Abstract
A systematic review of the literature on perioperative morbidity (POM) was done using Medline software with a combination of keywords like mortality, morbidity, and complications. In addition, we review the analysis of our hospital data of 261 Radical cystectomies (RCs) performed in an 11-year period and our latest clinical pathway for RC. Age range in our series was 50 to 81 years with 240 males and 21 females. RCs were performed by intraperitoneal method in 172 patients and by our extraperitoneal (EP) method in 89 patients. Urinary diversion was ileal conduit in 159 patients and neobladder in 102 patients. Blood loss ranged between 500 and 1500 ccs. Postoperative mortality occurred in eight patients (3%). Among the other early post-op complications, major urinary leak was seen in nine and minor in 11, requiring PCN in five patients and reoperation in four patients. Bowel leak or obstruction was seen in six and four patients, respectively, requiring reoperation in six patients. EP RC in our series showed some benefit in reduction of POM. The mortality of RC has declined but the POM still ranges from 11 to 68%, as reported in 23 series (1999-2008) comprising of 14 076 patients. Various risk factors leading to POM and some corrective measures are discussed in detail. However, most of these series are retrospective and lack standard complication reporting, which limits the comparison of outcomes. Various modifications in open surgical technique and laparoscopic and Robotic approaches are aimed at reduction in mortality and POM of RC.
Keywords: Clinical pathways, extraperitoneal approach, ileal conduit, morbidity, mortality, neobladder, radical cystectomy, surgery
INTRODUCTION
Radical cystectomy (RC) is a technically challenging operation and hence prompt postoperative recovery, short hospital stay, and reduction in morbidity and mortality are difficult to achieve.[1–5] The past two decades have witnessed several publications reporting the reduction in perioperative mortality of RC, reflecting the success of a multidisciplinary approach.[6–25] Before 1990, the perioperative mortality of RC ranged from 2.4 to 15% in large series (>100 patients) which is reduced to 0 to 3.9% during the past decade.[6–10,26–39] However, the perioperative morbidity (POM) has remained stable at 11 to 68%. Further late morbidity in contemporary series has been 19 to 58%.[2,26,35,36,39] All these authors have expressed their concern about the minimal impact on the incidence of POM which has defied modern technological advances. As a step, clinical care pathways have been designed to reduce postoperative ileus, early ambulation, and earlier discharge from the hospital.[25,40–45]
The current review will focus on the literature of POM in the contemporary era and will highlight our clinical pathway and experience. Furthermore, it is our endeavor to report the various factors which have helped us in reducing POM and mortality of open RC both by conventional (transperitoneal approach) and the modified (extraperitoneal [EP] retrograde approach) methods in our clinical setting and compare our schema of postoperative clinical care pathway with others.[46] Lastly, laparoscopic (LRC) or robot-assisted LRC are still evolving.[47–50]
MATERIALS AND METHODS
A systematic review of the literature (Medline) was performed using a combination of keywords like POM and mortality after cystectomy, early complications, and cystectomy. Hospital records of the 261 RCs performed till 1998 were reviewed and the data were analyzed in terms of perioperative complications and outcome. Of the 261, a cohort of 102 neobladder has been reported in 2003.[35] In 2002, we appended our patient selection criteria and postoperative clinical care pathways [Table 1].
Table 1.
Clinical care pathway (our institution)

RESULTS
Table 2 gives the data of 261 RCs performed in an 11-year period between January 1988 and December 1998. Most of patients were 50 to 70 years and only 21 patients were >70 years of age. Of the 261 patients, 240 were men and 21 women. Operative procedure was RC with ileal conduit in 159 patients while 102 a neobladder. All 24 women had an ileal conduit. RC was performed by standard intraperitoneal (IP) method in 172 patients, whereas remaining 89 patients had our EP retrograde approach. The hospital stay ranged from 15 to 60 days, with a median of 18. Perioperative mortality was 8/261 (3%). Blood loss was 750 to 1500 ml in majority, however, EP group showed a reduction of blood loss. Early complications occurred in 40 patients (15%) and were less in the EP group. Details of the complications and management are given in Table 2.
Table 2.
Radical cystectomy - our data with literature review
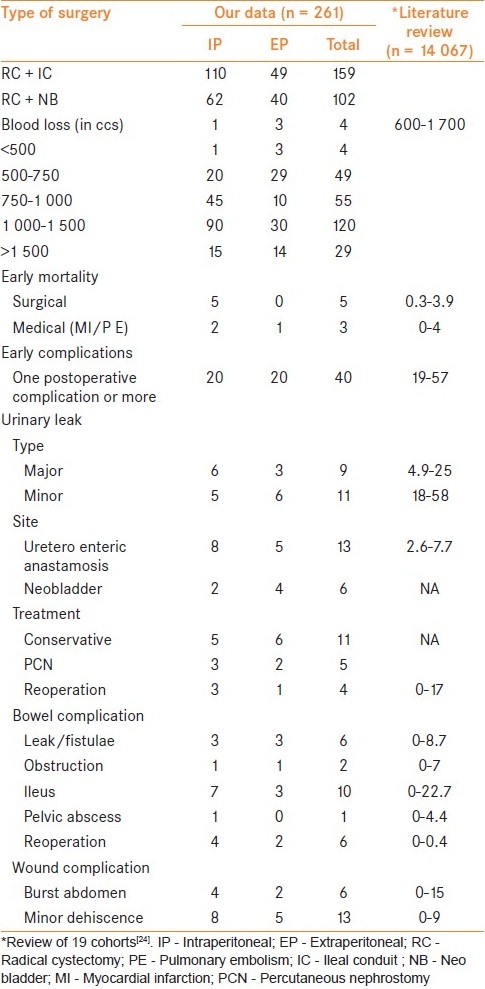
Literature review shows that between 1999 and 2009, a total of 23 series comprising 14 067 patients (series ranging from 96 to 6 577 patients) have been reported addressing early complications of RC [Table 2].[1–3,5,13,19,22,25–40] This also includes our previous report of 102 patients with a neobladder.[35] Table 2 summarizes the details of these series. All series have used different parameters to report the data and majority are retrospective. The only observation is that historically, before 1990, the mortality in larger series (>100patients) was 2.4 to 15% and has reduced to 0 to 3.4% in this decade, but the early morbidity which was 28 to 42% in that period has remained 11 to 68%.[6–10,24]
DISCUSSION
RC is a complex operation and has avoidable POM. Some authors have reported measures to help reduce or prevent them.[6–10] Various technical improvements in the surgical and anesthesia techniques, multi disciplinary approach for correction/control of comorbidities and early postoperative rehabilitation have produced salutary effect in reducing the mortality, but their impact on POM is at the best moderate.[11]
This review is aimed at focusing on some of the issues. They are patient selection criteria, surgical technique and its refinements, individual surgeon's preferences and skills, and institutional volumes. Most series are retrospective and lack standard reporting criteria.[11] Classification of the POM reporting is inconsistent in the majority of cohorts, hence it is difficult to derive conclusions.[4,12–14] At the most, meta-analysis can only provide suggestions rather than guidelines.[24] Few have reported prospective studies using Clavien system of complication reporting but there is need for standard guidelines.[11,13–16,18]
Patient selection criteria
Age >70 years and being a female have been labeled as higher risk factors.[13,19–21] However, with proper selection and risk balancing, there is a beneficial impact on reduction in morbidity.[20]
Comorbidities (including high BMI) have been included under the ASA score in the literature; however, it is logical to believe that greater than two comorbidities are not contraindication of surgery but will necessitate optimum correction (in ASA>2) and preventive measures to reduce morbidity.[13] Here, multidisciplinary approach and clinical care pathways [Table 1] are important.
Prior pelvic surgery, radiotherapy (preoperative or complete), and neoadjuvant chemotherapy may lead to some technical difficulties during surgery and postoperative period, but with careful planning and modern technique, these can be managed.[13,40] Cystectomy as a primary therapy or after neoadjuvant chemotherapy for bulky or locoregionally advanced tumors has a significant risk of POM.[15,51–54] We strongly feel that these situations need a careful and judicious management for achieving satisfactory result.
Surgeon and hospital volume
In last 5 years, there has been some debate on the issue of surgical learning curve both at personnel and institutional level vis-a-vis surgical volume.[55–58] Although this is a complex subject, it is accepted that an individual or institution which handles 10 RC per year and has all facilities for major surgeries can continue to undertake RC. However, orthotopic neobladder or continent pouches should be handled at larger volume centers. In the Indian context, we believe that all the teaching and large private hospitals can undertake RC with acceptable results.
Surgical issues
Impact of optimum surgical technique on reduction of POM of RC depends on the following: (i) Preoperative factors; (ii) Anesthesia and perioperative anesthetic management; (iii) Clinical Care pathways; (iv) Operative Technique.
Renal failure (serum creatinine >2.5/dl) and poor nutritional status necessitate correction wherever possible to reduce complications.[14] Generally in these patients, urinary diversion with an ileal conduit is preferable over complex procedures like orthotopic bladder substitution.[40] Older patients with chronic bowel disease and with significant comorbidities are better suited for ileal conduit.
In addition to preanesthetic optimization of medical comorbidities (cardiac, pulmonary, diabetes), epidural anesthesia has favorably affected perioperative complications.[59,60] We routinely use epidural anesthesia either alone (in majority of cases) or in combination with general anesthesia. By this combined approach, we had significantly lowered blood loss and reduced postoperative ICU admissions. In patients with ASA 2 and 3, our anesthesia team institutes short-term ventilator support in PACU (Post Anesthesia Care Unit) till the vital parameters including temperature, electrolytes, hematocrit, and biochemical profile are stabilized.
Clinical care pathways are referred to enhanced recovery protocols with standardized perioperative plan of care.[25,40–45] Generally, these pathways are directed towards the standardization of antibiotic and analgesic therapy, bowel preparation, nutrition, and surgical drain management. Furthermore, they are used as a guide, and minor deviation may be required in a given clinical scenario. Table 1 shows our latest clinical care pathway. In keeping with the modern trend, we have used less aggressive bowel preparation in our practice in last few years, with early return of peristalsis and early oral alimentation.
Understanding of surgical anatomy of the pelvis and adequate control of the dorsal venous complex (DVC) have not only helped us in reducing the blood loss but also POM of RC.[61] Ten years ago, we reported our ‘Extra peritoneal retro grade technique of RC’ (1999).[46] Initially like the steps of radical prostatectomy, DVC and urethra are sectioned early after EP pelvic node dissection on either side. Later, the prostate with SV and bladder are dissected off the rectum and Denonvillier's fascia cranially without opening the peritoneum. All the vascular pedicles of the bladder are ligated and cut. Cystectomy is completed by incising the peritoneum after sectioning the ureters. The bowel segment with its vascular pedicle is isolated (both for conduit or neobladder) and the peritoneum is closed around the mesentery. This bowel segment lies extraperitoneally and can be reconstructed as a conduit or neobladder and the ureters are implanted. Thus, bowel anastamosis is IP, and urinary pouch or conduit is EP [Figure 1]. This EP approach has shown significant reduction in ileus, resulting in effective management of minor urinary leaks without intervention [Table 2]. We have not used this approach when the disease is beyond the pelvic cavity, that is, sigmoid colon, retro peritoneal nodes, and urachal tumors. Others have reported ante grade technique (EP) with successful results in terms of reduction in morbidity.[62,63] In women, we have recently used this technique for sparing the gynecological tract effectively to reduce POM and to improve quality of life [Figure 2].[64]
Figure 1.
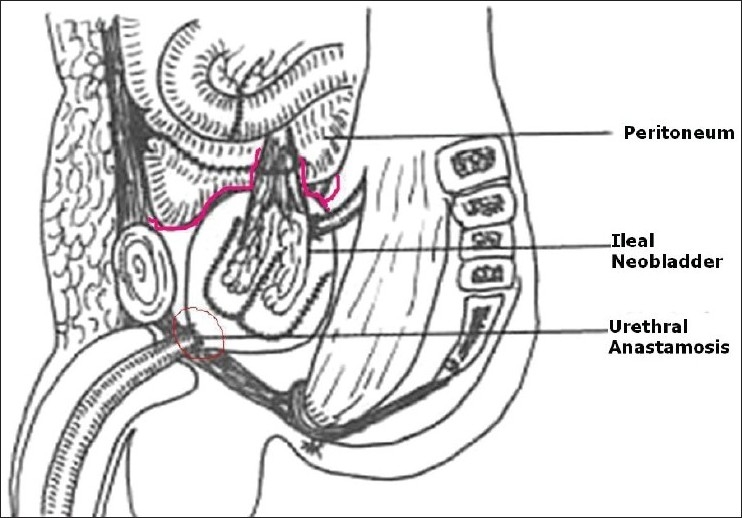
Extraperitoneal pouch in males 46
Figure 2.
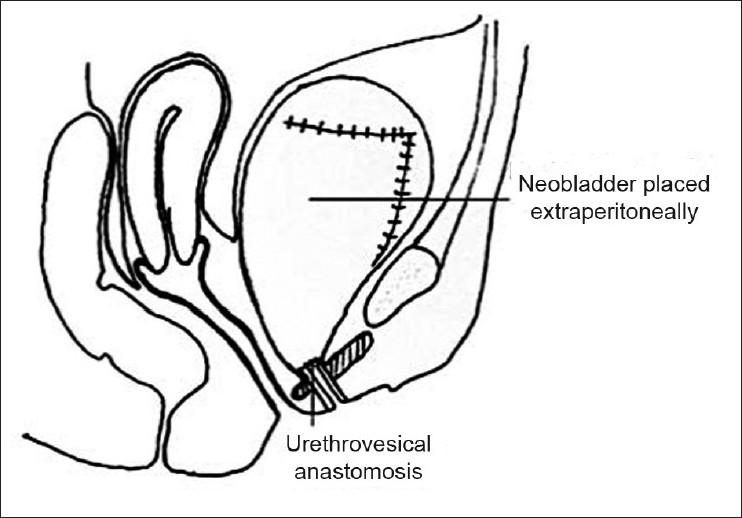
Gynec tract saving cystectomy in females 64
Perioperative complications
Detailed report of prevention and management of early and late complications following RC has been reported by Lawrentschuk et al.[24] We would like to briefly review some of the factors and our measures to prevent or reduce them.
Acute blood loss is common during or after RC and is difficult to predict.[1,3,5] In our setting, the majority of patients present with bulky disease and are nutritionally compromised, and hence require replacement of either whole blood or blood products intra and/or postoperatively [Table 2]. However with the EP approach, the blood loss has been reduced. Chang et al. in a prospective trial reported reduction of the blood loss with use of stapler device for controlling DVC and bladder pedicles, whereas others have advocated the use of the harmonic scalpel or ligasure.[23,65] Recently, we have used these new devices but cost constraints limit their use in our practice. In a recent review, LRC or robot-assisted LRC cystectomy has been shown to reduce the operative blood loss significantly and also the operative time.[66] We believe that these approaches will continue to expand and have great potential to be the preferred approach in future.
Meticulous dissection, proper hemostasis, and attention to vascular supply are the key issues in construction of the conduit or pouch and uretero-enteric anastamosis. Urinary leak is generally preceded by rise in pulse and temperature, toxic appearance, abdominal distension with excessive drainage, and reduced output and can be preempted by a high index of suspicion. Our EP approach which separates the bowel anastamosis from the reconstructed urinary segment has helped in reducing the urinary leak spreading into the peritoneal cavity progressing to sepsis and can be diagnosed easily by noting the increased drainage. Major leak can be managed by instituting measures like antibiotics, TPN, proper drainage of pelvic collection, and PCN. We routinely use stents across the uretero-enteric anastamosis and a suprapubic catheter in the neobladders. Both stents are brought out in the stoma bag in conduits and with SPC in pouches. Further, problem of mucus blockage is prevented by gentle irrigation of the stents and SPC washes. Mattei et al. in a controlled trial showed that stenting helps in the reduction of ileus, early pelvicalyceal dilatation, and metabolic acidosis.[67]
Shabsigh et al. proposed definition of Ileus as ‘Inability to tolerate solid food by postoperative day five, the need to place an NG tube or the need to stop oral intake due to abdominal distention, nausea or emesis.’[13] Modern concept of rapid recovery of small bowel motility and absorption within hours of surgery has propelled the early oral liquid diet, reducing the time of ileus. We believe that prolonged ileus could be due to a bowel or urinary leak and should be dealt with appropriately.
Recently, the use of staplers over hand-sewn enteric anastamosis has been shown to be superior in terms of reduction of operative time and morbidity.[68] Our preference is a hand-sewn anastamosis with acceptable results and cost benefit. Generally, management of bowel fistulae revolves around nutrition, diversion or reoperation, and treatment of sepsis.[69,70]
Routine use of appropriate combination antibiotic therapy pre and perioperatively and strict adherence to the clinical care pathway minimizes the incidence of sepsis. Pulmonary and peripheral vascular complications (DVT) can be reduced by active pulmonary physiotherapy, low molecular weight heparin, and intermittent pneumatic compression stocking (ICSI) during the early postoperative period. Similar measures are popular worldwide in most centers.[1,23,25,65] Lastly, early ambulation and frequent change of position during critical stages can prevent bed sores.
We perform meticulous closure of the fascia and rectus sheath using nonabsorbable sutures. A subcutaneous suction drain is placed in obese patients. Skin and subcutaneous layers are closed properly. Postoperatively, the wound is inspected regularly and early drainage of seroma is performed. The role of tension sutures is debatable.[70,71] However, early repair of wound dehiscence helps in preventing hernia.[72]
Prolonged pelvic drainage during the postoperative period is often due to a urinary leak. However, when the urine leak is ruled out, lymphorrhea could be preexisting filarial (sub clinical) disease in our practice and needs patience and symptomatic treatment. Lymphocoeles are generally detected after first or second follow-up visit, and may require percutaneous drainage.[30]
CONCLUSIONS
A lot of emphasis is given to the reduction of POM of RC. Newer techniques like minimal invasive (Lap) or Robotic RC are aimed at the same goal. However, both these approaches have a steep learning curve and cost issues. On the other hand, open RC with refinements of procedures has stood the test of time. Our EP approach with early DVC ligation, under vision dissection of the prerectal and paravesical region and minimal handling of bowel, has reduced ileus and associated morbidity. In case ureteric leak occurs, it can be managed with less invasive approaches.
RC due to its complexity necessitates a team approach with well-trained surgeon, anesthetist, intensivist, stoma care specialist, nutritionist, and physiotherapist. In India, traveling to ‘high volume centers’ may be difficult; hence, it is advisable for urological surgeon to attain the learning curve and form a team approach to reduce the POM of RC.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lee CT, Dunn RL, Chen BT, Joshi DP, Sheffield J, Montie JE. Impact of body mass index on radical cystectomy. J Urol. 2004;172:1281–5. doi: 10.1097/01.ju.0000138785.48347.aa. [DOI] [PubMed] [Google Scholar]
- 2.Meyer JP, Blick C, Arumainayagam N, Hurley K, Gillatt D, Persad R, et al. A three-centre experience of orthotopic neobladder reconstruction after radical cystectomy: revisiting the initial experience, and results in 104 patients. BJU Int. 2009;103:680–3. doi: 10.1111/j.1464-410X.2008.08204.x. [DOI] [PubMed] [Google Scholar]
- 3.Cookson MS, Chang SS, Wells N, Parekh DJ, Smith JA., Jr Complications of radical cystectomy for non muscle invasive disease: comparison with muscle invasive disease. J Urol. 2003;169:101–4. doi: 10.1016/S0022-5347(05)64045-1. [DOI] [PubMed] [Google Scholar]
- 4.Colombo R. Editorial comment on: Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009;55:175–6. doi: 10.1016/j.eururo.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 5.Maffezzini M, Campodonico F, Canepa G, Gerbi G, Parodi D. Current perioperative management of radical cystectomy with intestinal urinary reconstruction for muscle-invasive bladder cancer and reduction of the incidence of postoperative ileus. Surg Oncol. 2008;17:41–8. doi: 10.1016/j.suronc.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Charbit L, Beurton D, Cukier J. Mortality and morbidity after total cystectomy for cancer [in French] J Urol (Paris) 1984;90:39–46. [PubMed] [Google Scholar]
- 7.Johnson DE, Lamy SM. Complications of a single stage radical cystectomy and ileal conduit diversion: review of 214 cases. J Urol. 1977;117:171–3. doi: 10.1016/s0022-5347(17)58385-8. [DOI] [PubMed] [Google Scholar]
- 8.Skinner DG, Crawford ED, Kaufman JJ. Complications of radical cystectomy for carcinoma of the bladder. J Urol. 1980;123:640–3. doi: 10.1016/s0022-5347(17)56073-5. [DOI] [PubMed] [Google Scholar]
- 9.Thomas DM, Riddle PR. Morbidity and mortality in 100 consecutive radical cystectomies. Br J Urol. 1982;54:716–9. doi: 10.1111/j.1464-410x.1982.tb13632.x. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan JW, Grabstald H, Whitmore WF., Jr Complications of ureteroileal conduit with radical cystectomy: review of 336 cases. J Urol. 1980;124:797–801. doi: 10.1016/s0022-5347(17)55669-4. [DOI] [PubMed] [Google Scholar]
- 11.Donat SM. Standards for surgical complication reporting in urologic oncology: time for a change. Urology. 2007;69:221–5. doi: 10.1016/j.urology.2006.09.056. [DOI] [PubMed] [Google Scholar]
- 12.Hautmann RE. Editorial comment on: defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009;55:174. doi: 10.1016/j.eururo.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 13.Shabsigh A, Korets R, Vora KC, Brooks CM, Cronin AM, Savage C, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009;55:164–76. doi: 10.1016/j.eururo.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 14.Dmochowski R, Scarpero H. Surgical outcomes reporting-closer to reality. Eur Urol. 2007;52:1306–8. doi: 10.1016/j.eururo.2007.04.057. [DOI] [PubMed] [Google Scholar]
- 15.Hollenbeck BK, Miller DC, Taub D, Dunn RL, Khuri SF, Henderson WG, et al. Identifying risk factors for potentially avoidable complications following radical cystectomy. J Urol. 2005;174:1231–7. doi: 10.1097/01.ju.0000173923.35338.99. [DOI] [PubMed] [Google Scholar]
- 16.Novara G, De Marco V, Aragona M, Boscolo-Berto R, Cavalleri S, Artibani W, et al. Complications and mortality after radical cystectomy for bladder transitional cell cancer. J Urol. 2009;182:914–21. doi: 10.1016/j.juro.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 17.Clavien PA, Camargo CA, Jr, Croxford R, Langer B, Levy GA, Greig PD. Definition and classification of negative outcomes in solid organ transplantation. Application in liver transplantation. Ann Surg. 1994;220:109–20. doi: 10.1097/00000658-199408000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhoads KF, Konety BM, Dudley RA. Performance measurement, public reporting, and pay-for-performance. Urol Clin North Am. 2009;36:37–48. doi: 10.1016/j.ucl.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Hollenbeck BK, Taub DA, Miller DC, Dunn RL, Montie JE, Wei JT. The regionalization of radical cystectomy to specific medical centers. J Urol. 2005;174:1385–9. doi: 10.1097/01.ju.0000173632.58991.a7. [DOI] [PubMed] [Google Scholar]
- 20.Gamé X, Soulié M, Seguin P, Vazzoler N, Tollon C, Pontonnier F, Plante P. Radical cystectomy in patients older than 75 years: assessment of morbidity and mortality. Eur Urol. 2001;39:525–9. doi: 10.1159/000052498. [DOI] [PubMed] [Google Scholar]
- 21.Lee KL, Freiha F, Presti JC, Jr, Gill HS. Gender differences in radical cystectomy: complications and blood loss. Urology. 2004;63:1095–9. doi: 10.1016/j.urology.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 22.Clark PE, Stein JP, Groshen SG, Cai J, Miranda G, Lieskovsky G, et al. Radical cystectomy in the elderly: comparison of clinical outcomes between younger and older patients. Cancer. 2005;104:36–43. doi: 10.1002/cncr.21126. [DOI] [PubMed] [Google Scholar]
- 23.Boström PJ, Kössi J, Laato M, Nurmi M. Risk factors for mortality and morbidity related to radical cystectomy. BJU Int. 2009;103:191–6. doi: 10.1111/j.1464-410X.2008.07889.x. [DOI] [PubMed] [Google Scholar]
- 24.Lawrentschuk N, Colombo R, Hakenberg OW, Lerner SP, Mansson W, Sagalowsky A, et al. Prevention and Management of Complications following Radical Cystectomy for Bladder Cancer. Eur Urol. 2010;57:991–1001. doi: 10.1016/j.eururo.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 25.Arumainayagam N, McGrath J, Jefferson KP, Gillatt DA. Introduction of an enhanced recovery protocol for radical cystectomy. BJU Int. 2008;101:698–701. doi: 10.1111/j.1464-410X.2007.07319.x. [DOI] [PubMed] [Google Scholar]
- 26.Nieuwenhuijzen JA, de Vries RR, Bex A, van der Poel HG, Meinhardt W, Antonini N, et al. Urinary diversions after cystectomy: the association of clinical factors, complications and functional results of four different diversions. Eur Urol. 2008;53:834–44. doi: 10.1016/j.eururo.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Tolhurst SR, Rapp DE, O’Connor RC, Lyon MB, Orvieto MA, Steinberg GD. Complications after cystectomy and urinary diversion in patients previously treated for localized prostate cancer. Urology. 2005;66:824–9. doi: 10.1016/j.urology.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 28.Studer UE, Burkhard FC, Schumacher M, Kessler TM, Thoeny H, Fleischmann A, et al. Twenty years experience with an ileal orthotopic low pressure bladder substitute-lessons to be learned. J Urol. 2006;176:161–6. doi: 10.1016/S0022-5347(06)00573-8. [DOI] [PubMed] [Google Scholar]
- 29.Boström PJ, Kössi J, Laato M, Nurmi M. Risk factors for mortality and morbidity related to radical cystectomy. BJU Int. 2009;103:191–6. doi: 10.1111/j.1464-410X.2008.07889.x. [DOI] [PubMed] [Google Scholar]
- 30.Pycha A, Comploj E, Martini T, Trenti E, Mian C, Lusuardi L, et al. Comparison of complications in three incontinent urinary diversions. Eur Urol. 2008;54:825–34. doi: 10.1016/j.eururo.2008.04.068. [DOI] [PubMed] [Google Scholar]
- 31.Lowrance WT, Rumohr JA, Chang SS, Clark PE, Smith JA, Jr, Cookson MS. Contemporary open radical cystectomy: analysis of perioperative outcomes. J Urol. 2008;179:1313–8. doi: 10.1016/j.juro.2007.11.084. [DOI] [PubMed] [Google Scholar]
- 32.Novotny V, Hakenberg OW, Wiessner D, Heberling U, Litz RJ, Oehlschlaeger S, et al. Perioperative complications of radical cystectomy in a contemporary series. Eur Urol. 2007;51:397–402. doi: 10.1016/j.eururo.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Konety BR, Allareddy V, Herr H. Complications after radical cystectomy: analysis of population-based data. Urology. 2006;68:58–64. doi: 10.1016/j.urology.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 34.Knap MM, Lundbeck F, Overgaard J. Early and late treatment related morbidity following radical cystectomy. Scand J Urol Nephrol. 2004;38:153–60. doi: 10.1080/00365590310020060. [DOI] [PubMed] [Google Scholar]
- 35.Kulkarni JN, Pramesh CS, Rathi S, Pantvaidya GH. Long-term results of orthotopic neobladder reconstruction after radical cystectomy. BJU Int. 2003;91:485–8. doi: 10.1046/j.1464-410x.2003.04131.x. [DOI] [PubMed] [Google Scholar]
- 36.Chahal R, Sundaram SK, Iddenden R, Forman DF, Weston PM, Harrison SC. A study of the morbidity, mortality and long-term survival following radical cystectomy and radical radiotherapy in the treatment of invasive bladder cancer in Yorkshire. Eur Urol. 2003;43:246–57. doi: 10.1016/s0302-2838(02)00581-x. [DOI] [PubMed] [Google Scholar]
- 37.Malavaud B, Vaessen C, Mouzin M, Rischmann P, Schulman C. Complications for radical cystectomy: impact of the American Society of Anesthesiologists score. Eur Urol. 2001;39:79–84. doi: 10.1159/000052416. [DOI] [PubMed] [Google Scholar]
- 38.Rosario DJ, Becker M, Anderson JB. The changing pattern of mortality and morbidity from radical cystectomy. BJU Int. 2000;85:427–30. doi: 10.1046/j.1464-410x.2000.00454.x. [DOI] [PubMed] [Google Scholar]
- 39.Hautmann RE, de Petriconi R, Gottfried HW, Kleinschmidt K, Mattes R, Paiss T. The ileal neobladder: complications and functional results in 363 patients after 11 years of follow up. J Urol. 1999;161:422–8. doi: 10.1016/s0022-5347(01)61909-8. [DOI] [PubMed] [Google Scholar]
- 40.Mills RD, Studer UE. Metabolic consequences of continent urinary diversion. J Urol. 1999;161:1057–66. [PubMed] [Google Scholar]
- 41.Chang SS, Cookson MS, Baumgartner RG, Wells N, Smith JA., Jr Analysis of Early Complications after radical Cystectomy: Results of collaborative care pathway. J Urol. 2002;167:2012–6. [PubMed] [Google Scholar]
- 42.Olbert PJ, Baumann L, Hegele A, Schrader AJ, Hofmann R. Fast-track concepts in the perioperative management of patients undergoing radical cystectomy and urinary diversion: review of the literature and research results [in German] Urologie A. 2009;48:137–42. doi: 10.1007/s00120-008-1900-5. [DOI] [PubMed] [Google Scholar]
- 43.Park HK, Kwak C, Byun SS, Lee E, Lee SE. Early removal of nasogastric tube after cystectomy with urinary diversion: does postoperative ileus risk increase? Urology. 2005;65:905–8. doi: 10.1016/j.urology.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 44.Tabibi A, Simforoosh N, Basiri A, Ezzatnejad M, Abdi H, Farrokhi F. Bowel preparation versus no preparation before ileal urinary diversion. Urology. 2007;70:654–8. doi: 10.1016/j.urology.2007.06.1107. [DOI] [PubMed] [Google Scholar]
- 45.Pruthi RS, Chun J, Richman M. Reducing time to oral diet and hospital discharge in patients undergoing radical cystectomy using a perioperative care plan. Urology. 2003;62:661–5. doi: 10.1016/s0090-4295(03)00651-4. [DOI] [PubMed] [Google Scholar]
- 46.Kulkarni JN, Gulla RI, Tongaonkar HB, Kashyapi BD, Rajyaguru KB. Radical Cystectomy: an Extra peritoneal retrograde approach. J Urol. 1999;161:545–8. doi: 10.1016/s0022-5347(01)61946-3. [DOI] [PubMed] [Google Scholar]
- 47.Murphy DG, Challacombe BJ, Elhage O, O’Brien TS, Rimington P, Khan MS, et al. Robotic-assisted laparoscopic radical cystectomy with extracorporeal urinary diversion: initial experience. Eur Urol. 2008;54:570–80. doi: 10.1016/j.eururo.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 48.Naspro R. Editorial comment on: robotic-assisted laparoscopic radical cystectomy with extracorporeal urinary diversion: initial experience. Eur Urol. 2008;54:578. doi: 10.1016/j.eururo.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 49.Soloway MS. Editorial comment on: robotic-assisted laparoscopic radical cystectomy with extracorporeal urinary diversion: initial experience. Eur Urol. 2008;54:580. doi: 10.1016/j.eururo.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 50.Haber GP, Crouzet S, Gill IS. Laparoscopic and robotic assisted radical cystectomy for bladder cancer: a critical analysis. Eur Urol. 2008;54:54–64. doi: 10.1016/j.eururo.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 51.Nagele U, Anastasiadis AG, Merseburger AS, Corvin S, Hennenlotter J, Adam M, et al. The rationale for radical cystectomy as primary therapy for T4 bladder cancer. World J Urol. 2007;25:401–5. doi: 10.1007/s00345-007-0172-9. [DOI] [PubMed] [Google Scholar]
- 52.Shahin O, Thalmann GN, Rentsch C, Mazzucchelli L, Studer UE. A retrospective analysis of 153 patients treated with or without intravesical bacillus Calmette-Guerin for primary stage T1 grade3 bladder cancer: recurrence, progression and survival. J Urol. 2003;169:96–100. doi: 10.1016/S0022-5347(05)64044-X. [DOI] [PubMed] [Google Scholar]
- 53.Manoharan M, Reyes MA, Kava BR, Singal R, Kim SS, Soloway MS. Is adjuvant chemotherapy for bladder cancer safer in patients with an ileal conduit than a neobladder? BJU Int. 2005;96:1286–9. doi: 10.1111/j.1464-410X.2005.05822.x. [DOI] [PubMed] [Google Scholar]
- 54.Donat SM, Shabsigh A, Savage C, Cronin AM, Bochner BH, Dalbagni G, et al. Potential impact of postoperative early complications on the timing of adjuvant chemotherapy in patients undergoing radical cystectomy: a high-volume tertiary cancer center experience. Eur Urol. 2009;55:177–86. doi: 10.1016/j.eururo.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 55.Konety BR, Allareddy V, Modak S, Smith B. Mortality after major surgery for urologic cancers in specialized urology hospitals: are they any better? J Clin Oncol. 2006;24:2006–12. doi: 10.1200/JCO.2005.04.2622. [DOI] [PubMed] [Google Scholar]
- 56.Hollenbeck BK, Dunn RL, Miller DC, Daignault S, Taub DA, Wei JT. Volume-based referral for cancer surgery: informing the debate. J Clin Oncol. 2007;25:91–6. doi: 10.1200/JCO.2006.07.2454. [DOI] [PubMed] [Google Scholar]
- 57.Imkamp F, Herrmann TR, Rassweiler J, Sulser T, Stolzenberger R, Rebenault U. Laparoscopy in German urology: changing acceptance among urologists. Eur Urol. 2009;56:1074–81. doi: 10.1016/j.eururo.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 58.McCabe JE, Jibawi A, Javle PM. Radical cystectomy: defining the threshold for a surgeon to achieve optimum outcomes. Postgrad Med J. 2007;83:556–60. doi: 10.1136/pgmj.2007.058214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holt NF, Silverman DG, Prasad R, Dziura J, Ruskin KJ. Pre anesthesia clinics, information management, and operating room delays results of a survey of practicing anesthesiologists. Anesth Analg. 2007;104:615–8. doi: 10.1213/01.ane.0000255253.62668.3a. [DOI] [PubMed] [Google Scholar]
- 60.Dahm P, Tuttle-Newhall JE, Nimjee SM, Byrne RR, Yowell CW, Price DT. Indications for admission to the surgical intensive care unit after radical cystectomy and urinary diversion. J Urol. 2001;166:189–93. [PubMed] [Google Scholar]
- 61.Stenzl A, Nagele U, Kuczyk M, Sievert KD, Anastasiadis A, Siebold J, et al. Cystectomy - technical considerations in male and female patients. EAU Update Series. 2005;3:138–46. [Google Scholar]
- 62.Serel TA, Sevin G, Perk H, Kosar A, Soyupek S. Antigrade extra peritoneal approach to radical Cystectomy and ileal neobladder. Int Natl J Urol. 2003;10:25–8. doi: 10.1046/j.1442-2042.2003.00560.x. [DOI] [PubMed] [Google Scholar]
- 63.Huang J, Xu K, Yao Y, Guo Z, Lin T, Jiang C. Orthotopic Neo bladder similar original bladder. Chinese Med J. 2003;116:1943–5. [PubMed] [Google Scholar]
- 64.Kulkarni JN, Rizvi SJ, Acharya UP, Kumar KS, Tiwari P. Gynecologic Tract Sparing Extra Peritoneal Cystectomy with Neo bladder. Int Braz J Urol. 2008;34:180–90. doi: 10.1590/s1677-55382008000200008. [DOI] [PubMed] [Google Scholar]
- 65.Chang SS, Smith JA, Jr, Cookson MS. Decreasing blood loss in patients treated with radical cystectomy: a prospective randomizes trial using a new stapling device. J Urol. 2003;169:951–4. doi: 10.1097/01.ju.0000051372.67213.ca. [DOI] [PubMed] [Google Scholar]
- 66.Vira MA, Richstone L. Robotic cystectomy: Its time has come. J Urol. 2010;183:421–2. doi: 10.1016/j.juro.2009.11.070. [DOI] [PubMed] [Google Scholar]
- 67.Mattei A, Birkhaeuser FD, Baermann C, Warncke SH, Studer UE. To stent or not to stent perioperatively the ureteroileal anastomosis of ileal orthotopic bladder substitutes and ileal conduits? Results of a prospective randomized trial. J Urol. 2008;179:582–6. doi: 10.1016/j.juro.2007.09.066. [DOI] [PubMed] [Google Scholar]
- 68.Leung TT, MacLean AR, Buie WD, Dixon E. Comparison of stapled versus handsewn loop ileostomy closure: a meta-analysis. J Gastrointest Surg. 2008;12:939–44. doi: 10.1007/s11605-007-0435-1. [DOI] [PubMed] [Google Scholar]
- 69.Wainstein DE, Fernandez E, Gonzalez D, Chara O, Berkowsky D. Treatment of high-output enterocutaneous fistulas with a vacuum-compaction device.A ten-year experience. World J Surg. 2008;32:430–5. doi: 10.1007/s00268-007-9235-8. [DOI] [PubMed] [Google Scholar]
- 70.Mastroeni F, Aragona M, Caldarera E. Deep venous thrombosis in patients undergoing salvage radical cystectomy. Arch Esp Urol. 2001;54:839–41. [PubMed] [Google Scholar]
- 71.Gupta H, Srivastava A, Menon GR, Agrawal CS, Chumber S, Kumar S. Comparison of interrupted versus continuous closure in abdominal wound repair: a meta-analysis of 23 trials. Asian J Surg. 2008;31:104–14. doi: 10.1016/S1015-9584(08)60069-X. [DOI] [PubMed] [Google Scholar]
- 72.Riou JP, Cohen JR, Johnson H., Jr Factors influencing wound dehiscence. Am J Surg. 1992;163:324–30. doi: 10.1016/0002-9610(92)90014-i. [DOI] [PubMed] [Google Scholar]


