Abstract
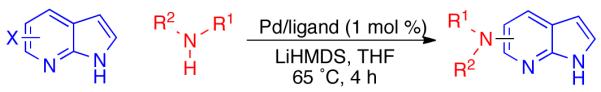
Simple and efficient procedures for the Pd-catalyzed cross-coupling of primary and secondary amines with halo-7-azaindoles (pyrrolo[2,3-b]pyridine) are presented. Previously, no general method was available to ensure the highly selective reaction of the heteroaryl halide in the presence of the unprotected azaindole N–H. Using palladium precatalysts recently reported by our group, such reactions are easily accomplished under mild conditions that can be applied to cross-coupling reactions with a wide array of aliphatic and aromatic amines.
Azaindoles have been receiving increased attention from the pharmaceutical and agrochemical industries over the last decade, due both to their potential as indole-isosteres and as interesting core structures in their own right.1,2 The substitution of a carbon by a nitrogen not only confers altered electronic properties but also adds a potential hydrogen bond acceptor, which can form the basis for altering the physicochemical or biological properties of a compound.3 The close proximity of hydrogen bond donor and acceptor sites also distinguishes azaindoles as important substructures in dyes, ligands for transition metals, and novel materials.2,4
Despite their utility, methods for the synthesis and functionalization of azaindole scaffolds remain limited. The majority of methods provide N–1, C–2 or C–3 substituted structures, with few offering general solutions to functionalization of the pyridine ring.2,5-7 A particularly interesting subset of these molecules are amino-substituted azaindoles, which appear in a variety of biologically active molecules (Figure 1)8-12 and can be particularly challenging or lengthy to prepare via reported methods. Amino-7-azaindoles are often accessed from the corresponding halide via SNAr displacement reactions, which typically require high temperatures, extended reaction times, and a large excess of the amine partner.13 Furthermore, 5-haloazaindoles are not suitable substrates for SNAr, nor are some amines. Alternative approaches employ the amino-substituted azaindole as the key intermediate, which can be challenging to prepare.14
Figure 1.
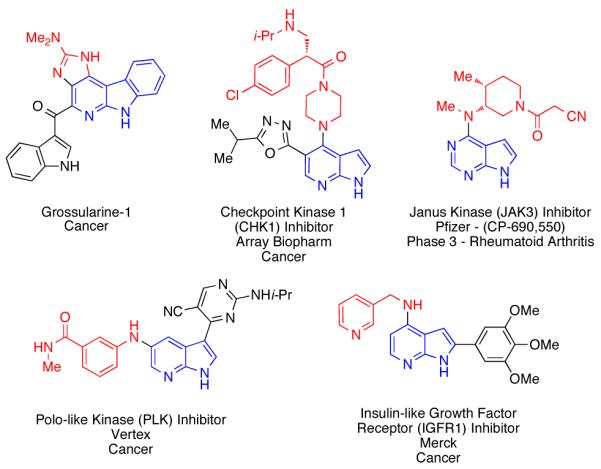
Pharmacologically active compounds containing the amino-azaindole motif.
Our approach to the synthesis of amino-azaindoles is to use Pd-mediated cross-coupling technology15-18 for the reaction of halo-azaindoles with amines. Although azaindoles have previously been reported to undergo arylation of the heterocyclic N–H nitrogen in the presence of copper or palladium catalysts,19,20 the cross-coupling of halo-azaindoles has received little attention.21-23 Haloazaindoles present a particular challenge for two reasons: the donor-acceptor system of the two adjacent nitrogens forms an excellent chelating ligand for metals, and there is the potential for homo-coupling between the halide and azaindole N-H.
Our recently reported precatalyst systems (Figure 2, P1 – P5),24,25 which are efficiently converted to an active monoligated Pd(0) complex when exposed to base, provided a potentially attractive solution to the first problem. We speculated that the pre-ligated palladium species would prove more resistant to undesirable azaindole coordination. To address the second issue of azaindole homo-coupling, we hypothesized that the use of a strong base such as LiHMDS, which would fully deprotonate both the amine and azaindole substrates, might reduce the rate of undesired transmetallation to Pd.26 Previous studies in our group support this hypothesis, having demonstrated that the cross-coupling of unprotected halo-indoles and simple amines could be achieved using LiHMDS as the base, in conjunction with with either an XPhos- (L3) or DavePhos- (L6) based catalyst system.27
Figure 2.
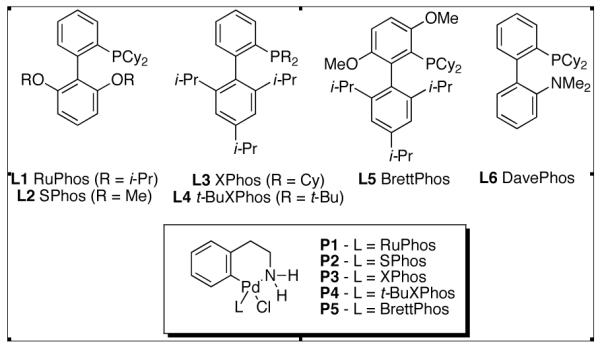
Biarylphosphine Ligands and Precatalysts
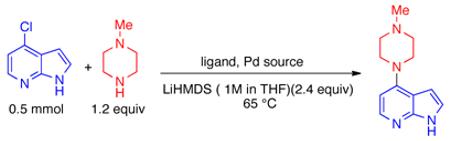
An examination of Pd precatalysts based on our biaryl phosphine ligands revealed that cross-coupling between 4-chloroazaindole and N-methylpiperazine occurred rapidly at catalyst loadings as low as 0.5 mol % (Table 1). A combination of RuPhos (L1) and RuPhos precatalyst (P1) proved to be the most effective system for this transformation, providing the product in 94% yield after 30 minutes (Table 1, entry 1), although SPhos (L2) and XPhos (L3) were also effective (Table 1, entries 2 and 3). Interestingly, Pd(OAc)2 can also be employed in lieu of a Pd precatalyst (Table 1, entry 5). However, the rate of formation of Pd(0) from phosphine and Pd(OAc)2 is highly amine dependent, thus precatalyst P1 was chosen for subsequent studies.28
Table 1.
Catalyst Systems used for the Coupling of 4-Chloroazaindole and N–Methylpiperazine
| entry | Pd source (mol %) |
ligand (mol %) |
time | yield |
|---|---|---|---|---|
| 1 | P1 (0.5) | L1 RuPhos (0.5) | 30 min | 94% |
| 2 | P2 (0.5) | L2 SPhos (0.5) | 30 min | 81% |
| 3 | P3 (0.5) | L3 XPhos (0.5) | 30 min | 90% |
| 4 | P4 (0.5) | L4 t-BuXPhos (0.5) | 30 min | 0% |
| 5 | Pd(OAc)2 (0.5) | L1 RuPhos (1) | 30 min | 80% |
| 6 | Pd2dba3 (0.25) | L1 RuPhos (1) | 30 min | 33% |
| 7 | Pd(OAc)2 (1) | Rac-BINAP (2) | 4 h | 0% |
| 8 | Pd(OAc)2 (1) | Xantphos (2) | 4 h | 0% |
Conditions: 4-chloroazaindole (0.5 mmol), N-methylpiperazine (0.6 mmol), LiHMDS (1.2 mmol, 1 M in THF).
Alternative base and solvent combinations were also examined (Table 2), as there have been isolated reports of employing NaOt-Bu or Cs2CO3 as base for the cross-coupling reaction of unprotected halo-azaindoles.21-23 We found that procedures using these bases were ineffective with our catalyst systems (Table 2, entries 1-5), with LiHMDS in THF proving optimal in terms of reaction time and temperature. Interestingly, the cross-coupling also proceeded at room temperature (Table 2, entry 9) although longer reaction times and higher catalyst loadings were required for complete conversion. Milder temperatures could be more convenient for parallel reactions, or for those involving sensitive substrates.
Table 2.
Examination of Base and Solvent Combinations for the Cross-Coupling of 4-Chloroazaindole.
| entry | base | solvent | temp (°C) | time | yield |
|---|---|---|---|---|---|
| 1 | Cs2CO3 | Toluene | 110 | 16 h | 0% |
| 2 | NaOt-Bu | Toluene | 110 | 16 h | 7% |
| 3 | K3PO4 | Toluene | 110 | 16 h | 0% |
| 4 | K2CO3 | Toluene | 110 | 16 h | 0% |
| 5 | K2CO3 | tBuOH | 110 | 16 h | 0% |
| 6 | LiHMDS | THF | 65 | 30 min | 94% a |
| 7 | LiHMDS | Dioxane | 80 | 4 h | 91%a |
| 8 | LiHMDS | Toluene | 80 | 4 h | 68% a |
| 9 | LiHMDS | THF | rt | 16 h | 42% a |
Conditions: 4-chloroazaindole (0.5 mmol), N-methylpiperazine (0.6 mmol), L1 (1 mol %), P1 (1 mol %), base (1.2 mmol), solvent (1 mL).
L1 (0.5 mol %), P1 (0.5 mol %).
We next applied this Pd-based cross-coupling process to a range of both aliphatic and aromatic secondary amines with 4-chloroazaindole. High yields and selectivities were observed in all cases examined (Scheme 1).29 By the addition of an extra equivalent of LiHMDS, amines containing a second protic functional group could be employed, allowing access to products containing phenols (1b), aliphatic alcohols (1e) or more hindered secondary amines (1c). Not unexpectedly, 4-bromoazaindole displayed reactivity similar to its chloro analog (1b).
Scheme 1.
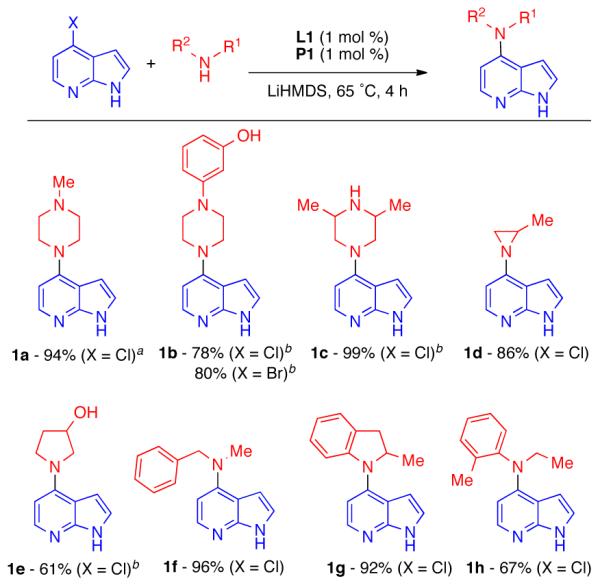
Cross-Couplings of 4-Azaindole with Secondary Amines.
Reaction conditions: ArX (0.5 mmol), amine (0.6 mmol), L1 (1 mol %), P1 (1 mol %), LiHMDS (1.2 mmol, 1 M in THF). Isolated yields are an average of at least two runs. aL1 0.5 mol %, P1 0.5 mol %, 30 min b3.6 equiv LiHMDS.
Without any alteration of the reaction conditions, we also found that 5-bromoazaindole (2a - 2f), 6-chloroazaindole (2f) and the related 4-chloro-7H-pyrrolo[2,3-d]pyrimidine (2g) were effective coupling partners with a similarly wide range of secondary amines (Scheme 2). 5-Bromo-3-chloro-7-azaindole (2h) underwent reaction exclusively at the 5-position, with no diamination or coupling of the chloride observed.30
Scheme 2.
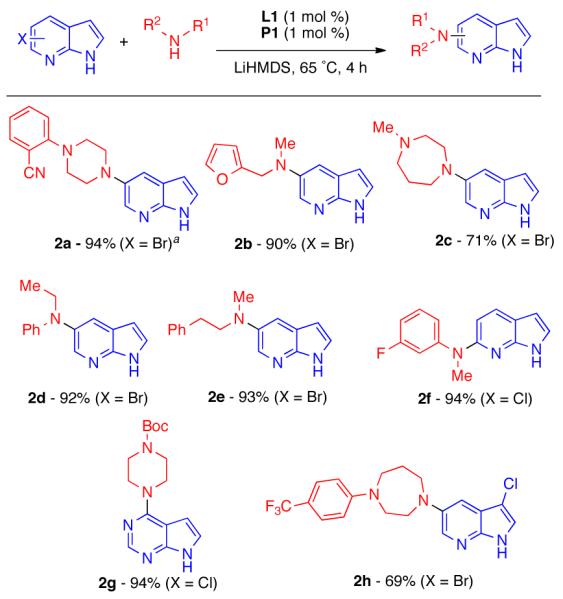
Cross-Coupling of 5- and 6-Haloazaindoles.
Reaction conditions: ArX (0.5 mmol), amine (0.6 mmol), L1 (1 mol %), P1 (1 mol %), LiHMDS (1.2 mmol, 1 M in THF). Isolated yields are an average of at least two runs. a3.6 equiv LiHMDS. bL1 (2 mol %), P1 (2 mol %).
Finally, we explored the cross-coupling of haloazaindoles with primary amines (Scheme 3). We have previously reported that catalyst systems based on BrettPhos (L5) are superior to other biarylphosphines for the cross-coupling of primary amines, and provide exceptional selectivity for mono-arylation.25 Using this system (P5), haloazaindoles underwent efficient cross-coupling with aliphatic, aromatic, and heteroaromatic amines without any other modification of the reaction parameters. While again, in some cases, Pd(OAc)2 can be used as the Pd source, the importance of using the precatalyst (P5) is highlighted in example 3a. In cases where the reacting amine lacks a β-hydrogen atom, Pd(OAc)2 cannot be reduced, and thus no cross-coupling occurs. We also found that the addition of extra ligand is not always necessary. For example, 3a was isolated in a yield of 88% in both the presence or absence of an extra equivalent of BrettPhos (L5).
Scheme 3.
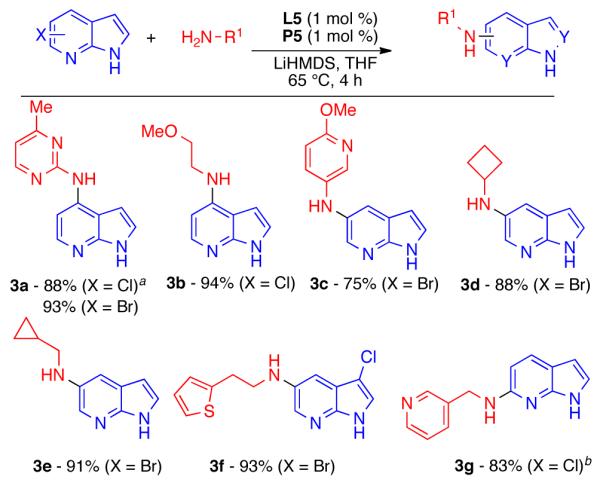
Cross-Coupling using Primary Amines
Reaction Conditions: ArX (0.5 mmol), amine (0.6 mmol), L5 (1 mol %), P5 (1 mol %), LiHMDS (1.2 mmol, 1 M in THF). Isolated yields are an average of at least two runs. aNo reaction when Pd(OAc)2 (1 mol %) used in place of P5. b Amine purified before use.
In summary, we have developed operationally simple and efficient methods for the cross-coupling of unprotected halo-7-azaindoles with a wide range of primary and secondary amines. Notably this reaction can be performed in the presence of a variety of protic functional groups. The success of these reactions further demonstrates the advantages of conducting Pd-catalyzed cross-coupling reactions using metallacyclic precatalysts such as P1 and P5, as these precatalysts permit rapid Pd activation in the presence of a wide range of substrates.
Supplementary Material
Acknowledgment
This work is supported by an educational donation provided by Amgen and from the National Institutes of Health Grant (GM-58160) to whom we are grateful.
Footnotes
Supporting Information Available Experimental procedures and spectral data for all products.
References
- (1).Lednicer D. Strategies for Organic Drug Synthesis and Design. 2 ed Hoboken; Wiley: 2009. [Google Scholar]
- (2).Popowycz F, Routier S, Joseph B, Merour JY. Tetrahedron. 2007;63:1031–1064. [Google Scholar]
- (3).Kulagowski JJ, Broughton HB, Curtis NR, Mawer IM, Ridgill MP, Baker R, Emms F, Freedman SB, Marwood R, Patel S, Ragan CI, Leeson PD. J. Med. Chem. 1996;39:1941–1942. doi: 10.1021/jm9600712. [DOI] [PubMed] [Google Scholar]
- (4).Hudson ZM, Wang S. Acc. Chem. Res. 2009;42:1584–1596. doi: 10.1021/ar900072u. [DOI] [PubMed] [Google Scholar]
- (5).Merour JY, Joseph B. Curr. Org. Chem. 2001;5:471–506. [Google Scholar]
- (6).Song JJ, Reeves JT, Gallou F, Tan ZL, Yee NK, Senanayake CH. Chem. Soc. Rev. 2007;36:1120–1132. doi: 10.1039/b607868k. [DOI] [PubMed] [Google Scholar]
- (7).Prokopov AA, Yakhontov LN. Pharmaceutical Chemistry Journal. 1994;28:30–51. [Google Scholar]
- (8).Blake J, Gunawardana IW, Le HY, Mohr PJ, Wallace EM, Wang B. Pyrrolo[2,3-b]pyridines as CHK1 and CHK2 kinase inhibitors for the treatment of various diseases and preparation thereof. 2009. WO 2009089352.
- (9).Mortimore M, Young SC, Everitt SRL, Knegtel R, Pinder JL, Rutherford AP, Durrant S, Brenchley G, Charrier JD, O’Donnell M. Preparation of 5-cyano-4-(pyrrolo[2,3-b]pyridin-3-yl)pyrimidines as polo-like kinase (PLK) inhibitors. 2008 WO2008/79346. [Google Scholar]
- (10).Ruggeri SG, Hawkins JM, Makowski TM, Rutherford JL, Urban FJ. Process for preparation of piperidinylaminopyrrolopyrimidines from activated pyrrolopyrimidines and piperidinylamines. 2007 WO 2007012953 A2. [Google Scholar]
- (11).Heinrich T, Blaukat A, Staehle W, Greiner H, Kordowicz M. Novel aza heterocycles serving as kinase inhibitors. 2006 WO 2006114180. [Google Scholar]
- (12).Choshi T, Yamada S, Sugino E, Kuwada T, Hibino S. J. Org. Chem. 1995;60:5899–5904. [Google Scholar]
- (13).Caldwell JJ, Cheung KM, Collins I. Tetrahedron Lett. 2007;48:1527–1529. [Google Scholar]
- (14).Schneller SW, Luo JK. J. Org. Chem. 1980;45:4045–4048. [Google Scholar]
- (15).Buchwald SL, Mauger C, Mignani G, Scholz U. Adv. Synth. Catal. 2006;348:23–39. [Google Scholar]
- (16).Tasler S, Mies J, Langa M. Adv. Synth. Catal. 2007;349:2286–2300. [Google Scholar]
- (17).Torborg C, Beller M. Adv. Synth. Catal. 2009;351:3027–3043. [Google Scholar]
- (18).Carey JS, Laffan D, Thomson C, Williams MT. Organic & Biomolecular Chemistry. 2006;4:2337–2347. doi: 10.1039/b602413k. [DOI] [PubMed] [Google Scholar]
- (19).Antilla JC, Klapars A, Buchwald SL. J. Am. Chem. Soc. 2002;124:11684–11688. doi: 10.1021/ja027433h. [DOI] [PubMed] [Google Scholar]
- (20).Old DW, Harris MC, Buchwald SL. Org. Lett. 2000;2:1403–1406. doi: 10.1021/ol005728z. [DOI] [PubMed] [Google Scholar]
- (21).Guillard J, Decrop M, Gallay N, Espanel C, Boissier E, Herault O, Viaud-Massuard MC. Bioorg. Med. Chem. Lett. 2007;17:1934–1937. doi: 10.1016/j.bmcl.2007.01.033. [DOI] [PubMed] [Google Scholar]
- (22).Thibault C, L’Heureux A, Bhide RS, Ruel R. Org. Lett. 2003;5:5023–5025. doi: 10.1021/ol036030z. [DOI] [PubMed] [Google Scholar]
- (23).Thutewohl M, Schirok H, Bennabi S, Figueroa-Perez S. Synthesis. 2006:629–632. [Google Scholar]
- (24).Biscoe MR, Fors BP, Buchwald SL. J. Am. Chem. Soc. 2008;130:6686–6687. doi: 10.1021/ja801137k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Fors BP, Watson DA, Biscoe MR, Buchwald SL. J. Am. Chem. Soc. 2008;130:13552–13554. doi: 10.1021/ja8055358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Biscoe MR, Barder TE, Buchwald SL. Angew. Chem., Int. Ed. 2007;46:7232–7235. doi: 10.1002/anie.200702122. [DOI] [PubMed] [Google Scholar]
- (27).Charles MD, Schultz P, Buchwald SL. Org. Lett. 2005;7:3965–3968. doi: 10.1021/ol0514754. [DOI] [PubMed] [Google Scholar]
- (28).Strieter ER, Buchwald SL. Angew. Chem., Int. Ed. 2006;45:925–928. doi: 10.1002/anie.200502927. [DOI] [PubMed] [Google Scholar]
- (29).In no instance was arylation of the azaindole N-H, nor the formation of amino-azaindoles through reaction with LiHMDS observed.
- (30).In the absence of Pd, the halo-azaindoles do not undergo reaction with N-methylpiperazine under the reaction conditions.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


