Abstract
Objective:
To evaluate the expression pattern of epidermal growth factor receptor (EGFR) in urinary bladder cancer and its association with human epidermal growth factor receptor 2 (HER2), epidermal growth factor (EGF), interleukin-6 (IL-6), and high risk human papilloma virus (HPV) types 16 and 18.
Materials and Methods:
Thirty cases of urothelial carcinoma were analyzed. EGFR, HER2, EGF, and IL-6 expressions in the tissue were evaluated by immunohistochemical staining. For HPV, DNA from tissue samples was extracted and detection of HPV was done by PCR technique. Furthermore, evaluation of different intracellular molecules associated with EGFR signaling pathways was performed by the western blot method using lysates from various cells and tissues.
Results:
In this study, the frequencies of immunopositivity for EGFR, HER2, EGF, and IL-6 were 23%, 60%, 47%, and 80%, respectively. No cases were positive for HPV-18, whereas HPV-16 was detected in 10% cases. Overall, expression of EGFR did not show any statistically significant association with the studied parameters. However, among male patients, a significant association was found only between EGFR and HER2.
Conclusions:
Overexpression of EGFR and/or HER2, two important members of the same family of growth factor receptors, was observed in a considerable proportion of cases. Precise knowledge in this subject would be helpful to formulate a rational treatment strategy in patients with urinary bladder cancer.
Keywords: Epidermal growth factor receptor, human epidermal growth factor receptor 2, human papilloma virus, immunohistochemistry, urinary bladder carcinoma
INTRODUCTION
The bladder is a common site for cancer development in the urinary tract. In India, according to the recent reports of the National Cancer Registry Programme, the overall incidence rate of the urinary bladder cancer is 2.25% (per 100,000 annually): 3.67% among males and 0.83% for females.[1] Urinary bladder cancer ranks ninth in worldwide cancer incidence (approximately 356,000 new cases each year); it is the seventh most common malignancy in men and 17th in women.[2,3] In the United States and Western Europe, the lifetime risk is about 1 in 25 and 1 in 80 for white males and females, respectively. Furthermore, approximately 145,000 patients die from this disease worldwide per year.[2] Majority of bladder tumors originate from transitional epithelium or urothelium, a multi-layered epithelium without squamous cells, which covers the inside of this organ. Epidemiological studies have shown that the cancer predominantly affects the aged males and the presence of mucosal irritants maybe important such as increased urinary excretion of carcinogenic substances and parasitic infestation. A number of factors, e.g., occupational exposure to chemicals, cigarette smoking, and urinary schistosomiasis are associated with increased risk.[3–7] It may be worthwhile to mention that the urinary bladder cancer is the most common malignancy in Egyptian males and has been attributed to schistosomiasis that is linked with squamous cell carcinoma. Interestingly, in recent time in Egypt, incidence of transitional cell carcinoma has been increasing, whereas squamous cell carcinoma has declined.[8] On the other hand, some reports have suggested a risk contributing role of factors like human papilloma virus (HPV) infection, obesity or coffee consumption,[9–11] however, more studies are needed on these issues for definite conclusions.
Cancer progression is associated with dysregulation of the signaling systems of various growth factors and pro-inflammatory cytokines such as epidermal growth factor (EGF), transforming growth factor-β (TGFβ) and interleukin-6 (IL-6).[12–14] Among four members of the family of erbB tyrosine kinase receptors, EGF-receptor (EGFR) and HER2/neu (or c-erbB-2) have been reported to be involved in the pathological processes of several cancers; and ligands like EGF, heparin-binding EGF (HB-EGF), TGFα and amphiregulin can bind to EGFR.[13] It is believed that HER2 has no known ligand; however, heterodimerization of HER2 with EGFR is possible, which probably confers aggressive tumor behaviors.[15] Several signaling molecules may participate in EGFR activation such as phosphatidylinositol-3 kinase (PI3K), extracellular signal regulated kinase (ERK), mitogen-activated protein kinase (MAPK), etc.[16,17] [Figure 1a]. Nevertheless, EGFR activation via ligand binding leads to downstream signaling that influences cell proliferation, angiogenesis, invasion and metastasis.[18] It is worth noting that different studies have shown a prognostic significance of EGFR overexpression in tumors of the urinary bladder. Mason et al. reported an association of the EGFR pathway with the bladder cancer risk and survival.[19] Similarly, Kramer et al. found that EGFR expression was associated with poor prognosis in bladder cancer cases.[20] Moreover, the results of a study on Greek subjects showed that simultaneous expression of EGFR and HER2 in transitional cell carcinomas was related to the advanced histological grade and stage of the disease.[21]
Figure 1.
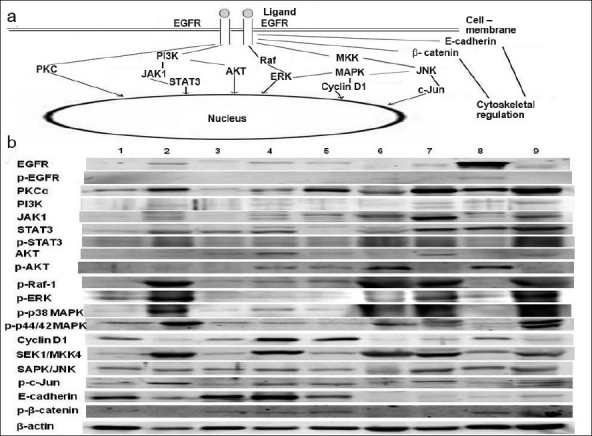
(a) Showing salient epidermal growth factor receptor (EGFR)-associated signaling pathways. EGFR: epidermal growth factor receptor, p-: phosphorylated, PKCα: protein kinase C α, PI3K: phosphatidylinositol 3-kinase, JAK1: janus kinase 1, STAT3: signal transducer and activator of transcription 3, ERK: extracellular signal-regulated kinase, MAPK: mitogen-activated protein kinase, MKK4: MAPK kinase 4, SAPK: stress activated protein kinase, JNK: c-jun amino-terminal kinase, Serine/threonine kinase (e.g., AKT, Raf). (b) Comparison between expressions of different EGFR-associated signaling molecules in various tissues and cell lines with reference to urinary bladder tissue. Lane 1: MCF-7 cells, Lane 2: Mouse mammary tissue, Lane 3: T47-D cells, Lane 4: LNCaP cells, Lane 5: PC-3 cells, Lane 6: TRAMP tumour tissue, Lane 7: Mouse urinary bladder tissue, Lane 8: SK-OV-3 cells, Lane 9: Mouse uterine tissue.
Considering the above-mentioned findings, the present study was conducted to evaluate the pattern of EGFR overexpression in urinary bladder cancer tissue and its correlation with related parameters such as HER2/neu, EGF as well as IL-6. Furthermore, efforts were made to detect HPV types 16 and 18 from the aforesaid cancer tissue and their association (if any) with the overexpression of EGFR. In addition, intracellular signaling molecules associated with EGFR were assessed in normal urinary bladder tissue from the experimental animals along with other normal and cancer cells/tissues in order to find out the predominant signaling molecules in normal condition.
MATERIALS AND METHODS
Clinical samples and immunochemical analysis
A total of 30 cases of transitional cell carcinoma of the urinary bladder were included in this study; the tumor tissue samples were collected from a single institute from 2000 to 2005. In the study, tumor cells recognizable as of transitional cell origin were considered, and thus tumors belonged to grade I (n = 12) and grade II (n = 18) were included. Among the above-mentioned cases, 24 were male and 6 were female; the mean ages of the patients were 60.97 ± 10.70 years. The immunohistochemical analysis was carried out on paraffin-embedded 5 μm thick tissue sections from tumors on poly-L-lysine coated slides.[22] In brief, tissue sections were incubated with primary antibody against EGFR (Sigma), HER2/c-erbB-2 (Boehringer Mannheim), EGF (Santa Cruz) and IL-6 (Vision Biosystems). Subsequently, sections were incubated with secondary antibody, followed by peroxidase-anti-peroxidase complex (PAP), and finally detection of immunoreactivity by substrate diaminobenzidine (DAB).
DNA extraction and detection of HPV DNA sequences by Polymerase Chain Reaction
High molecular weight genomic DNA from tumor tissue samples was isolated using standard Proteinase K digestion, phenol chloroform extraction, and ethanol precipitation method.[23,24] The quality and concentration of DNA was measured on standard spectrophotometric method. For the detection of HPV DNA, most common L1 consensus primers, MY 09/11 primers derived from HPV genome were employed. HPV-16 plasmid DNA as well as HPV-16 positive tumor DNA from uterine cervical cancer patient served as positive controls, whereas HPV negative cell line C33a DNA and HPV negative breast cancer sample served as negative control. Similarly, HPV-18 plasmid DNA and HPV-18 positive cervical tumor DNA were used as positive controls. For negative controls, the same DNA samples, which were used for HPV-16 analysis, were also utilized here. Amplification of β-globin gene served as internal controls to examine quality, integrity and successful amplification of tissue DNA.
Conventional PCR using L1 consensus MY 09/11, HPV-16 and HPV-18 primers
Approximately, 100-200 ηg genomic DNA was utilized for conventional PCR according to the routinely followed protocol[24,25] on a DNA Engine Tetrad (MJ Research, USA). Detection of HPV was carried out using consensus primers (MY09/MY11) located within the conserved L1 region of HPV genome (forward primer 5’-GCM CAG GGW CAT AAY AAT GG-3’, reverse primer 5’-CGT CCM ARR GGA WAC TGA TC-3’ where M= A+C, W= A+T, Y= C+T, R= A+G). HPV-16 URR gene sequences (forward primer 5´ –AAG GCC AAC TAA ATG TCA C -3´, reverse primer 5´-CTG CTT TTA TAC TAA CCG G -3´), HPV-18 (forward primer sequences 5’-TGA GGT ACC ATT GGA TAT TT-3´, reverse primer 5´-TAG CAA AAA GCT GCT TCA CGC-3’), and β-globin gene sequences (forward primer 5´-GAA GAG CCA AGG ACA GGT AC-3´, reverse primer 5´- CAA CTT CAT CCA CGT TAC ACC -3´) were used as internal controls.
Briefly, the method involved a 25-μl reaction mix containing 100-200 ηg DNA, 10 mM Tris-Cl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, 12.5 μM of each dNTP (dATP, dCTP, dGTP and dTTP), 5 pmoles of each oligonucleotide primer and 0.5 U Taq DNA polymerase (Perkin-Elmer Biosystems, Foster City, CA, USA). The temperature profile used for amplification constituted an initial denaturation at 95°C for 5 min followed by 39 cycles with denaturation at 95°C for 30 sec, annealing at 55°C for 30 sec and extension at 72°C for 30 sec, which was extended for 4-7 min in the final cycle. The oligonucleotide primers were synthesized in an automated Applied Biosystems DNA Synthesizer (Model 381A, Applied Biosystems Inc., Foster City, CA, USA) and HPLC purified.
Evaluation of intracellular signaling system
In normal urinary bladder tissue, to evaluate the important signaling molecules that are involved in EGFR system [Figure 1a], we examined different cancer cell lines (ATCC, Manassas, VA, USA) and tissues obtained from experimental animals. In fact, we did a comparative analysis of different signaling molecules, present in the lysates of the abovementioned cells/tissues, with reference to the normal urinary bladder tissue. For this purpose, we assayed MCF-7 and T47-D human breast cancer cells, mouse mammary tissue, LNCaP and PC-3 human prostate cancer cells, TRAMP tumour tissue, SK-OV-3 human ovarian cancer cells, mouse uterine tissue and mouse urinary bladder tissue.
MCF-7 and SK-OV-3 cells were grown in EMEM and McCoy's 5A medium, respectively. Both T47-D and LNCaP cells were cultured in RPMI-1640 medium, whereas PC-3 cells in F-12K medium. Media were supplemented with fetal bovine serum. Prostate cancer tissue was collected from TRAMP mouse (transgenic adenocarcinoma of the mouse prostate), which develops prostate tumour spontaneously. Furthermore, mammary tissue, urinary bladder tissue and uterine tissue were collected from C57BL6 mice. Lysates from different tissues and cancer cells were analyzed by the western blot method.[26] Except the primary antibodies against p-p38 MAPK, p-p44/42 MAPK, SEK1/MKK4, SAPK/JNK (Cell Signaling), and p-β-catenin (R and D Systems), all antibodies were from Santa Cruz.
Statistical analysis
Chi-square and Fisher exact test was employed to see the association between different parameters as suggested by Armitage et al. for small sample size.[27] Besides this, 95% confidence intervals (CI) were also calculated for the prevalence. Statistical software Epi Info version 6 was used for statistical analyses.
RESULTS AND DISCUSSION
Immunohistochemical staining exhibited brownish or golden brown color in positive tissue sections; predominantly membrane staining was observed for EGFR and HER2/neu, whereas EGF and IL-6 showed cytoplasmic staining [Figure 2]. The present study revealed 23% positivity for EGFR and 60% positivity for HER2; whereas 47% and 80% cases were positive for EGF and IL-6, respectively. In this study, overall, no statistical association was found between the expressions of EGFR and HER2, which are two important members of the same family of growth factor receptors [Table 1]. Interestingly, similar studies on patients with cancer of different sites from the same geographical location demonstrated different expression patterns of EGFR and HER2 [Table 2].[22,28] Nevertheless, in the current study, neither EGFR nor HER2 did exhibit any correlations with the expression of EGF and IL-6 or presence of HPV-16 [Table 1]. It may be worthwhile to mention that we tried to detect both high risk HPV types 16 and 18, but only 3 cases (10%) were positive for HPV-16 [Figure 3]. However, after division of the patients on the basis of gender, we found a significant association between EGFR and HER2 expressions in male group only (P < 0.05).
Figure 2.
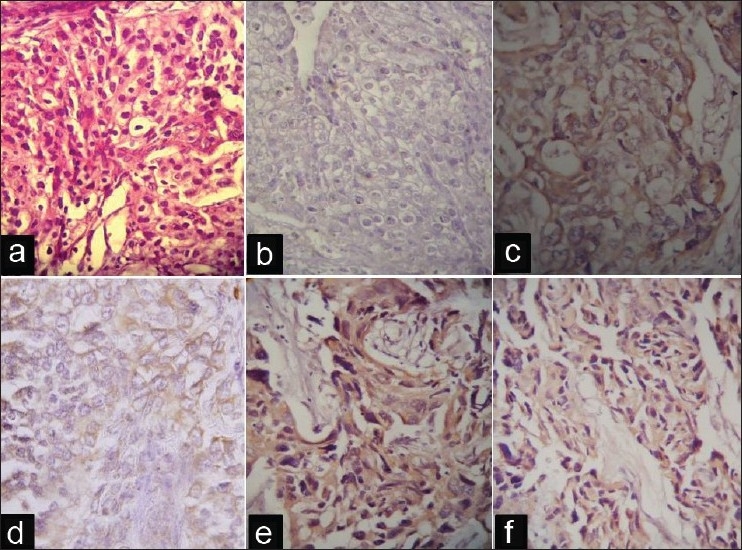
(a) Tumor tissue stained with hematoxylin and eosin. (b)Immunohistochemically negative tissue section. (c) Urinary bladder cancer tissue sections with overexpression of epidermal growth factor receptor. (d) Human epidermal growth factor receptor 2 (HER2). (e) Epidermal growth factor. and (f) interleukin-6.
Table 1.
Association pattern among the studied parameters in urinary bladder cancer cases
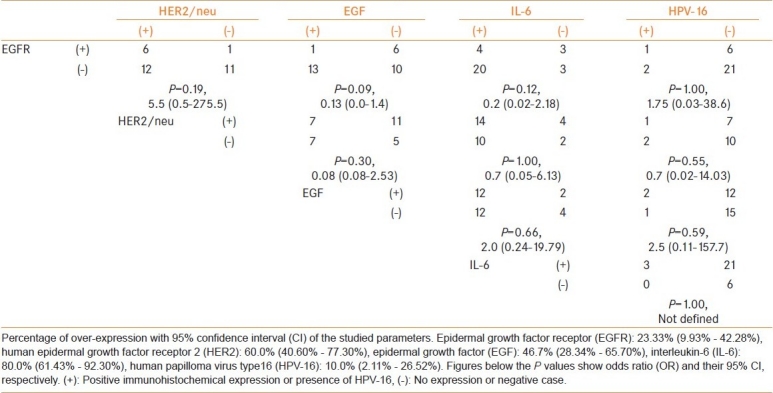
Table 2.
Percentage of expression of epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2) and their correlation in four different cancer sites
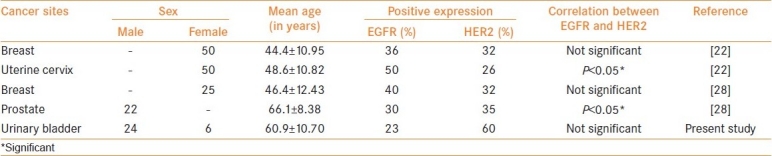
Figure 3.
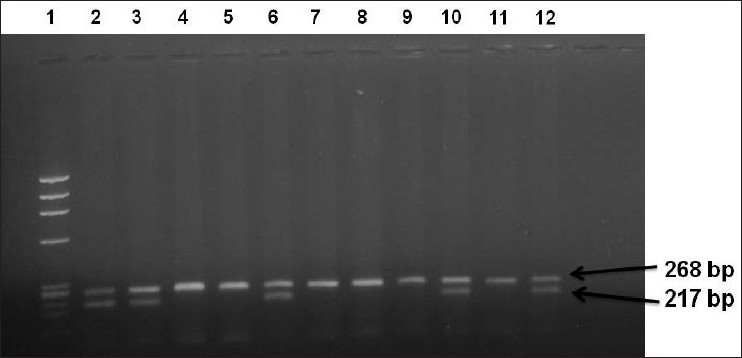
PCR amplification of β-globin and human papilloma virus type 16 (HPV-16) gene showing amplimer of 268 bp and 217 bp. Lane 1: Molecular weight DNA standard, Lane 2: Positive control (Plasmid DNA), Lane 3: Positive control (HPV-16 positive cervical cancer), Lane 4: Negative control (C33a DNA), Lane 5: Negative control (HPV negative breast cancer), Lane numbers 6, 10 and 12: Positive cases for HPV-16, Lane numbers 7, 8, 9 and 11: HPV-16 negative cases. N.B.: The amplimer of HPV-16 is 217 bp product and β-globin is 268 bp product (bp: base pair).
There are wide variations in the reports of different investigators regarding the frequencies of both EGFR and HER2 expressions in urinary bladder tumors. It has been documented that squamous cell carcinoma of the urinary bladder, which is frequently related to schistosomal etiology and an advanced stage, expresses enhanced levels of EGFR. Guo et al. analyzed 16 cases of squamous cell cancer and all cases were positive for EGFR.[29] However, Khaled et al. assessed 59 schistosomal bladder tumors; EGFR was detected in 66% cases.[30] On the other hand, Wang et al. noticed 27% positivity for EGFR (14 out of 52 cases) in small cell carcinoma of the urinary bladder.[31] In a study that included 67 patients with urothelial carcinomas, EGFR and HER2 were found to be positive in 63% and 22% patients, respectively.[32] Another study on 56 surgical specimens obtained from invasive bladder carcinomas exhibited a higher rate (75%) of EGFR expression.[33]
Like variations in EGFR positivity, differences in the expression rates of HER2 also have been observed among different reports. In a recent study on 40 patients with transitional cell carcinoma of the bladder, Matsubara et al. found that 43% tumors were HER2 positive.[34] On the contrary, Tapia et al. detected only 7% expression of HER2 protein.[35] Like our current study, Caner et al. and Tsai et al. reported positive staining for HER2 in 61% and 58% of bladder cancer cases, respectively.[36,37] On the other hand, de Pinieux et al. documented 23% (15 out of 64 cases) and Coogan et al. observed 26% (14/54) positive HER2 expression in human bladder transitional cell carcinoma.[38,39]
Our analyses were based on 30 cases, which is a limitation of the current study. Probably, for the small number of cases and inclusion of low grade tumors, the frequency of EGFR expression was lower in comparison with other reports. It is worth noting that a substantial number of studies have been carried out on breast cancer in connection with EGFR and HER2 including relevant targeted therapies. There are several parallels between the breast and bladder cancers. For instance, the risk of urinary bladder cancer has been reported to be higher among breast cancer survivors,[40] and breast cancer appears to be a common primary focus for metastases found in the bladder.[41] For both breast and bladder cancers, studies have implicated issues like environmental factors, sex hormones and insulin-like growth factors in the pathological processes.[42–47] Nevertheless, in comparison to breast cancer, clinical trials with molecularly targeted agents have been few in number and largely unsuccessful in bladder cancer.[48] Interestingly, a number of investigators have identified alterations in components of several signal transduction pathways in bladder cancer, which are also associated to EGFR. Aberrant activation of such pathways like signal transducer and activator of transcription 3 (S tat0 3), mitogen-activated protein kinase (MAPK), or phosphatidylinositol 3-kinase (PI3K) pathways perhaps plays crucial role in cancer cell growth and survival.[49–51] Proper evaluation of the complex interconnections among these signaling pathways and the major downstream effectors such as extracellular signal-regulated kinase (ERK) and AKT will greatly improve our understanding for better diagnosis and management.
Several investigators have suggested the possibility that expression of both EGFR and HER2 could be utilized for molecular targeted therapy in urinary bladder cancer.[30,33,34] Interestingly, our western blot analyses revealed an enhanced expression of some of the EGFR-related intracellular signaling molecules in normal bladder tissue from experimental animals [Figure 1b]. The condition may cause a gradual reduction in the effectiveness of molecular targeted therapy and lead to resistance to treatment eventually. Perhaps, a combination of conventional management along with the targeted therapy could be beneficial. Nevertheless, it seems that both EGFR and HER2 expressions indicate a poor prognosis[52] and thus hindering the activities of these growth factor receptors probably would help in the prognosis of bladder cancer patients. Different approaches to target these receptors have been devised such as tyrosine kinase inhibitors (e.g., gefitinib, lapatinib); monoclonal antibodies (e.g., cetuximab, trastuzumab); bispecific antibodies designed to target tumor antigens and cytotoxicity triggers; and monoclonal antibodies conjugated with cellular toxins, chemotherapeutic agents, or radioisotopes. Results of different ongoing studies on EGFR- or HER2-related targeted therapies in urinary bladder cancer may outline an effective treatment strategy in the near future.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bangalore: National Cancer Registry Programme (Indian Council of Medical Research); 2006. Consolidated report of population-based cancer registries 2001-2004. , Bangalore, 2006. [Google Scholar]
- 2.Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009;27:289–93. doi: 10.1007/s00345-009-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkin DM. The global burden of urinary bladder cancer. Scand J Urol Nephrol Suppl. 2008;218:12–20. doi: 10.1080/03008880802285032. [DOI] [PubMed] [Google Scholar]
- 4.Cassidy A, Wang W, Wu X, Lin J. Risk of urinary bladder cancer: A case-control analysis of industry and occupation. BMC Cancer. 2009;9:443. doi: 10.1186/1471-2407-9-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Vocht F, Sobala W, Wilczynska U, Kromhout H, Szeszenia-Dabrowska N, Peplonska B. Cancer mortality and occupational exposure to aromatic amines and inhalable aerosols in rubber tire manufacturing in Poland. Cancer Epidemiol. 2009;33:94–102. doi: 10.1016/j.canep.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Anderson B. Understanding the role of smoking in the aetiology of bladder cancer. Br J Community Nurs. 2009;14:385–92. doi: 10.12968/bjcn.2009.14.9.43805. [DOI] [PubMed] [Google Scholar]
- 7.Rollinson D. A wake up call for urinary schistosomiasis: Reconciling research effort with public health importance. Parasitology. 2009;136:1593–610. doi: 10.1017/S0031182009990552. [DOI] [PubMed] [Google Scholar]
- 8.Fedewa SA, Soliman AS, Ismail K, Hablas A, Seifeldin IA, Ramadan M, et al. Incidence analyses of bladder cancer in the Nile delta region of Egypt. Cancer Epidemiol. 2009;33:176–81. doi: 10.1016/j.canep.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badawi H, Ahmed H, Ismail A, Diab M, Moubarak M, Badawy A, et al. Role of human papillomavirus types 16, 18, and 52 in recurrent cystitis and urinary bladder cancer among Egyptian patients. Medscape J Med. 2008;10:232. [PMC free article] [PubMed] [Google Scholar]
- 10.Koebnick C, Michaud D, Moore SC, Park Y, Hollenbeck A, Ballard-Barbash R, et al. Body mass index, physical activity, and bladder cancer in a large prospective study. Cancer Epidemiol Biomarkers Prev. 2008;17:1214–21. doi: 10.1158/1055-9965.EPI-08-0026. [DOI] [PubMed] [Google Scholar]
- 11.Villanueva CM, Silverman DT, Murta-Nascimento C, Malats N, Garcia-Closas M, Castro F, et al. Coffee consumption, genetic susceptibility and bladder cancer risk. Cancer Causes Control. 2009;20:121–7. doi: 10.1007/s10552-008-9226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Boer WI, Houtsmuller AB, Izadifar V, Muscatelli-Groux B, Van der Kwast TH, Chopin DK. Expression and functions of EGF, FGF and TGFbeta-growth-factor family members and their receptors in invasive human transitional-cell-carcinoma cells. Int J Cancer. 1997;71:284–91. doi: 10.1002/(sici)1097-0215(19970410)71:2<284::aid-ijc25>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 13.De Luca A, Carotenuto A, Rachiglio A, Gallo M, Maiello MR, Aldinucci D, et al. The role of the EGFR signaling in tumor microenvironment. J Cell Physiol. 2008;214:559–67. doi: 10.1002/jcp.21260. [DOI] [PubMed] [Google Scholar]
- 14.Lukaszewicz M, Mroczko B, Szmitkowski M. [Clinical significance of interleukin-6 (IL-6) as a prognostic factor of cancer disease] Pol Arch Med Wewn. 2007;117:247–51. [PubMed] [Google Scholar]
- 15.Hirsch FR, Varella-Garcia M, Cappuzzo F. Predictive value of EGFR and HER2 overexpression in advanced non-small-cell lung cancer. Oncogene. 2009;28:S32–7. doi: 10.1038/onc.2009.199. [DOI] [PubMed] [Google Scholar]
- 16.Ettinger DS. Clinical implications of EGFR expression in the development and progression of solid tumors: Focus on non-small cell lung cancer. Oncologist. 2006;11:358–73. doi: 10.1634/theoncologist.11-4-358. [DOI] [PubMed] [Google Scholar]
- 17.Gee JM, Robertson JF, Gutteridge E, Ellis IO, Pinder SE, Rubini M, et al. Epidermal growth factor receptor/HER2/insulin-like growth factor receptor signalling and oestrogen receptor activity in clinical breast cancer. Endocr Relat Cancer. 2005;12:S99–111. doi: 10.1677/erc.1.01005. [DOI] [PubMed] [Google Scholar]
- 18.Kruser TJ, Wheeler DL. Mechanisms of resistance to HER family targeting antibodies. Exp Cell Res. 2010;316:1083–100. doi: 10.1016/j.yexcr.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Mason RA, Morlock EV, Karagas MR, Kelsey KT, Marsit CJ, Schned AR, et al. EGFR pathway polymorphisms and bladder cancer susceptibility and prognosis. Carcinogenesis. 2009;30:1155–60. doi: 10.1093/carcin/bgp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer C, Klasmeyer K, Bojar H, Schulz WA, Ackermann R, Grimm MO. Heparin-binding epidermal growth factor-like growth factor isoforms and epidermal growth factor receptor/ErbB1 expression in bladder cancer and their relation to clinical outcome. Cancer. 2007;109:2016–24. doi: 10.1002/cncr.22627. [DOI] [PubMed] [Google Scholar]
- 21.Gorgoulis VG, Barbatis C, Poulias I, Karameris AM. Molecular and immunohistochemical evaluation of epidermal growth factor receptor and c-erb-B-2 gene product in transitional cell carcinomas of the urinary bladder: A study in Greek patients. Mod Pathol. 1995;8:758–64. [PubMed] [Google Scholar]
- 22.Ray A, Naik SLD, Sharma BK. Distribution of prognostically unfavourable product of c-erbB-2 oncogene and EGF-R in carcinomas of the breast and uterine cervix. Indian J Physiol Pharmacol. 2002;46:423–33. [PubMed] [Google Scholar]
- 23.Das BC, Sharma JK, Gopalkrishna V, Das DK, Singh V, Gissmann L, et al. A high frequency of human papillomavirus DNA sequences in cervical carcinomas of Indian women as revealed by Southern blot hybridization and polymerase chain reaction. J Med Virol. 1992;36:239–45. doi: 10.1002/jmv.1890360402. [DOI] [PubMed] [Google Scholar]
- 24.Das BC, Sharma JK, Gopalkrishna V, Luthra UK. Analysis of polymerase chain reaction of the physical state of human papilloma type 16 DNA in cervical preneoplastic and neoplastic region. J Gen Virol. 1992;73:2327–36. doi: 10.1099/0022-1317-73-9-2327. [DOI] [PubMed] [Google Scholar]
- 25.Hedau S, Jain N, Husain SA, Mandal AK, Ray G, Shahid M, et al. Novel germline mutations in breast cancer susceptibility genes BRCA1, BRCA2 and p53 gene in breast cancer patients from India. Breast Cancer Res Treat. 2004;88:177–186. doi: 10.1007/s10549-004-0593-8. [DOI] [PubMed] [Google Scholar]
- 26.Ray A, Nkhata KJ, Grande JP, Cleary MP. Diet-induced obesity and mammary tumor development in relation to estrogen receptor status. Cancer Lett. 2007;253:291–300. doi: 10.1016/j.canlet.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Armitage P, Berry G, Matthews JN. Statistical methods in medical research. 4th ed. Oxford: Blackwell Science; 2002. Analysing means and proportions; pp. 83–146. [Google Scholar]
- 28.Jain D, Ray A, Bahadur AK, Chaturvedi KU, Sood R, Sharma S, et al. Status of epidermal growth factor receptors family in hormone-dependent carcinomas of the breast and prostate with reference to serum lipids and lipoproteins. Indian J Clin Biochem. 2001;16:42–51. doi: 10.1007/BF02867567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo CC, Gomez E, Tamboli P, Bondaruk JE, Kamat A, Bassett R, et al. Squamous cell carcinoma of the urinary bladder: A clinicopathologic and immunohistochemical study of 16 cases. Hum Pathol. 2009;40:1448–52. doi: 10.1016/j.humpath.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Khaled HM, Bahnassy AA, Raafat AA, Zekri AR, Madboul MS, Mokhtar NM. Clinical significance of altered nm23-H1, EGFR, RB and p53 expression in bilharzial bladder cancer. BMC Cancer. 2009;9:32. doi: 10.1186/1471-2407-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Zhang S, MacLennan GT, Eble JN, Lopez-Beltran A, Yang XJ, et al. Epidermal growth factor receptor protein expression and gene amplification in small cell carcinoma of the urinary bladder. Clin Cancer Res. 2007;13:953–7. doi: 10.1158/1078-0432.CCR-06-2167. [DOI] [PubMed] [Google Scholar]
- 32.Kiyoshima K, Oda Y, Kinukawa N, Naito S, Tsuneyoshi M. Overexpression of laminin-5 gamma2 chain and its prognostic significance in urothelial carcinoma of urinary bladder: Association with expression of cyclooxygenase 2, epidermal growth factor receptor and human epidermal growth factor receptor 2. Hum Pathol. 2005;36:522–30. doi: 10.1016/j.humpath.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Cardillo MR, Castagna G, Memeo L, De Bernardinis E, Di Silverio F. Epidermal growth factor receptor, MUC-1 and MUC-2 in bladder cancer. J Exp Clin Cancer Res. 2000;19:225–33. [PubMed] [Google Scholar]
- 34.Matsubara H, Yamada Y, Naruse K, Nakamura K, Aoki S, Taki T, et al. Potential for HER-2/neu molecular targeted therapy for invasive bladder carcinoma: Comparative study of immunohistochemistry and fluorescent in situ hybridization. Oncol Rep. 2008;19:57–63. [PubMed] [Google Scholar]
- 35.Tapia C, Glatz K, Novotny H, Lugli A, Horcic M, Seemayer CA, et al. Close association between HER-2 amplification and overexpression in human tumors of non-breast origin. Mod Pathol. 2007;20:192–8. doi: 10.1038/modpathol.3800729. [DOI] [PubMed] [Google Scholar]
- 36.Caner V, Turk NS, Duzcan F, Tufan NL, Kelten EC, Zencir S, et al. No strong association between HER-2/neu protein overexpression and gene amplification in high-grade invasive urothelial carcinomas. Pathol Oncol Res. 2008;14:261–6. doi: 10.1007/s12253-008-9027-y. [DOI] [PubMed] [Google Scholar]
- 37.Tsai YS, Tzai TS, Chow NH, Yang WH, Tong YC, Lin JS, et al. Prognostic values of p53 and HER-2/neu coexpression in invasive bladder cancer in Taiwan. Urol Int. 2003;71:262–70. doi: 10.1159/000072676. [DOI] [PubMed] [Google Scholar]
- 38.de Pinieux G, Colin D, Vincent-Salomon A, Couturier J, Amsellem-Ouazana D, Beuzeboc P, et al. Confrontation of immunohistochemistry and fluorescent in situ hybridization for the assessment of HER-2/ neu (c-erbb-2) status in urothelial carcinoma. Virchows Arch. 2004;444:415–9. doi: 10.1007/s00428-004-0986-4. [DOI] [PubMed] [Google Scholar]
- 39.Coogan CL, Estrada CR, Kapur S, Bloom KJ. HER-2/neu protein overexpression and gene amplification in human transitional cell carcinoma of the bladder. Urology. 2004;63:786–90. doi: 10.1016/j.urology.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 40.Bermejo JL, Sundquist J, Hemminki K. Bladder cancer in cancer patients: population-based estimates from a large Swedish study. Br J Cancer. 2009;101:1091–9. doi: 10.1038/sj.bjc.6605325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Velcheti V, Govindan R. Metastatic cancer involving bladder: A review. Can J Urol. 2007;14:3443–8. [PubMed] [Google Scholar]
- 42.Boysen T, Friborg J, Andersen A, Poulsen GN, Wohlfahrt J, Melbye M. The Inuit cancer pattern-the influence of migration. Int J Cancer. 2008;122:2568–72. doi: 10.1002/ijc.23367. [DOI] [PubMed] [Google Scholar]
- 43.de Vocht F, Sobala W, Wilczynska U, Kromhout H, Szeszenia-Dabrowska N, Peplonska B. Cancer mortality and occupational exposure to aromatic amines and inhalable aerosols in rubber tire manufacturing in Poland. Cancer Epidemiol. 2009;33:94–102. doi: 10.1016/j.canep.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 44.Sisci D, Surmacz E. Crosstalk between IGF signaling and steroid hormone receptors in breast cancer. Curr Pharm Des. 2007;13:705–17. doi: 10.2174/138161207780249182. [DOI] [PubMed] [Google Scholar]
- 45.Miyamoto H, Yang Z, Chen YT, Ishiguro H, Uemura H, Kubota Y, et al. Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst. 2007;99:558–68. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- 46.Teng J, Wang ZY, Jarrard DF, Bjorling DE. Roles of estrogen receptor alpha and beta in modulating urothelial cell proliferation. Endocr Relat Cancer. 2008;15:351–64. doi: 10.1677/erc.1.01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Metalli D, Lovat F, Tripodi F, Genua M, Xu SQ, Spinelli M, et al. The insulin-like growth factor receptor I promotes motility and invasion of bladder cancer cells through Akt- and mitogen-activated protein kinase-dependent activation of paxillin. Am J Pathol. 2010;176:2997–3006. doi: 10.2353/ajpath.2010.090904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dovedi SJ, Davies BR. Emerging targeted therapies for bladder cancer: A disease waiting for a drug. Cancer Metastasis Rev. 2009;28:355–67. doi: 10.1007/s10555-009-9192-9. [DOI] [PubMed] [Google Scholar]
- 49.Chen CL, Cen L, Kohout J, Hutzen B, Chan C, Hsieh FC, et al. Signal transducer and activator of transcription 3 activation is associated with bladder cancer cell growth and survival. Mol Cancer. 2008;7:78. doi: 10.1186/1476-4598-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Díaz De Ståhl T, Segersten U, Malmström PU. Molecular genetics of bladder cancer: An update. Minerva Urol Nefrol. 2008;60:205–16. [PubMed] [Google Scholar]
- 51.Knowles MA, Platt FM, Ross RL, Hurst CD. Phosphatidylinositol 3-kinase (PI3K) pathway activation in bladder cancer. Cancer Metastasis Rev. 2009;28:305–16. doi: 10.1007/s10555-009-9198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Black PC, Dinney CP. Growth factors and receptors as prognostic markers in urothelial carcinoma. Curr Urol Rep. 2008;9:55–61. doi: 10.1007/s11934-008-0011-6. [DOI] [PubMed] [Google Scholar]


