Abstract
Protein thiol modifications occur under both physiological and pathological conditions and can regulate protein function, redox signaling, and cell viability. The thiolation of proteins by glutathione appears to be a particularly important mode of post-translational modification that is increased under conditions of oxidative or nitrosative stress. Modification of proteins by glutathiolation has been shown to affect the structure and function of several susceptible proteins and protect them from subsequent oxidative injury. In many cases, the glutathiolated proteins are low in abundance, and de-thiolation occurs readily. Therefore, sensitive, reliable, and reproducible methods are required for measuring both the total levels of protein glutathiolation and for identifying glutathiolated proteins under given conditions. These methods necessitate the preservation or the controlled removal of the glutathione adducts during sample preparation for the accurate measurement of total S-glutathiolation and for identification of protein-glutathione adducts. In this article, we briefly review and provide protocols for chemical, mass spectrometric, immunological, and radioactive tagging techniques for measuring protein S-glutathiolation in cells and tissues.
1. Introduction
Glutathione (GSH) is a ubiquitous, cysteine-containing tripeptide (γ-Glu-Cys-Gly) that is abundant in most eukaryotic cells. The intracellular concentration of GSH varies between 0.1 to 10 mM. A complete lack of GSH is incompatible with long-term survival. Most of the functions of GSH depend upon its cysteine residue, which participates in several types of reactions including displacement, nucleophilic addition, and thiol-disulfide exchange. GSH provides reducing equivalents to glutathione peroxidases, and the oxidized glutathione (GSSG) generated by these reactions is reduced by GSSG reductases. By participating in these reactions, GSH helps to maintain cellular sulfhydryl residues in a reduced state. GSH also reacts with free radicals generating glutathionyl radicals that can combine with each other to form GSSG. In addition, GSH reduces dehydroascorbic acid (generated by radical-induced oxidation) to ascorbic acid. Because of these properties, GSH is viewed as the first line of defense against oxidants and the ultimate radical sink. In addition to participating in redox reactions, GSH also forms conjugates with metals and endogenous or xenobiotic electrophiles by participating in reactions catalyzed by glutathione-S-transferases (GSTs). Recent studies indicate that GSH is also an important participant and a regulator of the biological activity of nitric oxide (NO). Although NO does not directly react with thiols, upon auto-oxidation by molecular oxygen, it can form nitrosothiols. These compounds have been detected in vivo and are thought to be important mediators of NO action and NO-induced protein glutathiolation (Klatt et al., 2000).
The propensity of GSH to undergo thiol-disulfide exchange reactions favors ready reaction of the tripeptide with cysteinyl side chains of proteins. Proteins bound to GSH, previously called protein-GSH mixed-disulfides and now referred to as either glutathiolated or glutathionylated proteins, have been detected in several cells and tissues (Shackelford et al., 2005; Biswas et al., 2006; Hill et al., 2007). It was initially thought that glutathiolated proteins were mostly generated by the oxidation of protein cysteine residues by GSSG, where GSSG reacts with protein thiols via a thiol-disulfide exchange. Specific transmembrane transporters that extrude GSSG from the cell (such as the multidrug resistance protein and RLIP)may therefore be important in regulating the level of glutathiolated proteins. Recent evidence, however, indicates that the adduction of GSH to protein cysteines is primarily facilitated by transnitrosation reactions or sulfenic acids (West et al., 2006; Hill et al., 2007). As shown in Scheme 1, both NO and reactive oxygen species can promote the formation of S-glutathiolated proteins. The induction of protein S-glutathiolation by GSNO was first demonstrated by us for aldose reductase (Chandra et al., 1997). Incubation of the protein led to the stoichiometric adduction of a single GSH residue at the active site of the enzyme and resulted in complete inhibition of its catalytic activity. The enzyme was also found to be glutathiolated in vascular smooth muscle cells exposed to NO donors (Ramana et al., 2003). Later studies have shown that peroxynitrite arising from NO donors or pathological stimuli triggers S-glutathiolation of proteins such as the sarco/endoplasmic reticulum calcium ATPase (SERCA) (Adachi et al., 2004) and p21ras (Clavreul et al., 2006). Significantly, these proteins are glutathiolated in vivo and the modification of their cysteine residues alters protein function, suggesting that post-translational modification by glutathiolation may be a significant mechanism of redox regulation employed by NO. In this regard, it has been shown that an increase in endogenous NO synthesis either by the stimulation of endothelial NO synthase in aorta (West et al., 2006), overexpression of inducible NO synthase in the heart (West et al., 2006; Reinartz et al., 2008), or L-arginine treatment (West et al., 2008) increases protein S-glutathiolation, indicating that NO at physiological levels regulates protein function by inducing protein adduction to GSH. In addition, oxidation products of GSNO (e.g., glutathione sulfonic acid, glutathione disulfide S-oxide, and glutathione disulfide-S-dioxide) as well as protein sulfenic acids generated by reaction of protein thiols orglutathione with hydrogen peroxide have been suggested to be significant intracellular glutathiolating agents (Li et al., 2001; Bindoli et al., 2008). The view that glutathiolation is a regulated mode of signal transduction is supported further by the recent discovery of enzymatic pathways for protein de-glutathiolation. Several studies show that the de-glutathiolation of proteins is catalyzed by glutaredoxins and GSTs. The role of glutathiolation in signal transduction and regulation of protein function have been extensively reviewed elsewhere (Shackelford et al., 2005; Biswas et al., 2006; Hill et al., 2007).
Scheme 1.
Mechanisms of protein S-glutathiolation by nitric oxide and reactive oxygen species. In most cells, GSSG and nitrosoglutathione (GSNO) are likely to be the most significant glutathiolating agents. The cellular abundance of GSSG is regulated by processes that generate GSSG, such as the reduction of peroxides by glutathione peroxidases (GP). GSNO formed after direct reaction of NO with thiyl radicals or after reaction of glutathione with advanced nitrogen oxide species (e.g., N2O3) enters into transnitrosation reactions that also result in S-glutathiolated proteins. Peroxynitrite (ONOO−), formed from the reaction of NO with superoxide, is able to mediate the formation of both S-nitrosated (PS-NO) and sulfenic acid-modified (PS-OH) proteins and glutathione; these “activated” thiols react readily to form protein-glutathione adducts. Hydrogen peroxide also promotes PS-OH/GS-OH formation that leads to protein glutathiolation. Protein glutathiolation can facilitate redox cell signaling, regulate enzyme function, protect protein thiols from advanced protein oxidation, and, in some cases, promote cell death.
Although the physiological significance of protein glutathiolation has not been fully assessed, it is currently believed that the addition of GSH to protein sulfhydryls prevents excessive oxidation and thereby preserves protein integrity and function under conditions of oxidative stress. This is consistent with the increase in protein glutathiolation due to endogenously generated hydrogen peroxide (Adachi et al., 2004) and peroxynitrite (Clavreul et al., 2006), as well as exposure to oxidized LDL (Clavreul et al., 2006), cigarette smoke (Muscat et al., 2004), and hyperoxic conditions (Knickelbein et al., 1996). Protein glutathiolation due to NO generation may reflect the fact that S-nitrosated proteins are readily glutathiolated (West et al., 2006) and that glutathiolation may be an essential step in protein de-nitrosation (Baba et al., 2009). Also, recent evidence suggests that the functions of several enzymes and structural proteins are regulated by S-glutathiolation (Hill et al., 2007). It is important, therefore, that specific, sensitive, and reliable methods are used to study S-thiolation reactions and the proteins modified by GSH. Most studies have exploited the use of techniques or combinations thereof that: 1) quantify or estimate global changes in glutathiolated proteins, 2) identify proteins modified by GSH and their specific sites of adduction, and 3) determine how GSH modifications regulate protein function and physiological and pathological responses. The following is a brief description of several methods used to measure global changes in protein glutathiolation and identify glutathiolated proteins and sites of modification. We also discuss some of the new approaches for identifying proteins that are glutathiolated in cells or in animals in situ.
2. Chemical methods for the measurement of glutathiolated proteins
Early methods for measuring glutathiolated proteins were developed to quantify the total amount of GSH bound to proteins. The overall aim of these approaches was to demonstrate that GSH (or cysteine) forms a covalent attachment with proteins and that the levels of glutathiolated proteins (or mixed disulfides) change with specific diseases or pathological conditions associated with oxidative stress. These approaches are still useful to measure the extent of protein glutathiolation, although they are of limited value in identifying specific proteins or residues modified by GSH.
To quantify the amount of GSH bound to proteins, Harding reduced proteins by sodium borohydride and then quantified the GSH released by colorimetric measurements using DTNB (Harding 1970). The method is simple and straightforward, but requires large amounts of protein and is not-specific for GSH, because protein-bound cysteine is also released and reported in colorimetric measurements. To identify GSH specifically, the thiol liberated by sodium borohydrate was measured either by HPLC or by the glutathione reductase recycling assay. Nevertheless, the long procedures of reduction and subsequent HPLC analysis increased the likelihood of GSH autoxidation and the possibility of obtaining erroneous results leading to an underestimation of the extent of intracellular protein glutathiolation. Morever, reductants (either sodium borohydride or DTT) interfere with the measurement of GSH in both the recycling and HPLC methods. Therefore, to expedite sample preparation and to avoid reduction, Lou et al. (1986) developed a new method for measuring protein glutathiolation in which protein-bound GSH was cleaved and oxidized by performic acid. This procedure results in the formation of free non-protein bound glutathione- sulfonic acid (GSO3H) which can be quantified by anion-exchange chromatography. The method minimizes the potential for GSH autoxidation, is reproducible and reliable, and results in the quantitative release of GSH from proteins. However, it is not very sensitive and requires large amounts of tissue. To improve sensitivity, Kumari et al. (1994) modified this method by introducing an additional step in which the glutathione- sulfonic acid is derivatized with phenylisothiocyanate. The phenylthiocarbamyl derivative can then be separated and quantified by reverse-phase HPLC. This modification resulted in reduction of the volume required for lyophilization from 100–300 ml to 2 ml. This method, described in detail below, is at least 20× more sensitive than the non-derivatized measurement of sulfonic acid.
2.1. Release of protein-bound glutathione by oxidation
In this method, glutathiolated proteins are subjected to performic acid oxidation to cleave the S-S bond with simultaneous oxidation of GSH to glutathione -sulfonic acid. Excessive performic acid is removed by lyophilization, and the solution is deproteinized by ultrafiltration. The recovery of GSSG and glutathione-sulfonic acid is >90 %. Reagent glutathione-sulfonic acid is used as a standard.
2.1.1. Measurement of glutathione-sulfonic acid
Synthesis of reagent glutathione -sulfonic acid
Dissolve the appropriate amount of GSSGin 125 μl of performic acid in a prechilled test tube. This is prepared by adding 0.5 ml of 88% formic acid to 9.5 ml of 99% formic acid.
Vortex and allow the oxidation to continue for 2.5 h in an ice bath.
Add 2 ml de-ionized water and lyophilize the sample in a Speed Vac.
Add 100 μl of 2:2:1 methanol:water: triethylamine (TEA) and dry on a Speed Vac.
Add 80 μl of 7:1:1:1 methanol:water:TEA:phenylisothiocynate (PITC). The solution should be made fresh immediately before use and stored at −20 °C under nitrogen.
Dry and resuspend the sample in 100–200 ml water. Filter through a 0.2 μm filter.
Measurement of glutathione-sulfonic acid liberated from glutathiolated proteins
Homogenize the tissue in an appropriate volume of potassium phosphate (10 mM K-phosphate, pH 7.0, containing protease inhibitors).
Add trichloroacetic acid (TCA) to a final concentration of 10% to precipitate the proteins, and centrifuge at 13,000×g for 10 min.
Disrupt the pellet and wash 3× with 10% TCA, centrifuging after each wash.
Wash the pellet 1× with 1:1 methanol:ether, and dry the pellet at 40°C under nitrogen.
Resuspend the pellet in 125μl performic acid and vortex. Incubate on ice for 2.5 h, and add 0.2 ml deionized water.
Lyophilize the sample in a Speed Vac and dissolve in 1.0 ml deionized water.
Add sulfosalicyclic acid to a final concentration of 15%, and centrifuge at 13,000×g for 10 min.
Remove the supernatant and dry on Speed Vac. Reconstitute in 100 μl of 2:2:1 methanol:water: TEA and dry again on Speed Vac.
Add 80 ml of 7:1:1:1 methanol: water:TEA:PITC.
Vortex and incubate at room temperature for 20 min and dry on Speed Vac.
Resuspend the sample in 100 to 200 ml water and filter through a 0.2μm filter.
For HPLC, 10–50 ml of sample are injected into an ODS column equilibrated with 0.14 M sodium acetate, containing 0.1% TFA and 6% acetonitrile, pH adjusted to 6.4 with acetic acid. Sulfonic acids are eluted with an isocratic gradient at a flow rate of 0.5 ml/min, and the absorbance is measured at 251 nm using an absorbance detector. Using these conditions, a linear increase in peak area is observed from 0.05 to 3 nmols of GSO3H.
3. Detection of glutathiolated proteins by ESI/MS
Proteins adducted with GSH can be readily detected by a characteristic +305 Da shift in mass by electrospray mass spectrometry (ESI/MS). Because of its superior sensitivity and specificity, this technique has become the method of choice for measuring glutathiolation of purified proteins for structural or kinetic analysis. Although only small quantities of protein are required, best results are obtained with highly purified or homogenous protein solutions. Impurities decrease the signal-to-noise ratio and can interfere with accurate mass estimation. Proteins are usually modified in ionic medium and therefore the protein has to be exchanged into non-ionic medium for ESI/MS. This requires a rather high (0.2–1.0 mg) initial concentration of pure protein for accurate mass determination. Nevertheless, ESI/MS is a soft-ionization technique which does not disrupt the protein-SSG bond.
3.1.1. Mass spectrometric analysis
Incubate 0.5 to 1.0 mg protein with 0.1M DTT at 37°C for 1h in 100 mM phosphate buffer, pH7.0. This is essential to reduce all disulfide bonds and sulfenic acids incurred during storage. Long-term storage in β-mercaptoethanol is not advisable. At 4°C, the thiol has a half-life of 24 h and thus needs to be replenished constantly if the protein is to be stored for long periods. Storage with β-mercaptoethanol results in the formation of a mixed disulfide, which could significantly affect protein structure or function. Storage in 1 mM DTT is preferable, but does not ensure that the protein remains in a fully reduced state. Hence, it is advisable to reduce the protein immediately before use.
For glutathiolation, the reduced protein is incubated with 1 mM GSSG or 1 mM GSNO at 25°C for 1 h. Aliquots can be withdrawn at different times to measure changes in activity. With several proteins, we have found that GSNO is a better glutathiolating agent than GSSG. The disadvantage of using GSNO is that it could result in the formation of nitrosated proteins. Glutathiolation can be induced more specifically by GSSG; however, in some cases (for example actin), it may be necessary to “activate” the cysteine residue. For this, incubate 25–50 μM protein with a 20-molar excess of DTNB (20 mM in 1 % NaHCO3) and follow the reaction at 412 nm until 1 equivalent of TNB-is released (ε412 = 14.15 mM−1 cm−1). Excess reagent is removed by Sephadex G-25 filtration, and the eluted, activated protein sample is reacted with a 50-molar excess of GSH under spectrophotometric control to quantify the extent of glutathiolation. This procedure results in stoichometric induction of GSH adducts in protein samples and provide a sensitive measure of the extent of glutathiolation.
For desalting, load the protein on a Sephadex G-25 column equilibrated with N2-saturated 10 mM ammonium acetate.
For ESI/MS analysis, dilute the desalted protein with the flow injection solvent (acetonitrile:H2O:formic acid 50:50:1 v/v/v). The mixture is injected into the spectrometer at a rate of 10 μl/min. For Micromass LCZ spectrometer, the following conditions are routinely used in our laboratory: capillary voltage, 3.1kV; cone voltage, 27V; extractor voltage, 4V; source block temperature, 100°C; desolvation temperature, 200°C. Spectra can be acquired at 200 atomic mass units/s over a 20–2000 atomic mass unitrange. The instrument is calibrated with myoglobin. The spectra from each ion are then summed and deconvoluted with MaxEnt software (MaxEnt Solutions, Suffolk, UK).
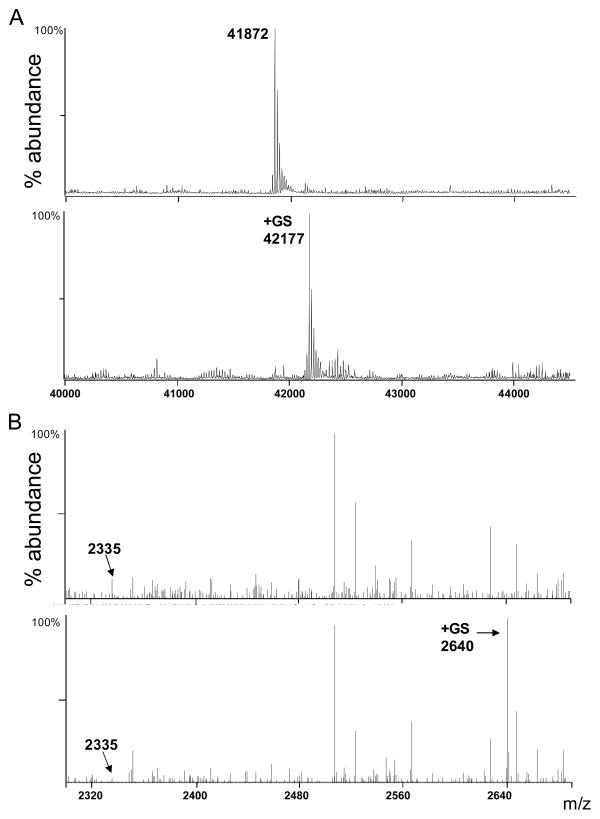
An example demonstrating stochiometric induction of a single GSH molecule in actin, using the procedure described above, is shown in Fig. 1A. An additional advantage of ESI/MS is that the site of modification can be readily identified following protease digestion. As shown in Fig. 1B, reduced and glutathiolated actin samples were digested with Glu-C. The digestion was stopped by addition of formic acid, the peptides were cleaned with C18 ZipTip, and the peptide mixture was analyzed by ESI/MS. The peptides from actin, found to be glutathiolated following incubation of the intact protein with GSNO shown in Table 1.
Figure 1.
Analysis of reduced and glutathiolated actin by mass spectrometry. (A) Deconvoluted ESI+/MS spectra of actin before (upper panel) and after (lower panel) modification by GSNO. The protein was first reduced by DTT and excess DTT was removed by Sephadex gel filtration. The reduced protein was then added to acetonitrile:water:acetic acid for spectrometric analysis (upper panel). The reduced protein was then incubated with 1 mM GSNO (in 20 mM Tris, pH 7.5) for 1 h. The protein was desalted and then analyzed by ESI/MS(lower panel). Note the +305 Da shift in the mass of the major ion, indicating the adduction of a single glutathione molecule to one molecule of actin. (B)ESI/MS spectra of native and GS-actin after hydrolysis with Glu-C. Two peaks (m/z 2640 and 3820) were observed only in the GS-actin spectrum and those corresponding to the addition of 305 Da to peaks from the sample of the native protein (m/z 2335 and 3515) were identified.
Table 1.
Actin peptides found to be susceptible to GSNO-mediated glutathione modification in vitro.
| Treatment | Sequence | MW(expected) | MW(observed) | Delta | Modification |
|---|---|---|---|---|---|
| Control | TTALVCDNGSGLVKAGFAGDDAPR | 2335.13 | 2335.13 | 0 | − |
| GSNO | TTALVCDNGSGLVKAGFAGDDAPR | 2335.13 | 2640.19 | 305.06 | + |
| Control | TTALVCDNGSGLVKAGFAGDDAPRAVFPSIVGRPR | 3514.82 | 3514.82 | 0 | − |
| GSNO | TTALVCDNGSGLVKAGFAGDDAPRAVFPSIVGRPR | 3514.82 | 3819.93 | 305.11 | + |
Actin samples were analyzed by ESI-MS before digestion. GSNO-treated actin was shown to be modified by one molecule of glutathione (Figure 1). The actin samples (without or with modification) were dried by SpeedVac, dissolved in 50 mM NH4HCO3 containing 8M urea, and digested with Glu-C (20 ng/5 μl in 50 mM NH4HCO3) at 30°C for 6 h, and digestion was stopped by adding 30 μl 5% formic acid. Peptides from these samples were cleaned with C18 ZipTip and analyzed by ESI-MS.
4. Identification of S-glutathiolated proteins in cells and tissues
4.1.1. Western Blot analysis
Western blot analysis has emerged as the technique of choice for the measurement of glutathiolated proteins. This approach is possible due to the availability of a specific IgG2a mouse monoclonal antibody that recognizes GSH-protein complexes (from ViroGen, Watertown, MA, USA). The protein A-purified antibody is used to detect glutathiolated proteins on Western blots under non-reducing conditions. Both one-dimensional and two-dimensional gel electrophoresis can be used to detect pure glutathiolated proteins or proteins glutathiolated in cells or tissues in situ. An additional variant of this technique is to purify glutathiolated proteins on a GSH-affinity column and then analyze the eluent by ESI/MS (Celli et al., 2003). However, enrichment on the glutathiolated proteins by a GSH-affinity column may require large amounts of protein and is likely to generate a mixture of different proteins which could need to be separated further either by 1D or 2D gel electrophoresis before mass spectrometric analysis.
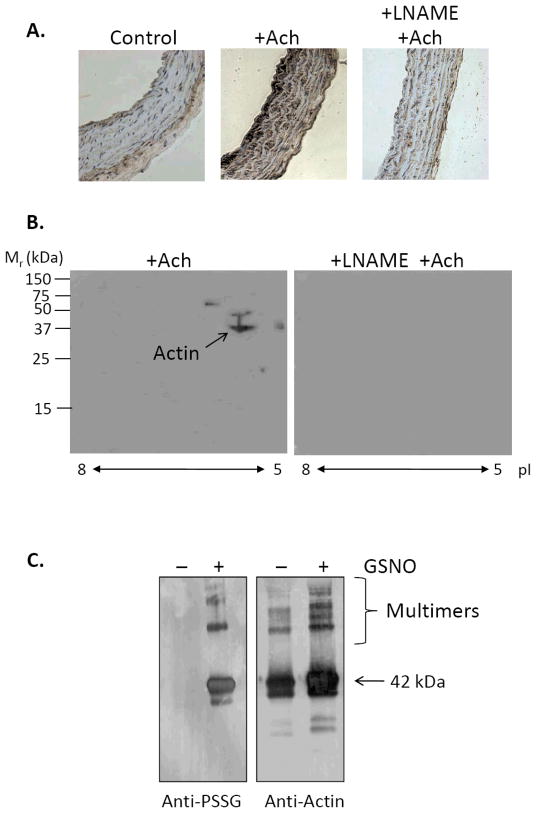
For measuring glutathiolated proteins in isolated proteins, N-ethylmaleimide (NEM; generally 5–25 mM) is added to the protein solution upon completion of, or to prevent further, protein thiolation reactions. For convenience, we add the proteins directly to Laemmli sample buffer containing 25 mM NEM prior to SDS-PAGE. For cells or tissues, NEM (25 mM) is added to both the lysis/homogenization buffer and the Laemmli sample buffer. It is also critical that NEM be present during the blocking step after transfer of the proteins to PVDF or nitrocellulose membranes; this step increases the detection of glutathiolated proteins several-fold by preventing the reduction of GSH adducts by thiol-containing proteins in the milk or albumin. For this, NEM (2.5 mM final concentration) is added to 5% blocking milk (or equivalent albumin blocking mixtures) and allowed to stir at room temperature for 30 min. After blocking for 2 h, the membranes are washed 3× with Tris-buffered saline containing 0.1% Tween-20 (TBS-Tween). The anti-protein-GSH monoclonal antibody is then diluted 1:1000 in TBS-Tween and incubated on a rocker for 2 h at room temperature or overnight at 4°C. Secondary antibodies are also diluted in TBS-Tween. An example of glutathiolated protein detection using this method is shown in Fig. 2C. In this image, immunoreactivity was visualized by chemifluorescence using a Typhoon 9400 Imager (Amersham Biosciences), and the intensity of the resulting bands was analyzed with ImageQuantTL software (Amersham Biosciences).
Figure 2.
Immunological detection of glutathiolated proteins. (A) Photomicrographs of aortic rings stained with the anti-glutathione antibody. Aortic rings were dissected from adult male rats, mounted ex vivo in a perfusion bath, and pre-contracted with 1 μM phenylephrine. The rings were either left untreated (control), stimulated with 1 μM acetylcholine (+Ach), or treated with acetylcholine in the presence of 100 μM L-NAME, a NO synthase inhibitor. Immediately after treatment, the rings were fixed and stained with the anti-glutathione antibody (1:200 dilution). (B) Two-dimensional Western blots of aortic rings precontracted with phenylephrine and relaxed by acetylcholine in the absence or presence of L-NAME. Extracts of aortic rings were subjected to 2D Western blot analysis using the anti-glutathione antibody. Note: The major immunopositive spot corresponds to actin (as indicated in the figure). (C) One-dimensional SDS-PAGE of rabbit skeletal muscle actin before (−) and after (+) treatment with 1 mM GSNO. The treated and untreated proteins were separated by SDS-PAGE, and Western blots were developed with the anti-glutathione (anti-PSSG) and anti-actin antibodies.
For 2D gel analysis, cells or tissues can be lysed or homogenized in low salt buffer (e.g., 5 mM Tris, pH 7.0) containing non-ionic detergents (e.g., 1% Triton X-100 or NP-40) and 25 mM NEM. The proteins can then be loaded on immobilized pH gradient strips and focused using typical protocols. As a general rule, one should load approximately 3× the amount of protein used in 1D gels; for example, if 10 μg protein is generally needed to detect glutathiolated proteins by 1D Western blotting, 30–40 μg protein should be loaded on the IPG strips. If the sample to be analyzed by 2D techniques contains too much salt, a “clean-up” step may be required. For this, the following protocol can be used:
Add trichloroacetic acid (TCA; 10% v/v) to tissue homogenates or cell lysates and allow to incubate on ice for 10 min.
Centrifuge the sample for 5 min at 13,000×g.
Wash the precipitated protein pellet 3x with acetone to remove remaining TCA. This step can also help remove lipids that cause streaking.
Resuspend the protein pellet in 10 mM Tris, pH 6.8, containing 8 M urea, 1 mM EDTA, and 1 mM NEM. In some cases, it may be required to incubate the sample overnight at 4°C for adequate resolubilization.
Measure protein by the Bradford method(Bradford 1976). Make the BSA standard and assay dilutions in the same buffer as in step 4 above.
Add the protein mixture (40 μg) to the appropriate amount of rehydration buffer (e.g., for 7 cm IPG strips from Biorad, a final volume of 125 μl is desirable) and focus the proteins on pH 3–10 or 5–8 IPG strips.
Prior to the 2nd dimension, equilibrate the strips in base equilibration buffer containing 25 mM NEM. If overlay agarose solution is used, include NEM in this solution as well. To obtain peptides for matrix assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF/MS), excise protein spots that were immunoreactive with anti-PSSG antibodies from parallel Sypro Ruby-stained gels and digest with trypsin using a modified version of the method described by Jensen et al (1999; West et al., 2006). The peptide masses obtained by MALDI-TOF/MS analysis can then be used in a database search (e.g., the National Center for Biotechnology Information) to identify the parent proteins. An example of 2D analysis of glutathiolated proteins using these methods is shown in Fig. 2B.
In some cases it may be necessary to immunoprecipitate proteins prior to ESI/MS or MALDI-TOF/MS analysis. For this, we homogenize the tissue in 50 mM Tris, pH 7.4, containing 250mM sucrose, 10 mM iodoacetic acid (IAA), and 1% protease inhibitor cocktail. The homogenates are then centrifuged at 14,000×g for 15min at 4°C, and the supernatant is incubated in the dark for 1 h at room temperature. The homogenate is then passed through a Sephadex G25 (PD-10) column to remove excess IAA, and the glutathiolated proteins are immunoprecipitated with the anti-PSSG Ab. Nonspecific mouse IgG is used as a control for the immunoprecipitations. The proteins are eluted by boiling the agarose beads in Laemmli buffer containing 25 mM NEM. The proteins are then separated by nonreducing SDS-PAGE and visualized by silver staining. The protein bands can then be excised for mass spectrometric analysis. Note that NEM or iodoacetamide (IAM) should be substituted for IAA if performing 2D analysis after the immunoprecipitation step; IAA imparts a negative charge that could cause proteins to focus to “false” isoelectric points.
4.1.2. Immunohistochemical staining
As shown in Fig. 2A, the anti-protein-GSH (PSSG) antibody can also be used to detect glutathiolated proteins in tissue sections using standard histology techniques(West et al., 2006). For this:
Fix cells in 100% acetone, wash with PBS, and then incubate in PBS containing 10% goat serum for 30 min.
Incubate the sections with the anti-PSSG antibody overnight(1:200 dilution), wash with PBS, and then incubate with fluorescent secondary antibodies (e.g., Alexa-488, Molecular Probes) for 2 h at room temperature.
Rinse the slides with PBS and mount with a coverslip using Fluorsave reagent (Calbiochem). For immunohistochemical analysis, rataortic rings can be fixed in formalin and stored in 70% EtOH.
Acquire fluorescent and phase contrast images using a water immersion objective and a high-resolution, high-sensitivity camera (e.g., Spot Insight QE). The images can be quantified using Metamorph software.
4.1.3. Radioactive tagging
An additional approach for identifying glutathiolated protein in cells is to radiolabel the GSH pool in cells before treating with a glutathiolating agent. The main advantage of this technique is that it is sensitive and robust and can be used under a variety of conditions. The main disadvantage is that it does not permit discrimination between proteins modified by GSH and those ligated to cysteines. However, radiolabel tagging followed by immunoprecipitation with the anti-PSSG antibody could be used to purify the glutathiolated protein for further analysis by ESI/MS or MALDI-TOF/MS and aid in identifying the specific cysteines modified. For radiolabel tagging:
Grow cells to 80–90 % confluency in 10 cm3dishes.
Remove the medium and wash twice with Kreb’s Heinslet buffer (KH buffer; 118mM NaCl, 4.7 mM KCl, 25 mM MgCl2, 3 mM CaCl2, 1.25 mM KH2PO4, 0.5 mM EDTA, 25 mM NaHCO3, 10 mM glucose pH 7.4).
Add cycloheximide (2 μg/mL) in KH buffer to prevent direct incorporation of the label in the cellular proteins.
After 60 min of incubation at 37°C in 5% CO2, add 20 μmol/mL of L-[35S]-cysteine to the cells and incubate for an additional 5 h to label the intracellular GSH pool.
To initiate glutathiolation, add either 100 μM H2O2, diamide (0.25 mM) or NO donors such as SNAP (1 mM prepared in 100 % DMSO). Add the same volume of the vehicle to control cells. Diamide can be used as a general positive control and requires only ~10 min of incubation to induce >50% maximal glutathiolation.
Incubate cells at 37 °C for 1 h
Remove KH buffer and wash with 5 ml of fresh KH buffer.
Lyse the cells in ice cold Tris-Tritonbuffer (1% Triton X-100, 0.5 % NP-40, 150 mM NaCl, 10 mM Tris, pH 7.0, 1 mMEDTA, 1 mM EGTA, and protease and phosphatase inhibitor cocktails) containing 25 mM NEM.
Centrifuge at 10,000 ×g for 5 min at 4°C. Save an aliquot of the supernatant to measure protein concentration. A detergent-compatible Lowry method (e.g., the DC Lowry assay, Biorad) works well for samples containing detergent and NEM.
If immunoprecipitation is required, remove excess NEM by gel filtration, and, to 500 μg of total lysate protein, add 2 volumes of immunoprecipitation buffer(2% Triton X-100, 300 mM NaCl, 20 mM Tris pH 7.4, 2 mM EDTA, 2 mM EGTA, 0.4 mM Na2O2V7, 0.4 mM PMSF, 1.0% NP-40, and 20 μL of protease inhibitor cocktail). Add the required antibody and incubate the samples with rocking at room temperature for 2 hor overnight at 4°C. After incubation, add 100 μL of protein-A agarose beads, followed by overnight incubation on a continuous shaker at 4°C to precipitate free and bound IgG. After the incubation, centrifuge the samples at 10,000×g for 5 min and wash 3–5× with immunoprecipitation buffer.
Resuspend the pellet in 50μL of Laemmli sample buffer containing NEM and centrifuge at 10,000×g for 5 min.
Separate proteins in the supernatant by SDS-PAGE.
Dry the gels and measure radioactivity by autoradiography.
To establish specificity, treat similarly prepared homogenates or lysates with 50 mM DTT. Repeat steps 7–13. The difference in radioactivity associated with specific proteins bands from those samples treated without or with 50 mM DTT reflects the extent of specific S-thiolation.
For most cells, simply incubating the cells with [35S]-cysteine is sufficient for adequate labeling of the thiol pool to induce detectable glutathiolation of the protein of interest. However, when the protein is only weakly glutathiolated, it may be necessary to radiolabel the thiol pool after depleting the intracellular thiols (Ward et al., 2000; Li et al., 2001). To deplete endogenous low-molecular weight thiols, replace the medium on 75% confluent cells with DMEM lacking sulfur-containing amino acids and containing 10% dialyzed serum for 16 h at 37°C. Next add the protein synthesis inhibitor cycloheximide and [35S]-cysteine as described above.
4.1.4. Biotin labeling
In this method, cells or tissues are incubated with biotinylated GSSG. The derivatized GSSG reacts with susceptible protein thiols to form protein-SSG-biotin adducts. The adducts can be detected by Western blotting with streptavidin or by other avidin-based techniques. Biotinylated proteins can also be localized in cells by fluorescence microscopy (Brennan et al., 2006). The extended spacer arm of biotin-SSG provides maximal accessibility of biotin for avidin conjugates. The biotin-SSG conjugate is membrane permeable and traverses the cell membrane to react with cytosolic proteins (Brennan et al., 2006).
Biotinylation of oxidized glutathione
Add 111.4 mg sulfosuccinimidyl-6-(biotinamido)hexanoate (Merck Biosciences Ltd., Nottingham, UK) to 61.2 mg GSSG in 1.8 ml water and adjust the pH to 7.2 with NaOH.
Let the mixture sit for 1 h at room temperature
Quench the reaction with 1 M Tris-HCl, pH 7.2 to a final volume of 2 ml.
Separate the mixture by HPLC using an ODS column. Monitor absorbance (190–400 nm) with a diode array detector.
Verify mass of the purified compound (Biotin-GSSG) by ESI/MS or MALDI-TOF/MS. The derivative ion should conform to an m/z value of 1290.85.
Modification of cellular proteins
Incubate cells in serum-free medium with 5 mM biotin-SSG for at least 10 min.
After incubation pellet cells by centrifugation, and discard the supernatant.
Lyse cells in lysis buffer containing 25 mM NEM. Alternatively, use Laemmli sample buffer containing NEM. Do not add reducing agents (DTT or 2-mercaptoethanol) to the medium.
For SDS-PAGE, separate the proteins on non-reducing 10% SDS-polyacrylamide gels. To establish specificity run a separate gel with 10% 2-mercaptoethanol added to the lysis/Laemmli buffer.
Transfer the samples to PVDF membranes using standard Western blotting protocols.
To visualize biotinylated proteins incubate the Western blots with streptavidin-HRP followed by the ECL reagent.
In addition, a cell-permeable, biotinylated GSH analog—biotinylated GSH ethyl ester (BioGEE; Invitrogen, Carlsbad, CA)—can be used to detect glutathiolated proteins under conditions of oxidative stress. Cells preincubated with BioGEE can be treated as desired and then either lysed for Western blot analysis or fixed and permeabilized for detection of protein glutathiolation with streptavidin conjugates by either flow cytometry or fluorescence microscopy. As with biotinylated GSSG, BioGEE can be used to extract and analyze glutathiolated proteins by immunoprecipitation and mass spectrometry. The primary advantages of using BioGEE over biotinylated GSSG include its increased cell permeability and its use for detecting S-glutathiolation due not only to increased GSSG, but also to increased S-oxidation and -nitrosation(see Scheme 1).
5. Conclusions
The choice of a specific method for detecting glutathiolated proteins depends upon the overall objective of the experiments. If changes in the total extent of protein glutathiolation are of interest, it is advisable to measure the free GSH liberated from the oxidation of protein-bound GSH by performic acid. The method is quantitative and reproducible and provides an accurate estimate of global changes in protein glutathiolation. This information is difficult to extract from immunoblotting techniques. If, however, the objective is to identify specific proteins that are modified by glutathiolation, the use of the anti-protein GSH antibody is recommended. It has been noted that the reactivity of the antibody depends upon the nature surrounding the epitope and that some glutathiolated proteins are not recognized or only weakly recognized by the antibody (Brennan et al., 2006). Nevertheless, the antibody technique allows detection of glutathiolated proteins in cells and tissues under conditions of oxidative or nitrosative stress, without the addition of exogenous reagents at arbitrary concentrations. The biotinylation method also allows for the detection of S-glutathiolated proteins by Western blotting. This method is advantageous because it could be readily adapted to purify and identify the modified proteins using avidin-based procedures. However, it requires the exogenous addition of GSSG and thus is not suitable for measuring glutathiolated proteins generated in tissues during intrinsic oxidative stress or due to increases in NO production. Hence, the biotinylation technique cannot be used to screen for the presence of glutathiolated proteins in diseased tissue samples. Regardless of the specific procedure for sample preparation, the identification of glutathiolated proteins is greatly facilitated by the use of mass spectrometry. Glutathiolated proteins can be immunoprecipitated from cell lysates and then separated by SDS-PAGE or directly resolved on 2D-dimensional gels and identified by either LC/MS or MALDI-TOF/MS. Further, MS/MS analysis can be employed to identify which specific sulfhydryl residues are modified by GSH. It is expected that the use of these sensitive methods will lead to a better recognition of the role of protein glutathiolation in cell signaling and function.
Acknowledgments
This work was partially supported by NIH grants GM71036, DK36118, HL-55477, HL-59378, HL-78825, and RR-024489.
References
- Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- Baba SP, Wetzelberger K, Hoetker JD, Bhatnagar A. Posttranslational glutathiolation of aldose reductase (AKR1B1): a possible mechanism of protein recovery from S-nitrosylation. Chem Biol Interact. 2009;178:250–258. doi: 10.1016/j.cbi.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindoli A, Fukuto JM, Forman HJ. Thiol chemistry in peroxidase catalysis and redox signaling. Antioxid Redox Signal. 2008;10:1549–1564. doi: 10.1089/ars.2008.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Chida AS, Rahman I. Redox modifications of protein-thiols: emerging roles in cell signaling. Biochem Pharmacol. 2006;71:551–564. doi: 10.1016/j.bcp.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brennan JP, Miller JI, Fuller W, Wait R, Begum S, Dunn MJ, Eaton P. The utility of N, N-biotinyl glutathione disulfide in the study of protein S-glutathiolation. Mol Cell Proteomics. 2006;5:215–225. doi: 10.1074/mcp.M500212-MCP200. [DOI] [PubMed] [Google Scholar]
- Celli N, Motos-Gallardo A, Tamburro A, Favaloro B, Rotilio D. Liquid chromatography-electrospray mass spectrometry study of cysteine-10 S-glutathiolation in recombinant glutathione S-transferase of Ochrobactrum anthropi. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;787:405–413. doi: 10.1016/s1570-0232(02)00706-7. [DOI] [PubMed] [Google Scholar]
- Chandra A, Srivastava S, Petrash JM, Bhatnagar A, Srivastava SK. Modification of aldose reductase by S-nitrosoglutathione. Biochemistry. 1997;36:15801–15809. doi: 10.1021/bi9714722. [DOI] [PubMed] [Google Scholar]
- Clavreul N, Adachi T, Pimental DR, Ido Y, Schoneich C, Cohen RA. S-glutathiolation by peroxynitrite of p21ras at cysteine-118 mediates its direct activation and downstream signaling in endothelial cells. FASEB J. 2006;20:518–520. doi: 10.1096/fj.05-4875fje. [DOI] [PubMed] [Google Scholar]
- Harding JJ. Free and protein-bound glutathione in normal and cataractous human lenses. Biochem J. 1970;117:957–960. doi: 10.1042/bj1170957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill BG, Bhatnagar A. Role of glutathiolation in preservation, restoration and regulation of protein function. IUBMB Life. 2007;59:21–26. doi: 10.1080/15216540701196944. [DOI] [PubMed] [Google Scholar]
- Jensen ON, Wilm M, Shevchenko A, Mann M. Sample preparation methods for mass spectrometric peptide mapping directly from 2-DE gels. Methods Mol Biol. 1999;112:513–530. doi: 10.1385/1-59259-584-7:513. [DOI] [PubMed] [Google Scholar]
- Klatt P, Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem. 2000;267:4928–4944. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- Knickelbein RG, Ingbar DH, Seres T, Snow K, Johnston RB, Jr, Fayemi O, Gumkowski F, Jamieson JD, Warshaw JB. Hyperoxia enhances expression of gamma-glutamyl transpeptidase and increases protein S-glutathiolation in rat lung. Am J Physiol. 1996;270:L115–122. doi: 10.1152/ajplung.1996.270.1.L115. [DOI] [PubMed] [Google Scholar]
- Kumari K, Khanna P, Ansari NH, Srivastava SK. High-performance liquid chromatography method for the determination of protein-glutathione mixed disulfide. Anal Biochem. 1994;220:374–376. doi: 10.1006/abio.1994.1352. [DOI] [PubMed] [Google Scholar]
- Li J, Huang FL, Huang KP. Glutathiolation of proteins by glutathione disulfide S-oxide derived from S-nitrosoglutathione. Modifications of rat brain neurogranin/RC3 and neuromodulin/GAP-43. J Biol Chem. 2001;276:3098–3105. doi: 10.1074/jbc.M008260200. [DOI] [PubMed] [Google Scholar]
- Lou MF, McKellar R, Chyan O. Quantitation of lensprotein mixed disulfides by ion-exchange chromatography. Exp Eye Res. 1986;42:607–616. doi: 10.1016/0014-4835(86)90050-3. [DOI] [PubMed] [Google Scholar]
- Muscat JE, Kleinman W, Colosimo S, Muir A, Lazarus P, Park J, Richie JP., Jr Enhanced protein glutathiolation and oxidative stress in cigarette smokers. Free Radic Biol Med. 2004;36:464–470. doi: 10.1016/j.freeradbiomed.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Chandra D, Srivastava S, Bhatnagar A, Srivastava SK. Nitric oxide regulates the polyol pathway of glucose metabolism in vascular smooth muscle cells. FASEB J. 2003;17:417–425. doi: 10.1096/fj.02-0722com. [DOI] [PubMed] [Google Scholar]
- Reinartz M, Ding Z, Flogel U, Godecke A, Schrader J. Nitrosative stress leads to protein glutathiolation, increased s-nitrosation, and up-regulation of peroxiredoxins in the heart. J Biol Chem. 2008;283:17440–17449. doi: 10.1074/jbc.M800126200. [DOI] [PubMed] [Google Scholar]
- Shackelford RE, Heinloth AN, Heard SC, Paules RS. Cellular and molecular targets of protein S-glutathiolation. Antioxid Redox Signal. 2005;7:940–950. doi: 10.1089/ars.2005.7.940. [DOI] [PubMed] [Google Scholar]
- Ward NE, Stewart JR, Ioannides CG, O’Brian CA. Oxidant-induced S-glutathiolation inactivates protein kinase C-alpha (PKC-alpha): a potential mechanism of PKC isozyme regulation. Biochemistry. 2000;39:10319–10329. doi: 10.1021/bi000781g. [DOI] [PubMed] [Google Scholar]
- West MB, Hill BG, Xuan YT, Bhatnagar A. Protein glutathiolation by nitric oxide: an intracellular mechanism regulating redox protein modification. FASEB J. 2006;20:1715–1717. doi: 10.1096/fj.06-5843fje. [DOI] [PubMed] [Google Scholar]
- West MB, Ramana KV, Kaiserova K, Srivastava SK, Bhatnagar A. L-Arginine prevents metabolic effects of high glucose in diabetic mice. FEBS Lett. 2008;582:2609–2614. doi: 10.1016/j.febslet.2008.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]