Abstract
We have recently found that exercise training is effective in the treatment of the Postural Orthostatic Tachycardia Syndrome (POTS). Whether this non-drug treatment is superior to “standard” drug therapies, such as β-blockade, is unknown. We tested the hypothesis that exercise training but not β-blockade treatment improves symptoms, hemodynamics, and renal-adrenal responses in POTS patients. Nineteen patients (18 women, 1 man) completed a double-blind drug trial (propranolol or placebo) for 4 weeks, followed by 3 months of exercise training. Fifteen age-matched healthy individuals (14 women, 1 man) served as controls. A 2-hour standing test was performed before and after drug treatment and training. Hemodynamics, catecholamines, plasma renin activity, and aldosterone were measured supine and during 2-hour standing. We found that both propranolol and training significantly lowered standing heart rate. Standing cardiac output was lowered after propranolol treatment (P=0.01), but was minimally changed after training. The aldosterone-to-renin ratio during 2-hour standing remained unchanged after propranolol treatment [4.1±1.7 (SD) pre vs. 3.9±2.0 post, P=0.46), but modestly increased after training (5.2±2.9 vs. 6.5±3.0, P=0.05). Plasma catecholamines were not affected by propranolol or training. Patient quality of life, assessed using the 36-item Short Form Health Survey, was improved after training (physical functioning score 33±10 pre vs. 50±9 post; social functioning score 37±9 vs. 48±6, both P<0.01), but not after propranolol treatment (34±10 vs. 36±11, P=0.63; 39±7 vs. 39±5, P=0.73). These results suggest that for patients with POTS, exercise training is superior to propranolol at restoring upright hemodynamics, normalizing renal-adrenal responsiveness, and improving quality of life.
Keywords: long-term orthostasis, renin-angiotensin-aldosterone system, hemodynamics, beta-blockade, quality of life
INTRODUCTION
We have recently demonstrated that carefully prescribed short-term exercise training improves or even cures the Postural Orthostatic Tachycardia Syndrome (POTS), also called “The Grinch Syndrome” because as a group, POTS patients have a heart that is “two sizes too small”.1 Whether this non-drug treatment is superior to “standard” drug therapies, such as β-blockade, is unknown. Although, β-blockers are used in POTS patients to empirically reduce tachycardia, the chronic effects of these medications are unclear.
The renin-angiotensin-aldosterone system (RAAS) plays a crucial role in the neurohumoral regulation of hemodynamics during prolonged orthostasis in humans.2 Recent studies have shown that POTS patients have a blunted aldosterone response to an increase in renin; namely, a reduced aldosterone-to-renin ratio during standing.3–5 Raj et al3 termed this dysregulation in POTS the “renin-aldosterone paradox”. The underlying pathophysiology is unknown, but may be related to sympathoexcitation and/or tachycardia. A similar reduced aldosterone-to-renin ratio has also been found in healthy individuals after a period of bed rest (i.e., simulated microgravity exposure), in which “cardiovascular deconditioning” (i.e., cardiac atrophy and hypovolemia) occurs.6–8 Numerous studies have demonstrated that real or simulated microgravity exposure elicits a “POTS-like” syndrome even in healthy fit people.9–11 Based on these findings, we speculated that the “deconditioning” phenotype rather than a secondary effect due to tachycardia contributes, at least in part, to the blunted adrenal response in POTS. If this model is true, we would expect to see an improvement in renal-adrenal function after “reconditioning” but not after lowering heart rate with β-blockade treatment.
The primary objective of this study was to test the hypothesis that in POTS patients, increases in physical fitness with exercise training can improve upright hemodynamics and increase the aldosterone-to-renin ratio during orthostasis, while lowering upright heart rate by β-blockers alone does not. We further hypothesized that exercise training but not β-blockade treatment improves patients' overall well-being. To accomplish these objectives, we compared the effects of exercise training and a non-selective β-blocker, propranolol on renal-adrenal and hemodynamic responses during prolonged (i.e., 2 hours) standing in patients with POTS. Patient quality of life was assessed using the 36-item Short Form Health Survey (SF-36)12 before and after propranolol treatment as well as exercise training.
METHODS
Participants
Nineteen POTS patients (18 women, 1 man) completed a 4-week double-blind drug trial (propranolol or placebo), followed by 3 months of exercise training. All patients met the inclusion without exclusion criteria for POTS,13 and had a heart rate rise ≥30 bpm or a rate that exceeded 120 bpm that occurred after 10 minutes of standing without any evidence of orthostatic hypotension.3 All were non-smokers. None was an endurance-trained athlete.14 All were screened with a careful medical history, physical examination, 12-lead electrocardiogram, and a 10-minute stand test. Patients had stopped taking medications that could affect the autonomic nervous system ≥2 weeks before screening and ≥4 weeks before testing. Fifteen age-matched healthy individuals (14 women, 1 man) served as controls. All were informed of the purpose and procedures used in the study and gave their written informed consent to a protocol approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas.
Measurements
Heart Rate and Blood Pressure
Heart rate was monitored from the electrocardiogram (Hewlett-Packard), and beat-to-beat arterial pressure was derived by finger photoplethysmography (Portapres). Arm cuff blood pressure was measured by electrosphygmomanometry (SunTech), with a microphone placed over the brachial artery to detect Korotkoff sounds. Respiratory excursions were detected by a nasal cannula.
Cardiac Output
Cardiac output was measured with the acetylene rebreathing technique,15 from the disappearance rate of acetylene in expired air, measured with a mass spectrometer (Marquette), after adequate mixing in the lung has been confirmed by a stable helium concentration. Stroke volume was calculated from cardiac output and the heart rate measured during rebreathing. Total peripheral resistance was calculated as the quotient of mean arterial pressure and cardiac output, multiplied by 80 (expressed as dyn·s·cm−5). Mean arterial pressure was calculated as [(systolic pressure – diastolic pressure)/3] + diastolic pressure.
Protocol
All the subjects were on an isocaloric diet consisting of 200 mEq sodium, 100 mEq potassium, and 1000 mg calcium 3 days prior to testing. Fluid intake was ad libitum and assessed by 24-hour urine output the day prior to testing to verify dietary compliance. Female subjects were tested during the mid-luteal phase (i.e., 19–22 days after the onset of menstruation) of their menstrual cycles to avoid the effects of sex hormone fluctuations on renal-adrenal and hemodynamic responses.5 Subjects were required not to exercise ≥24 hours prior to testing. They took a pregnancy test and showed negative results on each study day. Patient quality of life was assessed using the SF-36.
The experiment was performed in the morning or afternoon ≥2 hours after a light breakfast or lunch, and ≥72 hours after the last caffeinated or alcoholic beverage in a quiet, environmentally controlled laboratory with an ambient temperature of ≈25°C. The subject was placed in the supine position and an intravenous catheter was inserted into an antecubital vein. Hemodynamic variables were measured after ≥30 minutes in the supine resting position and every 10 minutes after the subject began 2-hour standing. Blood samples were collected after ≥1 hour in the supine position, and after 30 minutes, 1 and 2 hours of standing. Plasma renin activity and aldosterone were measured by radioimmunoassay techniques,16 while plasma catecholamine concentrations were measured by high-performance liquid chromatography.17 Plasma volume was measured by a modified carbon monoxide rebreathing technique.18, 19
Drug Treatment
Indistinguishable capsules of placebo (e.g., microcrystalline cellulose) and propranolol were prepared by the Investigational Drug Service at the Clinical Trials Department at the University of Texas Southwestern Medical Center. Long-acting propranolol (Par Pharmaceutical, NJ) was administrated orally 80 milligrams per day. Pill-counting was performed after 2 weeks of treatment in all the patients.
The 2-hour standing test and plasma volume measurement were repeated after 4 weeks of drug treatment. Patient quality of life was assessed using the SF-36 after treatment. Patients were always studied at the same time of the day.
Exercise Training
Details of the exercise intervention and some aspects of its clinical and physiological outcomes have been reported previously.1 A modified Astrand-Saltin incremental treadmill protocol was used to determine each patient's peak exercise capacity prior to training.20 The majority of the training sessions, particularly during the early phases were prescribed as “base training” with target heart rate equivalent to ~75–85% of maximal. Initially, patients trained 2 to 4 times per week for 30–45 minutes per session by using a recumbent bike, rowing, or swimming. The use of only semi-recumbent exercise at the beginning of the program was a critical strategy, allowing patients to exercise while avoiding the upright posture which elicits their symptoms. As the patients became relatively fit, the duration of the base training sessions was prolonged, and subsequently sessions of increased intensity (i.e., maximal steady-state) were added first once and then twice per week, and were always followed by recovery sessions. Upright exercise was added gradually as tolerated, though usually not until the second or third month. By the end of the training, patients were exercising 5–6 hours per week, and they were encouraged to use an upright bike, or walk on the treadmill, or jog. In addition to the endurance training, resistance training using weight lifting was also undertaken. Weight lifting started from once a week, 15–20 minutes per session, and gradually increased to twice a week, 30–40 minutes per session. Additionally, patients were encouraged to increase gradually their dietary salt intake to 6–8 grams per day and water intake of 3–4 liters per day, and elevate the head of the bed during sleeping at night.
The 2-hour standing test, plasma volume measurement, and patient quality of life assessment were repeated after 3 months of exercise training. Patients were studied at the same time of the day.
Statistical Analysis
Data are expressed as mean ± standard deviation unless otherwise noted. Physical characteristics between the groups were compared using Mann-Whitney rank-sum tests and within the groups were compared using Wilcoxon signed rank tests. Hemodynamic and renal-adrenal responses during 2-hour standing before and after treatment/training within and between the groups were analyzed using a two way repeated-measures analysis of variance (ANOVA), and the Holm-Sidak method was used post hoc for multiple comparisons. All statistical analyses were performed with a personal computer-based analysis program (SigmaStat, SPSS). A P value of <0.05 was considered statistically significant.
RESULTS
Physical Characteristics
Table 1 depicts subjects' characteristics. POTS patients had smaller plasma/blood volume, total hemoglobin mass, and red blood cell volume compared to healthy controls (all P<0.05). However, blood electrolytes as well as 24-hour urine output, osmolality, and urine electrolytes were not different between patients and controls (Table 2).
Table 1.
Subjects' Characteristics
| Variables | Healthy Controls (n=15) | POTS Patients |
|||||
|---|---|---|---|---|---|---|---|
| Exercise Training (n=19) | Propranolol (n=9) | Placebo (n=10) | |||||
| Pre | Post | Pre | Post | Pre | Post | ||
| Age (yr) | 31±10 | 27±9 | 28±9 | 27±9 | 27±9 | 27±9 | 27±9 |
| Height (cm) | 167±7 | 166±7 | 167±6 | 167±6 | 168±7 | 167±8 | 167±7 |
| Weight (kg) | 63±8 | 69±20 | 68±17 | 72±20 | 73±21 | 68±18 | 68±19 |
| BMI (kg/m2) | 23±2 | 25±5 | 24±5 | 26±5 | 26±6 | 24±5 | 24±5 |
| Peak Oxygen Uptake (ml/kg/min) | − | 26±4 | 29±5* | − | − | − | − |
| Plasma Volume (ml/kg) | 48±7 | 38±4† | 40±4*† | 38±6† | 38±4† | 39±6† | 40±5† |
| Blood Volume (ml/kg) | 72±12 | 58±7† | 61±7*† | 59±8† | 58±6† | 59±9† | 60±8† |
| Total Hemoglobin Mass (g/kg) | 9.09±2.77 | 7.49±0.89† | 7.90±1.08*† | 7.59±0.88† | 7.51±1.00† | 7.47±1.00† | 7.59±1.04† |
| Red Blood Cell Volume (ml/kg) | 23.9±5.7 | 19.8±2.7† | 20.8±3.0*† | 20.7±3.2† | 20.1±2.8† | 19.4±2.8† | 19.7±2.7† |
| Hematocrit (%) | 37.8±3.4 | 39.2±2.8 | 38.7±3.1 | 39.9±3.7 | 38.9±3.0 | 37.9±1.4 | 37.8±2.2 |
Values are mean±standard deviation. BMI, body mass index.
P<0.05 compared with pre-training within the group.
P<0.05 compared with healthy controls.
Table 2.
Blood and Urine Electrodes
| Variables | Healthy Controls (n=15) | POTS Patients |
|||||
|---|---|---|---|---|---|---|---|
| Exercise Training (n=19) | Propranolol (n=9) | Placebo (n=10) | |||||
| Pre | Post | Pre | Post | Pre | Post | ||
| Blood | |||||||
| pH | 7.40±0.04 | 7.40± 0.05 | 7.39± 0.03 | 7.39±0.03 | 7.41±0.04 | 7.42±0.05 | 7.44±0.04 |
| Na+ (mmol/l) | 138±1 | 137±3 | 138±2 | 137±2 | 136±2 | 138±2 | 137±2 |
| K+ (mmol/l) | 4.2±0.4 | 4.3±0.4 | 4.3±0.3 | 4.4±0.4 | 4.2±0.2 | 4.1±0.5 | 4.1±0.3 |
| Ca++ (mmol/l) | 1.2±0.1 | 1.1±0.1 | 1.1±0.1 | 1.1±0.1 | 1.1±0.1 | 1.1±0.1 | 1.1±0.1 |
| 24-hour Urine Output (ml) | 2142±805 | 2224±1037 | 2041±873 | 2088±1218 | 1690±866 | 2262±1207 | 2140±1198 |
| Osmolality (mOs/kg) | 401±152 | 417±207 | 437±153 | 495±293 | 507±242 | 398±223 | 393±154 |
| pH | 6.48±0.26 | 6.53±0.24 | 6.53±0.24 | 6.52±0.31 | 6.63±0.17 | 6.47±0.14 | 6.50±0.36 |
| Na+ (mmol/24 h) | 149±57 | 158±60 | 157±61 | 151±76 | 149±51 | 161±70 | 147±62 |
| K+ (mmol/24 h) | 54±20 | 59±25 | 54±26 | 53±30 | 52±28 | 62±27 | 50±26 |
| Ca++ (mmol/24 h) | 1.6±0.8 | 1.5±1.0 | 1.6±1.3 | 1.1±0.5 | 1.6±1.1 | 2.2±1.3 | 2.2±1.4 |
Values are mean±standard deviation.
Exercise Training
Three months of exercise training increased plasma/blood volume, total hemoglobin mass, and red blood cell volume in POTS patients (all P<0.05, Table 1). Blood electrolytes as well as 24-hour urine output, osmolality, and urine electrolytes remained unchanged after training (Table 2).
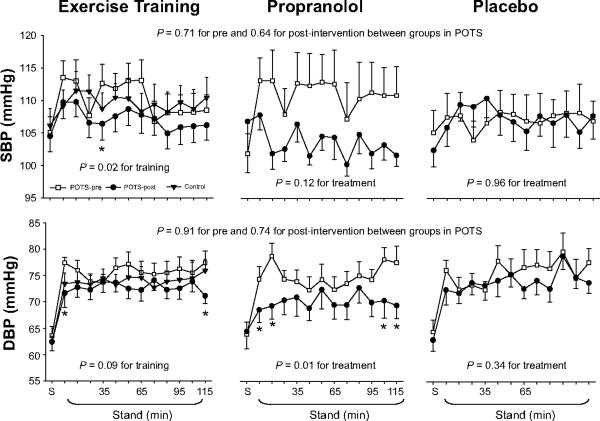
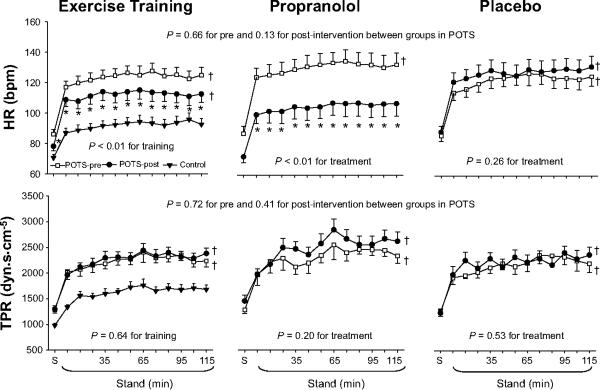
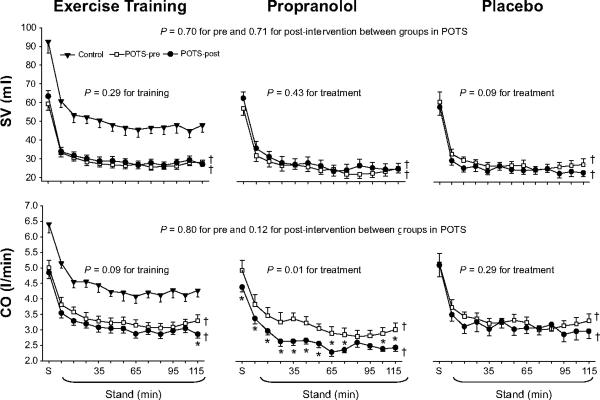
Systolic pressure was lower, while diastolic pressure tended to be lower during 2-hour standing after training (Figure 1). Standing heart rate was lower (Figure 2). Standing stroke volume remained stable, and standing cardiac output was slightly lower after training (Figure 3). Standing total peripheral resistance did not change after training (Figure 2).
Figure 1.
Systolic blood pressure (SBP) and diastolic blood pressure (DBP) responses during 2-hour standing in healthy controls (filled triangle) and in POTS patients before (open square) and after (filled circle) drug treatment or exercise training. S, supine. Values are expressed as mean±standard error. * P<0.05 compared with pre-treatment or pre-training within the group.
Figure 2.
Heart rate (HR) and total peripheral resistance (TPR) responses during 2-hour standing in healthy controls (filled triangle) and in POTS patients before (open square) and after (filled circle) drug treatment or exercise training. S, supine. Values are expressed as mean±standard error. *P<0.05 compared with pre-treatment or pre-training within the group. †P<0.05 compared with healthy controls.
Figure 3.
Stroke volume (SV) and cardiac output (CO) responses during 2-hour standing in healthy controls (filled triangle) and in POTS patients before (open square) and after (filled circle) drug treatment or exercise training. S, supine. Values are expressed as mean±standard error. *P<0.05 compared with pre-treatment within the group. †P<0.05 compared with healthy controls.
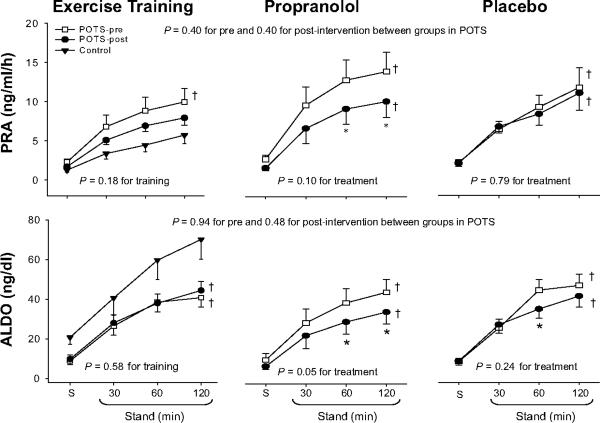
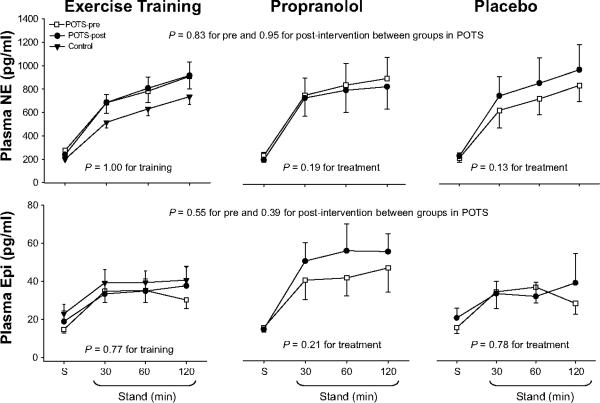
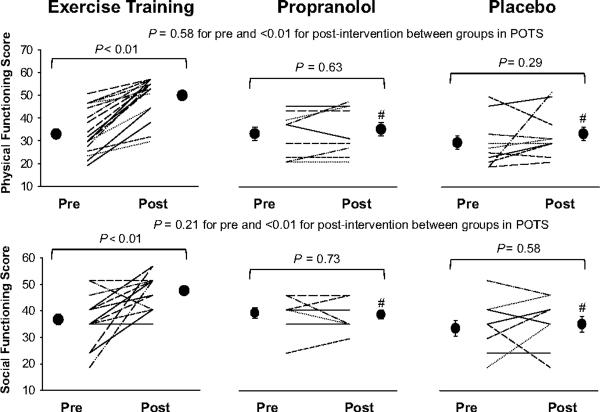
Training attenuated plasma renin activity increases modestly (21%), but had no impact on aldosterone increases during 2-hour standing (Figure 4). Thus, the aldosterone-to-renin ratio increased modestly after training (4.6±3.7 pre vs. 5.9±3.7 post in the supine position, 5.2±2.9 vs. 6.5±3.0 after 2 hours of standing; ANOVA P=0.05 for training). Training did not affect plasma catecholamine increases during 2-hour standing (Figure 5). Three patients had presyncope before training, but only one patient had presyncope after training. Patient quality of life was improved substantially after training in this subset (Figure 6), as has been reported previously.1
Figure 4.
Plasma renin activity (PRA) and aldosterone (ALDO) increases during 2-hour standing in healthy controls (filled triangle) and in POTS patients before (open square) and after (filled circle) drug treatment or exercise training. S, supine. Values are expressed as mean±standard error. *P<0.05 compared with before treatment within the group. †P<0.05 compared with healthy controls.
Figure 5.
Plasma norepinephrine and epinephrine concentration (NE and Epi) responses during 2-hour standing in healthy controls (filled triangle) and in POTS patients before (open square) and after (filled circle) drug treatment or exercise training. S, supine. Values are expressed as mean±standard error.
Figure 6.
Effects of exercise training, propranolol and placebo treatment on patient quality of life assessed by the SF-36. Values are expressed as individuals and mean±standard error.
Propranolol Treatment
Four weeks of propranolol treatment did not alter plasma/blood volume, total hemoglobin mass, or red blood cell volume in POTS patients (Table 1). Blood and urine electrolytes remained unchanged after treatment (Table 2). However, 24-hour urine output tended to be smaller (P=0.08), which may be attributable to a decrease in cardiac output and renal blood flow induced by β-adrenergic blockade. Urine osmolality was similar after treatment compared with before treatment (Table 2).
Systolic pressure trended lower, while diastolic pressure was significantly lower during 2-hour standing after propranolol treatment (Figure 1). Standing heart rate was significantly lower, but standing stroke volume was not augmented by the lower heart rate after treatment (Figures 2 and 3). Standing cardiac output was therefore markedly lower (Figure 3). Standing total peripheral resistance did not change after propranolol treatment (Figure 2).
Propranolol attenuated both plasma renin activity and aldosterone increases during 2-hour standing (Figure 4), and, therefore, the aldosterone-to-renin ratio remained unchanged after treatment (3.6±1.4 pre vs. 4.7±1.6 post in the supine position, 4.1±1.7 vs. 3.9±2.0 after 2 hours of standing; ANOVA P=0.46 for treatment). Propranolol did not affect plasma catecholamine increases during 2-hour standing (Figure 5). Two patients had presyncope before treatment, while three had presyncope after treatment. Patient quality of life remained unchanged after propranolol treatment (Figure 6).
Placebo had no impact on plasma/blood volume, total hemoglobin mass, red blood cell volume, hemodynamic and renal-adrenal responses, orthostatic tolerance, and quality of life in POTS patients (Tables 1–2, and Figures 1–6).
DISCUSSION
Our major findings are that (1) in POTS patients, both propranolol treatment and exercise training lowered standing heart rate; (2) propranolol attenuated plasma renin activity and aldosterone increases during 2-hour standing, and therefore, it did not change the aldosterone-torenin ratio; (3) conversely, exercise training attenuated plasma renin activity increases with preserved increases in aldosterone during prolonged standing, so that the aldosterone-to-renin ratio increased; and (4) patient quality of life was improved with training but not with propranolol treatment despite the markedly lower heart rate.
Thus, increases in physical fitness with exercise training improved the function of the RAAS and patients' overall well-being, while lowering heart rate by propranolol did not change the aldosterone-to-renin response or symptoms in POTS patients. It is suggested that the “deconditioning” phenotype rather than a secondary effect due to sympathoexcitation/tachycardia contributes, at least in part, to the blunted adrenal responsiveness in POTS, and exercise training appears to be a more effective therapy than simply lowering the heart rate with beta-blockade.
Exercise Training in POTS
In POTS patients, short-term exercise training lowered standing heart rate, which was attributable to a training-induced increase in baroreflex sensitivity.21 Blood pressure decreased to a lesser extent compared with propranolol treatment, stroke volume was preserved, cardiac output was slightly lowered, and total peripheral resistance remained unchanged during prolonged standing after training. These results suggest that exercise training is superior to propranolol at restoring upright hemodynamics. More importantly, patient quality of life was significantly improved after training, which was distinctly different from propranolol treatment. In addition to POTS, exercise training has also been found to be effective for chronic fatigue syndrome22 and fibromyalgia.23
Exercise training modestly attenuated plasma renin activity increases during 2-hour standing in POTS patients. There are at least two possible explanations for this observation. First, plasma volume expansion associated with training may have increased renal perfusion,24 resulting in a suppression of renin secretion possibly through adenosine production, shear-mediated nitric oxide synthase type 3 cGMP production, and myogenic-induced cellular depolarization permitting calcium entry.25–27 Second, training-induced overall decreases in sympathetic activation might account for the attenuation of plasma renin activity. Whether training altered sodium delivery in the kidney is unclear. We cannot measure sodium delivery directly, but urinary sodium reflects dietary sodium, which is a surrogate for sodium delivery over the time frame of the study. We found that both plasma sodium concentration and 24-hour sodium excretion were not different after training compared with before training, arguing against an alternation in sodium delivery contributing to the suppression of renin secretion in these patients.
Although exercise training attenuated plasma renin activity increases during prolonged standing in POTS patients, it did not change serum aldosterone during standing. Thus, the aldosterone-to-renin ratio increased, indicating that the adrenal responsiveness was improved with training. This result cannot be explained by plasma volume expansion. One previous study showed that the reduced aldosterone-to-renin ratio after simulated microgravity exposure was independent of plasma volume, since fluid loading after bed rest did not normalize this ratio.7 There are three well defined control mechanisms for aldosterone secretion; the RAAS, potassium, and adrenocorticitropin hormone (ACTH).28 In POTS patients, training-induced suppression of renin release would have caused a decrease in aldosterone secretion. Therefore, the RAAS did not seem to contribute to the increased aldosterone-to-renin ratio after training. Potassium was also not responsible for the increased aldosterone-to-renin ratio, because plasma potassium concentration and 24-hour urine potassium excretion did not differ after training compared with before training. It has been found that exercise training increases plasma ACTH levels in humans.29 ACTH, a pituitary peptide, has some stimulating effects on aldosterone probably by stimulating the formation of deoxycorticosterone, a precursor of aldosterone.30 It is therefore possible that training augmented the effect of ACTH on aldosterone production during standing; however, the relationship of ACTH pulsations to adrenal responsiveness is complex and beyond the scope of this study. Since dopamine suppresses aldosterone secretion, while atrial natriuretic factor (ANF) is an antagonist of aldosterone secretion,31–33 training-induced changes in dopamine and/or ANF levels also have contributed to the increased aldosterone-to-renin ratio in POTS patients. Unfortunately, we did not measure ACTH, dopamine, and ANF. Future studies are needed in this regard.
Although 3 months of training increased aldosterone-to-renin ratio in POTS patients, this ratio was still far below the levels of healthy sedentary individuals (i.e., 6.5±3.0 after training in POTS patients vs. 14.0±7.2 in healthy controls after 2 hours of standing;5P<0.001). It is possible that 3 months of training may not be long enough to restore the adrenal function to the normal level in these patients. Whether a longer duration of training can normalize the function of the RAAS in POTS patients needs to be determined in future studies.
Effects of Propranolol in POTS
Studies regarding the effectiveness of β-blockers in POTS have been limited and the results are inconsistent (see the online Data Supplement and Table S1 at http://hyper.ahajournals.org for details). Raj et al34 recently reported that acute administration of low-dose (20 mg) oral propranolol significantly attenuated tachycardia and improved symptoms in POTS, while a high dose (80 mg) of propranolol did not further improve, and may worsen, symptoms. We used long-acting propranolol (80 mg daily), which is similar to the low dose on a chronic basis. Upright heart rate was normalized to the level of healthy controls after 4 weeks of treatment, but patient quality of life remained unchanged, suggesting that chronic propranolol treatment cannot improve patients' overall well-being. It is likely that the markedly reduced cardiac output and blood pressure after propranolol treatment during upright posture could worsen fatigue and/or dizziness, especially if the heart rate rise in the untreated state is “appropriate.” Our results indicate that propranolol is no better than placebo on average, though we must acknowledge that some patients did improve symptoms, perhaps due to a decrease in palpitations.
Propranolol is a non-selective β-adrenergic blocker; as a consequence it blocks β1-receptors located on the juxtaglomerular cells of the kidney, resulting in a decrease in renin secretion.35 In addition, there is evidence that β-blockers may also inhibit intra-renal conversion of prorenin to renin.36 Whether propranolol can block renal baroreceptor-mediated renin release in humans is unknown. One previous investigation in hypertensive patients showed parallel suppression of angiotensin II and plasma renin activity during β-blocker therapy.37 Angiotensin II is a major physiologic stimulus for aldosterone production, a decrease in angiotensin II may cause a suppression of aldosterone release from the adrenal cortex.38 However, this assumption is not supported by the studies of Stewart et al39 and Mustafa et al40 showing that some POTS patients have inappropriately high plasma angiotensin II but low aldosterone levels. It has been suggested that suppression of aldosterone can cause the retention of potassium by the kidney. Since small changes in plasma potassium content can suppress renin release, it is likely that propranolol-induced hyperkalemia might also contribute to the inhibition of renin release by this drug.41
It was previously shown that in cirrhotic patients, propranolol treatment did not alter the ratio between the two main RAAS mediators, namely, the angiotensin-(1–7)-to-angiotensin II ratio,42 which has been used to evaluate the final functional effect of the RAAS. Consistent with this finding, we observed in POTS patients that the aldosterone-to-renin ratio remained unchanged after propranolol treatment. These results support the notion that non-selective β-adrenergic blockers suppress the RAAS but do not affect the final functional effect of this system in humans.
Perspectives
Patients with POTS have a blunted aldosterone-to-renin response during orthostasis. Results from our study suggest that the blunted adrenal function in these patients appears to be a consequence or signature of “cardiovascular deconditioning” rather than a secondary effect due to sympathoexcitation or tachycardia. Given the fact that exercise training but not propranolol treatment improved adrenal function, POTS symptoms, and most importantly, patient quality of life, it is reasonable to conclude that this non-drug therapy is superior to β-blockers. Since there are no effective pharmacologic therapies for POTS patients so far and many patients have disabling side effects with standard drug treatments, exercise training would appear to be the best initial therapy for this condition. It is important to emphasize that even the most symptomatic patients completed our training program and that training was facilitated by avoiding upright exercise in the early stage. This training program has been found to be effective for POTS patients in a research setting in this and our recent studies.1 Whether it is also effective in a community environment (i.e., outside the constraints of a controlled clinical trial) needs to be determined.
Supplementary Material
Acknowledgements
The time and effort put forth by the patients is greatly appreciated. The authors thank Robin P. Shook, Kazunobu Okazaki, Jeffrey L. Hastings, M. Dean Palmer, Daniel L. Creson, Colin L. Conner, Diane Bedenkop, and Peggy Fowler for their valuable laboratory assistance.
Sources of Funding This study was supported by the National Institutes of Health K23 grant (HL075283), National Space Biomedical Research Institute grant (CA00701), and the Clinical and Translational Research Center (formerly, the General Clinical Research Center) grant (RR00633).
Footnotes
Disclosures None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fu Q, Vangundy TB, Galbreath MM, Shibata S, Jain M, Hastings JL, Bhella PS, Levine BD. Cardiac Origins of the Postural Orthostatic Tachycardia Syndrome. J Am Coll Cardiol. 2010;55:2858–2868. doi: 10.1016/j.jacc.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowell LB. Human Cardiovascular Control. Oxford University Press; New York, NY: 1993. Neural-humoral adjustments to orthostasis and long-term control; pp. 81–117. [Google Scholar]
- 3.Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, Robertson D. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111:1574–1582. doi: 10.1161/01.CIR.0000160356.97313.5D. [DOI] [PubMed] [Google Scholar]
- 4.Garland EM, Raj SR, Black BK, Harris PA, Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology. 2007;69:790–798. doi: 10.1212/01.wnl.0000267663.05398.40. [DOI] [PubMed] [Google Scholar]
- 5.Fu Q, VanGundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Menstrual cycle affects renal-adrenal and hemodynamic responses during prolonged standing in the postural orthostatic tachycardia syndrome. Hypertension. 2010;56:82–90. doi: 10.1161/HYPERTENSIONAHA.110.151787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vernikos J, Dallman MF, Keil LC, O'Hara D, Convertino VA. Gender differences in endocrine responses to posture and 7 days of −6 degrees head-down bed rest. Am J Physiol. 1993;265:E153–E161. doi: 10.1152/ajpendo.1993.265.1.E153. [DOI] [PubMed] [Google Scholar]
- 7.Waters WW, Platts SH, Mitchell BM, Whitson PA, Meck JV. Plasma volume restoration with salt tablets and water after bed rest prevents orthostatic hypotension and changes in supine hemodynamic and endocrine variables. Am J Physiol Heart Circ Physiol. 2005;288:H839–H847. doi: 10.1152/ajpheart.00220.2004. [DOI] [PubMed] [Google Scholar]
- 8.Millet C, Custaud MA, Maillet A, Allevard AM, Duvareille M, Gauquelin-Koch G, Gharib C, Fortrat JO. Endocrine responses to 7 days of head-down bed rest and orthostatic tests in men and women. Clin Physiol. 2001;21:172–183. doi: 10.1046/j.1365-2281.2001.00315.x. [DOI] [PubMed] [Google Scholar]
- 9.Levine BD, Pawelczyk JA, Ertl AC, Cox JF, Zuckerman JH, Diedrich A, Biaggioni I, Ray CA, Smith ML, Iwase S, Saito M, Sugiyama Y, Mano T, Zhang R, Iwasaki K, Lane LD, Buckey JC, Jr., Cooke WH, Baisch FJ, Eckberg DL, Blomqvist CG. Human muscle sympathetic neural and haemodynamic responses to tilt following spaceflight. J Physiol. 2002;538:331–340. doi: 10.1113/jphysiol.2001.012575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamiya A, Iwase S, Kitazawa H, Mano T, Vinogradova OL, Kharchenko IB. Baroreflex control of muscle sympathetic nerve activity after 120 days of 6 degrees head-down bed rest. Am J Physiol Regul Integr Comp Physiol. 2000;278:R445–R452. doi: 10.1152/ajpregu.2000.278.2.R445. [DOI] [PubMed] [Google Scholar]
- 11.Convertino VA. Endurance exercise training: conditions of enhanced hemodynamic responses and tolerance to LBNP. Med Sci Sports Exerc. 1993;25:705–712. [PubMed] [Google Scholar]
- 12.Ware JEJ, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Health Institute, New England Medical Center; Boston, Mass: 1993. [Google Scholar]
- 13.Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, Suarez GA, Rummans TA. Postural tachycardia syndrome (POTS) Neurology. 1995;45:S19–S25. [PubMed] [Google Scholar]
- 14.Levine BD, Lane LD, Buckey JC, Friedman DB, Blomqvist CG. Left ventricular pressure-volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation. 1991;84:1016–1023. doi: 10.1161/01.cir.84.3.1016. [DOI] [PubMed] [Google Scholar]
- 15.Triebwasser JH, Johnson RL, Burpo RP, Campbell JC, Reardon WC, Blomqvist CG. Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviat Space Environ Med. 1977;48:203–209. [PubMed] [Google Scholar]
- 16.Boer P, Sleumer JH, Spriensma M. Confirmation of the optimal pH for measuring renin activity in plasma. Clin Chem. 1985;31:149–150. [PubMed] [Google Scholar]
- 17.van der Hoorn FA, Boomsma F, Man in 't Veld AJ, Schalekamp MA. Determination of catecholamines in human plasma by high-performance liquid chromatography: comparison between a new method with fluorescence detection and an established method with electrochemical detection. J Chromatogr. 1989;487:17–28. doi: 10.1016/s0378-4347(00)83003-0. [DOI] [PubMed] [Google Scholar]
- 18.Burge CM, Skinner SL. Determination of hemoglobin mass and blood volume with CO: evaluation and application of a method. J Appl Physiol. 1995;79:623–631. doi: 10.1152/jappl.1995.79.2.623. [DOI] [PubMed] [Google Scholar]
- 19.Gore CJ, Rodriguez FA, Truijens MJ, Townsend NE, Stray-Gundersen J, Levine BD. Increased serum erythropoietin but not red cell production after 4 wk of intermittent hypobaric hypoxia (4,000–5,500 m) J Appl Physiol. 2006;101:1386–1393. doi: 10.1152/japplphysiol.00342.2006. [DOI] [PubMed] [Google Scholar]
- 20.Balke B, Nagle FJ, Daniels J. Altitude and maximum performance in work and sports activity. JAMA. 1965;194:646–649. [PubMed] [Google Scholar]
- 21.Galbreath MM, Shibata S, Vangundy TB, Okazaki K, Fu Q, Levine BD. Effects of exercise training on arterial-cardiac baroreflex function in POTS. Clin Auton Res. 2011;21:73–80. doi: 10.1007/s10286-010-0091-5. [DOI] [PubMed] [Google Scholar]
- 22.Powell P, Bentall RP, Nye FJ, Edwards RH. Randomised controlled trial of patient education to encourage graded exercise in chronic fatigue syndrome. BMJ (Clinical research ed. 2001;322:387–390. doi: 10.1136/bmj.322.7283.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Martinez AM, De Paz JA, Marquez S. Effects of an exercise programme on self-esteem, self-concept and quality of life in women with fibromyalgia: a randomized controlled trial. Rheumatology international. 2011 doi: 10.1007/s00296-011-1892-0. in press. [DOI] [PubMed] [Google Scholar]
- 24.Fallo F. Renin-angiotensin-aldosterone system and physical exercise. J Sports Med Phys Fitness. 1993;33:306–312. [PubMed] [Google Scholar]
- 25.Wagner C, Pfeifer A, Ruth P, Hofmann F, Kurtz A. Role of cGMP-kinase II in the control of renin secretion and renin expression. J Clin Invest. 1998;102:1576–1582. doi: 10.1172/JCI4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Convertino VA, Keil LC, Greenleaf JE. Plasma volume, renin, and vasopressin responses to graded exercise after training. J Appl Physiol. 1983;54:508–514. doi: 10.1152/jappl.1983.54.2.508. [DOI] [PubMed] [Google Scholar]
- 27.Kurtz A, Wagner C. Role of nitric oxide in the control of renin secretion. Am J Physiol. 1998;275:F849–F862. doi: 10.1152/ajprenal.1998.275.6.F849. [DOI] [PubMed] [Google Scholar]
- 28.Williams GH, Dluhy RG. Aldosterone biosynthesis. Interrelationship of regulatory factors. Am J Med. 1972;53:595–605. doi: 10.1016/0002-9343(72)90156-8. [DOI] [PubMed] [Google Scholar]
- 29.Wittert GA, Livesey JH, Espiner EA, Donald RA. Adaptation of the hypothalamopituitary adrenal axis to chronic exercise stress in humans. Med Sci Sports Exerc. 1996;28:1015–1019. doi: 10.1097/00005768-199608000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Brown RD, Strott CA, Liddle GW. Site of stimulation of aldosterone biosynthesis by angiotensin and potassium. J Clin Invest. 1972;51:1413–1418. doi: 10.1172/JCI106937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carey RM, Thorner MO, Ortt EM. Effects of metoclopramide and bromocriptine on the renin-angiotensin-aldosterone system in man. Dopaminergic control of aldosterone. J Clin Invest. 1979;63:727–735. doi: 10.1172/JCI109356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carey RM, Thorner MO, Ortt EM. Dopaminergic inhibition of metoclopramide-induced aldosterone secretion in man. Dissociation of responses to dopamine and bromocriptine. J Clin Invest. 1980;66:10–18. doi: 10.1172/JCI109822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lang CC, Rahman AR, Struthers AD. Atrial natriuretic factor inhibits metoclopramide stimulated aldosterone release in man. British journal of clinical pharmacology. 1991;32:51–56. doi: 10.1111/j.1365-2125.1991.tb05612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raj SR, Black BK, Biaggioni I, Paranjape SY, Ramirez M, Dupont WD, Robertson D. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation. 2009;120:725–734. doi: 10.1161/CIRCULATIONAHA.108.846501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keeton TK, Campbell WB. The pharmacologic alteration of renin release. Pharmacol Rev. 1980;32:81–227. [PubMed] [Google Scholar]
- 36.Atlas SA, Sealey JE, Laragh JH, Moon C. Plasma renin and “prorenin” in essential hypertension during sodium depletion, beta-blockade, and reduced arterial pressure. Lancet. 1977;2:785–789. doi: 10.1016/s0140-6736(77)90723-1. [DOI] [PubMed] [Google Scholar]
- 37.Blumenfeld JD, Sealey JE, Mann SJ, Bragat A, Marion R, Pecker MS, Sotelo J, August P, Pickering TG, Laragh JH. Beta-adrenergic receptor blockade as a therapeutic approach for suppressing the renin-angiotensin-aldosterone system in normotensive and hypertensive subjects. Am J Hypertens. 1999;12:451–459. doi: 10.1016/s0895-7061(99)00005-9. [DOI] [PubMed] [Google Scholar]
- 38.Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13:S9–S20. doi: 10.18553/jmcp.2007.13.s8-b.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart JM, Taneja I, Medow MS. Reduced body mass index is associated with increased angiotensin II in young women with postural tachycardia syndrome. Clin Sci (Lond) 2007;113:449–457. doi: 10.1042/CS20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mustafa HI, Garland EM, Biaggioni I, Black BK, Dupont WD, Robertson D, Raj SR. Abnormalities of angiotensin regulation in postural tachycardia syndrome. Heart Rhythm. 8:422–428. doi: 10.1016/j.hrthm.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buhler FR, Laragh JH, Baer L, Vaughan ED, Jr., Brunner HR. Propranolol inhibition of renin secretion. A specific approach to diagnosis and treatment of renin-dependent hypertensive diseases. N Engl J Med. 1972;287:1209–1214. doi: 10.1056/NEJM197212142872401. [DOI] [PubMed] [Google Scholar]
- 42.Vilas-Boas WW, Ribeiro-Oliveira A, Jr., Ribeiro Rda C, Vieira RL, Almeida J, Nadu AP, Simoes e Silva AC, Santos RA. Effect of propranolol on the splanchnic and peripheral renin angiotensin system in cirrhotic patients. World J Gastroenterol. 2008;14:6824–6830. doi: 10.3748/wjg.14.6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.