Abstract
This phase 1/2 trial evaluated combination lenalidomide, bortezomib, pegylated liposomal doxorubicin, and dexamethasone (RVDD) in newly diagnosed multiple myeloma (MM) patients. Patients received RVDD at 4 dose levels, including the maximum tolerated dose (MTD). Patients with a very good partial response or better (≥ VGPR) after cycle 4 proceeded to autologous stem cell transplantation or continued treatment. The primary objectives were MTD evaluation and response to RVDD after 4 and 8 cycles. Seventy-two patients received a median of 4.5 cycles. The MTDs were lenalidomide 25 mg, bortezomib 1.3 mg/m2, pegylated liposomal doxorubicin 30 mg/m2, and dexamethasone 20/10 mg, as established with 3-week cycles. The most common adverse events were fatigue, constipation, sensory neuropathy, and infection; there was no treatment-related mortality. Response rates after 4 and 8 cycles were 96% and 95% partial response or better, 57% and 65% ≥ VGPR, and 29% and 35% complete or near-complete response, respectively. After a median follow-up of 15.5 months, median progression-free survival (PFS) and overall survival (OS) were not reached. The estimated 18-month PFS and OS were 80.8% and 98.6%, respectively. RVDD was generally well tolerated and highly active, warranting further study in newly diagnosed MM patients. This trial was registered at www.clinicaltrials.gov as NCT00724568.
Introduction
Recent studies have suggested that the achievement of a superior response to initial treatment is associated with improved outcomes in patients with multiple myeloma (MM).1–5 Three-drug combinations with bortezomib and/or thalidomide or lenalidomide appeared to be superior to most 2-drug combinations of the same agents in patients with newly diagnosed MM. The lenalidomide, bortezomib, and dexamethasone (RVD) regimen and the bortezomib, pegylated liposomal doxorubicin (PLD), and dexamethasone (VDD) regimens are among the most active in this setting, producing high rates of very good partial response (VGPR), complete response (CR), and near CR (nCR).4,6,7 Other 3-drug regimens producing high rates of CR and VGPR in patients with newly diagnosed MM include bortezomib, thalidomide, and dexamethasone (VTD)5,8,9; cyclophosphamide, thalidomide, and dexamethasone (CTD)10; bortezomib, cyclophosphamide, and dexamethasone (VCD)11–13; and bortezomib, doxorubicin, and dexamethasone (PAD).14 Furthermore, the 4-drug regimen of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide (VDCR) has recently been shown to be highly active and generally well tolerated in patients with newly diagnosed MM, although some additional toxicity was noted.15,16
We hypothesized that combining the 4 active drugs from the RVD and VDD regimens into one regimen, specifically RVDD (lenalidomide, bortezomib, PLD, and dexamethasone) might further increase CR/nCR and VGPR rates and thus potentially improve outcome. In preclinical studies, the RVDD combination demonstrated a 2- to 4-fold higher activity compared with 2- and 3-drug combinations of the component drugs,17 with earlier studies demonstrating synergy between these agents.18 Here we report the results of a prospective, multicenter phase 1/2 study of the RVDD regimen in patients with newly diagnosed MM. The primary objectives of this study were to determine the maximum tolerated dose (MTD) and to evaluate the VGPR rate (≥ 90% disease reduction) after 4 and 8 cycles.
Methods
Patient population
Eligible patients had to be ≥ 18 years of age, have newly diagnosed MM requiring first-line therapy according to the International Myeloma Foundation diagnostic criteria,19 have measurable disease per International Myeloma Working Group criteria,20 have an adequate Karnofsky performance status score (≥ 60) or an Eastern Cooperative Oncology Group (ECOG) performance status score of 0-2, and have either a left ventricular ejection fraction (LVEF) of ≥ 50% by multigated acquisition scan or an echocardiography with an LVEF ≥ the lower limit of normal for the testing facility. Patients were excluded if they had: grade ≥ 2 peripheral neuropathy, absolute neutrophil count (ANC) of < 1000 cells/mm3, hemoglobin level of < 8.0 g/dL, platelet count of > 50 000 (or unable to maintain platelet count of > 50 000 with platelet transfusions), creatinine level of > 2.5 mg/dL, aspartate aminotransferase of ≥ 2.5 × the upper limit of normal, total bilirubin level of ≥ 1.5 × the upper limit of normal, or serious comorbidities (eg, myocardial infarction < 6 months before enrollment, clinically relevant and active infection, severe chronic obstructive or chronic restrictive pulmonary disease, or documented hepatic cirrhosis).
This study was conducted in accordance with the Declaration of Helsinki and the International Conference of Harmonization for Good Clinical Practice. The protocol was approved by institutional review boards or by independent ethics committees. All participating patients provided written informed consent.
Study design and treatment
A total of 6 Multiple Myeloma Research Consortium centers (5 in the United States and 1 in Canada) participated in this open-label phase 1/2 study, with enrollment from May 2008 to October 2009. The primary end points were to determine the MTD for RVDD and to evaluate the ≥ VGPR rate after 4 and 8 cycles for patients who continued on treatment. Secondary end points were to determine the overall response rate, defined as partial response or better (≥ PR), time to progression, duration of response, progression-free survival (PFS), overall survival (OS), and safety. The flow of patients through this phase 1/2 trial is presented in Figure 1. Patients were treated with escalating doses of lenalidomide (15, 20, and 25 mg/d) on days 1-14 of each 21-day cycle for a total of 4-8 cycles; bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11; PLD at escalating doses (20 and 30 mg/m2) on day 4; and oral dexamethasone 20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12 during cycles 1-4, reduced to 10 mg on the same schedule for cycles 5-8.6 Patients who achieved ≥ PR could stop treatment at any point after 4 cycles and proceed to stem cell (SC) mobilization as per institutional guidelines and autologous SC transplantation (ASCT). After SC collection, patients were allowed to resume protocol treatment at the discretion of the investigator, but the study protocol did not explicitly require the continuation of treatment for patients who achieved ≥ PR but < VGPR; VGPR was a primary efficacy end point. Patients not undergoing ASCT could receive up to 8 cycles of RVDD. Patients with at least stable disease and acceptable toxicity who were not transplantation candidates or who opted not to undergo ASCT after 8 cycles could continue treatment on a maintenance schedule until disease progression, unacceptable toxicity, or consent withdrawal.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram.
Maintenance therapy consisted of lenalidomide at the dose level tolerated at the end of 8 cycles given on days 1-14; bortezomib at the dose level tolerated at the end of cycle 8 given on days 1 and 8; and dexamethasone 10 mg given on days 1, 2, 8, and 9 of each 21-day cycle (Figure 2). The protocol mandated dose-reduction steps for lenalidomide (5-mg decrements), bortezomib (1.3, 1.0, 0.7 mg/m2), PLD (30, 20, 15, 10 mg/m2), and dexamethasone (20, 10, 10 mg/d on a reduced schedule [ie, given only on days 1, 4, 8, and 11—a 50% dose reduction compared with the starting dose]); subsequent treatment discontinuation was initiated if adverse events (AEs) did not resolve after dose reductions. All patients received acyclovir or an equivalent antiviral therapy for herpes zoster prophylaxis, and for deep-vein thrombosis (DVT) prophylaxis they received aspirin (81 or 325 mg daily), low-molecular-weight heparin, or full anticoagulation with warfarin. The protocol did not mandate or recommend antibiotic prophylaxis. Use of an IV bisphosphonate was recommended.
Figure 2.
Response rates in the non-ASCT and ASCT groups of patients. For simplicity, CR and nCR rates were combined. In the non-ASCT group, the CR rate was 8.6% and at best response 26%, including 4% sCR, at 4 cycles. In the ASCT group, the CR rate was 22% and after ASCT 37%, including 22% sCR, at 4 cycles.
Dose escalation and determination of phase 2 dosing
Four dose levels were planned to determine the MTD (Table 1). In phase 1, patients were assigned to dose levels 1-4 according to the time-to-event continual reassessment method (TITE-CRM) algorithm.21,22 Prior probabilities of dose-limiting toxicity (DLT) were estimated to be 8%, 15%, 20%, and 25% for dose levels 1-4, respectively. The first patient was assigned to dose level 1. Subsequent dose assignments were made by calculating the posterior probability estimate for toxicity at each dose level. This was based on the prior probabilities for toxicity and the incidence of toxicity in patients already treated, weighted by the time those patients were observed. Weights were calculated as the proportion of the 21-day cycle observed, with full weight given to patients observed for the full 21-day cycle. Dose escalations, but not dose reductions, were restricted to one dose level between patients; intra-patient dose escalation was not permitted. At least 2 patients had to complete a full 21-day cycle of therapy at a dose level before allowing dose escalation. Eligible patients were continuously accrued to the trial as available and were assigned the dose level estimated to be closest to, but not exceeding, the 20% DLT target rate.
Table 1.
Patient disposition by dose level in the phase 1 and phase 2 portions, and number of patients experiencing DLT*
| Dose level | Lenalidomide dose, mg/d | Bortezomib dose, mg/m2 | Dexamethasone dose†, mg | PLD dose, mg/m2 | No. of patients enrolled | DLT, n |
|---|---|---|---|---|---|---|
| Phase 1: Dose escalation (n = 40) | ||||||
| 1 | 15 | 1.3 | 20 | 20 | 4 | 0 |
| 2 | 20 | 1.3 | 20 | 20 | 10 | 2 |
| 3 | 25 | 1.3 | 20 | 20 | 19 | 3 |
| 4 | 25 | 1.3 | 20 | 30 | 7 | 0 |
| Phase 2: After MTD determination (n = 32)‡ | ||||||
| 4 | 25 | 1.3 | 30 | 30 | 32 | NA |
NA indicates not applicable.
Two patients were not evaluable for DLT per protocol and were replaced.
20 mg/d dexamethasone cycles 1-4 and 10 mg/d dexamethasone cycles 5-8 and maintenance.
Including 7 patients treated at level 4 (MTD).
DLTs were defined as: grade ≥ 3 nonhematologic toxicity attributed to one or more of the study drugs; grade 4 hematologic toxicity (thrombocytopenia with platelet count of < 25 000 on more than one occasion; grade 4 neutropenia [ANC of < 500 cells/mm3] resulting in neutropenic fever with elevated temperature [defined as ≥ 100.5°F on one occasion]; grade 4 neutropenia without fever with confirmation within 5 days); or inability to receive the day 1 dose for cycle 2 because of continued drug-related toxicity from cycle 1 or drug-related toxicity newly encountered on day 1 of cycle 2. Dose modifications were not allowed during cycle 1 of the phase 1 portion of the study unless a patient experienced a DLT.
Assessments
AEs were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (NCI-CTCAE v3.0). Patients were evaluable for safety assessment if they received one dose of study treatment. Patients were assessed for treatment response by modified European Group for Blood and Marrow Transplantation23 and International Myeloma Working Group Uniform Criteria (modified to include nCR, defined as CR but immunofixation positive for M protein20,24) every cycle after cycle 2 and for full disease response after cycles 4 and 8.
Cytogenetic studies were performed using standard banding by metaphase analysis and FISH (Mayo Clinic Test 83358, plasma cell proliferative disorder; Rochester, MN). Probes included del(13q14), t(4;14), t(14;16), t(11;14), and del(17p).4
Statistical analysis
The phase 1 portion of the study was designed to accrue 40 patients with the expectation that most patients would be treated at, or near to, the MTD because of the TITE-CRM design. The phase 2 sample size included patients treated at the MTD in phase 1 and additional patients to meet a goal of 38 patients in total. The sample size was required to statistically distinguish the achievement of a 70% ≥ VGPR rate to the treatment vs a rate of 45% (which would not have been deemed worthy of further study) using a 2-sided χ2 test with 85% power and 5% type I error. The intent-to-treat population comprised all patients who received at least one dose of any of the component drugs of the RVDD regimen and did not exhibit hypersensitivity to PLD. Primary efficacy results were based on the intent-to-treat population. Exploratory analyses included the evaluation of outcome in transplantation (ASCT) vs nontransplantation (non-ASCT) patients and the evaluation of the subgroup of patients with cytogenetic abnormalities.
Results
Patients
A total of 74 patients were enrolled in this phase 1/2 study: 42 in phase 1 and 32 in phase 2 (Table 1). Seven patients were treated at dose level 4 (the MTD) during phase 1. Two patients (1 each at dose levels 2 and 3) were not evaluable for DLT per protocol and were replaced; 1 patient had a rapidly progressive myeloma and the other had a hypersensitivity reaction to PLD. Baseline demographic and disease characteristics are listed in Table 2.
Table 2.
Patient baseline demographics and disease characteristics
| Characteristic | All treated patients (n = 72) | Patients treated at MTD (n = 39) |
|---|---|---|
| Median age, y (range) | 59 (29-77)* | 60 (29-77) |
| Male | 41 (56.9) | 19 (48.7) |
| Race | ||
| White | 58 (80.6) | 31 (79.5) |
| Black | 6 (8.3) | 3 (7.7) |
| Other | 8 (11.1) | 5 (12.8) |
| MM subtype | ||
| IgG | 56 (77.8) | 32 (82.1) |
| IgA | 14 (19.4) | 7 (18.0) |
| κ light chain | 5 (6.9) | 0 |
| λ light chain | 4 (5.6) | 0 |
| ISS stage II/III | 38 (52.8) | 21 (53.8) |
| Durie-Salmon stage II/III | 59 (81.9) | 32 (82.1) |
| del(13)/del(13q)/hypodiploid, t(4;14), t(14;16), or 17p† | 33 (45.8) | 19 (48.7) |
| del(13)/hypodiploid, t(4;14), t(14;16), or 17p‡ | 19 (26.4) | 10 (25.6) |
| Karnofsky performance status ≤ 80% | 29 (40.3) | 19 (48.7) |
| Lytic lesions | ||
| None | 19 (26.4) | 11 (28.2) |
| 1-3 bones | 17 (23.6) | 11 (28.2) |
| > 3 bones | 36 (50.0) | 17 (43.6) |
Values are expressed as n (%) except for age.
Age (mean ± SD) of ASCT patients, 56 ± 10 years, n = 49; and non-ASCT patients, 65 ± 9 years, n = 23 (P = .0009).
Poor-prognosis abnormalities (patients without any of these abnormalities represent standard risk).4
High-risk abnormalities (excludes patients with del(13q) by FISH as the sole cytogenetic risk). n values for poor-prognosis and high-risk abnormalities were 67 for all patients and 37 for the phase 2 population.
Of 72 evaluable patients, 49 represented the ASCT group and 23 the non-ASCT group. Sixteen patients (70% of those not proceeding to ASCT) were not ASCT candidates because of either their age or comorbidities. The remaining non-ASCT patients included 1 patient who was not considered ready to proceed to ASCT by the treating physician and 7 who deferred or declined early ASCT. Non-ASCT candidates had higher-stage disease than either ASCT patients or ASCT candidates deferring transplantation (International Staging System [ISS] stage II/III, 69% vs 51% and 29%, respectively), although this was not statistically significant due to the small sample sizes (P = .22). However, high-risk cytogenetic abnormalities were more frequent in ASCT cases (51%) than either non-ASCT candidates (31%) or ASCT candidates deferring transplantation (29%); the difference again was not statistically significant (P = .32).
Determination of MTD
During the phase 1 study, 5 patients developed DLTs (2 at level 2 and 3 at level 3); consisting of 2 grade 3 asymptomatic neutropenia events on day 1 of cycle 2, 1 grade 3 elevation of transaminases, 1 grade 3 drug fever, and 1 grade 3 hypophosphatemia. There were no DLTs at levels 1 and 4. Based on the definition of MTD and the prior estimates for the probability of DLT at each dose level, the posterior probability estimates for DLTs were calculated as 4.7%, 9.7%, 13.7%, and 17.9% for levels 1-4, respectively. The maximum planned dose level 4 (lenalidomide 25 mg/d, bortezomib 1.3 mg/m2, PLD 30 mg/m2, and dexamethasone 20/10 mg/d) was selected as the MTD for the phase 2 study because it was the dose that was closest to, but did not exceed, the target rate of 20% of DLTs.
Treatment exposure and safety
The median treatment duration was 4.5 cycles (range, 2-31). Seventy patients (97%) completed ≥ 4 cycles and 20 patients (28%) completed ≥ 8 cycles; 11 patients (15%) remained on treatment. Forty-nine patients (68%) proceeded to ASCT after a median of 4 cycles (range, 3-12). Non-ASCT patients received a median of 13 cycles (range 2-31). Two patients (3%) discontinued treatment before study completion: 1 from the ASCT group after 3 cycles due to personal choice and 1 from the non-ASCT group who was not a transplantation candidate after 2 cycles because of age and progressive disease. None of the patients discontinued treatment before study completion due to safety concerns alone.
Overall, AEs proved manageable. The most common all-grade AEs were fatigue (83.3%), constipation (69.4%), sensory neuropathy (65.3%), and infections (56.9%) (Table 3). Grade ≥ 3 AEs included neutropenia (19.4%), infections (13.9%), thrombocytopenia (11.1%), and DVT/pulmonary embolism (2.8%). Importantly, 4 patients (5.6%) experienced grade 3 sensory neuropathy but none had grade 4. Eighteen patients (25%) had grade 1-2 palmar-plantar erythrodysesthesia and 1 patient (1%) experienced a grade 2 asymptomatic and reversible decrease in LVEF from 65% to 45%, which was attributed to PLD and required dose reduction and treatment delay. There was no treatment-related mortality. No significant decline of ANC or platelet count was observed in consecutive cycles.
Table 3.
AEs reported for≥15% of patients in the overall treated population (N = 72) and all grade 3 or 4 events
| AE | n (%) |
||
|---|---|---|---|
| All grades | Grade 3 | Grade 4 | |
| Fatigue | 60 (83.3) | 6 (8.3) | 0 |
| Constipation | 50 (69.4) | 1 (1.4) | 0 |
| Sensory neuropathy | 47 (65.3) | 4 (5.6) | 0 |
| Thrombocytopenia | 35 (48.6) | 7 (9.7) | 1 (1.4) |
| Nausea/diarrhea | 46 (63.9) | 1 (1.4) | 0 |
| Pneumonia/infections | 41 (56.9) | 10 (13.9) | 0 |
| Palmar-plantar erythrodysesthesia | 18 (25.0) | 0 | 0 |
| Neutropenia | 21 (29.2) | 12 (16.7) | 2 (2.8) |
| Cardiovascular | 11 (15.3) | 2 (2.8) | 0 |
| Painful neuropathy | 8 (11.1) | 0 | 0 |
| DVT/pulmonary embolism | 4 (5.6) | 1 (1.4) | 1 (1.4) |
Response to treatment
Overall, 96% of patients achieved ≥ PR, 67% ≥ VGPR, and 39% CR/nCR as their best response to RVDD (Table 4). Per-protocol-defined efficacy assessment of response rates at the end of cycles 4 and 8 are listed in Table 4. At the MTD, 95% of patients obtained ≥ PR, 64% ≥ VGPR, and 33% CR/nCR. Among the 33 patients with poor-risk chromosomal abnormalities, 97% had ≥ PR, 73% ≥ VGPR, and 48% CR/nCR as their best response, including 19% stringent CR (sCR).
Table 4.
Best response to treatment for all patients and responses achieved after 4 and 8 cycles of RVDD therapy
| Response | Best response to treatment, n (%) |
Responses after 4 and 8 cycles, n (%) |
||
|---|---|---|---|---|
| All (N = 72) | At MTD (n = 39) | At 4 cycles (n = 70) | At 8 cycles (n = 20)* | |
| CR + nCR | 28 (39) | 13 (33) | 20 (29) | 7 (35) |
| CR | 21 (29) | 11 (28) | 14 (20) | 5 (25) |
| sCR | 11 (15) | 5 (13) | 1 (1) | 3 (15) |
| nCR | 7 (10) | 2 (5) | 6 (9) | 2 (10) |
| ≥ VGPR | 48 (67) | 25 (64) | 40 (57) | 13 (65) |
| ≥ PR | 69 (96) | 37 (95) | 67 (96) | 19 (95) |
Of 20 patients who completed 8 cycles, 3 subsequently proceeded to ASCT.
Of the 49 patients who underwent ASCT, 46 were evaluable for response 3 months after transplantation. Of these patients, 39% had CR/nCR and 67% had ≥ VGPR after induction therapy with RVDD. After ASCT, 61% of these patients achieved CR/nCR and 85% had ≥ VGPR (Figure 2). For the nonevaluable patients, one had ASCT after documented disease progression before transplantation, one did not have data available at 3 months after transplantation, and one patient died after ASCT before the 3-month assessment.
Harvesting of SCs and engraftment
SC harvest was performed for 58 patients after completion of a median of 4 cycles (range, 3-8) of RVDD, as per institutional standards of care. Thirty-four patients collected stem cells after receiving cyclophosphamide + G-CSF (C + G), 12 after receiving G alone, 6 after receiving G + plerixafor (G + P), 2 after receiving C + G + P, 1 after receiving G + P + sargramostim, and for 3 patients the method was not recorded. Fifty-six (97%) of the 58 patients for whom SC harvest was attempted were successfully harvested for peripheral blood SCs. Two patients required a second attempt: 1 patient who initially failed G and had subsequently collected using G + P and 1 patient who failed G + P and had subsequently collected using etoposide + G. The mean and median total CD34+ cell count was 8.2 × 106 and 7.5 × 106 cells/kg, respectively, after a median of 4.5 days (range, 3-6). Forty-nine patients completed at least a single ASCT. There were no unexpected AEs during transplantation.
Time-to-event outcomes
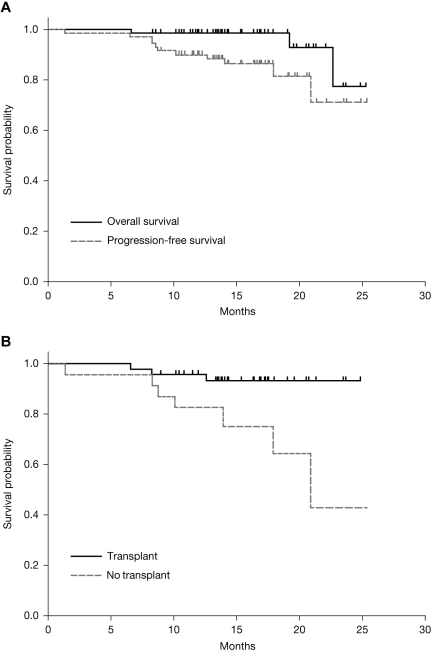
After a median 15.2 months (range, 6-25) of follow-up, the median PFS and OS were not reached (Figure 3A). The estimated 18-month PFS for all patients was 81.6% (95% confidence interval [CI], 65.5-90.6) and OS was 98.6% (95% CI, 90.5-99.8). At the time of manuscript preparation, 3 patients have died: 2 non-ASCT patients died after disease progression at 19.2 and 22.7 months after treatment initiation and 1 transplantation patient died while in disease remission at 6.6 months after treatment initiation (1.9 months after transplantation).
Figure 3.
Kaplan-Meier survival estimates. Shown are estimates for OS and PFS among all patients treated with RVDD (A) and PFS among patients who did or did not go on to transplantation (B).
After nonrandomized allocation of patients to ASCT or continued treatment, 18-month estimates for PFS were 93.3% (95% CI, 80.5-97.8) in ASCT patients compared with 64.3% (95% CI, 33.3-83.8) for non-ASCT patients, respectively, which was statistically significant (log-rank P = .01; Figure 3B). OS was similar in this population by ASCT status (log-rank P = .3). Subgroup analysis according to cytogenetic risk (defined in the footnote to Table 2) showed statistically comparable PFS and OS in both patient populations (not shown).
Discussion
Results from this phase 1/2 study of the RVDD regimen in patients with newly diagnosed MM show that RVDD is highly active and was generally well tolerated, both as a short-term, pretransplantation induction and as an extended treatment. There was no treatment-related mortality, although incorporation of PLD to RVD resulted in some additional AEs compared with RVD, including palmar-plantar erythrodysesthesia. Among patients who proceeded to ASCT and were evaluable for posttransplantation response, response rates further improved, reaching 85% of patients with ≥ VGPR and 61% of those with CR/nCR (including 37% CR and 24% sCR) at 3 months after ASCT. To the best of our knowledge, these rates represent some of the best reported at 3 months after ASCT. In patients who continued RVDD treatment beyond 4 cycles, the depth of response also further improved, reaching 65% ≥ VGPR and 35% CR/nCR (including 25% CR and 15% sCR) at the completion of 8 cycles. These rates are also among the best reported with this duration of initial treatment in newly diagnosed MM patients and are comparable to our experience with RVD.6
The phase 1 portion of the study showed that PLD can be safely added at a maximum planned dose4 to established doses of the RVD regimen6 without exceeding the study-defined maximum rate of DLTs. The TITE-CRM design used in the present study allowed for a rapid, continued escalation of dose levels in a multisite setting while preserving safety and accuracy in arriving at a dose level closest to, but not exceeding, the maximum planned rate of DLTs. After analysis of overall toxicity data, some increase in AEs was observed, including the degree of peripheral neuropathy and palmar-plantar erythrodysthesia compared with RVD. Cardiac dysfunction was rare and rates of DVT were similar. Moreover, this additional toxicity did not compromise overall safety and the favorable tolerability profile encountered supports the rationale of selecting a fourth drug partner to RVD with limited or no overlapping toxicity. In contrast, as reported in other recent studies, overlapping toxicities may significantly increase the rate of AEs, such as those seen with VDCR, but may also contribute to less than expected efficacy results of regimens, presumably due to treatment delays and discontinuations.15,16,25
The results presented herein further validate the very high quality of responses seen with the RVD regimen.6,15,16,26 Furthermore, our results suggest that the addition of a fourth drug, specifically PLD, may improve the depth and duration of response for all patients compared with RVD. Within the limitations of a historical comparison, it is notable that the RVDD regimen produced higher responses after 4 and 8 cycles compared with RVD (Table 5),6 suggesting a potentially more rapid cytoreduction; however, ultimately, the rate of best responses was comparable between the 2 trials overall. This may reflect a difference in the disposition of patients, including longer duration on treatment in RVD (10 cycles) compared with RVDD (4.5 cycles) because the depth of response improves with the duration of treatment.6 In this context, comparisons at equivalent time points may better reflect similarities or differences between these regimens.
Table 5.
Outcomes of RVD and RVDD regimens*
| Response after 4 cycles, % |
Response after 8 cycles, % |
Estimated PFS at 18 months, % | |||||
|---|---|---|---|---|---|---|---|
| ≥ PR | ≥ VGPR | CR/nCR | ≥ PR | ≥ VGPR | CR/nCR | ||
| RVD6 | 75 | 11 | 6 | 96 | 53 | 23 | 75 |
| RVDD | 96 | 57 | 29 | 95 | 65 | 35 | 82 |
Includes all patients (phase 1/2). Best-response rates for RVD after a median of 10 cycles: > PR, 100%; > VGPR, 67%; and CR/nCR, 39%. Best-response rates for RVDD after a median of 4.5 cycles: > PR, 96%; > VGPR, 67%; and CR/nCR, 39%.
Patients in the present study had an estimated 18-month PFS of 81.6% and an OS of 98.6%. This is similar, with some trend toward better PFS, to the results seen with RVD, for which 18-month PFS and OS estimates of 75% and 97%, respectively, were reported.6 Response rates before ASCT after ≥ 4 cycles were better for RVDD (39% of patients CR/nCR and 64% of those with ≥ VGPR) versus RVD (21% of patients with CR/nCR and 57% of those with ≥ VGPR). The ASCT group of patients in the RVDD study showed a 93.3% estimated 18-month PFS, similar to that seen with RVD. Although a randomized study would clearly be needed to better define the future role of RVDD in the treatment of newly diagnosed MM, preliminary results suggest that the addition of PLD to RVD results in a rapid reduction of disease burden.
Whereas the overall response rate was comparable at 4 cycles in the ASCT and non-ASCT groups, the non-ASCT group required a longer duration of treatment for a similar depth of response. In addition, in our analysis, a significant PFS benefit was seen in ASCT patients compared with non-ASCT patients (P = .01; Figure 3B). This finding was surprising and inconsistent with the observation seen in the RVD study, in which PFS was not statistically different between the ASCT and the non-ASCT groups. This may be related to differences in patient disposition between studies, because neither study randomly assigned ASCT-candidate patients to transplantation; therefore, the potential for selection bias is important. Indeed, patients not undergoing ASCT in our study were significantly older (65 vs 56 years) and had more comorbidities preventing them from undergoing transplantation. There was a higher proportion of patients with ISS stage II/III, but there was a comparable proportion of patients with high-risk cytogenetic abnormalities. In addition, this was a smaller subset of enrolled patients overall, with a smaller proportion of candidates deferring ASCT in the RVDD study compared with that seen in the RVD study. These reservations notwithstanding and recognizing the limitations of across-trial comparisons, this could still potentially be an important observation. A trend toward significantly shorter PFS for non-ASCT patients has been reported in high ISS patients, as defined by the International Staging System within the RVD study and other studies.6,27 The hypothesis that ASCT may improve the duration of response and PFS is currently being investigated in a prospective, randomized phase 3 study (IFM/DFCI2009; ClinicalTrials.gov identifier NCT01191060) comparing RVD induction followed by ASCT and consolidation plus continued lenalidomide as maintenance for up to 2 years in patients aged ≤ 65 years. While we are awaiting results from this study, the findings from our study appear to imply that addition of PLD may not substitute for a high-dose alkylator, although the regimen was generally well tolerated by older patients. If delayed ASCT or no ASCT is considered, a novel regimen without cytotoxic or alkylating agents may be more appropriate at this time. Conversely, RVDD may be better suited for treating younger ASCT candidates without significant comorbidities and without cardiac risk factors who wish to proceed to ASCT. In this regard, achieving a deeper response after a shorter duration of treatment may ultimately be beneficial, especially because the achievement of ≥ VGPR at the completion of induction treatment before ASCT is typically associated with improved PFS4 and OS.28
The hypothesis that the addition of a fourth drug to an active 3-drug regimen can improve efficacy has been investigated previously as the next logical step following up on several studies showing improved efficacy of 3-drug compared with 2-drug combinations in patients with newly diagnosed MM in the pre-ASCT setting.5,29,30 In the phase 1 portion of the EVOLUTION study, patients receiving VDCR showed promising responses, with response rates of 20% CR, 40% CR/nCR, and 68% ≥ VGPR.15 However, recently reported results from the phase 2 portion of this study, which enrolled both ASCT and non-ASCT candidates, showed no significant differences between the best-response rates, PFS, or OS of 3- and 4-drug combinations.16 Multiple factors may have contributed to this result. First, the treatment period in this study was 9 months and may have been too brief to detect a possible difference between the combinations. Second, the duration of treatment of each of the 3 arms and the timing of disposition to ASCT were not mandated in the EVOLUTION study, limiting the end point of comparison of these regimens to best-response rates. Finally, VDCR was associated with higher hematologic toxicities and worse nonhematologic side effects, including treatment-related mortality compared with the 3-drug regimens of RVD and VCD.15,16
In another approach, the GIMEMA investigators found that the 4-drug combination of bortezomib, melphalan, prednisone, and thalidomide followed by maintenance with bortezomib and thalidomide (VMPT-VT) significantly improved ≥ VGPR rates (59% vs 50%, respectively; P = .03) and CR rate (38% vs 24%; P < .001) when compared with the 3-drug combination of bortezomib, melphalan, and prednisone (VMP) without maintenance in the non-ASCT setting for older patients.31 In more recent results, differences in overall response rates and CR for VMPT-VT vs VMP were statistically significant (P = .01 and P < .001, respectively).25 This study also reported a 3-year PFS benefit (median follow-up 23.2 months; VMPT-VT 56% vs VMP 41%; P = .008). This difference reflected both the superior depth of response to initial cytoreduction and the significant maintenance effect with bortezomib and thalidomide, which was limited to the VMPT-VT arm. In the context of these 2 studies, our results appear to be more consistent with the results from the GIMEMA study, perhaps in part because our study also allowed prolonged maintenance. Nevertheless, these results support the hypothesis that novel 4-drug constructs may have the potential for higher efficacy than 3-drug novel regimens in selected patients.
Subsequent to laboratory studies demonstrating synergy, several investigators have recently started to explore the role of adding new, promising investigational agents to active 2- or 3-drug regimens.32 This is being explored as a possible alternative to adding a cytotoxic drug or an established novel agent to prior active regimens. Currently, various new dexamethasone + lenalidomide- or dexamethasone + bortezomib–based 3- or 4-drug combinations with new agents such as carfilzomib,33,34 panobinostat,35 vorinostat,27,36 and elotuzumab37 are being investigated in patients with relapsed/refractory MM and newly diagnosed MM, with promising results to date. These novel combinations have the potential to be evaluated in newly diagnosed MM patients.38
In conclusion, results from this phase 1/2 study show that the addition of PLD to the RVD combination appears to significantly augment the depth and duration of response in patients with newly diagnosed MM. These data provide the rationale for considering further studies with this 4-drug regimen, including randomized trials in this setting.
Acknowledgments
We thank Dominik Dytfeld and Brian Nordgren (University of Michigan, Ann Arbor) for contributing to data collection and analysis, and Diane Durecki from the University of Michigan for assistance with the preparation of the manuscript.
This study was supported by Celgene, Centocor Ortho Biotech Services, Millennium Pharmaceuticals, and the Multiple Myeloma Research Consortium. C.C.H. is a Paul Calabresi scholar on grant K12CA133250 from the National Cancer Institute. The content is solely the responsibility of the authors and does not represent the official views of the National Cancer Institute or the National Institutes of Health. The authors received editorial support from Adriana Stan, PhD, of Excerpta Medica in the preparation of this manuscript, which was funded by Celgene. The authors are fully responsible for the content and editorial decisions for this manuscript.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.J.J., K.A.G., J.C.B., C.L.T., D.-L.E., M.S.K., K.C.A., and P.G.R. designed the research; A.J.J., D.E.R., C.C.H., S.L., T.M.Z., E.L.C., R.L.S., J.P.L., N.S.R., T.A., and P.G.R. performed the research; M.A.M., C.K.H., and S.M.W. collected and assembled data; A.J.J., K.A.G., and P.G.R. analyzed and interpreted the data; K.A.G. performed statistical analyses; A.J.J., K.A.G., and P.G.R. wrote the manuscript; and all authors reviewed and approved the manuscript and had access to the primary clinical data.
Conflict-of-interest disclosure: A.J.J. is a consultant with Ortho Biotech, Celgene Corporation, Millennium Pharmaceuticals, Onyx Pharmaceuticals, BMS, and Exelixis; has received honoraria from Ortho Biotech, Celgene Corporation, Millennium Pharmaceuticals, BMS, and Exelixis (all of which were discontinued as of August 2010); is on the speakers' bureaus of Celgene, Ortho Biotech, and Millennium Pharmaceuticals (all of which were discontinued as of August 2010); and is on the advisory committee for Millennium Pharmaceuticals, BMS, and Onyx. D.E.R. has received research funding from Celgene Corporation, Johnson & Johnson, and Ortho Biotech; is on the advisory board of Celgene Corporation and Ortho Biotech; and has given unrestricted educational talks for Celgene Corporation and Ortho Biotech. S.L. is a consultant with Celgene Corporation, Millennium Pharmaceuticals, Novartis, BMS, and Onyx and is on the advisory board for Merck. T.M.Z. is a member of the board of directors, speakers' bureaus, or advisory committees for Celgene Corporation and Millennium Pharmaceuticals. N.S.R. is a consultant with Amgen, Celgene Corporation, and Novartis and has received research funding from AstraZeneca and Acetylon. T.A. is on the speakers' bureaus for Celgene Corporation and Millennium Pharmaceuticals. J.C.B. has ownership interest in and is an employee of Celgene Corporation. C.L.T. has ownership interest in Johnson & Johnson. D.-L.E. is employed by Millennium Pharmaceuticals and has ownership interest in Johnson & Johnson. S.L.K. is employed by the Multiple Myeloma Research Consortium. K.C.A. has ownership interest in Acetylon and is on the advisory boards of Celgene Corporation, Novartis, Millennium Pharmaceuticals, Onyx, BMS, and Merck. P.G.R. is on the advisory board of Millennium Pharmaceuticals, Celgene Corporation, Novartis, Johnson & Johnson, and BMS. K.A.G., C.C.H., E.L.C., R.L.S., J.P.L., M.A.M., C.K.H., S.M.W., and M.S.K. declare no competing financial interests.
Correspondence: Andrzej J. Jakubowiak, University of Michigan, 1500 E. Medical Center Dr, Room 4216 Cancer Center, SPC 5936, Ann Arbor, MI 48109-5936; e-mail: ajakubow@umich.edu.
References
- 1.Harousseau JL, Avet-Loiseau H, Attal M, et al. Achievement of at least very good partial response is a simple and robust prognostic factor in patients with multiple myeloma treated with high-dose therapy: long-term analysis of the IFM 99-02 and 99-04 trials. J Clin Oncol. 2009;27(34):5720–5726. doi: 10.1200/JCO.2008.21.1060. [DOI] [PubMed] [Google Scholar]
- 2.Harousseau JL, Palumbo A, Richardson PG, et al. Superior outcomes associated with complete response in newly diagnosed multiple myeloma patients treated with non-intensive therapy: analysis of the phase 3 VIST.A. study of bortezomib plus melphalan-prednisone versus melphalan-prednisone. Blood. 2010;116(19):3743–3750. doi: 10.1182/blood-2010-03-275800. [DOI] [PubMed] [Google Scholar]
- 3.Niesvizky R, Richardson PG, Rajkumar SV, et al. The relationship between quality of response and clinical benefit for patients treated on the bortezomib arm of the international, randomized, phase 3 APEX trial in relapsed multiple myeloma. Br J Haematol. 2008;143(1):46–53. doi: 10.1111/j.1365-2141.2008.07303.x. [DOI] [PubMed] [Google Scholar]
- 4.Jakubowiak AJ, Kendall T, Al-Zoubi A, et al. Phase II trial of combination therapy with bortezomib, pegylated liposomal doxorubicin, and dexamethasone in patients with newly diagnosed myeloma. J Clin Oncol. 2009;27(30):5015–5022. doi: 10.1200/JCO.2008.19.5370. [DOI] [PubMed] [Google Scholar]
- 5.Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376(9758):2075–2085. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 6.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116(5):679–686. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M, Delasalle K, Giralt S, Alexanian R. Rapid control of previously untreated multiple myeloma with bortezomib-lenalidomide-dexamethasone (BLD). Hematology. 2010;15(2):70–73. doi: 10.1179/102453310X12583347010133. [DOI] [PubMed] [Google Scholar]
- 8.Eom HS, Kim YK, Chung JS, et al. Bortezomib, thalidomide, dexamethasone induction therapy followed by melphalan, prednisolone, thalidomide consolidation therapy as a first line of treatment for patients with multiple myeloma who are non-transplant candidates: results of the Korean Multiple Myeloma Working Party (KMMWP). Ann Hematol. 2010;89(5):489–497. doi: 10.1007/s00277-009-0871-y. [DOI] [PubMed] [Google Scholar]
- 9.Wang M, Giralt S, Delasalle K, Handy B, Alexanian R. Bortezomib in combination with thalidomide-dexamethasone for previously untreated multiple myeloma. Hematology. 2007;12(3):235–239. doi: 10.1080/10245330701214236. [DOI] [PubMed] [Google Scholar]
- 10.Yang DH, Kim YK, Sohn SK, et al. Induction treatment with cyclophosphamide, thalidomide, and dexamethasone in newly diagnosed multiple myeloma: a phase II study. Clin Lymphoma Myeloma Leuk. 2010;10(1):62–67. doi: 10.3816/CLML.2010.n.007. [DOI] [PubMed] [Google Scholar]
- 11.Bensinger WI, Jagannath S, Vescio R, et al. Phase 2 study of two sequential three-drug combinations containing bortezomib, cyclophosphamide and dexamethasone, followed by bortezomib, thalidomide and dexamethasone as frontline therapy for multiple myeloma. Br J Haematol. 2010;148(4):562–568. doi: 10.1111/j.1365-2141.2009.07981.x. [DOI] [PubMed] [Google Scholar]
- 12.Reeder CB, Reece DE, Kukreti V, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia. 2009;23(7):1337–1341. doi: 10.1038/leu.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knop S, Liebisch P, Wandt H, et al. Bortezomib, IV cyclophosphamide, and dexamethasone (VelCD) as induction therapy in newly diagnosed multiple myeloma: Results of an interim analysis of the German DSMM Xia trial [abstract]. J Clin Oncol. 2009;27(15S):8516. [Google Scholar]
- 14.Popat R, Oakervee HE, Hallam S, et al. Bortezomib, doxorubicin and dexamethasone (PAD) front-line treatment of multiple myeloma: updated results after long-term follow-up. Br J Haematol. 2008;141(4):512–516. doi: 10.1111/j.1365-2141.2008.06997.x. [DOI] [PubMed] [Google Scholar]
- 15.Kumar SK, Flinn I, Noga SJ, et al. Bortezomib, dexamethasone, cyclophosphamide and lenalidomide combination for newly diagnosed multiple myeloma: phase 1 results from the multicenter EVOLUTION study. Leukemia. 2010;24(7):1350–1356. doi: 10.1038/leu.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar S, Flinn IW, Richardson PG, et al. Novel three- and four-drug combination regimens of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide, for previously untreated multiple myeloma: results from the multi-center, randomized phase 2 EVOLUTION study [abstract]. Blood. 2010;114(11):621. [Google Scholar]
- 17.Hari M, Hector-Word Z, Lebovic D, Soengas M, Jakubowiak A. Pre-clinical evaluation of bortezomib, doxorubicin, dexamethasone, and lenalidomide in multiple myeloma (MM) [abstract]. J Clin Oncol. 2008;26(15S):19513. [Google Scholar]
- 18.Mitsiades N, Mitsiades CS, Richardson PG, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101(6):2377–2380. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 19.Durie BG, Kyle RA, Belch A, et al. Myeloma management guidelines: a consensus report from the Scientific Advisors of the International Myeloma Foundation. Hematol J. 2003;4(6):379–398. [PubMed] [Google Scholar]
- 20.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 21.Cheung YK, Chappell R. Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics. 2000;56(4):1177–1182. doi: 10.1111/j.0006-341x.2000.01177.x. [DOI] [PubMed] [Google Scholar]
- 22.Normolle D, Lawrence T. Designing dose-escalation trials with late-onset toxicities using the time-to-event continual reassessment method. J Clin Oncol. 2006;24(27):4426–4433. doi: 10.1200/JCO.2005.04.3844. [DOI] [PubMed] [Google Scholar]
- 23.Bladé J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102(5):1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 24.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 25.Palumbo A, Bringhen S, Rossi D, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: a randomized controlled trial. J Clin Oncol. 2010;28(34):5101–5109. doi: 10.1200/JCO.2010.29.8216. [DOI] [PubMed] [Google Scholar]
- 26.Roussel M, Avet-Loiseau H, Moreau P, et al. Frontline therapy with bortezomib, lenalidomide, and dexamethasone (VRD) induction followed by autologous stem cell transplantation, VRD consolidation and lenalidomide maintenance in newly diagnosed multiple myeloma patients: primary results of the IFM 2008 phase II study [abstract]. Blood. 2010;116(11):624. [Google Scholar]
- 27.Dytfeld D, Griffith KA, Friedman J, et al. Superior overall survival of myeloma patients achieving very good partial response or better to initial treatment with bortezomib, pegylated liposomal doxorubicin, and dexamethasone, predicted after two cycles by a free light chain- and M-protein-based model: Extended follow-up of a phase II trial. Leuk Lymphoma. 2011 doi: 10.3109/10428194.2011.567316. In press. [DOI] [PubMed] [Google Scholar]
- 28.Richardson PG, Weber DM, Mitsiades CS, et al. Phase I study of combined vorinostat (V), lenalidomide (L), and dexamethasone (D) in patients (pts) with relapsed or refractory multiple myeloma (MM) [abstract]. J Clin Oncol. 2010;28(15S):8031. [Google Scholar]
- 29.Harousseau JL, Avet-Loiseau H, Facon T, et al. Bortezomib plus dexamethasone (VD) versus reduced-dose bortezomib plus thalidomide plus dexametasone (vTD) as induction treatment prior to autologous stem-cell transplantation (ASCT) in newly diagnosed multiple myeloma (MM) [abstract]. Blood. 2009;114(11):354. [Google Scholar]
- 30.Moreau P, Facon T, Attal M, et al. Comparison of reduced-dose bortezomib plus thalidomide plus dexamethasone (vTD) to bortezomib plus dexamethasone (VD) as induction treatment prior to ASCT in de novo multiple myeloma (MM): Results of IFM2007-02 study [abstract]. J Clin Oncol. 2010;28(15S):8014. [Google Scholar]
- 31.Boccadoro M, Bringhen S, Gaidano G, et al. Bortezomib, melphalan, prednisone, and thalidomide (VMPT) followed by maintenance with bortezomib and thalidomide (VT) for initial treatment of elderly multiple myeloma patients [abstract]. J Clin Oncol. 2010;28(15S):8013. doi: 10.1200/JCO.2010.29.8216. [DOI] [PubMed] [Google Scholar]
- 32.Richardson PG, Mitsiades C, Schlossman R, Munshi N, Anderson K. New drugs for myeloma. Oncologist. 2007;12(7):664–689. doi: 10.1634/theoncologist.12-6-664. [DOI] [PubMed] [Google Scholar]
- 33.Niesvizky R, Bensinger W, Vallone M, Gutierrez A, Kunkel L. PX-171-006: Phase Ib multicenter dose escalation study of carfilzomib (CFZ) plus lenalidomide (LEN) and low-dose dexamethasone (loDex) in relapsed and refractory multiple myeloma (MM): preliminary results [abstract]. J Clin Oncol. 2009;27(15S):8541. [Google Scholar]
- 34.Jakubowiak AJ, Dytfeld D, Jagannath S, et al. Carfilzomib, lenalidomide, and dexamethasone in newly diagnosed multiple myeloma: initial results of phase I/II MMRC trial [abstract]. Blood. 2010;116(11):862. [Google Scholar]
- 35.Mateos M, Spencer A, Taylor K, et al. Phase Ib study of oral panobinostat (LBH589) plus lenalidomide (LEN) plus dexamethasone (DEX) in patients (Pts) with relapsed (Rel) or Rel and refractory (Ref) multiple myeloma (MM) [abstract]. J Clin Oncol. 2010;28(15S):8030. [Google Scholar]
- 36.Siegel DS, Weber DM, Mitsiades CS, et al. Vorinostat in combination with lenalidomide and dexamethasone in patients with relapsed/refractory multiple myeloma: a phase I study [abstract]. J Clin Oncol. 2009;27(15S):8586. [Google Scholar]
- 37.Lonial S, Vij R, Harousseau J, et al. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma: a phase I/II study [abstract]. J Clin Oncol. 2010;28(15S):8020. doi: 10.1200/JCO.2011.37.2649. [DOI] [PubMed] [Google Scholar]
- 38.Richardson PG, Mitsiades CS, Schlossman R, et al. Bortezomib in the front-line treatment of multiple myeloma. Expert Rev Anticancer Ther. 2008;8(7):1053–1072. doi: 10.1586/14737140.8.7.1053. [DOI] [PubMed] [Google Scholar]