, Kong G, Dewhirst MW. A new temperature-sensitive liposome for use with mild hyperthermia: characterization and testing in a human tumor xenograft model. Cancer Research. 2000;60:1197–1201. [PubMed] [Google Scholar]
- 2.Troutman TS, Barton JK, Romanowski M. Biodegradable plasmon resonant nanoshells. Adv Mater. 2008;20:2604–2608. doi: 10.1002/adma.200703026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oldenburg SJ, Averitt RD, Westcott SL, Halas NJ. Nanoengineering of optical resonances. Chemical Physics Letters. 1998;288:243–247. [Google Scholar]
- 4.Wu G, Mikhailovsky A, Khant HA, Fu C, Chiu W, Zasadzinski JA. Remotely triggered liposome release by near-infrared light absorption via hollow gold nanoshells. J Am Chem Soc. 2008;130:8175–8177. doi: 10.1021/ja802656d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin Y, Gao X. Spectrally tunable leakage-free gold nanocontainers. J Am Chem Soc. 2009;131:17774–17776. doi: 10.1021/ja9076765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Troutman TS, Leung SJ, Romanowski M. Light-induced release from plasmon-resonant liposomes. Adv Mater. 2009;21:2334–2338. doi: 10.1002/adma.200900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung SJ, Troutman TS, Romanowski M. Plasmon resonant gold-coated liposomes for spectrally coded content release. Proc. of SPIE; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacques SL. Role of tissue optics and pulse duration on tissue effects during high-power laser irradiation. Appl Optics. 1993;32:2447–2454. doi: 10.1364/AO.32.002447. [DOI] [PubMed] [Google Scholar]
- 9.Romanowski M, Zhu XY, Kim K, Hruby VJ, O’Brien DF. Interaction of enkephalin peptides with anionic model membranes. Biochim Biophys Acta. 2002;1558:43–45. doi: 10.1016/s0005-2736(01)00421-7. [DOI] [PubMed] [Google Scholar]