Abstract
Heroin addiction is a wide-reaching problem with a spectrum of damaging social consequences. A vaccine capable of blocking heroin's effects could provide a long-lasting and sustainable adjunct to heroin addiction therapy. Heroin, however, presents a particularly challenging immunotherapeutic target as it is metabolized to multiple psychoactive molecules. To reconcile this dilemma we examined the idea of a singular vaccine with the potential to display multiple drug-like antigens; thus two haptens were synthesized, one heroin-like and another morphine-like in chemical structure. A key feature in this approach is that immunopresentation with the heroin-like hapten is thought to be immunochemically dynamic such that multiple haptens are simultaneously presented to the immune system. We demonstrate the significance of this approach though the extremely rapid generation of robust polyclonal antibody titers with remarkable specificity. Importantly, both the antinociceptive effects of heroin and acquisition of heroin self-administration were blocked in rats vaccinated using the heroin-like hapten.
Introduction
Injection drug abuse is a debilitating worldwide epidemic, comprised of an estimated 14 million global users.1 Of the commonly abused injection drugs, opiates can be considered as the primary source for abuse, as they accounted for 83% of injection drug hospital admissions in the United States in 1999.2 When considering the spectrum of negative effects from opiate abuse, heroin is especially destructive, with costs estimated at 22 billion dollars in the United States alone in 1996 attributed to productivity loss, criminal activity, medical care and social welfare.3 Additionally, heroin abuse and addiction can be viewed as a driving force in the spread of HIV, with an estimated 10% of all new HIV infections attributed to injection drug users.4 Thus, an effective therapy targeting the successful rehabilitation of opiate abusers represents an attractive goal to improve health throughout the population.
Treatment options for heroin addiction rehabilitation address both initial detoxification issues involved with heroin use cessation as well as assisting the addict in maintaining an abstinent lifestyle. However, these options suffer from serious side effects. For example, long lasting opioid agonists including methadone, levo-methadyl acetate and buprenorphine are used to prevent the negative consequences of withdrawal. But, heroin replacement therapy with agonistic compounds still exposes the patient to opiates, and the subject remains dependent and vulnerable to relapse. In addition, opiate replacement therapies are often unavailable to addicts, particularly in developing countries, due to lack of infrastructure to maintain a reliable supply or denial of replacement access altogether.5 Another treatment approach employing opioid antagonistic compounds such as naloxone or naltrexone blocks the body's endogenous opioids (endomorphins, enkephalins), potentially resulting in dysphoric symptoms for the patient, and as a result compliance is an issue.
In considering the array of therapies for heroin addiction, an additional tool would be valuable to assist addicts in maintaining abstinence. As such, we envisioned a heroin vaccine producing sufficiently high, specific antibody titers capable of binding heroin and/or its psychoactive metabolites before entry into the brain would minimize the reinforcing effects of the drug, and yield a potential, highly useful, additional treatment option that would avoid the negative side effects associated with naltrexone and naloxone provided that the vaccine has no affinity for endogenous opioids. This concept, termed immunopharmacotherapy, has been previously demonstrated in our laboratory to be successful in blunting the physiological effects of other abused drugs such as cocaine, methamphetamine and nicotine.6 In principle, antibodies generated by such a vaccine that are specific only for heroin and its psychoactive metabolites would act as an opiate antagonist without the aforementioned negative side effects associated with naltrexone/naloxone, and could be used in combination with synthetic opioid replacement therapy.
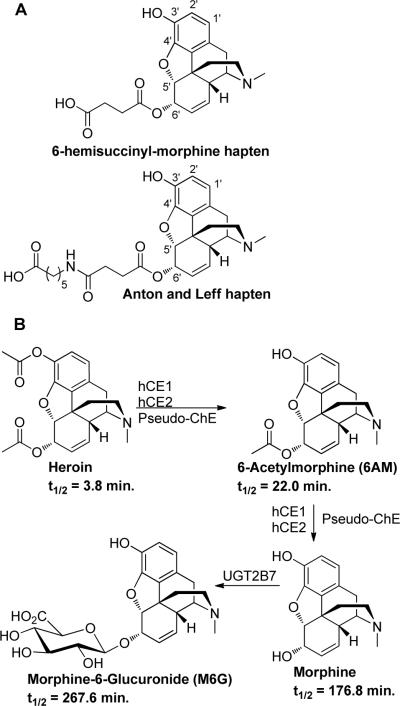
There are two reports detailing heroin vaccines, the first is that of Bonese et al.,7 who used a bovine serum albumin (BSA)-6-hemisuccinyl-morphine conjugate for vaccination of the rhesus monkey. Bonese et al. demonstrated that the 6-hemisuccinyl-morphine conjugate (Figure 1A), when used as an active vaccine over a twenty-week period, could block heroin self-administration. However, this blockade was also shown to be dose-dependent and could be overcome by higher doses of the drug. Unfortunately, this study was not followed up, despite the promising results that were obtained. Over 30 years later, Anton and Leff revisited the work of Bonese et al. again using a 6' ester linked morphine conjugate (Figure 1A).8 Critical details of the Anton study included adequate titers and the prevention of reacquisition of heroin self-administration after extinction training in rats. However, again like the Bonese et al. study, a total of four boosts were required over a 60-day period to reach adequate titers, and biweekly boosts were needed to keep titer levels high over a year period. In addition, this haptenic design displaying the opioid scaffold via conjugation through the 6' moiety presented an inherent lack of immunochemical focus. Thus, from the Anton study, heroin and all of its major psychoactive metabolites 6-acetylmorphine (6AM), morphine, morphine-6-glucuronide (M6G) and the non-psychoactive major metabolite, morphine-3-glucuronide, were sequestered with equal specificity. Taken in sum, we believed additional opportunities existed for heroin active vaccine design and development.
Figure 1.
A) 6-hemisuccinyl morphine hapten used by Wainer et al. and linker extended hapten of Anton and Leff. B) Metabolic pathways of heroin (including isozyme abbreviations) and metabolite half lives in humans.
An under-appreciated, and what we perceived to be major challenge in the construction of an effective hapten-protein conjugate for a heroin vaccine stems from the inherent susceptibility of heroin to be enzymatically degraded into the psychoactive metabolites 6AM and morphine. An additional psychoactive metabolite, M6G, stemming from phase II metabolism, is also formed in humans, but not rodents (Figure 1B).9 Critical from an immunopharmacodynamic standpoint is that while heroin and its psychoactive metabolites are structurally similar, they vary in lipophilicity, and thus the ability to cross the blood brain barrier (BBB). As such, the lipophilic molecules heroin and 6AM readily cross the BBB, while the less lipophilic morphine traverses the BBB slowly. Additionally, once heroin and 6AM cross the BBB, they are rapidly hydrolyzed to morphine, and can be considered as sequestered within the brain.10 Since antibodies are unable to cross the BBB, we hypothesized that for a heroin vaccine to be as efficacious as possible we needed to elicit a multi-drug immune response with 6AM and heroin being the primary targets in order to block the passage of these lipophilic psychoactive molecules into the brain.
To examine such an approach, the known protected environment induced once antigen is adsorbed onto Alum was implemented.11 We anticipated that rapid enzymatic heroin ester hydrolysis would be minimized while heroin was adsorbed onto the Alum surface, and that slow adjuvant desorption would provide a steady and chemically dynamic source of multiple drug-like antigens for presentation to the immune system.12 We hypothesized that heroin's structural integrity would be enhanced over its serum lability, and we expected to create a much improved immune response for 6AM. We also projected an immune response for morphine, yet to a lesser extent, as a result of the decreased hydrolytic susceptibility of heroin's 6' ester (Figure 1). To execute such a strategy, we selected the bridgehead nitrogen as our linker attachment point;13 and we underscore that engagement at this site is in stark contrast to previous heroin vaccines.7–8 Thus, the critical aspects of our haptenic linker approach are: (1) the use of an amide, instead of ester, functional group for attachment of hapten to carrier protein.6a, 14 (2) The display of crucial structural modality found within the heroin scaffolding such that both immune recognition as well as possible novel adjuvant effects are accessed. (3) The use of a modular linker to allow for facile comparison between different haptens.
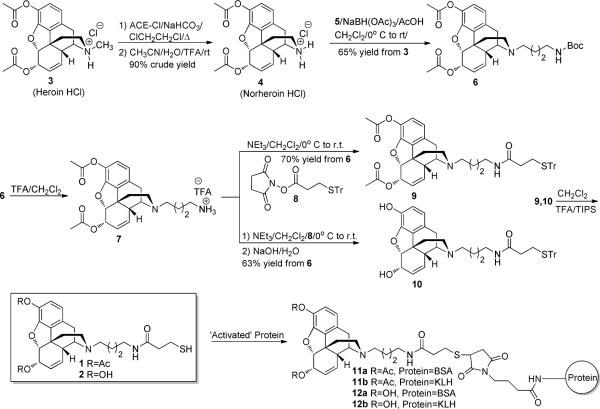
In lieu of our hapten design strategy, we were cognate of an inability to accurately monitor the time-scale for hydrolysis of both heroin esters once hapten-adjuvant adsorbance was finalized. Hence, to examine the immunological importance of heroin ester decomposition in our vaccine cocktail, we engaged morphine-like hapten-2, which lacks ester functionality and was expected to elicit antibodies that tightly bound the single drug antigen morphine rather than heroin or 6AM (Scheme 1).
Scheme 1.
Synthesis of haptens 1–2 and conjugation to KLH carrier protein.
RESULTS
Synthesis of Haptens 1–2 and Conjugation to Carrier Proteins
Synthesis of haptens 1–2 commenced from heroin hydrochloride salt 3, which was demethylated using a modification of Olofson's procedure.15 Thus, 3 was heated with α-chloroethyl chloroformate (ACE-Cl) and NaHCO3 to form the requisite intermediate carbamate. Following filtration to remove NaHCO3, the crude carbamate was decomposed in 6:1 CH3CN:H2O containing 0.1% trifluoroacetic acid (TFA) at room temperature to give norheroin 4 in 90% crude yield. This modification gave reliable yields of norheroin 4 without decomposition of heroin's 3'phenolic ester, which commonly occurred when the carbamate was decomposed by the original procedure of warming in MeOH. Reductive amination between N-Boc-δ-aminobutanal 516 and crude norheroin 4 using NaBH(OAc)3 gave Boc-protected amine 6 in 65% yield from heroin hydrochloride 3. Acidic deprotection of 6 yielded primary amine 7, which was coupled without purification with trityl-protected thiol 817 to give trityl protected heroin hapten 9 in 70% yield from Boc-protected amine 6. At this point, trityl protected morphine hapten 10 was synthesized in 63% yield from Boc-protected amine 6 by saponification of protected heroin hapten 9. The trityl protecting group of heroin/morphine haptens 9–10 was then removed under acidic conditions to give thiols 1–2, followed by preparative HPLC purification and conjugation to maleimide activated keyhole limpet hemocyanin (KLH) or BSA to give immunoconjugates 11a–12b (Scheme 1).
Vaccine Immunogenicity in Rats
To assess vaccine immunogenicity, three groups of male Wistar rats (n = 8 per group) were vaccinated with heroin-like immunoconjugate-11b, morphine-like immunoconjugate-12b, or KLH carrier protein (negative control). Rats were immunized with immunoconjugate in formulation with Alum adjuvant. Following a vaccination procedure developed in our laboratories to give optimized titer levels, six total immunizations were performed during the course of the study at days 0, 14, 28, 53, 108 and 151. Rats were bled immediately prior to boosting (t = 14, 28, 53 days), and at other regular intervals (t = 74, 105, 116, 130, 165 days), to monitor antibody titer levels, with serum obtained after the first injection (t = 14 days) showing significant amounts of antibody production as measured by ELISA. We observed a steady increase in titer levels for both vaccines, with maximum titer levels occurring after the third injection (second boost, t = 53 days). Titers were not observed for the KLH negative control. In comparing the vaccines, maximum antibody titer levels achieved after three injections (second boost) were highest with respect to the morphine-like vaccine (≈ 1:160,000), while slightly lower with the heroin-like vaccine (≈ 1:122,000). The difference between high and low responders for the heroin- and morphine-like vaccines at peak titer levels was ≈ 4–5 fold.
In order to assess antibody decay rate, titer levels were measured at 21 and 53 days (t = 74, 105 days after t = 0 days) after the fourth injection (third boost). As expected, after this time period antibody titer levels had decreased, but were still respectable for both vaccines (≈ 1:50,000 at t = 53 days). After interruption of the vaccination schedule to determine antibody decay rates, the rats were again immunized (fifth injection/fourth boost) with an increase in titer levels observed after 8 days, showing the vaccine's ability to rapidly regenerate titer levels even after a period of immunization absence (Figure 2).
Figure 2.
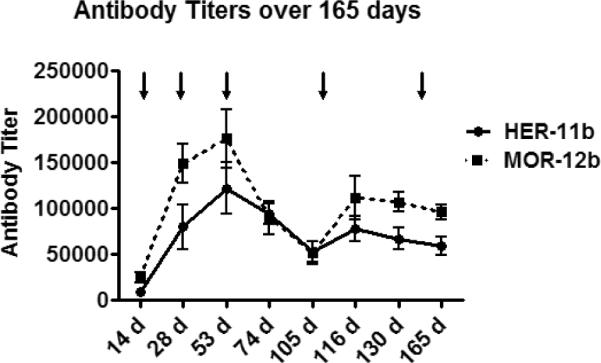
Vaccine titer levels over the course of 165 days. Vertical arrows represent booster injections at t = 14, 28, 53, 108 and 151 days. Data represented are the pooled sera mean value ± SEM.
Determination of Antibody Affinity
While high titer levels are a critical component for the construction of a successful vaccine, an additional facet that must be considered is the ability of the polyclonal antibodies generated to bind their target with high affinity. To determine antibody specificity for heroin, 6AM and morphine, competition ELISA was utilized. Thus, antisera from rats vaccinated with heroin-like immunoconjugate-11b bound 6AM with high affinity while heroin and morphine were bound with decreased affinity. Antisera from rats vaccinated with morphine-like immunoconjugate-12b bound morphine with high affinity, heroin with decreased affinity and did not bind 6AM (Table 1). For all vaccine groups there was no significant cross-reactivity with morphine-6-glucuronide, opioid peptides endomorphin-2/Leu-enkephalin, codeine, opioid agonist methadone and the opioid antagonists naltrexone and naloxone. The importance of the latter finding is that these vaccines could be used in combination with other heroin rehabilitation therapies.
Table 1.
Competition ELISA data obtained from immunoconjugates 11b and 12b. Data represented is the pooled mean sera value ± SEM.
| Conjugate | Heroin Kd (μM)1 | 6AM Kd (μM) | Morphine Kd (μM) |
|---|---|---|---|
| 11b | 4.19 ± 1.01 | 0.0356 ± 0.0010 | 11.2 ± 1.11 |
| 12b | 14.18 ± 6.62 | >100 | 1.18 ± 0.19 |
Sera from the third bleed was used for all competition ELISA experiments.
In order to obtain binding constants for morphine antibody-antigen interactions via an alternative method, a radioimmunoassay (RIA)18 was performed using 3H morphine. From the RIA, morphine binding affinities for antibodies from immunoconjugates 11b and 12b were 24.5 ± 0.8 nM and 16.6 ± 4.9 nM, respectively. Morphine binding capacity for 11b and 12b calculated from this data was 1.05 ± 0.03 and 9.48 ± 2.81 μM, respectively, corresponding to morphine specific antibody of 0.31 ± 0.01 and 2.84 ± 0.84 mg/mL. A limitation of this technique lies in the fact that 3H heroin and 3H 6AM are not readily available, preventing determination of binding affinity for heroin and 6AM by this method. Consequently, competition ELISA data was used as the guideline for antibody affinity to heroin, 6AM and morphine.
Ability of the Vaccines to Specifically Block the Effects of Heroin in Rats
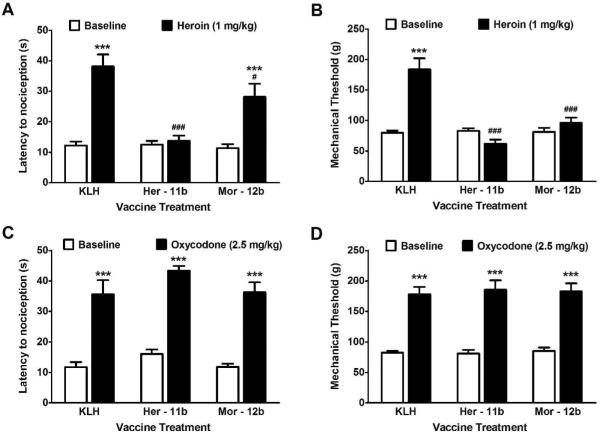
To test the robustness of the vaccine's immune response, mechanical and thermal nociceptive responses were measured following subcutaneous (s.c.) heroin administration using von Frey filaments and the hot plate test, respectively. Thus, after rats had received their fourth course of inoculations (third boost), all groups were administered an established dose of heroin (1 mg/kg, s.c.) that produced pronounced antinociceptive effects. KLH control rats showed marked increases in latency to demonstrate nociceptive behavior on the hot plate (Figure 3A). Heroin administration also resulted in KLH controls requiring increased force applied to the hindpaw before withdrawal response compared with baseline levels (p < 0.001). The rats vaccinated with heroin-like immunoconjugate-11b showed no significant difference from baseline response in either the thermal or mechanical sensitivity tests. The morphine-like immunoconjugate-12b vaccinated rats showed a significant thermal antinociceptive response to heroin (p < 0.001), though partially blunted compared to KLH controls (p < 0.05). Conversely, morphine vaccine rats did not show a heroin-induced antinociceptive effect in the von Frey test compared to baseline (Figure 3B).
Figure 3A–D.
Vaccination selectively blocks the thermal and mechanical antinociceptive effects of heroin. (A and B) Systemic injection of heroin (1 mg/kg, s.c.) produced robust decreases in both thermal (A) nociceptive sensitivity as measured by hot plate, and mechanical sensitivity (B) as measured by von Frey filament testing. This was fully reversed in the heroin-like vaccine (Her-11b) group. The morphine-like vaccine (Mor-12b) significantly blunted the thermal nociceptive effects of heroin compared to control, but was still significantly elevated from baseline and did not alter mechanical sensitivity. (C and D) Injection of the structurally similar opiate oxycodone produced similar thermal and mechanical insensitivity as seen with heroin. Neither the heroin-like (Her-11b) nor morphine-like (Mor-12b) vaccine altered antinociceptive responses to oxycodone. N = 7–8 per group, ***p < 0.001, 30 min post-drug versus baseline; #p < 0.05, ###p < 0.001, versus KLH response post-drug.
As a measure of vaccine drug specificity, nociceptive responses to oxycodone in vaccinated rats was tested. Oxycodone is a commonly prescribed analgesic drug that is structurally similar to both heroin and morphine, and provides an excellent challenge for hapten-antibody fidelity. Thus, an analgesic dose of oxycodone (2.5 mg/kg, s.c.) produced near-full thermal antinociceptive effects at 30 min (Figure 3C) regardless of vaccine pretreatment (p < 0.001). A similar pattern of findings was seen in mechanical sensitivity (Figure 3D), as all the vaccinated groups responded to the oxycodone with significantly increased thresholds compared with baseline levels (p < 0.001).
Analysis of Vaccine Ability to Block Acquisition of Heroin Self-Administration
Having confirmed the ability of our vaccines to blunt the antinociceptive effects of heroin, the ability of each vaccine to prevent acquisition of heroin self-administration in rats was assessed. Self-administration is the most accepted model of drug addiction, and rats will readily self-administer heroin intravenously without intervention, which can lead to drug dependence.19 Accordingly, any vaccine candidate(s) preventing acquisition of heroin self administration in rats could be an attractive possibility for transfer to human trials.
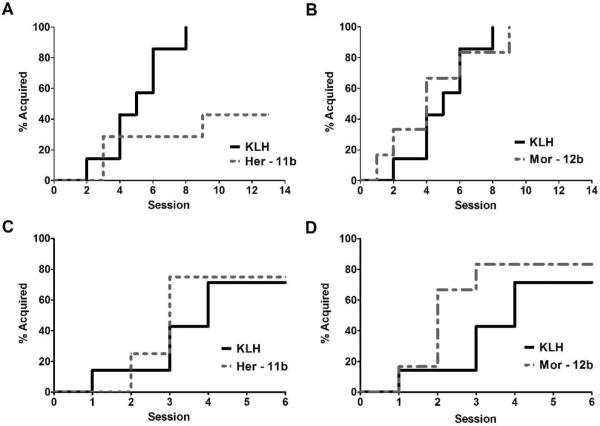
To conduct an acquisition study rats were given a 5th injection (4th boost) of their respective vaccine just prior to surgery to implant intravenous catheters. After a week of recovery, all rats were allowed one hour of access to heroin in the operant chambers. Presses on the active lever were monitored, and most rats pressed the lever at least once in the first session. Those that did not press on their own within the first 20 min during the first 3–4 sessions were given priming injections by guiding the animals to the active lever and depressing it directly in front of them. Those animals that did not press the active lever in subsequent sessions had a short wooden tongue depressor attached to the lever to promote pressing for the 5th and 7th sessions. As seen in Figure 4A–B, all KLH control animals began learning to press the active lever for heroin infusions within a week, defined as pressing three or more times for three consecutive sessions. Rats vaccinated with morphine-like immunoconjugate-12b showed similar ability to controls to acquire heroin self-administration. However, the majority of heroin-like immunoconjugate-11b vaccinated rats failed to maintain pressing for heroin (Figure 4A). A survival analysis on which animals acquired heroin self-administration showed the `heroin' vaccine significantly reduced the likelihood of acquisition [χ2 = 5.0, df = 1, p < 0.05].
Figure 4A–D.
(A and B) Acquisition of heroin self-administration is prevented in a subset of rats vaccinated with heroin-like immunoconjugate (Her-11b), but not morphine-like immunoconjugate (Mor-12b). The percentage of animals that obtain the acquisition criteria, maintaining at least 3 consecutive sessions of 3 infusions (60 μg/kg/infusion) or more, is significantly lower in a group of rats receiving heroin-like (Her-11b) vaccination compared to KLH controls (p < 0.05). Only 3 of 7 rats receiving Her-11b achieved criteria. Conversely, the morphine-like (Mor-12b) vaccine did not alter acquisition of heroin self-administration (p = 0.96). (C and D) Heroin- (Her-11b) and morphine-like (Mor-12b) vaccines do not alter the acquisition of self-administration of a natural reward. Animals were trained to press on an active for 0.1 ml of sweetened solution containing 0.125% w/v saccharine and 3% w/v glucose. When examining the percentage of animals that acquired the response criteria for the sweetened solution, defined as consecutive days of at least 30 or more responses, survival analysis revealed no significant differences based on vaccination treatment (Her-11b: p = 0.63, Mor-12b: p = 0.22 vs KLH control). N = 7–8 per group.
Neither Opioid Vaccine Alters Acquisition of Self-Administration of a Natural Reward
After a brief period without further testing or training, we sought to validate that the heroin-vaccinated rats were still capable of acquiring lever-pressing behavior to a highly palatable substance (sweetened water). As seen in Figure 4C–D, the majority of the rats from each of the vaccination groups showed similar and rapid acquisition of lever responding for this palatable solution, resulting in hundreds of responses in the 30 min period within less than five sessions. A few rats from each vaccine group showed minimal responding on the active lever (< 10 responses per session) and were not likely to meet criteria. However, it should be noted that all animals that failed to acquire responding for the sweetened water did meet criteria in the heroin acquisition. Also, due to the rapid acquisition by most subjects, no priming or additional prompting was necessary for acquisition in this test.
DISCUSSION
Heroin addiction is a complex pathological condition with a significant need for additional therapeutic choices for treatment and relapse prevention. As an additional treatment option, a vaccine for heroin addiction would use the body's immune system to blunt the psychoactive properties of heroin, allowing a motivated recovering addict the chance to continue the recovery process even in the case of a brief relapse. The hypothesis that such a vaccine could be developed was first employed in the seminal study of Bonese et al. and later revisited by Anton and Leff. While these studies have demonstrated such a vaccine to be effective, we felt there was ample opportunity to develop an improved vaccine against opioid abuse.
In terms of immunopharmacotherapy tactics, a hapten should be rationally designed to bind its target(s) with high specificity. To meet this requirement for heroin, a particularly challenging objective given its host of psychoactive metabolites, we departed from previous hapten designs and employed a bridgehead-nitrogen linked strategy for the generation of a heterologous immune response using heroin-like immunoconjugate 11b. To bring such a tactic to fruition, we hypothesized that rapid enzymatic decomposition of heroin's labile esters would be minimized once immunoconjugate-11b was adsorbed onto the protected environment of Alum. Under such a construct we envisioned the 3' and 6' esters to be slowly hydrolyzed at physiological pH and temperature. Thus, we considered immunization with heroin immunoconjugate-11b, while singular in its starting point, would eventually challenge the immune system with multiple hapten-like chemical epitopes including heroin, 6AM and morphine, leading to a honed heterologous antibody response to these three opioids. On the other hand, morphine-like hapten 12b was a `true' singular hapten without labile esters that we reasoned would elicit antibodies highly specific to morphine.
When considering vaccine candidates, critical guidelines for success include a rapid, high titer immune response from a minimum number of inoculations. Thus, both vaccine candidates 11b and 12b after only 28 days and two injections (1 boost) provided titer levels of ≈ 1:80,000 and 1:149,000, respectively. In addition, our maximal antibody titers from haptens 11b and 12b were ≈ 1:122,000 and 1:160,000, respectively, and these levels were reached after only three injections (2 boosts). While it would be difficult to contrast these titer levels to previous heroin vaccines as methodologies amongst each group varies, we can contrast these titer levels to previous work with drug of abuse vaccines from our own laboratory. Thus, cocaine, nicotine and methamphetamine vaccines have provided highest titer levels after multiple boosts of ≈ 1 : 18,000, 1 : 28,000 and 1 : 100,000, respectively, when using a similar vaccination protocol with KLH as the carrier protein.6a, 6f, 20
Antigen recognition is the cardinal feature of the immune system. For antidrug vaccines to be successful enhancement of the immune response is paramount. Our data highlight how both haptens provide access to rapid and very high titers. As a basis for this superior immune response we suggest the observation wherein continuous exposure to small doses of opiates can stimulate the immune system.21 As such, our vaccine design may possess bimodality wherein it not only allows for the display of multiple drug-like haptenic structures, but also embraces critical opiate structural functionality for receptor recognition; and we posit this presents an additional immunostimulant as it is desorbed from Alum. Our hapten adjuvant presentation may be contrasted with previous hapten designs that block the opiate motif at the 6' position, thus compromising opioid receptor recognition and any possible adjuvant effects.
Based upon our studies, we have not only achieved our goal of generating a high titer immune response, but have also surpassed the milestone of eliciting polyclonal antibodies capable of differentiating between extremely similar opiates, a critical point lacking from previous vaccines. Thus, as determined by competition ELISA, our vaccine based on heroin-like immunoconjugate-11b generates antibodies with outstanding affinity to 6AM, and excellent binding for heroin/morphine. Conversely, morphine-like immunoconjugate-12b generates antibodies with high affinity for morphine, but reduced binding for heroin and no affinity for 6AM. In addition, neither of these sets of polyclonal antibodies have any appreciable affinity to the structurally similar opioids morphine-6-glucuronide, codeine, naltrexone, oxycodone and naloxone, the opioid peptides endomorphin-2 and Leu-enkephalin, and the structurally dissimilar opioid agonist methadone. Confirmation of this approach can be seen in our antibody specificity; rats vaccinated with each immunoconjugate displayed significant antinociception after injection of oxycodone, a result that is particularly gratifying given its structural similarity to heroin and morphine.
In considering vaccine-drug fidelity, we hypothesized that instead of a broad immune response to the opiate scaffolding with equal affinity, a more successful vaccine candidate should preferentially bind the major lipophilic serum psychoactive components before crossing the BBB and gaining access to opioid receptors in the brain. This vaccine philosophy can be compared with cocaine, wherein immunopharmacotherapy can benefit from cocaine's ability to readily traverse the BBB.6b Accordingly, our heroin-like immunoconjugate is designed to take advantage of the slow release from the shielded environment of Alum, leading to multiple drug-like hapten display. Thus, this type of vaccine creates a high titer immune response to 6AM, and to a lesser extent heroin, which in turn prevents acquisition of heroin self-administration and other centrally-mediated heroin effects in rodents. On the other hand, our morphine-like immunoconjugate, which also produces a high titer immune response, but primarily to morphine, is not effective for prevention of heroin administration acquisition due to its inability to peripherally bind 6AM.
In conclusion, a vaccine for heroin addiction could prove to be a useful tool for combating heroin addiction, wherein it exploits a motivated recovering addict's own immune system to blunt heroin's psychoactive effects in case of relapse. Towards this heroin vaccine goal, we emphasize that heroin-like immunoconjugate 11b produces high, sustainable titers that can be rapidly reached via a minimum number of injections. We predict that if our vaccine provides protection within a clinical setting, then the recovery process could be more readily managed while requiring a lessened degree of noninvasive maintenance. In addition, if sufficiently selective antibody titers as seen in rats can be translated in humans, this vaccine could readily be used in tandem with opiate replacement therapy (where available) as a synergistic treatment option for addicts.
EXPERIMENTAL PROCEDURES
NMR spectra were recorded on Bruker spectrometers. Chemical shifts are reported in parts per million (ppm). For 1H NMR spectra (CDCl3), the residual solvent peak was used as the reference (7.26 ppm), while the central solvent peak was used as the reference (77.0 ppm in CDCl3) for 13C NMR. Preparative HPLC was performed using a Shimadzu LC-8A system equipped with a Grace-Vydac C18 column (2.2 cm × 15 cm). All HPLC experiments were monitored at 254 or 214 nm. Analytical thin layer chromatography (TLC) was performed using EMD pre-coated TLC plates, silica gel 60F-254, 0.25 mm layer thickness. TLC plates were visualized by exposure to UV light or submersion in aqueous potassium permanganate followed by heating on a hot plate. Preparative TLC was performed using EMD Silicagel 60F-254 plates (20 × 20 cm), 0.5 mm thickness. When necessary, reaction vessels were oven dried and cooled in a dessicator and performed under an inert argon atmosphere. Reagents were commercial grade and used without further purification. Heroin hydrochloride was obtained from NIDA, 6-acetyl morphine was prepared according to the literature procedure22 and Leuenkephalin/Endomorhin-1 were prepared by standard solid phase peptide synthesis.23 Analytical HPLC was used for analysis of compound purity; all purified compounds were of >95% purity, with the exception of Boc-protected compound 6.
(4aR, 7S, 7aR, 12bS)-3-(3-((tert-butoxycarbonyl)amino)propyl)-2,3,4,4a,7,7a-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-7,9-diyl diacetate (6)
To a solution of heroin hydrochloride 3 (21 mg/0.052 mmol) in 4 mL of dry 1,2-dichloroethane was added NaHCO3 (35 mg/0.42 mmol) and α-chloroethylchloroformate (93 μL/0.85 mmol) at room temperature. The resulting solution was heated to reflux for four hours with monitoring by TLC (9:1 CHCl3:MeOH). After this time, the solution was cooled and filtered before removal of the solvent under reduced pressure. The remaining residue was placed under high vacuum for two hours, followed by the addition of CH3CN:H2O (4:1, 0.1% TFA, 2 mL) and stirring for two hours at room temperature. Acetonitrile was removed under reduced pressure, and the aqueous solution lyophilized to give crude norheroin 4 as an amorphous solid (22 mg/90% crude yield). Crude norheroin 4 (22 mg/0.047 mmol) was dissolved in dry CH2Cl2 (5 mL) and cooled to 0° C, followed by the addition of aldehyde 5 (25 μL/0.24 mmol), acetic acid (14 μL/0.18 mmol) and NaBH(OAc)3 (120 mg/0.6 mmol) at the same temperature. The solution was allowed to stir at 0° C for two hours before allowing to slowly warm to room temperature overnight. After this time, additional aldehyde 5 (25 μL/0.24 mmol) and NaBH(OAc)3 (60 mg/0.3 mmol) were added and the solution stirred at room temperature for 6 hours before the addition of water (3 mL). The layers were separated, and the aqueous layer extracted with CH2Cl2 (3 × 5 mL). The combined organic layers were washed with brine (1× 5 mL), dried with MgSO4 and the solvent removed under reduced pressure. The resulting residue was purified by preparative TLC (9:1 CHCl3:MeOH) to give the pure product as an amorphous solid (18 mg/65% yield from 3). 1H NMR 500 MHz (CDCl3) δ 6.75 (d, J = 8.2 Hz, 1H), 6.56 (d, J = 8.2 Hz, 1H), 5.63 (d, J = 9.9 Hz, 1H), 5.43 (dt, J = 2.5, 10.8 Hz, 1H), 5.24 (br s, 1H), 5.15 (m, 1H), 5.11 (d, J = 6.6 Hz, 1H), 3.45 (s, 1H), 3.15 (m, 2H), 2.96 (d, J = 18.7 Hz, 1H), 2.77 (m, 1H), 2.67 (m, 1H), 2.54 (m, 2H), 2.36 (m, 1H), 2.32 (m, 1H), 2.27 (s, 3H), 2.12 (s, 3H), 2.08 (m, 1H), 1.87 (m, 1H), 1.55 (m, 4H), 1.44 (s, 9H); 13C NMR 125 MHz (CDCl3) δ 170.6, 168.6, 156.2, 149.5, 132.0, 131.9, 131.5, 129.5, 128.7, 122.2, 119.5, 88.7, 79.2, 68.0, 56.9, 54.3, 44.9, 43.3, 40.4, 40.2, 34.7, 29.3, 28.0, 24.6, 21.7, 20.8, 20.7. High resolution mass spectrometry (ESI) found 527.2761 [calculated for C29H39N2O7 (M + H+) 527.2752]. Purity (HPLC): 94%
(4aR, 7S, 7aR, 12bS)-3-(3-(3-tritylthio)propanamido)propyl)- 2,3,4,4a,7,7a-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-7,9-diyl diacetate (9)
To a solution of Boc-protected amine 6 (18 mg/0.034 mmol) in CH2Cl2 (1 mL) at room temperature was added a solution of CH2Cl2:TFA (1 mL:1 mL) in one portion. The resulting solution was stirred at room temperature for 2 hours, and then the solvents removed under reduced pressure to give 7 as a yellow solid. This solid was placed under high vacuum overnight, followed by dissolving in CH2Cl2 (4 mL) and cooling the resulting solution to 0°C. Triethylamine (17 μL/0.12 mmol) and activated ester 8 (18 mg/0.04 mmol) were added at the same temperature, and the resulting solution was stirred at 0 °C for two hours before allowing to warm to room temperature overnight. The solution was transferred to a separatory funnel, and washed once with brine (1 × 4 mL). The aqueous layer was extracted with CH2Cl2 (2 × 5 mL), the combined organic layers were dried with MgSO4, and the solvent removed under reduced pressure to give the crude product as a viscous oil which was purified by preparative TLC (9:1 CHCl3:MeOH) to give the product as an amorphous solid (18 mg/71% yield from 6). 1H NMR 500 MHz (CDCl3) δ 7.55-7.25 (m, 15H), 6.85 (d, J = 8.2 Hz, 1H), 6.65 (d, J = 8.2 Hz, 1H), 5.70 (m, 2H), 5.45 (dt, J = 2.4, 10.0 Hz, 1H), 5.23 (m, 1H), 5.16 (d, J = 6.6 Hz, 1H), 3.52 (m, 1H), 3.30 (m, 2H), 3.04 (d, J = 18.8 Hz, 1H), 2.81 (m, 1H), 2.72 (m, 1H), 2.63 (m, 2H), 2.57 (t, J = 7.2 Hz, 2H), 2.43 (m, 1H), 2.37 (m, 1H), 2.36 (2, 3H), 2.22 (s, 3H), 2.12 (t, J = 7.1 Hz, 2H), 2.05 (m, 1H), 1.90 (m, 1H), 1.65 (m, 4H); 13C NMR 125 MHz (CDCl3) δ 171.1, 170.6, 168.6, 149.4, 144.7, 132.0, 131.9, 131.6, 129.7, 129.5, 128.6,128.0 126.8, 122.0, 119.5, 88.7, 68.0, 66.8, 57.0, 54.3, 44.8, 43.4, 40.5, 39.3, 35.9, 35.1, 27.9, 27.5, 24.8, 21.8, 20.7, 20.6. High resolution mass spectrometry (ESI) found 757.3326 [calculated for C46H49N2O6S (M + H+) 757.3306].
N-(3-((4aR, 7S, 7aR, 12bS)-7,9-dihydroxy-4,4a,7,7a-tetrahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-3(2H)-yl)propyl-3-(tritylthio)propanamide (10)
To a solution of trityl protected heroin hapten 9 (12 mg/0.15 mmol) in MeOH (2 mL) was added 0.1 M NaOH (1 mL) at room temperature. The resulting solution was stirred at room temperature for 45 minutes before the removal of MeOH under reduced pressure. The remaining aqueous phase was extracted with EtOAc (6 × 5 mL), the combined organics were dried with MgSO4 and the solvent removed under reduced pressure to give the crude product as a viscous oil which was purified by preparative TLC (9:1 CHCl3:MeOH) to give the pure product as an amorphous solid (9.5 mg/92% yield). 1H NMR 600 MHz (CDCl3) δ 7.40-7.20 (m, 15H), 6.70 (d, J = 8.1 Hz, 1H), 6.58 (d, J = 8.1 Hz, 1H), 5.78 (br s, 1H), 5.75 (d, J = 11.0 Hz, 1H), 5.29 (d, J = 9.7 Hz, 1H), 4.96 (d, J = 5.9 Hz, 1H), 4.25 (m, 1H), 3.57 (m, 1H), 3.28 (m, 2H), 3.0 (d, J = 18.5 Hz, 1H), 2.69 (m, 1H), 2.54 (m, 2H), 2.50 (t, J = 7.3 Hz, 2H), 2.40 (m, 1H), 2.30–2.45 (m, 5H), 2.15 (t, J = 7.1 Hz, 2H), 1.90 (m, 1H), 1.70 (m, 4H); 13C NMR 150 MHz (CDCl3) δ 170.2, 145.3, 144.8, 138.1, 133.1, 130.9, 129.7, 128.1, 128.0, 126.9, 121.9, 120.1, 116.8, 91.8, 66.9, 66.6, 56.7, 54.3, 44.9, 43.8, 40.7, 39.4, 35.9, 30.9, 27.9, 27.4, 22.8, 21.4. High resolution mass spectrometry (ESI) found 673.3100 [calculated for C42H45N2O4S (M + H+) 673.3094].
(4aR, 7S, 7aR, 12bS)-3-(3-(3-mercaptopropanamido)propyl-2,3,4,4a, 7a-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-7,9-diyl diacetate (1)
5 mg of trityl protected hapten 9 was placed in a round bottom flask and put under high vacuum overnight, followed by purging the flask with Argon. CH2Cl2 (1 mL) was then added to the flask, followed by addition of a solution of CH2Cl2:TIPS:TFA (1 mL:33 μL:33 μL) at once at room temperature. The resulting solution was purged briefly with Argon, and stirred at room temperature for two hours before removal of the solvent under vacuum. The crude product was purified by preparative HPLC (0 to 10 min at 10% B, 10 to 50 min gradient to 90% B, 50 to 55 min gradient to 10% B, 55 to 60 min at 10% B) to yield fractions containing the pure thiol 1. CH3CN and TFA were removed under reduced pressure followed by lyophilization of the remaining aqueous solution to give the pure thiol as a white powder (≈ 2 mg). This thiol was sensitive to oxidation to disulfide, and was used immediately for protein conjugation.
N-(3-((4aR, 7S, 7aR, 12bS)-7,9-dihydroxy-4, 4a, 7, 7a-tetrahydro-1H-4, 12-methanobenzofuro[3,2-e]isoquinolin-3(2H)-yl)-3-mercaptopropanamide (2)
Synthesized using a procedure analogous to that for the synthesis of 1.
Construction of Heroin and Morphine Immunoconjugates 11a–12b
To a 0.1 mM solution of KLH or BSA (Pierce Protein Research Products) was rapidly added 10-fold excess of a solution of Sulfo-GMBS (N-[g-Maleimidobutyryloxy]sulfosuccinimide ester, Pierce Protein Research Products, 10 mM stock concentration, 1 mM final concentration) at room temperature. The resulting solution was gently shaken at room temperature for 30 minutes, followed by the removal of excess Sulfo-GMBS by dialysis (Slide-A-Lyzer cassette, 10,000 MWCO, Pierce, PBS buffer, pH = 7.4) at 4 °C (we found the use of `preactivated' KLH/BSA (Pierce) yielded significant amounts of protein denaturation during hapten conjugation, thus BSA/KLH was always `activated' following this standard procedure). To the solution of `activated' KLH obtained after dialysis was added heroin or morphine hapten 1–2 (2 mg hapten in 350 μL PBS + 30 μL DMSO, 1 mg hapten : 1mg of protein) at 4 °C, and the resulting solution gently shaken at the same temperature for four hours. After this time period, the crude immunoconjugate was purified by dialysis (PBS, pH = 7.0) at 4 °C to give pure immunoconjugate in 0.5–1.0 mg/mL concentration as measured by bicinchoninic acid (BCA) assay (Pierce). Coupling efficiency for BSA immunoconjugates 1–2 was monitored by MALDI-TOF MS, and found to be ≈ 22 copies of hapten per protein. It was assumed that coupling efficiency for KLH protein conjugates was similar, as KLH can not be analyzed by MALDI-TOF.
Active Immunization Protocol
All procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute. Four groups of n = 8 male wistar rats (n = 24 total; Charles River, Raleigh, NC) weighing between 225–275 g at the beginning of immunization were used. All rats were housed in groups of 3 per cage in a temperature-controlled (22°C) vivarium on a 12 h light/dark cycle (lights on at 6:00 h) with ad libitum access to food and water. Rats were assigned to morphine (MOR), heroin (HER) or control (KLH) vaccine groups. Rats were immunized with 0.1 mg of immunoconjugate 11b (HER) or 12b (MOR) in formulation with Alum adjuvant (Imject, Pierce) administered into 3 sites (2 s.c. and 1 i.p.). Six total immunizations were performed during the course of the study at days 0, 14, 28, 53, 108 and 151. On days 14, 28, 53, 75, 105, 116 and 130 roughly 0.2 mL of serum was collected to determine immune response as measured by ELISA. At day 165 the rats were sacrificed and their blood collected. While our protocol was not designed to specifically address vaccine toxicity, we did not notice any obvious signs of inflammation or irritation on subsequent booster injection sites. Weights of vaccinated animals were healthy and had no signs of chronic health issues.
ELISA and Competition ELISA
ELISAs to determine limiting dilution were performed using Costar 3690 ½ area high binding affinity plates. Plates were coated with BSA conjugated heroin or BSA conjugated morphine at 0.5μg/mL in pH 6.4 PBS and dried overnight at 37°C. Phosphate buffered saline of pH 6.4 was used throughout the ELISAs due to hydrolysis of the 3'-acetyl group of heroin in neutral to basic pHs at elevated temperature. Rat sera samples were diluted in 2% BSA serially across the plate to a final concentration of 1: 4,096,000. Plates were developed using a goat α rat HRP secondary antibody as well as TMB substrate (Pierce, Rockford, IL). Absorbance was read on a Spectramax M2e 250 spectrophotometer with endpoint readings at 450nm. O.D. values were plotted on a non-linear curve algorithm using PRISM software.
Competition ELISAs were performed using rat sera samples from bleed 3, due to the highest recorded antibody titers throughout the 165 day experiment. Sera samples were diluted to O.D.50 titer levels determined previously. 10mM drug stocks were prepared in DMSO. Stocks were diluted directly into coated 96 well costar 3690 plates to a final concentration range of 100μM - 49nM (<1% DMSO per well). Plates were developed as described previously. Absorbance was read at 450nm and O.D. values were plotted in PRISM in a one site fit log IC50 non-linear curve model to determine inhibition constants of drug competitors.
Radioimmunoassay (Equilibrium Dialysis)
Equilibrium dialysis was performed using Harvard Apparatus 96-well equilibrium dialyzer plates, MWCO 5kDa. All equilibrium dialysis was conducted at pH 7.0. To each side of the plate was added ~15,000 dpm of 3H Morphine (American Radiolabeled Chemicals, 80 Ci/mmol, 1mCi/mL) in 25 μL 2% BSA. On one side of the plate was added 75 μL of PBS buffer and 50 μL heroin/morphine of the appropriate dilution. On the other side of the plate was added 75 μL of diluted sera (due to supply constraints, sera from the 8th bleed was used for all equilibrium dialysis experiments) in 2% BSA and 50 μL of heroin/morphine of the appropriate dilution. The plate was then rotated for 22 hours, followed by removal of 75 μL from each side of the plate, and dissolution into 5 mL of scintiallation fluid (Ecolite, MP Biomedicals). Counts of radioactivity, as measured in dpm, were calculated (Beckman LS6500 Liquid Scintillation Counter). Percent inhibition was determined using the method of Muller18, with IC50 values determined using Prism software. IgG was assumed to have a molecular weight of 150 kDa and two binding sites per molecule.
Determination of the Vaccine's Ability to Blunt the Antinociceptive Effects of Heroin
Behavior experiments began after the 4th vaccine injection (3rd boost), when antibody titers were elevated. For the hot plate test (Ugo Basile, model-DS37), a steady temperature of 54 ± 0.2°C was used to evaluate thermal nociception. The animals were placed in an acrylic cylinder of 24-cm diameter on the heated metal surface, and the time between placement and hind paw licking or jumping (whichever occurred first) was recorded as nociceptive latency. A 45-s cut-off was established to prevent tissue damage. Rats were tested on the hot plate before (0; baseline) and 30 min after heroin (1 mg/kg; s.c.) or oxycodone (2.5 mg/kg; s.c.) injections. Mechanical nociceptive thresholds (von Frey's test) were measured according to King's method24. Briefly, rats were acclimated for 30 minutes in elevated acrylic cages with a wire mesh floor. A series of von Frey filaments were applied perpendicularly to the plantar surface of the hindpaw for 3 seconds. A sharp withdrawal of the hindpaw indicated a positive response. The stimulus was incrementally increased until a positive response was obtained, then decreased until a negative result was obtained in order to determine a pattern of responses for analysis by the non-parametric method of Dixon25.
Self Administration Studies
For heroin self-administration experiments, rats prepared with chronic intravenous Silastic catheters (Dow Corning, USA) into the right jugular vein and tested for self-administration in standard operant chambers (Med Associates Inc., St. Albans, VT) as previously described.26 The rats had to press one of the two levers (the active lever) on a fixed-ratio (FR) 1 schedule (each response resulted in fluid delivery) to obtain 0.1 ml (over 4 s) of heroin (60 μg/kg/infusion) in 1 h sessions. Reinforced responses were followed by a 20 s time-out period, in which a cue-light (above the active lever) was turned on and lever presses did not result in additional injections. Presses on the other lever (inactive lever) had no programmed consequences. Rats were tested for 14 sessions and the criterion for acquisition of heroin self-administration was a minimum of 3 reinforcements per session over 3 consecutive sessions. Rats were also tested for self-administration of a very palatable sweet solution in order to verify whether differences in acquisition of heroin self-administration among groups were specific for heroin or generalized to other reinforcers (i.e., differences in learning an operant task). For this experiment, animals were tested in similar operant chambers as for heroin, but a drinking cup placed at equidistance of the levers 6 cm from the floor was present for fluid delivery. In a 30 min session, presses in the active lever resulted in the delivery of 0.1 ml of a sweet solution (3% glucose + 0.125% saccharin in water). The active lever (left side) was opposite in relation to the active lever used for heroin self-administration (right side) in order to control for acquisition differences during the heroin experiment, i.e., the position of the active lever was novel for all the animals. Criteria for the acquisition of sweet solution intake were defined as 30 or more responses on the active lever in consecutive sessions.
Statistical Analysis
The results are presented as means and standard error of the mean (SEM). Hot plate and von Frey data were analyzed using analysis of variance (ANOVA) for repeated measures with group (KLH, HER and MOR) as a between-subject factor and time (baseline and post-injection) as a within-subject factor. The Newman-Keuls test was used for post-hoc comparisons of the means when appropriate. To analyze acquisition rates, a Kaplan-Meier survival analysis was performed on the number of sessions required to reach criterion followed by a logrank test (GraphPad Prism version 4.03, GraphPad Software, USA). The accepted level of significance for all tests was p<0.05.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge the support of the Scripps Research Institute, Skaggs Institute for Chemical Biology and the National Institute of Health under grant number R01-DA026625.
Abbreviations Used
- BSA
bovine serum albumin
- KLH
keyhole limpet hemocyanin
- 6AM
6-acetylmorphine
- M6G
morphine-6-glucuronide
- BBB
blood brain barrier
- ACE-Cl
α-chloroethyl chloroformate
Footnotes
SUPPORTING INFORMATION AVAILABLE. Spectral data (HPLC, 1H NMR, 13C NMR) for all previously unreported compounds is available.
REFERENCES
- 1.Aceijas C, Stimson G, Hickman M, Rhodes T. Global overview of injection drug use and HIV infection among injection drug users. AIDS. 2004;18:2296–2303. doi: 10.1097/00002030-200411190-00010. [DOI] [PubMed] [Google Scholar]
- 2.The DASIS Report Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Jun 21, 2002. Treatment admissions for injection drug abuse. [Google Scholar]
- 3.Mark TL, Woody GE, Juday T, Kleber HD. The economic costs of heroin addiction in the United States. Drug Alcohol Depend. 2001;61:195–206. doi: 10.1016/s0376-8716(00)00162-9. [DOI] [PubMed] [Google Scholar]
- 4.United Nations general assembly special session on HIV/AIDS. 2001. UN, June 25–27 Declaration of Commitment on HIV/AIDS. [Google Scholar]
- 5.(a) Gillies P, Tolley K, Wostenholme J. Is AIDS a disease of poverty? AIDS Care. 1996;8:351–363. doi: 10.1080/09540129650125768. [DOI] [PubMed] [Google Scholar]; (b) Tawil O, Verster A, O'Reilly K. Enabling approaches for HIV/AIDS prevention: can we modify the environment and minimize the risk? AIDS. 1995;9:1299–1306. [PubMed] [Google Scholar]
- 6.(a) Moreno AY, Azar MR, Warren NA, Dickerson TJ, Koob GF, Janda KD. A Critical Evaluation of a Nicotine Vaccine within a Self-Administration Behavioral Model. Mol Pharmaceut. 2010;7(2):431–441. doi: 10.1021/mp900213u. [DOI] [PubMed] [Google Scholar]; (b) Moreno A, Janda K. Immunopharmacotherapy: vaccination strategies as a treatment for drug abuse and dependence. Pharmacol Biochem Behav. 2009;92:199–205. doi: 10.1016/j.pbb.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kosten T, Owens M. Immunotherapy for the treatment of drug abuse. Pharmacol Ther. 2005:76–85. doi: 10.1016/j.pharmthera.2005.06.009. [DOI] [PubMed] [Google Scholar]; (d) Maurer P, Jennings GT, Willers J, Rohner F, Lindman Y, Roubicek K, Renner WA, Muller P, Bachmann MF. A therapeutic vaccine for nicotine dependence: preclinical efficacy and Phase I safety and immunogenicity. Eur J Immunol. 2005;35(7):2031–2040. doi: 10.1002/eji.200526285. [DOI] [PubMed] [Google Scholar]; (e) Carrera M, Ashley J, Wirsching P, Koob G, Janda K. A second-generation vacine suppresses psychoactive effects of cocaine in the rat. Proc Natl Acad Sci USA. 2001;98:1988–1992. doi: 10.1073/pnas.041610998. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Carrera M, Ashley J, Parsons L, Wirsching P, Koob G, Janda K. Suppression of psychoactive effects of cocaine by active immunization. Nature. 1995;378:727–730. doi: 10.1038/378727a0. [DOI] [PubMed] [Google Scholar]
- 7.Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR. Changes in heroin self-administration by a rhesus monkey after morphine immunization. Nature. 1974;252(5485):708–710. doi: 10.1038/252708a0. [DOI] [PubMed] [Google Scholar]
- 8.Anton B, Leff P. A novel bivalent morphine/heroin vaccine that prevents relapse to heroin addiction in rodents. Vaccine. 2006;24(16):3232–3240. doi: 10.1016/j.vaccine.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 9.(a) Rook E, Hillebrand M, Rosing H, van Ree J, Beijnen J. Poplulation pharmacokinetics of heroin and its major metabolites. Clin Pharmacokinet. 2006;45(4):401–417. doi: 10.2165/00003088-200645040-00005. [DOI] [PubMed] [Google Scholar]; (b) Osborne R, Thompson P, Joel S, Trew D, Patel N, Slevin M. The analgesic activity of morphine-6-glucuronide. Br J Clin Pharmac. 1992;34:130–138. doi: 10.1111/j.1365-2125.1992.tb04121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kuo CK, Hanioka N, Hoshikawa Y, Oguri K, Yoshimura H. Species difference of site-selective glucuronidation of morphine. J Pharmacobio-Dyn. 1991;14:187–193. doi: 10.1248/bpb1978.14.187. [DOI] [PubMed] [Google Scholar]
- 10.Oldendorf W, Hyman S, Braun L, Oldendorf S. Blood-brain barrier: penetration of morphine, codeine, heroin and methadone after carotid injection. Science. 1972;178:984–987. doi: 10.1126/science.178.4064.984. [DOI] [PubMed] [Google Scholar]
- 11.Skibinski D, O'Hagan D. Adjuvants. In: Rappuoli R, Bagnoli F, editors. In Vaccine Design: Innovative Approaches and Novel Strategies. Caister Academic Press; Norfolk, UK: 2011. pp. 139–169. [Google Scholar]
- 12.(a) Glenny AT, Pope CG, Waddington H, Wallace U. The antigenic value of toxiod preciptated by potassium alum. J Pathol Bacteriol. 1926;29:31–40. [Google Scholar]; (b) Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminum. Nat Rev. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris B, Robinson J, Piall E, Aherne G, Marks V. Development of a radioimmunoassay for morphine having minimal cross-reactivity with codeine. J Endocrin. 1975;64(1):6P–7P. [PubMed] [Google Scholar]
- 14.Matsushita M, Hoffman T, Ashley J, Wirsching P, Lerner R, Janda K. Cocaine catalytic antibodies: the importance of linker effects. Bioorg Med Chem. 2001;11:87–90. doi: 10.1016/s0960-894x(00)00659-4. [DOI] [PubMed] [Google Scholar]
- 15.Olofson R. New, useful reactions of novel haloformates and related reagents. Pure Appl Chem. 1988;60(11):1715–1724. [Google Scholar]
- 16.Boeglin D, Lubell WD. Aza-Amino Acid Scanning of Secondary Structure Suited for Solid-Phase Peptide Synthesis with Fmoc Chemistry and Aza-Amino Acids with Heteroatomic Side Chains. J Comb Chem. 2005;7(6):864–878. doi: 10.1021/cc050043h. [DOI] [PubMed] [Google Scholar]
- 17.Bray B, Kelly D, Mack P, Martin R, Wakelin L. DNA-binding compounds. III. Synthesis of a peptide-linked binuclear platinum(II)-terpyridine complex. Aust J Chem. 1990;43(3):629–634. [Google Scholar]
- 18.Muller R. Determination of affinity and specificity of anti-hapten antibodies by competitive radioimmunoassay. Meth Enzymol. 1983;92:589–601. doi: 10.1016/0076-6879(83)92046-3. [DOI] [PubMed] [Google Scholar]
- 19.Walker J, Chen S, Moffitt H, Inturrisi C, Koob G. Chronic opioid exposure produces increased heroin self-administration in rats. Pharmacol Biochem Behav. 2003;75(2):349–354. doi: 10.1016/s0091-3057(03)00094-7. [DOI] [PubMed] [Google Scholar]
- 20.Moreno A, Mayorov A, Janda K. Impact of Distinct Structures for the Development of a Methamphetamine Vaccine. J Am Chem Soc. 2011;133:6587–6595. doi: 10.1021/ja108807j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(a) Tomei E, Renaud FL. Effect of morphine on Fc-mediated phagocytosis by murin macrophages in vitro. J Neuroimmun. 1997;74:111–116. doi: 10.1016/s0165-5728(96)00213-5. [DOI] [PubMed] [Google Scholar]; (b) Roy S, Cain KJ, Chapin RB, Charboneau RG, Barke RA. Morpine modulates NFkB activation in macrophages. Biochem Biophys Res Comm. 1998;245:392–396. doi: 10.1006/bbrc.1998.8415. [DOI] [PubMed] [Google Scholar]
- 22.Neville G, Ekiel I, Smith I. High-resolution proton magnetic resonance spectra of morphine and its three O-acetyl derivatives. Mag Res Chem. 1987;25:31–35. [Google Scholar]
- 23.(a) Zinieris N, Leondiadis L, Ferderigos N. Nα-Fmoc Removal from Resin-Bound Amino Acids by 5% Piperidine Solution. J Comb Chem. 2004;7(1):4–6. doi: 10.1021/cc049872d. [DOI] [PubMed] [Google Scholar]; (b) Fransson R, Botros M, Sko□ld C, Nyberg F, Lindeberg G, Hallberg M, Sandstro□m A. Discovery of Dipeptides with High Affinity to the Specific Binding Site for Substance P1−7. J Med Chem. 2010;53(6):2383–2389. doi: 10.1021/jm901352b. [DOI] [PubMed] [Google Scholar]
- 24.King T, Vera-Portocarrero L, Gutierrez T, Vanderah T, Dussor G, Lai J, Fields H, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixon W. Efficient analysis of experimental obervations. Ann Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 26.Vendruscolo LF, Schlosburg JE, Misra KK, Chen S, Greenwell TN, Koob GF. Escalation patterns of varying periods of heroin access. Pharmacol Biochem Behav. 2011 doi: 10.1016/j.pbb.2011.03.004. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.