Abstract
“General intelligence” is purported to influence diverse domain-specific learning abilities in humans, and previous research indicates that an analogous trait is expressed in CD-1 outbred mice. In humans and mice, exploratory tendencies are predictive of general cognitive abilities, such that higher cognitive abilities are associated with elevated levels of exploration. However, in mice, repeated exposure to novel environments outside the home cage has been found to up-regulate exploratory tendencies but has no commensurate effect on general learning abilities, suggesting that exploratory tendencies do not causally influence general cognitive performance. This leaves open the question of what is responsible for the robust relationship observed between exploration and general learning abilities? In the present experiments, we find that differential rates of habituation (e.g., to a novel open field) between animals of high and low general learning abilities accounts for the relationship between exploration and learning abilities. First, we up-regulated exploration by exposing mice to a series of novel environments. Similar to its lack of effect on learning tasks, this up-regulation of exploration had no commensurate effect on habituation to novel objects or stimuli. Next we examined the relationship between general learning abilities and exploration under conditions where habituation had a high or low impact on exploratory behaviors. A strong correlation between general learning abilities and exploration was observed under conditions where the levels of habituation (to a novel object or an open field) between animals of high and low general learning abilities were allowed to vary. However, this same correlation was attenuated when the level of habituation attained by animals of high and low general learning abilities was asymptotic or held constant across animals. In total, these results indicate that the relationship between exploration and general learning abilities is accounted for by the impact of habituation (itself a form of learning) on behaviors indicative of exploration.
Keywords: Learning, General Intelligence, Exploration, Habituation, Open Field, Elevated Plus Maze
1. Introduction
In humans, general intelligence, or “g” is said to be the single most dominant cognitive trait ever identified [1]. Typically measured in humans by batteries of cognitive tests which yield an aggregate score known as an “intelligence quotient” (IQ), general intelligence is a single cognitive factor which exerts an influence over many domain-specific learning abilities such as spatial learning, auditory learning, and reading comprehension (among others). Thus, due to the influence of the underlying general cognitive factor, persons with high IQ scores tend to perform better on all cognitive tasks than persons with low scores.
While general cognitive abilities have been extensively studied from psychometric [2,3], brain imaging [4], and behavior-genetic perspectives [5], research into a physiological brain substrate for “g” has been limited. Towards that end, it has previously been reported by our laboratory that genetically heterogeneous CD1 mice express a trait that is psychometrically homologous to human intelligence [6,7]. In those studies, the performance of mice was assessed on as many as eight learning tasks that were explicitly intended to impinge on at least five distinct learning domains. Animals rates of acquisition across these diverse learning tasks tended to be positively correlated, and principal component analyses isolated a single factor that has accounted for as much as 32–48% of the variability of individual animals across all tasks in the battery.
The general factor that regulates broad learning abilities in mice appears to extend beyond merely learning, and is in fact correlated with performance on other tests of cognitive ability. For instance, Kolata et al. [8,9] demonstrated that an aggregate measure of general learning abilities was strongly correlated with abilities that are known correlates of human’s general intelligence. These include independent measures of both working memory capacity and selective attention [10–14]. Thus general learning abilities in mice might more aptly be described as a “general cognitive ability”.
General cognitive abilities in mice appear to be unrelated to obvious non-cognitive characteristics. For instance, the general learning abilities of CD1 mice were found to be unrelated to various measures of sensory/motor function, unlearned fear responses, and emotionality [15]. However, one exception to this seeming independence of general cognitive abilities and non-cognitive characteristics has been noted. It has been repeatedly observed that general learning abilities (as well as domain-specific learning abilities) are strongly predicted by various measures of exploratory tendencies, but not simple activity [6, 15–19], and similar relationships have also been observed in humans [20,21].
The observation that exploratory behaviors predict general cognitive abilities raises several possibilities. First, animals that are prone to exploration may do so owing to a lower level of stress reactivity, and may thus naturally be immune to the adverse effects of stress on learning [22]. It is also possible that levels of exploration directly impact the ability to learn, i.e., animals that are prone to exploration will make quicker or more effective contact with the environmental contingencies upon which learning is based. However, evidence suggests that neither of these possibilities accounts for the observed relationship between exploratory tendencies and general learning ability.
First, Grossman et al. [23] reported that a pharmacological reduction in stress responsivity (through administration of anxiolytic drugs) in mice promoted an increase in exploration, but produced no corresponding benefit on general learning abilities (and in fact induced mild impairments in some tasks). Second, Light et al. [24] exposed (i.e., “adapted”) mice (over a period of weeks) to a series of novel environments and observed that this experience supported a robust increase in exploratory behaviors. Despite this increased propensity for exploration, no beneficial effects of this adaptation to novelty were observed either on domain-specific or general learning abilities. In combination, these observations suggest that variations in the propensity for exploration, while related to general cognitive abilities, do not directly influence those abilities.
The above analyses were based on the possibility that variations in the propensity for exploration might causally influence an animal’s general cognitive performance. Since no evidence in support of this possibility was found, the question remains as to the nature of the relationship between exploration and general cognitive abilities. An alternative to the previous possibilities is that, rather than exploration driving learning, learning abilities might drive patterns of exploration. For instance, an animal’s rate of learning might influence performance in an open field (and similarly other tests of exploration) through its impact on the animals habituation to the periphery of the field. Presumably an animal which has effectively processed the periphery of the field would be more inclined to move into the internal portions of the field. One might imagine this influence as mediating the opposing forces of exploratory tendency and stress reactivity, shifting behavior towards exploration as the animal becomes more acclimated (or habituated) to the periphery. Thus an animal which processes information more efficiently would habituate to the periphery sooner and explore the interior of the field more extensively (early in a bout of exploration) than an animal which processes information more slowly.
The possibility that information processing rate underlies the relationship between general learning abilities and exploratory behaviors is intriguing because it has long been posited as a potential brain substrate for “g” [25]. This possibility, i.e., that general learning ability influences an animal’s pattern of exploration in an open field contrasts with the common presumption that open field performance (as well as other measures of exploration) is a “non-cognitive” variable [15,17]. It is this possibility that is the focus of the experiments reported here.
2. Experiment 1
In a previous report, we found that a behavioral manipulation (adaptation to novel environments) that increases exploratory tendencies in mice did not promote a commensurate increase in general learning abilities. This was true for animals adapted to novel environments in either pre-pubescence or young adulthood [24]. If habituation (e.g., to the walled areas of an open field) underlies the relationship between learning and exploration, adaptation to novel environments must have exerted its effect on exploration independently of an effect on rate of habituation. If so, adaptation to novel environments should increase exploration in a subsequent novel environment, but should have no effect on animals performance in explicit (delimited) tests of habituation. This possibility is assessed in Experiment 1. To ascertain if the age at which animals underwent adaptation to novelty could impact later habituation rates, animals were adapted to novel environments either in pre-pubescence or young adulthood. They were subsequently assessed in tests of exploration and habituation (no explicit tests of learning were conducted in this experiment).
2.1. Methods
2.1.1. Subjects
Forty-two male outbred CD-1 mice were obtained at 21 days of age from Harlan Sprague Dawley, Inc. They were housed individually and maintained on ad libitum food and water (except where noted) in a temperature-controlled vivarium on a 12-hour light/dark cycle. They were handled (removed from the home cage and held by the experimenter) for 60s/day for one week prior to behavioral training/testing, which began at 37 days postnatal. This handling insured that differential stress responses to the experimenters, and any associated effects on learning, were minimized.
2.1.2. Apparatus and Procedure
This experiment was a three-group (14 mice per group), between subjects design. Single-trial tasks such as fear conditioning were analyzed using between groups analysis of variance. The three groups were balanced for native exploratory tendencies after being tested in the open field (on postnatal Day 37), as described below. One group of animals (“juvenile-adapted”) began their adaptation to novel environments after one day of rest (at 39 days of age) while a second group (“adult-adapted”) began this treatment after 25 days of rest (at 62 days of age). A third group of animals (“control”) was handled equivalently (half starting at 39 days of age, half starting at 62 days of age) during novel environment adaptation. At post-natal day 77, all animals began post-adaptation testing in exploratory and habituation tasks.
2.1.2.1. Open Field
The open field was a square field (46 × 46 cm) with 13 cm high walls, constructed of white Plexiglas and located in a brightly-lit room (400 lux) with a background noise of 65 dBc. The field was divided into a 6×6 grid comprised of 7.65 cm2 quadrants, where 20 of the quadrants abutted the outer walls of the field (i.e., “wall” quadrants), and 16 quadrants were displaced from the wall (i.e., “open” quadrants).
Animals were placed in the center of the open field. After 15 sec, during which time the animal self-selected a starting location, their behavior was monitored for 4 min. Throughout this time the animals entries into walled and open quadrants were recorded. An entry was recorded whenever both front paws crossed the border of a quadrant. Total activity (i.e., quadrant entries regardless of category) was recorded, as was the percentage of entries into unwalled (open) quadrants of the field. This latter measure has been previously interpreted to reflect a behavior more relevant to exploratory tendencies (as opposed to non-specific activity). Animals were placed into one of three groups (juvenile novelty-adapted, adult novelty-adapted, and control), which were balanced based on the percentage of entries into unwalled quadrants of the field because this measure is predictive of later exploratory tendencies as well as general cognitive abilities.
2.1.2.2. Novel Environments
Twelve novel environments were designed to differ from each other on various dimensions, including the presumed level of stress the mouse would experience. They also varied from one another in elevation, size, illumination, scent of other mice and complexity. One group of mice (“juvenile-adapted”) was exposed to this set of novel environments (in various orders) after one day of rest following the open field test and another (“adult-adapted) was exposed to this set of novel environments (in various orders) after 25 days of rest following the open field.
Environment 1 (E1) was a dual-chamber box. One side of the box was 15×7.5 cm with a cardboard floor. The other side was 15×14 cm with a grid floor. Both sides were completely enclosed. The animal started in the larger side of the box, the room was lighted at 280 lux, and the cardboard in the small side was changed after each animal. Environment 2 (E2) was a round platform 45.7 cm in diameter with a 2.5 cm lip, elevated 58.5 cm from the floor, lighted at 350 lux, and cleaned between each mouse with a 33% alcohol solution. Environment 3 (E3) was another dual chamber, completely enclosed, lighted at 340 lux and made of plastic. One side was red and round (19 cm diameter) and the other was black and rectangular, 15×21.5 cm. The long side of the rectangular half adjoined the circular half 15 cm in from the circle’s edge, giving the environment a “mushroom” shape. The floor was covered in wood chips which were changed every four animals. Environment 4 (E4) was a rectangular 70×31.75 cm steel tray elevated 45.75 cm from the floor. This was cleaned with 33% alcohol solution after every animal. An indirect lamp produced dim lighting (5 lux).
Environment 5 (E5) was a 44.5×28 cm rectangular cardboard box with a layer of wood chips on the bottom lighted at 280 lux. The box contained a smaller Styrofoam box (10H × 15W × 10 cm D) as well as a 25.5 cm long clear plastic tube with a diameter of 3.8 cm. The smaller box was on one end of the larger box and the tube ran parallel to the length of the field in the center. Environment 6 (E6) was a rectangular 35.5×10.2 cm black/white discrimination box, half light (100 lux) and half dark (5 lux) with a 3 cm hole separating the two. The floor of the box was cardboard, changed between each animal. The rest of the apparatus was cleaned between mice with a 33% alcohol solution. Environment 7 (E7) was a tall plastic cylinder 22.86 cm diameter and 40.6 cm high, standing up on a white Plexiglas base covered with cardboard with the top open, over which a fan blew two 30.5×2.54 cm streamers. The cardboard floor was changed between each animal. This was lighted at 280 lux. Environment 8 (E8) was a 19 liter white bucket with 30.5 cm diameter and 36.8 cm height. A metal test tube rack was placed on the floor, which was cleaned between mice with a 33% alcohol solution. A 40W indirect lamp was used for dim (8 lux) lighting.
Environment 9 (E9) was a 259×7.6 cm straight alley made of metal, elevated 30.5 cm from the floor with a Styrofoam start box (16.5H×28W×34 cm D) closed on three sides, open on the top and the wall facing the alley. The top of the box was covered with a piece of translucent orange Mylar. The room was lighted at 400 lux and the apparatus was cleaned between animals with a 33% alcohol solution. Environment 10 (E10) was a perforated 22.9 cm diameter 12.7 cm high metal pail with a screen placed on top. This was lighted at 15 lux using indirect lamp light and cleaned between animals with a 33% alcohol solution. Environment 11 (E11) was a 25.4 × 25.4 cm square elevated platform on which a screen covered cylinder 15.25 cm in diameter and 5 cm high was placed. The room was lighted at 280 lux and the apparatus was cleaned between animals. Environment 12 (E12) was a black Plexiglass T-maze. The long portion of the maze was 96.5×11.4 cm and was bisected (to form a T) by the short portion which was 21.6×11.4 cm long. The short portion contained a covered start box that was 14×11.4 cm. The floor of the apparatus was white plastic in a square grid so that animal waste fell through to the table surface. This was not cleaned between animals and an indirect lamp was used to create a 2 lux environment at the floor of the apparatus.
After the final day of novel environment exposure, animals received either one day of rest (“adult-adapted”) or 24 days of rest (“juvenile-adapted”) followed by a re-assessment of exploration, this time measured in an elevated plus maze, at post-natal day 77, as described below.
2.1.2.3. Elevated Plus Maze
The elevated plus maze was used to assess the exploratory behaviors of mice after their exposure to novel environments (or control handling). The maze was constructed of grey Plexiglas in the shape of a “plus.” Each arm of the maze was 28 cm long and 6 cm wide, and the maze was elevated 30 cm above a white floor. Two opposing arms of the maze were enclosed in 8 cm high, grey Plexiglas walls and two of the arms were open. The maze was located in a 200 lux environment.
Animals were placed in the center of the maze facing a closed arm, and their behavior in the maze was recorded for 3 min. Of interest was the time to exit the first closed arm entered, total number of arm entries, percent time in closed and open arms, latency to enter the first open arm, percent of entries into open arms and the percent of re-entries into an arm previously exited. Generally, entries into open arms are considered to be stressful to animals, thus measures in the open arms provide indices of exploratory tendencies similar in nature to that of exploration of the open quadrants of the open field.
2.1.2.4. Nose Poke Habituation
A round open field of 61 cm diameter and 17.77 cm walls was constructed out of black plastic with an off-white floor. The wall of the field had two holes of .635 cm diameter 7.62 cm from the floor and 5.08 cm apart. Two black rubber plugs could be fit into these two holes in order to occlude nosepokes.
All animals received three days of acclimation to the apparatus (14 min/day) with both holes plugged. On the fourth and fifth days one of the two holes was unplugged and the animals were allowed to poke their noses through it freely for fourteen minutes. Two measures were taken on these days. The first was the number of one-minute bins required to reach a habituation criterion (two consecutive bins with 25% of the number of pokes as their peak trial), to index their rates of habituation within a session. The second was to assess the extent of their habituation by the end of the third day. To this end, a ratio was created between the total nose pokes made in the middle ten minutes of the third day and the number of nose pokes in the middle ten minutes of either of the first two days, whichever was greater. On the sixth day, both holes were unplugged and the ratio of pokes in the new hole to pokes in the old hole was observed. This last measure served as an index of the animals exploratory tendencies because the opening of the second hole is an opportunity to interact with a “novel” component of the environment, compared to continued exploration of the first, more familiar, hole.
2.1.2.5. Tone and Light Habituation
Unlearned responses and habituation of responding to a tone and flashing light were assessed in a Med Associates Modular Mouse Chamber (ENV-307). The end walls of the chamber (14 × 9 cm) were constructed of aluminum, while the front and rear walls (15 × 9 cm) and ceiling (14 × 15 cm) were clear Plexiglas. The chamber floor was constructed of stainless steel grids (.5 mm diameter), under which was a bed of wood chips like those used in the animals’ home cages. The test chamber was enclosed in a larger sound and light attenuating box. A window in the front panel of this box allowed observation of the animal. Background noise (72 dBc at the center of the chamber) was produced by a ventilation fan. Dim background illumination (2 Lux at the center of the chamber) was produced by diffuse light that originated outside of the environmental enclosure.
A speaker grid (4 cm w × 8 cm high) was centered in (and flush with the surface of) the front wall of the chamber. When operated, the speaker produced a 5,000 Hz tone (40 dBc above background). The filament of a 2.5 cm diameter light bulb (GE 306) was horizontally centered 3 cm behind the exterior of the chamber’s rear wall, 9 cm above the chamber’s grid floor. When operated, the bulb flashed on and off at a rate of 2.2 Hz, and raised the illumination of the chamber’s interior (during the on phase) to 1080 Lux.
Animals were exposed to the test chamber and tested in response to the tone in a single session. After 6 min in the chamber, a 10 sec tone was initiated, and time spent orienting to the tone was recorded for each animal. An orienting response was defined as a cessation of forward movement accompanied by either head waving (a horizontal, side-to-side movement of the head) and/or turning in place. These characteristic movements in response to a novel tone are not typically observed in the absence of the tone, thus time spent orienting during the 10 sec tone could be recorded without normalization. Four tone presentations were scheduled at 2 min intervals (beginning 6 min after an animal’s placement in the chamber). However, a tone was not initiated until an animal’s head was located near the center of the chamber and oriented in a direction away from the speaker grid. Consequently, inter-trial intervals ranged from 2 min to 2 min and 22 sec (across the entire sample of mice). Animals were removed from the test chamber immediately following the fourth trial.
Three days after the completion of testing in response to a tone, animals were tested in response to a flashing light. Procedures were identical to that for the tone test, although rearing constituted the orienting response to light. Rearing was recorded whenever the animal raised both front paws from the grid floor. Although the light could be localized behind one of the chamber’s walls, it was also reflected from the remaining walls, and thus rearing was recorded regardless of whether the rearing response was accompanied by the front paws contacting a wall. The flashing light was initiated when the animal’s head was near the center of the chamber and facing in a direction away from the light source. Particularly when exposed to a novel environment, mice exhibit frequent rearing. Thus measurements of rearing in response to the 10 sec flashing light were calculated relative to any spontaneous rearing that occurred during the 10 sec period immediately preceding the light. Neither the baseline period nor the light period was initiated until any ongoing rearing had ended (and consequently, the baseline and test period were not necessarily contiguous, although for this sample, these periods were never separated by more than 2.6 sec.) For this sample of 39 mice, the average time spent rearing during the baseline period across all 156 trials was 0.92 sec.
2.2. Results
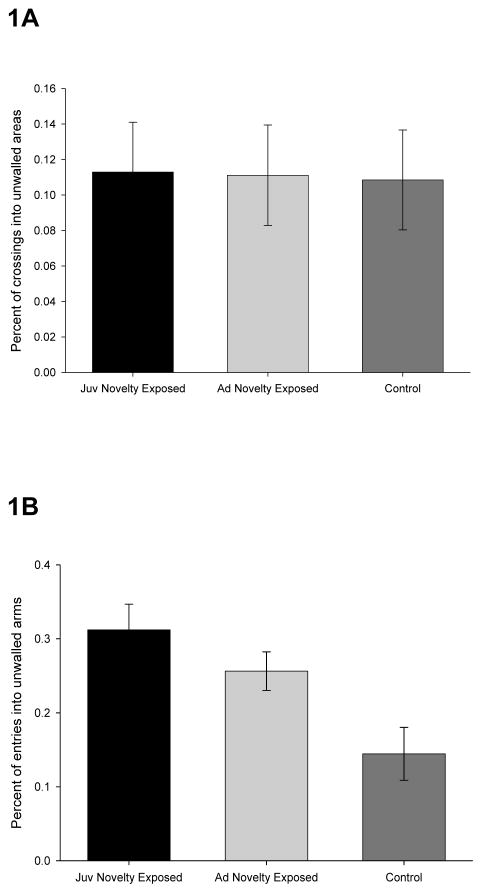
In the open field, assessed prior to exposure to novel environments, no difference among the three balanced groups was observed in either percent of entries into open quadrants (Figure 1A), F(2, 39) = .006, ns, or in total quadrant entries (i.e., open + walled quadrants; not illustrated), F(2, 39) = .015, ns. In the elevated plus maze, assessed after exposure to novel environments, mice exposed to the series of 12 novel environments were observed to have an increase in percent of entries into open arms (Figure 1B), F(2, 36) = 7.51, p < .005, reduced latencies to first enter an open arm (not illustrated), F(2, 36) = 7.87, p < .005, and increased total arm entries (not illustrated), F(2, 36) = 3.3527, p < .05.
Figure 1. Exposure to novel environments promotes an increase in exploratory behaviors.
A: Prior to exposure to a series of novel environments, three groups of animals were assessed in an open field. Percent of crossings into unwalled regions of the field are plotted as a function of group. Error bars indicate standard errors. B: Juvenile and young adult animals were exposed to a series of novel environments, while control animals received only handling. Animals were then assessed in an elevated plus maze. Percent of entries into open arms of the maze are plotted as a function of group. Error bars indicate standard errors.
Planned comparisons revealed increased exploration of open arms for both pre-pubescent (p < .001) and young adult (p < .05) animals that had been adapted to novel environments relative to controls. The same pattern of differences were found for latency to enter the first open arm, p < .005, for both juvenile and adolescent groups adapted to novel environments. These results indicate that exposure to a series of novel environments in both pre- and post-adolescent mice promoted an increase in behaviors indicative of exploration when those same animals are tested as young adults.
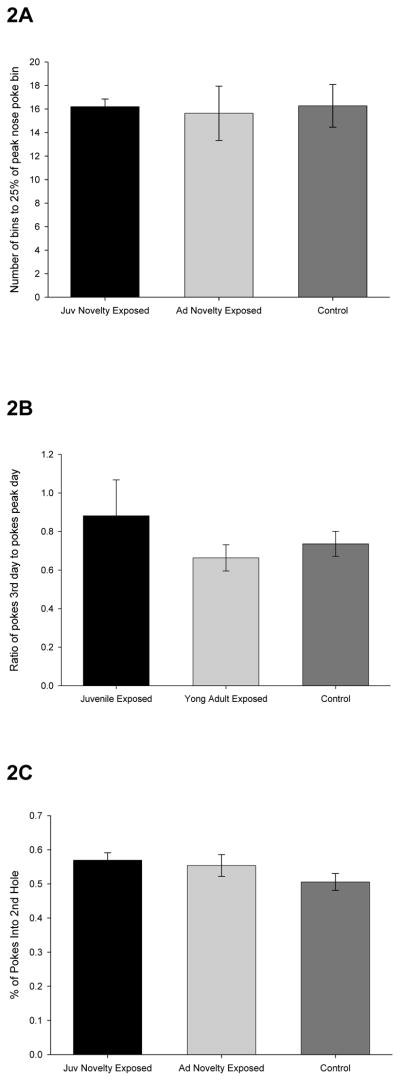
Having induced an increase in exploratory behaviors, we next asked if that increase in exploration was associated with an increase in rates of habituation. In nose poke habituation, when the first nose poke hole was opened, no differences were observed among groups in the number of bins taken to reach a pre-established habituation criterion (Figure 2A), F(2, 30) = .10, n.s., i.e., rates of habituation did not differ as a function of prior exposure to a series of novel environments. To examine whether habituation rates differed between sessions rather than within, the number of nose pokes in the middle ten minutes of the third session was compared to the number of nose pokes in the middle ten minutes of either of the first two sessions, whichever was greater. No differences were observed among groups on this measure (Figure 2B), F(2, 35) = .79, n.s. Finally, upon opening the second hole (after responding to the first hole had asymptoted), groups were not statistically different in terms of percentage of nose pokes made in the second (novel) hole (Figure 2C), F(2, 32) = 1.77, n.s.
Figure 2. Increases in exploration are not associated with increased rates of habituation.
After exposure to novel environments (which promoted an increase in exploration), rates of habituation were assessed in a nose poke apparatus. A: In the nose poke test (single hole open), number of bins to reach a level of nose poking that was 25% of that attained during initial exposure to the hole is plotted as a function of group. Error bars indicate standard errors. B: In the nose poke test (single hole open), ratio of the slope of the 3rd day to the slope of the peak day(1st or 2nd) plotted as a function of group. Error bars indicate standard errors. C: In the nose poke test (with a second, novel hole open), percent of pokes into the 2nd hole plotted as a function of group. Error bars indicate standard errors.
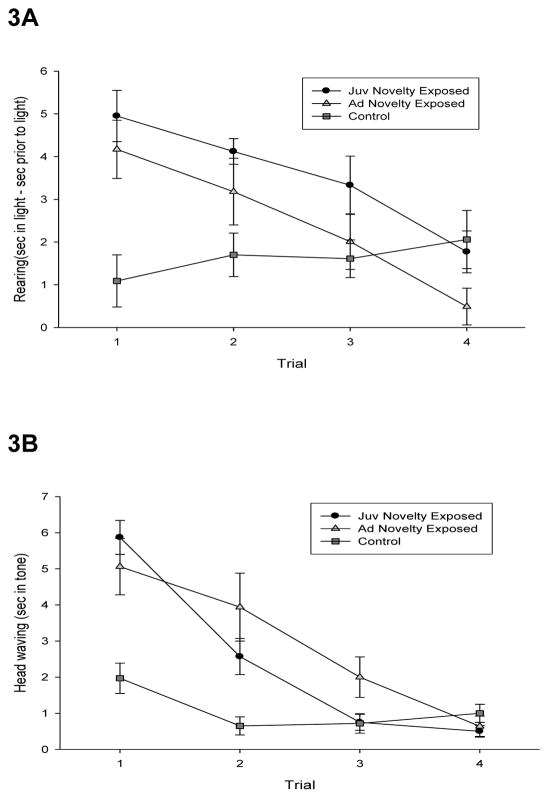
In the tone and light habituation tests, rates of habituation between control animals and animals exposed to the series of novel environments could not be compared because control mice responded to the test stimuli at a stable (unchanging), low levels throughout the period of testing. However, animals that had previously been adapted to novel environments exhibited significantly higher levels of responding to both the tone and the light. Like tests of exploration, this result suggests that adaptation to novel environments promoted orienting responses (a form of exploration) to the novel tone and light in the two groups so treated. On the first exposure to both the tone and the light, a main effect of exposure to novelty was found (Figures 3A and 3B), F(2, 32) = 22.69, p < .0001 and F(2, 32) = 18.78, p < .0001 respectively. While these data do not allow us to assess the degree to which exposure to novelty promotes habituation, it does provide independent confirmation of the effect of exposure to novelty on measures of exploration.
Figure 3. Exposure to novel environments promotes orienting responses to a novel light or tone.
After exposure to novel environments, orienting responses to a novel light and tone were assessed. A: Time spent rearing during a novel light less time spent rearing prior to the onset of the light plotted as a function of trial for each group. Error bars indicate standard errors. B: In the tone habituation test, seconds of head waving response to a novel tone plotted as a function of trial for each group. Error bars indicate standard errors.
2.3. Discussion
Prior to any treatment (exposure to novel environments), no differences among groups were found either in activity or behavioral patterns indicative of exploration in a standard open field test. Subsequently, animals were exposed to a series of novel environments (either in pre- or post-adolescence), and this treatment promoted an increase in behaviors indicative of exploration (on a later test in an elevated plus maze).
In addition to promoting exploration in an elevated plus maze, exposure to novel environments supported an increase in orienting responses toward both a novel tone and light stimulus. It is also noteworthy that in prior research, we observed that animals exposed to novel environments stepped from a novel elevated platform more quickly than did control animals [24]. In combination, these observations suggest that the effects of exposure to novel environments were not specific to tests in the elevated plus maze, and instead, had more broad impact on behaviors that can generally be described as indicative of exploration.
Most relevant to the present study is the observation that exposure to novel environments failed to increase rates of habituation in the nose poke apparatus. Therefore this manipulation can be concluded to have increased the propensity for exploration without having a commensurate effect on rates of habituation, effectively dissociating the two. This result leaves open the possibility that the often observed co-variation of exploration and general learning ability reflects an influence of learning on habituation (which might contribute to changes in patterns of exploration)
3. Experiment 2
3.1. Introduction
In order to directly assess the possibility that differential rates of habituation between fast and slow learners underlies the relationship between general learning abilities and exploration, it was necessary to equalize rates of habituation in fast and slow learners and to observe the resultant effect on the correlation between exploration and general learning performance. To this end, we observed the stability of the relationship between exploratory measures and general learning abilities under two different habituation conditions in two independent tasks. We hypothesized that when levels of habituation varied across individual mice (owing to differences in general learning ability), those variable levels of habituation would have commensurate effects on related exploratory behaviors. For instance, animals that habituate to the walled portions of a novel open field faster (i.e., learn faster) would be more prone to explore the unwalled portions of the field earlier in a bout of exploration. This could account for the common observation of a robust relationship between general learning abilities and measures of exploration in the open field. In contrast, if rates of habituation mediate the relationship between general learning abilities and exploration, when exploration is assessed at a point at which habituation is equal between fast and slow learners (e.g., later in a period of exposure to a novel field), the correlation between general learning performance and exploration should be attenuated. This can be thought of as a sequence of influences, where variability in general learning abilities influences habituation rates, which in turn influences variability in exploration. In this manner, eliminating variability in habituation would disrupt this chain of influences and would eliminate the relationship between general learning abilities and exploration.
Normalizing the level of habituation across animals can be achieved in one of two ways. First, all animals can be allowed to reach the similar asymptotic levels of habituation. In this manner individuals would presumably reach a habituative ceiling, thereby eliminating variability in habituation levels between mice and hence its effect on exploratory behaviors. Alternatively, exploratory behaviors can be measured when animals reach a specified level of habituation (i.e., reach a habituative criterion), regardless of how long it takes individuals to reach that level.
To achieve the first method of reducing variability in habituation levels, we administered an open field test which is substantially longer (12 min) than that used to observe a correlation between general learning ability and exploration in the field (i.e., 4 min). While a direct measure of habituation can not be obtained in this task, animals would presumably vary in the degree to which they would have habituated to the novel field early in a bout of exploration (e.g., at 4 min) relative to later in a bout of exploration (e.g., at 12 min). Thus we might anticipate that exploratory behavior during the first 4 min of a 12 min bout would better predict general learning performance than exploratory behavior in the last 4 min of a 12 min bout.
To achieve the second method of equating the level of habituation across individual animals, a nose poke exploration task was used. Here, animals explored two objects, across multiple sessions, by nose poking through a hole board. In the test session, one of the two objects was switched and exploration of the novel object was assessed. The level of habituation reached was left incomplete, although comparable levels were attained for all animals. This was accomplished by administering the critical test (i.e., replacement of one of two objects) after a number of training sessions which was known (based on pilot data) to produce incomplete levels of habituation. In addition, to eliminate variability (between animals) in habituation levels at the time of testing, animals were exposed to the test object after the same level of habituation (a pre-determined criterion) to the familiar objects was reached by each individual animal.
If differential rates of habituation (between animals of high or low general learning ability) underlie the relationship between general learning abilities and exploration, the relationship should be observed when levels of habituation are left variable across animals (i.e., during early time points in the open field or in initial exploration of a novel object) but should be eliminated when levels of habituation are more complete or are held constant across animals (i.e., during late time points in the open field or nose poke exploration assessed after all animals had reached a comparable habituation criterion).
3.2. Methods
3.2.1. Subjects
Twenty-four outbred CD-1 mice were obtained from Harlan Sprague Dawley, Inc. at 45 days of age and participated in both the open field and novel object exploration tests. For the open field experiment, 23 additional animals (for a total of 47 in the open field) were added to the analysis (these animals became available from an experiment that was being concurrently performed). Animals were acclimated to our laboratory until 62 days of age, during which time they were handled for 90 sec/day, 5 days/week. This handling insured that differential stress responses to the experimenters, and any associated effects on learning, were minimized. Animals were individually housed in clear boxes with floors lined with wood shavings in a humidity- and temperature-controlled vivarium adjacent to testing rooms. A 12hr/12hr light/dark cycle was maintained. All manipulations and testing occurred around the middle portion of the light cycle.
3.2.2. Apparatus and Procedure
3.2.2.1. Learning Battery
The order of testing was intended to provide a temporal separation between any two tasks that were motivated by either food or water deprivation (to minimize excessive physical strain and to mitigate potential cross-task influences due to motivational factors). In addition, the testing order was designed to separate tasks based on similar processes or motor requirements (e.g., mazes of a similar nature, activity vs. passivity). All animals were tested in the following order: Lashley maze, passive avoidance, odor discrimination, fear conditioning, water maze.
3.2.2.1.1. Lashley III Maze
The Lashley III maze consisted of a start box, four interconnected alleys, and a goal box containing a food reward. The maze was scaled for mice, and parameters were developed that supported rapid acquisition. Over trials, the latency of mice to locate the goal box decreased, as did their errors (i.e., wrong turns or retracing). The maze was constructed of black Plexiglas. A 2 cm wide x 0.1 cm deep white cup was located in the rear portion of the goal box, and 45 mg BioServe (rodent grain) pellets served as reinforcers. Illumination was 80 lux at the floor of the maze. The maze was isolated behind a shield of white Plexiglas to prevent the use of extra-maze landmark cues.
Food-deprived animals were acclimated and trained on two successive days. On the day prior to acclimation, when ad libitum food was removed near the end of the light cycle, all animals were provided with two food pellets in their home cages to familiarize them with the novel reinforcer. On the acclimation day, each mouse was placed in the four alleys of the maze, but the openings between the alleys were blocked so that the animals could not navigate the maze. Each animal was confined to the start and subsequent two alleys for 4 min, and for 6 min in the last (goal) alley, where three food pellets were present in the food cup. This acclimation period promotes stable and high levels of activity on the subsequent training day. On the training day, each animal was placed in the start box and allowed to traverse the maze until it reached the goal box and consumed the single food pellet present in the cup. Upon consuming the food, the animal was returned to its home cage for a 20 min interval (ITI), during which the apparatus was cleaned. After the ITI, the mouse was returned to the start box to begin the next trial, and the sequence was repeated for five trials. The errors (i.e., a turn in an incorrect direction, including those which result in path retracing) made before entering the goal box were recorded on each trial.
3.2.2.1.2. One-Trial Passive Avoidance
A chamber illuminated by dim (<20 lux) red light was used for training and testing. Animals were confined to a circular (“safe”) chamber (10 cm diameter, 8 cm high). The walls and floor of this chamber were white, and the ceiling was translucent orange. The floor was comprised of plastic rods (2 mm diameter) arranged to form a pattern of 1 cm square grids. A clear exit door (3 cm square) was flush with the floor of the safe compartment, and the door was able to slide horizontally to open or close the compartment. The bottom of the exit door was located 4 cm above the floor of a second circular chamber (20 cm diameter, 12 cm high). This “unsafe” chamber had a clear ceiling and a floor comprised of 4 mm wide aluminum planks that formed a pattern of 1.5 cm square grids oriented at a 45° angle relative to the grids in the safe compartment. When an animal stepped from the safe compartment through the exit door onto the floor of the unsafe compartment, a compound aversive stimulus comprised of a bright (550 lux) white light and a “siren” (60 dBc above the 50 dB background) was presented.
Animals learn to suppress movement to avoid contact with aversive stimuli. This “passive avoidance” response is exemplified in step-down avoidance procedures, where commonly, an animal is placed on a platform, whereupon stepping off of the platform it encounters a footshock. Following just a single encounter with shock, animals are subsequently reluctant to step off of the safe platform. The animals’ reluctance to leave the platform is believed not to reflect fear, because typical fear responses are not expressed in animals engaged in the avoidance response [26,27]. So as not to duplicate stimuli between tasks (see associative fear conditioning, above), upon stepping off the platform, animals here were exposed to a compound of bright light and a loud oscillating noise rather than shock. Like more common procedures, our variant of this task supports learning after only a single trial (i.e., subsequent, step-down latencies will be markedly increased).
Animals were placed on the platform behind the exit blocked by the Plexiglas door. After 4 min of confinement, the door was retracted and the latency of the animal to leave the platform and make contact with the grid floor was recorded. Prior to training, step-down latencies typically range from 8–20 sec. Upon contact with the floor, the door to the platform was closed and the aversive stimulus (light, noise, and vibration) was presented for 4 sec, at which time the platform door was opened to allow animals to return to the platform, where they were again confined for 5 min. This ITI is sufficiently long to demonstrate learning as opposed to recovery from the aversive stimuli [6]. At the end of this interval, the door was opened and the latency of the animal to exit the platform and step onto the grid floor (with no aversive stimulation) was recorded. The ratio of post-training to pre-training step-down latencies was calculated for each animal and served to index learning. It has been determined that asymptotic performance is apparent in group averages following 2–3 training trials; thus performance after a single trial reflects, in most instances, sub-asymptotic learning.
3.2.2.1.3. Associative Fear Conditioning
Two distinct experimental chambers (i.e., contexts; 32 l × 28 w × 28 cm h) were used, each of which was contained in a sound-and light-attenuating enclosure. These boxes were designated as “training” and “testing” contexts, and differed as follows: The training context was brightly illuminated (100 lux), had clear Plexiglas walls, no lick tube, and parallel stainless-steel rods (5 mm, 10 mm spacing) forming the floor. The test context was dimly illuminated (6 lux) the walls were covered with an opaque pattern of alternating black and white vertical stripes (3 cm wide), and the floor was formed from stainless-steel rods arranged at right-angles to form a grid of 8 mm squares. A water-filled lick tube protruded through a small hole in one wall of the test chamber, such that the tube’s tip was flush with the interior surface of the wall at a point 2 cm above the floor. Upon contacting the tube, the animal completed a circuit such that the number of licks per second could be recorded. This circuit was designed so that if an animal makes continuous contact with the tube (i.e., “mouthed” the tip), the circuit recorded 8 licks per second, a rate that approximates constant licking.
In this procedure, animals were exposed to a stimulus (i.e., a CS; white noise) that terminated at the onset of a mild footshock (i.e., a US). These white noise-shock (CS-US) pairings come to elicit conditioned fear responses when animals are subsequently presented with the white noise. This learned fear can be assessed in various ways. In the present studies, fear was indexed by CS-elicited suppression of ongoing drinking, as this measure is easily and precisely quantified. “Lick suppression” is conceptually analogous to the more commonly used measure of CS-elicited generalized “freezing” (i.e., during that time in which an animal freezes it will necessarily suppress its approach to and drinking from a lick tube). In our laboratory, lick suppression has proven to be of greater utility, given that the generalized freezing exhibited by mice is far less regular, and thus more ambiguous, than that typically observed in rats. To avoid any interaction with the training context, which itself acquires an association with shock (and the capacity to evoke fear), with the CS at the time of testing, training and testing were conducted in separate distinct contexts.
In the training chamber, a 0.6 mA constant-current scrambled footshock (US) was delivered through the grid floor. In both the training and test chambers, a 40 dB above background white noise CS was presented through speakers mounted at the center of the chamber’s ceiling.
Water bottles were removed from the animals’ cages near the offset of the light cycle on the day prior to acclimation. Water-deprived animals were then acclimated to the training and test chambers by placing them each in both contexts for 10 min on the day prior to training. Within several minutes of their first placement in the test context, water-deprived mice exhibit stable licking. When subsequently placed in the chamber, these animals will initiate licking within 5–10 sec and lick at relatively stable rates for the subsequent 3–5 min. Animals were given their water bottles for 90 minutes prior to the offset of the light cycle at the end of the acclimation day and the training day. Training occurred in the training context in a single 20 min session during which each animal was administered a white noise-shock pairing 7 and 14 minutes after entering the chamber. Each 10 sec white noise terminated with the onset of a 500 msec footshock. Asymptotic performance (as evidenced in group means) has been observed with these parameters after 4–6 such pairings. Thus, two pairings, in most instances, supports sub-asymptotic conditioned responding. At the end of the training session, animals were returned to their home cages for 60 min, after which they were re-acclimated to the test context for 10 min where they were allowed free access to the lick tubes. On the subsequent day (23–25 hours post-training), animals were tested. Each animal was placed in the test context whereupon after making 25 licks, the noise CS was presented continuously until the animal completed an additional 25 licks. The latency to complete the 25 licks during the pre-tone interval and in the presence of the one was recorded, with a 600 sec limit imposed on the second 25 licks, a limit not reached by any animal described here. With these measures, the ratio of latency to complete 25 licks in the presence of the CS to the latency to complete 25 licks prior to CS onset served as our index of learned fear.
3.2.2.1.4. Odor Discrimination and Choice
A black Plexiglas 60 cm square field with 30 cm high walls was located in a dimly lit (20 lux) testing room with a high ventilation rate (3 min volume exchange). Three 4L × 4W × 2 cm H aluminum food cups were placed in three corners of the field. A food reinforcer (30 mg portions of chocolate flavored puffed rice) was placed in a 1.6 cm deep, 1 cm diameter depression in the center of each cup. The food in two of the cups was covered (1.0 cm below the surface of the cup) with a wire mesh so that it was not accessible to the animal, while in the third cup (the “target” cup), the food was able to be retrieved and consumed. A cotton-tipped laboratory swab, located between the center and rear corner of each cup, extended vertically 3 cm from the cups’ surface.
Rodents rapidly learn to use odors to guide appetitively-reinforced behaviors. In a procedure based on one designed by Sara [28] for rats, mice learned to navigate a square field in which unique odor-marked (e.g., almond, lemon, mint) food cups were located in three corners. Although food was present in each cup, it was accessible to the animals in only one cup, the one marked by mint odor. An animal was placed in the empty corner of the field, after which it explored the field and eventually retrieved the single piece of available food. On subsequent trials, the location of the food cups was changed, but the accessible food was consistently marked by the same odor, mint. On successive trials, animals required less time to retrieve the food and made fewer approaches (i.e., “errors”) to those food cups in which food was unavailable. Using this procedure, errorless performance is typically observed within 3–4 training trials.
Immediately prior to each trial, fresh swabs were loaded with 25 ul of either lemon, almond, or mint odorants (McCormick flavor extracts). The mint odor was always associated with the target food cup. It should be noted that in pilot studies, the odor associated with food was counterbalanced across animals, and no discernible differences in performance could be detected in response to the different odors.
Food deprivation occurred in the same manner as it did in Lashley Maze. The night that food was removed from the animals’ cages, two chocolate flake reinforcers were placed in the home cage. The next day would normally be an acclimation day, but instead animals were given 60 minutes of free feeding at the same time of day they would have received it had they been acclimated to the apparatus. On the subsequent test day, animals received four training trials in the field with the three food cups present. On each trial, an animal was placed in the empty corner of the field. On Trial 1, the reinforcing food was available to the animal in the cup marked by mint odor. An additional portion of food was placed on the top surface of the same cup for the first trial only. The trial continued until the animal retrieved and consumed the food from the target cup, after which the animal was left in the chamber for an additional 20 sec and then returned to its home cage to begin a 6 min ITI. On Trials 2 through 4, the location of the food cups was re-arranged, but the baited cup remained consistently marked by the mint odor. On each trial, the latency to retrieve the food and errors were recorded. An error was recorded any time an animal made contact with an incorrect cup, or its nose crossed a plane parallel to the perimeter of an incorrect cup. Similarly, an error was recorded when an animal sampled (as above) the target cup but did not retrieve the available food.
3.2.2.1.5. Spatial Water Maze
A round black pool (140 cm diameter, 56 cm deep) was filled to within 24 cm of the top with water made opaque by the addition of a nontoxic, water soluble, black paint. A hidden 11 cm diameter perforated black platform was in a fixed location 1.5 cm below the surface of the water midway between the center and perimeter of the pool. The pool was enclosed in a ceiling-high black curtain on which five different shapes (landmark cues) were variously positioned at heights (relative to water surface) ranging from 24–150 cm. Four of these shapes were constructed of strings of white LED’s (spaced at 2.5 cm intervals) and included an “X”(66 cm arms crossing at angles of 40° from the pool surface), a vertical “spiral” (80 cm long, 7 cm diameter, 11 cm revolutions), a vertical line (31 cm) and a horizontal line (31 cm). The fifth cue was constructed of two adjacent 7W light bulbs (each 4 cm diameter). These cues provided the only illumination of the maze, totaling 172 lux at the water surface. A video camera was mounted 180 cm above the center of the water surface.
For this task, animals were immersed in a round pool of opaque water from which they can escape onto a hidden (i.e., submerged) platform. The latency for animals to find the platform decreased across successive trials. In this task, performance of animals can improve across trials despite the animals beginning each trial from a new start location. As demonstrated by Morris [29], rats performance in the water maze does not rely on fixed motor patterns (i.e., performance improves despite the animal’s irregular starting location) or the presence of discernable cues within the maze (e.g., visual, tactile, or olfactory signals). Instead, performance is dependent on the stability of extra-maze cues, or “landmarks”, and is said to reflect the animals’ representation of its environment as a “cognitive map.”
We have developed a protocol in which mice exhibit significant reductions in their latency to locate the escape platform within ten training trials. In our protocol, animals were confined in a clear Plexiglas cylinder on the safe platform for 5 min on the day prior to training. Second, a 10-minute inter-trial interval (ITI) was used, which is considerably longer than is typical (c.f., 90 sec). Lastly, the maze, surround, and water were black with visual cues that were constructed of patterns of lights.
On the day prior to training, each animal was confined to the escape platform for 300 sec. Training was conducted on the two subsequent days. On Day 1 of training, animals were started from one of the three unique locations on each of six trials. The pool was conceptually divided into four quadrants, and one starting point was located in each of the three quadrants that did not contain the escape platform. The starting point on each trial alternated between the three available quadrants. An animal was judged to have escaped from the water (i.e., located the platform) at the moment at which four paws were situated on the platform, provided that the animal remained on the platform for at least 5 sec. Each animal remained on the platform for a total of 20 sec, after which the trial was terminated. Trials were spaced at 10 min intervals, during which time the animals were held in their home cages. On each trial, a 90 sec limit on swimming was imposed, at which time any animal that had not located the escape platform was placed by the experimenter on to the platform, where it remained for 20 sec. Animals were observed from a remote (outside of the pool’s enclosure) video monitor, and animals’ performance was recorded on video tape for subsequent analysis. Day 2 of training proceeded the same as Day 1, albeit with four trials only. 60 minutes after the 10th trial the hidden platform was removed from the pool and animals were placed inside the maze for a 90 second probe trial.
3.2.2.2. Object Exploration & Habituation
In this task, animals were exposed to two objects (distinct Fisher Price, Little People animal and human figures) placed directly underneath two holes (spaced 2 cm under the opening) in a hole board consisting of an arena (35×52×18 cm rectangle with a black Plexiglas bottom and walls) positioned in front of a shield of white Plexiglas and illuminated by a dim (10 W) light placed behind the shield, so as to minimize the light. This task consisted of two conditions, differential habituation and asymptotic habituation, both of which occurred in this hole board apparatus. The order of the conditions was counter-balanced. Prior to each condition, animals were acclimated to the apparatus for 20 minutes on each of three consecutive days.
In the differential habituation condition, all animals were allowed to explore the same two objects for three trials of 300s followed by a 90s inter-trial interval (ITI), during which the animal was returned to its home cage and the apparatus and object were cleaned with a 67% alcohol solution. One day later, animals were returned to the apparatus for three more trials. On the first trial, the animal was allowed to explore the same two objects it experienced on the previous day. This was intended to mitigate any degradation of long-term memory as well as any initial burst of activity or dishabituation that could be present on the first trial. Prior work had determined that this amount of exposure supported sub-asymptotic levels of habituation to the two target objects. On the second and third trials, one of the two objects was replaced with a novel object, counter-balanced for which object was replaced. Of interest in this condition is the ratio of exploration of the novel object to exploration of the familiar object.
In the asymptotic habituation condition, all animals were exposed to the same objects for an extended period of time until each reached a set habituation criterion. Because animals habituate at different rates, this time period was different for all animals. Here, habituation was quantified after each trial until the animal reached a criterion of five successive trials over the course of two days in which the animal s mean time spent on object exploration (summed between both objects) is 15 seconds or less and equal to 28% or less of their total time spent on object exploration in their most active trial on the first day. Mice reached this criterion across a range of 4 to 11 days (notably, a minimum of one day more than was allotted in the differential [preasymptotic] habituation condition described above). On the following day, animals were exposed to a novel object in the same manner as in the differential habituation condition. Of interest in this condition is the number of trials taken to reach the asymptote criterion in addition to the measure taken to assess novel object exploration in the differential habituation condition.
3.2.2.3. Open Field
Assessment in the open field was performed as in Experiment 1, although here, behavior was monitored for 12 instead of 4 min. For data analysis, the 12 min was divided into three 4-min bins and time spent in open and walled quadrants for each of these time bins were recorded for each animal. Exploration during the first 4-min bin constituted the measure of open field exploration previously found to correlate with learning abilities. Measuring exploration in the second and third time bins enables comparison of the relationship between learning abilities and exploration in both early and later time points in the animals bout of exploration (where different levels of the habituation to the periphery of the field would presumably have been attained). Analysis involved converting animals individual percentages of crossings made in the interior of the field to z-scores relative to their experimental cohorts. To correlate open field performance with general learning abilities, factor scores (representative of aggregate learning performance) were also obtained relative to the animals experimental cohorts.
3.3. Results
Consistent with previous findings, positive correlations were found between animals performance on all learning tasks (Table 1). These data were subjected to a principal component factor analysis. A single factor accounted for approximately 40% of the variance in the performance of individuals across all learning tasks (Table 2). Although the sample size here is small, this degree of explanatory variance is comparable to that observed in prior studies [6,7] with larger sample sizes and different combinations of learning tasks. Factor scores were extracted from this analysis to serve as an index of animals aggregate performance across all tasks (i.e., their general learning abilities). (A factor score is closely analogous to an average z score of an animal s performance on each learning task, where the scores are weighted according to the loading of each individual task on the primary factor.)
Table 1.
Correlations (n=24) of Learning Tasks and Exploration in the open field.
| LM | WM | FC | PA | OD | OF | |
|---|---|---|---|---|---|---|
| LM | *0.56 | 0.22 | 0.23 | *0.65 | −0.28 | |
| WM | * 0.56 | 0.05 | 0.40 | *0.55 | −0.41 | |
| FC | 0.22 | 0.05 | 0.11 | 0.05 | −0.06 | |
| PA | 0.23 | 0.40 | 0.11 | 0.12 | −0.20 | |
| OD | *0.65 | *0.55 | 0.05 | 0.12 | 0.16 | |
| OF** | −0.28 | −0.41 | −0.06 | −0.20 | 0.16 |
= p < .05
Table 2.
Principal Components Analysis (n=24). Variables reflect performance of animals on five learning tasks as well as exploration in an open field.
| Factor 1 | Factor 2 | |
|---|---|---|
| Lashley Maze | 0.84 | −0.18 |
| Water Maze | 0.86 | 0.09 |
| Fear Conditioning | 0.23 | 0.12 |
| Passive Avoidance | 0.51 | 0.37 |
| Odor Discrimination | 0.71 | −0.65 |
| Open Field | 0.41 | 0.78 |
|
| ||
| eigenvalue | 2.44 | 1.22 |
| Percent variance explained | 40.8 | 20.4 |
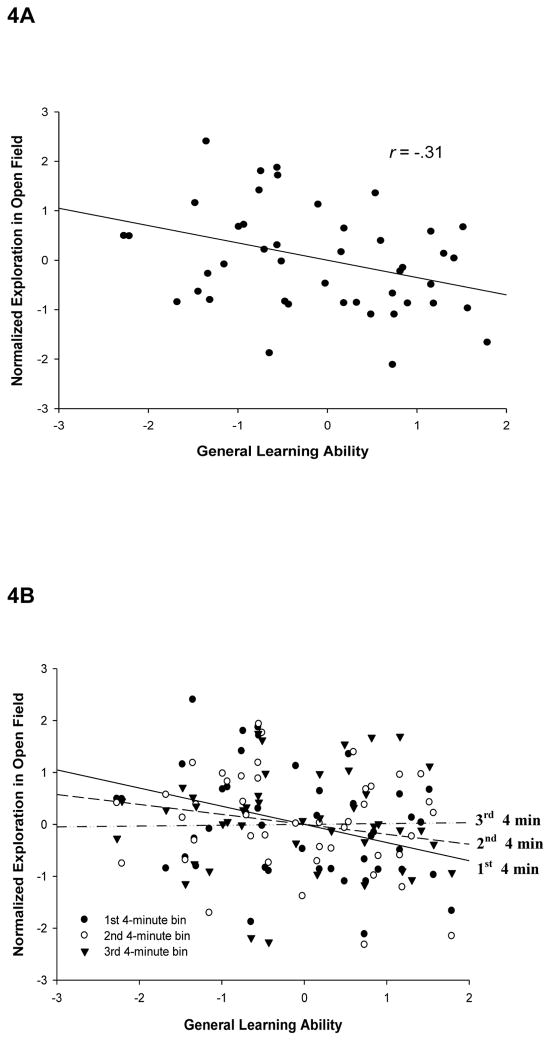
Consistent with results of previous analyses, the percentage of entries into open quadrants of a walled open field during an initial 4-min period was significantly correlated with individuals aggregate performance (i.e., factor scores) on all learning tasks, r(43) = −.31, p < .05 (Figure 4A). Note here that the negative correlation indicates that animals which performed more efficiently on the learning battery made a larger percentage of their open field crossings in the unwalled portions of the field, i.e., they engaged in more exploration. Furthermore, performance in this first 4 min loaded at .40 with animals performance on the learning tasks on the same principal factor (Table 2). However, across the full 12-minute session in the open field, this relationship diminished numerically and fell below the threshold of statistical significance in the subsequent two 4-min time bins, r(43) = −.14, n.s. and r(43) = .03, n.s. respectively (Figure 4B).
Figure 4. Exploration in an open field predicts general learning performance during intial, but not late, exposure to the novel field.
Animals were placed in a novel open field where exploration of the unwalled areas was observed across three consecutive 4 min blocks. Plotted is the ratio of crossings in the unwalled relative to walled areas of the field. A: Average learning rank plotted as a function of open field exploration (percent of crossings made into open quadrants) in the 4 min of exposure to the field. B: Average learning rank plotted as a function of open field exploration (percent of crossings made into open quadrants) in the first, second, and third 4 min blocks of time. While exploration was correlated with general learning performance during the first 4 min block, this correlation dissipated over the subsequent 4 min blocks.
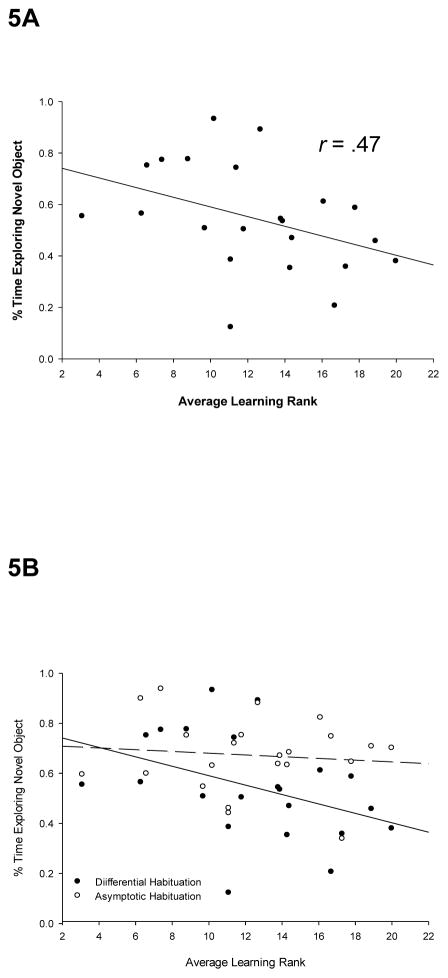
In the object exploration task, two measures of novel object exploration were taken. One of these measures was taken after an equal (subasymptotic) number of exposure trials for every animal, and therefore, the degree of habituation to the two sample objects can be assumed to vary across subjects at the time that the novel object was introduced. In this condition, a correlation was found between novel object exploration and general learning ability, r(22) = −.47, p < .02 (Fig 5A). Another measure of exploration of a newly-introduced object was taken after individual animals reached an equal habituation criterion to the familiar sample objects, requiring a different number of exposures (for each animal) to the two sample objects prior to the introduction of the novel object. In this manner, habituation levels to the familiar object was equated across all animals at the time at which the novel object was introduced. In contrast to the condition where habituation levels varied across animals, no correlation between novel object exploration and general learning abilities was observed when animals were equally habituated to the companion (familiar) object, r(22) = −.01, n.s., (Figure 5B).
Figure 5. Exploration of a novel object is correlated with general learning ability when the level of habituation to a comparison object varied across individual animals.
Animals were allowed to explore an object, then were simultaneously allowed access to a second, novel object. Plotted is the percent of time spent exploring the second, novel object. A: Average learning rank plotted as a function of exploration of a novel object at a time at which exploration (habituation) of the first object was incomplete. A significant correlation (r = .47) was observed. B: Average learning rank plotted as a function of exploration of the second, novel object after sub-asymptotic (differential) habituation to the comparison object or after the same habituation criterion to the comparison object was reached by all animals. A significant correlation was observed when the degree of habituation to the first object varied across animals, but not when habituation to that object was equal across animals.
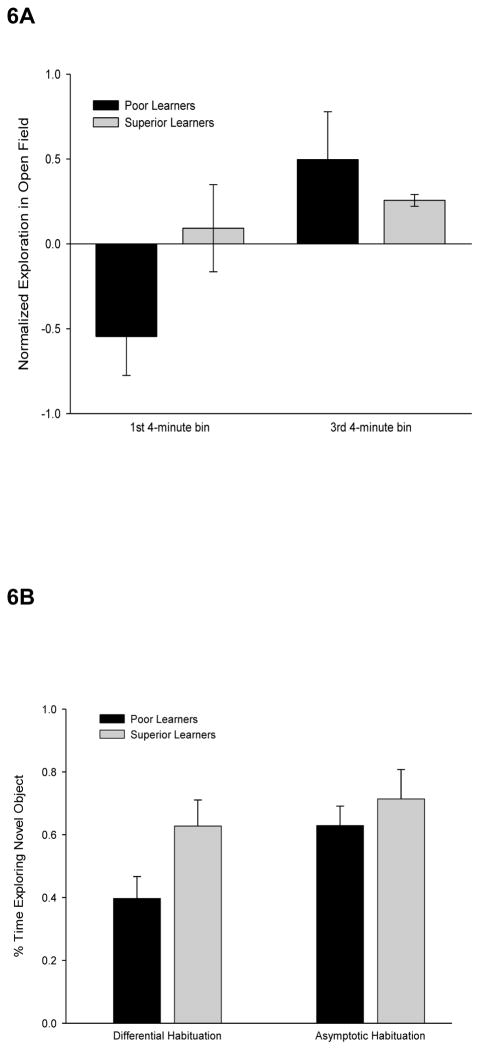
The dependence of the relationship between general learning abilities and exploration upon rates of habituation is further supported by the analysis of the top and bottom quartile of learners. In the first 4-min time bin of the open field test, the highest quartile of learners (n = 13) displayed significantly greater exploration of open quadrants than the lowest quartile (n = 13), t(24) = 2.87, p < .05 (Figure 6A). However, when exploration was measured in the last 4-min time bin, when habituation had presumably impacted the performance of both subsets of animals, both groups explored the open quadrants to a similar degree, t(24) = 0.42, n.s.
Figure 6. Animals of higher general learning performance are more exploratory early, but not late, when exposed to a novel field or object.
A: Top and bottom quartile of learners plotted for the first and third 4 min period of the open field test. Error bars indicate standard errors. B: Top and bottom quartile of learners response to a second, novel object early in the period of exposure to a comparison object (differential habituation) or when exploration of the companion object was asymptotic (and comparable) across animals. Error bars indicate standard errors.
A similar pattern of results were observed for novel object exploration in the hole board task. In the differential habituation condition (where the level of habituation varied across animals), the top quartile of learners (n = 5) exhibited significantly greater exploration of a novel object than the lowest quartile of learners (n = 5), t(8) = 2.80, p < .05 (Figure 6B). In contrast, in the asymptotic habituation condition (where the level of habituation was equated across animals), both groups of learners explored the novel object to a similar degree, t(8) = .78, n.s. Further, the level of exploration of the novel object was similar to that of the top quartile of learners in the differential habituation condition. In both cases, when habituation rates had a minimal influence on exploration, the lower quartile of learners explored no less than the top quartile of learners, indicating that faster rates of habituation drive better learners to explore more at an earlier time point.
3.4. Discussion
Based on the outcomes of prior experiments [23,24], here we proposed the possibility that differential rates of habituation (between animals of high and low general learning abilities) might mediate the relationship between general learning abilities and tests of exploration (such as in a novel open field). To assess this possibility, we proposed that levels of habituation could be manipulated such that they are either variable or held constant across a sample of mice, and suggested that only measures of exploration taken when habituation levels are variable across animals should correlate with those animals general learning abilities. In contrast, measures of exploration obtained when habituation levels are comparable across animals should be unrelated to general learning performance.
In two independent tasks, the present experiment provides evidence consistent with the above hypotheses. Early during a period of exposure to an open field (where levels of habituation are presumably most variable across mice), a relationship between general learning abilities and measures of exploration were correlated such that animals of higher general learning abilities exhibited more exploration (i.e., more time in the center, unwalled portions of the field). However, at later time points, where habituation to the walled portions of the field would have been more complete (and comparable across animals), the relationship between general learning abilities and exploration was no longer observed. In a nose poke exploration task, when levels of habituation to two sample objects were free to vary (owing to an equal but sub-asymptotic number of exposure sessions), a relationship existed between general learning abilities and measures of exploration of a novel object (that replaced one of the familiar objects). However, when levels of habituation to the familiar objects were equated across mice (by terminating exposure for each individual when a common habituation criterion was reached), the level of exploration of a new object did not differ across animals of high and low general learning abilities. Thus in both of these tasks, the relationship between general learning ability and exploration was only observed when habituation levels (either to a novel environment or object) varied across animals at the time of critical testing. This pattern of results provides strong evidence that habituation (itself a form of learning) mediates the relationship between general learning abilities and exploratory behaviors.
It is important to note that in the open field, the relationship between general learning abilities and exploration did not diminish over time due to a reduction in exploratory behaviors in animals with the highest general learning performance. Rather, after prolonged exposure to the open field, animals of higher general learning ability continued to express high levels of exploration, while animals of lower general learning ability increased exploratory behaviors to a level comparable to that of their faster learning counterparts. It should also be noted here that a similar pattern has been observed when animals received extensive training on other learning tasks. That is, animals learning performance on early trials (i.e., during acquisition) was more highly correlated with general learning abilities than was their performance on later (asymptotic) trials [7]. Based on both the present data and these earlier observations, it thus appears that patterns of “exploration” are, at least in part, a reflection of the rate at which animals learn about a novel environments (or objects), and rather than reflecting only a native behavioral tendency, is an expression (in part) of an animal s general learning ability.
4. General Discussion
We have demonstrated that a general cognitive factor exists in CD-1 outbred mice [6], and that the general learning ability of individual mice is consistently correlated with their degree of exploration in novel environments (including in an open field, elevated plus maze, and straight alleys [6,15,23]). A similar relationship has been observed in human infants [20,21]. Because the performance on various cognitive tasks load with exploration (an often asserted “noncognitive” behavior) on the same single factor (in a principal component analysis), we are left to question the exact nature of that factor. The experiments reported here represent the latest in a series of attempts to better understand the basis for the relationship between cognitive abilities and exploration [23,24]. For instance, we previously speculated that exploratory tendencies might modulate learning by regulating the manner in which animals interacted with the environmental contingencies upon which learning is based. However, when exploratory tendencies were up-regulated (by exposing animals to a series of novel environments), this increase in exploration had no commensurate effect on general cognitive performance [24], indicating that there was no direct causal influence of exploration on performance in our cognitive test battery.
Since it was determined that exploration did not directly influence general learning abilities, we next turned our attention to possible common mediators of both traits. Stress reactivity could commonly influence both learning test performance and tests of exploration since participation in any of these tests is to some degree anxiogenic to mice. However, systematic studies of multiple stress responses found no direct relationship between stress reactivity and general learning ability as determined by our batteries of learning tasks [15], indicating that this is not a likely mediator of this relationship. Further, using the administration of anxiolytic drugs, we determined that a reduction of stress reactivity had no consequent effect on general learning performance [23].
A third possibility that can account for the relationship between exploration and general cognitive abilities was that general cognitive abilities influence exploratory tendencies. Habituation (for instance, to a novel environment) is presumably modulated by general learning abilities (since it is itself a form of learning), and one could imagine that a mouse that habituates more quickly to the less anxiogenic areas of a novel environment would be more likely to enter the more anxiogenic portions of those same environments sooner, thus exhibiting a pattern of behavior that would suggest more “exploration”.
This mediation of the relationship between general learning abilities and exploration could feasibly occur in two directions: exploration could drive rates of habituation which could drive general learning abilities or general learning abilities could modulate habituation which could modulate exploration. The results of Experiment 1 here were inconsistent with the former possibility. In that experiment, we observed that a manipulation that produces robust increases in exploratory behaviors across a variety of tasks [24] produced no consequent increase in rates of habituation, indicating that exploration does not itself impact rates of habituation.
The results of Experiment 2 here served to assess the possibility that general cognitive abilities contribute to the regulation of habituation, which in turn modulates exploration. To this end, we observed the relationship between general cognitive abilities and measures of exploration under conditions (in an open field and novel-object exploration task) in which habituation was in its early stages (thus varying across animals) or was in its later stages or held constant across animals. The relationship between general learning ability and exploration was observed when the level of habituation (to the open field or familiar object) was variable across animals, but this relationship diminished as habituation became more complete or was equated across animals. Thus the relationship between general cognitive abilities and exploration exists only when the level of habituation varies across animals, indicating that it is a necessary mediator of the relationship between these two traits.
It is important to note that in both the later stages of exposure to a novel open field (when habituation levels were more complete and thus more comparable across animals), exploration was statistically similar between animals of high and low general learning ability. Furthermore, at these later stages of exploration, animals of lower general learning abilities exhibited levels of exploration that were comparable to those exhibited by animals of higher general learning ability early in the bout of exploration. This suggests that slower rates of habituation to the less stressful portions of novel environments prevented animals of lower learning capacity from exploring the more stressful portions of those environments at the same rate as animals of a higher learning capacity. In essence, when slower learners catch up to faster learners (re. their level of habituation to the walled portions of the field), they explore the unwalled portions of the field at the same level as their faster-learning cohorts. Therefore we can conclude that habituation rate, not native exploratory tendencies, forms the basis for the robust relation between measures of exploration and general learning abilities. This is consistent with the broader literature that finds that various measures of information processing are related to general intelligence [25].
The finding that open field performance correlates with general learning abilities early in the period of exposure to the field, but not later in that period of exposure is strikingly similar to the pattern of correlations observed between general learning abilities and either acquisition or asymptotic performance in a Lashley III maze [7]. In that task, performance on early (acquisition) trials was correlated with performance on other learning tasks, while those correlations diminished over trials and no longer existed on later trials when the task was well-learned. This suggests that Lashley III maze performance and exploration in the open field share a common influence. The above experiments provide strong evidence that this influence is rate of information processing, making information processing speed a good candidate for a neural mechanism for general learning abilities, and therefore general intelligence.
Nominally, the assertion that rate of information processing might regulate general cognitive performance (including that related to the rate of habituation) might seem to contradict previous publications from our laboratory (among others) which purport that the working memory system, and in particular its selective attention component, is a strong candidate process for the regulation of general intelligence [8,9, 30–38]. However, closer inspection reveals that we could feasibly be putting different labels on the same, or highly related, processes. Working memory can be defined as a system of temporary storage of incoming information. Selective attention can be defined as a related system of prioritizing the efficient storage of that information currently in working memory. Information processing can be defined as the neural encoding of incoming information. Thus all three heavily impinge on a common end product: the efficacy with which new information is stored and processed. Therefore these are highly interrelated processes, any or all of which could be or could strongly contribute to the construct characterized by general cognitive ability.
Research Highlights.
Performance across multiple cognitive tasks are commonly regulated by a “general” cognitive ability.
Exploratory tendencies predict general cognitive performance.
Exploration is in part determined by the rate at which animals habituate to novel environments .
Exploratory behavior n is in part determined by an animal’s general learning ability.
Acknowledgments
These experiments were supported by the National Institute of Aging (AG022698) and a Busch Foundation Grant to L.D.M. Thanks are extended to Ronald Gandelman and Tracey Shors for their helpful comments on this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Plomin R. Genetics and general cognitive ability. Nature. 1999;402(Suppl):C25–9. doi: 10.1038/35011520. [DOI] [PubMed] [Google Scholar]
- 2.Jensen AR. The g factor: the science of mental ability (human evolution, behavior, and intelligence) New York: Praeger; 1998. [Google Scholar]
- 3.Mackintosh NJ. IQ and human intelligence. Oxford: Oxford UP; 1998. [Google Scholar]
- 4.Duncan J, Seitz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, Newell FN, Emslie H. A neural basis for general intelligence. Science. 2000;289 (5478):457–60. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- 5.Kolata S, Light K, Wass CD, Colas-Zelin D, Roy D, Matzel LD. A dopaminergic gene cluster in the prefrontal cortex predicts performance indicative of general intelligence in genetically heterogeneous mice. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matzel LD, Han YR, Grossman H, Karnik MS, Patel D, Scott N, Specht SM, Ghandi CC. Individual differences in the expression of a “general” learning ability in mice. Journal of Neuroscience. 2003;23:6423–33. doi: 10.1523/JNEUROSCI.23-16-06423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolata S, Light K, Matzel LD. Domain-specific and domain-general learning factors are expressed in genetically heterogenous CD-1 mice. Intelligence. 2008;36(6):619–29. doi: 10.1016/j.intell.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolata S, Light K, Townsend DA, Hale G, Grossman H, Matzel LD. Variations in working memory capacity predict individual differences in general learning abilities among genetically diverse mice. Neurobiology of Learning and Memory. 2005;84(3):241–6. doi: 10.1016/j.nlm.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Kolata S, Light K, Grossman HC, Hale G, Matzel LD. Selective attention is a primary determinant of the relationship between working memory and general learning ability in outbred mice. Learning and Memory. 2007;14:22–8. doi: 10.1101/lm.408507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conway AR, Cowan N, Bunting M, Therriault DJ, Scott RB. A latent variable analysis of working memory capacity short-term memory capacity processing speed and general fluid intelligence. Intelligence. 2002;30:163–83. [Google Scholar]
- 11.Conway AR, Kane MJ, Engle RW. Working memory capacity its relation to general intelligence. Trends Cogn Sci. 2003;7:522–47. doi: 10.1016/j.tics.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Engle RW, Tuholski SW, Laughlin W, Conway AR. Working memory short-term memory and general fluid intelligence: A latent variable approach. Journal of Experimental Psychology: General. 1999;128:309–31. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- 13.Jarrod C, Towse JN. Individual differences in working memory. Neurosci. 2008;139:39–50. doi: 10.1016/j.neuroscience.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Sub HM, Oberauer K, Wittman WW, Wilhelm O, Schulze R. Working memory capacity explains reasoning ability-and a bit more. Intelligence. 2002;30:261–88. [Google Scholar]
- 15.Matzel LD, Townsend DA, Grossman H, Han YR, Hale G, Zappula M, Light K, Kolata S. Exploration in outbred mice covaries with general learning abilities irrespective of stress reactivity, emotionality, and physical attributes. Neurobiology of Learning and Memory. 2006;86:228–40. doi: 10.1016/j.nlm.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Galsworthy MJ, Amrein I, Kuptsov PA, Poletaeva II, Zinn P, Rau A, Rau A, Vyssotski A, Lipp HP. A comparison of wild-caught wood mice bank voles in the Intellicage: Assessing exploration daily activity patterns place learning paradigms. Behavioral Brain Research. 2005;157:211–7. doi: 10.1016/j.bbr.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Kazlauckas V, Schuh J, Dall’Igna OP, Pereira GS, Bonan C, Lara DR. Behavioral cognitive profile of mice with high low exploratory phenotypes. Behavior brain research. 2005;162:272–8. doi: 10.1016/j.bbr.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Poucet B, Chapuis N, Durup M, Thinus-Blanc C. A study of exploratory behavior as an index of spatial knowledge in hamsters. Animal Learning and Behavior. 1986;14:93–100. [Google Scholar]
- 19.Anderson B. Evidence in the rat for a general factor that underlies cognitive performance and that relates to brain size: Intelligence? Neuroscience Letters. 1993;153:98–102. doi: 10.1016/0304-3940(93)90086-z. [DOI] [PubMed] [Google Scholar]
- 20.Bornstein M, Sigman M. Continuity in mental development from infancy. Child Development. 1986;57:251–74. doi: 10.1111/j.1467-8624.1986.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 21.Vietze P, Coates D. Information-processing approaches to early identification of mental retardation. In: Wisniewski H, Snyder D, editors. Mental retardation: Research, Education, and Technology Transfer. New York: New York Academy of Sciences; 1986. pp. 266–276. [DOI] [PubMed] [Google Scholar]
- 22.McEwen BS. Effects of adverse experiences for brain structure and function. Biological Psychiatry. 2003;48:721–31. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- 23.Grossman H, Hale G, Light K, Kolata S, Townsend DA, Goldfarb Y, Kusnecov A, Matzel LD. Pharmacological modulation of stress reactivity dissociates exploratory tendencies and general learning abilities in outbred mice. Behavioral Neuroscience. 2007;121(5):949–64. doi: 10.1037/0735-7044.121.5.949. [DOI] [PubMed] [Google Scholar]
- 24.Light KR, Kolata S, Hale G, Grossman H, Matzel LD. Up-regulation of exploratory tendencies does not enhance general learning abilities in juvenile or young-adult outbred mice. Neurobiology of Learning and Memory. 2008;90(2):317–29. doi: 10.1016/j.nlm.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Sheppard LD, Vernon PA. Intelligence and speed of information processing: a review of 50 years of research. Personality and Individual Differences. 2008;44:535–51. [Google Scholar]
- 26.Morris RGM. Pavlovian conditioned inhibition of fear during shuttlebox avoidance behavior. Learning and Motivation. 1974;12:239–60. [Google Scholar]
- 27.Bolles RC. Avoidance and escape learning: Simultaneous acquisition of different responses. Journal of Comparative and Physiological Psychology. 1969;68:355–8. doi: 10.1037/h0027536. [DOI] [PubMed] [Google Scholar]
- 28.Sara SJ, Roullet P, Przybyslawski J. Consolidation of memory for odor-reward association: B-adrenergic receptor involvement in the late phase. Learning and Memory. 2001;6:88–96. [PMC free article] [PubMed] [Google Scholar]
- 29.Morris RGM. Spatial localization does not require the presence of local cues. Learning and Motivation. 1981;12:239–60. [Google Scholar]
- 30.Colom R, Rebollo I, Palacios A, Jaun-Espinosa M, Kyllonen PC. Working memory is (almost) perfectly predicted by g. Intelligence. 2004;32:277–96. [Google Scholar]
- 31.Daneman M, Carpenter PA. Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior. 1980;19:450–66. [Google Scholar]
- 32.Diamond A, Barnett WW, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007;318:1387–8. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. PNAS. 2008;105:6829–33. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klingberg T, Frossberg H, Westerberg H. Training of working memory in children with ADHD. J Clinical Exp Neuropsychology. 2002;24:781–91. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- 35.Light KR, Kolata S, Wass C, Denman-Brice Al, Zagalsky R, Matzel LD. Working memory training promotes general cognitive abilities in genetically heterogeneous mice. Current Biology. 2010;20(8):777–82. doi: 10.1016/j.cub.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oleson PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nature Neuroscience. 2004;7:75–9. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- 37.Rueda MR, Rothbart MK, McCandliss BD, Saccomanno L, Posner MI. Training, maturation, and genetic influences on the development of executive attention. PNAS. 2005;102:14931–6. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang YY, Posner MI. Attention training and attention state training. Trends in Cognitive Science. 2009;13:222–7. doi: 10.1016/j.tics.2009.01.009. [DOI] [PubMed] [Google Scholar]