Abstract
The diurnally active fruit flies prefer a major meal in the morning. Feeding the flies in the evening uncouples their metabolic cycle from circadian activity rhythms. A paper by Xu et al. in this issue of Cell Metabolism found that such uncoupled rhythms reduce egg laying.
Organisms have evolved intrinsic time-keeping mechanisms of ~24 h periodicity to anticipate and respond to the environmental changes associated with the earth’s day/night cycle. As an evolutionary trait, the circadian regulation of physiology must have improved metabolism and reproduction. Several studies have illustrated how circadian rhythms optimize metabolism. A study by Xu et al. (Xu, 2011) in this issue of Cell Metabolism elucidates how rhythmic gene expression in the Drosophila fat body imparts reproductive fitness.
The ability to align systemic processes with nutrient availability and day length improves fitness and fecundity in many species. In cyanobacteria, synchrony between the photoperiod and periodicity of the endogenous rhythms improves fitness (Ouyang et al., 1998). In plants, the circadian clock regulates the transition from the vegetative to the reproductive phase and thereby determines the flowering season (Sawa et al., 2007). As in plants, the circadian clock in insect populations native to different latitudes shows features that likely improve adaptation towards local climates (Pittendrigh et al., 1991). However, the regulatory complexity of organ systems in animals has precluded studies of whether and how circadian clocks impact broad physiological outcomes.
Circadian clocks in animals are cell-autonomous, hierarchical and adjusted by light and food. The clock residing in the central nervous system together with the light/dark cycle generates diurnal rhythms in activity and feeding. The feeding/fasting cycle interacts with peripheral organ oscillators. Such interactions among the circadian clock, the photoperiod and feeding time generate tissue-specific gene expression rhythms. These tissue-specific clocks oscillate with a defined phase relationship relative to each other and establish temporal relationships in organ function. Several aspects of physiology (such as reproduction) result from time-dependent interactions between different organs. It is expected that optimal interactions among tissue-specific oscillators may improve system-level functions.
Like humans, diurnal rhythms in Drosophila activity are driven by clock neurons in the brain. The fat body in flies is akin to the liver in mammals, harbors a circadian clock, shows temporal rhythms for dozens of transcripts and plays an important role in energy metabolism (Xu, 2011). Mutant flies with a ubiquitously-disrupted circadian clock lack rhythmic fat body transcripts. However, in flies with fat body specific clock disruption, the authors noted that a majority of the transcripts that normally cycle in the fat body either dampen or lose oscillations. To test whether the residual transcriptional rhythms in the fat body of these flies are driven by rhythmic feeding, the authors fed clock-deficient flies for only a few hours daily. As in the liver of circadian clock-deficient mice (Vollmers et al., 2009), periodic feeding drove rhythmic expression of several fat body transcripts. This dominant effect of feeding on fat body transcriptional rhythms prompted the authors to test the consequences of altered feeding time on tissue-specific rhythms in normal flies. They allowed adult flies to eat either around their preferred feeding time of dawn, or at dusk. Notably, while the daily food intake was comparable, rhythmic transcripts in the fat body of flies fed at dusk peaked several hours later than in dawn-fed flies. However, feeding time had no effect on rhythmic transcripts in the brain. Hence, an ”unnatural” feeding paradigm disrupts the synchrony between the brain and fat body oscillators.
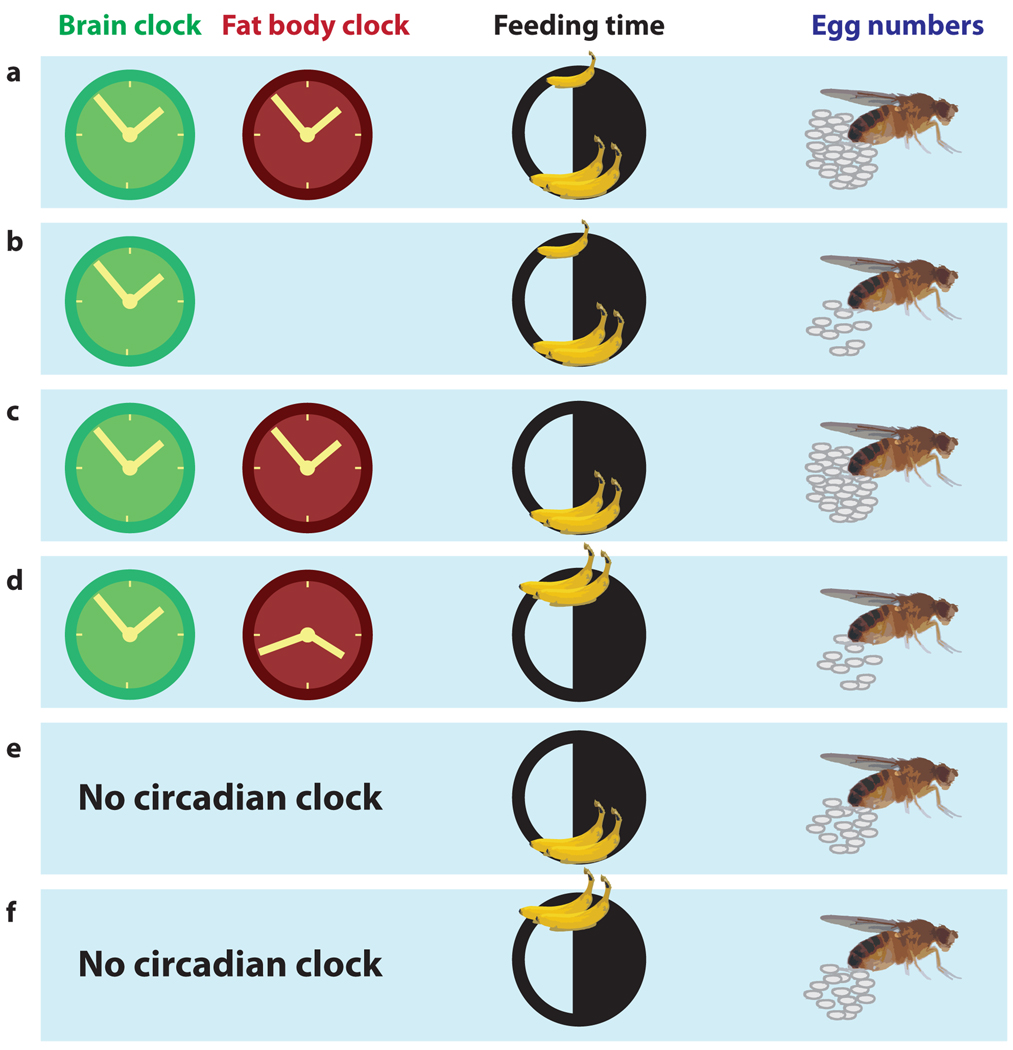
Gene ontology analysis of oscillating transcripts in the fat body revealed that they are involved in energy metabolism, pheromone production and reproduction. In Drosophila, reproduction is an energy intensive process, and is also rhythmic. A mated female lays ~100 eggs daily with peak egg-laying in the evening. As in mammals, Drosophila reproduction is a product of signaling from the brain and metabolic flux from peripheral organs. So, the authors hypothesized that suboptimal energy metabolism and desynchronized clocks might reduce fecundity as reflected in daily egg laying (Figure 1). Indeed, flies that lack a circadian clock everywhere or in the fat body alone laid fewer eggs, and the egg-laying rhythm was dampened indicating that the circadian clock improves fecundity. In addition, when flies with a functional circadian clock were fed at dusk, they also laid fewer eggs than flies fed at dawn. Importantly, the effect of feeding time on egg-laying requires a circadian clock because egg-laying in circadian mutant flies is unaltered by changing the feeding schedule.
Figure 1. Tissue-specific circadian clocks and feeding time impact reproductive output in Drosophila.
This figure summarizes the findings by Xu et al (Xu, 2011). (a) A wild-type female Drosophila that feeds ad libitum (a large meal in the morning and a smaller one later) has synchronized brain-specific and fat body-specific clocks and lays a certain number of eggs/day. (b) A genetic disruption of the fat body-specific clock results in fewer eggs. (c) Restriction of food availability to the preferred feeding time of dawn does not impact the number of eggs. (d) If the same amount of food is ingested when the fly usually feeds very little, the brain and fat body clocks become desynchronized and egg production is reduced. (e) Genetic disruption of the circadian clock in the entire organism reduces the number of eggs laid but is not impacted by (f) feeding times.
Collectively, the authors’ findings reveal that feeding is a critical input to the circadian clock in the Drosophila fat body and that optimum interaction between the fat body clock and the time of feeding improves female reproductive fitness. The underlying mechanisms are likely complex and may involve multiple tissues. The authors have alluded to the possibility that fat body-derived lipids and other metabolites important for ova formation are under circadian regulation and may play a role in this phenomenon. Remarkably, if male flies fed at the “wrong” time were mated to ad libitum fed females, female fecundity decreased. This suggests the effect might even lie in pheromone production, male behavior or gender interactions. The microarray analysis identified candidate rhythmic transcripts whose protein products can regulate the temporal flux of nutrients or hormones. Follow-up studies might dwell on examining the effects of manipulating the level and/or time of the expression of these transcripts on fecundity.
The contribution of transcriptional rhythms driven by the circadian clock and feeding time to fertility in farm animals, endangered species in captivity and humans may be more important than has been previously realized. Reproduction in vertebrates involves temporally orchestrated signaling in the hypothalamus-pituitary-gonadal (HPG) axis. The function of this axis is regulated by the circadian oscillator (Sellix and Menaker, 2010). ClockΔ19 mutant female mice with dampened circadian rhythms show defects in reproductive fitness (Miller et al., 2004), thus demonstrating an important role of circadian rhythms in vertebrate reproduction. Studies such as Xu et al. now prompt us to test whether feeding time affects metabolism or signaling in the HPG axis. Chronic abnormal feeding schedules might desynchronize rhythmic metabolism or temporal aspects of signaling in the HPG axis and adversely affect reproduction. This may explain the fertility decline in industrialized countries (World Fertility Patterns, 2010) where the population experiences prolonged nighttime activity and eating.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol. 2004;14:1367–1373. doi: 10.1016/j.cub.2004.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations. World Fertility Patterns 2009. United Nations Publications; 2010. [Google Scholar]
- Ouyang Y, Andersson CR, Kondo T, Golden SS, Johnson CH. Resonating circadian clocks enhance fitness in cyanobacteria. Proc Natl Acad Sci U S A. 1998;95:8660–8664. doi: 10.1073/pnas.95.15.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS, Kyner WT, Takamura T. The amplitude of circadian oscillations: temperature dependence, latitudinal clines, and the photoperiodic time measurement. J Biol Rhythms. 1991;6:299–313. doi: 10.1177/074873049100600402. [DOI] [PubMed] [Google Scholar]
- Sawa M, Nusinow DA, Kay SA, Imaizumi T. FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science. 2007;318:261–265. doi: 10.1126/science.1146994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellix MT, Menaker M. Circadian clocks in the ovary. Trends Endocrinol Metab. 2010;21:628–636. doi: 10.1016/j.tem.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci U S A. 2009;106:21453–21458. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, DiAngelo JR, Hughes ME, Hogenesch JB, Sehgal A. Interaction between circadian clocks and metabolic physiology: implications for reproductive fitness. Cell metabolism. 2011 doi: 10.1016/j.cmet.2011.05.001. 00, 00. [DOI] [PMC free article] [PubMed] [Google Scholar]