Abstract
Chain-transfer reactions from thiols to methacrylates are expected to delay gelation and possibly reduce stress at the bonded interface of dental restorations. Thiol additives with varying structures were combined with a dimethacrylate commonly used in dental materials. Polymerization stress/modulus development were monitored by a tensometer/rheometer, respectively, both coupled with RT-NIR. For all thiol-modified materials, conversion and modulus were 5–25 % higher than the control, and maximum reaction rate was 25–50 % lower. Gel point conversions were 12–22 % (control=5 %), and deceleration was observed at later stages in conversion (30–60 %; control=15 %). Consequently, even with increased conversion/modulus, stress values were either equal or reduced compared to the control. This approach does not require any modification in the bonding/photoactivation procedures, and seems promising for stress management not only in polymeric dental materials, but also for other applications of glassy, crosslinked photopolymers, as long as thiol volatility is addressed.
Keywords: Methacrylates, gel-point conversion, polymerization stress, chain-transfer reactions, networks
Introduction
Dimethacrylate polymerizations, which serve as the basis of most commercial dental restorative materials, typically progress rapidly and undergo crosslinking from early stages in conversion [1, 2]. Although this is an advantage from the clinically practical standpoint, allowing the in situ formation of strong, mechanically functional materials almost immediately, early crosslink formation and the fact that continued network development becomes reaction diffusion-controlled soon after gelation [3] leaves little opportunity for viscous flow during polymerization, which contributes to stress build up on bonded interfaces [4]. This is in addition to a relatively high molar shrinkage coefficient [5, 6] given by the difunctional transition to covalent bonds between monomer molecules. One of the approaches to circumvent this problem is to reduce shrinkage, which propelled the synthesis of a variety of alternative monomers, which either involve higher molecular weight (lower initial reactive group concentration) or structures that engage in ring-opening polymerization mechanisms that provide reduced molar shrinkage coefficients [7]. Other efforts have focused on the delay of gelation, the classical example being the thiol-ene reaction, in which the well established alternating addition/chain-transfer reactions of thiol to ene affords a step-growth process in which network formation is delayed with respect to chain-growth polymers [8]. That way, even in densely crosslinking systems, high molecular weight polymer is formed at much later stages in conversion, reducing the magnitude of the viscoelastic effects on internal and interfacial stress through flow during the extended pre-gel regime. Pure thiol-enes are impractical for dental restorative applications because the relatively flexible nature of the step-growth polymer backbone limits glass transition temperature (Tg), hence the proposition of their combination to glassy methacrylates in ternary systems [9–11]. In methacrylate polymerizations, chain transfer is a chain breaking mechanism in which the radical formed through the transfer serves as a new initiation site [3]. There has been evidence that ternary thiol-ene/methacrylate systems ally stress reduction, which is hypothesized to be due to delayed gelation, with good mechanical properties similar to conventional dimethacrylates [12]. Mechanistic kinetics studies have shown, however, that the ene component in this type of hybrid ternary formulation has little to no participation in early network formation, since its consumption is delayed until the methacrylate-thiol reaction is largely complete [9, 10]. In turn, the methacrylate conversion in this ternary system progresses at a slower rate compared with the methacrylate alone, indicating that chain transfer reactions with thiol are altering network development and associated kinetic behavior. It then follows that the consequent stress reduction obtained in the ternary system is expected to also be achieved in large part with a simpler thiol-methacrylate system. It is important to note that this approach differs completely from the proposed soft-start polymerization protocols [13, 14] as a means to delay gelation with respect to time rather than conversion. While extending the time to reach the gel point, the use of an initially low irradiance photocuring protocol actually increases chain lengths and leads to gelation at a lower conversion value than would be obtained at higher radical concentrations at higher levels of irradiance [15].
In methacrylate polymerizations, the addition of chain-transfer agents (thiols being very efficient in this role) in amounts as low as 0.1 wt % significantly reduces the rate of polymerization and the polymer chain length [16–18]. Due to the shorter chains, and consequently the higher concentration of chain ends, thiol-modified methacrylate networks could be expected to produce lower, but yet narrower Tg values, which translates into more homogeneous networks [19, 20]. However, because of chain transfer provides for enhanced mobility within the developing network, higher limiting conversion is also likely to result [3], which may compensate for the reduction in Tg values expected to be achieved. Also, if multifunctional thiols are used as the chain-transfer agents, the potential for crosslinking through chain ends is expected to contribute to network structure with improved mechanical properties compared to monofunctional thiols [9]. This study proposes a simple modification of dimethacrylate polymerizations through the addition of small to moderate amounts of thiol chain-transfer agents with the objective of delaying gelation and ultimately reducing polymerization stress. Other features of interest for dental materials applications include the potential for lower molar shrinkage due to monofunctional double bond consumption as well as decreased sensitivity to oxygen inhibition [11, 21]; however, these aspects are not the focus of this investigation. This study fills a gap in knowledge of ternary hybrid thiol-ene/methacrylate systems by simplifying the matrix phase to focus exclusively on the thiol/methacrylate interactions and how thiol structure, functionality and reactivity can be used to alter methacrylate reaction kinetics, final conversion and the physical/mechanical properties of polymer networks, including stress development, based on significant chain transfer contributions during photopolymerization.
Experimental
Material formulations
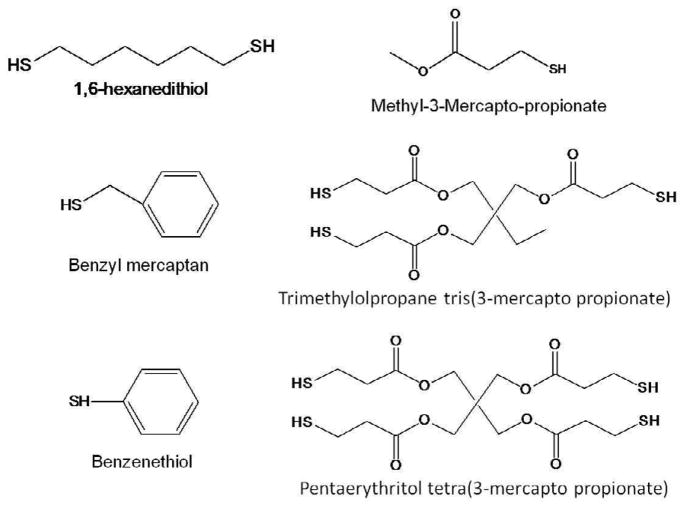
The thiol compounds (obtained from Aldrich, Milwaukee, WI) used in this study are shown in Figure 1. Ethoxylated bisphenol A methacrylate (BisEMA; Esstech, Essington, PA) was combined with thiols at 5, 10 or 20 mol% of thiol to methacrylate functionalities. The unmodified methacrylate monomer served as the control. The selected model additives span various types of chain end groups, number of thiol functionalities and spacer lengths/flexibilities. Dimethyl phenylacetophenone (DMPA, λmax; =365 nm; Aldrich) was added at 0.1 wt% as a simple, single-component photoinitiator. Unless specified otherwise, materials were photoactivated at 50 mW/cm2, using a mercury arc light source (Acticure 4000, EXFO, Ontario, Canada), filtered to 320–390 nm.
Figure 1.
Structures of thiols used in this study.
IR monitoring
The kinetics of methacrylate conversion and thiol consumption was followed with mid/near-IR during UV photoactivation [22]. The near-IR technique is very convenient since it does not require purging and therefore allows for remote monitoring of specimens being tested in different set ups, such as the tensometer [4] and the rheometer [23]. The range of the near-IR spectrum was extended to encompass both the methacrylate first overtone absorption band at 6165 cm−1 [22] and the thiol absorption band at 2575 cm−1 in the mid-IR region [21], allowing for their simultaneous monitoring. Maximum rate of polymerization (Rpmax) was obtained from the first derivative of the degree of conversion (DC) versus time kinetic curves. Plots of Rpmax normalized by the vinyl concentration (Rpmax/[M]) were also constructed.
Residual thiol determination
Discs of the thiol-modified polymer materials (15 mm in diameter and 1 mm in thickness) were placed in a Soxhlet extraction apparatus, through which methylene chloride was refluxed for 4 hours. The solvent was removed and the resulting sol fraction was analyzed by 1H-NMR for monomer and thiol residue, based on =CH2 resonances at approximately 5.1 – 6.5 ppm for the methacrylate in the monomer and based on –SH resonances at approximately 0.9 – 2.5 ppm for aliphatic thiols and 2.8 – 3.6 for benzenethiol [24].
Polymerization stress
Polymerization stress development was followed in real-time with methacrylate conversion using the American Dental Association - Health Foundation tensometer, as previously described [4]. Briefly, the material is placed between two glass rods, attached to the fixed base of the apparatus and to a deformable cantilever beam. As the material polymerizes and shrinks, it causes a deflection in the beam and the stress is then calculated based on the cross-sectional area of the specimen and a calibration curve of the beam constant obtained previously. Rate of stress development (Rs) was calculated as the first derivative of the stress versus time curve. Fiber optic cables provide remote monitoring of vinyl conversion through near-IR.
Gelation profiles coupled to polymerization kinetics and turbidity measurements
Samples of selected formulations were sandwiched between two 20 mm parallel quartz disc plates, attached to a rheometer (ARES, TA Instruments, New Castle, DE, USA) and tested in shear at a frequency of 100 rad/s with 10 % strain (ensuring that the test was carried out within the linear viscoelastic regime), while being photopolymerized at 0.07 mW/cm2 under nitrogen purge. An optical apparatus developed in our laboratory allowed both UV and NIR direct transmission access to specimens within the photorheometer so that methacrylate conversion was remotely followed concomitantly with modulus development (a detailed description of the instrumentation and procedure will be reported separately). The crossover between G′ (storage modulus) and G″ (loss modulus) was used to determine the gel point conversion [25].
Mechanical properties
Flexural strength and modulus were evaluated on 2×2×25 mm bars in three-point bending, with 20 mm span between the supports, tested at a cross-head speed of 1 mm/min (according to ASTM D-790)[26].
Statistical analysis
Two-way analysis of variance/Tukey’s test was used as the statistical method, once the results have shown normal distribution and homocedasticity. A sample size of three for the kinetic runs, tensometer and rheometer experiments and five for the flexural tests has been shown to provide sufficient power to the statistical analysis, at a 95 % confidence level.
Results and discussion
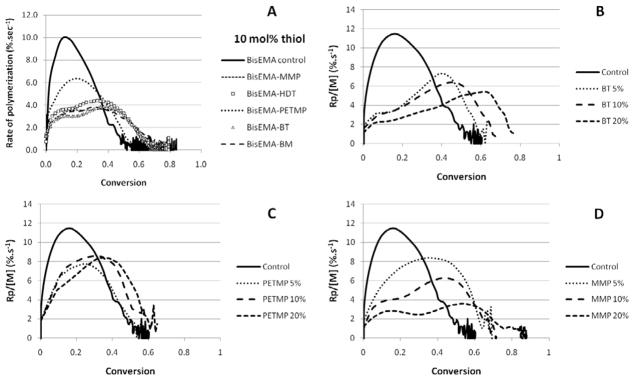
Due to their high reactivity and tendency for homopolymerization, methacrylates are not normally used as the vinyl monomer in classic thiol-ene reactions [8]. However, the potential for delayed gelation with the use of thiols as chain transfer agents is retained even in methacrylate polymerizations and makes this approach attractive as a means to potentially reduce shrinkage stress. Indeed, the conversion-dependent rate of polymerization plots (Figure 2) show generally extended conversions at deceleration with the addition of thiols. While this does not translate into a practical determination of the gel point (since the maximum rate of polymerization is typically observed after the gelation), the conversion at the onset of deceleration, when both propagation and termination become diffusion controlled, corresponds to vitrification [1, 3], as will be explored further.
Figure 2.
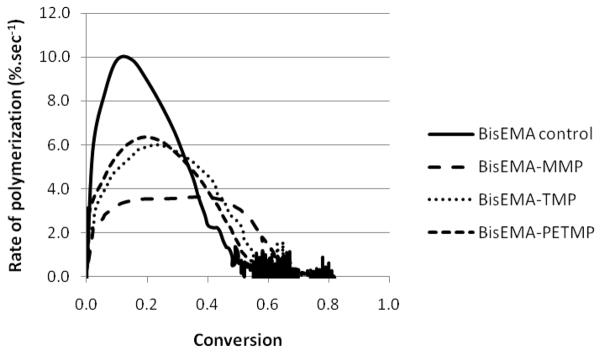
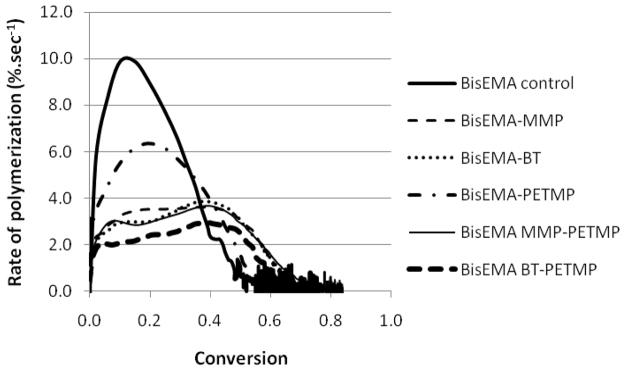
(A) Polymerization kinetics for BisEMA (control) and BisEMA combined to thiols of different functionalities at a 10:1 molar ratio: monothiol (MMP – methyl-3 mercaptopropionate), di-thiol (HDT –1,6-hexanedithiol), tetra-thiol (PETMP – pentaerythritol tetra-(3-mercaptopropionate)) and two aromatic thiols (benzene thiol and benzyl mercaptan, also monofunctional). (B–D) Polymerization kinetics for BisEMA (control) and BisEMA combined to thiols of different functionalities at different molar ratios: monothiol (MMP – methyl-3-mercaptopropionate), tetra-thiol (PETMP – pentaerythritol tetra-(3-mercaptopropionate)), aromatic thiol (BT - benzene thiol, also monofunctional). Materials were polymerized at 50 mW/cm2, using a 320–390 nm band-pass filter.
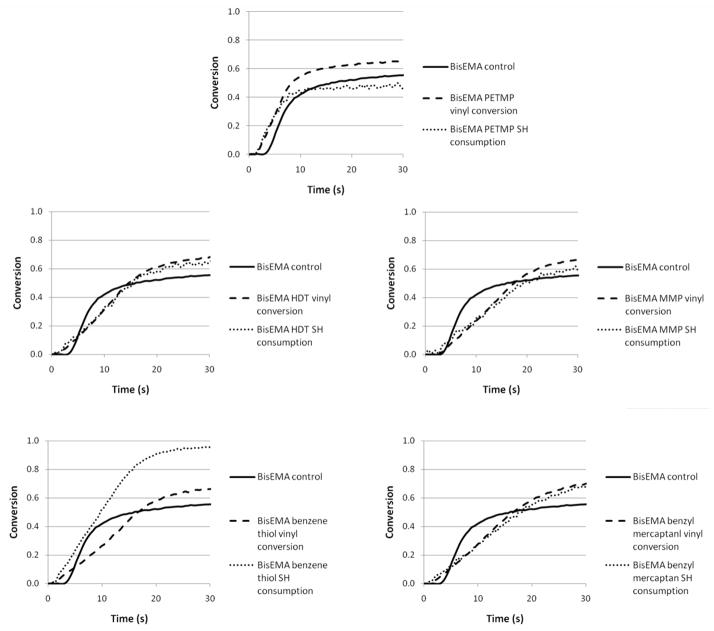
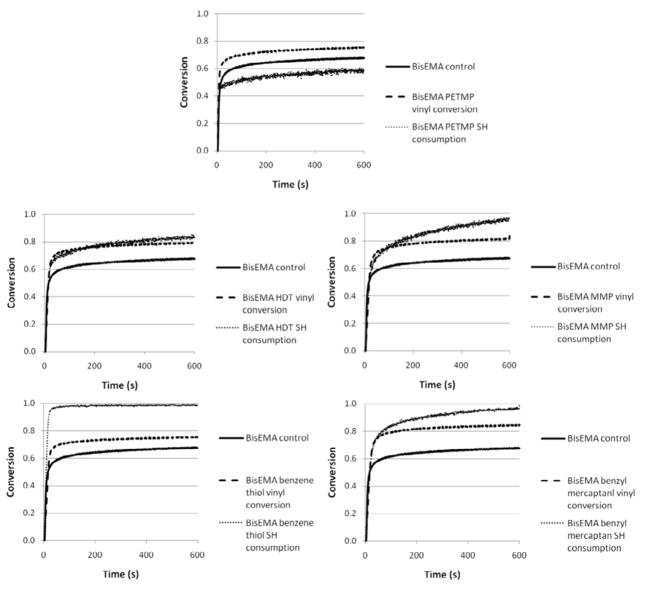
The polymerization for the unmodified methacrylate used as a control is affected by oxygen inhibition, a well described phenomenon for free-radical polymerizations [27], as is evidenced by the very slow progression for the first 4–5 seconds of irradiation (Figure 3). In general, the addition of thiols reduced the induction period (BisEMA-MMP being the exception), as can be observed in Figure 3. In a comparison of mono-thiols, a ranking of reactivity could be established with BT being the most reactive, followed by BM and MMP. Direct aromatic substitution of the thiol, as in BT, is expected to increase its chain transfer reactivity due to electronic stabilization. Thiol and vinyl conversion were simultaneous with similar rates for the first several seconds, except for the very reactive benzenethiol. For that material, polymerization rates and the delay in autodeceleration were comparable to other mono-thiols, but with a much faster –SH group consumption in relation to the vinyl, pointing to more efficient chain transfer reactions. For BT-modified photopolymerizations, at the point thiol conversion reached 90 %, vinyl conversion was only approximately 50 %. The limiting vinyl conversion reached 75 % with BT present compared with 68 % for the control. For PETMP, 90 % of the final thiol consumption was also reached by 50 % vinyl conversion, but in that case the thiol and vinyl final conversions values were lower (46 and 70 %, respectively), compared to BT, which was completely consumed. In every other case (MMP, BM and HDT), the thiol is consumed concomitantly with the vinyl up to approximately 80 % conversion, meaning that –SH groups are available to chain-transfer through the end of polymerization (Figures 3 and 4).
Figure 3.
Initial polymerization kinetics (0–30 s) for BisEMA alone or combined to different thiols. Materials were polymerized at 50 mW/cm2, using a 320–390 nm band-pass filter.
Figure 4.
Polymerization kinetics for BisEMA (control) and BisEMA combined to thiols of different functionalities at a 10:1 molar ratio: monothiol (MMP – methyl-3- mercaptopropionate), di-thiol (HDT – 1,6-hexanedithiol), tetra-thiol (PETMP – pentaerythritol tetra-(3-mercaptopropionate)) and two aromatic thiols (benzene thiol and benzyl mercaptan, also monofunctional). Materials were polymerized at 50 mW/cm2, using a 320–390 nm band-pass filter.
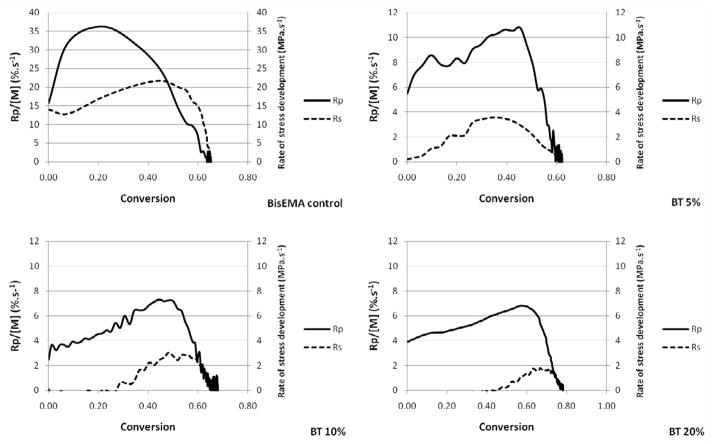
In Figure 2, the conversion is plotted against polymerization rate and the point in conversion where the rate starts to decelerate reveals the onset of severe diffusion limitations for propagation, corresponding to vitrification [3]. In dimethacrylate polymerizations, termination is predominantly reaction-diffusion controlled [28] and it has been reported that the addition of chain transfer agents to these reactions, even at amounts as minute as 0.1 wt% translates into a delay in the reaction-diffusion-controlled stage of the polymerization, i.e., the reduction in average chain length allows conversion to progress to a greater extent before significant mobility restrictions are imposed [16]. All mono-thiols were effective in increasing the point in conversion at which deceleration takes place based on mobility enhancement due to shorter chains and more chain ends. In spite of the different reactivities, MMP, BM and BT all led to deceleration at around 35–45 % vinyl conversion (Figure 2), much higher than the 10 and 19 % conversion levels observed for the control and PETMP, respectively. The efficient chain-transfer mechanism affords increased mobility, while conveniently allowing for high final conversions to be achieved within the same time as the control. As for the gel point, the conversion at the onset of autoacceleration, also called the gel-effect, provides a reasonable indication of network formation as the point at which termination becomes selectively restricted, but it cannot be used as an accurate substitute for true gel point determination (through rheology experiments). The gel point in bulk dimethacrylate polymerizations is known occur at less than 5 % conversion [29] while the rate maximum in typical dental resins has been measured at about 5 to 20 % conversion depending on initial resin viscosity and curing conditions [2]. Commercial dental composites display maximum polymerization rates at about 10 % conversion [30]. The gel point, as determined by the crossover of G′ and G″ [25], was 5 % for the pure dimethacrylate control, in close agreement with previously reported data, validating the experimental set up used here. Gelation is a bond percolation transition: at the gel point, the system is a polydisperse mixture of branched polymers, with one structure percolating the entire system (or, in other words, the stage when a volume-spanning single macromolecule is first formed) [31]. At that point, rigidity of the network starts to develop, so delayed gelation has been regarded as an efficient way of reducing stress through viscous flow [32]. However, it is later in conversion, as the increasing Tg of the developing network begins to overlap the effective cure temperature, which marks the onset of vitrification, that the majority of stress begins to develop [4]. Thiol modified network formation can be expected to develop Tg more slowly (with respect to conversion) and undergo vitrification over a narrower range of conversion compared with conventional dimethacrylate polymerizations. In viscoelastic systems, such as the dimethacrylates used here, this means that the modulus/stiffness of the materials remains relatively low between gelation and vitrification, which in turn allows for a greater amount of strain to be accommodated. By decreasing the breadth of thermal transition (tan delta peak), which means a more homogeneous network structure is formed, the limiting Tg may actually be somewhat reduced compared to that of a similar polymer representing a broader range of Tg (more heterogeneous network structure). The rheology experiments demonstrated significantly delayed gelation with the addition of thiols. While the crossover of G′/G″ occurred at approximately 5 % for the control, the addition of 10 mol% thiol delayed this event to 22, 13 and 12 % conversion, for BT, MMP and PETMP, respectively. The details of the rheological experiments will be reported in a separate study. This delay in modulus development, rather than viscous flow, reflects on delayed stress development and lower stress values overall, and has been demonstrated in ternary thiol-ene/acrylate systems [10]. Therefore, more than describing the kinetic profiles of vinyl and thiol consumption, the main goal of this paper was to identify compositions in which chain-transfer reactions would be efficient in delaying gelation/vitrification and ultimately, providing an effective reduction in the final stress state.
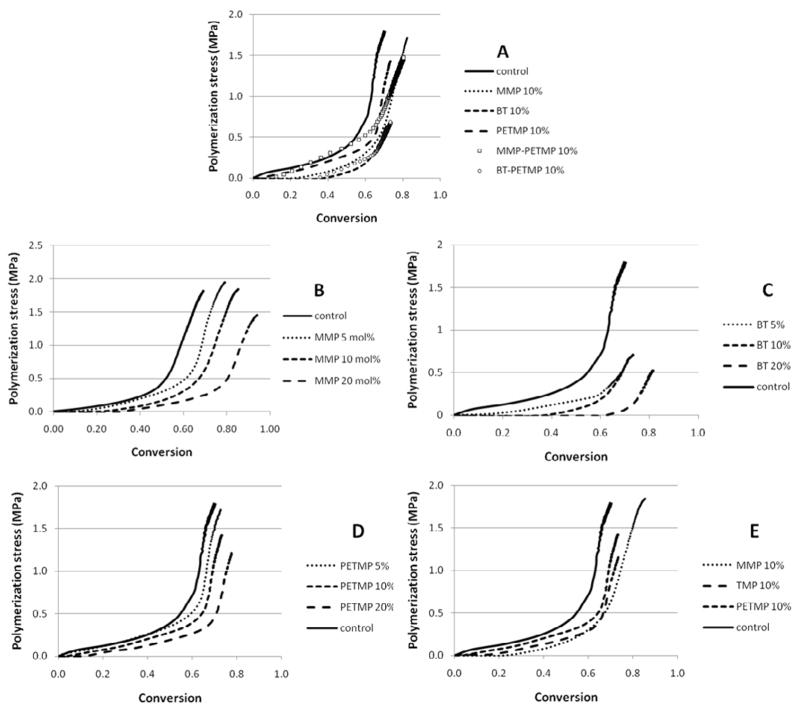
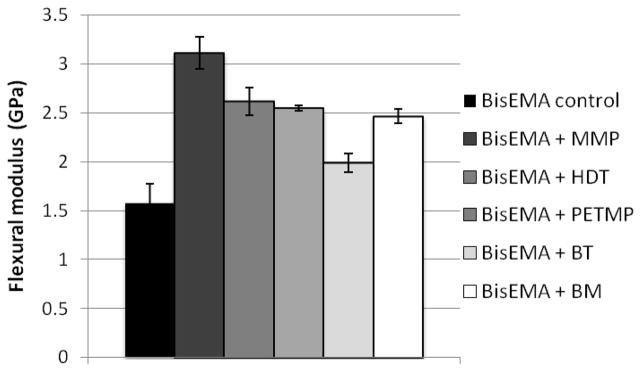
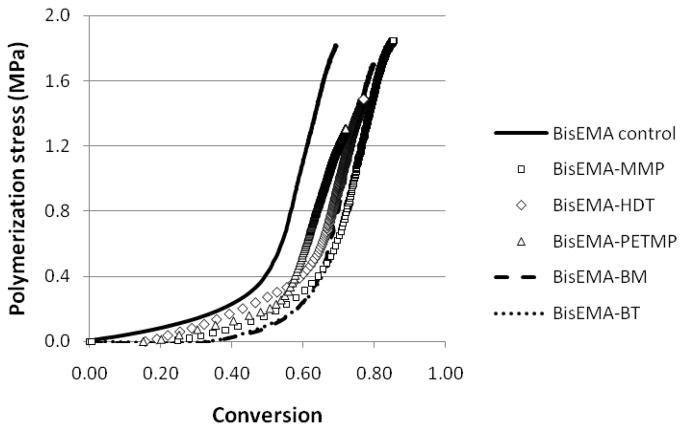
Stress is a function of the shrinkage and stiffness development, both of those dictated by conversion. Shrinkage was not evaluated here. However, the monofunctional character of methacrylate bonds involved in the chain transfer reaction to thiols and also the expected greater free volume due to the increase in the number of chain ends in the case of monothiols [33], are likely to contribute lower shrinkage; however, once the increase in limiting conversion is considered, an increase in the overall volumetric shrinkage might be expected. It should be emphasized that it is only post-gel shrinkage and particularly, shrinkage occurring as vitrification is approached that actually contributes meaningfully to stress development. The simultaneous, real-time evaluation of polymerization stress with the tensometer and conversion kinetics through NIR [4] allows for rational interpretation of how changes in material composition affect polymerization stress development. In Table 2, the values of Rpmax were normalized by the vinyl group concentration at each time point, since that provides a better account of the reaction kinetics with the actual reactive group availability, which obviously changes during the course of polymerization. For all thiols, at every concentration, stress starts to develop at conversion values that are lower than the point at which at Rpmax is observed (Table 2), showing that while the onset of autoacceleration may reasonably approximate gel point, Rpmax does not. As the initial thiol concentration increases, both the onset of stress and Rpmax are delayed to later stages in conversion. Beyond the gel point and through the rubbery regime, a steady increase in stress is sustained until later in conversion, when stress rises dramatically as vitrification is approached. The vitrification point can be reliably estimated by the onset of deceleration with respect to conversion. The conversion at which deceleration was detected from the kinetic data increased linearly with the thiol concentration for all thiols. The conversion values at the point where stresses increased sharply were systematically higher than the conversion at the onset of the kinetic deceleration. This indicates that stresses develop more markedly into the vitrified state, possibly enhanced by the fact that shrinkage development may be delayed in relation to conversion in glassy materials [34] and further, may involve thermal contraction effects. As a way to account for the differences in rate of polymerization (Rp) on stress development, plots of Rp x Rs (rate of stress development) were constructed to provide cleaner comparisons between the groups than the one provided by the simple conversions at the different stages of stress development. Consistently, Rsmax coincides in DC with Rpmax (normalized by the vinyl concentration), which is also the conversion at deceleration (Figure 5). For the control, stress rises dramatically around 50 % conversion (Figure 6, Table 2). As the thiol concentration increases, the point in conversion at which the dramatic rise in stress is observed also increases, reaching 66–75 % for the highest thiol concentrations (Figure 6, Table 2). Another way of comparing stress data when final conversion values differ among materials is by integrating the areas under the Rp/[M] x DC and Rs x DC curves, and then calculating the ratio of conversion to stress. This ratio increases with thiol concentrations, in the following order: BT>MMP=PETMP (Table 2). This provides evidence that, at the same overall thiol concentration, mono-thiols were more efficient than multi-thiols in delaying the onset of gelation/vitrification, and among the mono-thiols, BT was the most efficient in this role, as already discussed. For all thiols, increasing thiol concentration also increases limiting conversion and, in spite of that, decreases stress. Notably, bulk flexural modulus, as evaluated by three-point bending, was also significantly improved, most likely due to the higher conversion achieved, despite the greater local flexibility in the region of the chain transfer event. So, in spite of significantly higher modulus (Figure 7) and conversion (Figure 8) as well as potentially increased overall polymerization shrinkage, the experimental polymeric materials showed stress development either significantly lower or at least similar compared to the control (Figure 8).
Table 2.
Results from the stress test (DC: degree of conversion; Rpmax: maximum rate of polymerization). Values followed by the same superscript within the same column are not statistically different (2-way ANOVA, α=0.05).
| Group | Tensometer (30 mW/cm2) | Ratio DC/stress | |||
|---|---|---|---|---|---|
| DC (%) at onset of stress | DC (%) at Rpmax* | DC (%) at stress shoot up | DC (%) at decel.* | ||
| Control | 2.4 (0.5)f | 22.0 (4.0)d | 44.3 (5.5)d | 22.0 (4.0)e | 0.7 |
| BT 5% | 17.0 (0.2)d | 45.4 (0.6)c | 56.0 (0.0)c | 45.4 (0.6)cd | 5.6 |
| BT 10 % | 38.3 (0.0)b | 49.4 (0.6)bc | 57.3 (1.5)c | 49.4 (0.6)b | 5.6 |
| BT 20% | 62.7 (0.1)a | 57.5 (1.7)ab | 68.0 (1.0)ab | 64.9 (1.7)a | 5.8 |
| MMP 5% | 12.3 (2.5)de | 43.3 (4.0)c | 55.7 (3.1)c | 43.3 (4.0)cd | 0.6 |
| MMP 10% | 24.7 (3.2)c | 45.3 (6.1)c | 64.0 (3.5)b | 50.3 (6.1)bc | 0.7 |
| MMP 20% | 35.0 (2.0)b | 61.0 (1.5)a | 75.3 (1.5)a | 63.3 (1.5)a | 1.4 |
| PETMP 5% | 9.7 (1.5)e | 39.4 (2.4)c | 61.3 (1.2)bc | 38.6 (2.5)d | 0.8 |
| PETMP 10% | 10.0 (0.6)e | 44.1 (3.0)c | 62.0 (1.0)b | 44.1 (3.0)cd | 1.1 |
| PETMP 20% | 18.9 (2.7)d | 53.0 (4.9)ab | 66.7 (1.2)b | 53.0 (5.0)ab | 1.6 |
values normalized by the vinyl group concentration.
Figure 5.
Conversion as a function of rate of polymerization (normalized by the vinyl concentration) and rate of stress development for the benzene thiol groups (BT) at different concentrations. The graphs for methyl-3-mercaptopropionate and pentaerythritol tetra-(3-mercaptopropionate) (MMP and PETMP, respectively) are not shown. Materials were polymerized at 50 mW/cm2, using a 320–390 nm band-pass filter.
Figure 6.
Polymerization stress as a function of vinyl conversion for (A) BisEMA (control) and BisEMA combined to thiols of different functionalities at a 10:1 molar ratio (MMP – methyl-3-mercaptopropionate; BT - benzene thiol and PETMP – pentaerythritol tetra-(3-mercaptopropionate)) and combinations; (B – D) BisEMA (control) and MMP, BT and PETMP at different concentrations and (E) BisEMA (control) and MMP, Trimethylolpropane tris(3-mercaptopropionate) (TMP) and PETMP (mono, tri and tetra thiols, respectively) at 10:1 molar ratio. Materials were polymerized at 50 mW/cm2, using a 320–390 nm band-pass filter.
Figure 7.
Flexural strength for BisEMA (control) and BisEMA combined to thiols of different functionalities at a 10:1 molar ratio: monothiols (MMP – methyl-3-mercaptopropionate or DDT-dodecanethiol), di-thiol (HDT – 1,6-hexanedithiol), tetra-thiol (PETMP – PETMP – pentaerythritol tetra- (3-mercaptopropionate)) and two aromatic thiols (benzene thiol and benzyl mercaptan, also monofunctional). Materials were polymerized at 50 mW/cm2, using a 320–390 nm band-pass filter.
Figure 8.
Stress development plotted as a function of conversion for BisEMA (control) and BisEMA combined to thiols of different functionalities at a 10:1 molar ratio: monothiols (MMP – methyl-3- mercaptopropionate or MBT – 4-methyl benzenethiol), di-thiol (HDT – 1,6- hexanedithiol), tetra-thiol (PETMP – PETMP – pentaerythritol tetra-(3-mercaptopropionate)) and two aromatic thiols (benzene thiol and benzyl mercaptan, also monofunctional). Materials were polymerized at 50 mW/cm2, using a 320–390 nm band-pass filter.
The effect of the number of thiol functional groups on thiol-ene reactions has been extensively explored [8, 35]. Although it has been reported that the thiol functionality has little to no effect on thiol-ene polymerization kinetics, its influence on the onset of gel point was well established for liquid crystal display applications [35]. This approach has not been investigated for dimethacrylates, so here the number of thiol functionalities was also studied in a series of analogous mono-, tri- and tetra-thiols (MMP, TMP and PETMP, respectively) combined with BisEMA. Our major goal with this design was to identify possible differences in network structure, since these molecules are expected to provide a wide range of effective crosslinking potential. The tri- and tetra-functional thiols behaved similarly and were able to increase conversion at deceleration to 22 and 19 %, respectively (compared to 15 % for the control), with a maximum rate of polymerization that was 70 and 40 % lower than that of the control (for TMP and PETMP, respectively) (Table 1). The mono-functional thiol reached much higher conversion prior to autodecceleration (around 50 %) and presented an Rpmax 60 % lower than the control (Table 1, Figure 9), adding evidence to a likely effect of molecular mobility/diffusion ability on the chain-transfer potential of thiol additives, in addition to the much different steric access for chain-growth in comparison to step-growth processes. For PETMP, the vinyl consumption kinetic profile was very similar to the unmodified methacrylate (Figure 3). This is explained by the fact that for the multi-functional thiol molecules (and especially for the tetra-functional PETMP), as one of the –SH groups engages in chain-transfer and the molecule becomes covalently bound to the network, mobility/reactivity of the remaining thiol groups is significantly reduced. This means that the effective thiol concentration in those cases is likely underestimated in relation to the more mobile and more uniformly dispersed mono-thiol analogs. Even without assuming thiol reactivity differences, at a fixed thiol:methacrylate ratio, the effective intermolecular distance between thiols increases with the thiol functionality, which implies that some portions of the network would more closely resemble the dimethacrylate control.
Table 1.
Results from standard kinetic runs (DC: degree of conversion; Rpmax: maximum rate of polymerization). Values followed by the same superscript within the same column are not statistically different (2-way ANOVA, α=0.05).
| Group | Standard kinetic runs (30 mW/cm2) | ||||
|---|---|---|---|---|---|
| Final vinyl DC (%) | Final thiol DC (%) | DC (%) at Rpmax* | Rpmax* (%.s−1) | DC (%) at decel.* | |
| Control | 67.3 (0.5)gh | - | 15.4 (0.5)f | 11.5 (0.9)a | 15.4 (1.0)f |
| BT 5% | 69.0 (1.0)fg | 97.3 (3.1)a | 39.1 (0.5)c | 7.3 (0.2)d | 39.0 (1.5)c |
| BT 10 % | 74.3 (2.0)e | 100.0 (0.0)a | 45.0 (0.2)b | 6.4 (0.3)e | 47.0 (1.3)b |
| BT 20% | 84.0 (2.0)b | 96.3 (3.5)a | 61.0 (0.4)a | 5.4 (0.1)e | 61.0 (0.9)a |
| MMP 5% | 80.0 (1.0)d | 100.0 (0.0)a | 34.6 (0.3)d | 8.4 (0.1)bc | 38.0 (1.0)c |
| MMP 10% | 82.3 (0.5)c | 88.3 (3.2)b | 43.7 (0.2)b | 6.2 (0.6)e | 45.0 (1.6)b |
| MMP 20% | 91.7 (0.5)a | 72.0 (2.0)c | 60.6 (0.3)a | 8.4 (0.5)bc | 60.5 (0.8)a |
| PETMP 5% | 65.7 (0.5)h | 92.3 (3.1)ab | 23.0 (0.2)e | 7.7 (0.5)cd | 23.0 (0.2)e |
| PETMP 10% | 70.0 (0.0)fg | 54.7 (1.5)d | 30.0 (0.3)d | 8.6 (0.5)b | 30.0 (0.2)d |
| PETMP 20% | 73.0 (1.0)ef | 38.3 (0.3)e | 33.0 (0.5)d | 8.4 (0.4)bc | 38.0 (0.1)c |
values normalized by the vinyl group concentration.
Figure 9.
Polymerization kinetics for BisEMA (control) and BisEMA combined to thiols of different functionalities at a 10:1 molar ratio: monothiol (MMP – methyl-3 mercaptopropionate), tri-thiol (TMP – Trimethylolpropane Tris(3-mercaptopropionate)), tetra thiol (PETMP – pentaerythritol tetra-(3- mercaptopropionate)). Materials were polymerized at 50 mW/cm2, using a 320–390 nm band-pass filter.
Through the systematic evaluation of the isolated thiol species conducted so far, blends of thiols were designed, with the aim of optimizing gelation/vitrification control. One highly reactive thiol (BT), which efficiently delays the onset of gelation at the early stages of polymerization, was combined with a multifunctional, less reactive thiol (PETMP) that would provide crosslinking and additional chain-transfer at later stages. Alternatively, MMP was also combined to PETMP, since in this case the reactivity of the functional group itself is similar in both thiols. The overall methacrylate to thiol functional group ratio was kept at 10:1, with mono- and tetra-thiols each contributing half of the thiol functionalities. When 50 % of the tetra-functional thiol was replaced by BT, the delay in deceleration (at 41 % conversion) was greater than PETMP alone (at 19 %) and only slightly lower than BT (at 45 % conversion) (Figure 10, Table 1). Rpmax is reduced by 70 %, however. Because BT is much more reactive than PETMP, it is likely that it was responsible for delaying deceleration at the early stages of the reaction, giving PETMP extended opportunity to chain-transfer before getting entrapped into the network, in a synergistic effect. When MMP was partially substituted for PETMP, the material behaves similarly to the MMP-only composition (deceleration at 38 and 36%, respectively), and the synergistic effect is less noticeable as is the stress reduction (Figure 5).
Figure 10.
Polymerization kinetics for BisEMA (control) and BisEMA combined to thiols of different functionalities at a 10:1 molar ratio: monothiol (MMP – methyl-3- mercaptopropionate), tetra-thiol (PETMP – pentaerythritol tetra-(3-mercaptopropionate)), aromatic thiol (BT - benzene thiol, also monofunctional) and combinations. Materials were polymerized at 50 mW/cm2, using a 320–390 nm band-pass filter.
In general, the incorporation of the chain transfer agents to the dimethacrylate polymerization led to improvement in final conversion ranging from 10 to 20 %, with a strong dependence on thiol group functionality, as well as reactivity. However, in some cases, thiol conversion was incomplete at the limiting vinyl conversion. The extent of thiol consumption is a legitimate concern from the clinical standpoint, not only because of the odor, but also because of the diffusional mobility of such small molecules, which could facilitate their leaching, in the event of incomplete reactions. Different from mono-thiols, incomplete thiol group consumption for multifunctional thiols does not necessarily lead to leachable components. Therefore some residual thiol can be anticipated and tolerated with less concern for both odor and leachability. In the material containing the tetra-thiol (BisEMA-PETMP), thiol consumption reached 60 %, at an initial 10:1 functional group ratio (Table 1). For the mono-thiols, with relatively low molecular weight and great flexibility, small molecule diffusion allows for their nearly complete consumption, shown to be 96% or higher (Figure 7, Table 1). To confirm that either all thiol functionalities had been consumed or that minimally, all thiol molecules were covalently attached to the network, disc specimens were refluxed in dichloromethane using a Soxhlet apparatus. The analysis of the extracted components from thiol-methacrylate specimens by NMR shows no resonance at the sulfhydryl proton regions, even for higher thiol concentrations (up to 20 mol %). This was true for all thiols tested (BT, MMP and PETMP), which shows that even when the consumption was not complete, the thiols are tied into the network and are unlikely to be leachable. For the mono-thiols, the absence of a detectable NMR resonance might have been, apart from complete consumption during polymerization, also due to the volatility of those compounds, which may then not have survived the solvent extraction/evaporation process. PETMP, the only one whose consumption was not complete according to IR data, showed no volatility when tested under the same conditions of the solvent extraction, and therefore, the absence of that molecule in the extract confirms that it was fully incorporated into the network.
The results of this study provide an encouraging alternative for stress control, through a relatively simple approach, that should have generic application in methacrylate polymerizations. Efforts are underway to synthesize molecules that present long resin shelf-life at room temperature, as well as to circumvent the thiol odor issue prior to polymerization. By harnessing the effects of efficient chain-transfer reactions, low stress materials with higher conversion, mechanical properties and resistance to degradation in organic solvents can be designed.
Acknowledgments
The donation of part of the monomers used in this study monomer by Esstech and funding support from NIH/NIDCR (5R01DE014227) are greatly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Carmem S. Pfeifer, Craniofacial Biology Department, University of Colorado.
Nicholas D. Wilson, Craniofacial Biology Department, University of Colorado.
Zachary R. Shelton, Craniofacial Biology Department, University of Colorado.
Jeffrey W. Stansbury, Craniofacial Biology Department & Chemical and Biological Engineering Department, University of Colorado.
References
- 1.Lovell LG, Stansbury JW, Syrpes DC, Bowman CN. Macromolecules. 1999;32(12):3913–3921. [Google Scholar]
- 2.Dickens SH, Stansbury JW, Choi KM, Floyd CJE. Macromolecules. 2003;36(16):6043–6053. [Google Scholar]
- 3.Odian G. Principles of polymerization. 4. New York: Wiley-Interscience; 2004. [Google Scholar]
- 4.Lu H, Stansbury JW, Bowman CN. Dent Mater. 2004;20(10):979–986. doi: 10.1016/j.dental.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Dewaele M, Truffier-Boutry D, Devaux J, Leloup G. Dent Mater. 2006;22(4):359–365. doi: 10.1016/j.dental.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Patel MP, Braden M, Davy KWM. Biomaterials. 1987;8(1):53–56. doi: 10.1016/0142-9612(87)90030-5. [DOI] [PubMed] [Google Scholar]
- 7.Weinmann W, Thalacker C, Guggenberger R. Dent Mater. 2005;21(1):68–74. doi: 10.1016/j.dental.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Hoyle CE, Lee TY, Roper T. J Pol Scie Part a-Pol Chem. 2004;42(21):5301–5338. [Google Scholar]
- 9.Lee TY, Carioscia J, Smith Z, Bowman CN. Macromolecules. 2007;40(5):1473–1479. [Google Scholar]
- 10.Lee TY, Smith Z, Reddy SK, Cramer NB, Bowman CN. Macromolecules. 2007;40(5):1466–1472. [Google Scholar]
- 11.Senyurt AF, Wei HY, Hoyle CE, Piland SG, Gould TE. Macromolecules. 2007;40(14):4901–4909. [Google Scholar]
- 12.Bowman CN, Cramer NB, Sansbury JW. J Dent Res. 2011;90(4):402–416. doi: 10.1177/0022034510381263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim BS, Ferracane JL, Sakaguchi RL, Condon JR. Dent Mater. 2002;18(6):436–444. doi: 10.1016/s0109-5641(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 14.Pfeifer CS, Braga RR, Ferracane JL. Oper Dent. 2006;31(5):610–615. doi: 10.2341/05-118. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto A, Kawasaki N, Shimatani T. Macromolecules. 2000;33(5):1646–1650. [Google Scholar]
- 16.Berchtold KA, Hacioglu B, Lovell L, Nie J, Bowman CN. Macromolecules. 2001;34(15):5103–5111. [Google Scholar]
- 17.Berchtold KA, Lovell LG, Nie J, Hacioglu B, Bowman CN. Polymer. 2001;42(11):4925–4929. [Google Scholar]
- 18.Hacioglu B, Berchtold KA, Lovell LG, Nie J, Bowman CN. Biomaterials. 2002;23(20):4057–4064. doi: 10.1016/s0142-9612(02)00138-2. [DOI] [PubMed] [Google Scholar]
- 19.Chen F, Cook WD. Eur Pol J. 2008;44(6):1796–1813. [Google Scholar]
- 20.Dean KM, Cook WD, Lin MY. Eur Pol J. 2006;42(10):2872–2887. [Google Scholar]
- 21.Cramer NB, Bowman CN. J Pol Scie Part a-Pol Chem. 2001;39(19):3311–3319. [Google Scholar]
- 22.Stansbury JW, Dickens SH. Dent Mater. 2001;17(1):71–79. doi: 10.1016/s0109-5641(00)00062-2. [DOI] [PubMed] [Google Scholar]
- 23.Chiou BS, Khan SA. Macromolecules. 1997;30(23):7322–7328. [Google Scholar]
- 24.Silverstein RM, Webster FX, Kiemle DJ. Spectrometric identification of organic compounds. 7. Hoboken, NJ: John Wiley and sons; 2005. [Google Scholar]
- 25.Chambon F, Winter HH. J Rheology. 1987;31(8):683–697. [Google Scholar]
- 26.ASTM. 2011 doi: 10.1520/D0790-10. [DOI] [Google Scholar]
- 27.O’Brien AK, Cramer NB, Bowman CN. J Pol Scie Part a-Pol Chem. 2006;44(6):2007–2014. [Google Scholar]
- 28.Anseth KS, Decker C, Bowman CN. Macromolecules. 1995;28(11):4040–4043. [Google Scholar]
- 29.Cook WD, Brockhurst P. J Dent Res. 1980;59(5):795–799. doi: 10.1177/00220345800590050801. [DOI] [PubMed] [Google Scholar]
- 30.Trujillo M, Newman SM, Stansbury JW. Dent Mater. 2004;20(8):766–777. doi: 10.1016/j.dental.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Rubinstein M, Colby RH. Polymer Physics. 6. New York: Oxford University Press; 2008. [Google Scholar]
- 32.Carioscia JA, Lu H, Stanbury JW, Bowman CN. Dent Mater. 2005;21(12):1137–1143. doi: 10.1016/j.dental.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Pfeifer CS, Shelton ZR, Braga RR, Windmoller D, Machado JC, Stansbury JW. Eur Pol J. 2011;47(2):162–170. doi: 10.1016/j.eurpolymj.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anseth KS, Kline LM, Walker TA, Anderson KJ, Bowman CN. Macromolecules. 1995;28(7):2491–2499. [Google Scholar]
- 35.White TJ, Natarajan LV, Tondiglia VP, Bunning TJ, Guymon CA. Macromolecules. 2007;40(4):1112–1120. [Google Scholar]