Abstract
Background
Staging laparoscopy can visualize peritoneal and liver metastases in pancreatic cancer otherwise undetectable by preoperative imaging. However, false-negative rates may be as high as 18%–26%. The aim of the present study was to improve detection of metastatic pancreatic cancer with the use of fluorescence laparoscopy (FL) in a nude-mouse model with the tumors expressing green fluorescent protein (GFP).
Methods
The carcinomatosis mouse model of human pancreatic cancer was established by intraperitoneal injections of green fluorescent protein-expressing MiaPaca-2 human pancreatic cancer cells into 6-week-old female athymic mice. Two weeks later, mice underwent diagnostic laparoscopy. Laparoscopy was performed first under standard brightfield lighting, followed by fluorescent lighting. The number of metastatic foci identified within the four quadrants of the peritoneal cavity was recorded. After laparoscopy, the animals were sacrificed, opened, and imaged with the OV-100 Small Animal Imaging system as a positive control to identify metastasis. Tumors were collected and processed for histologic review.
Results
FL enabled visualization of pancreatic cancer metastatic foci not visualized with standard brightfield laparoscopy (BL). Under FL, in 1 representative mouse, 26 separate micrometastatic lesions were identified. In contrast, only very large tumors were seen using BL. Use of the OV-100 images, as positive controls, confirmed the presence of tumor foci. FL thus allowed identification and exact localization of submillimeter tumor foci. Such small-sized tumor foci were not distinguished from surrounding tissue under BL. All malignant lesions were histologically confirmed.
Conclusions
The use of FL enables the identification of tumor foci that cannot be seen with standard laparoscopy. The technology described in this report has important potential for the clinical development of FL.
Introduction
Although, currently, there is no universal consensus on the indications for staging laparoscopy in patients with pancreatic cancer, this diagnostic tool has been shown to improve preoperative detection of peritoneal and liver metastases undetectable on preoperative imaging.1,2 With improved accuracy in the identification of metastatic disease, earlier therapy can be directed to either curative resection or appropriate palliative care in patients for whom aggressive surgical resection will not provide any additional survival benefit. However, staging laparoscopy in its current form is not sufficiently adequate. Studies examining its accuracy in predicting the resectability of peripancreatic tumors have revealed false-negative rates of 18%–26%.3,4 To minimize these rates, efforts to systematically improve visualization and identification of tumor deposits are needed.
We have previously demonstrated the utility of fluorescence laparoscopy (FL) in the detection of primary pancreatic cancer in mouse models.5 We observed then that primary lesions that were embedded within the pancreatic tail and that might otherwise have been missed were rendered much more readily identifiable with FL than standard laparoscopy. In the present study, we demonstrate that FL can identify metastases in a mouse model of disseminated human pancreatic cancer, which standard laparoscopy cannot.
Materials and Methods
Cell culture
Human MiaPaCa-2 cells were stably transduced to express the green fluorescent protein (GFP) as previously described.6 These cells were maintained in Dulbecco's modified Eagle's medium (Gibco-BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), penicillin/streptomycin (Gibco-BRL), sodium pyruvate (Gibco-BRL), sodium bicarbonate (Cellgro, Manassas, VA), l-glutamine (Gibco-BRL), and minimal essential medium nonessential amino acids (Gibco-BRL). Cells were incubated at 37°C with 5% CO2.
Animal care
Female athymic nu/nu nude mice were maintained in a barrier facility on high-efficiency particulate air-filtered racks. The animals were fed with autoclaved, laboratory rodent diet (Teckland LM-485; Western ResearchProducts, Orange, CA). All surgical procedures were performed under anesthesia with intramuscular injection of a 100 μL mixture of 100 mg/kg ketamine and 10 mg/kg xylazine. For each procedure, 20 μL of 1 mg/kg buprenorphine was administered for pain control. Euthanasia was achieved by 100% CO2 inhalation, followed by cervical dislocation. All animal studies were approved by the UCSD Institutional Animal Care and Use Committee and conducted in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Animals.
Metastasis cancer model
MiaPaCa-2-GFP cells were harvested by trypsinization and washed three times with serum-free medium. Viability was verified to be greater than 95% using the Vi-Cell XR automated cell viability analyzer (Beckman Coulter, Brea, CA). The cells were resuspended at 1 million cells per 100 μL serum-free medium and placed on ice before intraperitoneal (IP) injections were performed using a 27-gauge needle in three 6-week-old female nude mice.7
Fluorescence laparoscopy
We have previously described the methods for achieving maximal fluorescence signals and differentiation from background and surrounding tissues by modifying a standard laparoscopic system (Stryker, Kalamazoo, MI).5 In the present experiment, a 480-nm interference short-pass excitation filter was placed between the light cable and the camera and a GG495 Schott glass filter was placed between the camera and the laparoscope. The excitation light source was a 300 W Xenon lamp (Stryker). A MultiCam 310C camera (UVP, Upland, CA), which allows variable exposure time and gain setting in the controlling software (VisionWorks LS; UVP), was used.
Standard laparoscopy
Two weeks after IP injection of the MiaPaCa-2-GFP cells, noninvasive whole-body imaging of the mice was performed under the OV-100 Small Animal Imaging System (Olympus Corp., Tokyo, Japan) to verify establishment of carcinomatosis. Next, the animals were anesthetized and a 22-gauge angiocatheter was introduced sterilely into the abdominal cavity at an angle to avoid injury to the underlying bowel. The catheter was secured to the abdominal wall with a suture and connected to the insufflation tubing of the laparoscopic tower. Insufflation was first initiated at 1 mmHg and then augmented to a final pressure of 2 mmHg. Next, a small incision was made higher up in the abdomen, through which a 3-mm 0-degree Karl-Storz laparoscope (Karl-Storz GmbH & Co., Tuttlingen, Germany) was introduced. A purse-string suture was placed around this incision to prevent its widening and to maintain proper insufflation. Upon termination of laparoscopy, the mice were sacrificed for tumor collection.
Tissue histology
At necropsy, each animal was imaged with its abdomen exposed under the OV-100. Fresh tissues were fixed in Bouin's solution and embedded in paraffin before sectioning and staining with hematoxylin and eosin (H&E) for standard light microscopy. H&E-stained permanent sections were examined using an Olympus BX41 microscope equipped with a Micropublisher 3.3 RTV camera (QImaging, Surrey, BC, Canada). All images were acquired using QCapture software (QImaging) without postacquisition processing. The slides were reviewed by a pathologist to confirm the identity of the fluorescent lesions.
Data processing
Images obtained during laparoscopy were not processed in any way. Representative frames were selected and presented in this report. Histology images were processed for brightness and contrast using Photoshop Element 4 (Adobe Systems, Inc., San Jose, CA).
Results
Two weeks after IP injection of MiaPaca-2-GFP human pancreatic cancer cells, 3 female athymic mice were anesthetized after prior confirmation of established carcinomatosis under noninvasive OV-100 imaging. Laparoscopic examination of the peritoneal cavity was performed first under standard brightfield lighting, examining all quadrants in a systematic manner, and the number of metastatic foci was recorded for each quadrant. This process was repeated under FL. Under both modes, insufflation was set to 2 mmHg, to allow for adequate visualization and manipulation of the laparoscope within the abdominal cavity.
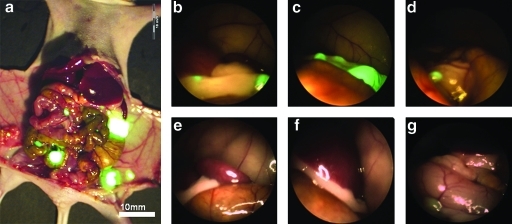
As seen in Figure 1, FL reveals micrometastases that are nearly invisible under standard brightfield laparoscopy (BL). These small lesions are either imperceptible or mistaken for normal structures under BL. The images demonstrate that visualization of large deposits under BL can be challenging and that of smaller tumor foci is nearly impossible.
FIG. 1.
Comparative identification of tumor foci under brightfield and fluorescence laparoscopy. (a) OV-100 open image from a representative mouse. View of left upper quadrant in a mouse specimen under FL (b–d) and BL (e–g). The green fluorescence of the metastatic lesion was unmistakable under FL, whereas under BL the tumor foci resembled normal tissue and were not identifiable. BL, brightfield laparoscopy; FL, fluorescence laparoscopy.
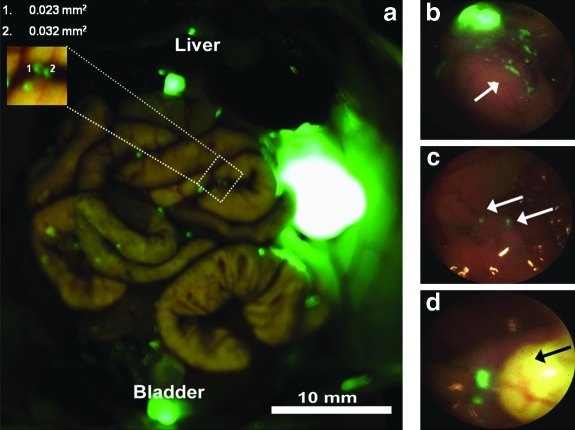
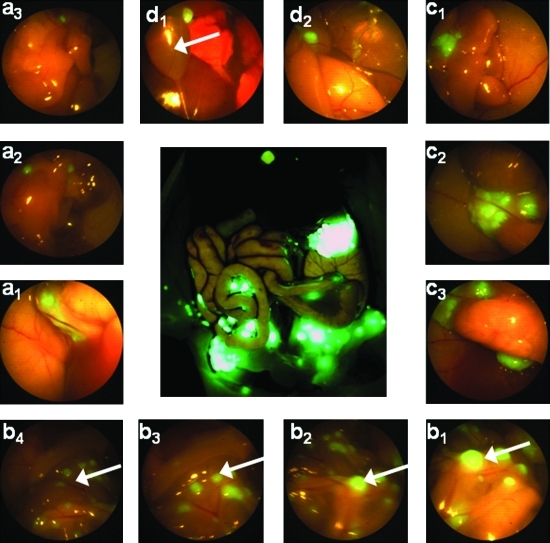
After completion of laparoscopic imaging, mice were sacrificed and the OV-100 was used as the positive control to measure and confirm presence of tumor at necropsy. OV-100 images were compared with video images of FL and BL imaging. As seen in the magnified area in Figure 2a, tumor foci as small as 0.023 mm2 were identified, as measured by Image J software. Note that autofluorescence of the urinary bladder and gallbladder in this representative mouse is easily distinguished from sites of tumor foci seen to the left of the gallbladder. FL identified tumor microfoci in all four quadrants as shown in Figure 3 of a representative mouse.
FIG. 2.
Fluorescence laparoscopy in a representative mouse. (a) OV-100 (positive control) imaging of the same mouse depicted in Figure 1 with measurements of the size of the smallest identified tumor foci as calculated by Image J software. The right upper corner of the figure illustrates the ability to identify tumor microfoci as small as 0.023 mm2. (b) Laparoscopic imaging of right upper quadrant; note the number of individual tumor microfoci (arrow) afforded by the high resolution of FL. (c) Fluorescence laparoscopic visualization of tumor microfoci (arrow) not visible with the OV-100. (d) Fluorescence laparoscopic view of the fluorescent gallbladder; tumor microfoci seen to the left of the gallbladder (arrow).
FIG. 3.
Fluorescence laparoscopy identifies tumor microfoci in four quadrants of a representative mouse. OV-100 fluorescence imaging is shown as a positive control, visualizing peritoneal carcinomatosis (center). Panels d1 and d2 show the right upper quadrant; panels c1–c3 show left upper quadrant; panels b1–b4 show carcinomatosis within the intra-abdominal (arrows); panels a1 and a3 show right upper quadrant. Note the slight autofluorescence of the gallbladder in panel d1 (arrow), which is easily distinguished from the adjacent tumor foci.
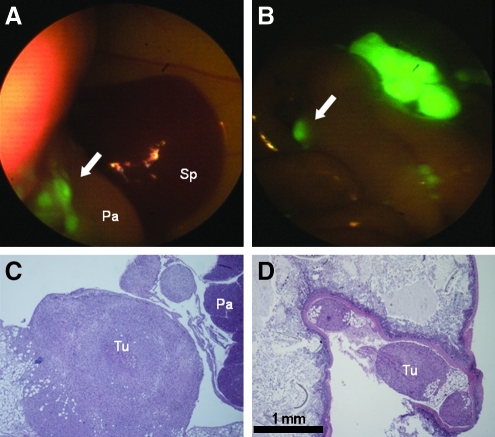
Using the modified laparoscopic setup, FL permitted rapid identification of tumor foci with resolution adequate to identify numerous tumor microfoci far beyond the capabilities of the BL (Table 1). In Mouse 1, 15 tumor microfoci were identified under FL versus 3 under BL. In Mouse 2, 16 tumor microfoci were identified under FL, whereas no visible tumor was identified under BL. Finally, Mouse 3 showed 26 tumor microfoci under FL, compared with 2 under BL. Although laparoscopy does not allow for direct size measurement of tumor foci, OV-100 imaging reveals that FL resolution allows detection of lesions smaller than 1 mm2. H&E stained tissue sections histologically confirmed that the identified fluorescent tumor foci were pancreatic adenocarcinoma (Fig. 4).
Table 1.
Comparative Identification of Tumor Foci Under Brightfield Laparoscopy, OV-100 (Positive Control), and Fluorescence Laparoscopy
| Brightfield laparoscopy | OV-100 | Fluorescence laparoscopy | |
|---|---|---|---|
| Mouse 1 | 2 | 13 | 15 |
| Mouse 2 | 0 | 14 | 16 |
| Mouse 3 | 2 | 2 | 26 |
OV-100 imaging was performed under low power. The average number±standard error of mean of tumor foci identified under BL was 1.33±0.66 compared with 19.0±3.5 for FL (p=0.033, paired t-test). The increased number of tumor foci identified using FL compared with BL demonstrates the power of this modality. BL, brightfield laparoscopy; FL, fluorescence laparoscopy.
FIG. 4.
Correlation of fluorescence laparoscopic images with histology in a pancreatic adenocarcinomatosis model. Histological confirmation of small tumor foci (arrows) identified by fluorescence imaging (A, B) as seen at low power (C, D; scale bar=1 mm). (A, C) Tumor nodules (arrow, Tu) infiltrating intra-abdominal fat adjacent to the normal pancreas (Pa), anterior to the spleen (Sp). (B, D) Small tumor nodules lodged in the mesentery of the bowel.
Discussion
We have previously developed a fluorescence laparoscope by modifying a standard laparoscope with the appropriate set of excitation and emission filters, to permit real-time identification and localization of fluorescently labeled primary tumors while maintaining adequate visualization of the surrounding tissue in an orthotopic mouse model.5 When applying this technology in our present study, we were able to quickly and easily detect GFP-expressing metastases in the mouse peritoneal cavity with much greater sensitivity and accuracy than with standard BL.
Fluorescence technology has been applied in open animal models of peritoneal carcinomatosis with success. Our laboratory has previously demonstrated the ability to illuminate tumors and metastases by the tumor-specific delivery of the GFP gene using a telomerase-dependent adenovirus.8 The fluorescent tumors were then resected by fluorescence-guided surgery. Keramidas et al. reported that the use of near-infared image-guided surgery improved the quality of surgery for peritoneal carcinomatosis.9 These authors showed that over 50% of peritoneal metastases were left behind using brightfield alone, whereas near-infared imaging enabled resection of twice the number of metastatic foci including those as small as 227 tumor cells. Recently, a tumor-activated probe was used to resect breast cancer in a mouse model.10
Gahlen et al. first reported using delta-aminolevulinic acid (ALA) for laparoscopic fluorescence diagnosis of peritoneal deposits of colorectal cancer in rats.11 In their study, the authors utilized a blue light to excite the photosensitizier protoporphyrin IX that was produced from tissues after IP infusion of ALA. This fluorescence light mode yielded 21% more lesions than were seen under standard white light. The benefits of 5-ALA–based photodynamic diagnosis have since been reproduced in more recent studies in both preclinical animal models and clinical applications, with sensitivity measured at around 92%.12,13 However, the use of blue light to detect the fluorescent signal in these studies translated to a dark and poorly visualized background.
In the present study, the utilization of a fluorescence laparoscope that permitted good visualization of the background while achieving identification of the fluorescent tumors avoided this problem of poorly visualized background. FL demonstrated such high-resolution identification of tumor microfoci that it led to a need to amplify the magnification of the OV-100 positive control to confirm their presence. In several instances, FL demonstrated that several larger tumor foci seen under low-magnification OV-100 as single foci were actually multiple adjacent tumor microfoci. Overall, GFP-expressing tumors were detected with 100% sensitivity. In comparison, the luciferase-expressing tumors in the Keramidas et al. study9 carried a 14% false-negative rate when compared with the control bioluminescent imaging of the animal. The high resolution achieved in our model will undoubtedly enable more accurate staging and greater surgical navigation of pancreatic cancer. Although the use of GFP-expressing tumors is convenient for a mouse model of pancreatic cancer, there are obvious leaps that need to occur before this method can be clinically applicable, which our laboratory is now carrying out.8
In another approach toward the clinical application of FL, our laboratory has used fluorophore-conjugated antibodies against the most clinically relevant tumor antigens carcinoembryonic antigen (CEA) and CA19-9 to illuminate pancreatic cancer in orthotopic mouse models.14,15 These fluorophore-conjugated antibodies are delivered via single intravenous injection into the tail vein in mouse models, which are ready for imaging after 24 hours. The obvious advantage of this method of fluorescence labeling is its direct clinical applicability. The aim of our present study was to provide a proof of principle that FL could detect fluorescently labeled tumor foci with high sensitivity. We expect that these results will have a formative impact on the clinical development and improved staging of pancreatic and other gastrointestinal cancers.
Acknowledgments
This work was supported in part by grants CA109949 and CA132971 from the National Cancer Institute (to M.B. and AntiCancer, Inc.) and T32 training grant CA121938 (to H.S.T.C. and C.A.M.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Contreras CM. Stanelle EJ. Mansour J. Hinshaw JL. Rikkers LF. Rettammel R. Mahvi DM. Cho CS. Weber SM. Staging laparoscopy enhances the detection of occult metastases in patients with pancreatic adenocarcinoma. J Surg Oncol. 2009;100:663–669. doi: 10.1002/jso.21402. [DOI] [PubMed] [Google Scholar]
- 2.Stefanidis D. Grove KD. Schwesinger WH. Thomas CR., Jr. The current role of staging laparoscopy for adenocarcinoma of the pancreas: a review. Ann Oncol. 2006;17:189–199. doi: 10.1093/annonc/mdj013. [DOI] [PubMed] [Google Scholar]
- 3.Arnold JC. Neubauer HJ. Zopf T. Schneider A. Benz C. Adamek HE. Riemann JF. Improved tumor staging by diagnostic laparoscopy. Z Gastroenterol. 1999;37:483–488. [PubMed] [Google Scholar]
- 4.Nieveen van Dijkum EJ. Romijn MG. Terwee CB. de Wit LT. van der Meulen JH. Lameris HS. Rauws EA. Obertop H. van Eyck CH. Bossuyt PM. Gouma DJ. Laparoscopic staging and subsequent palliation in patients with peripancreatic carcinoma. Ann Surg. 2003;237:66–73. doi: 10.1097/00000658-200301000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran Cao HS. Kaushal S. Lee C. Snyder CS. Thompson KJ. Horgan S. Talamini MA. Hoffman RM. Bouvet M. Fluorescence laparoscopy imaging of pancreatic tumor progression in an orthotopic mouse model. Surg Endosc. 2011;25:48–54. doi: 10.1007/s00464-010-1127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouvet M. Wang J. Nardin SR. Nassirpour R. Yang M. Baranov E. Jiang P. Moossa AR. Hoffman RM. Real-time optical imaging of primary tumor growth and multiple metastatic events in a pancreatic cancer orthotopic model. Cancer Res. 2002;62:1534–1540. [PubMed] [Google Scholar]
- 7.Katz MH. Takimoto S. Spivack D. Moossa AR. Hoffman RM. Bouver M. A novel red fluorescent protein orthotopic pancreatic cancer model for the preclinical evaluation of chemotherapeutics. J Surg Res. 2003;113:151–160. doi: 10.1016/s0022-4804(03)00234-8. [DOI] [PubMed] [Google Scholar]
- 8.Kishimoto H. Zhao M. Hayashi K. Urata Y. Tanaka N. Fujiwara T. Penman S. Hoffman RM. In vivo internal tumor illumination by telomerase-dependent adenoviral GFP for precise surgical navigation. Proc Natl Acad Sci USA. 2009;106:14514–14517. doi: 10.1073/pnas.0906388106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keramidas M. Josserand V. Righini CA. Wenk C. Faure C. Coll JL. Intraoperative near-infrared image-guided surgery for peritoneal carcinomatosis in a preclinical experimental model. Br J Surg. 2010;97:737–743. doi: 10.1002/bjs.6986. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen QT. Olson ES. Aguilera TA. Jiang T. Scadeng M. Ellies LG. Tsien RY. Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival. Proc Natl Acad Sci USA. 2010;107:4317–4322. doi: 10.1073/pnas.0910261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gahlen J. Stern J. Laubach HH. Pietschmann M. Herfarth C. Improving diagnostic staging laparoscopy using intraperitoneal lavage of delta-aminolevulinic acid (ALA) for laparoscopic fluorescence diagnosis. Surgery. 1999;126:469–473. [PubMed] [Google Scholar]
- 12.Collinet P. Sabban F. Cosson M. Farine MO. Villet R. Vinatier D. Mordon S. Laparoscopic photodynamic diagnosis of ovarian cancer peritoneal micro metastasis: an experimental study. Photochem Photobiol. 2007;83:647–651. doi: 10.1562/2006-04-13-RA-869. [DOI] [PubMed] [Google Scholar]
- 13.Löning M. Diddens H. Küpker W. Diedrich K. Hüttmann G. Laparoscopic fluorescence detection of ovarian carcinoma metastases using 5-aminolevulinic acid-induced protoporphyrin IX. Cancer. 2004;100:1650–1656. doi: 10.1002/cncr.20155. [DOI] [PubMed] [Google Scholar]
- 14.Kaushal S. McElroy MK. Luiken GA. Talamini MA. Moossa AR. Hoffman RM. Bouvet M. Fluorophore-conjugated anti-CEA antibody for the intraoperative imaging of pancreatic and colorectal cancer. J Gastrointest Surg. 2008;12:1938–1950. doi: 10.1007/s11605-008-0581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McElroy M. Kaushal S. Luiken GA. Talamini MA. Moossa AR. Hoffman RM. Bouvet M. Imaging of primary and metastatic pancreatic cancer using a fluorophore-conjugated anti-CA19-9 antibody for surgical navigation. World J Surg. 2008;32:1057–1066. doi: 10.1007/s00268-007-9452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]