SUMMARY
The type VI secretion system (T6SS) is a virulence mechanism common to several Gram-negative pathogens. In Vibrio cholerae, VgrG-1 is required for T6SS-dependent secretion. VgrG-1 is also secreted by T6SS and displays a C-terminal actin cross-linking domain (ACD). Using a heterologous reporter enzyme in place of the ACD, we show that the effector and secretion functions of VgrG-1 are genetically dissociable with the ACD being dispensable for secretion, but required for T6SS-dependent phenotypes. Furthermore, internalization of bacteria is required for ACD translocation into phagocytic target cells. Inhibiting bacterial uptake abolishes actin cross-linking while improving intracellular survival enhances it. Otherwise resistant nonphagocytic cells become susceptible to T6SS-mediated actin cross-linking when engineered to take up bacteria. Our results support a model for translocation of VgrG C-terminal effector domains into target cell cytosol by a process that requires trafficking of bacterial cells into an endocytic compartment where translocation is triggered by an unknown signal.
INTRODUCTION
Vibrio cholerae is a Gram-negative pathogen that causes the diarrheal disease cholera. It is a diverse species that includes over 200 serogroups, including O1 and O139 strains that cause epidemic and pandemic disease and non-O1/non-O139 strains that more typically cause sporadic outbreaks of gastroenteritis or extraintestinal infections (Rahman et al., 2008). The diversity displayed by V. cholerae is reflective of the range of environments it inhabits, including aquatic environments with their associated organisms (Abd et al., 2005; Chiavelli et al., 2001; Rawlings et al., 2007) and also the environment within a human host. V. cholerae has acquired a variety of horizontally transferred elements encoding human virulence factors including the CTX phage encoding cholera toxin (Waldor and Mekalanos, 1996) and a chromosomal island encoding a toxin co-regulated pilus and other intestinal colonization factors (Everiss et al., 1994; Taylor et al., 1987). However, virtually all strains of V. cholerae, including both clinical and environmental isolates, encode the genes for well-conserved ‘accessory virulence factors’ whose role in human disease is less clear but which may play essential roles in the environmental fitness of V. cholerae and thus its transmission in natural settings. These include HlyA hemolysin (Manning et al., 1984), HapA haemagglutinin/protease (Wu et al., 1996), and RtxA toxin (Fullner and Mekalanos, 2000). Together, these three virulence factors are thought to contribute to long-term colonization of adult mice (Olivier et al., 2007) but their role in human disease has not been fully evaluated. The type III secretion system islands (Tam et al., 2007) and the recently identified type VI secretion system (T6SS) (Pukatzki et al., 2006) are also virulence factors that are present in clinical and environmental strains of V. cholerae. All these products and gene clusters are associated with toxicity for eukaryotic cells and therefore may play a role in human disease or other pathobiological interactions with environmental organisms.
Genes encoding a putative T6SS are present in nearly 25% of all sequenced Gram-negative bacterial species, and are mainly restricted to pathogens (Bingle et al., 2008). T6SS has been implicated in virulence in V. cholerae (Pukatzki et al., 2006), Pseuodomonas aeruginosa (Mougous et al., 2006), Edwardsiella tarda (Rao et al., 2004; Zheng and Leung, 2007), Burkholderia species (Aubert et al., 2008; Pilatz et al., 2006; Schell et al., 2007) and Aeromonas hydrophila (Suarez et al., 2008) among others. A T6SS is defined by a canonical group of 15–20 genes and secretion via this pathway requires orthologs of clpV, icmF, dotU (Bonemann et al., 2009; Mougous et al., 2006; Pukatzki et al., 2006; Zheng and Leung, 2007), sciN which encodes an outer membrane lipoprotein (Aschtgen et al., 2008), genes that encode VipA/MglA and VipB/MglB orthologs which interact with each other (de Bruin et al., 2007) and with ClpV (Bonemann et al., 2009). Also genetically or functionally associated with T6SS clusters are hcp and vgrG genes. In V. cholerae, Hcp and VgrG proteins are both required for T6SS-dependent secretion and are themselves secreted in a T6SS-dependent fashion (Pukatzki et al., 2006). Thus, deletion of hcp-1 and hcp-2 in V. cholerae results in a secretion defect of VgrG proteins (Pukatzki et al., 2006) and deletion of vgrG-1 or vgrG- 2 results in a secretion defect of Hcp (Pukatzki et al., 2007). This reciprocal requirement for secretion suggests that Hcp and VgrG are secretion substrates that are transported through a putative core T6SS complex, and could also comprise components of an extracellular portion of the T6SS apparatus that can then shear off from bacterial cells.
In vitro secretion of Hcp has been observed in many T6SS-containing bacterial species (Aschtgen et al., 2008; Dudley et al., 2006; Mattinen et al., 2007; Mougous et al., 2006; Pukatzki et al., 2006; Schell et al., 2007; Suarez et al., 2008; Wu et al., 2008; Zheng and Leung, 2007), but secretion of VgrG homologs has been reported for only several of these organisms. EvpI is a VgrG ortholog whose secretion by Edwardsiella tarda requires many of the proteins found in its T6SS locus and secretion of Hcp and VgrG is also mutually dependent in E. tarda (Zheng and Leung, 2007). VgrG ortholog ECA3427 is secreted by Pectobacterium atrosepticum (Mattinen et al., 2007) and expression of five VgrG orthologs and various other T6SS components is regulated by quorum sensing in planta or exposure to host extracts (Liu et al., 2008; Mattinen et al., 2007; Mattinen et al., 2008).
VgrG-1 is required for T6SS-dependent cytotoxic effects of V. cholerae on eukaryotic cells including Dictyostelium discoideum amoebae and J774 macrophages (Pukatzki et al., 2007; Pukatzki et al., 2006). The ACD at the C-terminus of VgrG-1 is closely homologous to the ACD domain present within a secreted toxin of V. cholerae called RtxA (Fullner and Mekalanos, 2000; Sheahan et al., 2004), which inhibits actin polymerization by catalyzing intramolecular isopeptide bond formation between E270 and K50 residues of monomeric actin (Kudryashov et al., 2008). The appearance of covalently cross-linked actin in J774 cells incubated with T6SS+ RtxA- but not with T6SS- RtxA- V. cholerae provides strong evidence that the ACD of VgrG-1 can enter target cells by a T6SS-dependent process. Concentrated supernatants containing T6SS secreted substrates and purified, enzymatically active VgrG-1, do not cross-link actin in host cells, suggesting that contact between V. cholerae and the target cell is required for this translocation mechanism (Pukatzki et al., 2007).
Bioinformatic analysis predicts that VgrGs are homologs of the bacteriophage T4 cell-puncturing device called the ‘tail spike’ (Pukatzki et al., 2007). This complex is used by T4 bacteriophage to puncture the bacterial cell envelope during infection and is composed of a (gp5)3-(gp27)3 complex that adopts a needle shape with a central channel (Kanamaru et al., 2002). The N-terminal region of a VgrG ortholog from uropathogenic E. coli has since been crystallized and corresponds to gp27 (Leiman et al., 2009). VgrG proteins are fusion proteins between gp27- and gp5- like domains and are predicted to form a similar trimeric structure. Consistent with this model, interactions between VgrG- 1, −2 and −3 have been demonstrated in supernatant fluids of V. cholerae V52 (Pukatzki et al., 2007). Many VgrG proteins in other T6SS+ bacterial species contain various C-terminal domains that correspond to the position of the ACD of VgrG-1, but translocation of these putative effector domains and their cognate VgrG proteins has not been reported.
Here we report that the effector and secretion functions of VgrG-1 can be genetically dissociated. We find that the ACD is necessary for T6SS-dependent host cell cytotoxicity and impairment of phagocytosis. Furthermore, the ACD is not required for secretion and can be substituted with a heterologous reporter enzyme that is similarly secreted and translocated into target cells. In studying the mechanism of VgrG-1 translocation into target cells, we find that this process requires endocytosis of V. cholerae cells and the same requirements exist for a heterologous reporter enzyme. Our results support a model for the translocation of C-terminal effector domains of VgrG proteins into the cytosol of target cells by a process that requires trafficking of bacterial cells into an endocytic compartment where translocation is triggered by an unknown signal.
RESULTS
VgrG-1 ACD is dispensable for secretion but is required for T6SS-mediated host cell cytotoxic effects
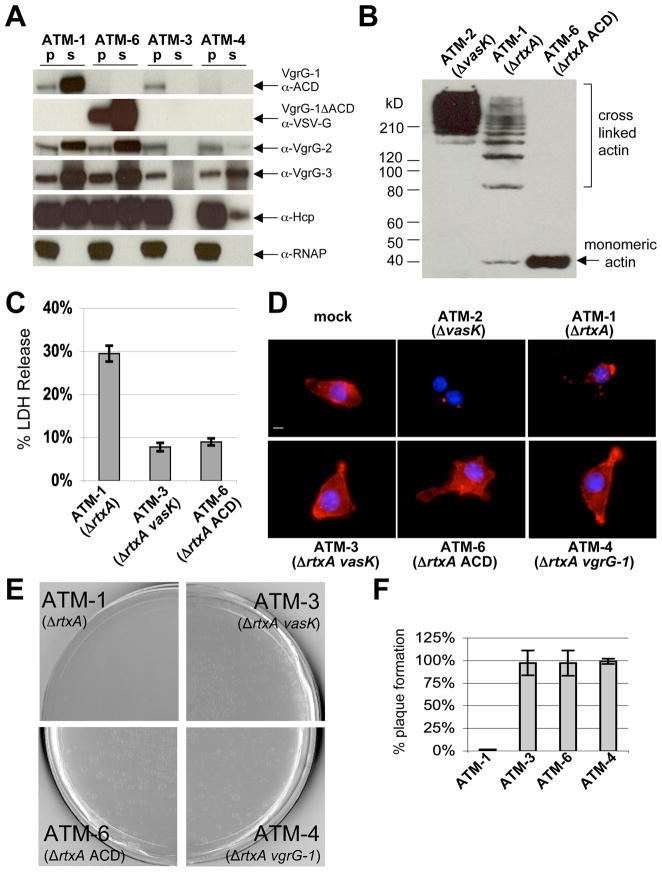
VgrG-1 is the first secreted T6SS protein with demonstrated effector function and vgrG-1 mutant ATM-4 is phenotypically avirulent towards J774 cells and Dictyostelium discoideum (Figure 1D, 1E, 1F). However, this mutation impairs secretion of the other T6SS secretion substrates (Figure 1A). To dissociate the effector and structural apparatus roles of VgrG-1, we constructed strain ATM-6, a V. cholerae V52 strain that lacks the ACD of vgrG-1. In this strain, endogenous vgrG-1 is replaced with a truncated vgrG-1 gene that encodes for amino acids 1–715 and contains a VSV-G epitope tag in place of the ACD. ATM-6 was able to secrete the truncated VgrG-1 and the other known T6SS substrates, VgrG-2, VgrG-3 and Hcp, at levels comparable to its parental T6SS+ RtxA-strain ATM-1 (Figure 1A). Although competent for secretion, ATM-6 is phenotypically avirulent to J774 cells and to D. discoideum. ATM-6 does not induce actin cross-linking (Figure 1B) and does cause cell rounding of J774 macrophages (data not shown) like ATM-1. It does not alter cellular actin morphology as seen after exposure of J774 cells to ATM-1 or ATM-2, which is a T6SS- RtxA+ strain that can cross-link actin (Figure 1D). Transient exposure to ATM-1 causes host cell death that occurs over the span of several days and lysis can be monitored by measuring lactate dehydrogenase (LDH) release one day after exposure (Figure 1C). Host cells similarly exposed to ATM-6 or ATM-3, a strain that lacks rtxA and vasK, an icmF homolog required for T6SS function (Figure 1A), do not cause host cell lysis (Figure 1C), and recover to proliferate normally (data not shown). Additionally, ATM-6 is not virulent towards D. discoideum which is demonstrated by the appearance of D. discoideum plaques that form on a lawn of ATM-6 (Figure 1E) and this plaque formation occurs at levels comparable to K. aerogenes, a commonly used feeder strain for D. discoideum (Figure 1F). These data indicate that the ACD is likely the critical translocated effector domain for T6SS-mediated phenotypes observed in host cells.
Figure 1. The ACD of VgrG-1 is required for host cell phenotypes.
A. Western blots of pellet and supernatant fractions of various V52 strains. B. Western blot of host cell actin from J774 cells incubated with various V52 strains at an MOI of 10 for 2 hours. C. Supernatants of J774 cells were analyzed for LDH release one day after exposure to V. cholerae. Values are from triplicate wells and are expressed as percentage of LDH released from lysed mock-treated cells. Error bars indicate +/− one standard deviation. D. Fluorescence microscopy of cells visualizing actin with rhodamine-phalloidin (red) and nuclei with DAPI (blue). Scale bar indicates 10 μm. E. Dictyostelium discoideum plaque assay with various V52 strains. F. Quantification of plaque formation normalized to plaque formation on K. aerogenes.
T6SS-mediated actin cross-linking requires endocytosis of V. cholerae into host cells
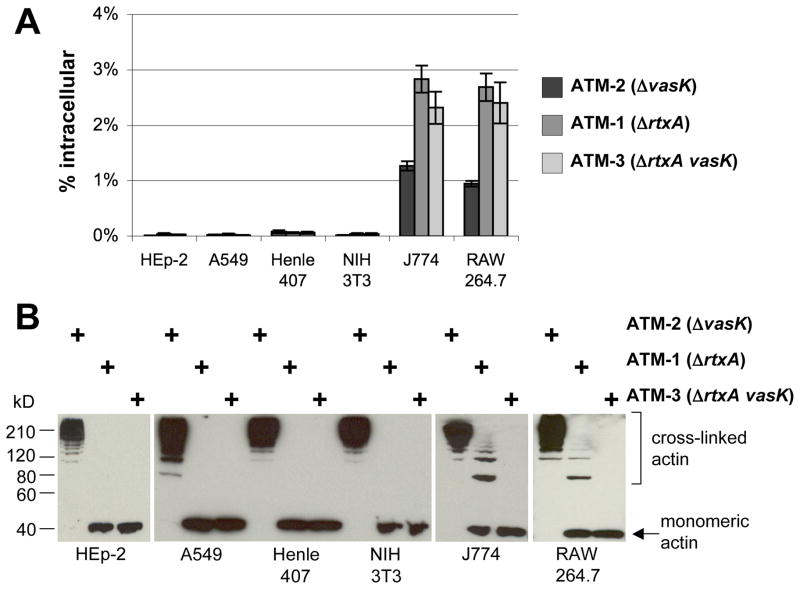
J774 macrophages and D. discoideum amoebae are both phagocytic cell types that are sensitive to V. cholerae T6SS-mediated cytotoxicity. To determine whether phagocytic activity contributes to host cell sensitivity, we surveyed a panel of cell lines for their ability to internalize V. cholerae and for their sensitivity to RtxA and T6SS-mediated actin cross-linking (Figure 2A-2B). Internalization was measured by assaying for V. cholerae cells protected from gentamicin, an antibiotic impermeable to host cells (Isberg and Falkow, 1985). HEp-2, A549, and Henle-407 cells are epithelial cell lines that did not measurably take up V52 strains. Similarly, 3T3 cells are fibroblast cells which also do not take up V52 cells (Figure 2A). When exposed to ATM-2, actin was cross-linked efficiently, indicating that these cell lines were fully sensitive to RtxA-mediated actin cross-linking. However, when incubated with ATM-1, host cell actin remained in monomeric form, indicating a resistance to T6SS-mediated actin cross-linking (Figure 2B). In contrast, two different macrophage cells lines, J774 and RAW 264.7, both internalized ATM-1 and ATM-3 (Figure 2A) and all other V52 strains listed in Table 1 to similar levels (data not shown), except for ATM-2 which is discussed below. Both macrophage cell lines are sensitive to T6SS-mediated actin cross-linking (Figure 2B).
Figure 2. T6SS mediated actin cross-linking correlates with endocytic uptake into host cells.
A. Gentamicin protection assay of various host cell lines exposed to various V52 strains at an MOI of 10 for 1 hour, followed by gentamicin treatment and enumeration of intracellular bacteria. Values are of triplicate wells and are expressed as percentage of initial inoculum recovered. Error bars indicate +/− 1 standard deviation. B. Actin cross-linking in host cells detected by western blot after exposure to various V52 strains at an MOI of 10 for 2 hours.
Table 1.
Bacterial strains constructed for this study
| Strain
|
Genotype
|
|---|---|
| ATM-1 | V52 ΔrtxAa ΔhlyAa ΔhapAa |
| ATM-2 | V52 ΔhlyA ΔhapA ΔvasKb |
| ATM-3 | V52 ΔrtxA ΔhlyA ΔhapA ΔvasK |
| ATM-4 | V52 ΔrtxA ΔhlyA ΔhapA ΔvgrG-1b |
| ATM-5 | V52 ΔrtxA ΔhlyA ΔhapA Δhcp-1b Δhcp-2b |
| ATM-6 | V52 ΔrtxA ΔhlyA ΔhapA vgrG-1ΔACD::VSV-Gc |
| ATM-7 | V52 ΔrtxA ΔhlyA ΔhapA vgrG-1::blac |
| ATM-8 | V52 ΔrtxA ΔhlyA ΔhapA ΔvasK vgrG-1::bla |
| ATM-9 | V52 ΔrtxA ΔhlyA ΔhapA Δhcp-1 Δhcp-2 vgrG-1::bla |
| ATM-10 | V52 ΔrtxA ΔhlyA ΔhapA ΔvgrG-2b vgrG-1::bla |
| ATM-11 | V52 ΔrtxA ΔhlyA ΔhapA vgrG-1ΔACD::blac |
accessory toxin in frame deletions as constructed in Tam et al., 2007
T6SS in frame deletions as constructed in Pukatzki et al., 2006, Pukatzki et al., 2007
new deletions and fusions were constructed as described in Experimental Procedures
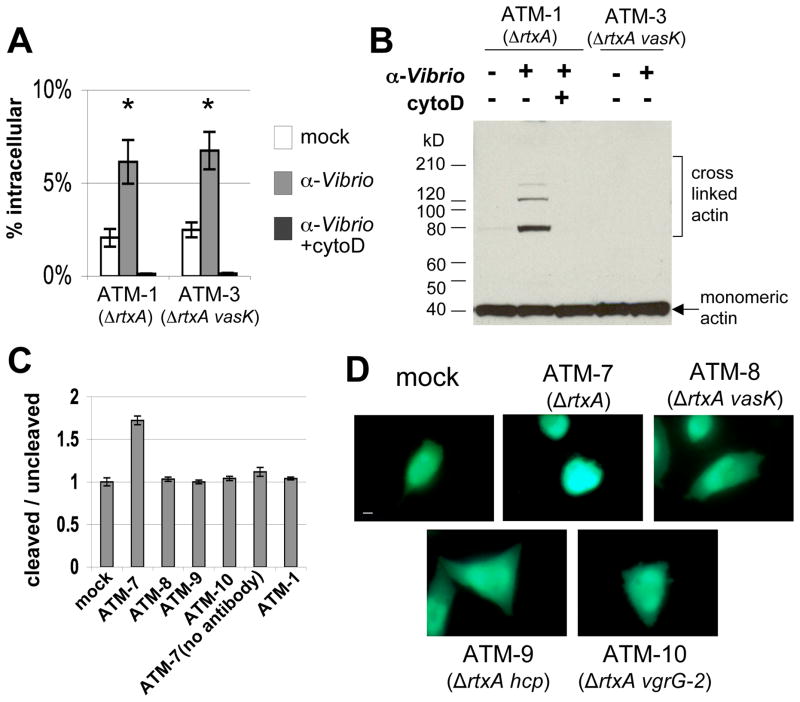
To determine whether J774 cells remain sensitive to T6SS actin cross-linking if internalization is inhibited, incubations were performed in the presence of cytochalasin D, which inhibits phagocytosis by inhibiting actin polymerization, resulting in depolymerized actin filaments. Cytochalasin D had little effect on RtxA-mediated actin cross-linking as has been previously reported (Fullner and Mekalanos, 2000). In contrast, T6SS-mediated actin cross-linking was abolished (Figure 3A). Inhibition of bacterial uptake by cytochalasin D was verified by gentamicin protection assay (Figure 3B). Furthermore, cytochalasin D had no effect on the catalytic activity of VgrG-1 ACD in vitro (Figure 3C). Thus, the ability of cytochalasin D to inhibit VgrG-1-mediated actin cross-linking indicates that bacterial internalization is likely required for delivery of the ACD into the target cell cytosol.
Figure 3. Cytochalasin D inhibits uptake into J774 cells and in vivo actin crosslinking.
A. Western blot against actin from J774 cells in the presence or absence of cytochalasin D. Exposure to V. cholerae was at an MOI of 10 for 2 hours. B. Gentamicin protection assays were performed to verify inhibition of uptake. One hour exposure to V. cholerae at an MOI of 10 was followed by gentamicin treatment and enumeration of intracellular bacteria. Values are of triplicate wells and are expressed as percentage of initial inoculum recovered. Error bars indicate +/− 1 standard deviation. C. In vitro actin cross-linking assay with purified VgrG-1 and actin with various concentrations of cytochalasin D or 0.1% DMSO. After incubation at 37°C for 1 hour, reactions were ran out on a 4–15% gradient SDS-PAGE gel and monitored by western blot.
Inhibition of endosomal acidification improves intracellular viability of V. cholerae, resulting in increased T6SS-mediated actin cross-linking
A variety of bacterial toxins ‘sense’ their internalization by target cells by undergoing conformational changes induced by endosomal acidification (Glomski et al., 2002; Qa’Dan et al., 2000). To determine whether endosomal pH triggers T6SS-mediated translocation of the ACD into target cells, we incubated J774 cells with V. cholerae in the presence of bafilomycin A, a specific inhibitor of vacuolar ATPases (Yoshimori et al., 1991). To exaggerate the effects of bafilomycin A on intracellular bacteria, a time course of J774 cells exposed to V. cholerae at a low multiplicity of infection was performed. Higher order actin multimers formed more quickly and were more abundant in incubations with bafilomycin A-treated cells compared to untreated cells (Figure 4A). Because this drug does not affect in vitro actin cross-linking by VgrG-1 (Figure 4D) and is not known to affect rates of endocytosis, the effect of bafilomycin A on actin cross-linking is likely due to efficient inhibition of phagosome acidification. This would have a protective effect on V. cholerae since it is an acid sensitive organism (Merrell and Camilli, 1999), resulting in increased translocation of ACD. Indeed, we observed a significant increase in the number of intracellular bacteria in incubations performed in the presence of bafilomycin A (Figure 4B). This effect is abrogated by cytochalasin D treatment since it blocks endocytosis altogether (Figure 4B, 4C). Furthermore, translocation apparently requires active bacterial protein synthesis after internalization given that chloramphenicol blocks the appearance of cross-linked actin in J774 cells exposed to ATM-1 (data not shown). Enhancement of actin cross-linking by bafilomycin A is thus consistent with its ability to improve bacterial viability and function by maintaining a neutral vacuolar pH rather than enhancing ACD translocation per se.
Figure 4. Bafilomycin A increases ATM-1 induced actin cross-linking in J774 cells.
A. Time course of J774 cells exposed to ATM-1 at an MOI of 1 in the presence or absence of bafilomycin A. Actin was visualized by western blot. B. Gentamicin protection assay of J774 in the presence or absence of bafilomycin A and with the addition of cytochalasin D. One hour long incubations at an MOI of 10 were followed by gentamicin treatment and enumeration of intracellular bacteria. Values are of triplicate wells and are expressed as percentage of initial inoculum recovered. Error bars indicate +/− 1 standard deviation. Values between different treatment conditions are statistically significant by Student’s t-test (p < 0.05). C. Actin cross-linking in host cells in the presence of 0.1% DMSO, bafilomycin A alone or bafilomycin A with cytochalasin D. Exposures at MOI of 10 and were 2 hours long. Actin was visualized by western blot. D. In vitro actin cross-linking assay with purified VgrG-1 and actin with various concentrations of bafilomycin A or 0.1% DMSO. After incubation at 37°C for 1 hour, reactions were ran out on a 4–15% gradient SDS-PAGE gel and monitored by western blot.
Translocation of VgrG-1-Bla fusion proteins into J774 cells
Many VgrG orthologs in other T6SS+ bacteria display C-terminal extension domains which might correspond to effectors domains similarly transported into target cells (Pukatzki et al., 2007). Thus, heterologous protein domains fused to the C-terminus of VgrG-1 might also be translocated in the cytosol of target cells. Accordingly, we constructed two strains that encode fusions between endogenous vgrG-1 or vgrG-1ΔACD and the blaM gene that encodes β-lactamase enzyme (Bla). β-lactamase activity can be measured using CCF2, a FRET substrate that emits green fluorescence, but emits blue fluorescence after cleavage by β-lactamase (Zlokarnik et al., 1998). The Bla domain replaces the C-terminal ACD domain of VgrG-1 in strain ATM-11 and extends from the C-terminus of the ACD in strain ATM-7. This vgrG-1::bla gene was also introduced into T6SS mutant backgrounds, creating strains ATM-8 (ΔvasK vgrG-1::bla), ATM-9 (Δhcp vgrG-1::bla) and ATM-10 (ΔvgrG-2 vgrG-1::bla). All these strains produced β-lactamase fusion proteins, but only VgrG-1-Bla and VgrG-1-ΔACD-Bla were secreted in vitro by ATM-7 and ATM-11 (Figure 5A). Additionally, secreted VgrG-1-Bla and VgrG-1-ΔACD-Bla were able to cleave a free acid form of CCF (Figure 5B), indicating β-lactamase activity was intact in the fusion proteins. Extracellular transport of VgrG-1- ΔACD-Bla also confirms that the ACD domain of VgrG-1 is not required for secretion function of the T6SS apparatus.
Figure 5. Translocation of VgrG-1-Bla or VgrG-1ΔACD-Bla into J774 cells.
A. Western blot against β-lactamase of pellet and supernatant fractions of various V52 strains producing VgrG-1-Bla or VgrG-1ΔACD-Bla. B. In vitro CCF2-FA cleavage by supernatants of various V52 strains grown in triplicate. Fluorescence was measured with an excitation wavelength of 405 nm and emission wavelengths of 460 nm (cleaved blue channel) and 530 nm (uncleaved green channel). Values are expressed as ratios of cleaved signal to uncleaved signal. Error bars indicate +/− one standard deviation. C. Translocation of VgrG-1-Bla or VgrG-1ΔACD-Bla into J774 cells incubated with various V52 strains. Cells were pre-treated with bafilomycin A, incubated with V52 strains for 2 hours at an MOI of 50, and then loaded with CCF2/AM. Cells were harvested and analyzed on a fluorescence plate reader as for in vitro CCF2-FA assay. Ratios were normalized to mock and error bars indicate +/− 1 standard deviation. D. Gentamicin protection assay on host cells incubated with V. cholerae as for translocation assay and subsequent treated with gentamicin and enumeration of intracellular bacteria. Values are of triplicate wells and are expressed as percentage of initial inoculum recovered. Error bars indicate +/− 1 standard deviation. E. Fluorescence microscopy of J774 cells loaded with CCF2/AM. Scale bar indicates 10 μm. ATM-7, ATM-8, ATM-9, ATM-10 all contain the vgrG-1-bla fusion gene and ATM-11 contains the vgrG-1-ΔACD-bla gene.
We next tested whether VgrG-1-Bla fusion proteins could be translocated into target cells. To monitor translocation of β-lactamase, we used CCF2/AM, an esterified form of CCF2 that accumulates in host cells and fluoresces green; after cleavage by cytosolic β-lactamase its product emits blue fluorescence (Charpentier and Oswald, 2004; Marketon et al., 2005). In order to optimize translocation into J774 cells we took advantage of the increased translocation elicited by treatment of cells with bafilomycin A observed earlier for the natural ACD reporter of VgrG-1 and also treated host cells with a higher MOI. After J774 cells were incubated with various VgrG-1-Bla fusion strains, translocation of β-lactamase into the cytosol was monitored by quantitatively measuring substrate cleavage and by fluorescence microscopy. All non-secreting T6SS mutants had CCF2/AM cleaved/uncleaved ratios similar to mock-treated cells and incubations with ATM-1, which lacks the β-lactamase fusion, while ATM-7 had a significantly higher cleaved/uncleaved ratio which was inhibited by cytochalasin D (Figure 5C). Substrate cleavage in incubations with ATM-7 varied with multiplicity of infection and bafilomycin A pre-treatment time (Supplementary Figure 1A, 1B). Substrate cleavage was also monitored by fluorescence microscopy (Figure 5E).
ATM-11, which expresses and secretes VgrG-1-ΔACD-Bla, is also able to translocate the β-lactamase fusion protein into host cells and had a significantly higher cleaved/uncleaved ratio than strain ATM-7. This could be due to an anti-phagocytic effect caused by the ACD of VgrG-1-Bla which cross-links actin in host cells and thus inhibits subsequent uptake of bacterial cells (Figure 5D). This anti-phagocytic effect was observed after shorter incubations with strain ATM-2, which produces RtxA (Figure 2A), during which time robust actin cross-linking occurs. However, with T6SS-mediated actin cross-linking, this effect becomes apparent only after longer exposure to V. cholerae. By eliminating the actin cross-linking activity in strain ATM-11, more bacteria are taken up into J774 cells over the course of the incubation, thus more intracellular bacteria are able to translocate VgrG-1-ΔACD-Bla.
Opsonophagocytosis by CHO-FcγRII cells
To determine whether induction of bacterial uptake into a non-phagocytic cell line could stimulate translocation of VgrG-1 into an otherwise resistant cell line, we used Chinese hamster ovary cells that express Fc-gamma receptors (CHO-FcγRII), a cell line which is engineered to induce opsonophagocytosis in an IgG dependent manner (Joiner et al., 1990). Gentamicin protection assays show that uptake of ATM-1 and ATM-3 is significantly higher than in incubations lacking opsonizing antibodies (Figure 6A). Actin cross-linking occurs after exposure to ATM-1 in the presence of anti-Vibrio antibody (Figure 6B), but not after exposure to vasK mutant ATM-3. Similar to results seen with J774 cells, cytochalasin D inhibited uptake of ATM-1 and ATM-3 and actin cross-linking induced by ATM-1 (Figure 6A, 6B).
Figure 6. Opsonophagocytosis by CHO-FcRγII cells induces translocation of VgrG- 1.
A. Gentamicin protection assay of CHO-FcγRII cells incubated with various V52 strains in the presence or absence of α-Vibrio antibody and with cytochalasin D. Values are expressed as percentage of initial inoculum recovered and error bars indicate +/− 1 standard deviation. Groups marked with asterisks are significantly higher than remaining groups (Student’s t-test, p<0.05). B. Western blot against actin from CHO-FcγRII cells incubated with various V52 strains at an MOI of 50 for 30 minutes under various conditions, then treated with gentamicin for an additional 1.5 hours. Actin was visualized by western blot. C. Translocation of VgrG-1-Bla into CHO-FcγRII cells by various V52 strains. Cells were pre-treated with α-Vibrio antibody, incubated with V. cholerae at an MOI of 50 for 2 hours, loaded with CCF2/AM, then harvested for quantification. Fluorescence was measured with an excitation wavelength of 405 nm and emission wavelengths of 460 nm (cleaved blue channel) and 530 nm (uncleaved green channel). Values are expressed as ratios of cleaved signal to uncleaved signal and are normalized to mock. Error bars indicate +/− one standard deviation. D. Fluorescence microscopy of CHO-FcγRII cells incubated with various V52 strains and then loaded with CCF2/AM. Scale bar indicates 10 μm. ATM-7, ATM-8, ATM-9, ATM-10 all encode vgrG-1-bla fusion gene.
Translocation into CHO-FcγRII cells was also monitored by using β-lactamase fusions to VgrG-1 and CCF2/AM substrate. Incubations with T6SS mutants expressing the VgrG-1-Bla fusion protein, had cleaved/uncleaved ratios similar to mock-treated cells and incubation with ATM-1, a T6SS+ strain that lacks the β-lactamase fusion. However, incubation with ATM-7 in the presence of opsonizing antibodies had significantly higher cleaved/uncleaved ratios, while incubation in the absence of opsonizing antibody had cleaved/uncleaved ratios similar to mock-treated cells (Figure 6C). Cells were imaged and CHO-FcγRII cells emitted blue fluorescence when exposed to ATM-7 in the presence of anti-Vibrio antibodies (Figure 6D), indicating cytosolic translocation of the C-terminal β-lactamase domain on VgrG-1-Bla had occurred.
DISCUSSION
Bacterial pathogens utilize a variety of mechanisms to manipulate host cell function and the actin cytoskeleton is a common target (Barbieri et al., 2002). The ACD of RtxA toxin and VgrG-1 of V. cholerae represents another biochemical paradigm for microbial manipulation of actin function (Fullner and Mekalanos, 2000; Kudryashov et al., 2008; Pukatzki et al., 2007). Detection of actin cross-linking was a particularly useful tool to monitor T6SS function and allowed us to determine that bacterial endocytosis is required for translocation into host cells. Even nonphagocytic cell lines can be rendered sensitive to T6SS-mediated effects by opsonophagocytosis of V. cholerae via expression of the FcγRII receptor. This induced sensitivity is blocked by treatment with cytochalasin D, suggesting that adherence of V52 to CHO-FcγIIR cells via IgG-FcγIIR interaction is not sufficient to induce translocation. Even without this artificial interaction, V52 and derivative strains adhere to both phagocytic and nonphagocytic host cells (data not shown), suggesting that the correlation between endocytic uptake and translocation is not due to impaired interaction with the host cell surface. In contrast, Legionella pneumophila can translocate Type IV secretion effectors into CHO-FcγRII cells at wild type levels even in the presence of cytochalasin D, suggesting that membrane proximity and/or adherence is sufficient to induce translocation (Cambronne and Roy, 2007). This distinction suggests that the one or more putative signals could trigger translocation within the endosome and may represent a strategy to ensure that translocation occurs only in that specific microenvironment. It has yet to be determined whether V. cholerae intracellular survival is affected by the action of the ACD or whether eventual escape from host cells can occur after their actin cytoskeleton has been disrupted. To some extent this may depend on how fast the phagocytic cell acidifies its endosome or whether V. cholerae is phagocytosed in a physiological state of comparable acid tolerance (Merrell and Camilli, 1999). Since T6SS-dependent translocation of the ACD requires close contact between bacterium and target cell, it seems quite clear that this has not evolved as a mechanism to disrupt the function of host cells at a distance but rather plays its predominant role in close cell-to-cell interactions. The endocytosis requirement for T6SS suggests that phagocytes are the natural target for V. cholerae T6SS, which could include predatory phagocytes encountered in the environment or phagocytic cells of the mammalian host immune system. T6SS-mediated virulence mechanisms could function in both of these environments similar to Legionella pneumophila virulence factors facilitating replication within both amoebae and human macrophages (Swanson and Hammer, 2000). After uptake of V. cholerae by J774 cells, T6SS mediates cross-linking of actin, which then results in impaired phagocytic function and eventual host cell death. These effects could provide protection to bystander V. cholerae within an infection of a mammalian host or within an environmental reservoir. Indeed, interaction between V. cholerae with free living aquatic amoebae Acanthamoeba castellani has been reported (Abd et al., 2005). However, determining the biological role of the T6SS of V. cholerae will require other experimental approaches and appropriate animal models that are currently under development.
T6SS-mediated virulence targeted at phagocytes seems to be a common theme for T6SS+ bacterial organisms since many of these phenotypes involve intracellular behavior or interaction with phagocytes. Mutations in evpC, an hcp homolog, resulted in reduced replication rates of Edwardsiella tarda in gourami fish phagocytes (Rao et al., 2004). A homolog of IcmF, SciS, from Salmonella typhimurium is required for limiting intracellular growth in macrophages (Parsons and Heffron, 2005). Components of T6SS were reported to be upregulated in Burkholderia pseudomallei after invasion of macrophages (Shalom et al., 2007). These phenotypes and modes of regulation indicate that signals in the endocytic compartment of host cells could trigger the expression and function of T6SS systems in organisms others than V. cholerae. The concept that T6SS-dependent virulence factors are specialized for use in the endosomal microenvironment is somewhat reminiscent of specialized T3SS loci that function only after entry of bacteria into endosomes, as has been observed in Salmonella species (Holden, 2002).
The results reported in this study indicate that the ACD of VgrG-1 is not required for secretion in vitro or for translocation into host cells, but is required for all known T6SS phenotypes observed in V. cholerae incubation with eukaryotic cells. This indicates that VgrG-1 is a bona fide effector of T6SS-dependent cellular pathobiology rather than solely a component of the T6SS apparatus. Using β-lactamase fusions to VgrG-1, we demonstrated T6SS-mediated intracellular delivery of an ectopic reporter domain using the conserved ‘core’ structure of VgrG-1. Our data support a more general model for T6SS-mediated delivery of effector domains located at the C-termini of VgrG orthologs. Previously we reported that in excess of 500 VgrG orthologs exist in bacterial genome sequence databases and many of these VgrG orthologs carry C-terminal extensions, some of which are predicted to have interesting biochemical properties based on their structural similarity to other eukaryotic and prokaryotic proteins (Pukatzki et al., 2007). Thus, these C-terminal domains could be translocated into target cells only after phagocytosis of bacteria capable of expressing a functional T6SS. However, not all T6SS+ organisms contain VgrGs with putative C-terminal effector domains, suggesting that there may be T6SS effectors that are not VgrG orthologs (Pukatzki et al., 2009). It remains to be determined whether VgrG proteins possess innate translocation activity or can serve also as a channel for translocation of other effectors. Attempts to show that secreted VgrG-1 and Hcp proteins display cytotoxicity for phagocytic cells have so far failed (Pukatzki et al., 2006), although it is possible that such secreted complexes are capable of mediating translocation of the ACD only in the microenvironment of the endosomal lumen.
Because the well conserved core of VgrG-related proteins is predicted to form a trimeric needle-like complex that resembles bacteriophage T4 tail spike, a membrane penetrating device, it is possible that VgrG proteins might play an active role in breaching the target cell membrane. In the model of T4 bacteriophage infection of E. coli, the force generated from conformational changes in the baseplate and contraction of the tail sheath drives the tail tube and tail spike through host cell membrane (Kostyuchenko et al., 2005). In addition to VgrG, there are other T6SS proteins that are structurally similar to bacteriophage tail components. The structure of Hcp resembles bacteriophage tail tube proteins of bacteriophage T4 and lambda (Leiman et al., 2009; Pell et al., 2009). Another T6SS protein resembles gp25, a bacteriophage T4 tail protein that is present at the interface of the tail tube and tail spike (Leiman et al., 2009). Given the mechanism by which T4 bacteriophage infects host cells and the structural similarities between components of T6SS and T4 bacteriophage tail, we favor a model in which T6SS-dependent phenotypes truly reflect the activity of a T6SS membrane penetrating, translocation machine rather than simply extracellular secretion within the endosomal compartment. Mechanistic studies of the structure and function of the bacterial associated T6SS core complex and mechanism of translocation are needed to test this model.
EXPERIMENTAL PROCEDURES
Bacterial strains and cell culture
Vibrio cholerae strains were grown in LB supplemented with 100 μg per ml streptomycin. In frame deletions and β-lactamase fusions were introduced into V52 using previously described methods (Skorupski and Taylor, 1996). In frame deletions of accessory toxins rtxA, hlyA, and hapA were constructed as in Tam et al., 2007. In frame deletions of T6SS components vasK, hcp-1, hcp-2, vgrG-1, and vgrG-2 were constructed as in Pukatzki et al., 2006 and Pukatzki et al., 2007. Strain ATM-6 was constructed by allelic exchange of vgrG-1ΔACD-VSV-G, which encodes amino acids 1–715 of VgrG-1 fused to VSV-G epitope tag. The blaM gene encodes a TEM-1 type β-lactamase and was cloned from pVTM30 (Tam et al., 2007) to produce vgrG-1::bla fusion gene, which was introduced into ATM-7, ATM-8, ATM-9, and ATM-10 by allelic exchange. Strain ATM-11 was constructed similarly with vgrG-1ΔACD::bla. All resulting strains are listed in Table 1. J774, RAW264.7, NIH 3T3 and CHO-FcγRII cells were maintained in DMEM. HEp-2 and A549 cells were maintained in RPMI. Henle-407 cells were maintained in EMEM. All media were supplemented with 2 mM L-glutamine, penicillin at 100U per ml, 100 μg per ml streptomycin, and 10% heat-inactivated fetal bovine serum. Cells were maintained at 37°C and 5% CO2.
In vitro secretion assay
Various bacterial strains were back-diluted 1:50 into LB broth from overnight cultures. Cultures were grown for approximately 2 hours on a roller drum at 37°C. Pellet fractions were collected and concentrated 2 fold in LDS loading buffer (Invitrogen). Supernatants were filtered, precipitated with trichloroacetic acid, and resuspended in LDS loading buffer resulting in a 100-fold concentration, except for α-Hcp western blots which were not concentrated. VSV-G antibody was obtained from Sigma-Aldrich. RNAP α-subunit antibody was obtained from Neoclone. β-lactamase antibody was obtained from Abcam. VgrG-1, VgrG-2, VgrG-3, and Hcp polyclonal antibodies were generated by New England Peptide (Gardner, MA). Synthetic peptides encoding unique amino acid sequences of Hcp-1, VgrG-1, VgrG-2, and VgrG-3, were prepared and conjugated to keyhole limpet hemocyanin (KLH). The conjugated peptides were injected into New Zealand White rabbits, boosted twice, and then tested on crude cell extracts and culture supernatants from Vibrio cholerae V52 and corresponding mutants by western blot to ensure specificity.
In vitro β-lactamase activity assay
Bacteria were grown in triplicate cultures as for in vitro secretion assays. CCF2-FA (Invitrogen) was added to cell-free supernatant fractions and the mixture was incubated in the dark for 30 minutes at room temperature. Fluorescence was quantified on a SpectraMax Gemini XS fluorescent plate reader with an excitation wavelength of 405 nm and emission wavelengths of 460 nm for the cleaved blue channel and 530 nm for the uncleaved green channel. Values were corrected for LB blank readings and expressed as a ratio of cleaved to uncleaved signal.
Dictyostelium discoideum plaque assay
100 μl of overnight bacterial cultures were plated with dilutions of Dictyostelium discoideum on SM/5 plates as previously described (Pukatzki et al., 2007). Plates were incubated at 22°C for 3 days before plaque formation was assessed. For quantification, bacterial strains were plated in triplicate and the number of plaques was normalized to plaques formed on Klebsiella aerogenes lawns.
Incubation with host cells
Host cells were seeded in 12 well plates at a density of 1–2 × 105 per well in drug free media. The next day, cells were pre-treated with 0.1% DMSO, 5 μg per ml cytochalasin D, or 100 nM bafilomycin A for 1 hour unless otherwise indicated. Triplicate incubations were performed at a multiplicity of infection of 10, unless otherwise indicated. For gentamicin protection assays, incubations were allowed to proceed for 1 hour, followed by 1 hour treatment with 100 μg per ml gentamicin. Host cells were washed with PBS, lysed with 1% saponin and plated for colony forming units on LB agar plates. For detection of in vivo actin cross-linking, incubations were allowed to proceed for 2 hours, unless otherwise indicated. Host cells were harvested, pooled and resuspended in LDS loading buffer (Invitrogen) for analysis by western blot using α-actin antibody (Sigma-Aldrich).
CHO-FcγRII cells
CHO-FcγRII cells were incubated with 1:100 anti-Vibrio antibody (Tam et al., 2007) for at least 1 hour before incubation with V. cholerae. Bacteria were added at an MOI of 10 for gentamicin protection assays or an MOI of 50 for actin cross-linking assays and plates were centrifuged at 1000 rpm for 5 minutes. Cells were incubated with V. cholerae for 30 minutes at 37°C, then treated with 100 μg per ml gentamicin for 1.5 hours. Cells were washed with PBS, lysed with 1% saponin and plated at LB agar plates to enumerate colony forming units. Alternatively, cells were harvested for western blot analysis of actin.
Translocation assay
Host cells were seeded as described above. For fluorescence microscopy experiments, cells were seeded into glass bottom 12 well plates (Mattek). J774 cells were pre-treated with 100 nM bafilomycin A (Sigma-Aldrich) for 4 hours, then incubated with various V52 strains at an MOI of 50 for 2 hours. CHO-FcγRII cells were incubated as described above for actin cross-linking assays, except incubations were allowed to proceed for 2 hours. Host cells were then washed with PBS and loaded with CCF2-AM for 1 hour. For fluorescence microscopy, images were acquired with Nikon inverted epifluorescence microscope. For quantification by fluorescence plate reader, cells were washed after CCF2-AM loading, then allowed to incubate for an additional 30 minutes. Cells were then harvested in 100 μl HBSS, transferred to an opaque 96-well plate and fluorescence was quantified as described for in vitro β-lactamase activity assay.
LDH release assay
J774 cells were incubated with various bacterial strains in triplicate at an MOI of 10 for 2 hours, at which time wells were washed with PBS and treated with 100 μg per ml gentamicin overnight. The next day, supernatant samples or lysed mock-treated cells were collected and analyzed with cytotoxicity detection kit (Roche). Values are expressed as percentage of LDH released from lysed mock-treated wells.
Visualization of actin cytoskeleton by fluorescence microscopy
J774 cells were seeded onto glass-bottom 12 well plates (Mattek). The next day, J774 cells were incubated with various bacterial strains at an MOI of 10 for 2 hours, at which time wells were washed with PBS and fixed with 3.7% paraformaldehyde. Cells were permeabilized with 0.1% Triton-X, stained with rhodamine-phalloidin (Invitrogen) and DAPI (Sigma-Aldrich) and mounted. Images were acquired on a Nikon inverted epifluorescence microscope.
In vitro actin cross-linking assay
This assay was performed as previously described (Cordero et al., 2006; Pukatzki et al., 2007) except for the addition of DMSO, cytochalasin D, or bafilomycin A at the concentrations indicated. Actin derived from rabbit skeletal muscle (Cytoskeleton) was used as substrate for in vitro cross-linking reactions that were incubated at 37°C for 1 hour.
Supplementary Material
Acknowledgments
Thanks to Andree Hubber and Craig Roy for the kind gift of CHO-FcγRII cells and helpful advice. Thanks to David Raskin, Vincent Tam, and Ann Thanawastien for critical reading of the manuscript. Thanks to Daniel Grau for helpful discussions. This work was supported by the National Institutes of Health Grant AI-18045 and AI-026289.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abd H, Weintraub A, Sandstrom G. Intracellular survival and replication of Vibrio cholerae O139 in aquatic free-living amoebae. Environ Microbiol. 2005;7:1003–1008. doi: 10.1111/j.1462-2920.2005.00771.x. [DOI] [PubMed] [Google Scholar]
- Aschtgen MS, Bernard CS, De Bentzmann S, Lloubes R, Cascales E. SciN is an outer membrane lipoprotein required for type VI secretion in enteroaggregative Escherichia coli. J Bacteriol. 2008;190:7523–7531. doi: 10.1128/JB.00945-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert DF, Flannagan RS, Valvano MA. A novel sensor kinase-response regulator hybrid controls biofilm formation and type VI secretion system activity in Burkholderia cenocepacia. Infect Immun. 2008;76:1979–1991. doi: 10.1128/IAI.01338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri JT, Riese MJ, Aktories K. Bacterial toxins that modify the actin cytoskeleton. Annu Rev Cell Dev Biol. 2002;18:315–344. doi: 10.1146/annurev.cellbio.18.012502.134748. [DOI] [PubMed] [Google Scholar]
- Bingle LE, Bailey CM, Pallen MJ. Type VI secretion: a beginner’s guide. Curr Opin Microbiol. 2008;11:3–8. doi: 10.1016/j.mib.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Bonemann G, Pietrosiuk A, Diemand A, Zentgraf H, Mogk A. Remodelling of VipA/VipB tubules by ClpV-mediated threading is crucial for type VI protein secretion. EMBO J. 2009;28:315–325. doi: 10.1038/emboj.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambronne ED, Roy CR. The Legionella pneumophila IcmSW complex interacts with multiple Dot/Icm effectors to facilitate type IV translocation. PLoS Pathog. 2007;3:e188. doi: 10.1371/journal.ppat.0030188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier X, Oswald E. Identification of the secretion and translocation domain of the enteropathogenic and enterohemorrhagic Escherichia coli effector Cif, using TEM-1 beta-lactamase as a new fluorescence-based reporter. J Bacteriol. 2004;186:5486–5495. doi: 10.1128/JB.186.16.5486-5495.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiavelli DA, Marsh JW, Taylor RK. The mannose-sensitive hemagglutinin of Vibrio cholerae promotes adherence to zooplankton. Appl Environ Microbiol. 2001;67:3220–3225. doi: 10.1128/AEM.67.7.3220-3225.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero CL, Kudryashov DS, Reisler E, Satchell KJ. The Actin cross-linking domain of the Vibrio cholerae RTX toxin directly catalyzes the covalent cross-linking of actin. J Biol Chem. 2006;281:32366–32374. doi: 10.1074/jbc.M605275200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin OM, Ludu JS, Nano FE. The Francisella pathogenicity island protein IglA localizes to the bacterial cytoplasm and is needed for intracellular growth. BMC Microbiol. 2007;7:1. doi: 10.1186/1471-2180-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley EG, Thomson NR, Parkhill J, Morin NP, Nataro JP. Proteomic and microarray characterization of the AggR regulon identifies a pheU pathogenicity island in enteroaggregative Escherichia coli. Mol Microbiol. 2006;61:1267–1282. doi: 10.1111/j.1365-2958.2006.05281.x. [DOI] [PubMed] [Google Scholar]
- Everiss KD, Hughes KJ, Peterson KM. The accessory colonization factor and toxin-coregulated pilus gene clusters are physically linked on the Vibrio cholerae 0395 chromosome. DNA Seq. 1994;5:51–55. doi: 10.3109/10425179409039704. [DOI] [PubMed] [Google Scholar]
- Fullner KJ, Mekalanos JJ. In vivo covalent cross-linking of cellular actin by the Vibrio cholerae RTX toxin. EMBO J. 2000;19:5315–5323. doi: 10.1093/emboj/19.20.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glomski IJ, Gedde MM, Tsang AW, Swanson JA, Portnoy DA. The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J Cell Biol. 2002;156:1029–1038. doi: 10.1083/jcb.200201081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden DW. Trafficking of the Salmonella vacuole in macrophages. Traffic. 2002;3:161–169. doi: 10.1034/j.1600-0854.2002.030301.x. [DOI] [PubMed] [Google Scholar]
- Isberg RR, Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985;317:262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- Joiner KA, Fuhrman SA, Miettinen HM, Kasper LH, Mellman I. Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc receptor-transfected fibroblasts. Science. 1990;249:641–646. doi: 10.1126/science.2200126. [DOI] [PubMed] [Google Scholar]
- Kanamaru S, Leiman PG, Kostyuchenko VA, Chipman PR, Mesyanzhinov VV, Arisaka F, Rossmann MG. Structure of the cell-puncturing device of bacteriophage T4. Nature. 2002;415:553–557. doi: 10.1038/415553a. [DOI] [PubMed] [Google Scholar]
- Kostyuchenko VA, Chipman PR, Leiman PG, Arisaka F, Mesyanzhinov VV, Rossmann MG. The tail structure of bacteriophage T4 and its mechanism of contraction. Nat Struct Mol Biol. 2005;12:810–813. doi: 10.1038/nsmb975. [DOI] [PubMed] [Google Scholar]
- Kudryashov DS, Durer ZA, Ytterberg AJ, Sawaya MR, Pashkov I, Prochazkova K, Yeates TO, Loo RR, Loo JA, Satchell KJ, et al. Connecting actin monomers by iso-peptide bond is a toxicity mechanism of the Vibrio cholerae MARTX toxin. Proc Natl Acad Sci U S A. 2008;105:18537–18542. doi: 10.1073/pnas.0808082105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiman PG, Basler M, Ramagopal U, JBB, Sauder JM, Pukatzki S, Burley SK, Almo SC, Mekalanos JJ. Type VI secretion apparatus and phage-tail associated protein complexes share a common evolutionary origin. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0813360106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Coulthurst SJ, Pritchard L, Hedley PE, Ravensdale M, Humphris S, Burr T, Takle G, Brurberg MB, Birch PR, et al. Quorum sensing coordinates brute force and stealth modes of infection in the plant pathogen Pectobacterium atrosepticum. PLoS Pathog. 2008;4:e1000093. doi: 10.1371/journal.ppat.1000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning PA, Brown MH, Heuzenroeder MW. Cloning of the structural gene (hly) for the haemolysin of Vibrio cholerae El Tor strain 017. Gene. 1984;31:225–231. doi: 10.1016/0378-1119(84)90213-0. [DOI] [PubMed] [Google Scholar]
- Marketon MM, DePaolo RW, DeBord KL, Jabri B, Schneewind O. Plague bacteria target immune cells during infection. Science. 2005;309:1739–1741. doi: 10.1126/science.1114580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattinen L, Nissinen R, Riipi T, Kalkkinen N, Pirhonen M. Host-extract induced changes in the secretome of the plant pathogenic bacterium Pectobacterium atrosepticum. Proteomics. 2007;7:3527–3537. doi: 10.1002/pmic.200600759. [DOI] [PubMed] [Google Scholar]
- Mattinen L, Somervuo P, Nykyri J, Nissinen R, Kouvonen P, Corthals G, Auvinen P, Aittamaa M, Valkonen JP, Pirhonen M. Microarray profiling of host-extract-induced genes and characterization of the type VI secretion cluster in the potato pathogen Pectobacterium atrosepticum. Microbiology. 2008;154:2387–2396. doi: 10.1099/mic.0.2008/017582-0. [DOI] [PubMed] [Google Scholar]
- Merrell DS, Camilli A. The cadA gene of Vibrio cholerae is induced during infection and plays a role in acid tolerance. Mol Microbiol. 1999;34:836–849. doi: 10.1046/j.1365-2958.1999.01650.x. [DOI] [PubMed] [Google Scholar]
- Mougous JD, Cuff ME, Raunser S, Shen A, Zhou M, Gifford CA, Goodman AL, Joachimiak G, Ordonez CL, Lory S, et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science. 2006;312:1526–1530. doi: 10.1126/science.1128393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier V, Salzman NH, Satchell KJ. Prolonged colonization of mice by Vibrio cholerae El Tor O1 depends on accessory toxins. Infect Immun. 2007;75:5043–5051. doi: 10.1128/IAI.00508-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons DA, Heffron F. sciS, an icmF homolog in Salmonella enterica serovar Typhimurium, limits intracellular replication and decreases virulence. Infect Immun. 2005;73:4338–4345. doi: 10.1128/IAI.73.7.4338-4345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pell LG, Kanelis V, Howell PL, Davidson AR. Phage Lambda Major Tail Protein: A Common Evolution for All Long-Tailed Phages and the Type VI Bacterial Secretion System. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0900044106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilatz S, Breitbach K, Hein N, Fehlhaber B, Schulze J, Brenneke B, Eberl L, Steinmetz I. Identification of Burkholderia pseudomallei genes required for the intracellular life cycle and in vivo virulence. Infect Immun. 2006;74:3576–3586. doi: 10.1128/IAI.01262-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukatzki S, Ma AT, Revel AT, Sturtevant D, Mekalanos JJ. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc Natl Acad Sci U S A. 2007;104:15508–15513. doi: 10.1073/pnas.0706532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukatzki S, Ma AT, Sturtevant D, Krastins B, Sarracino D, Nelson WC, Heidelberg JF, Mekalanos JJ. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc Natl Acad Sci U S A. 2006;103:1528–1533. doi: 10.1073/pnas.0510322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukatzki S, McAuley SB, Miyata ST. The type VI secretion system: translocation of effectors and effector-domains. Curr Opin Microbiol. 2009 doi: 10.1016/j.mib.2008.11.010. in press. [DOI] [PubMed] [Google Scholar]
- Qa’Dan M, Spyres LM, Ballard JD. pH-induced conformational changes in Clostridium difficile toxin B. Infect Immun. 2000;68:2470–2474. doi: 10.1128/iai.68.5.2470-2474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MH, Biswas K, Hossain MA, Sack RB, Mekalanos JJ, Faruque SM. Distribution of genes for virulence and ecological fitness among diverse Vibrio cholerae population in a cholera endemic area: tracking the evolution of pathogenic strains. DNA Cell Biol. 2008;27:347–355. doi: 10.1089/dna.2008.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, Yamada Y, Tan YP, Leung KY. Use of proteomics to identify novel virulence determinants that are required for Edwardsiella tarda pathogenesis. Mol Microbiol. 2004;53:573–586. doi: 10.1111/j.1365-2958.2004.04123.x. [DOI] [PubMed] [Google Scholar]
- Rawlings TK, Ruiz GM, Colwell RR. Association of Vibrio cholerae O1 El Tor and O139 Bengal with the Copepods Acartia tonsa and Eurytemora affinis. Appl Environ Microbiol. 2007;73:7926–7933. doi: 10.1128/AEM.01238-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell MA, Ulrich RL, Ribot WJ, Brueggemann EE, Hines HB, Chen D, Lipscomb L, Kim HS, Mrazek J, Nierman WC, et al. Type VI secretion is a major virulence determinant in Burkholderia mallei. Mol Microbiol. 2007;64:1466–1485. doi: 10.1111/j.1365-2958.2007.05734.x. [DOI] [PubMed] [Google Scholar]
- Shalom G, Shaw JG, Thomas MS. In vivo expression technology identifies a type VI secretion system locus in Burkholderia pseudomallei that is induced upon invasion of macrophages. Microbiology. 2007;153:2689–2699. doi: 10.1099/mic.0.2007/006585-0. [DOI] [PubMed] [Google Scholar]
- Sheahan KL, Cordero CL, Satchell KJ. Identification of a domain within the multifunctional Vibrio cholerae RTX toxin that covalently cross-links actin. Proc Natl Acad Sci U S A. 2004;101:9798–9803. doi: 10.1073/pnas.0401104101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorupski K, Taylor RK. Positive selection vectors for allelic exchange. Gene. 1996;169:47–52. doi: 10.1016/0378-1119(95)00793-8. [DOI] [PubMed] [Google Scholar]
- Suarez G, Sierra JC, Sha J, Wang S, Erova TE, Fadl AA, Foltz SM, Horneman AJ, Chopra AK. Molecular characterization of a functional type VI secretion system from a clinical isolate of Aeromonas hydrophila. Microb Pathog. 2008;44:344–361. doi: 10.1016/j.micpath.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson MS, Hammer BK. Legionella pneumophila pathogesesis: a fateful journey from amoebae to macrophages. Annu Rev Microbiol. 2000;54:567–613. doi: 10.1146/annurev.micro.54.1.567. [DOI] [PubMed] [Google Scholar]
- Tam VC, Serruto D, Dziejman M, Brieher W, Mekalanos JJ. A type III secretion system in Vibrio cholerae translocates a formin/spire hybrid-like actin nucleator to promote intestinal colonization. Cell Host Microbe. 2007;1:95–107. doi: 10.1016/j.chom.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Taylor RK, Miller VL, Furlong DB, Mekalanos JJ. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987;84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldor MK, Mekalanos JJ. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- Wu HY, Chung PC, Shih HW, Wen SR, Lai EM. Secretome analysis uncovers an Hcp-family protein secreted via a type VI secretion system in Agrobacterium tumefaciens. J Bacteriol. 2008;190:2841–2850. doi: 10.1128/JB.01775-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Milton D, Nybom P, Sjo A, Magnusson KE. Vibrio cholerae hemagglutinin/protease (HA/protease) causes morphological changes in cultured epithelial cells and perturbs their paracellular barrier function. Microb Pathog. 1996;21:111–123. doi: 10.1006/mpat.1996.0047. [DOI] [PubMed] [Google Scholar]
- Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H (+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991;266:17707–17712. [PubMed] [Google Scholar]
- Zheng J, Leung KY. Dissection of a type VI secretion system in Edwardsiella tarda. Mol Microbiol. 2007;66:1192–1206. doi: 10.1111/j.1365-2958.2007.05993.x. [DOI] [PubMed] [Google Scholar]
- Zlokarnik G, Negulescu PA, Knapp TE, Mere L, Burres N, Feng L, Whitney M, Roemer K, Tsien RY. Quantitation of transcription and clonal selection of single living cells with beta-lactamase as reporter. Science. 1998;279:84–88. doi: 10.1126/science.279.5347.84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.