Abstract
Keratins, the major structural protein of all epithelia, are a diverse group of cytoskeletal scaffolding proteins that form intermediate filament networks, providing structural support to keratinocytes that maintain the integrity of the skin. Expression of keratin genes is usually regulated by differentiation of the epidermal cells within the stratifying squamous epithelium. Amongst the 54 known functional keratin genes in humans, about 21 different genes including hair and hair follicle-specific keratins have been associated with diverse hereditary disorders. The exact phenotype of each disease mostly reflects the spatial level of expression and types of the mutated keratin genes, the positions of the mutations as well as their consequences at sub-cellular levels. The identification of specific mutations in keratin disorders is the basis of our understanding that lead to reclassification, improved diagnosis with prognostic implications, prenatal testing and genetic counseling in severe cutaneous keratin genodermatoses. A disturbance in cutaneous keratins as a result of mutation(s) in the gene(s) that encode keratin intermediate filaments (KIF) causes keratinocytes and cutaneous tissue fragility, accounting for a large number of genetic disorders in human skin and its appendages. These diseases are characterized by a loss of structural integrity in keratinocytes expressing mutated keratins in vivo, often manifested as keratinocytes fragility (cytolysis), intra-epidermal blistering, hyperkeratosis, and keratin filament aggregation in severely affected tissues. Examples include epidermolysis bullosa simplex (EBS), keratinopathic ichthyosis (KPI), pachyonychia congenital (PC), monilethrix, steatocystoma multiplex and ichthyosis bullosa of Siemens (IBS). These keratins also have been identified to have roles in cell growth, apoptosis, tissue polarity, wound healing and tissue remodeling.
Keywords: epidermal keratin, epidermal differentiation, intermediate filaments, genodermatoses, keratin gene mutation, keratinopathic genodermatoses, skin fragility, epidermolysis bullosa, epidermolytic ichthyosis, Gene therapy, pharmacologic therapy
Structure and function of the skin
The Human skin is the largest organ of the body, being strategically located at the interface between the interior and exterior, providing the first line of defense against external insults. Histologically, the skin reveals a built-in synchrony in the outer most epidermis and delineates the existence of a tremendous degree of communication and coordination between adjacent and non-adjacent cell layers in the tissue culminating to mediate a multiplicity of functions. The skin is composed of a multilayered, non-vascularized, stratified squamous epidermal tissue overlying the thick fibrous connective dermal tissue, which then sits on the hypodermis (Figure 1a). The multilayered epidermal sheet comprises four distinct cell layers. The basal layer contains the partially characterized epidermal keratinocyte stem cell population. The basal keratinocytes divide and are committed to terminal differentiation, forming the suprabasal and upper layers of the epidermis. The thicker spinous (suprabasal) layers overly the basal layer, and as they move up during differentiation the cells gradually become flattened as they enter the granular layer where the cells collapse and commence nuclear and organelle degradation and active lipid and protein secretion. This protein and lipid secretion together with cell flattening is critical in maintaining the barrier function of the uppermost, cornified layer that are constantly desquamated for tissue homeostasis. This cornified layer composed of flattened corneocytes and lipid/protein complexes provides the barrier against water loss and external insults.
Figure 1. Skin structure and keratin intermediate filament in triton extracted cultured keratinocyte cytoskeleton.
(a) Cross section of the human skin tissue showing the three distinct skin components, (b) Triton X-100 extracted keratinocyte cytoskeleton in culture stained with keratin 14 antibody (LL002; green filaments) shows a dense network of keratin intermediate filament bundles. The protein scaffold linked to its associated complexes forms the main resilience structure of the epithelial keratinocytes. (Reproduced from [133] with permission from the publisher)
It is increasingly becoming clear that mutations in many cutaneous-associated keratin genes lead to a variety of genetic skin diseases, characterized by compromised specified cell-tissue integrity, thus impairing the ability of the skin to form a proper barrier and withstand constant physical insults [1, 2].
Epithelial keratin biology
a) Intermediate filaments (IFs) and the keratin IF (KIFs)
The cell cytoskeleton of all multicellular organism consists of three abundant filament systems which play important roles in the organization, mechanical integrity and strength of tissue cells: the actin microfilaments (MFs; 7–10nm diameter), intermediate filaments (IFs; 10–12 nm diameter), and interconnected microtubules (MTs; 25 nm diameter). Each filament system is built from a family of proteins with cell-tissue specific regulation of expression, with each protein family being encoded by the corresponding gene family. IFs are by far the most complex of the cytoskeletal proteins with at least 60 different IF proteins subcategorized into six broad types based on tissue-specific expression, sequence similarity and protein structure (for details see www.interfil.org).
The keratin intermediate filaments (KIFs) are the most abundant structural IF protein constituent in the cytoplasm of keratinocytes which forms the epidermis. KIFs are encoded by large and conserved multigene family coding proteins that form a network of 10-to12-nm wide KIFs. KIFs network represents about three-quarters of known IF in humans and builds into a dense, three-dimensional transcellular and highly dynamic cytoskeleton network structure spanning between the nucleus, extending to the cell periphery, where they anchor and interact with cell-cell (desmosomes) and cell-matrix (hemidesmosomes) adhesion complexes (Figure 1b) [3, 4]. This organization provides structural stability, flexibility, and ensures the mechanical integrity of the different epithelial cells and their specific tissues. The keratins vary in size between 40–70 kDa and are divided into two sizes based on molecular weight: the smaller or low molecular weight acidic type I (40–64 kDa, with PI: 4.7–6.1) and the larger or high molecular weight neutral-basic type II (52–70 kDa, with PI: 5.4–8.4) subgroups of IF proteins [5]. A novel consensus nomenclature for mammalian keratin genes and proteins has been established and grouped into three categories: (1) epithelial keratins, (2) hair keratins, (3) keratin pseudogenes [6, 7]. The nomenclature for both genes and proteins includes 28 type I (K9, K10, K12–K20, K23–K28, K31–K40) and 26 type II (K1–K8; K71–K86) keratins, which together forms two clusters of 27 genes each. In the human genome, the genes encoding type I and type II keratins are mainly clustered at two different loci on chromosomal regions 17q12-q21 and 12q11-q13, respectively. The epidermal type I keratin genes e.g. KRT1, KRT2 and KRT5, each comprise 9 exons, whereas the genes coding for epidermal Type II keratins e.g. KRT10 and KRT14, each consist of 8 exons (see www.interfil.org;[8]).
b) Structural and assembly properties of KIFs
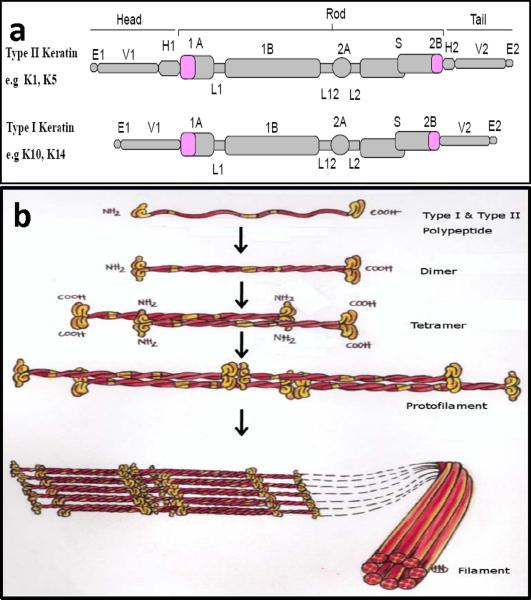
Similar to all IFs, keratins share a head-rod-tail structural domain organization, with the basic polypeptide structure consisting of a central α-helical coiled-coil rod domain of ≈ 310 to 315 amino acids in size. The central alpha-helical rod domain is composed of four helical segments (1A, 1B, 2A and 2B) that are interrupted by three short non-helical flexible linkers regions (L1, L12 and L2), and are flanked by variable, non-helical amino-terminal head and carboxy-terminal tail domains (Fig. 2a). The rod domain is composed of repeats of seven amino acid residues (a-b-c-d-e-f-g)n termed “heptad repeats”. Positions “a” and “d” are occupied by hydrophobic residues that are considered crucial for the coiled-coil formation. In addition, near the middle of the 2B domain is a discontinuity in the heptad repeat (helix inversion), where the heptad pattern is interrupted, giving rise to the “stutter” region. This region might play specific roles in the elongation and rotational characteristics of keratins [9]. This helical segment is highly conserved among IF and does not participate in the formation of the coiled-coil dimer [10, 11]. The start of the 1A rod domain and the end of the 2B rod domain, the so-termed helix initiation (HIP) and helix termination peptides (HTP) respectively, comprise ~20 amino acid sequence motifs that are most highly conserved among the different keratins. These motifs play a critical role in the overlapping interactions during KIF assembly and thus mutations in these motifs interfere with the early stages of filament elongation [12, 13]. Because of this, the helix boundary motifs are mutational “hot spots” in almost all inherited keratin disorders. The terminal head domain regions consist of the subdomains variable (V1) and homologous (H1) and the terminal tail domain regions of subdomains H2 and V2, and the end (E) domains (Fig. 2). Variations in the head and tail domains account for much of the diversity among the individual keratin proteins within one group, and results in type II keratins being larger and more extensible than their type I counterpart [14]. The end domains mediate interactions with other filaments and cellular proteins and serve as substrates for posttranslational modifications that regulate structure, organization and function [15, 16].
Figure 2. Molecular structure and assembly of keratin intermediate filaments (KIF).
(a) Schematic representation of type I and type II keratin polypeptide domain structural organization. Of the 54 different human keratin genes, each keratin molecule consists of a central alpha helical rod domain which is composed of four helical segments, 1A, IB, 2A and 2B that are interrupted by three flexible non-helical linker domains L1, L12 and L2. The rod domain begins and ends with highly conserved sequence motifs, helix initiation (HIP) and helix termination (HTP) peptides and is flanked by head and tail domains, respectively, (b) Keratin intermediate filaments assembly; Keratin polymerization obligatorily begins with the formation of coiled-coil obligate heterodimer structures involving winding around each other of the central rod domains of type I and type II polypeptides, a requirement underlying the pair wise transcriptional regulation of keratin genes in vivo. The heterodimers then associate (side-by-side) and assemble in an overlapping staggered and antiparallel fashion to form stable tetramers. Tetramers then associate end-to-end to form protofilaments and finally, four protofibrils laterally build keratin intermediate filaments. Each filament contains approximately eight protofilaments wound around each other in a rope-like structure, forming the 10–12 nm wide KIF network. (Reproduced from [133] with permission from the publisher)
The filamentous stage of keratins constitute of heteropolymeric pairing of one type I and one type II keratin molecule. Heterodimer formation is achieved by coiled-coiled association of the corresponding rod domains where the two participating monomers exhibit a parallel, in-register alignment. The heterodimers align laterally in an overlapping and antiparallel fashion to form tetramers which then polymerize to elongated chains and form KIFs through lateral packing [17] (Fig. 2b).
General features of inherited cutaneous keratin disorders
Besides providing structural function, accumulating evidence from human disorders and mouse genetics over the years have shown much more complex functional roles for the keratin cytoskeleton, culminating to the involvement in additional five broadly defined functions: apoptosis regulator, cytoarchitecture, stress response, protein synthesis and organellar and vesicle (re)distributor functions [18].
Keratins form a complex signaling array platform by interacting with various kinases, adaptor and apoptotic proteins to regulate several functions involved in cell growth and cell cycle progression [19, 20]. They are known to regulate apoptosis via several mechanisms [21], act as a phosphate “sink” for stress-activated kinases in order to prevent the activation of pro-apoptotic substrates [22] or may regulate key effectors of the stress-induced metabolic responses through phosphorylation of specific epitopes on keratins [19, 23]. Additionally, during physiological wound healing conditions, keratins may inculcate different intracellular signaling pathways that culminate in regulation of protein synthesis and cell size.
Progress in our understanding of the molecular basis of pathologies of cutaneous keratins has formed the basis for re-classification, improved diagnosis, pre-implantation genetics with prognostic implications, whereas the discovery of specific mutations has facilitated genetic counseling and prenatal testing for severely affected families [24, 25]. Increased knowledge regarding the regulatory functions of keratins and the molecular dissection of the pathologies may provide potentially new targets for the development of novel therapeutic strategies to counteract the consequences of these incapacitating keratinopathic genodermatoses.
Keratin-related genetic cutaneous disorders
Several human diseases are known to be caused by defects in genes which encode IF proteins. In the human epidermis and associated skin appendages, pathogenic mutations in the coding sequence of keratins and their associated linker proteins have been discerned as the molecular basis of a vast majority of cutaneous disorders. These wide ranges of abnormal genetic skin, appendages and membrane fragility pathologies are commonly termed genodermatoses, and are known to be caused by mutation in several intermediate filaments and associated linker proteins. These include about 21 different keratin genes, as well as hair and hair follicle-specific keratins (see the intermediate filament database www.interfil.org; [8]). Since the last 2 decades, when the first indications that dominant-negative mutations in basal keratins K5 or K14 elicit the human skin blistering disorder EBS (reviewed in [25–27]), our understanding of the molecular basis of several other genodermatoses due to defects in keratin genes have considerably advanced. In most conditions, the associated pathology results from fragile keratinocytes expressing the mutated keratin protein. These diseases commonly termed keratinopathic genodermatosis are individually rare (typically, less than 1:25-50,000 live births), but can be devastating and equally incapacitating to affected patients, incurably affecting their quality of life, and are occasionally lethal in severe episodes. For most of these disorders, there exist a good correlation between the type of mutated keratin gene, the nature and position of the mutation in the polypeptide, the extent to which the mutation alters the properties of keratin assembly, and the severity of the clinical phenotype.
Over 90% of pathogenic mutations in keratinopathies are missense mutations with a small number of small in-frame insertion vs. deletion mutations and a few intronic splice site defects leading to larger in-frame deletions. At the protein level the consequences of mutant polypeptides are the expression at normal or near-normal levels with substitutions, deletions or insertion of a different amino acid. The heterodimers of the protein formed from one mutant protein and the wild-type keratin partner then integrate the keratin network rendering the cytoskeleton susceptible to collapse upon mild or no physical stress [27, 28]. Additionally, knowledge about mutations in KIF and their associated linker proteins including our understanding of their site specific gene expression and the reflection of the sites of phenotypic expression pattern have delineated various subtypes and variants of each keratin-related disorders, including epidermolysis bullosa simplex (K5, K14), keratinopathic ichthyosis (Kl, K2, K10), palmoplantar keratoderma (K9), type I pachyonychia congenita (K6a and K16),7 type II pachyonychia congenita (K6b and K17), and monilethrix (K81, K83 and K86) etc,(see www.interfil.org and [6, 7, 14, 25]).
Epidermolysis bullosa (EB)
EB represents a large and heterogeneous group of genetically defined mechano-bullous skin fragility disorders characterized by increased fluid filled blister formation or erosion of the skin and mucous membrane occurring in response to mild or no physical trauma. About 1 in 20,000 individuals are affected by one of the EB types. Electron microscopy and immunofluorescence antigen mapping have been fundamental in our understanding of these genodermatoses and revised classification which established four major subtypes. Based on the level of skin cleavage within the cutaneous basement membrane zone (BMZ) [29], we now distinguish between: (i) the intra-epidermal EB simplex with cytolysis and fluid filled blister formed intra-epidermally within the basal keratinocytes, usually caused by mutations in either keratin 5 or keratin 14 gene, (ii) the intra-lamina lucida, junctional EB, with the split occurring at the level of the lamina lucida and resulting in blistering with no obvious structural abnormality of tonofilaments, which is caused by defects in laminin-332, collagen XVII, or a6ß4 integrin, (iii) the sub-lamina densa, dystrophic EB, with cleavage at the superficial dermis, underneath the lamina densa at the level of the anchoring fibrils (which link the epidermis and dermis), caused by mutations in the gene that encodes collagen VII, often with documented abnormality in tonofilament and (iv) mixed types, Kindler syndrome. The intra-epidermal EBS is further separated into two subgroups, the basal and suprabasal types, respectively, including the newly described entities, such as EBS caused by desmoplakin or plakophilin mutations [29]. To date, all EB subtypes have been characterized at the ultra-structural and molecular levels with more than 1000 mutations being described in more than 21 genes encoding for structural proteins in the human skin and its appendages, as well as can involve of mucous membranes. Amongst these are more than 150 KRT5 and KRT14 mutations (see IF database www.interfil.org; [8] and www.hgmd.cf.ac.uk).
Epidermolysis bullosa simplex (EBS)—diseases of K5/K14 mutations
Epidermolysis bullosa simplex (EBS) is a group of rare predominantly autosomal dominant genetic skin diseases affecting approximately 1:25000–50 000 live births of the population[27, 29]. EBS is the first identified and best studied variant of keratin disorders and has become the prototype for understanding disease pathology and genotype–phenotype correlations within a broad spectrum of keratin disorders. In EBS, two major subtypes have been defined: suprabasal and basal EBS [29]. Within the scope of this review, only subtypes caused by keratin mutations will be described. The suprabasal EBS types such as lethal acantholytic EB and plakophilin deficiency caused by mutations in desmoplakin and plakophilin-1, respectively, including the basal EBS subtypes; EBS with muscular dystrophy (plectin mutations), EBS with pyloric atresia (mutations in plectin and α6β4 integrin) and EBS Ogna (plectin mutations) will not be further discussed (for details of theses subtypes see www.interfil.org; [25]). EBS is the most common subtype of EB with clinical manifestations usually present at birth, characterized by intra-epidermal blistering due to cell degeneration within the basal layer of the epidermis and often with involvement of mucosal epithelia. Blistering is associated with mechanical stress and the blisters tend to heal without scarring. EBS is usually caused by mutations in keratin KRT5 or KRT14, and the pathogenic mutations usually occur within regions of the keratin genes that encode “hotspots” in the protein structure, namely the H1 domain of the head region (only for type II keratins), two segments (1A and 2B) of the rod domain, and the central linker region L12[8]. Upon mild physical trauma, the keratin filament network is easily compromised, resulting in structural failure of the affected epithelial keratinocytes and loss of tissue integrity (reviewed in [14, 30]). The degree of severity of the clinical phenotype has been directly linked to the position of the pathogenic mutation along the keratin polypeptide backbone, although more recent reports provide some exceptions to this, whereby also milder disease phenotypes are caused by pathogenic mutations in the conserved hot spot region of the KRT genes [31, 32]. However, other additional factors may as well affect and exacerbate disease severity [33, 34]. Based on the clinical severity, recent reclassification distinguishes four major EBS subgroups: a) the generalized Dowling-Meara EBS (EBS-DM; OMIM 131760), b) other generalized non-DM EBS (gen non-DM EBS; OMIM 131900), c) the localized EBS (EBS-Loc; OMIM 131800) and d) EBS with mottled pigmentation (EBS-MP; OMIM 131960) [29].
In both generalized forms, the most severe Dowling-Meara subtype and the milder non- Dowling-Meara subtype, also previously known as the Koebner form, present generalized and pronounced blistering at birth, while the localized EBS is milder with blistering confined to palmar and plantar regions of the body. Nevertheless, other not yet identified genetic or epigenetic modifiers and environmental factors, such as patient lifestyle and climate condition, clearly influence the phenotypic expression as different subtypes of EBS have been associated with the same mutation in several instances [35–37].
The generalized Dowling-Meara subtype (EBS-DM) is the most severe form being manifested at birth with erythema, widespread blistering, erosions and areas of denuded skin presenting spontaneous clusters of blisters also called “herpetiform” at multiple sites of the body which improves with age (Fig. 3 a). Progressive palmoplantar keratoderma becomes the chief complaint in adulthood. Other hallmarks include callosite formation (Fig. 3 b), secondary bacterial infections and sepsis, involvement of mucous membranes, nail dystrophy, healing of lesions without scarring and involvement of the oral mucosa. Inflammation especially of haemorrhagic blisters may be followed by transient milia formation, as well as healing of skin with hypo- and hyperpigmentation [27]. Diagnostic criteria include ultrastructural examination of skin biopsies showing the characteristic clumps or electron dense aggregates composed of K5 and K14 KIFs protein in the cytoplasm of basal keratinocytes harboring the mutation [38].
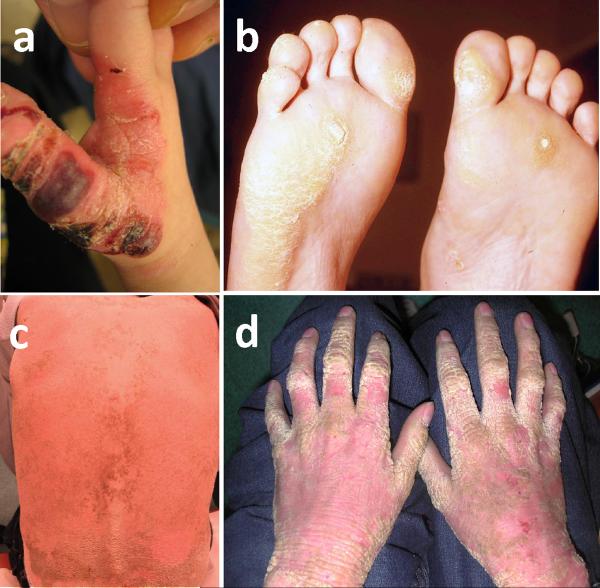
Figure 3. Clinical features of epidermolysis bullosa simplex (EBS) and epidermolytic ichthyosis (EI).
(a) A child's hand with severe generalized Dowling-Meara form of EBS, characterized by widespread herpetiform blister that heals without scar formation, (b) An Adult with severe EBS-DM whose feet present with painful plantar callosities, (c) Epidermolytic ichthyosis patient showing sharp massive hyperkeratosis of the lower back and (d) a diffuse hyperkeratosis of the hand and flexures with an erythromatous background. (Reproduced from [133] with permission from the publisher)
The pathogenic mutations in EBS-DM are usually missense mutations which reside in the highly conserved helix boundary domains (the HIP of the 1A segment and the HTP of the 2B segment). More than one-third of EBS-DM cases are caused by a unique mutation in the KRT14 gene that affecting a highly conserved arginine (Argl25) located within the HIP of K14, a known “hot spot” that is most likely due to a conserved hypermutable CpG dinucleotide in all type I keratins. Here, the cysteine (TGC) or histidine (CAC) often substituted for arginine codon (CGC) when mutated.
The generalized non-DM EBS (gen-non-DM EBS), is a more moderate subtype characterized at birth or in early infancy by generalized blistering, without clustering. The clinical presentation in majority of the cases is moderate, without any extra-cutaneous involvement, but with palms, soles and extremities being mostly affected and often in response to minor trauma and induced by increased ambient temperature. The disease-associated mutations in the gen non-DM EBS are more centrally located in the rod domain and sometimes more widely distributed along both KRT5 and KRT14 genes, including the non-helical linker segments (reviewed in [14]).
The localized EBS (EBS-loc), a clinically mild phenotype and the commonest form previously known as EBS-Weber Cockayne (EBS-WC), is characterized by late appearing skin blistering restricted to areas of greater friction or trauma such as hands and feet. Children tend not to be affected until they start to walk or crawl and the blistering worsen's with warm humid weather. Secondary infections of blistering lesions on the feet are the most common complication. Some affected individuals suffer from focal keratoderma (thickening of the skin of hands and feet). Mutations in EBS-loc are most frequently found in four clusters lying outside of the helix boundaries of K5 or K14, including the non-helical L12 linker motif, or in the the amino-terminal homologous domain (H1) of K5, or in the 2B segment of K14 (see www.interfil.org; [8]. However, exceptions do exist and patients with mild phenotype (EBS-loc) have been identified with mutations in the conserved 1A and 2B helix hotspots [31, 32, 39]. Ultrastructural abnormalities of the cytoskeleton are far less severe than those seen in EBS-DM and some cases of gen-non-DM EBS.
In EBS, genotype–phenotype correlations are quite well established. In the majority of cases the clinical severity relates to the location of the mutations and the degree to which these mutations perturb keratin structural assembly. Generally, six mutation hotspots are known to exist: mutations in EBS-DM are generally restricted to the helix boundary peptides of K5 and K14 which mark the importance of these structures for KIF assembly and elongation. In milder forms of EBS, the underlying mutations occur outside the helix boundary motifs, namely the H1 domain of K5, the second half of the 1A domain, the L12 domain and the central 2B domain of both proteins. Since such mutations do not interfere with the elongation process during filament assembly, ultrastructural examination reveals apparently normal filament, but consists of structurally weakened filaments that break upon mild mechanical stress. Conservative amino acid changes in the helix boundary motifs as well as complete disintegration of the amino acid sequences by frame shift mutations may also result in milder disease phenotypes [40]. Thus, based on the location of a mutation in K5 and K14 one can possibly predict the resulting phenotype [41,42].
Autosomal recessive epidermolysis bullosa simplex, E6S-AR
Although EBS is generally transmitted in an autosomal dominant mode, about 5% of EBS cases have been identified with inherited recessive mutations [43]. More than 10 different KRT14 mutations have been associated with recessive EBS (EBS-AR) including nonsense mutations, missense mutations, splice site mutations, deletion and deletion/insertion mutations [44]. In some cases of recessive EBS, compound heterozygous mutations have been described in K5 [9]. For further reading regarding other forms of EBS is summarized in Table 1 and see reviews in [14, 25,27].
Table 1.
Inherited cutaneous keratin disorders due to keratin mutations
| Human cutaneous keratin disorder | Mode of inheritance | Type II keratin | Type I keratin | Tissue specific expressions | Clinical characteristics |
|---|---|---|---|---|---|
| Epidermolysis bullosa simplex | AD | K5 | K14 | Basal keratinocytes of the epidermis | Localized, generalized and /or herpetiformblistering, PPK |
| Localized EBS (EBS-Loc) | ......“..... | Localized blistering in friction areas as in palms and soles, | |||
| Generalized non-Dowling meara EBS (gen-nonDM-EBS) | ......“..... | ....“...... | Generalized, “non herpetiform” mild blistering | ||
| EBS Dowling Meara (EBS-DM) | ......“..... | Generalized “herpetiform” blistering, may involve nail oral and other mucosa | |||
| Epidermolysis bullosa simplex (EBS-AR) | AR | K14 | .....“..... | Generalized blistering, ichthyotic plaques and may involve extra-cutaneous area, absent /reduced KIF in basal keratinocytes | |
| EBS with migratory circinate erythema (EBS-CE) | K5 | ......“..... | Generalized blistering, circinate erythroderma, brown hyperpigmentation, reduced KIF in basal keratinocytes | ||
| EBS with mottled pigmentation (EBS-MP) | K5 | K14 | ......“..... | Generalized blistering, reticulate/mottled brown hyperpigmentation, KIF aggregation and increased melanosomes in basal keratinocytes | |
| Dowling-Degos disease (DDD) | K5 | ......“..... | Hyperkeratotic papules, reticulate, progressive and disfiguring hyperpigmentation, primarily in the flexural areas | ||
| Naegeli-Franceschetti-Jadassohn syndrome (NFJS)/ Dermatopathia pigmentosa reticularis (DPR) | AD null | K14 | ......“..... | both share reticulate hyperpigmentation of skin, thickening of the palms and soles (PPK), signs of ectodermal dysplasia, such as abnormal sweating and absence of dermatoglyphics | |
| Epidermolytic ichthyosis (EI) | AD | K1 | K10 | Suprabasal keratinocytes of the epidermis | Erythroderma, blister formation, development of hyperkeratosis, PPK |
| Linear epidermal nevus (LEN) | AD | K1 | K10 | Epidermal hamartomas showing somatic mosaicism of epidermolytic hyperkeratosis | |
| Epidermolytic ichthyosis (EI) | AR | K10 | Erythroderma, blister formation, hyperkeratosis | ||
| Ichthyosis hystrix Curth-Macklin (IHCM) | K1 | Severe and spiky palmoplantar hyperkeratosis | |||
| Diffuse non-epidermolytic PPK (DNEPPK) | K1 | Upper suprabasal keratinocytes of palmoplantar skin | Demarcated diffuse PPK, | ||
| Palmoplantar keratoderma with tonotubules (PPK) | K1 | Upper suprabasal keratinocytes of palmoplantar skin | Diffuse PPK, erythematous margin | ||
| Ichthyosis bullosa of Siemens (IBS) | K2 | Upper suprabasal keratinocytes of cornifying epithelia | Superficial blistering and shedding or desquamation of the epidermis, with age dark brown hyperkeratosis | ||
| Epidermolytic palmoplantar keratoderma (EPPK) | K9 | Suprabasal layers of palmoplantar epidermis (palms and soles) | Diffuse PPK, erythematous margin, +- EHK/ suprabasal KIF clumping | ||
| Pachyonychia congenita type I (PCI) | K6a | K16 | Suprabasal orogenital mucosa; palmoplantar epidermis; epidermal appendages (nail) | Nail dystrophy, mild PPK, multiple pilosebaceous cysts, oral leukokeratosis | |
| Pachyonychia congenita type II (PCII) | K6b | K17 | Nail dystrophy and may involve hair follicle, and eccrine glands, mild PPK, pilosebaceous cysts, | ||
| Steatocystoma multiplex (SM) | K17 | Several and widespread distribution of cystic tumors | |||
| Focal non-epidermolytic PPK (FNEPPK) | K16 | Focal PPK with oral, genital, and/or follicular lesions | |||
| Monilethrix | AR, co-dominance | K81, 86, 83 | Hair shaft, (clumps of the structural protein s) | Hair dystrophy, varying degrees of alopecia | |
| Pseudofolliculitis barbae (PFB) | K75i | Hair follicle root sheath | Follicular infections, ingrowth hair follicles, affects mainly black individuals | ||
| Ectodermal dysplasia of hair and nail type | K85 | Hair shaft | Congenital onychodystrophy, brittle hair, and hypotrichosis |
AD autosomal dominant, AR autosomal recessive, KIF keratin intermediate, PPK palmoplantar keratoderma
mutations are risk factors but not a causative association
Keratinopathic Ichthyosis (KPI)-diseases of K1//K2/K10 mutations
Keratinopathic Ichthyosis (KPI), represent a family of superficial keratin keratodermas such as Epidermolytic ichthyosis (EI; K1/K10 mutations) and superficial epidermolyic ichthyosis (SEI; K2 mutations in Ichthyosis Bullosa of Siemens) [45].
Epidermolytic Ichthyosis (EI)—disorders of KRT1 and KRT10 genes mutations
Epidermolytic ichthyosis (EI; OMIM 113800) is a form of congenital ichthyosis with a prevalence of 1 in 200,000–300,000 people [46]. EI, also previously known as bullous congenital ichthyosiform erythroderma (BCIE) or Epidermolytic hyperkeratosis (EHK), is a relatively rare congenital skin fragility disease which is usually autosomal dominantly inherited [45]. EI is caused by mutations in the genes that encode for epidermal suprabasal keratin 1 or 10 expressed in keratinocytes of the suprabasal layers of the epidermis.
These disorders are characterized at birth with generalized erythroderma (redness of the skin) (Fig. 3 c), severe blistering and widespread hyperkeratosis (thickening of the uppermost layer of the skin), erosions and peeling of the skin even with mild trauma (Fig. 3 d). Superficial ulcerations develop on the flexural surfaces. Because of the disruption of the epithelial barrier, neonates with EI are at risk of developing severe infection, electrolyte imbalances, and sepsis. EI is often associated with rapid healing of denuded areas with recurrent episodes of blistering on the background of erythroderma that may persist throughout life. Later on in adulthood, blistering becomes infrequent; hyperkeratotic plaques with verrucous scales, mainly involving flexural and intertriginous areas develop, but can also appear on the scalp, neck and infragluteal folds. In most of the patients, palmoplantar hyperkeratosis is present and bacterial colonization of the macerated scales causes a distinct foul odor [47–51]. The cutaneous pathology in EI results from the expression of abnormal K1 or K10 proteins [52]. Since these two keratins provide structural stability to keratin intermediate filaments in the suprabasal keratinocytes, the blistering is more superficial than in EBS, and there is increased proliferation of suprabasal keratinocytes leading to ichthyosiform lesions. Ultrastructurally, the basal cells are normal, but irregularly shaped pathognomonic KIFs clumps are identified in suprabasal keratinocytes, giving a dense peri-nuclear shell-like appearance as primary event, and with secondary suprabasal cytolysis, blister formation and hyperkeratosis [43].
Most of the pathogenic mutations are missense mutations that usually occur within highly conserved regions of the alpha-helical rod domains and the non-helical HI domain of K1 and K10. Milder variants of the disease are associated with mutations in the L12 linker region or outside the helix boundary motifs, similar to mild EBS[53]. Therefore, the positions of the mutations along the keratin polypeptides and the level of expression of the mutated KRT1 and KRT10 genes, could explain the clinical features of this disorder as well [54–56].
Rare dinucleotide alterations in KRT10 that lead to substitution of two adjacent amino acids [54, 57] and spontaneous de novo point mutation, deletion, deletion/insertion and splice site mutations in KRT1 and KRT10 have been described in about half of the reported nucleotide changes [56, 58]. Similar to EBS-DM, a genetic “hot spot” has been identified as well in EI that affects an evolutionarily highly conserved arginine residue (p.Arg156). The nature of the mutations may predict the disease phenotype as it has been ascertained that KRT1 mutations are associated with palmoplantar keratoderma whereas KRT10 mutations lead to the non-palmoplantar variants [46]. Such an association appears to be true for KRT1 mutations; but exceptions do exist where KRT10 mutations have been identified in patients with severe EI and palmoplantar keratoderma [58, 59]. An interesting missense mutation in the HTP of K1, p.Ile479Thr, is associated with a mild ichthyosis-like phenotype in some cases [55] and epidermolytic palmoplantar keratoderma alone in other families [60].
Genotype–phenotype correlations have taught us that a much more complex link exists and that the genetic background may also modulate the phenotype., This is best illustrated by the findings that the conserved codon 156 mutations in KRT10, mostly associated with a severe phenotype, may also result in a mild form of EI [61]. This is analogous to the EBS phenotypes where mutations in the same location are associated with different phenotypes for mutations affecting K5 p.Iso183 [32, 62] as well as the K5 p.Val186 mutations [63, 64].
Even though EI is usually autosomal dominantly inherited, recently several reports showed that this disorder can as well be inherited in a recessive way by involving either a donor splice site, termination codon or nonsense mutations [65–67]. A recessive nonsense KRT10 mutation that leads to the loss of K10 expression has been identified in a consanguineous family with a severe EI phenotype, characterized by sparse keratin filaments with amorphous and homogenous-like keratin aggregates [40]. Recessive EI has a characteristic ultrastructural picture of EI consisting of sparse keratin filaments with a nearly homogenous and amorphous structure of keratin clumps. Other cases of recessive BCIE describes a homozygous nonsense mutation in KRT10, with heterozygous individuals being non-phenotypic carriers thus, clearly demonstrates that a normal K10 allele is sufficient to maintain a normal KIF network [67].
The nevoid variant of EI, also termed epidermal nevus of the epidermolytic hyperkeratotic type, exists with ichthyosiform lesions often distributed along the Blaschko lines, alternating with normal skin. The discovery of heterozygous KRT10 missense mutations in skin lesions and it absence in normal skin suggested the occurrence of postzygotic, spontaneous mutations during embryogenesis [68, 69]. In addition to this also a KRT1 mutation has been identified [70]. Patients with the nevoid variant of EI having children with full-blown EI have been shown to have underlying gonadal as well as cutaneous mosaicism. Moreover mosaicism has been described in EBS and in palmoplantar verrucous nevus (reviewed in [14]). Details of other forms of epidermolytic keratoderma are reviewed in [14,25].
The annular variant of EI also termed cyclic ichthyosis with epidermolytic hyperkeratosis (EHK) also exists and is a rare disease with only seven families reported. At birth, affected individuals show classic EI erythroderma and superficial erosions, with improved clinical symptoms during early infancy. It is characterized by flares of polycyclic psoriasiform plaques that persist for several weeks to several months with only benign localized disease in adulthood. Except for hyperkeratosis on palms and soles, the skin between the flares is often normal. Mutations in KRT1 and KRT10 have been identified suggesting a variant of EI as well [55,57, 71, 72].
Ichthyosis hystrix of Curth-Macklin (IHCM)-disorder of mutations in the V2 domain of K1
Ichthyosis hystrix is a distinct autosomal dominantly acting disorder with severe, widespread/localized and verrucous hyperkeratotic lesions without blister formation seen in EI or other disorders of cornification, e.g. erythrokeratodermia variabilis. It is clinically heterogeneous [73], with or without palmoplantar keratoderma [74, 75]. Ultrastructurally, cells are bi-nucleated with tonofilament shells surrounding the nucleus in differentiated keratinocytes without any signs of keratin clumping or epidermolysis.
Mutations in KRT1 have been linked to this pathology, mostly affecting the V2 domain, which dramatically alter the biochemical properties of the variable (V2) domain, apparently not inhibiting KIF formation. Instead, they cause intracellular misdistribution of loricrin [73, 76]. A heterozygous frameshift mutation in the V2 domain of K1 in close proximity to the described mutations results in striate palmoplantar keratoderma (SPPK), a heterogenetic disorder, associated with pathogenic mutations in K1 and its associated desmosomal proteins, desmoplakin and desmoglein 1. This frameshift mutation leads to the partial loss of the glycine loop motif in the V2 domain of K1 [77], pointing to a phenotypic heterogeneity among K1 disorders due to abnormal tail domain, and suggesting the existence of distinct pathogenic pathways.
Ichthyosis Bullosa of Siemens (IBS) - disorders linked to KRT2 mutations
Ichthyosis bullosa of Siemens (IBS), a more superficial epidermolytic ichthyosis (SEI), is an autosomal dominant keratinization disorder, with similar but milder clinical features compared to EI. IBS is characterized by the absence of congenital erythroderma and hyperkeratosis mostly localized to flexural areas, and with more superficial epidermal fragility and desquamation that lead to characteristic denuded areas (molting or Mauserung phenomenon). Aggregates of keratin filament bundles and cytolysis are confined to the upper spinous / granular layers of the epidermis coinciding with the epidermal tissue expression pattern of K2. The majority of mutations affect the HTM of K2, but mutations in the 1A and 2B domain of K2 have been identified with a probable p.Glu487Lys mutational “hot spot”. It is often clinically very difficult to clearly distinguish mild EI from severe IBS, and molecular analysis is definitive for prediction of diagnosis. However, K2 mutations have been identified in families previously misdiagnosed as IE [78], whereas a heterozygous missense mutation in KRT1 (p.Glu478Asp) has been identified in a family with mild EI showing clinical and histological features similar to IBS [79].
Palmoplantar keratoderma (PPK) - disorder of palmoplantar K9 mutations
Palmoplantar keratodermas (PPK), a group of heterogeneous keratinopathic genodermatoses characterized by hyperkeratotic skin confined to palms and soles, often clinically grouped into three distinct patterns: diffuse, focalize and punctate. Based on the identification of the underlying genetic defects in these disorders, a revised classification now exist as a result of the previous difficulties in classifying based on phenotypic and morphological criteria.
Epidermolytic PPK (EPPK) is an autosomal dominant disorder that develops within the first months after birth. It manifests as diffuse hyperkeratosis of the palms and soles and showing sharp demarcations with erythematous margin. The degree of hyperkeratosis often varies between families and among individuals of the same family. Other features seen include knuckle pad-like keratoses over the flexures of the finger joints and clubbing of the nails [80]. Another form, the Unna-Thost form, often clinically identical to Vorner disease, can be distinguished histologically by the absence of EHK and ultrastructurally by the absence of suprabasal KIF clumping [55]. The expression pattern of K9 which is limited to suprabasal keratinocytes of the glabrous skin epidermis (palms and soles) has been primed for EPPK. Several pathogenic KRT9 mutations have been identified in PPK families, which were similar to those described by Thost and Vorner [81]. The majority of pathogenic mutations in EPPK are missense or small inframe insertion vs. deletion mutations in the helix boundary motifs of the K9 polypeptide, often located at the beginning of the 1A rod domain of K9. A possible mutational hotspot exists at p.Arg163, often mutated either to tryptophan, glutamine or proline and two mutations, one insertion and one substitution mutation have been identified in the 2B region of K9 [82, 83]. A missense mutation in the helix termination EBS and in palmoplantar verrucous nevus (reviewed in [14]). Details of other forms of epidermolytic keratoderma are reviewed in [14, 25].
The annular variant of EI also termed cyclic ichthyosis with epidermolytic hyperkeratosis (EHK) also exists and is a rare disease with only seven families reported. At birth, affected individuals show classic EI erythroderma and superficial erosions, with improved clinical symptoms during early infancy. It is characterized by flares of polycyclic psoriasiform plaques that persist for several weeks to several months with only benign localized disease in adulthood. Except for hyperkeratosis on palms and soles, the skin between the flares is often normal. Mutations in KRT1 and KRT10 have been identified suggesting a variant of EI as well [55, 57, 71, 72].
Ichthyosis hystrix of Curth-Macklin (IHCM)-disorder of mutations in the V2 domain of K1
Ichthyosis hystrix is a distinct autosomal dominantly acting disorder with severe, widespread/localized and verrucous hyperkeratotic lesions without blister formation seen in EI or other disorders of cornification, e.g. erythrokeratodermia variabilis. It is clinically heterogeneous [73], with or without palmoplantar keratoderma [74, 75]. Ultrastructurally, cells are bi-nucleated with tonofilament shells surrounding the nucleus in differentiated keratinocytes without any signs of keratin clumping or epidermolysis.
Mutations in KRT1 have been linked to this pathology, mostly affecting the V2 domain, which dramatically alter the biochemical properties of the variable (V2) domain, apparently not inhibiting KIF formation. Instead, they cause intracellular misdistribution of loricrin [73, 76]. A heterozygous frameshift mutation in the V2 domain of K1 in close proximity to the described mutations results in striate palmoplantar keratoderma (SPPK), a heterogenetic disorder, associated with pathogenic mutations in K1 and its associated desmosomal proteins, desmoplakin and desmoglein 1. This frameshift mutation leads to the partial loss of the glycine loop motif in the V2 domain of K1 [77], pointing to a phenotypic heterogeneity among K1 disorders due to abnormal tail domain, and suggesting the existence of distinct pathogenic pathways.
Ichthyosis Bullosa of Siemens (IBS) - disorders linked to KRT2 mutations
Ichthyosis bullosa of Siemens (IBS), a more superficial epidermolytic ichthyosis (SEI), is an autosomal dominant keratinization disorder, with similar but milder clinical features compared to EI IBS is characterized by the absence of congenital erythroderma and convincing evidence that mutation in basal keratin genes can cause both blistering and pigmentary disorders of the skin.
Keratin mutations in skin appendages (Hair and Nail) and other genodermatoses
1a.) Hair keratins and diseases
Monilethrix- disease of K81/K83/K86 mutations
Monilethrix is an autosomal dominant congenital hair disease with high penetrance but variable expression, clinically characterized by dystrophic hair confined to either a small area or to almost total alopecia. Hair shaft deformation is characterized by elliptical nodes, regularly separated by dystrophic constrictions, with affected hair being more susceptible to fracture and breakage at the constricted sites. This results in diverse degrees of alopecia with short and sparse scalp hair, and occasional hair regrowth, during puberty or pregnancy. Ultrastructurally, affected hair shows defects in the cortex and clumps of the structural proteins of the hair shaft [7, 92], pointing to the trichocyte keratins as the prime disease candidates. The first mutations were identified in KRT86 (previously KRTHB6), and HTM mutation in K86 (Hb6; 90%) as well as HTM mutation in K81 (Hb1;100%) have been identified [93]. Although keratin K83 (Hb3) is not only co-expressed with K81 (Hb1) and K86 (Hb6) in the mid-cortex and shares an almost identical rod domain sequence, only one mutation in the 2B region (p.Glu407Lys) has been identified so far in a single monilethrix family with co-dominant manner [94, 95]. Multiple cases and pedigrees with autosomal recessive inheritance have been reported and mutations in desmoglein 4 (DSG4), a cadherin subfamily member expressed in the hair cortex and upper cuticle, have been disclosed [96–98]. Initially, mutated DSG4 has been associated with localized autosomal recessive hypotrichosis.
Pseudofolliculitis barbae – predisposition disease of K75 polymorphism
Pseudofolliculitis barbae (PFB), is a common hair disorder in which shaving leads to ingrowths of hair follicles and a tendency for follicular infections, with predilection sites including the neck and submental region of the face. This occurs mainly in black males owing to a genetic predisposition to curled hair. Molecular analysis in severely affected males and asymptomatic females revealed an unusual single-nucleotide polymorphism, which gives rise to a disruptive p.Ala161Thr substitution in the 1A alpha-helical segment of K75 (K6hf), partially responsible for the phenotypic expression and representing an additional genetic risk factor for PFB [99].
Ib.) Pachyonychia congenital (PC)-disorder of the nail K6/K17/K17 mutations
Pachyonychia congenita (PC), a group of autosomal dominant nail dysplasias characterized by hypertrophic nail dystrophy, exist in two forms. In pachyonychia congenita type I (PC-1; Jadassohn-Lewandowsky type), hypertrophic nail dystrophy is accompanied by severe focal keratoderma especially on the foot soles and often with oral leukokeratosis. In pachyonychia congenita type II (PC-2; Jackson-Lawler type), nail dystrophy is accompanied by mild palmoplantar keratoderma and multiple pilosebaceous cysts caused by hyperkeratosis of the infundibulum and accompanying sebaceous gland that typically develop after puberty. Natal teeth and hair abnormalities (twisted hair) are associated features but with no full penetrance [100]. Two milder phenotypic variants of PC-1 and PC-2 are now being recognized, focal non-epidermolytic palmoplantar keratoderma (FNEPPK) and steatocystoma multiplex.
In PC-1, mutations in the genes encoding for K6a and K16 which are normally regarded as stress response and wound healing induced keratins have been identified. The tissue distribution of these keratins in nail bed, nail fold as well as in palmoplantar skin and oral mucosa matches well the epithelial fragility phenotype. Keratins K6b and K17 mutated in pachyonychia congenita type II are found in the nail bed, hair follicle, eccrine glands and in palmoplantar skin, causing hyperkeratosis and overgrowth of the nail [101–103].
Similar to most KRT genes, most of the mutations in PC are missense mutations with a smaller number of in-frame insertions and deletions. In PC-1 two-third of the known mutations occurs in the KRT6A gene and one-third in KRT16. Two-third K6a mutations occur in the 1A domain, and one-third in the 2B domain, with a possible hotspot region at the p.Asn171 in PC-1. In K16, most pathogenic mutations are located in HIP with recent reports at p.Asnl25Ser. A missense mutation, p.Lys354Asn has been reported in the 2B domain, causing delayed onset of the clinical signs of PC-1 [104]. Some K16 mutations have been reported to cause FNEPPK focal keratoderma [105,106].
K17 mutations have consistently been associated with PC-2 and are by far more frequent compared to those of K6b, the pair-wise co-expressed partner of K17. A deletion in the 1A domain (p.Asn172del) and a missense mutation in the 2B domain (p.Glu472Lys) have been reported in K6b. Most mutations in K17 are localized in the 1A domain. Here, with two codons mutations, p.Asn92 and p.Arg94, are accounting for more than half of the cases, whereas the rest is accounted for by the missense mutation in the 1A domain of K17 (p.Asn107Asp) [107–111]. Interestingly both mutations, p.Lys354Asn in K16 and p.Asn107Asp in K17, are associated with late-onset PC-1 and PC-2 respectively, and occur in regions similar to the regions in K5 and K14 that are often associated with milder localized EBS phenotypes.
Ectodermal dysplasia of the hair and nail type keratins
Ectodermal dysplasia is a family of disorders with abnormal development of skin appendages such as hair, nails, teeth and sweat glands. There exist about 200 known ectodermal dysplasias with only about 30 having been characterized at the molecular level.
Most of the hair and nail type ectodermal dysplasias have associated abnormalities, such as keratoderma or ichthyosis, skeletal anomalies, cardiac irregularities, mental or psychomotor retardation. A homozygous substitution mutation (p.Arg78His) in the V1 region of the hair matrix and cuticle keratin gene K85 (Hb5) has been identified in affected individuals while heterozygous carriers of the mutation appeared normal [112]). K85 (Hb5) is expressed very early in the lowermost matrix and cuticle of the hair follicle as well as in the basal compartment and the lower keratogenous zone of the apical and ventral nail matrix. Thus, the absence of the protein from the steps of hair and nail formation can explain the severe hair and nail phenotype [7].
Animal and in vitro models of keratin-related cutaneous diseases
A significant increase in the knowledge that governs the biology and pathophysiology of keratinopathic genodermatoses has benefited tremendously from the development and use of knockout and transgenic mice models [113–115], and more recently in vitro models of such disorders. Animal models of keratin diseases have been useful for understanding the function of keratins, to identify and delineate molecular defects in human keratinopathic-disorders, and to develop potential therapies. Three main groups of animals exist: animals in which a specific or multiple keratin genes have been deleted [116–118]; animals expressing keratins into which mutations have been introduced [115, 119]); animals in which the expression pattern of keratin has been altered either by over-expression of normal keratin [120] or by the use of promoters to direct keratin expression to a different tissue or group of cells [121]. Most human keratin disorders are dominant disorders resulting from missense mutations, most transgenic mice are keratin knockouts and have recessive phenotypes as mouse and human phenotype may not be directly comparable.
In vitro models of inherited keratin disorders provide useful systems for the elucidation of the cellular, morphological, biochemical and biophysical mechanisms involved in the pathology of keratin disorders as well as represent an initial step towards developing novel therapeutic strategies. The introduction of mutant keratins into keratinocytes can result in the formation of keratin aggregates but often with little correlation between the severity of phenotype in vitro and in vivo. Alternative strategy is to create primary and immortalized cell lines from patients with known keratin mutations, where the mutant keratin is expressed at physiological levels. Primary patient-derived keratin-defective keratinocytes have recently been used as model for studying the impact of heat-stress induced keratin aggregation [62].
However, primary keratinocytes exhibited finite replicative lifespans in culture and develop heterogeneous behavior with increasing passage numbers, a limitation that makes them suboptimal for long-term reproducible and more extensive functional evaluations, especially when testing new treatment rationales. Complementing such knowledge with biochemical, biophysical and pharmacological assays using patient-derived cells culture models will rapidly enhance development of therapy.
a) Establishment of immortalized keratin-mutant keratinocyte lines
Immortalized cells are invaluable tools required at early stage for devising therapeutic strategies, and the phenotypic rescue of inherited skin disorders using genetically manipulated cultured cells have shown proofs of principle [122–124]. To date, several immortalized patient-derived keratin defective cell lines have been established as long-term reproducible cellular models of EBS and EI, [125–128].
Future challenges remain to rapidly make use of such established models to further explore the pathomechanisms, test new treatment options and to develop tools with which to curb current treatment limitations for these disorders.
Effects of keratin mutations and physical stress on cytoskeleton resilience in keratin-defective cells
Analysis of primary and immortalized keratin mutant keratinocytes demonstrated some common features as well as certain divergence related to the phenotypic variation seen in the patients in vivo. These changes could presumably be due to an increased physiological stress burden on the severely mutant cells, incurred by the requirements of handling mutated keratins [62,127,129].
It has also been observed that when keratin-defective cells are grown in conditions favoring desmosomal connections (serum containing medium) as well as at increased confluency; they are more resistant to the effect of physical stress. This emphasizes the necessity of uniformity in cellular confluency when performing such experiments. Cells grown in culture have been extremely useful for the establishment of different functional assays such as heat, mechanical and osmotic shock, which explore the effects of stress-induced KIF remodeling. Basal/ suprabasal keratinocyte expressing keratin mutations reveal pathologic keratin positive aggregates in both EBS and EI disease models in vitro (Figure 4 a and b) [125, 128–132]. Moreover, the effect of stress on mutant keratin aggregation in both basal and suprabasal keratin defective cell lines were shown to be similar to those of their respective primary cells [126]. These data infer that immortalization did not seem to interfere with the intrinsic properties of the patient-derived primary keratinocytes and point out the thus the importance of the data provided by experiments on these cell lines.
Figure 4. Triton extracted monolayer cultures of keratinocytes cytoskeleton and 3-D epidermis engineered with keratinocytes harboring mutations.
Heat stress of keratin cytoskeleton reveals pathognomonic keratin-aggregates in keratinocytes harboring (a) K5_p.475Gly>Glu mutation in severe generalized EBS-DM keratinocyte cell line (EB11) when stained with keratin 5 antibody, (b) K10_p.l56Arg>Gly mutation in a moderate EI-phenotype keratinocyte cell line (EH21) when stained with keratin 10 antibody. Keratin 10 expression was induced in submerged culture by calcium induced differentiation of keratinocytes. (c) Haematoxylin staining of organotypic epidermis generated using immortalized epidermolytic ichthyosis (EI: EH31) keratinocyte cell lines derived from a severely affected patient's organotypic epidermis and without heat-stress. The reconstructed epidermis here shows well-defined basal layer, superficial cleft or cytolysis at the suprabasal layers (arrows) leaving unperturbed basal and cornified layers, a histological feature that mimics the in vivo phenotype of the patient, (d) Haematoxylin staining of organotypic epidermis generated using immortalized epidermolysis bullosa simplex (EB11) keratinocyte cell lines derived from a severely affected EBS-DM patient's organotypic epidermis and after heat-stress. The organotypic epidermis shows well defined basal layer with some basal cell cytolysis or cleft formation reminiscent of in vivo severe generalized EBS phenotype. All organotypic epidermis was harvested after 12 day post-lifted at air-liquid interface.
b) In vitro tissue engineering of keratin defective epidermis
Since keratin diseases are tissue fragility disorders, which may not be adequately reproduced in monolayer cultures, attempt to mimic the disease phenotype in reconstructed epidermis in vitro, has been made prior to testing the effects of new drugs on animal models and clinical development. These cells were able to differentiate and reconstitute a 3-D engineered epidermal tissue in vitro on Mili-cell culture inserts or deepidermalized dermis. It was shown that cells from severe patients recapitulated the histological and phenotypic alterations reminiscent of in vivo phenotype [126, 133], (Figure 4c), a phenomenon consistent with a previous report in an in vitro recessive dog model of EI [126, 134, 135]. Nonetheless, these models represent useful tools for future development of clinical remedies for keratin-related skin disorders.
Problems related to treatment of keratin genodermatoses and prospects for emerging therapies
At present there is no curative therapy for keratin-related cutaneous disorders and some may argue that current therapies are tedious, only moderately effective and involve significant risks of side-effects. However, therapy for some disorders has improved considerably over the years and this is particularly true for some forms of EB, keratinopathic Ichthyosis and keratin related nail and hair dystrophies.
Unfortunately, it was thought that most of the hurdles lie in the lack of in-depth understanding of a detailed molecular signature of the defect. This is not the case now as significant knowledge, is available yet, treatment options are still below expectation. There is an obvious need for more research on the disease mechanisms and new therapies for these discomforting genodermatoses, and best restoration of the normal function of the skin and its appendages. The severe forms of keratinization disorders represent a serious handicap, requiring life-long therapy and affecting the patient's life situation. Associated complications, such as heat intolerance owing to chronic skin infections mainly in EI patients for instance, add to the incapacitation of the patients' and often require medical attention. Therefore, medical and ethical considerations, concomitant with funding agencies should support the attempt to develop more “experimental” treatments, including cutaneous gene transfer, small molecule based approaches to overcome treatment limitation thus helping this group of patients. Clearly, many different approaches must be tried. For example, in the case of dominant negative gene mutations such as in EBS, KPI (EI and SEI) and PC discussed above, it should at least in theory, be possible to silence a mutated keratin allele by applying RNAi technology. It was hoped that some encouraging in vitro results will lead to clinical trials [136] and have now been proven viable for different clinical phase trails [137–140]. In the case of recessive disorders where a protein is missing, the principle of ex vivo gene transfer using cultured keratinocytes that are re-transplanted to the patient might be possible [141]. Therefore, although systemic gene therapy exploiting viral vectors is more risky, it probably represents one of the many ways forward. As the molecular dissection of these genodermatosis has been raveled in the last two and a half decades, it certainly will be exciting to follow the progress in this field over the next half to one decade.
Concluding remarks
Our understanding of how various keratin genes are involved in causing inherited disorders of the skin and its appendages have increased tremendously over the last two and a half decades, revealing a spectrum of diverse pathogenic mechanisms of abnormal structural keratin proteins. It is hoped that this new knowledge will lead to many novel therapies for specific subtypes of keratinopathic genodermatoses, including perhaps somatic gene therapy for the most severely affected patients. Keratinopathies can indeed be a very disabling condition requiring laborious treatment several times a day, but it may also be a relatively mild disorder that only occasionally requires application of emollients or prevention of exacerbating conditions (heat moisture or other factors). From both a diagnostic and therapeutic point of view, the many different subtypes of genodermatoses represent a challenge for a caring physician, who must learn to understand the underlying pathology as well as to scientists whose relentless efforts will soon be benefited by the general patient public. For instance, a paradoxical combination of skin barrier failure and massive hyperkeratosis in some types of epidermolytic ichthyosis requires special attention when prescribing therapy. However, biomedical scientist and clinicians have collaborated enormously to handle these hurdles. It is likely that the choice of the best treatment for each individual will become increasingly simpler as more high-technology approaches and products appear on the market. These choices will largely depend by advantageous exploitation of the possible biophysics of the skin in normal and pathological situation.
Highlight.
This review presents an update of a broad spectrum of different keratin-related genodermatoses. We also highlight the models available for their future treatments. The clinical, ultrastructural, molecular genetics and biochemical characteristics are described, with special emphasis to epidermolysis bullosa simplex and keratinopathic ichthyosis. Better understanding of the molecular pathogenesis of these disorders by the aid of biophysical and biochemical investigations could lead to the development of novel therapies for several skin/cutaneous diseases.
Acknowledgements
US PHS Grants AR 059742 to HM. IAS was supported by T32 AR 055893
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Tabolli S, Pagliarello C, Uras C, Di Pietro C, Zambruno G, Castiglia D, Sampogna F, Abeni D. Family burden in epidermolysis bullosa is high independent of disease type/subtype. Acta Derm Venereol. 2010;90:607–611. doi: 10.2340/00015555-0947. [DOI] [PubMed] [Google Scholar]
- [2].Pagliarello C, Tabolli S. Factors affecting quality of life in epidermolysis bullosa. Expert Rev Pharmacoecon Outcomes Res. 2010;10:329–338. doi: 10.1586/erp.10.28. [DOI] [PubMed] [Google Scholar]
- [3].Coulombe PA, Hutton ME, Vassar R, Fuchs E. A function for keratins and a common thread among different types of epidermolysis bullosa simplex diseases. J Cell Biol. 1991;115:1661–1674. doi: 10.1083/jcb.115.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gu X, Xu F, Wang X, Gao X, Zhao Q. Molecular cloning and expression of a novel CYP26 gene (cyp26d1) during zebrafish early development. Gene Expr Patterns. 2005;5:733–739. doi: 10.1016/j.modgep.2005.04.005. [DOI] [PubMed] [Google Scholar]
- [5].Moll R, Franke WW, Volc-Platzer B, Krepler R. Different keratin polypeptides in epidermis and other epithelia of human skin: a specific cytokeratin of molecular weight 46,000 in epithelia of the pilosebaceous tract and basal cell epitheliomas. J Cell Biol. 1982;95:285–295. doi: 10.1083/jcb.95.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schweizer J, Bowden PE, Coulombe PA, Langbein L, Lane EB, Magin TM, Maltais L, Omary MB, Parry DA, Rogers MA, Wright MW. New consensus nomenclature for mammalian keratins. J Cell Biol. 2006;174:169–174. doi: 10.1083/jcb.200603161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schweizer J, Langbein L, Rogers MA, Winter H. Hair follicle-specific keratins and their diseases. Exp Cell Res. 2007;313:2010–2020. doi: 10.1016/j.yexcr.2007.02.032. [DOI] [PubMed] [Google Scholar]
- [8].Szeverenyi I, Cassidy AJ, Chung CW, Lee BT, Common JE, Ogg SC, Chen H, Sim SY, Goh WL, Ng KW, Simpson JA, Chee LL, Eng GH, Li B, Lunny DP, Chuon D, Venkatesh A, Khoo KH, McLean WH, Lim YP, Lane EB. The Human Intermediate Filament Database: comprehensive information on a gene family involved in many human diseases. Hum Mutat. 2008;29:351–360. doi: 10.1002/humu.20652. [DOI] [PubMed] [Google Scholar]
- [9].Yasukawa K, Sawamura D, McMillan JR, Nakamura H, Shimizu H. Dominant and recessive compound heterozygous mutations in epidermolysis bullosa simplex demonstrate the role of the stutter region in keratin intermediate filament assembly. J Biol Chem. 2002;277:23670–23674. doi: 10.1074/jbc.M200974200. [DOI] [PubMed] [Google Scholar]
- [10].Kirfel J, Magin TM, Reichelt J. Keratins: a structural scaffold with emerging functions. Cell Mol Life Sci. 2003;60:56–71. doi: 10.1007/s000180300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Steinert PM, North AC, Parry DA. Structural features of keratin intermediate filaments. J Invest Dermatol. 1994;103:19S–24S. doi: 10.1111/1523-1747.ep12398900. [DOI] [PubMed] [Google Scholar]
- [12].Steinert PM. Structure, function, and dynamics of keratin intermediate filaments. J Invest Dermatol. 1993;100:729–734. doi: 10.1111/1523-1747.ep12475665. [DOI] [PubMed] [Google Scholar]
- [13].Steinert PM, Marekov LN, Fraser RD, Parry DA. Keratin intermediate filament structure. Crosslinking studies yield quantitative information on molecular dimensions and mechanism of assembly. J Mol Biol. 1993;230:436–452. doi: 10.1006/jmbi.1993.1161. [DOI] [PubMed] [Google Scholar]
- [14].Arin MJ. The molecular basis of human keratin disorders. Hum Genet. 2009;125:355–373. doi: 10.1007/s00439-009-0646-5. [DOI] [PubMed] [Google Scholar]
- [15].Izawa I, Inagaki M. Regulatory mechanisms and functions of intermediate filaments: a study using site- and phosphorylation state-specific antibodies. Cancer Sci. 2006;97:167–174. doi: 10.1111/j.1349-7006.2006.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Omary MB, Ku NO, Tao GZ, Toivola DM, Liao J. “Heads and tails” of intermediate filament phosphorylation: multiple sites and functional insights. Trends Biochem Sci. 2006;31:383–394. doi: 10.1016/j.tibs.2006.05.008. [DOI] [PubMed] [Google Scholar]
- [17].Parry DA. Hard alpha-keratin intermediate filaments: an alternative interpretation of the low-angle equatorial X-ray diffraction pattern, and the axial disposition of putative disulphide bonds in the intra- and inter-protofilamentous networks. Int J Biol Macromol. 1996;19:45–50. doi: 10.1016/0141-8130(96)01099-9. [DOI] [PubMed] [Google Scholar]
- [18].Gu LH, Coulombe PA. Keratin function in skin epithelia: a broadening palette with surprising shades. Curr Opin Cell Biol. 2007;19:13–23. doi: 10.1016/j.ceb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- [19].Kim S, Coulombe PA. Intermediate filament scaffolds fulfill mechanical, organizational, and signaling functions in the cytoplasm. Genes Dev. 2007;21:1581–1597. doi: 10.1101/gad.1552107. [DOI] [PubMed] [Google Scholar]
- [20].Ku NO, Strnad P, Zhong BH, Tao GZ, Omary MB. Keratins let liver live: Mutations predispose to liver disease and crosslinking generates Mallory-Denk bodies. Hepatology. 2007;46:1639–1649. doi: 10.1002/hep.21976. [DOI] [PubMed] [Google Scholar]
- [21].Marceau N, Schutte B, Gilbert S, Loranger A, Henfling ME, Broers JL, Mathew J, Ramaekers FC. Dual roles of intermediate filaments in apoptosis. Exp Cell Res. 2007;313:2265–2281. doi: 10.1016/j.yexcr.2007.03.038. [DOI] [PubMed] [Google Scholar]
- [22].Ku NO, Omary MB. A disease- and phosphorylation-related nonmechanical function for keratin 8. J Cell Biol. 2006;174:115–125. doi: 10.1083/jcb.200602146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kim S, Kellner J, Lee CH, Coulombe PA. Interaction between the keratin cytoskeleton and eEF1Bgamma affects protein synthesis in epithelial cells. Nat Struct Mol Biol. 2007;14:982–983. doi: 10.1038/nsmb1301. [DOI] [PubMed] [Google Scholar]
- [24].Uitto J, Pfendner E, Jackson LG. Probing the fetal genome: progress in non-invasive prenatal diagnosis. Trends Mol Med. 2003;9:339–343. doi: 10.1016/s1471-4914(03)00137-0. [DOI] [PubMed] [Google Scholar]
- [25].Uitto J, Richard G, McGrath JA. Diseases of epidermal keratins and their linker proteins. Exp Cell Res. 2007;313:1995–2009. doi: 10.1016/j.yexcr.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cabral A, Voskamp P, Cleton-Jansen AM, South A, Nizetic D, Backendorf C. Structural organization and regulation of the small proline-rich family of cornified envelope precursors suggest a role in adaptive barrier function. J Biol Chem. 2001;276:19231–19237. doi: 10.1074/jbc.M100336200. [DOI] [PubMed] [Google Scholar]
- [27].Coulombe PA, Kerns ML, Fuchs E. Epidermolysis bullosa simplex: a paradigm for disorders of tissue fragility. J Clin Invest. 2009;119:1784–1793. doi: 10.1172/JCI38177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McLean WH, Smith FJ, Cassidy AJ. Insights into genotype-phenotype correlation in pachyonychia congenita from the human intermediate filament mutation database. J Investig Dermatol Symp Proc. 2005;10:31–36. doi: 10.1111/j.1087-0024.2005.10205.x. [DOI] [PubMed] [Google Scholar]
- [29].Fine JD, Eady RA, Bauer EA, Bauer JW, Bruckner-Tuderman L, Heagerty A, Hintner H, Hovnanian A, Jonkman MF, Leigh I, McGrath JA, Mellerio JE, Murrell DF, Shimizu H, Uitto J, Vahlquist A, Woodley D, Zambruno G. The classification of inherited epidermolysis bullosa (EB): Report of the Third International Consensus Meeting on Diagnosis and Classification of EB. J Am Acad Dermatol. 2008;58:931–950. doi: 10.1016/j.jaad.2008.02.004. [DOI] [PubMed] [Google Scholar]
- [30].Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bowden PE, Knight AG, Liovic M. A novel mutation (p.Thrl98Ser) in the 1A helix of keratin 5 causes the localized variant of Epidermolysis Bullosa Simplex. Exp Dermatol. 2009 doi: 10.1111/j.1600-0625.2008.00820.x. [DOI] [PubMed] [Google Scholar]
- [32].Glasz-Bona A, Medvecz M, Sajo R, Lepesi-Benko R, Tulassay Z, Katona M, Hatvani Z, Blazsek A, Karpati S. Easy method for keratin 14 gene amplification to exclude pseudogene sequences: new keratin 5 and 14 mutations in epidermolysis bullosa simplex. J Invest Dermatol. 2009;129:229–231. doi: 10.1038/jid.2008.223. [DOI] [PubMed] [Google Scholar]
- [33].Roth W, Reuter U, Wohlenberg C, Bruckner-Tuderman L, Magin TM. Cytokines as genetic modifiers in K5−/− mice and in human epidermolysis bullosa simplex. Hum Mutat. 2009;30:832–841. doi: 10.1002/humu.20981. [DOI] [PubMed] [Google Scholar]
- [34].Löffek S, Wöll S, Hohfeld J, Leube RE, Has C, Bruckner-Tuderman L, Magin TM. The ubiquitin ligase CHIP/STUB1 targets mutant keratins for degradation. Hum Mutat. 2010 doi: 10.1002/humu.21222. 10.1002/humu.21222. [DOI] [PubMed] [Google Scholar]
- [35].Covello SP, Irvine AD, McKenna KE, Munro CS, Nevin NC, Smith FJ, Uitto J, McLean WH. Mutations in keratin K9 in kindreds with epidermolytic palmoplantar keratoderma and epidemiology in Northern Ireland. J Invest Dermatol. 1998;111:1207–1209. doi: 10.1046/j.1523-1747.1998.00445.x. [DOI] [PubMed] [Google Scholar]
- [36].Covello SP, Smith FJ, Sillevis Smitt JH, Paller AS, Munro CS, Jonkman MF, Uitto J, McLean WH. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475–480. doi: 10.1046/j.1365-2133.1998.02413.x. [DOI] [PubMed] [Google Scholar]
- [37].Rugg EL, Horn HM, Smith FJ, Wilson NJ, Hill AJ, Magee GJ, Shemanko CS, Baty DU, Tidman MJ, Lane EB. Epidermolysis bullosa simplex in Scotland caused by a spectrum of keratin mutations. J Invest Dermatol. 2007;127:574–580. doi: 10.1038/sj.jid.5700571. [DOI] [PubMed] [Google Scholar]
- [38].Anton-Lamprecht I. Ultrastructural identification of basic abnormalities as clues to genetic disorders of the epidermis. J Invest Dermatol. 1994;103:6S–12S. doi: 10.1111/1523-1747.ep12398887. [DOI] [PubMed] [Google Scholar]
- [39].Liovic M, Bowden PE, Marks R, Komel R. A mutation (N177S) in the structurally conserved helix initiation peptide motif of keratin 5 causes a mild EBS phenotype. Exp Dermatol. 2004;13:332–334. doi: 10.1111/j.0906-6705.2004.00171.x. [DOI] [PubMed] [Google Scholar]
- [40].Müller FB, Huber M, Kinaciyan T, Hausser I, Schaffrath C, Krieg T, Hohl D, Korge BP, Arin MJ. A human keratin 10 knockout causes recessive epidermolytic hyperkeratosis. Hum Mol Genet. 2006;15:1133–1141. doi: 10.1093/hmg/ddl028. [DOI] [PubMed] [Google Scholar]
- [41].Irvine AD, McLean WH. Human keratin diseases: the increasing spectrum of disease and subtlety of the phenotype-genotype correlation. Br J Dermatol. 1999;140:815–828. doi: 10.1046/j.1365-2133.1999.02810.x. [DOI] [PubMed] [Google Scholar]
- [42].Irvine AD, McLean WH. The molecular genetics of the genodermatoses: progress to date and future directions. Br J Dermatol. 2003;148:1–13. doi: 10.1046/j.1365-2133.2003.05220.x. [DOI] [PubMed] [Google Scholar]
- [43].Porter RM, Lane EB. Phenotypes, genotypes and their contribution to understanding keratin function. Trends Genet. 2003;19:278–285. doi: 10.1016/s0168-9525(03)00071-4. [DOI] [PubMed] [Google Scholar]
- [44].Has C, Chang YR, Volz A, Hoeping D, Kohlhase J, Bruckner-Tuderman L. Novel keratin 14 mutations in patients with severe recessive epidermolysis bullosa simplex. J Invest Dermatol. 2006;126:1912–1914. doi: 10.1038/sj.jid.5700312. [DOI] [PubMed] [Google Scholar]
- [45].Oji V. Revised nomenclature and classification of inherited ichthyoses. Results of the First Ichthyosis Consensus Conference; Sorèze, France. 23–4 January (2009); [DOI] [PubMed] [Google Scholar]
- [46].DiGiovanna JJ, Bale SJ. Clinical heterogeneity in epidermolytic hyperkeratosis. Arch Dermatol. 1994;130:1026–1035. [PubMed] [Google Scholar]
- [47].Chipev CC, Korge BP, Markova N, Bale SJ, DiGiovanna JJ, Compton JG, Steinert PM. A leucine----proline mutation in the H1 subdomain of keratin 1 causes epidermolytic hyperkeratosis. Cell. 1992;70:821–828. doi: 10.1016/0092-8674(92)90315-4. [DOI] [PubMed] [Google Scholar]
- [48].Chipev CC, Yang JM, DiGiovanna JJ, Steinert PM, Marekov L, Compton JG, Bale SJ. Preferential sites in keratin 10 that are mutated in epidermolytic hyperkeratosis. Am J Hum Genet. 1994;54:179–190. [PMC free article] [PubMed] [Google Scholar]
- [49].Rothnagel JA, Longley MA, Holder RA, Kuster W, Roop DR. Prenatal diagnosis of epidermolytic hyperkeratosis by direct gene sequencing. J Invest Dermatol. 1994;102:13–16. doi: 10.1111/1523-1747.ep12371723. [DOI] [PubMed] [Google Scholar]
- [50].Yang JM, Chipev CC, DiGiovanna JJ, Bale SJ, Marekov LN, Steinert PM, Compton JG. Mutations in the H1 and 1A domains in the keratin 1 gene in epidermolytic hyperkeratosis. J Invest Dermatol. 1994;102:17–23. doi: 10.1111/1523-1747.ep12371725. [DOI] [PubMed] [Google Scholar]
- [51].McLean WH, Eady RA, Dopping-Hepenstal PJ, McMillan JR, Leigh IM, Navsaria HA, Higgins C, Harper JI, Paige DG, Morley SM, et al. Mutations in the rod 1A domain of keratins 1 and 10 in bullous congenital ichthyosiform erythroderma (BCIE) J Invest Dermatol. 1994;102:24–30. doi: 10.1111/1523-1747.ep12371726. [DOI] [PubMed] [Google Scholar]
- [52].Ishidayamamoto A, Iizuka H, Manabe M, Oguin WM, Hohl D, Kartasova T, Kuroki T, Roop DR, Eady RAJ. Altered distribution of keratinization markers in epidermolytic hyperkeratosis. Arch Dermatol Res. 1995;287:705–711. doi: 10.1007/BF01105793. [DOI] [PubMed] [Google Scholar]
- [53].Syder AJ, Yu QC, Paller AS, Giudice G, Pearson R, Fuchs E. Genetic mutations in the K1 and K10 genes of patients with epidermolytic hyperkeratosis. Correlation between location and disease severity. J Clin Invest. 1994;93:1533–1542. doi: 10.1172/JCI117132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].McLean WH, Morley SM, Higgins C, Bowden PE, White M, Leigh IM, Lane EB. Novel and recurrent mutations in keratin 10 causing bullous congenital ichthyosiform erythroderma. Exp Dermatol. 1999;8:120–123. doi: 10.1111/j.1600-0625.1999.tb00358.x. [DOI] [PubMed] [Google Scholar]
- [55].Sybert VP, Francis JS, Corden LD, Smith LT, Weaver M, Stephens K, McLean WH. Cyclic ichthyosis with epidermolytic hyperkeratosis: A phenotype conferred by mutations in the 2B domain of keratin K1. Am J Hum Genet. 1999;64:732–738. doi: 10.1086/302278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tal O, Bergman R, Alcalay J, Indelman M, Sprecher E. Epidermolytic hyperkeratosis type PS-1 caused by aberrant splicing of KRT1. Clin Exp Dermatol. 2005;30:64–67. doi: 10.1111/j.1365-2230.2004.01661.x. [DOI] [PubMed] [Google Scholar]
- [57].Joh GY, Traupe H, Metze D, Nashan D, Huber M, Hohl D, Longley MA, Rothnagel JA, Roop DR. A novel dinucleotide mutation in keratin 10 in the annular epidermolytic ichthyosis variant of bullous congenital ichthyosiform erythroderma. J Invest Dermatol. 1997;108:357–361. doi: 10.1111/1523-1747.ep12286491. [DOI] [PubMed] [Google Scholar]
- [58].Virtanen M, Smith SK, Gedde-Dahl T, Jr., Vahlquist A, Bowden PE. Splice site and deletion mutations in keratin (KRT1 and KRT10) genes: unusual phenotypic alterations in Scandinavian patients with epidermolytic hyperkeratosis. J Invest Dermatol. 2003;121:1013–1020. doi: 10.1046/j.1523-1747.2003.12534.x. [DOI] [PubMed] [Google Scholar]
- [59].Arin MJ, Longley MA, Anton-Lamprecht I, Kurze G, Huber M, Hohl D, Rothnagel JA, Roop DR. A novel substitution in keratin 10 in epidermolytic hyperkeratosis. J Invest Dermatol. 1999;112:506–508. doi: 10.1046/j.1523-1747.1999.00557.x. [DOI] [PubMed] [Google Scholar]
- [60].Terron-Kwiatkowski A, Terrinoni A, Didona B, Melino G, Atherton DJ, Irvine AD, McLean WH. Atypical epidermolytic palmoplantar keratoderma presentation associated with a mutation in the keratin 1 gene. Br J Dermatol. 2004;150:1096–1103. doi: 10.1111/j.1365-2133.2004.05967.x. [DOI] [PubMed] [Google Scholar]
- [61].Haruna K, Suga Y, Mizuno Y, Hasegawa T, Kourou K, Matsuba S, Muramatsu S, Ikeda S. R156C mutation of keratin 10 causes mild form of epidermolytic hyperkeratosis. J Dermatol. 2007;34:545–548. doi: 10.1111/j.1346-8138.2007.00328.x. [DOI] [PubMed] [Google Scholar]
- [62].Chamcheu JC, Virtanen M, Navsaria H, Bowden PE, Vahlquist A, Törmä H. Epidermolysis bullosa simplex due to KRT5 mutations: mutation-related differences in cellular fragility and the protective effects of trimethylamine N-oxide in cultured primary keratinocytes. Br. J. Dermatol. 2010;162:980–989. doi: 10.1111/j.1365-2133.2009.09615.x. [DOI] [PubMed] [Google Scholar]
- [63].Liovic M, Stojan J, Bowden PE, Gibbs D, Vahlquist A, Lane EB, Komel R. A novel keratin 5 mutation (K5V186L) in a family with EBS-K: a conservative substitution can lead to development of different disease phenotypes. J Invest Dermatol. 2001;116:964–969. doi: 10.1046/j.1523-1747.2001.01334.x. [DOI] [PubMed] [Google Scholar]
- [64].Chang YR. Genetic and biological characterization of keratin 5 and 14 mutations in EBS. 2007 [Google Scholar]
- [65].Covaciu C, Castori M, De Luca N, Ghirri P, Nannipieri A, Ragone G, Zambruno G, Castiglia D. Lethal autosomal recessive epidermolytic ichthyosis due to a novel donor splice-site mutation in KRT10. Br J Dermatol. 2010 doi: 10.1111/j.1365-2133.2010.09665.x. [DOI] [PubMed] [Google Scholar]
- [66].Terheyden P, Grimberg G, Hausser I, Rose C, Korge BP, Krieg T, Arin MJ. Recessive epidermolytic hyperkeratosis caused by a previously unreported termination codon mutation in the keratin 10 gene. J Invest Dermatol. 2009;129:2721–2723. doi: 10.1038/jid.2009.131. [DOI] [PubMed] [Google Scholar]
- [67].Tsubota A, Akiyama M, Kanitakis J, Sakai K, Nomura T, Claudy A, Shimizu H. Mild recessive bullous congenital ichthyosiform erythroderma due to a previously unidentified homozygous keratin 10 nonsense mutation. J Invest Dermatol. 2008;128:1648–1652. doi: 10.1038/sj.jid.5701257. [DOI] [PubMed] [Google Scholar]
- [68].Moss C, Jones DO, Blight A, Bowden PE. Birthmark due to cutaneous mosaicism for keratin 10 mutation. Lancet. 1995;345:596. doi: 10.1016/s0140-6736(95)90510-3. [DOI] [PubMed] [Google Scholar]
- [69].Paller AS, Syder AJ, Chan YM, Yu QC, Hutton E, Tadini G, Fuchs E. Genetic and clinical mosaicism in a type of epidermal nevus. N Engl J Med. 1994;331:1408–1415. doi: 10.1056/NEJM199411243312103. [DOI] [PubMed] [Google Scholar]
- [70].Tsubota A, Akiyama M, Sakai K, Goto M, Nomura Y, Ando S, Abe M, Sawamura D, Shimizu H. Keratin 1 gene mutation detected in epidermal nevus with epidermolytic hyperkeratosis. J Invest Dermatol. 2007;127:1371–1374. doi: 10.1038/sj.jid.5700712. [DOI] [PubMed] [Google Scholar]
- [71].Sheth N, Greenblatt D, McGrath JA. New KRT10 gene mutation underlying the annular variant of bullous congenital ichthyosiform erythroderma with clinical worsening during pregnancy. Br J Dermatol. 2007;157:602–604. doi: 10.1111/j.1365-2133.2007.08054.x. [DOI] [PubMed] [Google Scholar]
- [72].Suga Y, Duncan KO, Heald PW, Roop DR. A novel helix termination mutation in keratin 10 in annular epidermolytic ichthyosis, a variant of bullous congenital ichthyosiform erythroderma. J Invest Dermatol. 1998;111:1220–1223. doi: 10.1046/j.1523-1747.1998.00451.x. [DOI] [PubMed] [Google Scholar]
- [73].Sprecher E, Ishida-Yamamoto A, Becker OM, Marekov L, Miller CJ, Steinert PM, Neldner K, Richard G. Evidence for novel functions of the keratin tail emerging from a mutation causing ichthyosis hystrix. J Invest Dermatol. 2001;116:511–519. doi: 10.1046/j.1523-1747.2001.01292.x. [DOI] [PubMed] [Google Scholar]
- [74].Bonifas JM, Bare JW, Chen MA, Ranki A, Neimi KM, Epstein EH., Jr. Evidence against keratin gene mutations in a family with ichthyosis hystrix Curth-Macklin. J Invest Dermatol. 1993;101:890–891. doi: 10.1111/1523-1747.ep12371714. [DOI] [PubMed] [Google Scholar]
- [75].Niemi KM, Virtanen I, Kanerva L, Muttilainen M. Altered keratin expression in ichthyosis hystrix Curth-Macklin. A light and electron microscopic study. Arch Dermatol Res. 1990;282:227–233. doi: 10.1007/BF00371641. [DOI] [PubMed] [Google Scholar]
- [76].Richardson ES, Lee JB, Hyde PH, Richard G. A novel mutation and large size polymorphism affecting the V2 domain of keratin 1 in an African-American family with severe, diffuse palmoplantar keratoderma of the ichthyosis hystrix Curth-Macklin type. J Invest Dermatol. 2006;126:79–84. doi: 10.1038/sj.jid.5700025. [DOI] [PubMed] [Google Scholar]
- [77].Whittock NV, Smith FJ, Wan H, Mallipeddi R, Griffiths WA, Dopping-Hepenstal P, Ashton GH, Eady RA, McLean WH, McGrath JA. Frameshift mutation in the V2 domain of human keratin 1 results in striate palmoplantar keratoderma. J Invest Dermatol. 2002;118:838–844. doi: 10.1046/j.1523-1747.2002.01750.x. [DOI] [PubMed] [Google Scholar]
- [78].Rothnagel JA, Traupe H, Wojcik S, Huber M, Hohl D, Pittelkow MR, Saeki H, Ishibashi Y, Roop DR. Mutations in the rod domain of keratin 2e in patients with ichthyosis bullosa of Siemens. Nat Genet. 1994;7:485–490. doi: 10.1038/ng0894-485. [DOI] [PubMed] [Google Scholar]
- [79].Tsubota A, Akiyama M, Sakai K, Yanagi T, McMillan JR, Higashi A, Shimizu H. Congenital ichthyosiform erythroderma mimicking ichthyosis bullosa of Siemens. Br J Dermatol. 2008;158:191–194. doi: 10.1111/j.1365-2133.2007.08244.x. [DOI] [PubMed] [Google Scholar]
- [80].Kuster W, Zehender D, Mensing H, Hennies HC, Reis A. Vorner keratosis palmoplantaris diffusa. Clinical, formal genetic and molecular biology studies of 22 families. Hautarzt. 1995;46:705–710. doi: 10.1007/s001050050326. [DOI] [PubMed] [Google Scholar]
- [81].Kuster W, Reis A, Hennies HC. Epidermolytic palmoplantar keratoderma of Vorner: re-evaluation of Vorner's original family and identification of a novel keratin 9 mutation. Arch Dermatol Res. 2002;294:268–272. doi: 10.1007/s00403-002-0328-9. [DOI] [PubMed] [Google Scholar]
- [82].Coleman CM, Munro CS, Smith FJ, Uitto J, McLean WH. Epidermolytic palmoplantar keratoderma due to a novel type of keratin mutation, a 3-bp insertion in the keratin 9 helix termination motif. Br J Dermatol. 1999;140:486–490. doi: 10.1046/j.1365-2133.1999.02715.x. [DOI] [PubMed] [Google Scholar]
- [83].Kon A, Ito N, Kudo Y, Nomura K, Yoneda K, Hanada K, Hashimoto I, Takagaki K. L457F missense mutation within the 2B rod domain of keratin 9 in a Japanese family with epidermolytic palmoplantar keratoderma. Br J Dermatol. 2006;155:624–626. doi: 10.1111/j.1365-2133.2006.07358.x. [DOI] [PubMed] [Google Scholar]
- [84].Hatsell SJ, Eady RA, Wennerstrand L, Dopping-Hepenstal P, Leigh IM, Munro C, Kelsell DP. Novel splice site mutation in keratin 1 underlies mild epidermolytic palmoplantar keratoderma in three kindreds. J Invest Dermatol. 2001;116:606–609. doi: 10.1046/j.1523-1747.2001.13041234.x. [DOI] [PubMed] [Google Scholar]
- [85].Terron-Kwiatkowski A, Paller AS, Compton J, Atherton DJ, McLean WH, Irvine AD. Two cases of primarily palmoplantar keratoderma associated with novel mutations in keratin 1. J Invest Dermatol. 2002;119:966–971. doi: 10.1046/j.1523-1747.2002.00186.x. [DOI] [PubMed] [Google Scholar]
- [86].Candi E, Tarcsa E, Digiovanna JJ, Compton JG, Elias PM, Marekov LN, Steinert PM. A highly conserved lysine residue on the head domain of type II keratins is essential for the attachment of keratin intermediate filaments to the cornified cell envelope through isopeptide crosslinking by transglutaminases. Proc Natl Acad Sci U S A. 1998;95:2067–2072. doi: 10.1073/pnas.95.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kimonis V, DiGiovanna JJ, Yang JM, Doyle SZ, Bale SJ, Compton JG. A mutation in the VI end domain of keratin 1 in non-epidermolytic palmar-plantar keratoderma. J Invest Dermatol. 1994;103:764–769. doi: 10.1111/1523-1747.ep12412771. [DOI] [PubMed] [Google Scholar]
- [88].Wevers A, Kuhn A, Mahrle G. Palmoplantar keratoderma with tonotubular keratin. J Am Acad Dermatol. 1991;24:638–642. doi: 10.1016/0190-9622(91)70099-n. [DOI] [PubMed] [Google Scholar]
- [89].Grimberg G, Hausser I, Muller FB, Wodecki K, Schaffrath C, Krieg T, Oji V, Traupe H, Arin MJ. Novel and recurrent mutations in the 1B domain of keratin 1 in palmoplantar keratoderma with tonotubules. Br J Dermatol. 2009;160:446–449. doi: 10.1111/j.1365-2133.2008.08831.x. [DOI] [PubMed] [Google Scholar]
- [90].Terron-Kwiatkowski A, van Steensel MA, van Geel M, Lane EB, McLean WH, Steijlen PM. Mutation S233L in the 1B domain of keratin 1 causes epidermolytic palmoplantar keratoderma with “tonotubular” keratin. J Invest Dermatol. 2006;126:607–613. doi: 10.1038/sj.jid.5700152. [DOI] [PubMed] [Google Scholar]
- [91].Lugassy J, Hennies HC, Indelman M, Khamaysi Z, Bergman R, Sprecher E. Rapid detection of homozygous mutations in congenital recessive ichthyosis. Arch Dermatol Res. 2008;300:81–85. doi: 10.1007/s00403-007-0815-0. [DOI] [PubMed] [Google Scholar]
- [92].Langbein L, Schweizer J. Keratins of the human hair follicle. Int Rev Cytol. 2005;243:1–78. doi: 10.1016/S0074-7696(05)43001-6. [DOI] [PubMed] [Google Scholar]
- [93].Winter H, Rogers MA, Langbein L, Stevens HP, Leigh IM, Labreze C, Roul S, Taieb A, Krieg T, Schweizer J. Mutations in the hair cortex keratin hHb6 cause the inherited hair disease monilethrix. Nat Genet. 1997;16:372–374. doi: 10.1038/ng0897-372. [DOI] [PubMed] [Google Scholar]
- [94].Horev L, Glaser B, Metzker A, Ben-Amitai D, Vardy D, Zlotogorski A. Monilethrix: mutational hotspot in the helix termination motif of the human hair basic keratin 6. Hum Hered. 2000;50:325–330. doi: 10.1159/000022937. [DOI] [PubMed] [Google Scholar]
- [95].van Steensel MA, Steijlen PM, Bladergroen RS, Vermeer M, van Geel M. A missense mutation in the type II hair keratin hHb3 is associated with monilethrix. J Med Genet. 2005;42:e19. doi: 10.1136/jmg.2004.021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Schaffer JV, Bazzi H, Vitebsky A, Witkiewicz A, Kovich OI, Kamino H, Shapiro LS, Amin SP, Orlow SJ, Christiano AM. Mutations in the desmoglein 4 gene underlie localized autosomal recessive hypotrichosis with monilethrix hairs and congenital scalp erosions. J Invest Dermatol. 2006;126:1286–1291. doi: 10.1038/sj.jid.5700237. [DOI] [PubMed] [Google Scholar]
- [97].Shimomura Y, Sakamoto F, Kariya N, Matsunaga K, Ito M. Mutations in the desmoglein 4 gene are associated with monilethrix-like congenital hypotrichosis. J Invest Dermatol. 2006;126:1281–1285. doi: 10.1038/sj.jid.5700113. [DOI] [PubMed] [Google Scholar]
- [98].Zlotogorski A, Marek D, Horev L, Abu A, Ben-Amitai D, Gerad L, Ingber A, Frydman M, Reznik-Wolf H, Vardy DA, Pras E. An autosomal recessive form of monilethrix is caused by mutations in DSG4: clinical overlap with localized autosomal recessive hypotrichosis. J Invest Dermatol. 2006;126:1292–1296. doi: 10.1038/sj.jid.5700251. [DOI] [PubMed] [Google Scholar]
- [99].Winter H, Schissel D, Parry DA, Smith TA, Liovic M, Birgitte Lane E, Edler L, Langbein L, Jave-Suarez LF, Rogers MA, Wilde J, Peters G, Schweizer J. An unusual Ala12Thr polymorphism in the 1A alpha-helical segment of the companion layer-specific keratin K6hf: evidence for a risk factor in the etiology of the common hair disorder pseudofolliculitis barbae. J Invest Dermatol. 2004;122:652–657. doi: 10.1111/j.0022-202X.2004.22309.x. [DOI] [PubMed] [Google Scholar]
- [100].Itin PH, Fistarol SK. Palmoplantar keratodermas. Clin Dermatol. 2005;23:15–22. doi: 10.1016/j.clindermatol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- [101].Bowden PE, Haley JL, Kansky A, Rothnagel JA, Jones DO, Turner RJ. Mutation of a type II keratin gene (K6a) in pachyonychia congenita. Nat-Genet. 1995;10:363–365. doi: 10.1038/ng0795-363. [DOI] [PubMed] [Google Scholar]
- [102].De Berker D, Wojnarowska F, Sviland L, Westgate GE, Dawber RP, Leigh IM. Keratin expression in the normal nail unit: markers of regional differentiation. Br J Dermatol. 2000;142:89–96. doi: 10.1046/j.1365-2133.2000.03246.x. [DOI] [PubMed] [Google Scholar]
- [103].McLean WH, Rugg EL, Lunny DP, Morley SM, Lane EB, Swensson O, Dopping-Hepenstal PJ, Griffiths WA, Eady RA, Higgins C, et al. Keratin 16 and keratin 17 mutations cause pachyonychia congenita. Nat Genet. 1995;9:273–278. doi: 10.1038/ng0395-273. [DOI] [PubMed] [Google Scholar]
- [104].Connors JB, Rahil AK, Smith FJ, McLean WH, Milstone LM. Delayed-onset pachyonychia congenita associated with a novel mutation in the central 2B domain of keratin 16. Br J Dermatol. 2001;144:1058–1062. doi: 10.1046/j.1365-2133.2001.04199.x. [DOI] [PubMed] [Google Scholar]
- [105].Shamsher MK, Navsaria HA, Stevens HP, Ratnavel RC, Purkis PE, Kelsell DP, McLean WH, Cook LJ, Griffiths WA, Gschmeissner S, et al. Novel mutations in keratin 16 gene underly focal non-epidermolytic palmoplantar keratoderma (NEPPK) in two families. Hum Mol Genet. 1995;4:1875–1881. doi: 10.1093/hmg/4.10.1875. [DOI] [PubMed] [Google Scholar]
- [106].Smith FJ, Fisher MP, Healy E, Rees JL, Bonifas JM, Epstein EH, Jr., Tan EM, Uitto J, McLean WH. Novel keratin 16 mutations and protein expression studies in pachyonychia congenita type 1 and focal palmoplantar keratoderma. Exp Dermatol. 2000;9:170–177. doi: 10.1034/j.1600-0625.2000.009003170.x. [DOI] [PubMed] [Google Scholar]
- [107].Smith FJ. Nail that mutation-keratin 17 defect in late-onset pachyonychia. J Invest Dermatol. 2004;122:x–xi. doi: 10.1111/j.0022-202X.2004.22437.x. [DOI] [PubMed] [Google Scholar]
- [108].Smith FJ, Corden LD, Rugg EL, Ratnavel R, Leigh IM, Moss C, Tidman MJ, Hohl D, Huber M, Kunkeler L, Munro CS, Lane EB, McLean WH. Missense mutations in keratin 17 cause either pachyonychia congenita type 2 or a phenotype resembling steatocystoma multiplex. J Invest Dermatol. 1997;108:220–223. doi: 10.1111/1523-1747.ep12335315. [DOI] [PubMed] [Google Scholar]
- [109].Smith FJ, Liao H, Cassidy AJ, Stewart A, Hamill KJ, Wood P, Joval I, van Steensel MA, Bjorck E, Callif-Daley F, Pals G, Collins P, Leachman SA, Munro CS, McLean WH. The genetic basis of pachyonychia congenita. J Investig Dermatol Symp Proc. 2005;10:21–30. doi: 10.1111/j.1087-0024.2005.10204.x. [DOI] [PubMed] [Google Scholar]
- [110].Smith FJ, Sandilands A, McLean WH. Molecular genetics methods for human intermediate filament diseases. Methods Cell Biol. 2004;78:131–161. doi: 10.1016/s0091-679x(04)78006-1. [DOI] [PubMed] [Google Scholar]
- [111].Xiao SX, Feng YG, Ren XR, Tan SS, Li L, Wang JM, Shi YZ. A novel mutation in the second half of the keratin 17 1A domain in a large pedigree with delayed-onset pachyonychia congenita type 2. J Invest Dermatol. 2004;122:892–895. doi: 10.1111/j.0022-202X.2004.22408.x. [DOI] [PubMed] [Google Scholar]
- [112].Naeem M, Wajid M, Lee K, Leal SM, Ahmad W. A mutation in the hair matrix and cuticle keratin KRTHB5 gene causes ectodermal dysplasia of hair and nail type. J Med Genet. 2006;43:274–279. doi: 10.1136/jmg.2005.033381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Fuchs E, Coulombe PA. Of Mice and Men - Genetic Skin Diseases of Keratin. Cell. 1992;69:899–902. doi: 10.1016/0092-8674(92)90607-e. [DOI] [PubMed] [Google Scholar]
- [114].Rothnagel JA, Greenhalgh DA, Gagne TA, Longley MA, Roop DR. Identification of a calcium-inducible, epidermal-specific regulatory element in the 3'-flanking region of the human keratin 1 gene. J Invest Dermatol. 1993;101:506–513. doi: 10.1111/1523-1747.ep12365886. [DOI] [PubMed] [Google Scholar]
- [115].Vassar R, Coulombe PA, Degenstein L, Albers K, Fuchs E. Mutant keratin expression in transgenic mice causes marked abnormalities resembling a human genetic skin disease. Cell. 1991;64:365–380. doi: 10.1016/0092-8674(91)90645-f. [DOI] [PubMed] [Google Scholar]
- [116].Baribault H, Price J, Miyai K, Oshima RG. Mid-gestational lethality in mice lacking keratin 8. Genes Dev. 1993;7:1191–1202. doi: 10.1101/gad.7.7a.1191. [DOI] [PubMed] [Google Scholar]
- [117].Hesse M, Franz T, Tamai Y, Taketo MM, Magin TM. Targeted deletion of keratins 18 and 19 leads to trophoblast fragility and early embryonic lethality. EMBO J. 2000;19:5060–5070. doi: 10.1093/emboj/19.19.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wong P, Colucci-Guyon E, Takahashi K, Gu C, Babinet C, Coulombe PA. Introducing a null mutation in the mouse K6alpha and K6beta genes reveals their essential structural role in the oral mucosa. J Cell Biol. 2000;150:921–928. doi: 10.1083/jcb.150.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Cao T, Longley MA, Wang XJ, Roop DR. An inducible mouse model for epidermolysis bullosa simplex: implications for gene therapy. J Cell Biol. 2001;152:651–656. doi: 10.1083/jcb.152.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Bader BL, Franke WW. Cell type-specific and efficient synthesis of human cytokeratin 19 in transgenic mice. Differentiation. 1990;45:109–118. doi: 10.1111/j.1432-0436.1990.tb00464.x. [DOI] [PubMed] [Google Scholar]
- [121].Santos M, Paramio JM, Bravo A, Ramirez A, Jorcano JL. The expression of keratin k10 in the basal layer of the epidermis inhibits cell proliferation and prevents skin tumorigenesis. J Biol Chem. 2002;277:19122–19130. doi: 10.1074/jbc.M201001200. [DOI] [PubMed] [Google Scholar]
- [122].Magin TM, Kaiser HW, Leitgeb S, Grund C, Leigh IM, Morley SM, Lane EB. Supplementation of a mutant keratin by stable expression of desmin in cultured human EBS keratinocytes. J Cell Sci. 2000;113:4231–4239. doi: 10.1242/jcs.113.23.4231. [DOI] [PubMed] [Google Scholar]
- [123].Ortiz-Urda S, Lin Q, Green CL, Keene DR, Marinkovich MP, Khavari PA. Injection of genetically engineered fibroblasts corrects regenerated human epidermolysis bullosa skin tissue. J Clin Invest. 2003;111:251–255. doi: 10.1172/JCI17193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Ortiz-Urda S, Lin Q, Yant SR, Keene D, Kay MA, Khavari PA. Sustainable correction of junctional epidermolysis bullosa via transposon-mediated nonviral gene transfer. Gene Ther. 2003;10:1099–1104. doi: 10.1038/sj.gt.3301978. [DOI] [PubMed] [Google Scholar]
- [125].Chamcheu JC, Pavez Lorié E, Akgul B, Bannbers E, Virtanen M, Gammon L, Moustakas A, Navsaria H, Vahlquist A, Törmä H. Characterization of immortalized human epidermolysis bullosa simplex (KRT5) cell lines: trimethylamine N-oxide protects the keratin cytoskeleton against disruptive stress condition. J. Dermatol. Sci. 2009;53:198–206. doi: 10.1016/j.jdermsci.2008.11.003. [DOI] [PubMed] [Google Scholar]
- [126].Chamcheu JC, Pihl-Lundin I, Mouyobo CE, Gester T, Virtanen M, Moustakas A, Navsaria H, Vahlquist A, Torma H. Immortalized keratinocytes derived from epidermolytic ichthyosis patients reproduce the disease phenotype: a useful in vitro model for testing new treatments. Br J Dermatol. 2010 doi: 10.1111/j.1365-2133.2010.10092.x. [DOI] [PubMed] [Google Scholar]
- [127].Chipev CC, Steinert PM, Woodworth CD. Characterization of an immortalized cell line from a patient with epidermolytic hyperkeratosis. J Invest Dermatol. 1996;106:385–390. doi: 10.1111/1523-1747.ep12343322. [DOI] [PubMed] [Google Scholar]
- [128].Morley SM, D'Alessandro M, Sexton C, Rugg EL, Navsaria H, Shemanko CS, Huber M, Hohl D, Heagerty AI, Leigh IM, Lane EB. Generation and characterization of epidermolysis bullosa simplex cell lines: scratch assays show faster migration with disruptive keratin mutations. Br. J. Dermatol. 2003;149:46–58. doi: 10.1046/j.1365-2133.2003.05493.x. [DOI] [PubMed] [Google Scholar]
- [129].D'Alessandro M, Russell D, Morley SM, Davies AM, Lane EB. Keratin mutations of epidermolysis bullosa simplex alter the kinetics of stress response to osmotic shock. J Cell Sci. 2002;115:4341–4351. doi: 10.1242/jcs.00120. [DOI] [PubMed] [Google Scholar]
- [130].Liovic M, Lee B, Tomic-Canic M, D'Alessandro M, Bolshakov VN, Lane EB. Dual-specificity phosphatases in the hypo-osmotic stress response of keratin-defective epithelial cell lines, Exp. Cell Res. 2008;314:2066–2075. doi: 10.1016/j.yexcr.2008.02.020. [DOI] [PubMed] [Google Scholar]
- [131].Russell D, Andrews PD, James J, Lane EB. Mechanical stress induces profound remodelling of keratin filaments and cell junctions in epidermolysis bullosa simplex keratinocytes. J. Cell Sci. 2004;117:5233–5243. doi: 10.1242/jcs.01407. [DOI] [PubMed] [Google Scholar]
- [132].Russell D, Ross H, Lane EB. ERK involvement in resistance to apoptosis in keratinocytes with mutant keratin. J. Invest. Dermatol. 2010;130:671–681. doi: 10.1038/jid.2009.327. [DOI] [PubMed] [Google Scholar]
- [133].Chamcheu JC. Disease-causing Keratin Mutations and Cytoskeletal Dysfunction in Human Skin: Invitro Models and new Pharmacologic Strategies for Treating Epidermolytic Genodermatoses, Medical Sciences, Dermatology and Venereology. Uppsala University; Uppsala: 2010. p. 85. [Google Scholar]
- [134].Barnhart KF, Credille KM, Ambrus A, Dunstan RW. Preservation of phenotype in an organotypic cell culture model of a recessive keratinization defect of Norfolk terrier dogs. Exp Dermatol. 2005;14:481–490. doi: 10.1111/j.0906-6705.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- [135].Chamcheu JC, Virtanen M, Navsaria H, Bowden PE, Vahlquist A, Torma H. Epidermolysis bullosa simplex due to KRT5 mutations: mutation-related differences in cellular fragility and the protective effects of trimethylamine N-oxide in cultured primary keratinocytes. Br J Dermatol. 2010;162:980–989. doi: 10.1111/j.1365-2133.2009.09615.x. [DOI] [PubMed] [Google Scholar]
- [136].Hickerson RP, Smith FJ, McLean WH, Landthaler M, Leube RE, Kaspar RL. SiRNA-mediated selective inhibition of mutant keratin mRNAs responsible for the skin disorder pachyonychia congenita. Ann N Y Acad Sci. 2006;1082:56–61. doi: 10.1196/annals.1348.059. [DOI] [PubMed] [Google Scholar]
- [137].Hickerson RP, Leake D, Pho LN, Leachman SA, Kaspar RL. Rapamycin selectively inhibits expression of an inducible keratin (K6a) in human keratinocytes and improves symptoms in pachyonychia congenita patients. J Dermatol Sci. 2009;56:82–88. doi: 10.1016/j.jdermsci.2009.07.008. [DOI] [PubMed] [Google Scholar]
- [138].Hickerson RP, Smith FJ, Reeves RE, Contag CH, Leake D, Leachman SA, Milstone LM, McLean WH, Kaspar RL. Single-nucleotide-specific siRNA targeting in a dominant-negative skin model. J Invest Dermatol. 2008;128:594–605. doi: 10.1038/sj.jid.5701060. [DOI] [PubMed] [Google Scholar]
- [139].Leachman SA, Hickerson RP, Hull PR, Smith FJ, Milstone LM, Lane EB, Bale SJ, Roop DR, McLean WH, Kaspar RL. Therapeutic siRNAs for dominant genetic skin disorders including pachyonychia congenita. J Dermatol Sci. 2008;51:151–157. doi: 10.1016/j.jdermsci.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Leachman SA, Hickerson RP, Schwartz ME, Bullough EE, Hutcherson SL, Boucher KM, Hansen CD, Eliason MJ, Srivatsa GS, Kornbrust DJ, Smith FJ, McLean WI, Milstone LM, Kaspar RL. First-in-human mutation-targeted siRNA phase Ib trial of an inherited skin disorder. Mol Ther. 2010;18:442–446. doi: 10.1038/mt.2009.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Khavari PA, Rollman O, Vahlquist A. Cutaneous gene transfer for skin and systemic diseases. J Intern Med. 2002;252:1–10. doi: 10.1046/j.1365-2796.2002.00995.x. [DOI] [PubMed] [Google Scholar]