Abstract
Background
Atrial fibrillation (AF) often coexists with myocardial infarction (MI), yet its prognostic influence is controversial. Prior reports studied the role of AF during the early hospitalization for acute MI on the risk of death and could not address the timing of AF in relation to the MI (i.e. prior, during, post). Further, as data come mostly from clinical trials, their applicability to the community is uncertain. The aims of our study were to assess the occurrence of AF among MI patients, determine whether it has changed over time, and quantify its impact and the impact of its timing on mortality after MI.
Methods and Results
This was a community-based cohort of 3220 patients hospitalized with incident (first-ever) MI from 1983 to 2007 in Olmsted County, Minnesota. AF was identified by diagnostic codes and ECG. Outcomes were all-cause and cardiovascular death. AF prior to MI was identified in 304 patients and 729 developed AF after MI (218 (30%) within 2 days, 119 (16%) between 3 and 30 days, and 392 (54%) >30 days post-MI). The cumulative incidence of AF after MI at 5 years was 19% and did not change over calendar year of MI. During a mean follow-up of 6.6 years, 1638 deaths occurred. AF was associated with an increased risk of death (HR (95% CI) 3.77 (3.37–4.21)), independently of clinical characteristics at the time of MI and heart failure. This risk differed markedly according to the timing of AF and was the greatest for AF occurring >30 days post-MI (HR (95% CI) 1.63 (1.37–1.93) for AF within 2 days, 1.81 (.45–2.27) for AF between 3 and 30 days, and 2.58 (2.21–3.00) for AF > 30 days post MI).
Conclusions
In the community, AF is frequent in the setting of MI. AF carries an excess risk of death, which is the highest for AF developing more than 30 days post-MI.
Keywords: atrial fibrillation, myocardial Infarction, mortality
Introduction
Atrial fibrillation (AF), the most commonly encountered clinical arrhythmia, often coexists with myocardial infarction (MI).1 The prognostic impact of AF occurring in the setting of MI remains controversial, with some studies reporting an independent adverse impact on mortality,2–8 whereas others failed to detect such an association.9–13 This putative increased risk has been hypothesized as reflecting the fact that AF may be a surrogate or marker of heart failure, elevated filling pressures and atrial volume overload.7 However, this association is seemingly independent of age, heart failure and ventricular dysfunction.5 Importantly, most published data emanate from randomized clinical trials, which have uncertain applicability to the community.14 Population studies or registries that are more likely to reflect “real life“ practice report on AF ascertained during the initial hospitalization for acute MI.7, 10–12, 15, 16 These studies did not ascertain AF after hospital dismissal, and thus could not address the role of the timing of post-MI AF on the risk of death. This is important as it is unknown if the excess risk of death conferred by AF is of similar magnitude irrespective of whether it occurs prior, at the time of the MI, or after the MI. Finally, while we and others recently demonstrated a dramatic change in the epidemiology of MI,17, 18 there is limited data on whether the epidemiology of AF that coexists with MI changed in parallel.16
The Rochester Epidemiology Project, which provides complete ascertainment of subsequent health care events in disease-based community cohorts including out-patient encounters, constitutes a robust platform from which to address these aforementioned gaps in knowledge. Thus, the aims of our study were to comprehensively assess the occurrence of AF among community patients who experience a first MI, determine whether it had changed over time, quantify the excess risk of death conferred by AF in MI patients and determine if this excess risk is modulated by the timing of AF.
Methods
Study Setting
This study was conducted with approval from all institutional review boards. In Olmsted County, few providers (chiefly Mayo Clinic and Olmsted Medical Center) deliver nearly all medical care to county residents. With the exception of a higher proportion employed in health care, the characteristics of this population are similar to those of US whites.19 Each provider uses a medical record which captures information for all encounters and can be retrieved because the Mayo Clinic maintains indices based on all diagnoses and procedures.20 Since 1966, similar indices have been implemented for non-Mayo providers through the Rochester Epidemiology Project, resulting in the linkage of medical records from all sources of care. This provides a unique infrastructure to analyze disease occurrence and outcomes at the population level, with comprehensive access to the records of all hospitalizations and outpatient clinic visits.
Myocardial Infarction Ascertainment
All patients aged 18 years or older hospitalized in Olmsted County for an incident MI between 1983 and 2007 were included in the present study. MI was defined according to validated criteria including cardiac pain, biomarkers, and Minnesota coding of the electrocardiograms.21, 22 The procedures used to assemble the MI incidence cohort and their reliability have been previously described.23, 24 Abstractors verified patients’ residency in Olmsted County and incident status of MI by complete review of the community medical records. Clinical characteristics recorded included demographic data, cardiovascular risk factors and MI severity indicators. Comorbidities were ascertained through manual data collection from the review of the entire medical record and summarized using the Charlson Index. 25 Heart failure (HF) was defined by the Framingham criteria.26 Recurrent ischemia was defined as hospitalization for recurrent MI or unstable angina using physicians’ diagnosis. Medications used at dismissal, including angiotensin converting enzyme inhibitors/angiotensin II receptor blockers (ACE inhibitors/ARB), beta blockers and aspirin, and reperfusion/revascularization procedures used during hospitalization were recorded.
Atrial Fibrillation Ascertainment
We defined and categorized AF using an adaptation of the 2006 guidelines from the American College of Cardiology, American Heart Association and European Heart Association. 27 Patients were classified into 3 groups: those with no AF, those with known prior AF and those with first ever documented AF any time at or after MI onset. Patients with atrial flutter were considered as having AF. Prior AF was ascertained using the following codes from the 9th version of the International Classification of Diseases28 (ICD-9) 427.3 (atrial fibrillation and flutter), 427.31 (atrial fibrillation) and 427.32 (atrial flutter). First ever documented AF at or after MI was defined as first occurrence at the time of the MI or any time after MI onset in the absence of a prior diagnosis, based on 12-lead electrocardiographic recordings (ECG) retrieved from an electronic ECG database. AF was defined as the absence of P waves, atrial activity represented by fibrillatory waves and irregular time elapsing between 2 consecutive R waves (RR) intervals. Atrial flutter on ECG recordings had to meet the following criteria: presence of regular P waves with a rate of 250–350/min and regular or irregular RR intervals. ECG diagnoses were assessed by cardiologists or trained electrocardiogram interpreting technicians.29 Patients with first ever documented AF post MI were further classified according to the timing of AF: early for AF occurring at the time of the MI or within 2 days after MI onset, intermediate for AF occurring between 3 and 30 days after the MI, and late for AF occurring beyond 30 days after the MI.
Ascertainment of Death
Follow-up was performed using all inpatient and outpatient medical records and completed until the date of death, or if alive, to the date of the most recent clinical evaluation. Death was ascertained from several sources. In addition to the deaths noted during clinical care, all death certificates for Olmsted County residents are obtained annually from the county office. The Mayo Clinic registration office records the obituaries and notices of death in the local newspapers. Finally, data on all Minnesota deaths are obtained from the State of Minnesota annually. Causes of death were classified as cardiovascular, cancer or other based on ICD-9 codes.28 The American Heart Association categories were used to define cardiovascular deaths.30
Statistical Analysis
Patient characteristics are presented as frequency or mean ± standard deviation (SD). Associations between patient characteristics and time to AF were examined with proportional hazards regression modeling. Trends in patient characteristics with timing of AF post MI were analyzed with the Mantel-Haenszel chi-square test for categorical variables and linear regression models using a 2-level categorical variable for timing of AF for continuous variables. Survival free of AF was assessed treating death as a competing risk.31 To test for secular trends in AF, proportional hazards regression was used to test for an association between occurrence of AF post MI and calendar year of MI. Proportional hazards regression was also used to examine the association between outcome (overall death, 30 day mortality, death among 30-day survivors and cardiovascular death) and AF categories, individually and adjusting for baseline characteristics. AF prior to the MI was modeled with an indicator variable. First ever documented AF post-MI was modeled as a time-dependent covariate with indicator variables representing early AF (at the time of the MI or within 2 days post MI), intermediate AF (3–30 days post MI) and late AF (> 30 days post MI) using left-truncated histories with data represented in the counting process style. Heart failure and recurrent ischemic events were modeled as time-dependent covariates. Missing values did not exceed 5% for any variable used in the regression analyses. The proportional hazards assumption was tested using scaled Schoenfeld residuals32 and found to be valid. All p-values were from two-tailed significance tests with 0.05 selected as the threshold of statistical significance. Analyses were performed using SAS statistical software, version 9.1 (SAS Institute Inc., Cary, NC) and Splus version 8 (TIBCO Software Inc., Palo Alto, CA).
Results
The MI incidence cohort and the occurrence of atrial fibrillation
Between 1983 and 2007, 3227 Olmsted County residents were hospitalized with an incident MI. Data for AF diagnosis were not available for 7 patients, resulting in a cohort of 3220 patients that form the basis of all subsequent analyses.
The mean age (SD) at the time of MI was 68 (15) years and 58% of the patients were men. The median duration of medical history available in the medical record prior to the incident MI was 39 years. Over that extensive period of observation, 304 persons had a history of AF prior to their incident MI (Figure 1).
Figure 1.
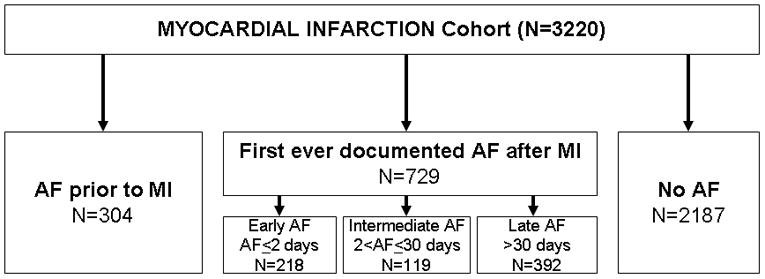
Classification of atrial fibrillation among subjects experiencing a myocardial infarction. Prior AF refers to AF occurring for the first time before the MI. New AF refers to AF detected for the first time during or after the MI. AF, atrial fibrillation; MI, myocardial Infarction.
Among the remaining patients, 729 patients had first ever documented AF post MI and 2187 patients did not develop AF during a mean follow-up of 6.6 years (limits, 0 to 25.9 years), which equated to an incidence of AF of 42 per 1000 person-years. The cumulative incidence of AF at 5 years with death as a competing risk was 19%. The occurrence of AF was not equally distributed during follow-up as 218 (30%) events occurred at the time of the MI or within 2 days after MI, 119 (16%) in the intermediate period of 3 to 30 days after MI, and 392 (54%) occurred beyond 30 days, with a gradual decline in AF over the duration of follow-up (Figure 2). The occurrence of AF post MI did not change over the study period (p=0.36 for calendar year of MI, adjusted for age, sex, and reperfusion or revascularization during the hospitalization for the incident MI).
Figure 2.
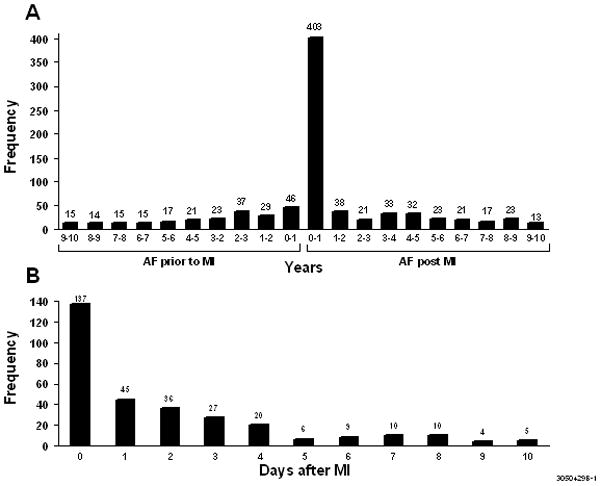
Time between the first occurrence of atrial fibrillation and myocardial infarction. Figure 2A: The period of observation has been truncated at 10 years. Figure 2B: The period of observation is the first 10 days post-MI. AF, atrial fibrillation; MI, myocardial Infarction.
Risk factors for the occurrence of AF
Factors associated with first ever documented AF at or after MI were older age, female sex, hypertension, diabetes and comorbidities including chronic kidney disease as estimated by creatinine clearance. Among MI characteristics, anterior location of the MI, higher Killip class, and lower ejection fraction were associated with newly identified AF at or after MI, while the presence of Q waves was marginally associated with the presence of AF (Table 1). Patients who developed AF early after MI were more likely to be older, of female sex, have a lower BMI, greater comorbidity burden including chronic kidney disease as estimated by creatinine clearance, and higher Killip class than those developing AF later (p value <0.05 for all comparisons).
Table 1.
Characteristics of Patients with Myocardial Infarction According to the Presence of Atrial Fibrillation
| Total N=3220 |
Prior AF N=304 |
New AF N=729 |
No AF N=2187 |
|
|---|---|---|---|---|
| Age (years), mean±SD | 68±15 | 79±11 | 72±13 | 65±15 |
| Men | 1852 (58) | 148 (49) | 378 (52) | 1326 (61) |
| BMI (kg/m2), mean±SD | 28±7 | 27±7 | 28±6 | 28±6 |
| Former or current smoker | 1940 (60) | 159 (52) | 415 (57) | 1366 (63) |
| Familial coronary disease | 651 (21) | 42 (15) | 126 (18) | 483 (23) |
| Hyperlipidemia | 1384 (43) | 131 (43) | 307 (42) | 946 (43) |
| Hypertension | 1919 (60) | 236 (78) | 474 (65) | 1209 (55) |
| Diabetes mellitus | 692 (22) | 82 (27) | 181 (25) | 429 (20) |
| Comorbidity Index | ||||
| 0 | 1243 (39) | 36 (12) | 238 (33) | 969 (44) |
| 1–2 | 1122 (35) | 121 (40) | 272 (37) | 729 (33) |
| ≥3 | 850 (26) | 146 (48) | 219 (30) | 485 (22) |
| Creatinine clearance (ml/min), mean±SD | 59 ±27 | 50±21 | 54±20 | 62±29 |
| MI characteristics and severity indicators | ||||
| Anterior location | 1125 (37) | 114 (39) | 273 (39) | 738 (36) |
| Presence of Q wave | 1620 (54) | 142 (52) | 391 (57) | 1087 (54) |
| ST elevation | 1066 (34) | 63 (21) | 260 (36) | 743 (35) |
| Killip class > 1 | 1038 (32) | 148 (49) | 274 (38) | 616 (28) |
| Ejection fraction ≤50% | 695 (51) | 75 (51) | 173 (58) | 447 (49) |
| Peak CK-MB, median (25th, 75th percentile) | 6.2 (2.2–17.1) | 4.3 (1.6–9.9) | 5.8 (2.1–17.6) | 6.8 (2.5–17.6) |
AF, atrial fibrillation; HR, hazard ratio; MI, myocardial infarction; SD, standard deviation; BMI, body mass index; CK, creatine kinase. Results presented as n (%) unless otherwise specified. Missing values were less than 8% for all variables except for ejection fraction where 58% were missing.
AF and mortality after MI
Over the follow-up period, 1638 deaths occurred, 314 of these within the first month after MI, equating to a 30-day case fatality rate of 10% (95% CI 9%-11%). At 5 years, mortality was 34% (95% CI 32%-36%) within the entire MI incidence cohort. Most deaths were from cardiovascular causes [n=933 (57%)], while 218 deaths (13%) were attributed to cancer, and 428 (26%) to other causes. The cause of death could not be determined in 59 (4%) persons.
The occurrence of AF at any time after MI was associated with a large increase in overall mortality (HR 3.77; 95% CI 3.37-4.21). Importantly, the excess risk of death conferred by AF differed markedly according to its timing (p<0.001, Table 2). Using patients with no AF as the referent, the risk of death was similar among patients with AF prior to the MI and those with newly identified AF occurring either early or within 3 to 30 days after the MI (p=0.24). Conversely, the risk of death was markedly higher for AF occurring more than 30 days after the MI, equating to a more than five-fold increase in the risk of death. These associations were only partially attenuated by adjustment for age, sex, and comorbidities and for the occurrence of heart failure during follow-up. All results were similar when recurrent ischemic events were included in the models and after further adjustment for reperfusion or revascularization during the hospitalization for the incident MI and dismissal medications. No clinically relevant interactions between AF and age, sex, and calendar year (i.e. year of the index MI) were detected.
Table 2.
Atrial fibrillation and risk of all-cause death and cardiovascular death after myocardial infarction. Results are presented as hazard ratios and 95% confidence intervals.
| Prior AF | Early AF | Intermediate AF | Late AF | P value* | |
|---|---|---|---|---|---|
| Death | |||||
| Unadjusted | 3.50 (3.03, 4.05) | 3.02 (2.55, 3.58) | 2.95 (2.36, 3.68) | 5.25 (4.51, 6.10) | <0.001 |
| Model 1† | 1.68 (1.44, 1.95) | 1.82 (1.54, 2.17) | 2.20 (1.76, 2.75) | 3.25 (2.79, 3.78) | <0.001 |
| Model 2‡ | 1.46 (1.26, 1.70) | 1.63 (1.37, 1.93) | 1.81 (1.45, 2.27) | 2.58 (2.21, 3.00) | <0.001 |
| Death within 30 days post MI | |||||
| Unadjusted | 2.42 (1.78, 3.30) | 3.72 (2.70, 5.12) | 7.68 (4.91,12.01) | Not applicable | <0.001 |
| Model 1† | 1.21 (0.88, 1.66) | 2.19 (1.58, 3.04) | 5.86 (3.74, 9.17) | Not applicable | <0.001 |
| Model 2‡ | 1.13 (0.82, 1.55) | 2.02 (1.46, 2.80) | 4.99 (3.18, 7.82) | Not applicable | <0.001 |
| Death among 30 day survivors | |||||
| Unadjusted | 3.99 (3.38, 4.73) | 2.84 (2.32, 3.48) | 2.52 (1.94, 3.28) | 5.22 (4.46, 6.11) | <0.001 |
| Model 1† | 1.88 (1.58, 2.24) | 1.73 (1.41, 2.13) | 1.81 (1.39, 2.35) | 3.21 (2.74,3.76) | <0.001 |
| Model 2‡ | 1.59 (1.33, 1.89) | 1.51 (1.23, 1.85) | 1.47 (1.13, 1.92) | 2.54 (2.17, 2.98) | <0.001 |
| Cardiovascular death among 30 day survivors | |||||
| Unadjusted | 3.88 ( 3.07, 4.91) | 3.33 (2.55, 4.33) | 3.41 (2.48, 4.70) | 5.77 (4.66, 7.16) | <0.001 |
| Model 1† | 1.91 (1.49, 2.43) | 2.04 (1.56, 2.67) | 2.49 (1.81, 3.44) | 3.62 (2.92, 4.49) | <0.001 |
| Model 2‡ | 1.55 (1.22, 1.98) | 1.72 (1.32, 2.25) | 1.94 (1.40, 2.68) | 2.70 (2.17, 3.36) | <0.001 |
P value comparing the equality of prior, early, intermediate and late AF.
Adjusted for age, sex, and comorbidity as measured by the Charlson index
Adjusted for age, sex, heart failure and comorbidity as measured by the Charlson index
When considering death within 30 days after the MI, similar associations were seen for prior and early AF as was observed for all deaths. However, the association between intermediate AF (within 3-30 days post MI) and 30-day mortality was much stronger; patients with intermediate AF had a 5-fold increased risk of death within 30 days after adjustment. Among 30-day survivors, the associations between AF and death were similar to those observed for all deaths. For cardiovascular death, results for prior AF were similar to those obtained for overall death, while stronger associations were observed for AF post MI, regardless of its timing (Table 2).
Further adjustment for Q-wave MI, ST-segment elevation MI, Killip class and peak CK-MB resulted in similar associations between the AF categories and each outcome. To examine secular trends in the associations between AF categories and each outcome, the interaction between AF categories and year of MI were tested and found to be not significant (p>0.10 for all outcomes).
Discussion
Our population-based data pertaining to a large MI incidence cohort indicate that, in the community, AF and MI often coexist and that approximately one-half of first-ever documented AF cases post MI develop in the first month after MI onset. There was no secular trend in the risk of AF after MI. AF after MI is associated with a large excess risk of death. Importantly, the excess risk imparted by AF varies markedly according to its timing with the highest risk of death being noted for AF occurring more than 30 days after the incident MI.
AF and MI as co-occurring events
Our study showed that AF and MI frequently coexist as approximately 1 out of 10 subjects who present with MI have a documented history of AF and 1 out of 4 subjects without prior AF will develop AF at or after the incident MI. The incidence of AF after MI reported herein was 42 per 1000 person-years. This far exceeded the age- and sex-adjusted AF incidence of 3.68 per 1000 person-years reported in the general Olmsted County population, from which this MI cohort was drawn33 as well as the incidence of AF reported in the Atherosclerosis Risk In Communities (ARIC) study.34
Previous studies reported heterogeneous frequencies of new onset AF (4% to 18%) that were lower than what we report herein. As these studies only captured AF during the initial MI hospitalization, they present an incomplete appraisal of its burden.35, 36 Data from studies that reported on the occurrence of AF within 30 days after the MI are consistent with the present report.9, 10, 12, 37, 38
Little is known on temporal trends of AF in patients with MI. The Worcester Heart Attack Study, which included data between 1990 and 2005, reported a decrease in the occurrence of AF during the initial hospitalization for MI during the 1990s and an increase since 2000,16 interpreting this fluctuation as reflecting an increasingly older population with a greater prevalence of comorbidities. However, the duration of hospitalization for MI, which also varied markedly during that time period, could also confound these trends. Conversely, the present data, where the categorization of timing of AF post-MI does not depend on the duration of hospitalization, which covers the entire length of follow-up beyond the initial hospitalization and which applies to an extended time period (25 years), did not detect any temporal change in the occurrence of AF post MI. The extended follow-up enabled us to examine the pattern of occurrence of AF, which is characterized by an early surge during the initial days after MI. Importantly, MI characteristics and severity indicators were for the most part not associated with the occurrence of AF, an observation that challenges the historical notion that AF denotes a more extensive and more severe infarction.2, 9, 39, 40 This hypothesis is further supported by the aforementioned temporal stability of AF post MI contrasting with profound changes in the epidemiology of MI noted during the same time period.17, 18
AF and Mortality
Previous studies on the impact of AF on survival in patients with MI reported discrepant results with some studies showing no adverse effect on mortality,9–13 whereas others reported an increased risk of death with AF.2, 6, 7, 16, 37, 41 Our study clearly demonstrates that AF in MI patients is associated with an increased risk of death even after adjustment for relevant confounders. Further, two other important findings deserve emphasis. First, patients presenting with acute MI and a history of AF have increased mortality compared with patients without AF. Second, AF developing more than 30 days post-MI was associated with the highest mortality risk with over a 2-fold increase in the risk of death compared to patients without AF. It has been suggested that the timing of AF onset may represent different mechanisms and, accordingly, may differentially influence outcomes.42–45
Limitations and strengths
Potential limitations of the current study need to be considered when interpreting the data. Most of these are shared by all studies addressing this topic, underscoring the challenges in studying the epidemiology of AF. AF can be discovered either in the presence of symptoms triggering an ECG or in the absence of any symptom by an ECG requested by a care provider in the presence of a heart rate abnormality or for an unrelated reason (such as a pre-anesthesia examination for example). ECGs were not routinely ascertained for this study; however, there is no apparent reason for symptomatic AF to be detected differentially before and after MI, although the timing of AF in relation to the MI may be misclassified due to delays in seeking medical care after symptom onset. This may not be the case for asymptomatic AF. Conversely, the increased “occurrence” of AF in the immediate post MI period could partially reflect previously undetected AF recognized during the hospitalization for MI. Atrial flutter and atrial fibrillation have a complex relationship that has mechanistic similarities but also differences. They often coexist and share similar thromboembolic risk.46–47 In our study, atrial fibrillation and flutter were combined as distinguishing between them was beyond the scope of this project.
While the racial and ethnic composition of Olmsted County may preclude generalizing findings to groups not adequately represented in this population, epidemiological studies in Olmsted County underscore that the results are generalizable to a large portion of the United States population and that cardiovascular disease trends measured in Olmsted County parallel national trends, further supporting its generalizability.48 Migration out of Olmsted County is rare among patients who experience an MI. Among subjects identified under the auspices of our study, 92% remained Olmsted County residents or remained within 30 miles of the city of Rochester and continued to receive care in Olmsted County such that complete follow-up was available through the medical record. Medications beyond discharge were not collected as part of this study.
Our study has several important strengths. Our population-based design reflects the experience of an entire community and thus is less subject to selection biases.14 This, in turn, optimizes the clinical relevance of our data. The internal validity of the present data are quite robust because our ascertainment identified all consecutive incident MIs in the community validated through rigorous criteria, and follow-up was extensive and comprehensive with few missing data. This allowed the comprehensive characterization of the occurrence of AF over an extended period of time, including any time before the MI and any time after hospital discharge.
Conclusions
These data from a large MI community cohort indicate that AF and MI often coexist and that AF frequently develops in the first month after MI. AF is associated with a large excess risk of death after MI, which varies markedly according to its timing with the highest risk of death being noted for AF occurring more than 30 days after the incident MI. This underscores the importance of long-term follow-up after MI.
Acknowledgments
Sources of Funding
Supported in part by grants from the Public Health Service and the National Institutes of Health (RO1 HL 59205) and the National Institute on Aging (AG034676). Dr Jabre is supported by INSERM, U970, Paris-Descartes University, France, and the French Emergency Physician Society (SFMU).
Footnotes
Disclosures
None.
References
- 1.Schmitt J, Duray G, Gersh BJ, Hohnloser SH. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J. 2009;30:1038–1045. doi: 10.1093/eurheartj/ehn579. [DOI] [PubMed] [Google Scholar]
- 2.Crenshaw BS, Ward SR, Granger CB, Stebbins AL, Topol EJ, Califf RM. Atrial fibrillation in the setting of acute myocardial infarction: the GUSTO-I experience. Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries. J Am Coll Cardiol. 1997;30:406–413. doi: 10.1016/s0735-1097(97)00194-0. [DOI] [PubMed] [Google Scholar]
- 3.Kober L, Swedberg K, McMurray JJ, Pfeffer MA, Velazquez EJ, Diaz R, Maggioni AP, Mareev V, Opolski G, Van de Werf F, Zannad F, Ertl G, Solomon SD, Zelenkofske S, Rouleau JL, Leimberger JD, Califf RM. Previously known and newly diagnosed atrial fibrillation: a major risk indicator after a myocardial infarction complicated by heart failure or left ventricular dysfunction. Eur J Heart Fail. 2006;8:591–598. doi: 10.1016/j.ejheart.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Lopes RD, Pieper KS, Horton JR, Al-Khatib SM, Newby LK, Mehta RH, Van de Werf F, Armstrong PW, Mahaffey KW, Harrington RA, Ohman EM, White HD, Wallentin L, Granger CB. Short- and long-term outcomes following atrial fibrillation in patients with acute coronary syndromes with or without ST-segment elevation. Heart. 2008;94:867–873. doi: 10.1136/hrt.2007.134486. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen OD, Bagger H, Kober L, Torp-Pedersen C. The occurrence and prognostic significance of atrial fibrillation/-flutter following acute myocardial infarction. TRACE Study group. TRAndolapril Cardiac Evalution. Eur Heart J. 1999;20:748–754. doi: 10.1053/euhj.1998.1352. [DOI] [PubMed] [Google Scholar]
- 6.Pizzetti F, Turazza FM, Franzosi MG, Barlera S, Ledda A, Maggioni AP, Santoro L, Tognoni G. Incidence and prognostic significance of atrial fibrillation in acute myocardial infarction: the GISSI-3 data. Heart. 2001;86:527–532. doi: 10.1136/heart.86.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rathore SS, Berger AK, Weinfurt KP, Schulman KA, Oetgen WJ, Gersh BJ, Solomon AJ. Acute myocardial infarction complicated by atrial fibrillation in the elderly: prevalence and outcomes. Circulation. 2000;101:969–974. doi: 10.1161/01.cir.101.9.969. [DOI] [PubMed] [Google Scholar]
- 8.Wong CK, White HD, Wilcox RG, Criger DA, Califf RM, Topol EJ, Ohman EM. New atrial fibrillation after acute myocardial infarction independently predicts death: the GUSTO-III experience. Am Heart J. 2000;140:878–885. doi: 10.1067/mhj.2000.111108. [DOI] [PubMed] [Google Scholar]
- 9.Asanin M, Perunicic J, Mrdovic I, Matic M, Vujisic-Tesic B, Arandjelovic A, Vasiljevic Z, Ostojic M. Prognostic significance of new atrial fibrillation and its relation to heart failure following acute myocardial infarction. Eur J Heart Fail. 2005;7:671–676. doi: 10.1016/j.ejheart.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Behar S, Zahavi Z, Goldbourt U, Reicher-Reiss H. Long-term prognosis of patients with paroxysmal atrial fibrillation complicating acute myocardial infarction. SPRINT Study Group. Eur Heart J. 1992;13:45–50. doi: 10.1093/oxfordjournals.eurheartj.a060046. [DOI] [PubMed] [Google Scholar]
- 11.Eldar M, Canetti M, Rotstein Z, Boyko V, Gottlieb S, Kaplinsky E, Behar S. Significance of paroxysmal atrial fibrillation complicating acute myocardial infarction in the thrombolytic era. SPRINT and Thrombolytic Survey Groups. Circulation. 1998;97:965–970. doi: 10.1161/01.cir.97.10.965. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg RJ, Yarzebski J, Lessard D, Wu J, Gore JM. Recent trends in the incidence rates of and death rates from atrial fibrillation complicating initial acute myocardial infarction: a community-wide perspective. Am Heart J. 2002;143:519–527. doi: 10.1067/mhj.2002.120410. [DOI] [PubMed] [Google Scholar]
- 13.Kinjo K, Sato H, Sato H, Ohnishi Y, Hishida E, Nakatani D, Mizuno H, Fukunami M, Koretsune Y, Takeda H, Hori M. Prognostic significance of atrial fibrillation/atrial flutter in patients with acute myocardial infarction treated with percutaneous coronary intervention. Am J Cardiol. 2003;92:1150–1154. doi: 10.1016/j.amjcard.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Steg PG, Lopez-Sendon J, Lopez de Sa E, Goodman SG, Gore JM, Anderson FA, Jr, Himbert D, Allegrone J, Van de Werf F. External validity of clinical trials in acute myocardial infarction. Arch Intern Med. 2007;167:68–73. doi: 10.1001/archinte.167.1.68. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg RJ, Seeley D, Becker RC, Brady P, Chen ZY, Osganian V, Gore JM, Alpert JS, Dalen JE. Impact of atrial fibrillation on the in-hospital and long-term survival of patients with acute myocardial infarction: a community-wide perspective. Am Heart J. 1990;119:996–1001. doi: 10.1016/s0002-8703(05)80227-3. [DOI] [PubMed] [Google Scholar]
- 16.Saczynski JS, McManus D, Zhou Z, Spencer F, Yarzebski J, Lessard D, Gore JM, Goldberg RJ. Trends in atrial fibrillation complicating acute myocardial infarction. Am J Cardiol. 2009;104:169–174. doi: 10.1016/j.amjcard.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roger VL, Killian JM, Weston SA, Jaffe AS, Kors J, Santrach PJ, Tunstall-Pedoe H, Jacobsen SJ. Redefinition of myocardial infarction: prospective evaluation in the community. Circulation. 2006;114:790–797. doi: 10.1161/CIRCULATIONAHA.106.627505. [DOI] [PubMed] [Google Scholar]
- 18.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 19. [Accessed December 15, 2010.];2000 Census. 2000 http://factfinder.census.gov.
- 20.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 21.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 22.Prineas R, Crow R, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings. Littleton, Massachusetts: John Wright-PSG, Inc; 1982. [Google Scholar]
- 23.Roger VL, Jacobsen SJ, Weston SA, Goraya TY, Killian J, Reeder GS, Kottke TE, Yawn BP, Frye RL. Trends in the incidence and survival of patients with hospitalized myocardial infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med. 2002;136:341–348. doi: 10.7326/0003-4819-136-5-200203050-00005. [DOI] [PubMed] [Google Scholar]
- 24.Roger VL, Weston SA, Gerber Y, Killian JM, Dunlay SM, Jaffe AS, Bell MR, Kors J, Yawn BP, Jacobsen SJ. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121:863–869. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 26.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22:6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 27.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 28.Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death: Based on the Recommendations of the Ninth Revision Conference. Geneva, Switzerland: World Health Organization; 1975. [Google Scholar]
- 29.Hammill SC, Andrist EM, Thorkelson LA. Electrocardiogram interpreting technician: training and role in a contemporary electrocardiogram practice. J Electrocardiol. 2008;41:442–443. doi: 10.1016/j.jelectrocard.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 31.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 32.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 33.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 34.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau DH, Huynh LT, Chew DP, Astley CM, Soman A, Sanders P. Prognostic impact of types of atrial fibrillation in acute coronary syndromes. Am J Cardiol. 2009;104:1317–1323. doi: 10.1016/j.amjcard.2009.06.055. [DOI] [PubMed] [Google Scholar]
- 36.Sugiura T, Iwasaka T, Takahashi N, Nakamura S, Taniguchi H, Nagahama Y, Matsutani M, Inada M. Atrial fibrillation in inferior wall Q-wave acute myocardial infarction. Am J Cardiol. 1991;67:1135–1136. doi: 10.1016/0002-9149(91)90879-p. [DOI] [PubMed] [Google Scholar]
- 37.Berton G, Cordiano R, Cucchini F, Cavuto F, Pellegrinet M, Palatini P. Atrial fibrillation during acute myocardial infarction: association with all-cause mortality and sudden death after 7-year of follow-up. Int J Clin Pract. 2009;63:712–721. doi: 10.1111/j.1742-1241.2009.02023.x. [DOI] [PubMed] [Google Scholar]
- 38.Madias JE, Patel DC, Singh D. Atrial fibrillation in acute myocardial infarction: a prospective study based on data from a consecutive series of patients admitted to the coronary care unit. Clin Cardiol. 1996;19:180–186. doi: 10.1002/clc.4960190309. [DOI] [PubMed] [Google Scholar]
- 39.Sakata K, Kurihara H, Iwamori K, Maki A, Yoshino H, Yanagisawa A, Ishikawa K. Clinical and prognostic significance of atrial fibrillation in acute myocardial infarction. Am J Cardiol. 1997;80:1522–1527. doi: 10.1016/s0002-9149(97)00746-7. [DOI] [PubMed] [Google Scholar]
- 40.Serrano CV, Jr, Ramires JA, Mansur AP, Pileggi F. Importance of the time of onset of supraventricular tachyarrhythmias on prognosis of patients with acute myocardial infarction. Clin Cardiol. 1995;18:84–90. doi: 10.1002/clc.4960180210. [DOI] [PubMed] [Google Scholar]
- 41.Lehto M, Snapinn S, Dickstein K, Swedberg K, Nieminen MS. Prognostic risk of atrial fibrillation in acute myocardial infarction complicated by left ventricular dysfunction: the OPTIMAAL experience. Eur Heart J. 2005;26:350–356. doi: 10.1093/eurheartj/ehi064. [DOI] [PubMed] [Google Scholar]
- 42.Hod H, Lew AS, Keltai M, Cercek B, Geft IL, Shah PK, Ganz W. Early atrial fibrillation during evolving myocardial infarction: a consequence of impaired left atrial perfusion. Circulation. 1987;75:146–150. doi: 10.1161/01.cir.75.1.146. [DOI] [PubMed] [Google Scholar]
- 43.Kyriakidis M, Barbetseas J, Antonopoulos A, Skouros C, Tentolouris C, Toutouzas P. Early atrial arrhythmias in acute myocardial infarction. Role of the sinus node artery. Chest. 1992;101:944–947. doi: 10.1378/chest.101.4.944. [DOI] [PubMed] [Google Scholar]
- 44.Nielsen FE, Andersen HH, Gram-Hansen P, Sorensen HT, Klausen IC. The relationship between ECG signs of atrial infarction and the development of supraventricular arrhythmias in patients with acute myocardial infarction. Am Heart J. 1992;123:69–72. doi: 10.1016/0002-8703(92)90748-k. [DOI] [PubMed] [Google Scholar]
- 45.Rechavia E, Strasberg B, Mager A, Zafrir N, Kusniec J, Sagie A, Sclarovsky S. The incidence of atrial arrhythmias during inferior wall myocardial infarction with and without right ventricular involvement. Am Heart J. 1992;124:387–391. doi: 10.1016/0002-8703(92)90602-r. [DOI] [PubMed] [Google Scholar]
- 46.Simpson CS, Mitchell LB, Klein GJ. Similarities and differences between atrial flutter and atrial fibrillation. Can J Cardiol. 2005;21 (Suppl B):67B–70B. [PubMed] [Google Scholar]
- 47.Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter mechanisms and clinical implications. J Am Coll Cardiol. 2008;51:779–786. doi: 10.1016/j.jacc.2007.08.066. [DOI] [PubMed] [Google Scholar]
- 48.Gerber Y, Jacobsen SJ, Frye RL, Weston SA, Killian JM, Roger VL. Secular trends in deaths from cardiovascular diseases: a 25-year community study. Circulation. 2006;113:2285–2292. doi: 10.1161/CIRCULATIONAHA.105.590463. [DOI] [PubMed] [Google Scholar]


