Abstract
A recurrent exon 1 nonsense mutation in the DMD gene, p.Trp3X (c.9G>A), was first ascertained in a proband with no symptoms until age 20 and who walked until the age of 62. Six other unrelated kindreds carrying a p.Trp3X mutation were subsequently ascertained, five from North America and one from Italy. In six of the seven kindreds, the proband presented in childhood incidental to elevated creatine kinase levels detected in the context of other illnesses, or in the setting of cramps with or without rhabdomyolysis. Genetic analysis by high density SNP genotyping demonstrates that the six North American families share a 3.7 Mbp haplotype surrounding the p.Trp3X allele, signifying that this is a founder mutation in these individuals. The size of the founder haplotype and the structure of shared genome-wide segments suggests that the minimal age of this mutation is >6 generations. The discovery of the first DMD founder mutation, associated with a mild Becker phenotype, suggests that the prevalence of hypomorphic dystrophin mutations should be re-examined with the use of improved genomic analysis.
Keywords: DMD, Duchenne muscular dystrophy, Becker muscular dystrophy, founder allele
1. Introduction
The Duchenne (DMD [MIM 310200]) and Becker (BMD [MIM 300376]) forms of muscular dystrophy are the most common inherited disorders of muscle. These allelic X-linked recessive disorders are both caused by loss-of-function mutations in the 79 exon, 2.2 Mbp dystrophin gene (MIM 300377). DMD is a severe childhood myopathy with an estimated incidence of 1:3,500 male births, from which patients suffer loss of ambulation before the age of 12 years and often die in their early 20's of cardiac or respiratory failure. BMD is a clinically similar but less severe form affecting 1:12,000 male births [1], and the severity of BMD patients is more variable than that of DMD patients, ranging from patients who are wheelchair-dependent at the age of 16 years, to those who remain asymptomatic until the fifth or sixth decade of life [2]. Asymptomatic X-linked hyperCKemia has also been described as a dystrophinopathy phenotype [3], although it can be viewed as the mildest form of a BMD phenotype. The high prevalence and clinical variability of both DMD and BMD reflect the mutation rate of the dystrophin gene due to its extreme size and the correlation between loss-of-function alleles, residual dystophin protein levels and clinical phenotypes. In DMD patients, the dystrophin Dp427m protein isoform is typically absent in muscle, while in BMD patients, dystrophin protein is present but abnormal in quantity or size [4, 5]. This difference in residual protein levels is a consequence of the DMD mutational spectrum, which is characterized by a near complete absence of missense mutations, despite the large target size (Dp427m, 3685 amino acids) versus an abundance of deletion mutations of one or more exons (60-65% DMD phenotypes), truncating point mutations (25-30% DMD phenotypes) and duplications of one or more exons (5-10% DMD phenotypes) [6].
The BMD mutation spectrum is less well characterized, but generally conforms to the ‘reading-frame rule’ hypothesis where this class of mutations leads to the generation of internally deleted, but partly functional dystrophins [7]. The internally deleted dystrophins produced from ‘in-frame’ deletion mutations in patients with BMD conforming to the reading-frame rule lack portions of the middle rod domain formed by 24 spectrin-like repeats. BMD patients with exceptions to this reading-frame rule include those with ‘out-of-frame’ deletions in the 5′ region of the dystrophin gene, such as exon 3-7 deletions. Patients with this mutation show the entire range of clinical severity from BMD to DMD [8-11]. Numerous molecular mechanisms, such as cryptic promoter utilization, ribosomal frameshifting, and translation reinitiation, have been suggested as having a role in restoring the reading frame for these exceptional classes [12, 13].
We have previously reported a patient with BMD (42970) with a muscle isoform exon 1 nonsense mutation (c.9G>A, p.Trp3X) who was ascertained from a large cohort screen for point mutations by DNA sequencing [14]. The molecular mechanism underlying residual dystrophin expression in patients with this mutation (and other exon 1 mutations that disrupt the reading frame) has been shown to result from initiation of translation at two exon 6 AUG codons [15], located between the tandem calponin-homology sub-domains (CH1 and CH2) within the N-terminal actin-binding domain (ABD, residues 1-246). These results may explain how mutations in the first exons of the DMD gene are ameliorated by internal initiation, and suggest that dystrophin with a single N-terminal CH2 domain has significant function. Here, we investigate whether the recurrent c.9G>A, p.Trp3X mutations ascertained independently in seven unrelated families are due to independent mutations or are the result of a single founder mutation derived from a recent common ancestor.
2. Patients and Methods
2.1 Families included for study
The probands of families I, II, and III were enrolled within the United Dystrophinopathy Project (UDP), a large multi-center natural history and genotype/phenotype study (http://dystrophy.genetics.utah.edu). Families IV, V and VI were originally identified by primary clinicians and subsequently enrolled in the UDP, and family VII was identified at the Dino Ferrari Centre, Department of Neurological Sciences, University of Milan. This study was approved by the Institutional Review Board of the University of Utah and by the I.R.S. of the “I.R.C.C.S. Foundation Ospedale Maggiore Policlinico, Mangiagalli and Regina Elena, Milan, Italy”. All DNA samples were purified from peripheral blood drawn after written informed consent had been obtained.
2.2 Genetic Analysis
The c.9G>A, p.Trp3X (NM_004006) mutation was identified or confirmed in each patient by DNA sequencing using an established sequencing protocol [14]. Genome-wide SNP analysis used standard protocols for the Affymetrix GeneChip® Human Mapping 250K Nsp assay (Santa Clara, CA, USA), which has been optimized to detect 262,000 SNPs in each sample. Genomic DNA from each male patient I-2 (41934), II-1 (43043), II-2 (43640), III-1 (43800), IV-1 (43676), V-2 (43738), and V-3 (43741), VI-1 (43889), and VII (DR0007) was digested with the restriction enzyme Nsp I. Adaptor ligation, single-primer PCR amplification, fragmentation, labeling and hybridization were performed according to the manufacturers' protocols, and the genotypes were called from probe intensities using the BRLMM algorithm. Genomic DNA from III-1 (43800), VI-1 (43889) and VII (DR0007) were also genotyped on the Affymetrix Genome-Wide Human SNP Array 6.0 which includes more than 906,600 SNPs.
PLINK [16] was used to filter the SNP dataset for missing genotypes and to find extended stretches of homozygosity in the X chromosome data and in the whole genome allowing for a certain amount of missing genotypes and occasional heterozygote calls. We used the PLINK method of moments approach to estimate the probability of sharing 0, 1 or 2 alleles IBD for any two individuals. The HHanalysis program [17] was modified to include the X chromosome along with the default analysis of autosomes. The type A and the type B false positive rate was calculated using the patients' genotype data and regions with conserved homozygosity haplotypes (RCHH) cutoff value of 6.0 cM was chosen as optimal, although the results were robust for RCHH values from 3.0 to 8.0 cM. The control pool for calculating P values for segmental homozygous haplotype sharing consisted of 53 unrelated European-American samples.
3. Results
3.1 p.Trp3X kindreds
The clinical features of the probands and affected male relatives (n=12) from all seven kindreds are summarized in Table 1, and more detailed versions of the clinical histories are contained in the Supplemental Data. As noted in Table 1, in six of the seven families (families II – VII) the probands ranged from age 4 to age 16 when they were last examined; all had presented with elevated serum CK (often incidentally detected), post-exertional myalgias, or a combination of the two. Six of the nine males examined at age 16 or younger (ages 2 – 16) showed no weakness; the remaining three showed only trace to mild proximal weakness.
Table 1.
Summary of Trp3X patient data in the seven affected families.
| Patients, family | Proband/relative | Geographical origin | Age at presentation | Presenting complaint | Current degree of weakness (age at exam, yrs) | Dystrophin immuno-staining | CK level (IU/L) | Maternal genotype |
|---|---|---|---|---|---|---|---|---|
| I-1 (42970) |
proband | Utah | 20 | Weakness | profound limb-girdle weakness (67) [Loss of ambulation at age 62] |
n.d. | n.d. | n.d. |
| I-2 (43194) |
brother | Utah | n/a | n/a | trace pelvic girdle weakness (age 58); denies symptoms via phone (age 61) | n.d. | n.d. | n.d. |
| II-1 (43043) |
proband | Michigan | 4 | incidental hyperCKemia | none (4) | diminished | 523 ↑ | carrier |
| II-2 (43640) |
brother | Michigan | 2 | incidental hyperCKemia | none (2) | n.d. | 5080 ↑ | carrier |
| III-1 (43800) |
proband | Missouri | 7 | Bilateral calf pain, post-exercise; elevated CK | trace deltoid weakness (7) | diminished | 8000 ↑ | n.d. |
| IV-1 (43676) |
proband | Missouri | 4 | incidental hyperCKemia | Minimal deltoid weakness, minimal heel cord contractures (4) | occasional fibers severely reduced | 4558 ↑ | n.d. |
| 43831, V-1 | proband | Wisconsin | “childhood” | myalgias, elevated CK (childhood); rhabdomyolysis (age 14) | none (14) | diminished | 19,189 ↑ | carrier |
| V-2 (43738) |
maternal cousin | Wisconsin | 12 | postexertional limb cramping and “stiffness” | none (16) | n.d. | n.d. | carrier |
| V-3 (43741) |
maternal cousin | Wisconsin | 2.5 | incidental hyperCKemia | none (4.5) | n.d. | “elevated” | carrier |
| VI-1 (43889) |
proband | Pennsylvania | 13 | incidental hyperCKemia, postexertional myalgia | none (14) | n.d. | 712 ↑ | carrier |
| VI-2 (43910) |
Maternal grandfather | Pennsylvania | “childhood” | severe post-exertional myalgias in childhood | none (73) | n.d. | n.d. | n.d. |
| VII DR0007 |
proband | Milan, Italy | 6 | incidental hyperCKemia; running difficulties, frequent falling | mild proximal weakness (12) | diminished | 2932 ↑ | carrier |
Three older patients suggest that the clinical course associated with the mutation remains mild. The proband in family I (patient 42970; ascertained in Utah) is now 67 years old. He first noted weakness at age 20 years, when he was running an obstacle course in the Navy, and was unable to do it in the allotted time. By age 28, he was unable to walk up a flight of stairs while carrying a box of supplies. His weakness progressed through adulthood; although he walked with assistive devices, he never used a wheelchair until the age of 62 years, when he became functionally non-ambulant. His younger brother (patient 43194) was examined by one of us (KMF) at age 58 years. At that time, although he noted occasional nocturnal post-exertional muscle cramps, his examination revealed only trace hip flexor and extensor weakness; he has never used a wheelchair. Similarly, the maternal grandfather of the proband in family VI (patient 43910) also carries the mutation, and at age 73 denies any current symptoms but recalls severe post-exertional myalgias in childhood; his examination shows a mild Trendelenberg gait but no weakness to manual muscle testing. Echocardiogram was performed in patient 42970 and patient 43910; both are normal.
3.2 X chromosome allele sharing
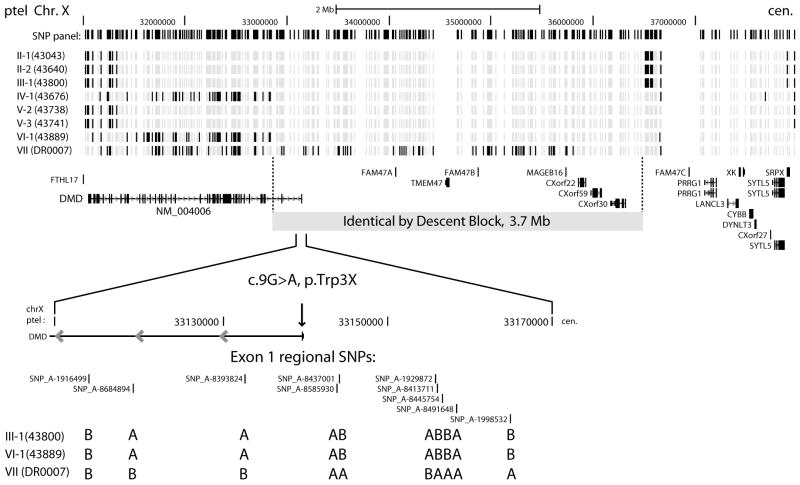
We were unable to establish a genealogical relationship between any of the families, who are all of European-American or European heritage. Although not definitive, more extensive genealogic research carried out by members of families I and II demonstrated no common ancestors between them. To determine whether the p.Trp3X mutation represents a founder allele, we used Affymetrix 250K NspI mapping arrays to detect the pattern of SNP marker sharing throughout the genome and at the DMD gene. If the p.Trp3X mutation resulted from a single event in a common ancestor of the seven families, we would expect identical by descent (IBD) allele sharing of the surrounding chromosomal region. The fine structure of SNP allele sharing in the DMD region is shown in Figure 1, where the 5′ end of the DMD gene overlaps the telomeric end of a shared IBD segment in 6 out of 7 families. This shared segment begins in intron 2 of the Dp427m transcript isoform and extends 3.7 Mbp towards the centromere. The c.9G>A, p.Trp3X mutation is contained within this IBD block and the most parsimonious conclusion is that this mutation is derived from a single event in a distant common ancestor of the North American families I through VI, while family VII of Italian origin does not share the haplotype block surrounding the mutation (Figure 1) and therefore represents an independent event.
Figure 1.
DMD region IBS/IBD blocks determined by high density SNP genotyping of the seven p.Trp3X families. The allelic state of 390 SNP markers spanning ∼ 6.9 Mbp of the Xp21.1 region are shown for pairs of individuals, with patient 43194 (family I) as the reference. Light grey positions are alleles shared identical by state and black positions are non-shared alleles in this pairwise comparison. The location of the large IBD block (3.7 Mbp) is shown in relation to RefSeq annotations and the position of the p.Trp3X mutation in the NM_004006 transcript. The allele calls flanking the exon 1 c.9G>A, p.Trp3X mutation are shown in detail (A and B allele calls from Affymetrix 6.0 data) for families III, VI and VII.
The number of shared contiguous SNP blocks on the X chromosome that are identical by state (IBS) or identical by descent (IBD) between individuals from the six North American families is 1,351 (average block size = 67 kb), indicating that recombination has reduced the segmental sharing to the range expected for common “haplotype blocks” separated by recombination hotspots [18]. One of the two largest IBS/IBD blocks overlaps the DMD gene (3.7 Mbp, 195 SNP markers) and the other spans the centromere (8.9 Mbp, 66 SNP markers). The actual size of centromere-spanning block contains significant uncertainty due to sparse SNP density surrounding this region and the padding of a 3.0 Mbp centromeric gap of “unfinished” sequence. Conversion of distance units from megabases to centimorgans using sex-averaged recombination rates from the genetic map [19] reverses the apparent block size (3.7 Mbp = 6.7 cM versus 8.8 Mbp = 0.9 cM); thus, the p.Trp3X IBD block overlapping the DMD gene is the largest euchromatic X chromosomal segment shared by the six North American families.
3.3 Empirical detection of ancestry
Information on the age of origin for the p.Trp3X mutation can be derived from the length of the chromosomal region in linkage disequilibrium (LD) and the strength of the LD. However, applying direct methods for estimating allele age, such as those described by Risch et al. [20] or Stephens et al. [21], on the small number of p.Trp3X chromosomes ascertained here without a wider estimate of the population frequency of p.Trp3X would place a wide error margin on these estimates. These methods also rely on estimates of the intensity of past selection, presumably weakly negative. It has been suggested that even with detailed knowledge of genetic and demographic parameters, precise estimates of allele age and selection intensity cannot be accurately calculated because of the stochastic nature of recombination and genetic drift [22].
As an alternative to estimating the allele age by LD methods, we used genome-wide metrics to evaluate the genetic relatedness among the seven patients. The size distribution of shared X chromosome IBD segments and the length of the p.Trp3X DMD IBD block are consistent with an allele age >6 generations old, leading to a testable prediction of distant genetic relatedness by estimation of genome-wide identity-by-state and identity-by-descent probabilities for all pairs of study participants. We estimated the cryptic relatedness between study participants based on sharing of genotypes measured with Affymetrix 250K NspI arrays by estimating identity by descent (IBD) using the PLINK software [16]. Table 2 shows the genome-wide probabilities of sharing 0, 1, or 2 alleles IBD for any two patients from the seven p.Trp3X families, including the non-founder family VII of Italian origin. As expected, the two pairs of full sibs (family II: 43043 vs. 43640 and family V: 43738 vs. 43741) show the estimated proportion of alleles shared IBD at ∼0.5. That only several pairs of individual from different families show values ranging from 0.01 to 0.06 for the proportion of alleles shared IBD supports the conclusion that these families are only distantly related.
Table 2.
Pairwise IBD estimation calculated from genome-wide IBD and IBS
| number and probability of sharing 0, 1, or 2 alleles IBD1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Individual 1 | Individual 2 | IBS0 | P(Z=0) | IBS1 | P(Z=1) | IBS2 | P(Z=2) | proportion IBD2 |
| I-2 (43194) | II-1 (43043) | 12502 | 1 | 72426 | 0 | 66829 | 0 | 0 |
| I-2 (43194) | II-2 (43640) | 12324 | 1 | 73153 | 0 | 66280 | 0 | 0 |
| I-2 (43194) | III-1 (43800) | 12883 | 1 | 71329 | 0 | 67545 | 0 | 0 |
| I-2 (43194) | IV-1 (43676) | 12603 | 1 | 71437 | 0 | 67717 | 0 | 0 |
| I-2 (43194) | V-2 (43738) | 12558 | 1 | 73111 | 0 | 66088 | 0 | 0 |
| I-2 (43194) | V-3 (43741) | 12266 | 1 | 72835 | 0 | 66656 | 0 | 0 |
| I-2 (43194) | VI-1 (43889) | 13495 | 1 | 70630 | 0 | 67632 | 0 | 0 |
| I-2 (43194) | VII (DR0007) | 14307 | 1 | 70283 | 0 | 67167 | 0 | 0 |
| II-1 (43043) | II-2 (43640) | 2986 | 0.25 | 51417 | 0.56 | 97354 | 0.19 | 0.47 |
| II-1 (43043) | III-1 (43800) | 12964 | 1 | 72749 | 0 | 66044 | 0 | 0 |
| II-1 (43043) | IV-1 (43676) | 11965 | 0.95 | 74129 | 0.05 | 65663 | 0.00 | 0.03 |
| II-1 (43043) | V-2 (43738) | 12305 | 1 | 75165 | 0 | 64287 | 0 | 0 |
| II-1 (43043) | V-3 (43741) | 11916 | 0.92 | 75407 | 0.08 | 64434 | 0 | 0.04 |
| II-1 (43043) | VI-1 (43889) | 13354 | 1 | 72428 | 0 | 65975 | 0 | 0 |
| II-1 (43043) | VII (DR0007) | 14083 | 1 | 72245 | 0 | 65429 | 0 | 0 |
| II-2 (43640) | III-1 (43800) | 12813 | 1 | 73288 | 0 | 65656 | 0 | 0 |
| II-2 (43640) | IV-1 (43676) | 11924 | 0.95 | 73470 | 0.05 | 66363 | 0 | 0.02 |
| II-2 (43640) | V-2 (43738) | 12296 | 1 | 75274 | 0 | 64187 | 0 | 0 |
| II-2 (43640) | V-3 (43741) | 11925 | 0.93 | 75098 | 0.07 | 64734 | 0 | 0.04 |
| II-2 (43640) | VI-1 (43889) | 13206 | 1 | 72923 | 0 | 65628 | 0 | 0 |
| II-2 (43640) | VII (DR0007) | 14017 | 1 | 72442 | 0 | 65298 | 0 | 0 |
| III-1 (43800) | IV-1 (43676) | 12699 | 1 | 71302 | 0 | 67756 | 0 | 0 |
| III-1 (43800) | V-2 (43738) | 12478 | 1 | 73590 | 0 | 65689 | 0 | 0 |
| III-1 (43800) | V-3 (43741) | 12582 | 1 | 73252 | 0 | 65923 | 0 | 0 |
| III-1 (43800) | VI-1 (43889) | 11715 | 0.92 | 74323 | 0.08 | 65719 | 0 | 0.04 |
| III-1 (43800) | VII (DR0007) | 12047 | 0.98 | 72208 | 0.02 | 67502 | 0 | 0.01 |
| IV-1 (43676) | V-2 (43738) | 12390 | 1 | 74076 | 0 | 65291 | 0 | 0 |
| IV-1 (43676) | V-3 (43741) | 12176 | 1 | 73174 | 0 | 66407 | 0 | 0 |
| IV-1 (43676) | VI-1 (43889) | 13634 | 1 | 70921 | 0 | 67202 | 0 | 0 |
| IV-1 (43676) | VII (DR0007) | 14280 | 1 | 70384 | 0 | 67093 | 0 | 0 |
| V-2 (43738) | V-3 (43741) | 3002 | 0.25 | 50818 | 0.55 | 97937 | 0.20 | 0.48 |
| V-2 (43738) | VI-1 (43889) | 13780 | 1 | 72055 | 0 | 65922 | 0 | 0 |
| V-2 (43738) | VII (DR0007) | 14075 | 1 | 72034 | 0 | 65648 | 0 | 0 |
| V-3 (43741) | VI-1 (43889) | 13758 | 1 | 72087 | 0 | 65912 | 0 | 0 |
| V-3 (43741) | VII (DR0007) | 14088 | 1 | 72370 | 0 | 65299 | 0 | 0 |
| VI-1 (43889) | VII (DR0007) | 10726 | 0.88 | 70285 | 0.11 | 70746 | 0.00 | 0.06 |
method-of-moments approach was used to estimate the global IBD estimates of P(Z), where Z is the IBD state
proportion of alleles shared IBD = P(IBD=2)+0.5*P(IBD=1)
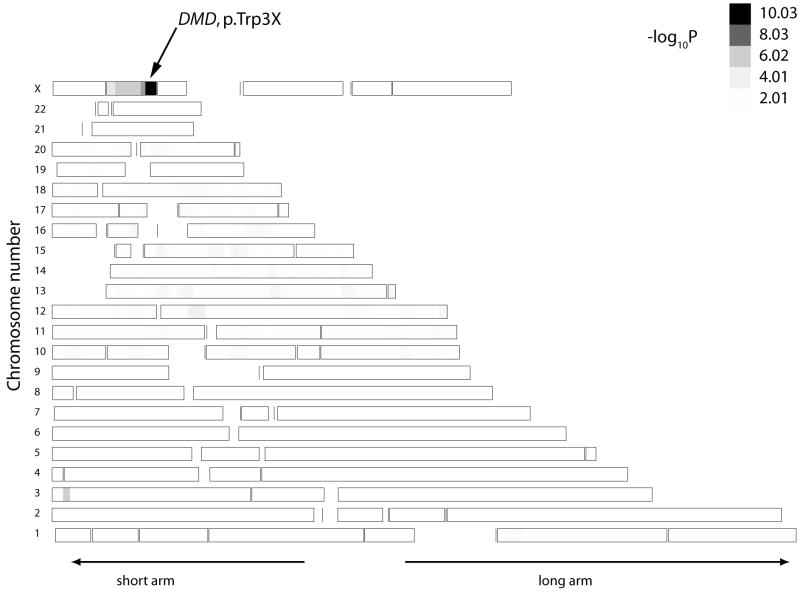
A more direct genome-wide test that searches for shared chromosomal segments derived from a common ancestor is homozygosity haplotype (HH) analysis. This method has successfully been applied in mapping single disease gene locations using high density SNP data [17] from a small number of patients when certain conditions are met. Application of this technique to the p.Trp3X mutation relies on the assumption that a common ancestor brought the disease gene into this patient population within the last several hundred years (6 to 30 generations ago) and the technique is also robust to an admixture of founder and non-founder alleles in the patient pool. Figure 2 shows the result of HH analysis using Affymetrix 250K NspI mapping data from one member of each of the seven p.Trp3X families versus 53 unrelated controls drawn from the same study population. HH analysis robustly detected the correct location of the DMD gene and the p.Trp3X mutation with a −log10(P) value of 10.01. Removing the non-founder Trp3X patient from family VII increases the −log10(P) value to 11.01 supporting robustness to admixture in the patient pool. Given that HH analysis works optimally when 6 ≤ m + n ≤ 30, where m and n are the generations away from the common ancestor, this genome-wide analysis suggests that the p.Trp3X has been inherited from a distant common ancestor.
Figure 2.
Genome-wide heat map of shared chromosomal segments among seven p.Trp3X patients. The 6.7 cM-length region containing the p.Trp3X mutation at chromosome X: 32.8 – 36.5 Mbp has the greatest genome-wide −log10P value (10.03). The patient pool contained the p.Trp3X patients and the control pool consisted of 53 unrelated European-American subjects. The P values for segmental homozygous haplotype sharing were calculated with the HHanalysis program using the representative strategy modified to analyze both autosomal and X regions.
4. Discussion
Our report highlights the fact that BMD founder mutations in the DMD gene may exist in large and diverse populations. This is the first such founder mutation reported in the DMD gene, but analogous recent founder mutations have been reported in autosomal dominant forms of hereditary colon cancer syndromes. Of note is a founder mutation in MSH2 that dates back to the 1700's and has been observed in 9 independently ascertained American families across the USA with hereditary nonpolyposis colorectal cancer (HNPCC). The geographic distribution and ascertainment frequency suggests that this mutation may account for a significant proportion of HNPCC cases in the white USA population [23]. Also, a recent founder mutation embedded in a 6.7 Mbp haplotype surrounding the APC gene has been found in attenuated form of familial adenomatous polyposis [24]. Through genealogical analysis of two large kindreds, this APC mutation has been traced back to a founding couple in the early 1600s, and the mutation has been independently ascertained in 13 other families across the US population. The conserved haplotype surrounding the p.Trp3X mutation and the independent ascertainment of the six BMD families analyzed here suggest that this founder mutation may be similar in frequency and demographic distribution to those found in the late onset colorectal cancer syndromes.
The availability of comprehensive mutation analysis of the dystrophin gene from leukocyte-derived DNA samples has improved the diagnosis and genetic counseling of dystrophinopathies. For Duchenne muscular dystrophy, the observation that 1/3 of sporadic mutations occur in the maternal germ line or ovum supports the lethality of the phenotype as expected from Haldane's theory of the balance between selection and mutation in X-linked lethals. Even deleterious X-linked mutations persist for only a small number of generations and affect only a small number of people [25]. Our finding of a founder mutation for mild Becker muscular dystrophy in the US population suggests that the observed frequency may be due to genetic drift and demographic effects, such as population expansion. Using ‘long-range haplotype’ methods, it has been recently demonstrated that a signal of positive selection resides near DMD intron 12 (SNP rs808540) in the HapMap African sample [26]. Prior evidence from a sampling a 2.4 kb segment in DMD intron 7 indicated positive selection in a small number of individuals from Africa [27]. Although the findings presented here are the result of ancestral haplotype sharing surrounding a nonsense mutation resulting in a mild BMD phenotype, it is possible that the N-terminal region of human dystrophin is still experiencing positive selection based on its actin-binding properties, perhaps through a heterozygote advantage. Internal initiation at exon 6 AUG124 leads to a single N-terminal CH2 domain instead of the typical tandem CH1-CH2 high-affinity actin-binding domain, perhaps contributing to alternate modes of actin-binding activity by dystrophin.
The p.Trp3X mutation is one example of the general class of dystrophin mutations that lead to partial activity through residual dystrophin protein levels. Further characterization of the phenotypes and population prevalence of mutations exemplified by p.Trp3X may help further elucidate the function of dystrophin in muscular and neuronal tissues. With improved mutation surveillance, we can anticipate that similar alleles may be found at appreciable population frequencies and may be important in the overall burden of skeletal and cardiac phenotypes associated with the dystrophinopathies.
Supplementary Material
Acknowledgments
The authors wish to thank O. Gurvich and T. Tuohy for discussions about the mechanism of p.Trp3X amelioration, A. Bringard and J. Tyce for administrative assistance, and to acknowledge the study coordinator assistance of K. Hart, C. Moural, and K. Hak and the technical assistance of E. Meenan, A. Aoyagi, B. Duval, C. Hamil and M. Mahmoud. This work is supported by the National Institute of Neurologic Diseases and Stroke (R01 NS043264 [KMF, MTH, RBW]; the National Center for Research Resources (M01-RR00064, to the University of Utah, Dr. L. Betz, P.I.); by the Association Francaise Contre les Myopathies (KMF); by the Jett Foundation; and by the Parent Project Muscular Dystrophy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Emery AE. The muscular dystrophies. Lancet. 2002;359(9307):687–95. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 2.Bushby KM, Gardner-Medwin D. The clinical, genetic and dystrophin characteristics of Becker muscular dystrophy. I. Natural history. J Neurol. 1993;240(2):98–104. doi: 10.1007/BF00858725. [DOI] [PubMed] [Google Scholar]
- 3.Saengpattrachai M, Ray PN, Hawkins CE, Berzen A, Banwell BL. Grandpa and I have dystrophinopathy?: approach to asymptomatic hyperCKemia. Pediatr Neurol. 2006;35(2):145–9. doi: 10.1016/j.pediatrneurol.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Tuffery-Giraud S, Saquet C, Thorel D, Disset A, Rivier F, Malcolm S, et al. Mutation spectrum leading to an attenuated phenotype in dystrophinopathies. Eur J Hum Genet. 2005;13(12):1254–60. doi: 10.1038/sj.ejhg.5201478. [DOI] [PubMed] [Google Scholar]
- 5.Deburgrave N, Daoud F, Llense S, Barbot JC, Recan D, Peccate C, et al. Protein-and mRNA-based phenotype-genotype correlations in DMD/BMD with point mutations and molecular basis for BMD with nonsense and frameshift mutations in the DMD gene. Hum Mutat. 2007;28(2):183–95. doi: 10.1002/humu.20422. [DOI] [PubMed] [Google Scholar]
- 6.Dent KM, Dunn DM, von Niederhausern AC, Aoyagi AT, Kerr L, Bromberg MB, et al. Improved molecular diagnosis of dystrophinopathies in an unselected clinical cohort. Am J Med Genet A. 2005;134(3):295–8. doi: 10.1002/ajmg.a.30617. [DOI] [PubMed] [Google Scholar]
- 7.Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H, Kunkel LM. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988;2(1):90–5. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- 8.Malhotra SB, Hart KA, Klamut HJ, Thomas NS, Bodrug SE, Burghes AH, et al. Frame-shift deletions in patients with Duchenne and Becker muscular dystrophy. Science. 1988;242(4879):755–9. doi: 10.1126/science.3055295. [DOI] [PubMed] [Google Scholar]
- 9.Chelly J, Gilgenkrantz H, Lambert M, Hamard G, Chafey P, Recan D, et al. Effect of dystrophin gene deletions on mRNA levels and processing in Duchenne and Becker muscular dystrophies. Cell. 1990;63(6):1239–48. doi: 10.1016/0092-8674(90)90419-f. [DOI] [PubMed] [Google Scholar]
- 10.Winnard AV, Klein CJ, Coovert DD, Prior T, Papp A, Snyder P, et al. Characterization of translational frame exception patients in Duchenne/Becker muscular dystrophy. Hum Mol Genet. 1993;2(6):737–44. doi: 10.1093/hmg/2.6.737. [DOI] [PubMed] [Google Scholar]
- 11.Muntoni F, Gobbi P, Sewry C, Sherratt T, Taylor J, Sandhu SK, et al. Deletions in the 5′ region of dystrophin and resulting phenotypes. J Med Genet. 1994;31(11):843–7. doi: 10.1136/jmg.31.11.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gangopadhyay SB, Sherratt TG, Heckmatt JZ, Dubowitz V, Miller G, Shokeir M, et al. Dystrophin in frameshift deletion patients with Becker muscular dystrophy. Am J Hum Genet. 1992;51(3):562–70. [PMC free article] [PubMed] [Google Scholar]
- 13.Winnard AV, Mendell JR, Prior TW, Florence J, Burghes AH. Frameshift deletions of exons 3-7 and revertant fibers in Duchenne muscular dystrophy: mechanisms of dystrophin production. Am J Hum Genet. 1995;56(1):158–66. [PMC free article] [PubMed] [Google Scholar]
- 14.Flanigan KM, von Niederhausern A, Dunn DM, Alder J, Mendell JR, Weiss RB. Rapid direct sequence analysis of the dystrophin gene. Am J Hum Genet. 2003;72(4):931–9. doi: 10.1086/374176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurvich OL, Maiti B, Weiss RB, Aggarwal G, Howard MT, Flanigan KM. DMD exon 1 truncating point mutations: amelioration of phenotype by alternative translation initiation in exon 6. Hum Mutat. 2009;30(4):633–40. doi: 10.1002/humu.20913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyazawa H, Kato M, Awata T, Kohda M, Iwasa H, Koyama N, et al. Homozygosity haplotype allows a genomewide search for the autosomal segments shared among patients. Am J Hum Genet. 2007;80(6):1090–102. doi: 10.1086/518176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, et al. The structure of haplotype blocks in the human genome. Science. 2002;296(5576):2225–9. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 19.Kong A, Gudbjartsson DF, Sainz J, Jonsdottir GM, Gudjonsson SA, Richardsson B, et al. A high-resolution recombination map of the human genome. Nat Genet. 2002;31(3):241–7. doi: 10.1038/ng917. [DOI] [PubMed] [Google Scholar]
- 20.Risch N, de Leon D, Ozelius L, Kramer P, Almasy L, Singer B, et al. Genetic analysis of idiopathic torsion dystonia in Ashkenazi Jews and their recent descent from a small founder population. Nat Genet. 1995;9(2):152–9. doi: 10.1038/ng0295-152. [DOI] [PubMed] [Google Scholar]
- 21.Stephens JC, Reich DE, Goldstein DB, Shin HD, Smith MW, Carrington M, et al. Dating the origin of the CCR5-Delta32 AIDS-resistance allele by the coalescence of haplotypes. Am J Hum Genet. 1998;62(6):1507–15. doi: 10.1086/301867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slatkin M. A Bayesian method for jointly estimating allele age and selection intensity. Genet Res. 2008;90(1):129–37. doi: 10.1017/S0016672307008944. [DOI] [PubMed] [Google Scholar]
- 23.Lynch HT, Coronel SM, Okimoto R, Hampel H, Sweet K, Lynch JF, et al. A founder mutation of the MSH2 gene and hereditary nonpolyposis colorectal cancer in the United States. Jama. 2004;291(6):718–24. doi: 10.1001/jama.291.6.718. [DOI] [PubMed] [Google Scholar]
- 24.Neklason DW, Stevens J, Boucher KM, Kerber RA, Matsunami N, Barlow J, et al. American founder mutation for attenuated familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2008;6(1):46–52. doi: 10.1016/j.cgh.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lange K, Gladstien K, Zatz M. Effects of reproductive compensation and genetic drift on X-linked lethals. Am J Hum Genet. 1978;30(2):180–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Sabeti PC, Varilly P, Fry B, Lohmueller J, Hostetter E, Cotsapas C, et al. Genome-wide detection and characterization of positive selection in human populations. Nature. 2007;449(7164):913–8. doi: 10.1038/nature06250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nachman MW, Crowell SL. Contrasting evolutionary histories of two introns of the duchenne muscular dystrophy gene, DMD, in humans. Genetics. 2000;155(4):1855–64. doi: 10.1093/genetics/155.4.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.