Abstract
Metastasis is the most deadly aspect of cancer and results from several interconnected processes including cell proliferation, angiogenesis, cell adhesion, migration, and invasion into the surrounding tissue. The appearance of metastases in organs distant from the primary tumor is the most destructive feature of cancer. Metastasis remains the principal cause of the deaths of cancer patients despite decades of research aimed at restricting tumor growth. Therefore, inhibition of metastasis is one of the most important issues in cancer research. Several in vitro, in vivo, and epidemiological studies have reported that the consumption of green tea may decrease cancer risk. (−)-Epigallocatechin-3-gallate, major component of green tea, has been shown to inhibit tumor invasion and angiogenesis which are essential for tumor growth and metastasis. This article summarizes the effect of green tea and its major polyphenolic compounds on cancer and metastasis against most commonly diagnosed cancer sites.
Keywords: Cancer, EGCG, Green tea, Metastasis, Tumor growth
1 Introduction
Cancer is recognized worldwide to be a major health problem. A total of 1,479,350 new cancer cases and 562,340 deaths from cancer were projected to occur in the United States in 2009 [1]. The main reason for such a high mortality from cancer is due to the highly invasive behavior of cancer cells, which usually results in cancer progression and metastasis. Metastasis is the process whereby neoplastic cells spread from a primary site where the primary tumor originated to distant organs and is responsible for the majority of deaths related to cancer. The metastatic process involves tumor cell invasion from the primary tumor, intravasation, arrest, and extravasation of the circulatory system, followed by angiogenesis and growth at a distant site [2, 3]. For successful manifestation of metastasis, all steps in the metastatic cascade must be completed. Therefore, the blockade of any single step in the metastatic cascade would be expected to slow metastasis. Progression towards a metastatic phenotype requires a concerted effort between different molecules that have been implicated in advancing one or more steps of the metastatic cascade [4]. It is a complex process which is dependent on both host and tumor properties for the dissemination of malignant cells throughout the body and their survival to form secondary growths. Metastatic cancer cells require properties that allow them not only to adapt to a foreign microenvironment but to subvert it in a way that is conducive to their continued proliferation and survival.
Despite improvements in diagnosis, surgical techniques, patient care, and adjuvant therapies, most deaths from cancer are due to metastasis that is resistant to conventional therapies, direct organ damage by the growing lesions, paraneoplastic syndromes, or from the complications of treatment [5]. Several reasons account for treatment failure in patients with metastases. The major obstacle to effective treatment is the heterogeneity of the tumor cells which contain subpopulations of cells with different angiogenic, invasive, and metastatic properties. Although metastases can have a clonal origin, genetic instability results in rapid biological diversification and the regeneration of heterogeneous subpopulations of cells. The specific organ environment can influence the biological behavior of metastatic cells, including their response to systemic therapy as the metastases can be located in lymph nodes and different organs [2]. The outcome of cancer metastasis depends on multiple interactions between metastatic cells and homeostatic mechanisms that are unique to one or another organ microenvironment. The specific organ microenvironment determines the extent of cancer cell proliferation, angiogenesis, invasion, and survival. Therefore, the therapy of metastasis should be targeted against tumor cells and the host factors that contribute to and support the progressive growth and survival of metastatic cancer cells.
Tea, derived from the plant Camellia sinensis, is the most globally consumed beverage as green, black, or Oolong tea. [6, 7]. It is estimated that about 2.5 million tons of tea leaves are produced throughout the world each year with 20% produced as green tea, which is mainly consumed in Asia, some parts of North Africa, the United States, and Europe [8]. The most significant effects on human health have been attributed to green tea. It contains characteristic polyphenolic compounds, (−)-epigallocatechin-3-gallate (EGCG), (−)-epigallocatechin (EGC), (−)-epicatechin-3-gallate (ECG), and (−)-epicatechin (EC). [9]. Catechin, gallocatechin, epigallocatechin digallate, epicatechin digallate, 3-O-methyl EC and EGC, catechin gallate (CG), and gallocatechin gallate are present in smaller quantities. EGCG has an antioxidant activity about 25–100 times more effective than that of vitamins C and E and appears to be the most potent of all the catechins [10]. The anti-cancer effects of EGCG have been reported to be linked to the modulation of multiple signaling pathways, finally resulting in the downregulation of expression of proteins involved in the invasiveness of cancer cells [11]. In this review article, we discuss the modulation of signaling pathways responsible for invasive behavior and metastasis of different cancer types by green tea [Tables 1 and 2].
Table 1.
Effect of green tea on metastasis of cancer in cell culture systems
| Type of cancer | Mechanism | References |
|---|---|---|
| Skin Cancer | Inhibition of melanoma cell migration, invasion, spread of cells on fibronectin, laminin, collagen, and Matrigel, inhibition of the tyrosine phosphorylation of FAK and MMP-9 activity | [16] |
| Impairment of the adhesion of murine melanoma cells to laminin | [17] | |
| Upregulation of the expression of E-cadherin time and concentration dependently in human malignant melanoma cell line | [18] | |
| Prostate Cancer | Dose-dependent inhibition of cell growth with induction of apoptosis | [19] |
| Combination of EGCG and Apo2L/TRAIL caused induction of apoptosis, upregulation of PARP, modulation of pro- and antiapoptotic Bcl2 family of proteins, inhibition in the invasion and migration of prostate cancer cells, inhibition of VEGF, uPA, angiopoietin-1,-2, decrease in MMP-2, -3, and -9, and upregulation of TIMP1 | [20] | |
| Inhibition of the degradation of gelatin, type IV collagen in reconstituted basement membrane, and activation of MMP-2 but not pro-MMP-9 | [24] | |
| Breast Cancer | Dose-dependent downregulation of EGFR phoshoporylation, EGFR mRNA expression and protein level, decrease in ERK1/2, phosph-ERK1/2, in vitro cell growth, MMP-2 and -9, and increase in TIMP-1 and -2 | [27] |
| Inhibition of migration/invasion by suppressing the HRG-stimulated activation of ErbB2/ErbB3/Akt | [28] | |
| Decrease in MMP-2, FAK, MT1-MMP, NF-κB, VEGF, reduction in the adhesion of breast cancer cells to ECM, fibronectin, vitronectin and reduction in the expression of integrin receptors α5, β1, αv, and β3 | [29] | |
| Inhibition of AP-1, NF-κB, uPA, uPA-R, vitronectin, and integrin receptor | [30] | |
| Lung Cancer | Inhibition of MMP-2 and -9 and alteration of the intermediate filaments of vimentin | [31] |
| Inhibition of the invasion of human lung carcinoma cells and downregulation of MMP-9 and NF-κB | [32] | |
| Inhibition of invasion of highly metastatic mouse Lewis lung carcinoma cells, downregualtion of MMP-2 and -9 and type IV collagenases | [39] | |
| Reduction in the number of lung colonies of mouse Lewis lung carcinoma cells, inhibition of penetration of the cells through the basement membrane in a spontaneous metastasis system | [40] | |
| Liver Cancer | Suppression of the invasion and the migration of human hepatocellular carcinoma cells and activities of MMP-2 and -9 | [42] |
| Inhibition of the proliferation and metastasis of liver cancer cells with the scavenging of reactive oxygen species | [43] | |
| Gastrointestinal Cancer | Suppression of the activation of Met in the presence of HGF in human colon cancer cells | [45] |
| Increased ubiquitination of bFGF and trypsin-like activity of the 20S proteasome, thereby resulting in the degradation of bFGF protein in colon cancer cells | [46] | |
| Increase in both intracellular and extracellular pro-MMP-7 protein and mRNA expression levels, activation of ERK1/2, JNK1/2 and p38 MAPK, phosphorylation of c-JUN and induction of pro-MMP-7 production in human colorectal cancer cells | [48] | |
| Inhibition of viability, capillary tube formation and migration of HUVECs | [49] | |
| Dose-dependent antiproliferative effect, decreased expression of MMP-9 and inhibition of invasion through Matrigel in pancreatic cancer cells | [50] |
Table 2.
Effect of green tea on metastasis of cancer in animal models
| Type of cancer | Mechanism | References |
|---|---|---|
| Skin Cancer | Inhibition of tumor growth and metastasis in a mouse melanoma model, enhancement of the mean survival of the treated groups | [12] |
| Suppression of tumor growth with inhibition of MMP-9 and VEGF secretion in athymic nude mice implanted with human melanoma cells | [13] | |
| In SKH-1 hairless mice, reduction of UVB-induced tumor incidence, tumor multiplicity, and tumor growth. Reduction of MMP-2 and -9, CD31, VEGF, and PCNA, increased activation of caspase-3, enhancement of TIMP, and inhibition of angiogenic factors and recruitment of cytotoxic T cells in the tumor microenvironment | [14] | |
| Inhibition of protein expression and activity of MMP-2 and -9 decreased expression of CD31 and PCNA and increased expression of TIMP | [15] | |
| Prostate Cancer | Inhibition of tumor growth and invasion | [19] |
| Inhibition of tumor growth, MMP-9, VEGF secretion and mitosis in tumors of athymic nude mice | [21] | |
| Inhibition of PCa progression associated with reduction of S100A4 and restoration of E-cadherin in TRAMP mice | [22] | |
| Reduction in IGF-I with increase in IGFBP-3 in the dorsolateral prostate in TRAMP mice. Inhibition of PI3K, p-Akt, ERK1/2, VEGF, uPA, MMP-2 and -9 | [23] | |
| Inhibition of tumor weight and metastasis, reduction in serum concentrations of testosterone and DHT in a mouse model of orthotopic androgen-sensitive human prostate cancer | [25] | |
| Inhibition of prostate cancer development and increased survival in male TRAMP mice. Delay in primary tumor incidence and tumor burden, significant decrease in prostate and genitourinary weight, inhibition in serum IGF-1, increase in IGFBP-3, reduction in PCNA, and inhibition of distant site metastases to lymph nodes, lungs, liver and bone | [26] | |
| Breast Cancer | Reduction of tumor growth, increase in Bax/Bcl-2 ratio, reduction in PCNA, activation of caspase-3, inhibition of metastasis of tumor cells to lungs and increase in the survival period of BALB/c mice | [31] |
| Increased natural killer cell activity and reduction in the number of lung-metastatic colonies in SAMP10 mice | [33] | |
| Lung Cancer | Inhibition of MMP-2 and -9 secretion, invasion of human lung carcinoma cells through Matrigel in a dose-dependent fashion in athymic nude mice | [37] |
| Reduction of lung metastases, primary tumor growths and increased survival rate in mice bearing melanomas | [16] | |
| Liver Cancer | Inhibition of metastasis of melanoma cells to the liver and increase of the survival time in C57BL/6 mice | [41] |
| Suppression of the increase in liver weight and hepatic metastasis of ovarian sarcoma cells transplanted subcutaneously in mice | [44] | |
| Gastrointestinal Cancer | Inhibition of intestinal tumor formation with reduced bFGF expression in APC (Min/+) mice | [46] |
| Reduction in the size of tumors in nude mice implanted with human colon cancer cells. Inhibition of MMP-9 and VEGF secretion and mitotic index in the tumor tissues | [47] | |
| Reduction in Ki-67, PCNA, vWF, VEGF, CD31, VEGFR-2, ERK1/2, JNK1/2, p38, MMP-2, MMP-7, MMP-9, and MMP-12 and induction of apoptosis, caspase-3 activity, and p21/WAF1 in tumors of athymic nude mice implanted with human pancreatic cells | [49] | |
| Inhibition of pancreatic cancer incidence, process of pancreatic carcinogenesis, and tumor promotion of transplanted pancreatic cancer in hamsters | [51] |
2 Green tea and metastasis of skin cancer
The skin cancer incidence is increasing by epidemic proportions. A trimethoxy derivative of ECG, 3-O-(3,4,5-trimethoxybenzoyl)-(−)-epicatechin (TMECG), is a prodrug that is selectively activated by the specific melanocyte enzyme tyrosinase. The treatment of melanoma cells with TMECG affected cellular folate transport and the gene expression of dihydrofolate reductase. It also inhibited tumor growth and metastasis in a mouse melanoma model, significantly enhancing the mean survival of the treated groups [12]. Treatment with a diet containing lysine, proline, arginine, ascorbic acid, and green tea extract to athymic nude mice implanted with human melanoma A2058 cells strongly suppressed tumor growth with inhibition of MMP-9 and VEGF secretion [13]. In SKH-1 hairless mice, oral administration of green tea polyphenols (GTP) reduced ultraviolet (UV)B-induced tumor incidence, tumor multiplicity, and tumor growth. The group treated with UVB and given GTP had reduced expression of the matrix metalloproteinases (MMP)-2 and -9, which have crucial roles in tumor growth and metastasis, enhanced expression of tissue inhibitor of MMP (TIMP), reduced expressions of CD31 and vascular endothelial growth factor (VEGF), increased expression of proliferating cell nuclear antigen (PCNA), more cytotoxic CD8(+) T cells, and increased activation of caspase-3 in the tumors as compared with group treated with UVB alone. It was concluded that administration of GTP caused inhibition of angiogenic factors and recruitment of cytotoxic T cells in the tumor microenvironment [14]. UVB-induced skin tumors with and without treatment of EGCG and age-matched skin biopsies from SKH-1 hairless mice were used to identify potential molecular targets of skin cancer prevention by EGCG. Topical application of EGCG in UV-induced tumors resulted in the inhibition of protein expression and activity of MMP-2 and -9, increased expression of TIMP, and decreased expression of CD31 and PCNA [15].
EGCG dose-dependently inhibited B16-F3m melanoma cell migration and invasion and inhibited the spread of melanoma cells on fibronectin, laminin, collagen, and Matrigel in a dose-dependent manner. EGCG significantly inhibited the tyrosine phosphorylation of focal adhesion kinase (FAK) and the activity of MMP-9. EGCG also caused reduction in the lung metastases in mice bearing B16-F3m melanomas, but a combination of EGCG and dacarbazine was more effective than EGCG alone in reducing the number of pulmonary metastases and primary tumor growths and in increasing the survival rate of melanoma-bearing mice [16]. EGCG and ECG were found to inhibit melanoma cell adhesion in the culture medium. The adhesion of murine melanoma cells to laminin was impaired on pretreatment with EGCG [17]. EGCG significantly upregulated the expression of E-cadherin time and concentration dependently in human malignant melanoma A375 cell line. EGCG inhibited the invasion of human malignant melanoma cell line which correlated with the upregulation of E-cadherin expression [18].
3 Green tea and metastasis of prostate cancer
In U.S. men, prostate cancer (PCa) is the most common non-cutaneous malignancy. Treatment of PCa PC-3 cells with Traditional Botanical Supplement-101 (TBS-101), a botanical agent containing standardized botanical extracts of Panax ginseng, cranberry, green tea, grape skin, grape seed, Ganoderma lucidum, and chamomile resulted in dose-dependent inhibition of cell growth with concomitant induction of apoptosis. On treatment with TBS-101, mice bearing moderate or large tumors showed significant inhibition of tumor growth and invasion while control group mice had significant tumor growth and lymph node metastasis. No toxicity was reported in healthy or tumor-bearing mice with high doses of TBS-101 [19]. Study from our laboratory has shown for the first time that EGCG sensitizes TRAIL-resistant PCa LNCaP cells to TRAIL-mediated apoptosis through modulation of intrinsic and extrinsic apoptotic pathways. Combination of EGCG and Apo2L/TRAIL caused induction of apoptosis accompanied by the upregulation of poly (ADP-ribose) polymerase (PARP) cleavage and modulation of pro- and antiapoptotic Bcl2 family of proteins. Pretreatment of cells with EGCG resulted in modulation of death-inducing signaling cascade complex involving DR4/TRAIL R1, Fas-associated death domain, and FLICE-inhibitory protein. There was also synergistic inhibition in the invasion and migration of PCa cells mediated through inhibition in the protein expression of VEGF, uPA, and angiopoietin-1 and -2. Additionally, on treatment of cells with a combination of EGCG and TRAIL, there was decrease in the activity and protein expression of MMP-2, -3, and -9 and upregulation of TIMP1 [20].
The effects of a diet containing lysine, proline, arginine, ascorbic acid, and green tea extract on the growth of tumors induced by implanting human PCa PC-3 cells in athymic nude mice and on the expression of MMPs, VEGF, Ki-67, and fibronectin in these tumors, as well as the production of mucin, were investigated. It was found that there was inhibition of tumor growth and inhibition of MMP-9 and VEGF secretion and mitosis in tissues of group treated with the nutrient mixture of the diet [21]. The expression of metastasis-promoting Mts1 gene (S100A4) was assessed in GTP-treated transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Freshly prepared 0.1% GTP solution in tap water was supplied thrice a week to experimental animals as the sole source of drinking fluid for 24 weeks, while the control group of animals received the same tap water throughout the study. The animals were sacrificed at 0, 8, 16, and 24 weeks of GTP feeding and were analyzed for S100A4 and E-cadherin. An increase in the expression of S100A4 at mRNA and protein level in dorsolateral prostate, but not in nontransgenic mice, was found with the progression of age and PCa growth in TRAMP mice. There was inhibition of PCa progression in mice fed with GTP which was associated with reduction of S100A4 and restoration of E-cadherin [22].
Our laboratory study demonstrated the role of insulin growth factor (IGF)-I/IGF binding protein (IGFBP)-3 signaling and its downstream and other associated events during chemoprevention of PCa by GTP in TRAMP mice. There were increased levels of IGF-I, phosphatidylinositol 3′-kinase (PI3K), phosphorylated Akt, and extracellular signal-regulated kinase (ERK)1/2 with concomitant decrease in IGFBP-3 in dorsolateral prostate of TRAMP mice during the course of cancer progression. By continuous GTP infusion for 24 weeks to mice, there was substantial reduction in the levels of IGF-I and significant increase in the levels of IGFBP-3 in the dorsolateral prostate. This modulation of IGF/IGFBP-3 was found to be associated with an inhibition of protein expression of PI3K, phospho-Akt, and ERK 1/2 with concomitant inhibition of markers of angiogenesis and metastasis such as VEGF, uPA, and MMP-2 and -9 [23]. The effects of EGCG were investigated on the expression and activity of PSA in PCa cells. EGCG was found to inhibit degradation of gelatin, degradation of type IV collagen in reconstituted basement membrane (Matrigel), and activation of MMP-2 but not pro-MMP-9 in a cell-free system in a dose-dependent manner at concentrations lower than the cytotoxic serine-protease inhibitor phenylmethyl sulfonyl fluoride and close to levels measured in the serum following ingestion of green tea [24]. The synergistic effects between soy and tea components on prostate tumor progression in a mouse model of orthotopic androgen-sensitive human PCa were identified. Soy phytochemical concentrate (SPC), black tea, and green tea significantly reduced tumorigenicity, while SPC and black tea also significantly reduced final tumor weights. Green tea did not reduce final tumor weight, but it elevated serum dihydrotestosterone (DHT) concentration. There was inhibition of prostate tumorigenicity, final tumor weight, and metastases to lymph nodes in vivo by combination of SPC and black tea while the combination of SPC and green tea inhibited final tumor weight and metastasis and significantly reduced serum concentrations of both testosterone and DHT in vivo. Inhibition of tumor progression was associated with reduced tumor cell proliferation and tumor angiogenesis [25].
We have reported that oral infusion of GTP at a human achievable dose, i.e., equivalent to six cups of green tea per day, significantly inhibited PCa development and increased survival in male TRAMP mice. GTP provided as the sole source of drinking fluid to TRAMP mice from 8 to 32 weeks of age caused significant delay in primary tumor incidence and tumor burden, significant decrease in prostate and genitourinary weight, significant inhibition in serum IGF-I, and restoration of IGFBP-3 levels and marked reduction in the protein expression of PCNA in the prostate compared with water-fed TRAMP mice. More importantly, GTP infusion was found to result in almost complete inhibition of distant site metastases to lymph nodes, lungs, liver, and bone. There was also significant apoptosis of PCa cells resulting in the reduced dissemination of cancer cells, thereby causing inhibition of PCa development, progression, and metastasis of PCa to distant organ sites by GTP consumption [26].
4 Green tea and metastasis of breast cancer
Metastasis of breast cancer is the major reason for the high mortality of breast cancer patients and is directly linked to the invasive behavior of breast cancer cells. Cancer metastasis consists of several interdependent processes including cancer cell adhesion, cancer cell migration, and invasion of cancer cells. Recently, the effects of EGCG treatment on growth and invasion in a breast carcinoma cell line resistant to tamoxifen (MCF-7Tam) and parental MCF-7 were reported. Treatment with EGCG caused dose-dependent downregulation of epidermal growth factor receptor (EGFR) phoshoporylation and EGFR mRNA expression and protein level in MCF-7Tam cells. There was also decrease in ERK1/2, phospho-ERK1/2, in vitro cell growth, MMP-2 and -9, and extracellular MMP-inducer while increase in TIMP-1 and -2 after EGCG treatment [27]. EGC has been shown to inhibit heregulin (HRG)-β1-induced migration/invasion of MCF-7 human breast carcinoma cells to approximately the same extent as EGCG. It was found that EGCG inhibited this migration/invasion by suppressing the HRG-stimulated activation of EGFR-related protein B2 (ErbB2)/ErbB3/Akt, whereas the disruption of the HRG-stimulated activation of ErbB2/ErbB3 but not Akt was involved in the inhibition of migration/invasion by EGC. It was concluded that EGC and EGCG could play important role against the promotion of metastasis of breast cancer cells [28].
Treatment with EGCG reduced the activity, protein expression, and mRNA expression level of MMP-2. It also caused reduction on the expression of FAK, membrane type-1-MMP, nuclear factor-kappa B (NF-κB), and VEGF and reduced the adhesion of MCF-7 cells to extracellular matrix, fibronectin, and vitronectin. EGCG treatment also led to a reduction in the expression of integrin receptors α5, β1, αv, and β3 as observed by real time RT-PCR [29]. GTP has been reported to inhibit cell growth and invasive behavior of human breast cancer MDA-MB-231 cells. It also caused inhibition of constitutively active transcription factors AP-1 and NF-κB, which further suppressed secretion of uPA from breast cancer cells. GTP treatment resulted in the inhibition of formation of signaling complexes responsible for cell adhesion and migration viz., uPA, uPA-receptor, vitronectin, and integrin receptor by inhibiting the invasive behavior of breast cancer cells [30].
The effects of GTP on growth and metastasis of highly metastatic mouse mammary carcinoma 4T1 cells were examined in in vitro and in vivo systems. Treatment of metastatic mouse mammary carcinoma 4T1 cells with EGCG resulted in inhibition of cell proliferation, induction of apoptosis, decrease in the protein expression of Bcl-2, increase in Bax, cytochrome c release, Apaf-1, and cleavage of caspase-3 and PARP proteins. Treatment of EGCG-rich GTP in drinking water to 4T1 cells bearing BALB/c mice resulted in reduction of tumor growth, increase in Bax/Bcl-2 ratio, reduction in PCNA, and activation of caspase-3 in tumors. GTP treatment also caused inhibition of metastasis of tumor cells to lungs and increase in the survival period of animals [31]. The association between consumption of green tea prior to clinical cancer onset and various clinical parameters assessed at surgery among 472 patients with stage I, II, and III breast cancer were examined. There was decrease in the numbers of auxiliary lymph node metastases among premenopausal patients with stage I and II breast cancer and increased expression of progesterone receptor and estrogen receptor among postmenopausal patients on increased consumption of green tea. In a 7-year follow-up of stage I and II breast cancer patients, increased consumption of green tea was correlated with decreased recurrence of stage I and II breast cancer. The recurrence rate was 16.7% in those patients consuming ≥5 cups and 24.3% in patients consuming ≤4 cups/day with 0.564 relative risk of recurrence after adjustment for other lifestyle factors. This showed that increased consumption of green tea prior to clinical cancer onset was significantly associated with improved prognosis of stage I and II breast cancer, and this association may be related to a modifying effect of green tea on the clinical characteristics of the cancer [32].
5 Green tea and metastasis of lung cancer
Lung cancer is the most common cancer in the world and represents a major public health problem. The preventive effect of green tea catechins intake on lung tumor metastasis was examined in senescence-accelerated mice prone (SAMP) 10. Green tea catechins intake increased natural killer cell activity, which is an indicator of immune surveillance potential and was reduced in control group mice with age. Mice were given intravenous injection of the melanoma cells and the early accumulation of lung-metastatic K1735M2 melanoma cells and the subsequent experimental lung metastasis was investigated after treatment with green tea catechins. The accumulation at 6 and 24 h after injection of melanoma cells and the number of lung-metastatic colonies were significantly reduced in mice treated with green tea catechins as compared to mice in control group suggesting that green tea catechin intake prevented the experimental tumor metastasis in aged SAMP10 mice via inhibition of the reduction in immune surveillance potential with age [33]. The effect of cytokines, mitogens, and inhibitors on MMP-2 and -9 expressions in human lung cancer A549 cells malignant melanoma MSTO-211H cells was investigated. EGCG inhibited MMP-2 and -9 expressions in both cell lines [34]. It has been shown that EGCG had an inhibitory effect on bronchial tumor cells migration in 2D and 3D cell culture models. Treatment with EGCG also inhibited MMP-2 mRNA and protein expression and altered the intermediate filaments of vimentin [35].
The effects of a nutrient mixture consisting of ascorbic acid, lysine, proline, arginine, and green tea extract were investigated on lung metastasis by B16F0 melanoma cells in C57BL/6 female mice. Pulmonary metastatic colonies were counted after 2 weeks of nutrient mixture supplementation. Pulmonary colonization was reduced by 63% in mice receiving nutrient mixture in diet, whereas, it was reduced by 86% in mice receiving nutrient mixture by intraperitoneal and intravenous injections and completely inhibited in mice injected with melanoma cells pretreated with nutrient mixture [36]. This nutrient mixture supplementation to athymic nude mice implanted with human lung cancer A549 cells suppressed the tumor growth without adverse effects. It also inhibited the secretion of both MMP-2 and -9 with reduction in the invasion of human lung carcinoma cells through Matrigel in a dose-dependent fashion [37]. Treatment with EGCG reduced lung metastases in mice bearing B16-F3m melanomas while a combination of EGCG and dacarbazine was more effective in reducing the number of pulmonary metastases and primary tumor growths and increased the survival rate of melanoma-bearing mice [16]. EGCG inhibited the invasion of lung carcinoma 95-D cells in invasion assay and downregulated the expression of MMP-9 and NF-κB in a dose-dependent manner [38]. Theaflavin, theaflavin digallate, and EGCG inhibited invasion of highly metastatic mouse Lewis lung carcinoma LL2-Lu3 cells. They also inhibited MMP-2 and -9 from the culture medium of these tumor cells suggesting that these compounds inhibit tumor cell invasion by inhibiting type IV collagenases [39]. In a spontaneous metastasis system, the administration of green tea infusion reduced the number of lung colonies of mouse Lewis lung carcinoma cells which was attributed to the inhibitory effects of the green tea infusion and its constituent catechins on the penetration of the cells through the basement membrane [40].
6 Green tea and metastasis of liver cancer
Hepatocellular carcinoma is a growing health problem worldwide and only few promising treatment options are available at present, stressing the urgent need for novel therapeutic approaches. The effect of a nutrient mixture containing lysine, proline, arginine, ascorbic acid, and green tea extract on tumor growth and hepatic metastasis were investigated in athymic nude male mice inoculated with 10(6) B16FO melanoma cells. Metastasis was studied in C57BL/6 mice receiving melanoma cells by intrasplenic injection, as well as a regular or 0.5% nutrient mixture-supplemented diet for 2 weeks. Nutrient mixture inhibited the growth of melanoma cells, and the lesions were consistent with malignant melanoma. Mice were also injected with melanoma cells in the spleen. Control group animals developed large black spleens and livers indicating growth in the spleen and metastasis to the liver, while mice supplemented with nutrient mixture showed less growth in spleen and reduced metastasis to the liver. The survival time was also greater in mice receiving nutrient mixture supplementation than animals on the regular diet [41].
EGCG, EGC, and CG were reported as the major phenolic phytochemicals found in red pine leaves. It was found that red pine leaf extract, EGCG, and CG suppressed the invasion and the migration of human hepatocellular carcinoma cells SK-Hep-1 cells. Red pine leaf extract, EGCG, and CG suppressed the activities of both MMP-2 and MMP-9 with EGC exhibiting a lower efficacy on both MMPs with EGC exhibiting a lower efficacy [42]. There was much higher inhibition of hepatocellular carcinoma SMMC-7721 cell proliferation and migration by mixture of EGCG and ascorbic acid as compared with EGCG or ascorbic acid alone. Fluorographic analysis of oxidative stress revealed that ascorbic acid enhanced the antioxidant activity of EGCG by decreasing the intracellular oxidative stress. It was concluded in the study that the combination of EGCG and ascorbic acid strongly suppressed the proliferation and metastasis of liver cancer cells, possibly with a mechanism associated with the scavenging of reactive oxygen species [43]. The effect of the combination of theanine with doxorubicin was investigated against hepatic metastasis of M5076 ovarian sarcoma cell line transplanted subcutaneously in mice. The liver weight increased to twice the normal level because of hepatic metastasis in the control group whereas treatment with theanine and doxorubicin suppressed the increase in liver weight and hepatic metastasis. Theanine enhanced the inhibition of hepatic metastasis induced by doxorubicin as demonstrated by liver weights and metastasis scores. Theanine also increased the intracellular concentration of doxorubicin remaining in ovarian sarcoma cells proving that theanine caused enhancement of the suppressive efficacy of doxorubicin on hepatic metastasis in vivo [44].
7 Green tea and metastasis of colon cancer
Colorectal cancer is the second most deadly cancer in the United States. Current treatment options offer partial success when diagnosed early; however, radiation and chemotherapy are generally ineffective once metastasis occurs. Human colon cancer cell lines HCT116 and HT29 were used to examine the relationships between Met activation, EGCG treatment, and generation of H2O2. EGCG markedly suppressed the activation of Met in the presence of hepatocyte growth factor. The concentrations of ≤10 µM EGCG generated low amounts of H2O2, whereas higher H2O2 concentrations were required to directly increase the phosphorylation of Met. The activation of Met by EGCG occurred in the presence or absence of catalase showing that EGCG might be a beneficial therapeutic agent in the colon, inhibiting Met signaling and helping to attenuate tumor spread/metastasis, independent of H2O2-related mechanisms [45].
It has been reported that basic fibroblast growth factor (bFGF) protein was quickly degraded in the presence of EGCG, but proteasome inhibitor suppressed this degradation. There was increased ubiquitination of bFGF and trypsin-like activity of the 20S proteasome by EGCG, thereby resulting in the degradation of bFGF protein. EGCG was also found to inhibit intestinal tumor formation in APC (Min/+) mice, compared with vehicle-treated mice, in association with reduced bFGF expression [46]. Diet of nutrient mixture containing amino acids, ascorbic acid, and green tea extract inhibited growth and reduced the size of tumors in nude mice implanted with human colon HCT 116 cells. In the control group tissues, increased mitotic index and MMP-9 and VEGF secretion were found, whereas, nutrient supplementation diet inhibited MMP-9 and VEGF secretion and mitotic index in the tumor tissues [47]. EGCG treatment increased both intracellular and extracellular pro-MMP-7 protein levels in dose- and time-dependent manner HT-29 human colorectal cancer cells with a significant upregulation of its mRNA expression. EGCG also activated ERK1/2, JNK1/2, and p38 MAPK, triggered the phosphorylation of c-JUN, and induced c-JUN/c-FOS, thereby increasing the DNA-binding activity of activator protein-1 (AP-1). EGCG-induced pro-MMP-7 production was attenuated by N-Acetyl-L-cysteine, superoxide (O2−) dismutase and catalase, suggesting an involvement of oxidative stress in these events. EGCG treatment also induced pro-MMP-7 expression in human colorectal adenocarcinoma Caco-2 cell line [48].
8 Green tea and metastasis of pancreatic cancer
It is difficult to detect pancreatic cancer in the early stage despite the development of more sophisticated diagnostic techniques and surgical resection provides the only option. Treatment with EGCG inhibited viability, capillary tube formation, and migration of human umbilical vein endothelial cells (HUVECs). There was reduction in Ki-67, PCNA, Von Willebrand factor, VEGF, CD31, VEGFR-2, ERK1/2, JNK1/2, p38, MMP-2, MMP-7, MMP-9, and MMP-12 and induction of apoptosis, caspase-3 activity, and p21/WAF1 in tumors of athymic nude mice implanted with human pancreatic cancer AsPC-1 cells suggesting that EGCG inhibited pancreatic cancer growth, invasion, metastasis, and angiogenesis [49]. In pancreatic cancer cell line MIA PaCa-2, nutrient mixture containing green tea exhibited a dose-dependent antiproliferative effect, decreased expression of MMP-9, and inhibition of invasion through Matrigel [50].
In hamsters, the inhibitory effect of green tea extract on the process of pancreatic carcinogenesis induced by N-nitrosobis-(2-oxypropyl)amine and on tumor promotion after transplantation of N-nitrosobis-(2-hydroxypropyl)amine (BHP)-induced pancreatic cancer were investigated. In the control group, seven of the 13 hamsters were found to have pancreatic tumors, while six of the 18 hamsters had pancreatic tumors in the green tea extract group. The average number of tumors in the control group was 1.0/hamster with pancreatic cancer incidence of 54% compared with the green tea extract group which had average number of tumors of 0.5/hamster with 44% incidence of pancreatic cancer. The number of pancreatic cancers, including invasive carcinoma, carcinoma in situ, and incidence of atypical ductal hyperplasia, which is thought to be an early pancreatic cancer, was significantly lower in the green tea extract group than in the control group. In a different experiment, 1 mm3 pieces of BHP-induced pancreatic cancer were transplanted into the back of hamsters and were given tap water and green tea extract. Till 11 weeks after transplantation, tumor growth was similar in both groups, but inhibition of tumor growth became evident after 11 weeks in the green tea extract treated group. The average tumor volume in the green tea extract group was significantly lower than that in the control group at 13 weeks demonstrating that green tea extract had an inhibitory effect on the process of pancreatic carcinogenesis and on tumor promotion of transplanted pancreatic cancer in hamsters [51].
9 Green tea and metastasis of miscellaneous cancers
The effects of EGCG on the methylation status of the reversion-inducing cysteine-rich protein with Kazal motifs (RECK) gene and cancer invasion in oral squamous cell carcinoma cell lines were investigated. EGCG treatment of oral cancer cells partially reversed the hypermethylation status of the RECK gene and significantly enhanced the expression level of RECK mRNA with inhibition of MMP-2 and MMP-9 levels. EGCG also suppressed cancer cell-invasive ability by decreasing the number of invasive foci as well as invasion depth in 3D collagen invasion model [52]. A molecular epidemiologic study was conducted at Jiangsu Province of China, on histologically confirmed esophageal squamous cell carcinoma patients to investigate the association between aberrant hypermethylation of MGMT gene and clinical characteristics as well as MTHFR C677T genetic polymorphisms in esophageal squamous cell carcinoma. The aberrant hypermethylation rate of MGMT gene was 27.2% in cancer tissues and 11.2% in precancerous normal tissues among esophageal squamous cell carcinoma patients, while no hypermethylation was found in normal esophageal tissues from healthy adult subjects. In patients with lymph node metastasis, the methylation rate of MGMT gene in cancer tissues was significantly higher than in those without lymph node metastasis. Variant allele of MTHFR C677T was found to be associated with hypermethylation of MGMT gene after adjusting by potential confounders [53]. The inhibitory effects of EGCG on ephrin-A1-mediated cell migration and angiogenesis were reported. It was shown that ephrin-A1 mediated endothelial cell migration and regulated vascular remodeling in tumor neo-vascularization in vitro. Treatment with EGCG inhibited ephrin-A1-mediated endothelial cell migration, tumor angiogenesis, and phosphorylation of EphA2 and ERK-1/2 in a dose-dependent manner [54].
Expression of the metastasis-associated 67-kDa laminin receptor (LR) confers EGCG responsiveness to cancer cells at physiologically relevant concentrations [55]. The 67 LR is expressed on several tumor cells, and the expression level of this protein powerfully correlates with the risk of tumor invasion and metastasis [56, 57]. The antiangiogenic activities of EGCG are linked to 67 LR by the identification of a role for the 67 LR in retinal angiogenesis and its potent upregulation in malignant mesothelioma by gene expression profiling associated with tumor endothelial cells [58]. The effects of green tea extract on cell viability, cell proliferation, cell cycle dynamics, VEGF, and expression of VEGF receptors fms-like tyrosine kinase (Flt)-1 and fetal liver kinase (Flk)-1/kinase insert domain containing receptor (KDR) were studied in vitro using HUVECs. Treatment of cells with green tea extract did not affect cell viability but significantly reduced cell proliferation dose-dependently and caused a dose-dependent accumulation of cells in the G1 phase. There was also decrease in the expression of Flt-1 and Flk-1/KDR/in HUVECs on treatment with green tea extract suggesting that it affects tumor angiogenesis and metastasis through reduction in the expression of VEGF receptors [59]. EGCG suppressed the gelatin-degrading activities due to MMP-2 and MMP-9 in the culture medium of human fibrosarcoma HT1080 cells which were consistent with the decreased levels of MMP-2 and MMP-9 mRNAs. The suppression of the level of MMP-9 transcript on treatment with EGCG was correlated with its suppression of MMP-9 promoter activity. EGCG treatment also caused inhibition of the phosphorylation of ERK1/2 and p38 MAPK activity showing that suppression of ERK phosphorylation by EGCG was involved in the inhibition of MMP-2 and MMP-9 mRNAs, leading to the reduction of their enzyme activities in the cancer cells [60]. The effect of EGCG on the tube formation of HUVECs on Matrigel was investigated. EGCG treatment both prior to plating and after plating endothelial cells on Matrigel caused inhibition of tube formation and reduce the migration of endothelial cells in Matrigel plug model. Zymography revealed that EGCG-treated culture supernatants modulated the gelatinolytic activities of secreted proteinases demonstrating that EGCG may be exerting its inhibitory effect by regulating proteinases. Thus, these experiments showed that EGCG acts as an angiogenesis inhibitor by modulating protease activity during endothelial morphogenesis [61].
10 Conclusions and perspectives
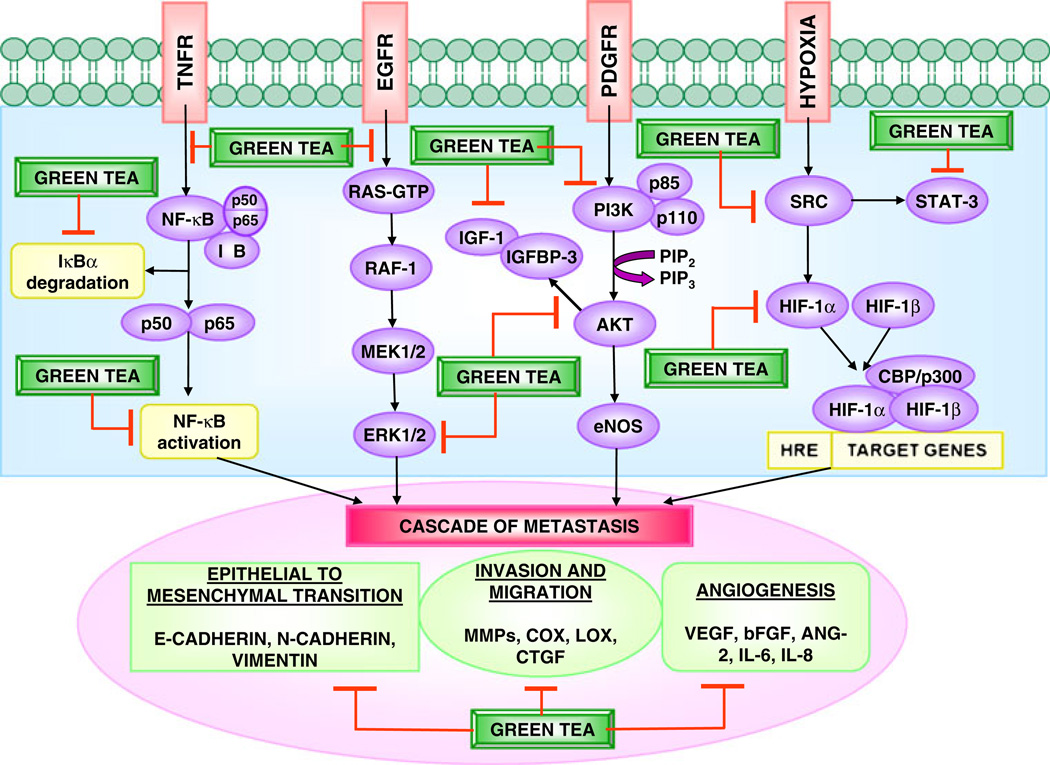
The possible cancer-preventive activity of green tea constituents has been studied extensively and the amount of experimental evidence documenting the properties of green tea, which affects multiple signaling pathways (Fig. 1) against metastasis of cancer is increasing rapidly. Metastasis is responsible for most deaths due to cancer and therefore, therapeutic strategies to prevent development of metastases have potential to impact on cancer mortality. However, a better understanding of the biology and molecular events of the metastatic process is required for the development of these therapies. In successfully treating cancer, focus should be on combating metastasis formation and growth. Significant improvements in early detection of cancer and development of effective novel therapeutic strategies targeting metastasis will help improve patient outcome. A better approach for the treatment of cancer seems to be the development of strategies to treat tumor cells and to modulate the host microenvironment. For better understanding of the interaction of green tea, employment of more specific and sensitive methods with more representative models of metastasis in conjunction with the development of good predictive biomarkers are required. Well-designed clinical and intervention trials will give the clear picture about the protective effects of green tea against metastasis of cancer.
Fig. 1.
Modulation of signaling pathways and cascade of metastasis by green tea
Acknowledgments
The original work from the author’s (HM) laboratory outlined in this review was supported by United States Public Health Service Grants RO1 CA 78809, RO1 CA 101039, RO1 CA 120451, and P50 DK065303.
References
- 1.Jemal A, et al. Cancer statistics, 2009. CA: A Cancer Journal for Clinicians. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nature Medicine. 2006;12(8):895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 3.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nature Reviews Cancer. 2002;2(8):563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 4.Fidler IJ, Radinsky R. Search for genes that suppress cancer metastasis. Journal of the National Cancer Institute. 1996;88(23):1700–1703. doi: 10.1093/jnci/88.23.1700. [DOI] [PubMed] [Google Scholar]
- 5.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. The American Journal of Clinical Nutrition. 2000;71(6 Suppl):1698S–16702S. doi: 10.1093/ajcn/71.6.1698S. [DOI] [PubMed] [Google Scholar]
- 7.Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxidants Redox Signaling. 2008;10(3):475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 8.Chacko SM, et al. Beneficial effects of green tea: a literature review. Chinese Medicine. 2010;5(6):13. doi: 10.1186/1749-8546-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan N, Mukhtar H. Tea polyphenols for health promotion. Life Sciences. 2007;81(7):519–533. doi: 10.1016/j.lfs.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao Y, Cao R, Brakenhielm E. Antiangiogenic mechanisms of diet-derived polyphenols. The Journal of Nutritional Biochemistry. 2002;13(7):380–390. doi: 10.1016/s0955-2863(02)00204-8. [DOI] [PubMed] [Google Scholar]
- 11.Khan N, et al. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Research. 2006;66(5):2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-del-Campo L, et al. Melanoma activation of 3-o-(3, 4, 5-trimethoxybenzoyl)-(−)-epicatechin to a potent irreversible inhibitor of dihydrofolate reductase. Molecular Pharmacology. 2009;6(3):883–894. doi: 10.1021/mp800259k. [DOI] [PubMed] [Google Scholar]
- 13.Roomi MW, et al. In vivo and in vitro antitumor effect of ascorbic acid, lysine, proline and green tea extract on human melanoma cell line A2058. In Vivo. 2006;20(1):25–32. [PubMed] [Google Scholar]
- 14.Mantena SK, et al. Orally administered green tea polyphenols prevent ultraviolet radiation-induced skin cancer in mice through activation of cytotoxic T cells and inhibition of angiogenesis in tumors. The Journal of Nutrition. 2005;135(12):2871–2877. doi: 10.1093/jn/135.12.2871. [DOI] [PubMed] [Google Scholar]
- 15.Mantena SK, Roy AM, Katiyar SK. Epigallocatechin-3-gallate inhibits photocarcinogenesis through inhibition of angiogenic factors and activation of CD8+ T cells in tumors. Photochemistry and Photobiology. 2005;81(5):1174–1179. doi: 10.1562/2005-04-11-RA-487. [DOI] [PubMed] [Google Scholar]
- 16.Liu JD, et al. Inhibition of melanoma growth and metastasis by combination with (−)-epigallocatechin-3-gallate and dacarbazine in mice. Journal of Cellular Biochemistry. 2001;83(4):631–642. doi: 10.1002/jcb.1261. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki Y, Isemura M. Inhibitory effect of epigallocatechin gallate on adhesion of murine melanoma cells to laminin. Cancer Letters. 2001;173(1):15–20. doi: 10.1016/s0304-3835(01)00685-1. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, et al. Inhibition of invasion and up-regulation of E-cadherin expression in human malignant melanoma cell line A375 by (−)-epigallocatechin-3-gallate. Journal of Huazhong University of Science and Technology, Medical Sciences. 2008;28(3):356–359. doi: 10.1007/s11596-008-0330-3. [DOI] [PubMed] [Google Scholar]
- 19.Evans S, et al. The effect of a novel botanical agent TBS-101 on invasive prostate cancer in animal models. Anticancer Research. 2009;29(10):3917–3924. [PubMed] [Google Scholar]
- 20.Siddiqui IA, et al. Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated apoptosis and synergistically inhibits biomarkers associated with angiogenesis and metastasis. Oncogene. 2008;27(14):2055–2563. doi: 10.1038/sj.onc.1210840. [DOI] [PubMed] [Google Scholar]
- 21.Roomi MW, et al. In vivo antitumor effect of ascorbic acid, lysine, proline and green tea extract on human prostate cancer PC-3 xenografts in nude mice: evaluation of tumor growth and immunohistochemistry. In Vivo. 2005;19(1):179–183. [PubMed] [Google Scholar]
- 22.Saleem M, et al. Prognostic significance of metastasis-associated protein S100A4 (Mts1) in prostate cancer progression and chemoprevention regimens in an autochthonous mouse model. Clinical Cancer Research. 2005;11(1):147–153. [PubMed] [Google Scholar]
- 23.Adhami VM, et al. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Research. 2004;64(23):8715–8722. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- 24.Pezzato E, et al. Prostate carcinoma and green tea: PSA-triggered basement membrane degradation and MMP-2 activation are inhibited by (−)epigallocatechin-3-gallate. International Journal of Cancer. 2004;112(5):787–792. doi: 10.1002/ijc.20460. [DOI] [PubMed] [Google Scholar]
- 25.Zhou JR, et al. Soy phytochemicals and tea bioactive components synergistically inhibit androgen-sensitive human prostate tumors in mice. The Journal of Nutrition. 2003;133(2):516–521. doi: 10.1093/jn/133.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta S, et al. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(18):10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farabegoli F, Papi A, Orlandi M. -(−)Epigallocatechin-3-gallate downregulates EGFR, MMP-2, MMP-9 EMM-PRIN and inhibits the invasion of MCF-7 tamoxifen resistant cells. Bioscience Reports. 2010 doi: 10.1042/BSR20090143. [DOI] [PubMed] [Google Scholar]
- 28.Kushima Y, et al. Inhibitory effect of (−)-epigallocatechin and (−)-epigallocatechin gallate against heregulin beta1-induced migration/invasion of the MCF-7 breast carcinoma cell line. Biological & Pharmaceutical Bulletin. 2009;32(5):899–904. doi: 10.1248/bpb.32.899. [DOI] [PubMed] [Google Scholar]
- 29.Sen T, et al. Multifunctional effect of epigallocatechin-3-gallate (EGCG) in downregulation of gelatinase-A (MMP-2) in human breast cancer cell line MCF-7. Life Sciences. 2009;84(7–8):194–204. doi: 10.1016/j.lfs.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 30.Slivova V, et al. Green tea polyphenols modulate secretion of urokinase plasminogen activator (uPA) and inhibit invasive behavior of breast cancer cells. Nutrition and Cancer. 2005;52(1):66–73. doi: 10.1207/s15327914nc5201_9. [DOI] [PubMed] [Google Scholar]
- 31.Baliga MS, Meleth S, Katiyar SK. Growth inhibitory and antimetastatic effect of green tea polyphenols on metastasis-specific mouse mammary carcinoma 4T1 cells in vitro and in vivo systems. Clinical Cancer Research. 2005;11(5):1918–1927. doi: 10.1158/1078-0432.CCR-04-1976. [DOI] [PubMed] [Google Scholar]
- 32.Nakachi K, et al. Influence of drinking green tea on breast cancer malignancy among Japanese patients. Japanese Journal of Cancer Research. 1998;89(3):254–261. doi: 10.1111/j.1349-7006.1998.tb00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimizu K, et al. Preventive effect of green tea catechins on experimental tumor metastasis in senescence-accelerated mice. Biological & Pharmaceutical Bulletin. 2010;33(1):117–121. doi: 10.1248/bpb.33.117. [DOI] [PubMed] [Google Scholar]
- 34.Roomi MW, et al. Modulation of MMP-2 and MMP-9 by cytokines, mitogens and inhibitors in lung cancer and malignant mesothelioma cell lines. Oncology Reports. 2009;22(6):1283–1291. doi: 10.3892/or_00000566. [DOI] [PubMed] [Google Scholar]
- 35.Hazgui S, et al. Epigallocatechin-3-gallate (EGCG) inhibits the migratory behavior of tumor bronchial epithelial cells. Respiratory Research. 2008;9(1):33. doi: 10.1186/1465-9921-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roomi MW, et al. Inhibition of pulmonary metastasis of melanoma b16fo cells in C57BL/6 mice by a nutrient mixture consisting of ascorbic acid, lysine, proline, arginine, and green tea extract. Experimental Lung Research. 2006;32(10):517–530. doi: 10.1080/01902140601098552. [DOI] [PubMed] [Google Scholar]
- 37.Waheed Roomi M, et al. In vivo and in vitro antitumor effect of a unique nutrient mixture on lung cancer cell line A-549. Experimental Lung Research. 2006;32(9):441–453. doi: 10.1080/01902140601047658. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Wei D, Liu J. Repressions of MMP-9 expression and NF-kappa B localization are involved in inhibition of lung carcinoma 95-D cell invasion by (−)-epigallocatechin-3-gallate. Biomedicine & Pharmacotherapy. 2005;59(3):98–103. doi: 10.1016/j.biopha.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Sazuka M, et al. Inhibition of collagenases from mouse lung carcinoma cells by green tea catechins and black tea theaflavins. Bioscience, Biotechnology, and Biochemistry. 1997;61(9):1504–1506. doi: 10.1271/bbb.61.1504. [DOI] [PubMed] [Google Scholar]
- 40.Sazuka M, et al. Inhibitory effects of green tea infusion on in vitro invasion and in vivo metastasis of mouse lung carcinoma cells. Cancer Letters. 1995;98(1):27–31. [PubMed] [Google Scholar]
- 41.Roomi MW, et al. Suppression of growth and hepatic metastasis of murine B16FO melanoma cells by a novel nutrient mixture. Oncology Reports. 2008;20(4):809–817. [PubMed] [Google Scholar]
- 42.Lee SJ, et al. Phenolic phytochemicals derived from red pine (Pinus densiflora) inhibit the invasion and migration of SK-Hep-1 human hepatocellular carcinoma cells. Annals of the New York Academy of Sciences. 2007;1095:536–544. doi: 10.1196/annals.1397.058. [DOI] [PubMed] [Google Scholar]
- 43.Wei DZ, et al. Inhibition of liver cancer cell proliferation and migration by a combination of (−)-epigallocatechin-3-gallate and ascorbic acid. Journal of Chemotherapy. 2003;15(6):591–595. doi: 10.1179/joc.2003.15.6.591. [DOI] [PubMed] [Google Scholar]
- 44.Sugiyama T, Sadzuka Y. Combination of theanine with doxorubicin inhibits hepatic metastasis of M5076 ovarian sarcoma. Clinical Cancer Research. 1999;5(2):413–416. [PubMed] [Google Scholar]
- 45.Larsen CA, Dashwood RH. Suppression of Met activation in human colon cancer cells treated with (−)-epigallocatechin-3-gallate: minor role of hydrogen peroxide. Biochemical and Biophysical Research Communications. 2009;389(3):527–530. doi: 10.1016/j.bbrc.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sukhthankar M, et al. A green tea component suppresses posttranslational expression of basic fibroblast growth factor in colorectal cancer. Gastroenterology. 2008;134(7):1972–1980. doi: 10.1053/j.gastro.2008.02.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roomi MW, et al. In vivo antitumor effect of ascorbic acid, lysine, proline and green tea extract on human colon cancer cell HCT 116 xenografts in nude mice: evaluation of tumor growth and immunohistochemistry. Oncology Reports. 2005;13(3):421–425. [PubMed] [Google Scholar]
- 48.Kim M, et al. (−)-Epigallocatechin-3-gallate promotes pro-matrix metalloproteinase-7 production via activation of the JNK1/2 pathway in HT-29 human colorectal cancer cells. Carcinogenesis. 2005;26(9):1553–1562. doi: 10.1093/carcin/bgi104. [DOI] [PubMed] [Google Scholar]
- 49.Shankar S, et al. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Frontiers in Bioscience. 2008;13:440–452. doi: 10.2741/2691. [DOI] [PubMed] [Google Scholar]
- 50.Roomi MW, et al. Antitumor effect of a combination of lysine, proline, arginine, ascorbic acid, and green tea extract on pancreatic cancer cell line MIA PaCa-2. International Journal of Gastrointestinal Cancer. 2005;35(2):97–102. doi: 10.1385/IJGC:35:2:097. [DOI] [PubMed] [Google Scholar]
- 51.Hiura A, Tsutsumi M, Satake K. Inhibitory effect of green tea extract on the process of pancreatic carcinogenesis induced by N-nitrosobis-(2-oxypropyl)amine (BOP) and on tumor promotion after transplantation of N-nitrosobis-(2-hydroxypropyl) amine (BHP)-induced pancreatic cancer in Syrian hamsters. Pancreas. 1997;15(3):272–277. doi: 10.1097/00006676-199710000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Kato K, et al. Effects of green tea polyphenol on methylation status of RECK gene and cancer cell invasion in oral squamous cell carcinoma cells. British Journal of Cancer. 2008;99(4):647–654. doi: 10.1038/sj.bjc.6604521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xue HC, et al. Correlation of aberrant methylation of MGMT gene to MTHFR C677T genetic polymorphisms in esophageal squamous cell carcinoma. Ai Zheng. 2008;27(12):1256–1262. [PubMed] [Google Scholar]
- 54.Tang FY, Chiang EP, Shih CJ. Green tea catechin inhibits ephrin-A1-mediated cell migration and angiogenesis of human umbilical vein endothelial cells. The Journal of Nutritional Biochemistry. 2007;18(6):391–399. doi: 10.1016/j.jnutbio.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 55.Tachibana H, et al. A receptor for green tea polyphenol EGCG. Nature Structural & Molecular Biology. 2004;11(4):380–381. doi: 10.1038/nsmb743. [DOI] [PubMed] [Google Scholar]
- 56.Martignone S, et al. Prognostic significance of the 67-kilodalton laminin receptor expression in human breast carcinomas. Journal of the National Cancer Institute. 1993;85(5):398–402. doi: 10.1093/jnci/85.5.398. [DOI] [PubMed] [Google Scholar]
- 57.Menard S, et al. New insights into the metastasis-associated 67 kD laminin receptor. Journal of Cellular Biochemistry. 1997;67(2):155–165. [PubMed] [Google Scholar]
- 58.Singhal S, et al. Gene expression profiling of malignant mesothelioma. Clinical Cancer Research. 2003;9(8):3080–3097. [PubMed] [Google Scholar]
- 59.Kojima-Yuasa A, et al. Green tea extract inhibits angiogenesis of human umbilical vein endothelial cells through reduction of expression of VEGF receptors. Life Sciences. 2003;73(10):1299–1313. doi: 10.1016/s0024-3205(03)00424-7. [DOI] [PubMed] [Google Scholar]
- 60.Maeda-Yamamoto M, et al. Association of suppression of extracellular signal-regulated kinase phosphorylation by epigallocatechin gallate with the reduction of matrix metalloproteinase activities in human fibrosarcoma HT1080 cells. Journal of Agricultural and Food Chemistry. 2003;51(7):1858–1863. doi: 10.1021/jf021039l. [DOI] [PubMed] [Google Scholar]
- 61.Singh AK, et al. Green tea constituent epigallocatechin-3-gallate inhibits angiogenic differentiation of human endothelial cells. Archives of Biochemistry and Biophysics. 2002;401(1):29–37. doi: 10.1016/S0003-9861(02)00013-9. [DOI] [PubMed] [Google Scholar]