Abstract
We have developed a cancer vaccine in which autologous tumor is fused with dendritic cells (DCs) resulting in the presentation of tumor antigens in the context of DC mediated costimulation. In clinical trials, immunologic responses have been observed, however responses may be muted by inhibitory pathways. The PD1/PDL1 pathway is an important element contributing to tumor mediated immune suppression. In this study, we demonstrate that myeloma cells and DC/tumor fusions strongly express PD-L1. Compared to a control population of normal volunteers, increased PD-1 expression was observed on T cells isolated from patients with myeloma. Interestingly, following autologous transplantation, T cell expression of PD-1 returned to levels seen in normal controls. We examined the effect of PD-1 blockade on T cell response to DC/tumor fusions ex-vivo. Presence of CT-011, an anti-PD1 antibody, promoted the vaccine induced T cell polarization towards an activated phenotype expressing Th1 as compared to Th2 cytokines. A concomitant decrease in regulatory T cells and enhanced killing in a cytotoxicity assay was observed. In summary, we demonstrate that PD-1 expression is increased in T cells of patients with active myeloma, and that CT-011 enhances activated T cell responses following DC/tumor fusion stimulation.
Introduction
PD-1 is a member of the B7 receptor family and is expressed by immune effector cells including T cells, B cells and NK cells1-4. Ligation of this receptor results in T cell inactivation and apoptosis and plays a key role in modulating the delicate balance between tolerance and immune activation5. In animal models, the inappropriate upregulation of PD-1 expression by T cells has been shown to promote an exhausted phenotype facilitating the development of chronic viral infection5-9. In contrast, PD-1 blockade results in the restoration of functionally active T cells and clearance of infection6. The PD-1/PDL-1 pathway is also being evaluated as a central mechanism by which tumors escape host immunity10,11. Tumor expression of PDL-1 has been demonstrated in several disease models and is thought to exert a significant role in promoting T cell tolerance by binding PD-1 on activated T cells and suppressing their capacity to secrete stimulatory cytokines1,12-16. In addition, tumor expression of /PDL-1 has been shown to directly inhibit T cell mediated lysis by the inactivation of tumor reactive CTLs1,10. The precise impact of hematological malignancies on this pathway including the T cell expression of PD-1 remains to be elucidated.
A major area of investigation in cancer therapy is the development of cellular immunotherapy that effectively targets malignant cells including those resistant to standard chemotherapy. The unique efficacy of this treatment approach for hematological malignancies is highlighted by the experience with allogeneic hematopoietic stem cell transplantation which is uniquely curative for a subset of patients due to the graft versus disease effect mediated by alloreactive lymphocytes17,18. However, allogeneic transplantation is associated with significant morbidity and mortality secondary to the lack of specificity of the allo-reactive response which results in graft versus host disease (GVHD). Tumor cells express unique antigens that may be selectively targeted by host immunity19-22. However, they evade recognition by the lack of effective antigen presentation and the inhibition of effector cells. Key elements in promoting the immunosuppressive milieu of malignancy include the upregulation of inhibitory pathways such as PDL-1/PD-1 and the recruitment of inhibitory cells including regulatory T cells1,10,23-25. In contrast, a promising strategy is the use of cancer vaccines to elicit tumor specific immune responses by the presentation of tumor antigens in the context of stimulatory signals.
We have developed a tumor vaccine in which the most potent antigen presenting cells, dendritic cells (DCs) are chemically fused with autologous multiple myeloma cells. DCs represent a complex network of cells characterized by the expression of costimulatory molecules necessary to initiate primary immune responses26. In animal models, vaccination with DC/tumor fusions results in the eradication of disease in tumor bearing animals27-29. In a phase I clinical study, vaccination with DC/myeloma fusions was well tolerated and resulted myeloma specific immune response and disease stabilization in a majority of patients, despite the presence of advanced disease30. To further augment vaccine efficacy, it is vital to overcome the inhibitory effects of tumor mediated immune suppression.
In this study, we quantified the presence of PDL-1 expression on myeloma cells and DC/myeloma fusions and PD-1 expression on T cells isolated from patients with active disease. We examined the effect of PD-1 blockade on vaccine response using CT-011 (CureTech Ltd) a clinical grade humanized anti PD-1 antibody (Previously CT-AcTibody or BAT), which has been shown to promote the expansion of activated memory T cells and anti-tumor immunity. We have shown DC/myeloma fusions uniformly express PDL-1 providing an inhibitory signal that potentially blunts vaccine response. In addition, T cell expression of PD-1 is upregulated in patients with advanced multiple myeloma and further increased following vaccine induced stimulation. In contrast, PD-1 blockade reverses the vaccine mediated upregulation of PD-1 expression. We demonstrated that PD-1 blockade in conjunction with DC/tumor fusion cell stimulation results in a skewing toward a Th1 phenotype, decreased regulatory T cell expansion, and enhanced killing of autologous tumor.
Methods
Generation of DC, T cell and DC/myeloma fusion preparations
Peripheral blood was obtained from patients with multiple myeloma and normal volunteers in accordance with a protocol approved by the institutional review board. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density centrifugation. PBMCs were incubated in RPMI 1640 complete medium containing 2 mM L-glutamine (Mediatech, Herndon, VA) and supplemented with heat inactivated 10% human AB male serum (Sigma, St. Louis, MO), 100 U/ml penicillin and 100 μg/ml streptomycin (Mediatech) for 1 h at 37°C in a humidified 5% CO2 incubator. The nonadherent fraction rich in T cells was removed. The monocyte enriched adherent fraction was cultured in complete medium containing GM-CSF (1000 U/ml) (Berlex, Wayne/Montville, NJ) and IL-4 (1000 U/ml) (R&D Systems (Minneapolis, MN)) for 5 days to generate immature DCs. The DC preparation underwent maturation by culturing the cells for an additional 48-72 h in the presence of TNFα (25 ηg/ml). The cell preparation underwent flow cytometric analysis to confirm the presence of DC associated costimulatory and maturation markers (CD86 and/or CD83) and absence of myeloma antigens (CD38 and/or CD138).
Myeloma cells were obtained from bone marrow aspirates collected from patients with multiple myeloma in accordance with a protocol approved by the institutional review board. Bone marrow mononuclear cells were cultured in RPMI 1640 culture media containing 2 mmol/l L-glutamine (Lonza, Walkersville, MD) and supplemented with heat-inactivated 10% human serum, 100 U/ml penicillin and 100 μg/ml streptomycin (Mediatech). Myeloma preparations were stained with mouse anti-human CD38, CD138, CD86 and CD83 directly conjugated to FITC (BD Biosciences, San Jose, CA) to document expression of tumor associated markers CD38 or CD138, and the absence of DC associated markers CD83 and CD86.
Plasma cells were mixed with DC preparations at ratios of 1:1 - 1:3 (dependent on cell yields) and washed 3 times in serum-free RPMI 1640 culture media. After the final wash, the cell pellet was resuspended in 1 ml of 50% PEG solution. After 2 min at room temperature, the PEG solution was progressively diluted and cells were washed twice with serum free media. The DC-tumor fusions were cultured in RPMI complete media in the presence of GM-CSF, IL-4, and TNFα. Fusion cells were identified for subsequent analysis by FACS gating around the population of cells that co-express unique tumor and DC associated antigens as described below.
Characterization of PDL-1 expression on DCs, myeloma, and, DC/myeloma fusion cells by flow cytometric analysis
DC preparations were incubated with mouse anti-human PDL-1 directly conjugated to PE (eBioscience, San Diego, CA) and a matching isotype control for 45 minutes at 4°C. Patient derived myeloma cells isolated from bone marrow aspirates were stained with mouse anti-human CD38 or CD138 directly conjugated to FITC (BD Biosciences, San Jose, CA), and mouse anti-human PDL-1 directly conjugated to PE for 45 minutes at 4°C. Cells were fixed in 2% paraformaldehyde (Sigma) and underwent flow cytometric analysis using FACScan (Becton Dickinson, San Jose, CA) and CellQuest Pro software© (Becton Dickinson).
DC/myeloma fusion cell preparations were subjected to dual staining to quantify the percentage of cells that co-expressed unique DC (CD86-PE-Cy5) (BD Biosciences, San Jose, CA) and tumor antigens (CD38 or CD138-FITC) (BD Biosciences, San Jose, CA). Dual staining fusion cells were isolated by FACS gating and stained with PE-conjugated mouse anti-human antibodies directed against PDL-1. The percentage of fusion cells expressing PDL-1 was determined by multichannel flow cytometric analysis.
PD-1 expression on T cells isolated from patients with multiple myeloma
Nonadherent peripheral blood mononuclear cells and bone marrow mononuclear cells were obtained from patients with myeloma at various stages of disease presentation including at time of presentation, relapse, and following therapy mediated cytoreduction. The myeloma cell preparation was incubated with mouse anti-human anti-CD4 or anti-CD8 directly conjugated to FITC, and mouse anti-human anti-PD1 directly conjugated to PE (eBioscience, San Diego, CA). Cells were washed, fixed in 2% paraformaldehyde (Sigma), and analyzed by multichannel flow cytometry.
Effect of non-antigenic and antigenic stimulation of T cells on expression of PD-1
T cells were activated for by exposure to the immobilized monoclonal antibodies, anti-CD3 (clone-UCHT1; Pharmingen) and anti-CD28 (clone-CD28.2; Pharmingen; CD3i/CD28i) for 1-4 days. Twenty-four-well non-tissue culture-treated plates (Falcon, Fisher) were coated with each of the antibodies (1 ug/ml in PBS) at 0.5 ml/well and left overnight at 4°C. The plates were washed in 1x PBS, and T cell preparations were added at a density of 2×106 cells/well. T cells were stimulated with anti-CD3/CD28 (48 hours). Alternatively, T cells were stimulated by the mitogen PHA (2 ug/ml) for 4 days, or DC/myeloma fusion cells at a ratio of 10:1 for 5 days. PD-1 expression on T cells before and after stimulation was assessed by flow cytometric analysis.
Effect of PD-1 blockade on T cell polarization and cytokine expression following stimulation by DC/myeloma fusions
DC/myeloma fusions were co-cultured for 5 days with autologous T cells at a 1:10 ratio in the presence or absence of 5ug/ml anti-PD1 (CT-011, CureTech Ltd., Israel). Co-cultures were then pulsed with GolgiStop (1 μg/ml; Pharmingen) for 4-6h at 37°C prior to analysis. Cells were harvested and cultured with murine anti-human FITC conjugated anti-CD4 or anti-CD8 (BD Biosciences). Cells were then permeabilized by incubation in Cytofix/Cytoperm plus™ (containing formaldehyde and saponin) (Pharmingen) for 45 min at 4°C, washed twice in Perm/Wash™ solution (Pharmingen), and incubated with PE-conjugated IFNγ (Caltag Laboratories), IL-10 (Caltag Laboratories), or a matched isotype control antibody for 45 min. Cells were washed in 1x Perm/Wash™ solution and fixed in 2% paraformaldehyde (Sigma). Labeled cells were analyzed by flow cytometry using FACScan (Becton Dickinson, San Jose, CA) and CellQuest Program.
Effect of PD-1 blockade on vaccine mediated expansion of regulatory and activated T cells
Autologous T cell preparations were co-cultured with DC/myeloma fusions for 5 days at a 10:1 ratio in the presence and absence of 5ug/ml anti-PD-1 (CT-011, CureTech Ltd., Israel). The cell preparations were incubated with FITC conjugated anti-CD4, tricolor conjugated anti-CD25 (Caltag Laboratories), and PE-conjugated anti-CD69 (BD Biosciences). Alternatively, cells were permeabilized and cultured with PE conjugated antibody directed against FoxP3 (eBioscience). Cells were subsequently analyzed by multichannel flow cytometry.
Effect of PD-1 blockade on CTL response following stimulation with DC/myeloma fusions
DC/myeloma fusions were cocultured with autologous T cells at a ratio of 1:10 for 5 days in the presence and absence of 5ug/ml anti-PD1 (CT-011, CureTech Ltd, Israel). Cell mediated cellular cytotoxicity was evaluated using the GranToxiLux cell-based fluorogenic cytotoxicity assay (OncoImmunin, Inc., Gaithersburg MD). Autologous tumor cells were used as target cells. Target cells were incubated in PE labeled Medium T (1μL of reconstituted TFL2 in PBS at 1:3000 ratio) at 2×106 cells/ml for 45 minutes at 37°C. Labeled cells were washed twice in PBS. T cells stimulated by fusions alone or in the presence of PD-1 blockade were co-incubated with labeled target cells in the presence of a fluorogenic granzyme B substrate for 1-2 hours at 37°C. Cells were washed and analyzed by flow cytometry. Dead target cells are identified by cells that dually stain for granzyme B and PE label (right upper quadrant). As a control, killing of targets by unstimulated T cells was assessed.
Statistical analysis
Results are expressed as mean ± SEM. 2 tailed students t test was used for comparisons, and values of p<0.05 were considered as significant
Results
Expression of PDL-1 on plasma cells, DCs, and DC/tumor fusion cells
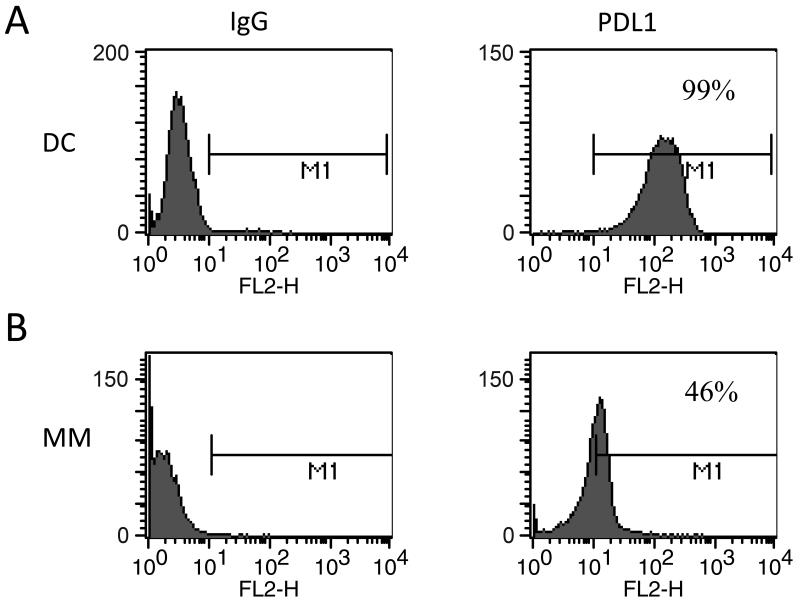
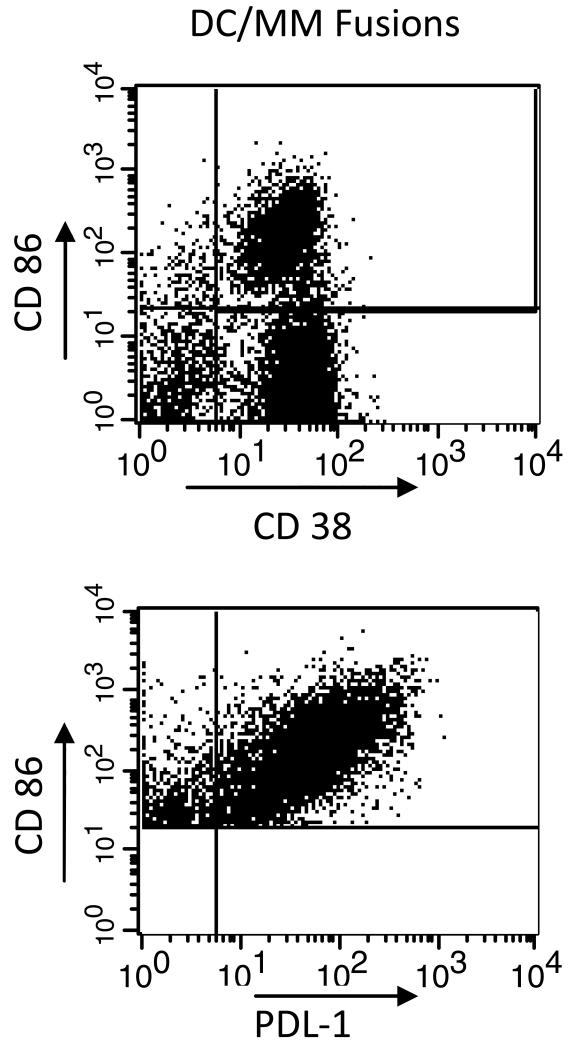
Expression of PDL-1 on antigen presenting cells plays an important role in blunting activated T cell responses. To assess the role that the PD-1/PDL-1 pathway may play in limiting activated immune responses to the DC/tumor vaccine, we evaluated expression of PDL-1 on ex-vivo generated DCs, myeloma cells, and DC/tumor fusions. DCs were generated by culture of adherent PBMCs for 1 week with GM-CSF and IL-4 to generate immature DCs, followed by maturation in the presence of TNFα for 48 hours. Ex-vivo generated dendritic cells were shown to strongly express the costimulatory molecules CD80 and CD86 and to lack expression of myeloma associated antigens CD38 and CD138. Tumor cells were obtained from bone marrow aspirates of patients with multiple myeloma. Patient derived plasma cells were shown to express CD38 and CD138, and lacked expression of CD86. Tumor cells were fused with DCs by coculture in 50% solution of polyethylene glycol. Mean expression of PDL-1 was 97% on DCs generated from normal volunteers (n=5, Figure 1), 94% on DCs generated from patients with multiple myeloma (n=4), and 45% on patient derived myeloma cells (n=6, Figure 1). In addition, mean expression of PDL-1 was 90% on DC/myeloma fusions (n=2, Figure 2). In the physiologic setting, PDL-1 expression on antigen presenting cells plays a role in limiting the expansion of auto-reactive T cell populations and preventing auto-immunity. Expression of PDL-1 on DC/myeloma fusion cells may provide an inhibitory signal that blunts activated T cell responses to fusion mediated stimulation.
Figure 1. Expression of PDL-1 on DCs and plasma cells.
A. DCs were generated from adherent mononuclear cells isolated from leukopak collections obtained from normal donors. DCs were cultured with GM-CSF and IL-4 for 5 days and then underwent maturation by exposure to TNFα for 48-72 hours. DC preparations were stained with PE conjugated anti-PDL-1 and analyzed by flow cytometric analysis. A representative example is shown.
B. Plasma cells were isolated from bone marrow aspirates of patients with active multiple myeloma. Cells were stained with FITC conjugated anti-CD38 and PE conjugated anti-PDL-1. Expression of PDL-1 on gated CD38+ cells was assessed by flow cytometric analysis.
Figure 2. Expression of PDL-1 on DC/myeloma fusions.
Fusion cells were generated by co-culture of DCs and plasma cells in the presence of PEG. Fusion cell preparations were stained with PE-cy5 conjugated CD86, FITC conjugated CD38, and PE conjugated PDL-1. Fusion cells were quantified by gating on cells dually staining for tumor derived CD38 and DC derived CD86 (upper panel). Dual staining cells were gated on, and expression of PDL-1 on the fusion cell population was assessed (lower panel). A representative example is shown.
Expression of PD-1 on T cells isolated from patients with multiple myeloma
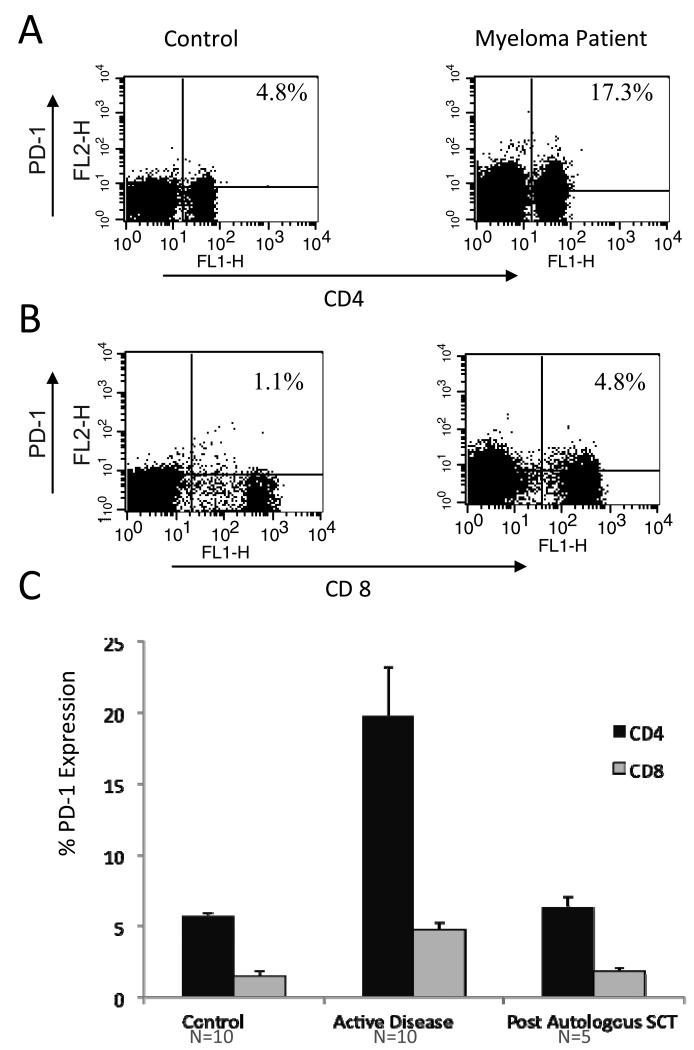
In infectious disease models, upregulation of T cell expression of PD-1 is associated with an exhausted phenotype facilitating the development of chronic viral infection. We evaluated expression of PD-1 on T cells derived from patients with myeloma with active disease or after cytoreduction following autologous stem cell transplantation. Nonadherent PBMCs obtained from patients with multiple myeloma and normal volunteers were cultured in RPMI supplemented with 10U/ml IL-2, and expression of PD-1 on CD4+ T cells was assessed by flow cytometric analysis. We demonstrate that PD-1 expression is markedly upregulated in patients with advanced multiple myeloma. Compared to a control population of normal volunteers where PD-1 expression on CD4+ T cells was 6% (n=10), a significant increase in PD1 expression was observed on CD4+ T cells isolated from patients with advanced myeloma 20% (n=10, p=<0.05), Figure 3A, 3B). Interestingly, PD-1 expression on CD4+ T cells isolated from patients who obtained a complete or very good partial response to transplant returned to levels observed in normal volunteers (mean expression of PD-1 on CD4+ T cells 6%, n=5, Figure 3C). Similarly, increased PD-1 expression was observed on CD8+ T cells isolated from patients with advanced myeloma (5%, n=10) compared to normal controls (1.4%, n=10, p<0.05). These findings suggest that upregulation of PD-1 expression may play a central role in tumor mediated immune suppression.
Figure 3. Expression of PD-1 on T cells isolated from myeloma patients.
A. Nonadherent peripheral blood mononuclear cells isolated from normal controls (left) and myeloma patients (right) were incubated with FITC conjugated anti-CD4 and PE conjugated anti-PD1. Expression of PD-1 on CD4+ T cells was assessed by FACS analysis. A representative example is shown.
B. Nonadherent peripheral blood mononuclear cells isolated from normal controls (left) and myeloma patients (right) were incubated with FITC conjugated anti-CD8 and PE conjugated anti-PD1. Expression of PD-1 on CD8+ T cells was assessed by FACS analysis. A representative example is shown.
C. Expression of PD-1 was assessed on CD4+ and CD8+ T cell populations isolated from normal controls, patients with active myeloma, and myeloma patients who were cytoreduced following autologous transplant. Nonadherent peripheral blood mononuclear cells were incubated with FITC conjugated anti-CD4 or anti-CD8 and PE conjugated anti-PD1 and assessed by FACS analysis as described above. Mean values with associated standard errors of the mean are shown.
Effect of antigen specific and nonspecific stimulation on T cell expression of PD-1
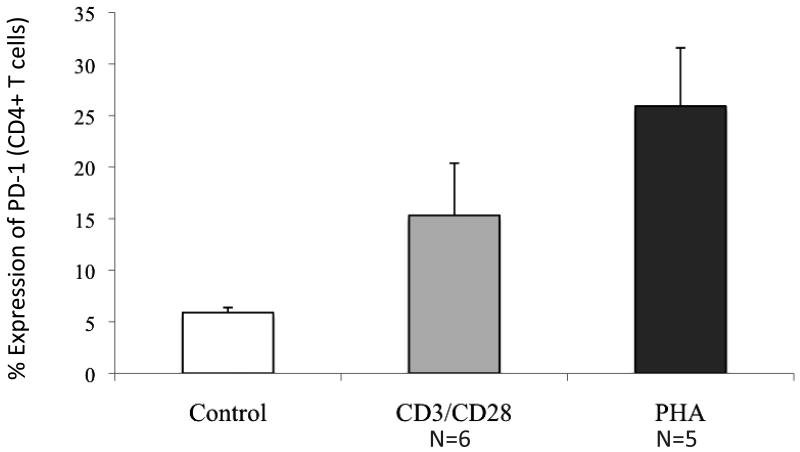
We subsequently assessed the effect of both non-antigenic and antigen mediated stimulation of T cells on PD-1 expression. Following nonspecific T cell activation by exposure to anti-CD3/CD28, mean PD-1 expression on CD4+ T cells increased from 5.1% to 14% (n=6, p<0.05, Figure 4). Similarly, mean PD-1 expression on CD4+ T cells increased from 6% to 23% following stimulation with PHA (n=5, p<0.05, Figure 4). These results suggest that antigen independent stimulation of T cells results in an upregulation of PD-1 expression.
Figure 4. Expression of PD-1 on stimulated T cell populations.
T cells were stimulated with anti-CD3/CD28 for 48 hours, or the mitogen PHA (2 ug/ml) for 4 days. Stimulated T cell populations were stained with FITC conjugated anti-CD4 and PE conjugated anti-PD-1. PD-1 expression on T cells before and after stimulation was assessed by flow cytometric analysis. Mean values with associated standard errors of the mean are shown.
We evaluated whether stimulation with DC/tumor fusion cells also results in increased PD-1 expression. While co-culture of tumor cells and T cells did not result increased PD-1 expression on CD4+ T cells (mean 6% and 8.7% expression pre and post co-culture respectively, n=4, p=NS), following stimulation with DC/myeloma fusions for 5 days, PD-1 expression on CD4+ T cells increased from 8% to 13% (n=3, p=0.05). In contrast, stimulation with DC/myeloma fusions in conjunction with PD-1 blockade was not associated with upregulation of PD-1 expression (mean CD4+ T cell expression of PD1 9%, n=2). To confirm that the decrease in PD-1 expression observed in fusion stimulated T cells in the presence of CT-011 was not due to a technical artifact of CT-011 blocking the ability to detect PD1 expression, we evaluated the expression of PD1 on unstimulated T cells in the presence and absence of CT-011. Nonadherent cells were isolated by ficoll density centrifugation and cultured in the presence or absence of CT-011 for 2 hours. Mean expression of PD-1 on CD4+ T cells cultured with and without CT-011 was 12.3% and 11.7% respectively (n=4), confirming that the presence of CT-011 does not interfere with flow cytometric detection of PD1 expression.
Effect of PD-1 blockade on T cell polarization following DC/myeloma fusion stimulation
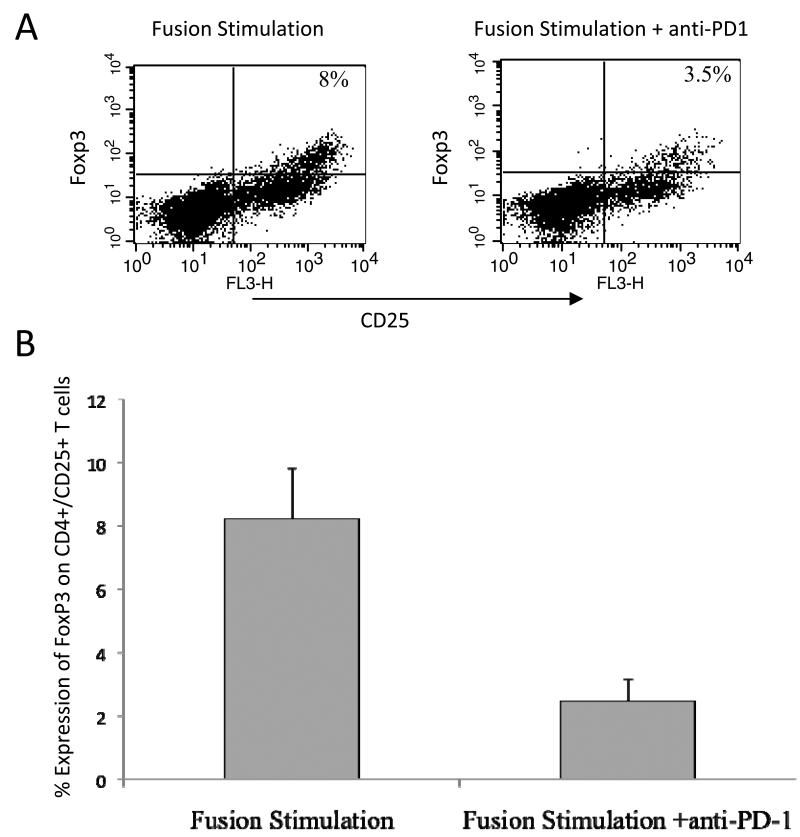
We have previously demonstrated that T cell stimulation with DC/myeloma fusion cells results in the concomitant expansion of activated and regulatory T cells. However, fusion mediated stimulation in conjunction with PD-1 blockade resulted in the suppression of regulatory T cell expansion. The percentage of CD4+/CD25+/FOXP3 T cells following stimulation of T cells with DC/myeloma fusions alone or with CT-011 was 12% and 5%, respectively (n=5, p<0.05, Figure 5).
Figure 5. Effect of PD-1 blockade in conjunction with DC/myeloma fusion stimulation on regulatory T cell populations.
Autologous T cells were co-cultured with DC/myeloma fusions for 5 days at a 10:1 ratio in the presence and absence of 5ug/ml anti-PD1. The cell preparations were incubated with FITC conjugated anti-CD4 and tricolor conjugated anti-CD25. The cells were permeabilized, stained with PE conjugated anti-FoxP3, and analyzed by multichannel flow cytometry. CD4/CD25+ T cells were isolated by FACS gating and expression of Foxp3 was determined. A representative example (A) and mean values of 5 experiments with associated standard errors of the mean (B) are shown.
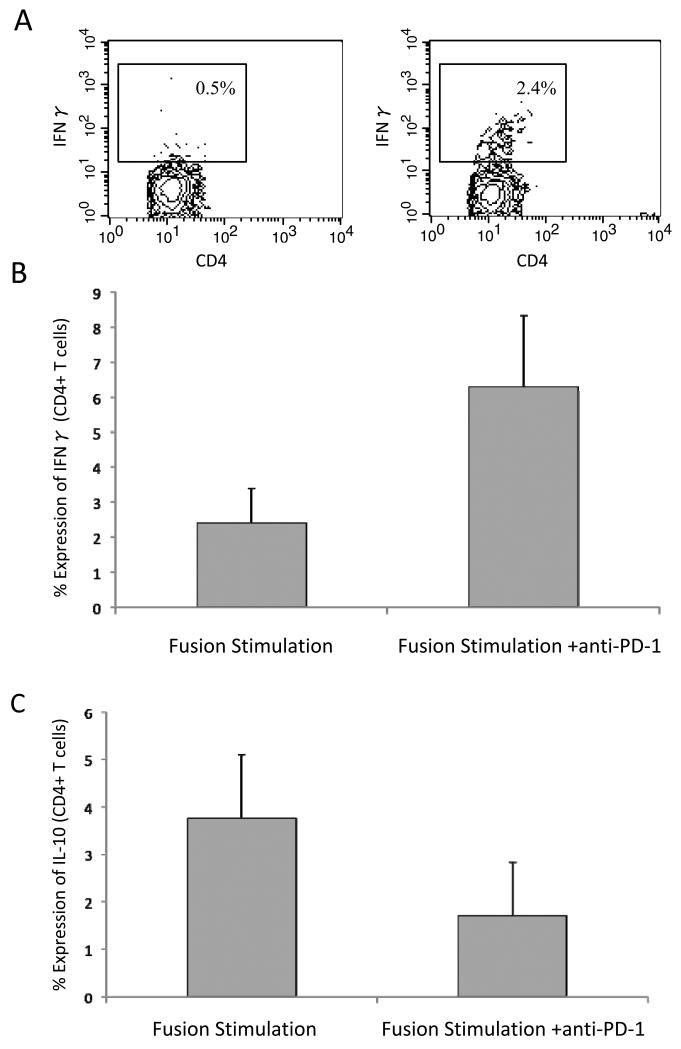
We further examined the effect of PD-1 blockade on fusion mediated stimulation of activated versus inhibitory T cell populations by quantifying the percentage of cells that expressed interferon gamma (IFNγ) as compared to IL-10. IFNγ secretion by CD4+ T cells in response to stimulation by DC/myeloma fusion cells increased 3 fold in the presence of PD-1 blockade (n=5, p<0.05, figure 6). In addition, a 2.2 fold decrease in IL-10 secretion by CD4+ cells following DC/myeloma fusion stimulation was noted in the presence of PD-1 blockade (n=5, p<0.05, figure 6C). These findings demonstrate that PD-1 blockade enhances vaccine efficacy by biasing toward Th1 cytokines, and limiting the expansion of suppressive T cell populations.
Figure 6. Cytokine secretion by fusion stimulated T cell populations in the presence and absence of PD-1 blockade.
A. Stimulated T cell preparations were stained for FITC conjugated CD4. Cells were then washed, permeabilized, and incubated with PE conjugated anti-human IFN γ or a matched isotype control antibody. Intracellular expression of IFNγ was determined by flow cytometric analysis. A representative example is shown.
B. Stimulated T cell preparations were stained for FITC conjugated CD4. Cells were then washed, permeabilized, and incubated with PE conjugated anti-human IFN γ or a matched isotype control antibody. Intracellular expression of IFNγ was determined by flow cytometric analysis. Mean values of 5 experiments with associated standard error of the means are shown.
C. Stimulated T cell preparations were stained for FITC conjugated CD4. Cells were then washed, permeabilized, and incubated with PE conjugated anti-human IL-10 or a matched isotype control antibody. Intracellular expression of IL-10 was determined by flow cytometric analysis. Mean values of 5 experiments with associated standard error of the means are shown.
Effect of PD-1 blockade on CTL response following stimulation with DC/myeloma fusions
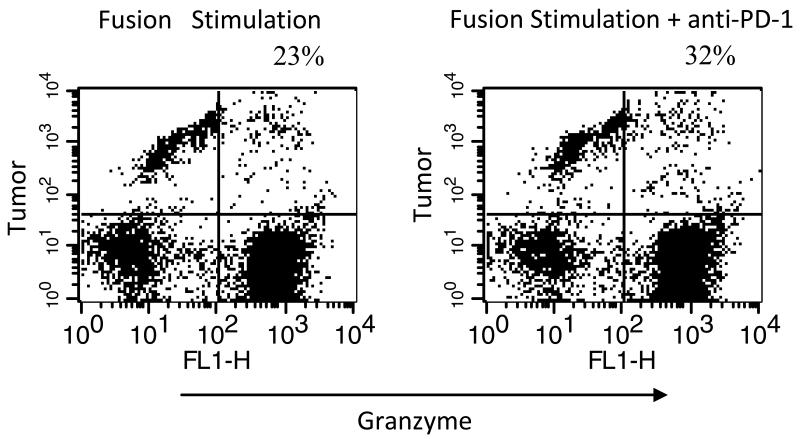
DC/myeloma fusion cells potently stimulate the expansion of myeloma specific CTLs with the capacity to lyse autologous tumor targets. We demonstrated that CTL activity was further increased in the presence of PD-1 blockade at the time of vaccine mediated T cell activation and coculture of activated effector cells and myeloma targets. Mean CTL lysis was 25% and 29% for T cells stimulated with DC/myeloma cells alone, and in the presence of PD-1 blockade respectively (n=6, p<0.05, Figure 7).
Figure 7. Effect of PD-1 blockade on CTL response following stimulation with DC/myeloma fusions.
DC/myeloma fusions were cocultured with autologous T cells at a ratio of 1:10 for 5 days in the presence and absence of PD-1 blockade. Autologous tumor cells were used as target cells, labeled with PE labeled Medium T. T cells stimulated by fusions alone (left) or in the presence of PD-1 blockade (right) were co-incubated with labeled target cells in the presence of a fluorogenic granzyme B substrate. Cells were washed and analyzed by flow cytometry. Dead target cells are identified by cells that dually stain for granzyme B and PE label (right upper quadrant). A representative example is shown.
Discussion
The PDL-1/PD-1 pathway plays a key role in modulating the crucial balance between immune activation and tolerance5. Upregulation of this pathway has been demonstrated in the setting of chronic infection and the immunosuppressive milieu that characterizes patients with malignancy. Increased signaling is associated with an “exhausted” T cell phenotype incapable of mounting an appropriate response to foreign antigen. The precise role of this pathway in blunting anti-tumor immunity has not been well defined in the setting of hematological malignancies.
In the present study, we have demonstrated high levels of PDL-1 expression on myeloma cells, suggesting that this pathway may be exploited in the setting of multiple myeloma to promote immune tolerance and facilitate disease growth. PDL-1 expression by malignant cells has been observed in several solid tumor models including renal, stomach, breast, and lung carcinoma13,15,31,32. PDL-1 expression has been shown to disrupt T cell activation and CTL mediated lysis of tumor targets in this setting. PDL-1 expression on antigen presenting cells, including dendritic cell populations, may limit the development of auto-immunity in the physiologic setting. In this study, we demonstrate that DC/myeloma fusion cells strongly express PDL-1, which may play a role in blunting activated immune responses to vaccination. We have observed that while DC/tumor fusion mediated stimulation of T cells in vitro results in an activated T cell response, there is a concomitant rise in regulatory T cells, which may limit vaccine efficacy33. In clinical studies, we have demonstrated that regulatory T cells persist in the post-vaccination period. Expression of PDL-1 by the fusion vaccine provides a negative signal to the costimulatory complex, likely blunting T cell activation and potentially contributing to the concomitant expansion of regulatory T cells observed following vaccine mediated stimulation. In this study, we evaluate PDL-1 expression on patient derived tumor cells, ex-vivo generated dendritic cells, and DC/tumor fusion cell populations. TNFα matured DCs were used for fusion generation, as we and have shown potent immunologic responses in this setting in clinical trials.
We evaluated the expression of PD-1 on circulating T cell populations isolated from patients with advanced multiple myeloma. We have demonstrated that expression of PD-1 is markedly increased in patients with active myeloma as compared to normal volunteers. In a melanoma model, it was recently shown that PD-1 expression on tumor infiltrating lymphocytes (TIL) is significantly higher than on a T cells isolated from the peripheral blood or normal tissue of the same individuals34. PD-1+ TIL were shown to have impaired effector function, suggesting that PD-1 expression on T cells limits their capacity to mount an effective anti-tumor immune response. In addition, in a recent publication, Benson et al demonstrate that NK cells isolated from patients with multiple myeloma express higher levels of PD1 compared to NK cells derived from normal controls35.
Of note, we demonstrate that while PD-1 is highly expressed on T cells isolated from patients with advanced myeloma, PD-1 expression is decreased on T cells isolated from patients in a minimal disease setting following autologous transplantation. PD-1 is upregulated as a negative feeback signal in response to chronic stimulation. One can hypothesize that the low levels of PD1 expressed on CD4+ T cells following autologous transplantation suggest that chemotherapy induced cytoreduction may alter the immunosuppressive milieu of patients with myeloma by reducing the effect of inhibitory pathways. In patients with active disease, chronic antigenic stimulation may result in increased expression of PD-1, contributing to tumor induced immunosuppression. In keeping with this, previous studies have demonstrated that levels of regulatory T cells are increased in the setting of malignancy and correlate with disease burden24.
The critical role that PD-1 plays in muting activated T cell responses is illustrated by the autoimmune phenotypes that develop in PD-1 knockout mice, including diabetes, glomerulonephritis, arthritis, and cardiomyopathy36-39. We demonstrated that PD-1 expression is increased following nonspecific activation of T cells. This likely represents a homeostatic mechanism to prevent T cell hyperstimulation and corresponding immune mediated damage. Physiologically, the PD-1/PDL-1 pathway plays an important role in preventing the development of auto-immunity. Similarly, it has been shown that PD-1 expression is upregulated on T cells in the face of chronic viral infection, preventing the clearance of virally infected cells. While upregulation of PD-1 is not unique to the setting of tumor mediated stimulation, we demonstrate that PD1 is upregulated on patients with advanced hematologic malignancies, suggesting that this pathway does play a role in mediated tumor induced immune suppression. Similarly, we demonstrated an increase in T cell PD-1 expression following stimulation with DC/myeloma fusion cells, suggesting that upregulation of this pathway may prevent sustained response to vaccination.
We demonstrated that PD-1 blockade prevented the vaccine mediated increase in T cell expression of PD-1. Presence of CT-011 promoted the vaccine induced polarization of T cells towards an activated phenotype expressing Th1 as compared to Th2 cytokines. An increase in IFNγ as compared to IL-10 expression was noted. A concomitant decrease in regulatory T cells was observed. Similar findings have been noted with the use of cytokine adjuvants such as IL-12, toll like receptor agonists and CPG oligonucleotides which have been shown to promote Th1 responses to tumor vaccination33,40-42. Regulatory T cell depletion has been shown to increase vaccine efficacy in both pre-clinical models and clinical trials43-48. In keeping with these findings, PD-1 blockade was associated with increased CTL mediated killing of myeloma targets. These results are complementary to Benson‘s recent findings that PD-1 blockade enhances NK cell mediated killing of myeloma cells35.
The clinical relevance of PD-1 blockade in augmenting vaccine response remains to be studied. Initial clinical studies of PD-1 blockade as an immunomodulatory therapy alone have been pursued in various malignancies49,50. Several different antibodies are currently being studied in the clinic as single agents or in combination with standard of care therapy. Efficacy of this treatment approach is based on the premise that expansion of native anti-tumor immunity would result in disease regression. In a phase I study of patients with hematological malignancies including multiple myeloma, single agent CT-011 therapy was well tolerated and associated with clinical benefit in 33% of patients49. The combination of PD-1 blockade with active vaccination strategies is a logical extension of this strategy. We are currently initiating a protocol in which patients with multiple myeloma will undergo serial infusions of CT-011 in conjunction with vaccination with DC/myeloma fusions following autologous transplantation. In this clinical trial, patients will receive 3 doses of DC/MM fusion cells, in conjunction with a dose of CT-011 administered one week after each vaccine. In providing an essential stimulatory signal for the expansion of memory effector cells and reversing a key element of tumor mediated immune suppression, we hypothesize that therapy with CT-011 in conjunction with the DC/MM fusion vaccine will expand tumor specific T cells, and importantly, will result in the expansion of memory T cell populations which are critical for durable immunity.
A potential concern with PD-1 blockade is the development of autoimmunity by suppression of this key regulatory pathway. Of note, antibodies directed against CTLA-4 have been associated with inflammatory colitis and other manifestations of autoimmunity in a subset of patients51. In fact, disease response correlates with the autoimmune complications of therapy. Whether PD-1 blockade will effectively facilitate the expansion of activated T cells and induce clinically meaningful anti-tumor immunity without autoimmunity remains to be seen.
Acknowledgements
Funding to conduct the study was obtained from the DOD (grant # W81XWH-09-1-0296 to DA), the NIH (K12 Clinical Research Career Development Program Award grant # 5 K12 CA087723 to JR), and by research funding obtained from CureTech, Ltd. to DA and JR.
Sources of Support: Funding to conduct the study was obtained from the DOD (grant # W81XWH-09-1-0296 to David Avigan, MD), the NIH (K12 Clinical Research Career Development Program Award grant # 5 K12 CA087723 to Jacalyn Rosenblatt, MD), and by research funding obtained from CureTech, Ltd. to David Avigan, MD and Jacalyn Rosenblatt, MD.
Footnotes
Authorship Contributions: Jacalyn Rosenblatt, MD and David Avigan, MD were involved in experimental design, obtaining patient derived samples, data analysis, and manuscript preparation. Brett Glotzbecker, MD, Heidi Mills, Baldev Vasir, PhD, Kerry Wellenstein and Whitney Keefe were involved in conducting experiments and data analysis. Dimitrios Tzachanis, MD/PhD, James D. Levine, MD and Robin M. Joyce, MD were involved in obtaining patient derived samples. Michael Schickler, PhD and Rinat Rotem-Yehudar, PhD provided reagents. Donald Kufe, MD was involved in experimental design and manuscript preparation.
Financial Disclosure: Michael Schickler, PhD and Rinat Rotem-Yehudar, PhD are employees of CureTech, Ltd., which provided CT-011. Jacalyn Rosenblatt, MD and David Avigan, MD received research funding from CureTech, Ltd. All other authors have declared there are no conflicts of interest to disclose.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 2.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett F, Luxenberg D, Ling V, et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J Immunol. 2003;170(2):711–718. doi: 10.4049/jimmunol.170.2.711. [DOI] [PubMed] [Google Scholar]
- 5.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 7.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12(10):1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 8.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 9.Urbani S, Amadei B, Tola D, et al. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80(22):11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11(8):2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 12.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101(49):17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108(1):19–24. doi: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13(7):2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 15.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10(15):5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 16.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104(9):3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gahrton G, Tura S, Ljungman P, et al. Allogeneic bone marrow transplantation in multiple myeloma. European Group for Bone Marrow Transplantation. N Engl J Med. 1991;325(18):1267–1273. doi: 10.1056/NEJM199110313251802. [DOI] [PubMed] [Google Scholar]
- 18.Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86(5):2041–2050. [PubMed] [Google Scholar]
- 19.Takahashi T, Makiguchi Y, Hinoda Y, et al. Expression of MUC1 on myeloma cells and induction of HLA-unrestricted CTL against MUC1 from a multiple myeloma patient. J Immunol. 1994;153(5):2102–2109. [PubMed] [Google Scholar]
- 20.Brossart P, Schneider A, Dill P, et al. The epithelial tumor antigen MUC1 is expressed in hematological malignancies and is recognized by MUC1-specific cytotoxic T-lymphocytes. Cancer Res. 2001;61(18):6846–6850. [PubMed] [Google Scholar]
- 21.Lim SH, Wang Z, Chiriva-Internati M, Xue Y. Sperm protein 17 is a novel cancer-testis antigen in multiple myeloma. Blood. 2001;97(5):1508–1510. doi: 10.1182/blood.v97.5.1508. [DOI] [PubMed] [Google Scholar]
- 22.Szmania S, Tricot G, van Rhee F. NY-ESO-1 immunotherapy for multiple myeloma. Leuk Lymphoma. 2006;47(10):2037–2048. doi: 10.1080/10428190600742292. [DOI] [PubMed] [Google Scholar]
- 23.Mumprecht S, Schurch C, Schwaller J, Solenthaler M, Ochsenbein AF. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood. 2009;114(8):1528–1536. doi: 10.1182/blood-2008-09-179697. [DOI] [PubMed] [Google Scholar]
- 24.Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169(5):2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 25.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167(3):1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 26.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 27.Gong J, Chen D, Kashiwaba M, Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med. 1997;3(5):558–561. doi: 10.1038/nm0597-558. [DOI] [PubMed] [Google Scholar]
- 28.Gong J, Nikrui N, Chen D, et al. Fusions of human ovarian carcinoma cells with autologous or allogeneic dendritic cells induce antitumor immunity. J Immunol. 2000;165(3):1705–1711. doi: 10.4049/jimmunol.165.3.1705. [DOI] [PubMed] [Google Scholar]
- 29.Gong J, Chen D, Kashiwaba M, et al. Reversal of tolerance to human MUC1 antigen in MUC1 transgenic mice immunized with fusions of dendritic and carcinoma cells. Proc Natl Acad Sci U S A. 1998;95(11):6279–6283. doi: 10.1073/pnas.95.11.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avigan D, Rosenblatt J, Vasir B, et al. Phase I Study of Vaccination with Dendritic Cell Myeloma Fusions. Blood (ASH Annual Meeting Abstracts) 2007;110 [Google Scholar]
- 31.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66(7):3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 32.Ghebeh H, Mohammed S, Al-Omair A, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8(3):190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vasir B, Wu Z, Crawford K, et al. Fusions of dendritic cells with breast carcinoma stimulate the expansion of regulatory T cells while concomitant exposure to IL-12, CpG oligodeoxynucleotides, and anti-CD3/CD28 promotes the expansion of activated tumor reactive cells. J Immunol. 2008;181(1):808–821. doi: 10.4049/jimmunol.181.1.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benson DM, Jr., Bakan CE, Mishra A, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 116(13):2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 38.Ansari MJ, Salama AD, Chitnis T, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198(1):63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salama AD, Chitnis T, Imitola J, et al. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med. 2003;198(1):71–78. doi: 10.1084/jem.20022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong J, Koido S, Chen D, et al. Immunization against murine multiple myeloma with fusions of dendritic and plasmacytoma cells is potentiated by interleukin 12. Blood. 2002;99(7):2512–2517. doi: 10.1182/blood.v99.7.2512. [DOI] [PubMed] [Google Scholar]
- 41.Igartua M, Pedraz JL. Topical resiquimod: a promising adjuvant for vaccine development? Expert Rev Vaccines. 9(1):23–27. doi: 10.1586/erv.09.135. [DOI] [PubMed] [Google Scholar]
- 42.Smits EL, Cools N, Lion E, et al. The Toll-like receptor 7/8 agonist resiquimod greatly increases the immunostimulatory capacity of human acute myeloid leukemia cells. Cancer Immunol Immunother. 59(1):35–46. doi: 10.1007/s00262-009-0721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbon CM, Yang M, Wands GD, et al. Consecutive low doses of cyclophosphamide preferentially target Tregs and potentiate T cell responses induced by DNA PLG microparticle immunization. Cell Immunol. 262(2):150–161. doi: 10.1016/j.cellimm.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Poehlein CH, Haley DP, Walker EB, Fox BA. Depletion of tumor-induced Treg prior to reconstitution rescues enhanced priming of tumor-specific, therapeutic effector T cells in lymphopenic hosts. Eur J Immunol. 2009;39(11):3121–3133. doi: 10.1002/eji.200939453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann N Y Acad Sci. 2009;1174:99–106. doi: 10.1111/j.1749-6632.2009.04939.x. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto M, Kamigaki T, Yamashita K, et al. Enhancement of anti-tumor immunity by high levels of Th1 and Th17 with a combination of dendritic cell fusion hybrids and regulatory T cell depletion in pancreatic cancer. Oncol Rep. 2009;22(2):337–343. [PubMed] [Google Scholar]
- 47.Prasad SJ, Farrand KJ, Matthews SA, Chang JH, McHugh RS, Ronchese F. Dendritic cells loaded with stressed tumor cells elicit long-lasting protective tumor immunity in mice depleted of CD4+CD25+ regulatory T cells. J Immunol. 2005;174(1):90–98. doi: 10.4049/jimmunol.174.1.90. [DOI] [PubMed] [Google Scholar]
- 48.Casares N, Arribillaga L, Sarobe P, et al. CD4+/CD25+ regulatory cells inhibit activation of tumor-primed CD4+ T cells with IFN-gamma-dependent antiangiogenic activity, as well as long-lasting tumor immunity elicited by peptide vaccination. J Immunol. 2003;171(11):5931–5939. doi: 10.4049/jimmunol.171.11.5931. [DOI] [PubMed] [Google Scholar]
- 49.Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(10):3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 50.Brahmer JR, Topalian S, Wollner I, et al. Safety and activity of MDX-1106 (ONO-4538), an anti-PD-1 monoclonal antibody, in patients with selected refractory or relapsed malignancies. J Clin Oncol. 2008 May 20;26(suppl) abstr 3006. [Google Scholar]
- 51.Yang JC, Hughes M, Kammula U, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30(8):825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]