Abstract
Introduction:
A total of 356,557 new cases were diagnosed annually worldwide in 2009, it was estimated that 52,810 new patients were to be diagnosed with bladder cancer and there were 10,180 projected deaths from the disease in the USA. Despite being the fourth commonest cancer in men, we do not have an early detection/screening program for bladder cancer. The review was aimed at looking at the evidence for the rationale for an early detection program for bladder cancer.
Materials and Methods:
A detailed search on bladder cancer epidemiology, diagnosis, pathology, tumor markers, treatment outcomes, screening, morbidity and mortality of bladder cancer was carried out on Pubmed central/Medline. Original articles, review articles, monograms, book chapters on bladder cancer, text books on urological oncology, oncology and urology were reviewed. The latest information for new articles before publication was last accessed in June 2010.
Discussion and Conclusions:
Bladder cancer is the fourth commonest cancer in men, the annual death rate from this disease is significant and every year there is an increase in its incidence globally. The prognosis of bladder cancer is stage and grade dependent; the lower the stage (T2 or less) the better is the survival. Delay in the diagnosis and treatment does alter the overall outcome. Therefore, there is a clear need for early detection of bladder cancer and screening program. Although we do not have an ideal marker for bladder cancer, it is time we maximize the potential of markers such as UroVysion, NMP22 along with cytology to start such a program. May be as a first step the early detection and screening program could be started in high-risk population. It is not worth waiting till we find the best marker as it would be unfair to our patients. The fear of unnecessary tests and treatment in bladder cancer after its detection in screening program is without any substance. The cost-effectiveness of such a program is certainly comparable to that is used for colon or breast and for prostate as well.
Keywords: Biomarkers, bladder cancer, early detection, high-risk population, PSA, screening, survival in bladder cancer
INTRODUCTION
Worldwide, bladder cancer is the fourth most common cancer in men and eighth in the women, with transitional cell carcinoma (TCC) comprising up to 90% of all primary bladder tumors.[1] It is the second most common malignancy affecting the urinary system. Bladder cancer is three to four times more common in men than in women. A total of 356,557 new cases are diagnosed annually worldwide with more than 60,000 new cases diagnosed each year in the USA.[2] Age-standardized (world) mortality rates are 2-10 per 100,000 males and 0.5-4 per 100,000 females.[2] In 2009, it was estimated that 52,810 new patients were to be diagnosed with bladder cancer and there were 10,180 projected deaths from the disease in the USA[1]
The majority is TCC/urothelial carcinoma (around 90%) and the rest are squamous cell carcinoma, adenocarcinoma and rare variety like small cell carcinoma. Herein, we will be discussing TCC/urothelial carcinoma.
Of the bladder cancer, around 70-75% are non-muscle invasive (Ta/T1) while 25-30% are muscle invasive (T2 and higher) at presentation. Around 20-40% of the non-muscle invasive bladder cancers progress to muscle invasion. Despite advances in surgical techniques and chemotherapy the 10-year disease-free survival of muscle-invasive disease in many large series is 50-60%.[3] Most deaths due to bladder cancer are directly related to the higher stage of the disease i.e., T2 or higher and higher grade.[4] At initial diagnosis almost 50% of the patients with high-grade bladder cancer have muscle invasive disease, of these 50% have distant metastasis with in 2 years and almost 50-60% of these patients die with 5 years despite treatment.[5] In order to improve the prognosis of bladder cancer early detection is of paramount importance.
As of today, there is no screening program for the early detection of bladder cancer. The diagnosis is mainly in symptomatic patients and on occasion with routine health check ups where an abnormality in urine dipstick test or imaging is found. There have been few attempts to conduct screening programs for a high-risk population, but there is no universal agreement on the cost-benefit ratio of these programs. The present article is aimed at reviewing the need of early detection of bladder cancer, the current tools we have for detecting bladder cancer and their effectiveness and how best we can utilize their potential in early detection and therefore overall outcome in bladder cancer. It is time that we need to have an early detection program for bladder cancer, in order to improve ‘our performance’ in the treatment of this disease.
MATERIALS AND METHODS
A detailed search on bladder cancer epidemiology, diagnosis, pathology, tumor markers, treatment outcomes, screening, morbidity and mortality of bladder cancer was carried out on Pubmed central/Medline. Original articles, review articles, monograms, book chapters on bladder cancer, text books on urologic oncology, oncology and urology were reviewed. The latest information for new articles before publication was last accessed in June 2010.
DISCUSSION
Need for the early detection
The clinical stage and grade are the two most important determinants of the fate of bladder cancer. The depth of tumor invasion in the bladder wall, the key component of stage, is time dependent.[6] Although difficult to prove in prospective studies, it is pretty clear that the delays encountered in the process of diagnosis and treatment of bladder cancer lead to unsatisfactory outcome. The delay could be multifactorial. It could be unawareness of the important facts about this disease amongst the general population and non-urology physicians, delay in reaching the physician, delay in the referral and administrative (hospital) delay in the treatment. Decision making as to when to treat aggressively and identifying the high-risk group as per risk stratification can also be a deciding factor in the treatment outcome of the bladder cancer. Delay caused by improper judgment in identifying the ‘pussy cats from tigers’ in bladder cancer and ill judgment in the ‘timing’ of the treatment can also have an adverse effect on survival.[7] In a retrospective study of 10 years, Wallace et al, have highlighted the various types of delays in the diagnosis and treatment of bladder cancer and concluded that the delay in the treatment of T1 and muscle-invasive bladder cancer perhaps had resulted in adverse effects.[6] Some of the recent studies have highlighted less than optimal referral patterns for hematuria in current practice.[8–10] This inaccuracy can lead to delay in the diagnosis and may lead to the more advanced stage at diagnosis and ultimately culminate in a worse prognosis. So the delay does matter.
There is always an argument (by those who feel that early detection may not translate in to improved survival) saying that despite the advances in the progress of surgery and chemotherapy the 5-year disease-free survival rate in muscle-invasive bladder cancer has not improved and is still in the range of 50-60%.[3] There could be many reasons for this. Non standardization of the technique of cystectomy and regional lymph node clearance, no universal agreement on perioperative chemotherapy, lack of further progress in new drug development and lack of knowledge of the biological behavior of bladder cancer could well be the reasons behind a poor outcome. While analyzing the data, the experts unanimously agree on the impact of upstaging on the final histology as a key determinant toward the outcome of the disease.[3,11] At times this upstaging could be as high as 42% and has direct impact on the disease-free survival.[12] Therefore, an early detection, appropriate and timely treatment in bladder cancer is desirous and is very vital to improve the outcome in bladder cancer.
The tools we have for early detection
So, currently what tools do we have that can be used in the early detection program for bladder cancer? For the diagnosis of bladder cancer, we mainly rely on urine cytology and cystoscopy. Although cystoscopy is the mainstay of the diagnosis, it is an invasive procedure. Cytology has high specificity but has low sensitivity especially in low-grade, low-stage tumors. It has the advantage of being cheap, office based but is highly dependent on the expertise of the cyto-pathologist and cannot be done as a home-based assay. Many urine-based markers are being used in clinical practice, but none of them have shown better specificity than urine cytology, but have shown definitely a better sensitivity than urine cytology. This is highlighted in an extensive review and meta-analysis by Lotan et al.[13]
The Table 1 highlights some of the markers that are in current use. As highlighted in the table, there are many tests that have a better sensitivity than cytology even in low-grade tumors and but fall short of higher specificity. Despite the pros and cons of these biomarkers/tests one has to accept that these tests do help in the diagnosis and surveillance of the bladder cancer. Agreed, none of them is an ‘ideal marker’, but how many malignancies have a perfect marker?
Table 1.
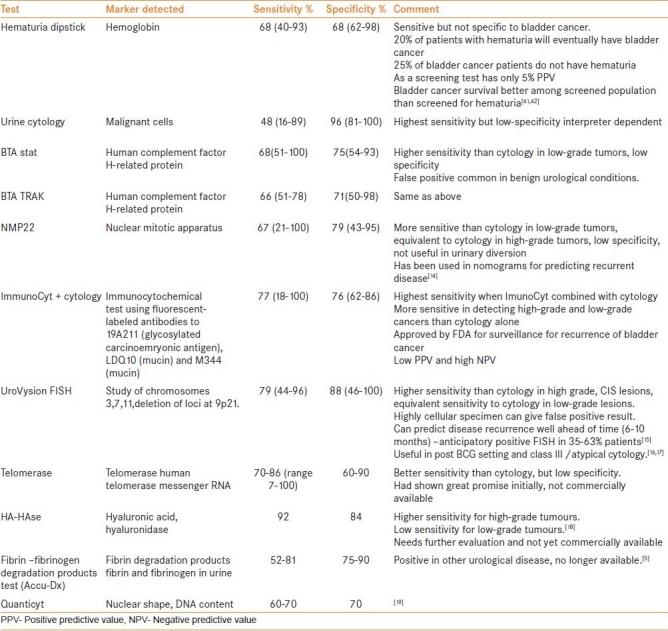
An ideal tumor marker or a test in detecting bladder cancer should be a one that is non-invasive, has highest specificity and sensitivity, easy to perform, rapid, reproducible, office based and cost-effective and should detect bladder cancer even before it becomes visible on cystoscopy. A test with an area under the Receiver Operating Characteristic curve (ROC curve) of 0.5 has low utility while a perfect test has an area under ROC curve of 1. The International Consensus Panel on Bladder Tumor Markers defined the characteristics of an ideal tumor marker to be technical ease of assaying, low intra-assay and inter-assay variability and a high level of accuracy.[24] Although currently none of the markers mentioned in Table 1 fulfill the criteria of ‘ideal marker’, UroVysion (Abbot Laboratories, Abbot Park, IL) and telomerase have shown a great promise in sensitivity and specificity.
International Consensus Panel on Bladder Tumor Markers infer from the so far available data that many tumor markers have higher sensitivity to detect bladder cancer. Although cytology has better specificity, many markers have shown better sensitivity than cytology and some markers in limited numbers of studies have shown specificity equivalent to that of cytology. The panel believes that several bladder tumor markers are more accurate in detecting bladder cancer than prostate-specific antigen (PSA).[24]
BCAN Think Tank session recently concluded that though the practicality of the markers has been challenged, continued research efforts directed at developing and evaluating markers for screening are warranted.[25]
Is it really true that ‘none of the markers are up to the mark’? –A critical analysis
I think it is unfair to say that none of ‘the markers are up the mark’ unless we critically look into certain important issues.
The first issue is that of ‘false positive’ results. There are certain situations where the marker is positive but no tumor is found on white light cystoscopy. This phenomenon has been observed in UroVysion FISH test more predominantly and to moderate extent in cytology and to lesser extent in BTA test and NMP22 (Matritech Inc., Newton, MA) test. This should not be seen as the downside of the marker. If the marker/test can predict the disease well in advance, there is nothing like it, in fact it should be the advantage in both the screening and surveillance. This phenomenon of ‘anticipatory positive ‘test in UroVysion FISH was first reported by Sarosdy et al,[26] and has been confirmed in many other studies. In a review by Greene and Konety et al,[15] a positive UroVysion FISH test even in the absence of confirmatory cystoscopical or cytological findings could predict disease recurrence in 35-63% of patients within the next 6-10 months. If blue light cystoscopy was used in these situations of positive tests/marker, may be the results could have been different, knowing the improved visibility of flat lesions and tumors with blue light cystoscopy. But this phenomenon of anticipatory positive test cannot be downplayed and in fact should be taken as an advantage, both in screening and surveillance. Friedrich et al, have also found this phenomenon with a test using immunocytology with mAbs against Lewis X. They found this test to have greater than 90% sensitivity and suggested its use in surveillance of high-risk population.[16,27]
The second issue is of interpretation of various studies on biomarkers in bladder cancer. In a review, Van Tiborg et al, from the Netherlands question the validity of interpretations of major studies on tumor markers in bladder cancer. The authors felt that the figures of sensitivity and specificity of these markers need to be carefully looked /interpreted as they are compared with so-called ‘gold standard-cystoscopy’. The authors remind us that cystoscopy does not offer 100% sensitivity, so the results of these markers/tests should be interpreted with caution. They suggest a proper evaluation of urine-based tests based on longitudinal tests.[28] Another angle to the poor sensitivity of the markers/test was highlighted by Boman et al,[29] they found that smaller size recurrences could be one of the factors for so called ‘poor performance’ of these tests. These important issues need to be kept in mind before we ‘sweep aside’ these markers for early detection of bladder cancer.
Can we improve the performance of existing markers?
Rather than relying on only one marker if one believes in the statement that ‘no marker is up to the mark’, attempts have been made to use more than one marker in improving the sensitivity and specificity, therefore the diagnosis.
In a systematic review of 42 major studies on bladder cancer markers, Glas et al, found that cytology has the best specificity at 94% (95% CI: 90-96%); telomerase had specificity closer to cytology–specificity 86% (71-94%). Telomerase had the best sensitivity of 75% (71-79%) but was not significantly better than that of BTA stat –70% (66-74%). This observation was based on the detailed analysis of 42 major studies on bladder cancer and the method of bivariate analysis was used. The authors claimed this being a better meta-analysis than others, but could have flaws in interpretation. Based on the findings in this study, the authors suggested that a combination of cytology and telomerase might be an option in inching toward a better ‘combined marker test’ in the future; however, there is no current data to evaluate this statement.[23]
There have been attempts to combine NMP22 and cytology to improve the detection of bladder cancer in a hematuria clinic. A combination of Bard BTA test and urine cytology in a setting of hematuria clinic improved the bladder cancer detection rate as compared to only BTA test or cytology in a series of 143 patients. (M Khochikar, Waterfall N B: Paper presented at European Association of Urology, Paris, France. September 1-4 1996)
A combination of ImmunoCyt + cytology has shown promising results in both low-grade and high-grade disease. The overall sensitivity of this combination ranges from 40% to 92% and specificity from 62% to 84%.[30–32] Studies by Mian et al, 942 patients,[33] Pfister et al, - a French multicenter study of 694 patients[34] and Messing et al, a multicenter study in USA 341 patients[35] confirm the findings of improved sensitivity and specificity with this combination when used in the follow-up of the patients with bladder cancer. This combination of ImmunoCyt and cytology has been studied to replace the cystocopy in the surveillance of Ta, low-grade lesions by Lodde et al.[19,36] In this study, the markers were done six-monthly and cystoscopy annually, this lower intensity surveillance did not result in any cases of missed disease progression, the negative predictive value of this combination test was 95%. If such a combination is useful in even low-grade recurrence, why not use such a combination in screening/early detection of bladder cancer?
A two-stage approach has been suggested by Halling et al. Based on their study of 265 patients, where they compared BTA stat, hemoglobin dipstick, telomerase, UroVysion assay, they found UroVysion had the highest sensitivity and specificity. The specificity for UroVysion, telomerase, BTA stat and hemoglobin dipstick was 96, 91, 74 and 51%, respectively. The authors suggested two-stage testing/screening procedure. Stage 1 would involve use of an inexpensive test with a relatively high sensitivity (for example BTA stat or hemoglobin dipstick) and would be followed by stage 2 in which a test with high specificity and sensitivity (for example UroVysion) can be used. The authors felt that such a two-stage approach would be cost-effective and efficient mean of diagnosing/follow-up of patients.[37]
There are some ‘new entries’ on the horizon of bladder cancer diagnosis such as CYFRA 21-1 test (the soluble fragment of cytokeratin is measured), BLCA-4, a nuclear matrix protein detection test, Urinary levels of Survinin-a protein belonging to inhibitor of apoptotic gene family and DNA and micro satellite analysis and many others.
The Food and Drug Administration in the USA has approved Bladder Check (NMP22) and UroVysion for use in screening of bladder cancer.
Is screening beneficial?
There have been short-term and long-term studies that have looked into the usefulness of a screening program in bladder cancer.[17] A community-based study in the UK by Britton et al, looked into the bladder cancer screening by the detection of occult urinary bleeding.[38] They tested 2356 men aged >60 yrs with repeated hematuria dipstick tests. Four seventy-four (20%) had a positive dipstick and 317 patients agreed for further investigations. Bladder cancer was found in 17 patients all of them had non-muscle-invasive disease. The outcome at 3 years was excellent.[39] In the subsequent 4 years many patients had progression of their cancer. They found that none of the patients with low-grade non-invasive disease died from the disease or progressed to muscle-invasive stage, but three of the nine patients with high grade or invasive disease died from bladder cancer. The authors highlighted the fact that patients can be detected early and at an apparently curative stage of the disease by screening.[40] Messing et al, tested 1575 men aged >50 years in Wisconsin for hematuria at home screening.[41] They found 21 bladder cancers amongst 258 subjects (16.4%) that were tested positive for hematuria. In a further study, they compared these patients in the screened group with patients in Wisconsin tumor registry in 1998 (509 men). They found no difference in the low and high-grade cancers between the screened group and controls; however, there was higher incidence of invasive high-grade cancer in unscreened men (60%) than in screened men (10%, P=0.002). At a long-term follow-up of 14 years, no one died from bladder cancer in the screened group whereas 20.4% of unscreened men died from bladder cancer.[42] This study not only highlights the usefulness of the screening but also highlights the fact that screened group has better survival than that among individuals diagnosed with symptomatic bladder cancer.
Screening in high-risk population
Although the advantages of screening in community at >50 or >60 yrs of age have been highlighted by the above-mentioned two studies, the screening in high-risk population is expected to yield better results and more benefits. The association of cigarette smoking, exposure to aromatic amines, aniline dyes, exposure from rubber industry with bladder cancer are well known.[43]
There have been many studies highlighting the role of screening in high-risk group. A study by Lotan et al, found that the positive predictive value (PPV) of NMP-22 test was higher in men (24%) than women (18.4%), PPV increased with smoking (35.4%), gross hematuria (51.2%) and both factors (70.6%).[44] Sarosdy et al, used FISH assay to study the incidence of bladder cancer amongst the smokers depending on the degree of smoking. It was a multicenter, blinded trial in patients with hematuria. They found a higher PPV with FISH assay in patients with >40 pack-years of smoking (65%) vs. 13.6 –24.2% in those with <20 or a 20-40 pack-year smoking history.[45] Hemstreet et al, studied the biomarkers in workers exposed to benzidine. They evaluated 1788 workers exposed to benzidine and 373 unexposed workers over 6-year period. The urine samples were tested for DNA ploidy, the bladder tumor-associated antigen p300 and G-actin. Bladder cancer was detected in 28 exposed workers and in two unexposed workers. The DNA ploidy had 87.5% sensitivity and 86.5% specificity; p300 had 50% sensitivity and 97.9% specificity. The risk of developing bladder cancer was higher in workers positive for either DNA ploidy or p300 biomarkers than in workers that were negative for both markers and risk was much higher in workers that were positive for both markers.[46] Fire fighters have been found to have higher chance of bladder cancer.[47] Early results from a bladder cancer screening study of fire-fighters in the city of San Francisco using dipstick hematuria and NMP22 test suggest that the projected rate of prevalence of bladder cancer is 1060 per 100 000 fire fighters, which is much higher than in general population in that geographical area (32 per 100 000) based in seer data.[48]
Recently Wu et al,[49] have used epidemiological and genetic data from a large case-control study to build a prediction model for bladder cancer. Significant risk factors in this epidemiological model included pack-years smoked and exposures to diesel, aromatic amines, dry-cleaning fluids, radioactive materials and arsenic. This model yielded a good discriminatory ability (area under curve: AUC 0.70) and when the genetic factor (mutagen sensitivity) was incorporated the AUC increased to 0.80. Although this model has to be validated in external population, epidemiological-genetic model perhaps would be an answer.
There are some ongoing screening studies on bladder cancer. A study at University of Texas Southwestern is assessing the screening value of the NMP22 Bladder Check test in patients aged >50 yrs with >10 pack-year smoking history. MD Anderson SPORE study is looking at men >55 years with a >40 pack-year smoking history, a 10-day dipstick test gets carried out and a dipstick positive test individual gets cystoscopy plus three marker tests (NMP22 Bladder check, UroVysion and ImmunoCyt).
Cost-effectiveness of screening
One of the important issues that gets consideration in early detection/screening is the cost-effectiveness of such a program. In absence of ‘a test’ that will be utilized for screening or early detection, it is no surprise that we do not have many studies that have worked on cost and cost-benefit ratio in bladder cancer in general. However, as the survival is better in non-muscle-invasive bladder cancer as against muscle-invasive bladder cancer and more importantly the treatment cost is different in these two conditions, early detection /screening that will detect early stage cancer is going to be cost-effective. Although there are no randomized studies on screening vs. no screening in bladder cancer, statistical models can be used looking at the cost-effectiveness of screening in bladder cancer. Lotan et al, recently studied the cost-effectiveness of using NMP22 in screening bladder cancer. They incorporated NMP22 test Bladder Check testing in a cost analysis model of bladder cancer. They found that screening high-risk individuals for bladder cancer using NMP22 could result in saving of $101,000 per 3 life years saved.[50] Using a Markov model, they found that in a population with >1.6% cancer incidence, screening with NMP22 Bladder Check would be cost-effective. Svatek et al, also looked into the economic impact of screening bladder cancer using bladder tumor markers.[51] The authors constructed a decision tree analysis to evaluate the total cost of screening a low and high-risk population for bladder cancer using NMP22.The cost per bladder cancer detected was $783,913 for age of 50-59 years, $269,028 for age of 60-69 years and $139,305 for age of 70-79 years in all men regardless the degree of risk. They found that screening only patients who are at high risk for bladder cancer (annual incidence 6%) would yield a cost per cancer detected of $3.310. The authors felt that application of NMP22 to entire population would not be cost-effective, application to appropriate high risk could achieve cost per cancer detected comparable to currently used screening program for prostate, colon and breast cancer.
So, what is the way out?
The incidence of bladder cancer has certainly increased in the last two decades. Bladder cancer has been occupying the fourth position consistently in the incidence of cancer in the USA since 2000-2009.[1] Annual incidence has increased from 6% in 2000 to 7% in 2009. The estimated new cases (both men and women) were 53,200 in year 2000, in year 2005 there were 63,210 new cases and in 2009 the estimated new cases were 70,980 with 14.330 estimated deaths[1] With such a rise in incidence and estimated deaths in a fourth leading cancer, are we not going to think about early detection and screening?
We have discussed in length about what tools we have in order to have early detection and screening program for bladder cancer. We have convincing evidence that the incidence of bladder cancer is high and so are deaths due to bladder cancer. We also have evidence suggesting that delay in the diagnosis and treatment does matter. We have a number of biomarkers that have been extensively studied in clinical practice. Currently we may not have an ideal marker, but we have some that have potential in terms of diagnosis, surveillance and screening.[24,52] I think we should begin early detection and screening program with what we have.
With whatever controversy we have around PSA, this marker has made a significant difference in the way which prostate cancer is diagnosed, treated and followed up. The clouds of controversy are still around about its effectiveness in screening,[53] but many guidelines have recommended its use with proper counseling and public education. (AUA guidelines in prostate cancer screening and PSA)
Can these existing markers be ‘PSA for bladder cancer’? This was first highlighted by Soloway.[54] Soloway reviewed the potentials of various markers and thought that there could be a place for such a marker in early detection and screening of bladder cancer. The author emphasized the fact that high percentage of patients with life-threatening bladder cancer (muscle invasive) do not have a history of TCC and the initial diagnosis is invasive bladder cancer, a similar situation that we faced 20 years ago for prostate cancer. As PSA has done a turnaround in prostate cancer, a similar marker for bladder cancer can change the things for better for bladder cancer. Similar view has been echoed by Konety et al,[21] Lokeshwar et al.[18,24]
PSA is not an ideal marker for carcinoma of the prostate, still it is widely used. We have accepted this marker, as this is the best we have. Then why can not we make use of markers such as UroVysion, NMP22 or telomerase who are the better performers amongst the biomarkers we have. (Ref Table 1). May be, we could use combination tests such as UroVysion +cytology, NMP22 + cytology to improve the yield. We do variety of maneuvers for prostate cancer by doing % free PSA, PSA velocity, PSA density to improve the performance of PSA, a better yield and avoid unnecessary biopsies. A combination of DRE and PSA, DRE, TRUSP and PSA is another maneuver to diagnose early prostate cancer. If this is possible in prostate cancer, why not try similar mechanisms that would increase the positive yield in bladder cancer? May be, as suggested by Halling et al,[37] we can do a stepwise program: Step 1-dipstick urine test and step 2- UroVysion + cytology or NMP22 with cytology. Imaging such as ultrasonography also could be also added in such program in step 2 like TRUSP in prostate cancer.
Questions that are raised while considering the use of a biomarker or its use in screening program are - would this screening test lead to further unnecessary testing and over-treatment? Would this be cost-effective and improve the survival?[55] I do not think a screening program for bladder cancer would lead to unnecessary testing and over treatment. Further testing could be in the form of cystoscopy and imaging that has very low or negligible morbidity. Similarly there is unlikely to be any over-treatment based on screening program for bladder cancer. Cost-effectiveness issue has been discussed earlier[50,51] and as discussed before early treatment for bladder cancer certainly improves the outcome.[41,42]
Patients with bladder cancer are also keen to have a ‘perfect test’ that will not miss their cancer. In a study by Yossepowitch and Herr et al, patients with bladder cancer expected a sensitivity of >90% of a test if that is used to replace or forgo cystoscopy. So, clearly there is a demand for a test than can replace/avoid an invasive test like cystoscopy.[56] Public education and awareness of bladder cancer is extremely important if we want to be successful in early detection and screening program for bladder cancer. The Bladder Cancer Advocacy Network was launched in 2005 as the first national patient advocacy organization dedicated to the advancement of bladder cancer education, public awareness and research related to the prevention, diagnosis and treatment of bladder cancer in USA.[57] It has been noted that in USA the funding that bladder cancer received in the fiscal year 2007 was just $24 million, a figure well below the four most common cancers, each of those received $200 million,[57] and research budget for bladder cancer is declining since 2002 and is the lowest for bladder cancer. May be more public education and awareness of bladder cancer would change the scenario and there need for early detection /screening will be emphasized.
There is a clear need for the early detection/screening program for bladder cancer knowing its incidence and mortality. It is not right to wait for an ideal marker for the bladder cancer, we have to use what we have best (UroVysion, NMP22) either solo or in combination with cytology, may be in a stepwise program. This is likely to improve the survival and quality of life in bladder cancer patients. We may have to have multi-institutional trials to prove this point, but clearly we have to start somewhere and though already late this is the right time for it.
CONCLUSIONS
Bladder cancer is the fourth commonest cancer in men, the annual death rate from this disease is significant and every year there is an increase in its incidence globally. The prognosis of bladder cancer is stage and grade dependent; lower the stage (T2 or less) better is the survival. Delay in the diagnosis and treatment does alter the overall outcome. Therefore, there is a clear need for early detection of bladder cancer and screening program. Although we do not have an ideal marker for bladder cancer, it's high time we maximize the potential of markers such as UroVysion, NMP22 along with cytology to start such program. May be as a first step the early detection and screening program could be started on high-risk population It is not worth waiting till we find the best marker, it would be unfair to our patients. The fear of unnecessary tests and treatment in bladder cancer after its detection in screening program is without any substance. The cost-effectiveness of such a program is certainly comparable to that is used for colon or breast and for that matter prostate as well.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Thalmann GN, Stein JP. Outcomes of radical cystectomy. BJU Int. 2008;102:1279–88. doi: 10.1111/j.1464-410X.2008.07971.x. [DOI] [PubMed] [Google Scholar]
- 4.Raghavan D, Shipley WU, Garnick MB, Russell PJ, Richie JP. Biology and management of bladder cancer. N Engl J Med. 1990;332:1129–38. doi: 10.1056/NEJM199004193221607. [DOI] [PubMed] [Google Scholar]
- 5.Lokeshwar VB, Soloway MS. Current bladder tumour tests: Does their projected utility fulfill clinical necessity? J Urol. 2001;165:1067–77. [PubMed] [Google Scholar]
- 6.Wallace DM, Bryan RT, Dunn JA, Begum G, Bathers S. West Midlands Urological Research Group. Delay and survival in bladder cancer. BJU Int. 2002;89:868–78. doi: 10.1046/j.1464-410x.2002.02776.x. [DOI] [PubMed] [Google Scholar]
- 7.Stöckle M, Alken P, Engelmann U, Jacobi GH, Riedmiller H, Hohenfellner R. Radical cystectomy -often too late? Eur Urol. 1987;13:361–7. doi: 10.1159/000472824. [DOI] [PubMed] [Google Scholar]
- 8.Singh R, Saleemi A, Walsh K, Popert R, O’Brien T. Near misses in bladder cancer-an airline safety approach in urology. Ann R Coll Surg Engl. 2003;85:378–81. doi: 10.1308/003588403322520717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson EK, Daignault S, Zhang Y, Lee CT. Patterns of hematuria referral to urologists: Does a gender disparity exist? Urology. 2008;72:498–502. doi: 10.1016/j.urology.2008.01.086. [DOI] [PubMed] [Google Scholar]
- 10.Nieder AM, Manoharan M, Vyas S. Evaluation and work-up of hematuria among primary care physicians in Miami-Dade County: An anonymous questionnaire based survey. J Urol. 2007;177:357. [Google Scholar]
- 11.Bassi P, Ferrante GD, Piazza N, Spinadin R, Pappagallo G, Pagano F. Prognostic factors of outcome after radical cystectomy for bladder cancer: A retrospective study of a homogeneous patient cohort. Urology. 1999;161:1494–7. [PubMed] [Google Scholar]
- 12.Shariat SF, Karakiewicz PI, Palapattu GS, Lotan Y, Rogers CG, Amiel GE, et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: A contemporary series from the Bladder Cancer research Consortium. J Urol. 2006;176:2414–22. doi: 10.1016/j.juro.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Lotan Y, Roehrborn CG. Sensitivity and specificity of commonly available bladder tumour markers versus cytology: Results of a comprehensive literature review and Meta analyses. Urology. 2003;61:109–18. doi: 10.1016/s0090-4295(02)02136-2. [DOI] [PubMed] [Google Scholar]
- 14.Shariat SF, Zippe C, Lüdecke G, Boman H, Sanchez-Carbayo M, Casella R, et al. Nomograms including nuclear matrix protein 22 for prediction of disease recurrence and progression in patients with Ta,T1 or CIS transitional cell carcinoma of the bladder. J Urol. 2005;173:1518–25. doi: 10.1097/01.ju.0000154696.48217.75. [DOI] [PubMed] [Google Scholar]
- 15.Greene KL, Konety B. Urinary markers for bladder cancer.AUA update series. Vol. 26. Linthicum, MD: American Urological association, Education and Research Inc; 2007. pp. 325–35. [Google Scholar]
- 16.Mengual L, Marín-Aguilera M, Ribal MJ, Burset M, Villavicencio H, Oliver A, et al. Clinical utility of fluorescent in situ hybridization for the surveillance of bladder cancer patients treated with bacillus Calmette-Guerin therapy. Eur Urol. 2007;52:752–9. doi: 10.1016/j.eururo.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Song MJ, Lee HM, Kim SH. Clinical usefulness of fluorescence in situ hybridization for diagnosis and surveillance of bladder cancer. Cancer Genet Cytogenet. 2010;198:144–50. doi: 10.1016/j.cancergencyto.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Lokeshwar V, Hautmann S, Schroeder G. HA-Hase test: An accurate test for monitoring bladder cancer recurrence and follow up. J Urol. 2000;163:132. [Google Scholar]
- 19.Wiener HG, Mian C, Haitel A, Pycha A, Schatzl G, Marberger M. Can urine bound diagnostic tests replace cystoscopy in the management of bladder cancer? J Urol. 1998;159:1876–80. doi: 10.1016/S0022-5347(01)63184-7. [DOI] [PubMed] [Google Scholar]
- 20.Konety BR. Molecular markers in bladder cancer: A critical appraisal. Urol Oncol. 2006;24:326–37. doi: 10.1016/j.urolonc.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Konety BR, Lotan Y. Urolthelial bladder cancer: Biomarkers for detection and screening. BJU Int. 2008;102:1234–41. doi: 10.1111/j.1464-410X.2008.07965.x. [DOI] [PubMed] [Google Scholar]
- 22.Olaf PJ, Vrooman J, Witjes A. Urinary Markers in bladder cancer. Eur Urol. 2008;53:909–16. doi: 10.1016/j.eururo.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Glas AS, Roos D, Deutekom M, Zwinderman AH, Bossuyt PM, Kurth KH. Tumor markers in the diagnosis of primary bladder cancer: A systematic review. J Urol. 2003;169:1975–82. doi: 10.1097/01.ju.0000067461.30468.6d. [DOI] [PubMed] [Google Scholar]
- 24.Lokeshwar VB, Habuchi T, Grossman HB, Murphy WM, Hautmann SH, Hemstreet GP, 3rd, et al. Bladder tumour markers beyond cytology: International consensus panel on bladder tumour markers. Urology. 2005;66:35–63. doi: 10.1016/j.urology.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 25.Robinson VL, Porter M, Messing E, Fradet Y, Kamat AM, Lotan Y. BCAN Think Tank session 2: Molecular detection of bladder cancer: The path to progress. Urol Oncol. 2010;28:334–7. doi: 10.1016/j.urolonc.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 26.Sarosdy MF, Schellhammer P, Bokinsky G, Kahn P, Chao R, Yore L, et al. Clinical evaluation of a multi-target fluorescent in situ hybridization assay for detection of bladder cancer. J Urol. 2002;168:1950–4. doi: 10.1016/S0022-5347(05)64270-X. [DOI] [PubMed] [Google Scholar]
- 27.Friedrich MG, Hellstern A, Hautmann SH, Graefen M, Conrad S, Huland E, et al. Clinical use of urinary markers for the detection and prognosis of bladder carcinoma: A comparison of immunocytology with monoclonal antibodies against Lewis X and 486p3/12 with the BTA STAT and NMP22 tests. J Urol. 2002;168:470–4. doi: 10.1016/s0022-5347(05)64660-5. [DOI] [PubMed] [Google Scholar]
- 28.Van Tiborg AA, Bangma CH, Zwarthoff EC. Bladder cancer biomarkers and their role in surveillance and screening. Int J Urol. 2009;16:23–30. doi: 10.1111/j.1442-2042.2008.02174.x. [DOI] [PubMed] [Google Scholar]
- 29.Boman H, Hedelin H, Holmang S. Four bladder tumour markers have a disappointingly low sensitivity for small size and low-grade recurrence. J Urol. 2002;167:80–3. [PubMed] [Google Scholar]
- 30.Vriesema JL, Atsma F, Kiemeney LA, Peelen WP, Witjes JA, Schalken JA. Diagnostic efficacy of the ImmunoCyt test to detect superficial bladder cancer recurrence. Urology. 2001;58:367–71. doi: 10.1016/s0090-4295(01)01217-1. [DOI] [PubMed] [Google Scholar]
- 31.Lodde M, Mian C, Negri G, Berner L, Maffei N, Lusuardi L, et al. Role of uCyt+ in the detection and surveillance of urothelial carcinoma. Urology. 2003;61:243–7. doi: 10.1016/s0090-4295(02)02073-3. [DOI] [PubMed] [Google Scholar]
- 32.Piaton E, Daniel L, Verriele V, Dalifard I, Zimmermann U, Renaudin K, et al. Improved detection of urothelial carcinomas with fluorescence immunocytochemistry (uCyt+assay) and urine cytology: Results of a French prospective multicenter study. Lab Invest. 2003;83:845–52. doi: 10.1097/01.lab.0000074893.70675.2e. [DOI] [PubMed] [Google Scholar]
- 33.Mian C, Maier K, Comploj E, Lodde M, Berner L, Lusuardi L, et al. uCyt+/immunoCyt in the detection of recurrent urothelial carcinoma: An update on 1991 analyses. Cancer. 2006;108:60–5. doi: 10.1002/cncr.21712. [DOI] [PubMed] [Google Scholar]
- 34.Pfister C, Chautard D, Devonec M, Perrin P, Chopin D, Rischmann P, et al. Immunocyt test improves the diagnostic accuracy of urinary cytology: Results of a French multicenter study. J Urol. 2003;169:921–4. doi: 10.1097/01.ju.0000048983.83079.4c. [DOI] [PubMed] [Google Scholar]
- 35.Messing EM, Teot L, Korman H, Underhill E, Barker E, Stork B, et al. Performance of urine test in patients monitored for recurrence of bladder cancer: A multicenter study in United States. J Urol. 2005;174:1238–41. doi: 10.1097/01.ju.0000173918.84006.4d. [DOI] [PubMed] [Google Scholar]
- 36.Lodde M, Mian C, Comploj E, Palermo S, Longhi E, Marberger M, et al. uCy+test: alternative to cystoscopy for less –invasive follow up of patients with low risk of urothelial carcinoma. Urology. 2006;67:950–4. doi: 10.1016/j.urology.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 37.Halling KC, King W, Sokolova IA, Karnes RJ, Meyer RG, Powell EL, et al. A comparison of BTA stat, hemoglobin dipstick, telomerase and Vysis UroVysion assays for the detection of urothelial carcinoma in urine. J Urol. 2002;167:2001–6. [PubMed] [Google Scholar]
- 38.Britton JP, Dowell AC, Whelan P, Harris CM. A community study of bladder cancer screening by the detection of occult urinary bleeding. J Urol. 1992;148:788–90. doi: 10.1016/s0022-5347(17)36720-4. [DOI] [PubMed] [Google Scholar]
- 39.Whelan P, Britton JP, Dowell AC. Three-year follow up of bladder tumours found on screening. Br J Urol. 1993;72:893–6. doi: 10.1111/j.1464-410x.1993.tb16292.x. [DOI] [PubMed] [Google Scholar]
- 40.Mayfield MP, Whelan P. Bladder tumours detected on screening: Results at 7 years. Br J Urol. 1998;82:825–8. doi: 10.1046/j.1464-410x.1998.00879.x. [DOI] [PubMed] [Google Scholar]
- 41.Messing EM, Young TB, Hunt VB, Newton MA, Bram LL, Vaillancourt A, et al. Hematuria home screening: Repeat testing results. J Urol. 1995;154:57–61. doi: 10.1016/s0022-5347(01)67224-0. [DOI] [PubMed] [Google Scholar]
- 42.Messing EM, Madeb R, Young T, Gilchrist KW, Bram L, Greenberg EB, et al. Long term outcome of hematuria home screening for bladder cancer in men. Cancer. 2006;107:2173–9. doi: 10.1002/cncr.22224. [DOI] [PubMed] [Google Scholar]
- 43.Silverman DT, Hartge P, Morrison AS, Devesa SS. Epidemiology of bladder cancer. Hematol Oncol Clin North Am. 1992;6:1–30. [PubMed] [Google Scholar]
- 44.Lotan Y, Shariat SF. NMP 22 Study Group. Impact of risk factors on the performance of the nuclear matrix protein 22 point of care test for bladder cancer detection. BJU Int. 2008;101:1362–7. doi: 10.1111/j.1464-410X.2008.07473.x. [DOI] [PubMed] [Google Scholar]
- 45.Sarosdy MF, Kahn PR, Ziffer MD, Love WR, Barkin J, Abara EO, et al. Use of a multitarget fluorescent in situ hybridization assay to diagnose bladder cancer in patients with hematuria. J Urol. 2006;176:44–7. doi: 10.1016/S0022-5347(06)00576-3. [DOI] [PubMed] [Google Scholar]
- 46.Hemstreet GP, 3rd, Yin S, Ma Z, Bonner RB, Bi W, Rao JY, et al. Biomarker risk assessment and bladder cancer detection in a cohort exposed to benzidine. J Natl Cancer Inst. 2001;93:427–36. doi: 10.1093/jnci/93.6.427. [DOI] [PubMed] [Google Scholar]
- 47.Ma F, Fleming LE, Lee DJ, Trapido E, Gerace TA, Lai H, et al. Mortality in Florida professional firefighters, 1972 to 1999. Am J Ind Med. 2005;47:509–17. doi: 10.1002/ajim.20160. [DOI] [PubMed] [Google Scholar]
- 48.Greene KG, Konety BR, Stroller M. Results from the San Frasisco Firefighters bladder cancer screening study. J Urol. 2008;179:323. [Google Scholar]
- 49.Wu X, Lin J, Grossman HB, Huang M, Gu J, Etzel CJ, et al. Projecting individualized probabilities of developing bladder cancer in white individuals. J Clin Oncol. 2007;25:4974–81. doi: 10.1200/JCO.2007.10.7557. [DOI] [PubMed] [Google Scholar]
- 50.Lotan Y, Svatek RS, Sagalowsky AI. Should we screen for bladder cancer in high –risk population? A Cost per life year saved analysis. Cancer. 2006;107:982–90. doi: 10.1002/cncr.22084. [DOI] [PubMed] [Google Scholar]
- 51.Svatek RS, Sagalowsky AI, Loan Y. Economic impact of screening for bladder cancer using bladder tumour markers: A decision analysis. Urol Oncol. 2006;24:338–43. doi: 10.1016/j.urolonc.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 52.Simon MA, Lokeshwar VB, Soloway MS. Current bladder cancer tests: Unnecessary or beneficial? Crit Rev Oncol Hematol. 2003;47:91–107. doi: 10.1016/s1040-8428(03)00074-x. [DOI] [PubMed] [Google Scholar]
- 53.Murphy AM, McKiernan JM, Olsson CA. Controversies in prostate cancer screening. J Urol. 2004;172:1822–4. doi: 10.1097/01.ju.0000140500.65341.9a. [DOI] [PubMed] [Google Scholar]
- 54.Soloway M. Do we have a prostate specific antigen for bladder cancer? J Urol. 1999;161:447–8. doi: 10.1016/s0022-5347(01)61920-7. [DOI] [PubMed] [Google Scholar]
- 55.NCI. NCI Cancer Screening overview. [last accessed on 2010Jun]. Available from: http://www.cancer.gov/cancerinfo/pdg/screening/overview .
- 56.Yossepowitch O, Herr HW, Donat SM. Use of urinary biomarkers for bladder cancer surveillance: Patient perspectives. J Urol. 2007;177:1277–82. doi: 10.1016/j.juro.2006.11.066. [DOI] [PubMed] [Google Scholar]
- 57.Joel DeCastro G, Steinberg D. Are we making significant progress in the diagnosis and management of bladder cancer? J Urol. 2010;183:1667–8. doi: 10.1016/j.juro.2010.02.2376. [DOI] [PubMed] [Google Scholar]


