Abstract
Although cocaine is illegal in most countries of the world, addiction is common and increasing in many populations, and the effectiveness of current treatment options for those afflicted has been very limited. The availability of an anti-cocaine vaccine could offer help to those who wish to quit their addiction. A number of vaccines differing in their chemical nature have been developed, and one has advanced into clinical trials. This review will discuss the successes and limitations of the various vaccines and the results of clinical trials of the vaccine using succinyl norcocaine conjugated to cholera toxin B. This latter vaccine shows considerable promise for those individuals whose antibody response is adequate..
Keywords: Substance Abuse, Vaccination, Cocaine, Immunotherapy
History of Cocaine Use
For thousands of years, indigenous peoples in South America have chewed the leaves of the coca plant as a stimulant, enabling them to work at high altitudes and providing a sense of well-being. In modern times, the active ingredient of these leaves is extracted and purified, giving us the highly addictive drug cocaine (1) (Figure 1a). By the turn of the twentieth century, it’s addictive properties had become clear, at least in Europe and the United States (2). Cocaine was restricted to prescription only use in the United States by the Harrison Narcotics Act of 1914, and its overall use declined in the U.S. so that between 1920 and about 1947, cocaine was not seriously abused or trafficked in the general population. However, as cocaine abuse began to spread in the 1960’s, efforts were made to stop the Andean trade in cocaine, particularly by United States, but the spread of production and smuggling in South America accelerated, along with a vast increase in the number of users in the U.S. from the early 1970’s (3). Although it is illegal in most countries of the world, a brutal criminal enterprise has grown up around the production, distribution, sale and use of cocaine, leading to much loss of innocent life and providing the traffickers with untold wealth (4). As a result, the “War on Drugs” has been spectacularly ineffective as demonstrated in government statistics on drug use (5). The price of cocaine is lower and the availability greater than it was 25 years ago, and addiction has spread, affecting not only individuals, but their families and communities (6).
Figure 1.
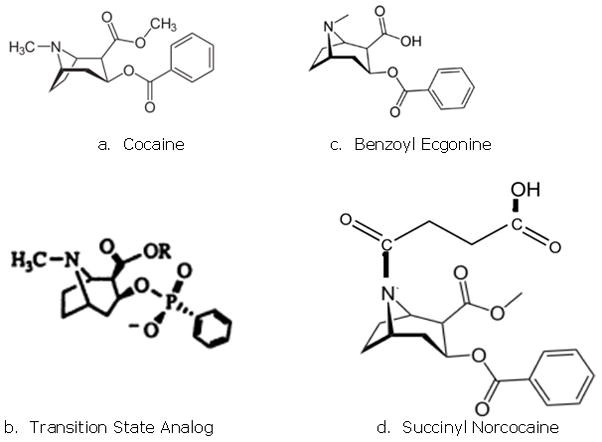
Structure of cocaine and cocaine derivatives
Effects of Cocaine
Cocaine can be injected, snorted or, in the form of the free base (known as crack), smoked. It crosses the blood-brain barrier and binds strongly to the dopamine transporter causing dopamine to accumulate in the synaptic cleft, affecting dopamine rich areas of the brain especially, such as the nucleus accumbens and the prefrontal cortex. The release of dopamine in the nucleus accumbens region of the brain is believed to lead to cocaine’s rewarding effects (7). Stimulation of the dopamine transporter produces regional changes in brain metabolism (8), and chronic administration in rodents and humans has been shown to decrease dopamine D2 receptor expression in the nucleus accumbens, which has been shown to be associated with self administration behavior in rats (9). Frequent use in humans can lead to addictive behavior in some individuals, depending on numerous factors including genetic predilection (10, 11).
Breaking the cocaine habit has proved very difficult for most addicts (12), but the recent development of vaccines against cocaine may offer help. Normally the body does not make an immune response against small molecules like cocaine which by themselves are unable to activate immune recognition mechanisms. , If cocaine is attached to a foreign protein such as cholera toxin, however, then the immune system can make antibodies that bind to cocaine, as well as antibodies recognizing the foreign protein as one would expect, but which are not relevant to the effects on the drug. When the cocaine molecule is bound by an anti-cocaine antibody in the bloodstream, the complex is too large to cross the blood/brain barrier, therefore the cocaine is prevented from causing its well-known pleasurable and reinforcing effects. Antibody bound cocaine is released slowly, depending on the antibody affinity, and can be metabolized by hydrolysis in the bloodstream or in tissues. Animal studies have shown that mice vaccinated with an anti-cocaine vaccine do not exhibit the behaviors associated with cocaine ingestion (e.g., increased locomotor activity), indicating that anti-cocaine antibodies are present in their blood in sufficient amounts (see below) to sequester to drug.
Anti-cocaine Vaccines
The first report of the preparation and use of an anti-cocaine vaccine was published in 1992 (13). Rats that were vaccinated with a cocaine-keyhole limpet hemocyanin (KLH) conjugate and subsequently given cocaine showed a reduced analgesic effect by the hotplate method, but this work was not followed up, and some concern was voiced about the preparation of the vaccine (14).
In 1993, Chandrakumar (15) and Landry (16) published the preparation of vaccines designed to produce catalytic antibodies. This involved synthesizing stable derivatives of cocaine whose structure mimicked that of the transition state of the chemical reaction (i.e., an intermediate, higher free energy structure of the chemical, often one in which the reacting molecule is strained or distorted or has an unfavorable electronic structure) through which the benzoyl group of cocaine is hydrolyzed (Figure 1b). These transition state analogs were then attached to immunogenic proteins and used to vaccinate mice. Although it was reported that the mice made high levels of antibodies, their behavior following a dose of cocaine was not assayed. Instead, monoclonal antibodies were generated that released of benzoic acid from free cocaine. In 1995, Basmadjian et al. (17) made vaccines from transition state analogs which had linkers coming from three different places on the cocaine molecule: the carboxylic acid position, the tropane nitrogen, and the phenyl ring (Figure 2, a, b, c). They demonstrated that the polyclonal antibodies produced in mice catalyzed the hydrolysis of radioiodinated cocaine in vitro. Landry expanded on this work in 1996 (18), and generated monoclonal antibodies from the immunized mice that were successfully analyzed for effectiveness. In none of the cases discussed above were studies carried out to examine how the vaccinated mice responded to doses of cocaine, in spite of high titer antisera being reported. Catalytic antibodies continue to be studied (19), but no clinical trials for vaccines based on this model have been initiated as yet.
Figure 2.
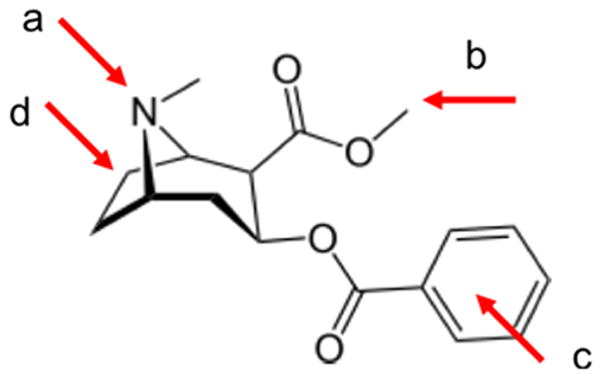
Sites for attachment of linkers to cocaine for conjugation to proteins
In 1995, Janda reported the preparation of a new anti-cocaine vaccine (GNC-KLH) by conjugating a cocaine derivative (which he named GNC) to KLH (20). GNC has a linker group attached to the carboxylic acid of benzoyl ecgonine (Figure 1c.). His group measured the anti-cocaine antibody titers in vaccinated rats and observed the psychoactive effects following a cocaine challenge to vaccinated and control rats, as well as the cocaine concentration in their cerebral tissue. They concluded that the antibodies bound the cocaine in the serum, preventing the drug from accessing the brain and thus preventing behavioral effects. Further studies with the same vaccine demonstrated protection against relapse to cocaine self administration behavior in rats (21), and a monoclonal Ab was generated (GNC92H2) that was later studied in mice as an immunotherapy against cocaine overdose (22). This monoclonal was also humanized (23) and the crystal structure obtained to demonstrate the contact points of cocaine binding to the antibody (24). Janda further refined the chemical structure of the GNC hapten by changing the ester groups to amides (named GND) (25), leading to a vaccine designated GND-KLH that was reported to be more stable and efficacious than GNC-KLH (26). Attempts by Janda to create an improved anti-cocaine or catalytic antibody vaccine by attaching a linker to the tropane nucleus of cocaine (Figure 2d) were unsuccessful in that they produced antibodies that bound cocaine poorly (27). Even though the animal studies were promising, none of Janda’s vaccines have proceeded to clinical trials.
Ettinger produced a vaccine in 1997 by coupling cocaine to KLH through a photoactivation method (28). This method does not allow one to tell where the linker is attached to the cocaine, as it acts by inserting the linker (which is conjugated to the KLH) between any carbon-carbon single bond on the cocaine. In spite of this ambiguity, Ettinger showed that the vaccine produced anti-cocaine antibodies and blunted the effects of cocaine in rats. Further work by the same group suggested that the anti-cocaine antibodies could be overwhelmed by large cocaine doses, an ongoing concern for any vaccination strategy (29). As discussed in more detail elsewhere (30), this implies that high level, persistent antibody responses will be required to block cocaine effects, but that addicts motivated to stop their drug abuse will be most likely to respond to vaccines successfully.
Other anti-cocaine vaccines continue to be produced. Hrafnkelsdottir et al., in 2005, with a very nice description of the chemistry methods, conjugated succinyl norcocaine (Figure 1d) to KLH and used it with an adjuvant, RhinoVax, as an intranasal vaccine (31). They also vaccinated mice subcutaneously with the same vaccine adjuvanted with alum. They found that effects on cocaine distribution to the brain were similar in intranasally and subcutaneously vaccinated mice. They postulated that mucosal antibodies could play a role in preventing access of cocaine to the brain via the olfactory bulb.
In 2004, Danger (32) generated monoclonal antibodies from mice vaccinated against cocaethylene, a more active metabolite of cocaine, where the methyl ester has been changed to an ethyl ester (see Figure 2b), This metabolite is formed when cocaine and ethanol are taken together, as is often the case in substance abusers. Danger’s group used cocaethylene as the hapten with the linking moiety on the phenyl ring (Figure 2c). The monoclonals prepared from vaccinated animals recognized both cocaine and cocaethylene with reasonable affinity. In 2006, Danger’s group immunized rabbits with six different cocaine haptens coupled separately to three different carriers: bovine serum albumin (BSA), tetanus toxoid, and KLH (33). The cocaine haptens consisted of benzoyl ecgonine (Figure 2b), an amide derivative of benzoyl ecgonine, a derivative of norcocaine, and three cocaine derivatives which had linking moieties on the phenyl ring. No syntheses were described for any of these haptens. The affinities of the polyclonal antibodies produced were above 109M−1, which is considered to lead to stable drug-antibody binding.
Monoclonal Anti-cocaine Antibodies
Crystal structures have been obtained for cocaine bound to many of the anti-cocaine monoclonal antibodies obtained by various groups (24, 34–36). The article by Pozharshi et al. (36) succinctly sums up the findings of these efforts thus: “binding of a small ligand can be achieved in diverse ways, both in terms of a binding site structure/topology and protein–ligand interactions.” This statement confirms the prediction that numerous different antibodies are capable of binding cocaine, and different vaccine structures may be capable of eliciting complementary antibody populations that may enhance sequestration of the drug in circulation, decreasing and slowing of penetration into the brain, depending in part, on their binding characteristics.
Monoclonal antibodies are, of course, individual molecules selected from the many different antibodies produced in a polyclonal response to a vaccine, and so such single molecules could behave very differently from the population of antibodies produced in vivo. One issue of considerable concern is the tightness of antibody binding to cocaine (i.e., the antibody affinity for the drug). Usually this is determined by equilibrium binding studies with values expressed as the affinity (in units of concentration−1) or its inverse, the dissociation constant (Kd in units of concentration, e.g., nM) (31). However, drug binding in the context of substance abuse provides some additional concerns due to the potentially repeated use of the abused compound. It is well established that antibody binding prolongs the half-life of drug in circulation, although the antibody half-life is not affected, since the small molecule interactions with single combining sites do not alter antibody structure. If binding is very tight, the drug cannot be released and metabolized, and furthermore, the antibody will be effectively blocked from subsequently binding readministered drug. Thus, the rates of antibody binding and dissociation should be considered in vaccine design. Recent studies in our lab using surface plasmon resonance detection of antibody binding and release have demonstrated that a butyl-norcocaine conjugate vaccine induced antibodies against cocaine in mice that on average have association rates around the 5 × 105 L·M−1·sec−1, while the dissociation rates showed an off rate 2.5 × 10−3 sec−1. As a result, the calculated Kd for these samples was 5 nM. This would imply that 75% of the bound drug would be released (and potentially rebound) over approximately 9 minutes, permitting enzymatic degradation of some of the drug, as well as slowed distribution into tissues.
Clinical Trials Using TA-CD, a Succinyl Norcocaine-Cholera Toxin B Vaccine
In 1996, Fox and Kantak reported on a cocaine vaccine where succinyl norcocaine (SNC) (Figure 1c) was conjugated to BSA (37). Although the description of the chemistry of the synthesis of succinyl norcocaine and its attachment to BSA was minimal, mass spectral analysis showed that haptenation levels of 20–27 SNC molecules per molecule of BSA were obtained, a salient bit of information which was not reported for any of the vaccines referenced above. In 2001, they followed up with extensive studies in rats (38, 39) using a vaccine consisting of SNC conjugated to cholera toxin B, a well-studied immunogen, - Significantly, this vaccine, named TA-CD, was prepared by a commercial company (Cantab Pharmaceuticals, UK) and included alum, and adjuvant approved for human use. The TA-CD vaccine was quickly taken into clinical trials by Kosten in 2002 (40). The Phase I trial was randomized, double blind and placebo controlled, involving subjects who were abstinent cocaine users and at first confined in a residential drug treatment program. The vaccine was given three times at monthly intervals in doses of 13, 82 or 709 μg. Out of 24 subjects who completed the 3 month protocol, 15 were able to be followed for one year after the initial vaccination. The vaccine was well tolerated, and all the subjects developed cocaine-specific antibodies which were detectable after the second vaccination, and increased after the third dose. The antibody response peaked around two months after the last vaccination, and then slowly decreased to baseline by 1 year. There was considerable variation among subjects in all dosage groups, and the levels of antibody produced were lower that would be desirable to reduce cocaine abuse relapse. However, the results were positive enough to encourage further trials.
The next trial was designed to further test the safety and dose response of the TA-CD vaccine as well as assessing its efficacy for reducing cocaine abuse (41). The carefully screened subjects were persons in early recovery and were seen weekly for counseling sessions, providing urine samples for drug testing 3 times a week. Over 12 weeks, ten subjects received four 100 μg vaccinations, and eight others received four 400 μg vaccinations. There were no adverse effects. The higher dose group had higher titers of antibody, and maintained their levels longer than the low dose group, but antibody levels in both groups had waned almost to baseline by 6 months. Booster responses gave the expected increase in titers, but suggested that two boosters over 2 to 4 weeks would most likely be needed to increase the antibody levels back to their initial peak responses. Encouragingly, the higher dose group had fewer reported uses of cocaine during the study.
A larger trial was carried out by Martell et al. in 2009 using 114 outpatients who were in a methadone maintenance program, and who participated in weekly counseling sessions (42). It had been estimated from animal studies that a level of 43 μg/mL or greater of anti-cocaine antibodies in human serum would be enough to capture cocaine in circulation and reduce its euphoric effects, for ordinary recreational doses of the drug (43). These trials showed that when the anti-cocaine vaccine elicited a sufficient amount of antibodies in an individual, that person was more likely to have fewer positive urine tests for cocaine use. Interestingly, quantitative urine testing showed that few subjects took large doses of cocaine to overcome the antibody blockade, often attributing the lack of cocaine effects as being due to poor quality drug from their supplier (42). Disappointingly however, the vaccine only gave high enough antibody levels in about a third of the participants for reasons that are not entirely clear.
In a study of 10 cocaine dependent men not seeking treatment, Haney et al. randomized them to one of two different dose levels (82 μg and 369 μg) of TA-CD and then compared these two groups on the effects of cocaine before and after vaccination given at weeks 1,3 5, and 9 (44). As in the other trials, the antibody response was highly variable, the larger dose gave higher antibody levels, and the titer waned after the vaccinations stopped. Those with high levels of antibody reported lower ratings of the “Good Drug Effect” and “Cocaine Quality”, and the subjective effects of the drug were blunted. Haney had previously reported that no other anti-cocaine medication had given similarly robust results when tested under similar conditions as the vaccine study (45).
The Future of Anti-cocaine Vaccines
These trials are encouraging in that they demonstrate that anti-cocaine antibodies can be produced at least in some individuals at a level where a “usual” dose of cocaine (43) will be bound, preventing passage of the drug to the brain. However, they also indicate the need for improved vaccine design, adjuvants, and/or vaccination strategies. Unlike vaccinations against flu where the body makes more antibodies when it subsequently encounters a flu virus, the ingestion of cocaine, a small molecule, does not stimulate the immune system to increase the number of anti-cocaine antibodies: only booster shots of the anti-cocaine conjugate vaccine will do so. This is due to the inability of small molecules to crosslink surface antigen specific antibodies expressed on memory B cells to activate them, and their failure to interact with helper T cells that are required to restimulate anamnestic responses (46). Motivating addicts to stick to a course of vaccinations and boosters could be daunting (see number of dropouts reported in the clinical trial data), but it might be considerably easier than persuading them to take a medication daily, or even more frequently for prolonged periods.
David Jackson at the University of Melbourne in Australia has developed a totally synthetic self-adjuvanting vaccine (47) that has proved in initial studies to be effective in eliciting antibodies to cocaine in a mouse model in our laboratory. These relatively small vaccine constructs include a lipid component derived from bacterial lipopolysaccharide attached to a short peptide (e.g., tripalmitoyl-S-glyceryl-cysteine), which is also covalently bound to a T cell peptide epitope and the antigen or hapten of interest. Such vaccines are strong stimulators of toll receptor 2 and activate NFκB, a central nuclear signaling molecule that regulates the expression of pro-inflammatory cytokines. When this type of vaccine is developed further for human use, it would have the advantage of ease of manufacture and ready adjustments in peptide sequence and hapten-linker structure.
A novel conjunction of an anti-cocaine vaccine and an improved version of human butyryl cholinesterase is being developed by Steven Brimijoin at the Mayo Clinic (48). The natural enzyme hydrolyzes cocaine to benzoic acid and ecgonine, but is insufficiently fast to have a substantial impact on acutely administered drug at the doses ordinarily used. The efficiency of the enzyme has been improved several thousand fold, however, and when passively administered, can completely block cocaine activity (49). While this can be useful at high doses for acute toxicity, the half life of the enzyme is short (hours) and so passive administration can have no role in the treatment of addiction. However, if he enzyme can delivered by a viral vector and expressed from its coding sequence in vivo, even at lower concentrations it may be able to hydrolyze the cocaine in the serum if the antibody binding capacity were exceeded, and further, it could then degrade cocaine molecules as they were released from the antibodies, preventing the released drug from entering the brain (50).
A better anti-cocaine vaccine or new methods will not be a panacea, however. Vaccination will always need to be done in conjunction with other interventions such as rehabilitation and therapy in order to be of genuine help to the addict who is motivated to quit this terrible addiction.
References
- 1.History of Cocaine [online] Available at: http://www.a1b2c3.com/drugs/coc01.htm.
- 2.Ruetsch YA, Boni T, Borgeat A. From cocaine to ropivacaine: the history of local anesthetic drugs. Curr Top Med Chem. 2001;1:175–182. doi: 10.2174/1568026013395335. [DOI] [PubMed] [Google Scholar]
- 3.Gootenberg P. Andean Cocaine: The Making of a Global Drug. Chapel Hill, NC: University of North Carolina Press; 2008. [Google Scholar]
- 4.Drug Related Crime [online] Available at: http://www.whitehousedrugpolicy.gov/publications/factsht/crime/index.html.
- 5.Results from the 2007 National Survey on Drug Use and Health: National Findings (NSDUH Series H-34, DHHS Publication No. SMA 08-4343). Rockville, MD. [online]. Available at. http://oas.samhsa.gov.
- 6.Goldstein RA, DesLauriers C, Burda A, Johnson-Arbor K. Cocaine: history, social implications, and toxicity: a review. Semin Diagn Pathol. 2009;26:10–17. doi: 10.1053/j.semdp.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Bressan RA, Crippa JA. The role of dopamine in reward and pleasure behaviour--review of data from preclinical research. Acta Psychiatr Scand Suppl. 2005:14–21. doi: 10.1111/j.1600-0447.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 8.Thanos PK, Michaelides M, Benveniste H, Wang GJ, Volkow ND. The effects of cocaine on regional brain glucose metabolism is attenuated in dopamine transporter knockout mice. Synapse. 2008;62:319–324. doi: 10.1002/syn.20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thanos PK, Michaelides M, Umegaki H, Volkow ND. D2R DNA transfer into the nucleus accumbens attenuates cocaine self-administration in rats. Synapse. 2008;62:481–486. doi: 10.1002/syn.20523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li MD, Burmeister M. New insights into the genetics of addiction. Nat Rev Genet. 2009;10:225–231. doi: 10.1038/nrg2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thanos PK, Habibi R, Michaelides M, et al. Dopamine D4 receptor (D4R) deletion in mice does not affect operant responding for food or cocaine. Behav Brain Res. 2010;207:508–511. doi: 10.1016/j.bbr.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preti A. New developments in the pharmacotherapy of cocaine abuse. Addict Biol. 2007;12:133–151. doi: 10.1111/j.1369-1600.2007.00061.x. [DOI] [PubMed] [Google Scholar]
- 13.Bagasra O, Forman LJ, Howeedy A, Whittle P. A potential vaccine for cocaine abuse prophylaxis. Immunopharmacology. 1992;23:173–179. doi: 10.1016/0162-3109(92)90023-6. [DOI] [PubMed] [Google Scholar]
- 14.Gallacher G. A potential vaccine for cocaine abuse prophylaxis? Immunopharmacology. 1994;27:79–84. doi: 10.1016/0162-3109(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 15.Chandrakumar NS, Carron CP, Meyer DM, Beardsley PM, Nash SA, Tam LL, Rafferty M. Phenylphosphonate monoester analogs of cocaine. Potential haptens for the generation of catalytic antibodies. Bioorg Med Chem Lett. 1993;3:309–312. [Google Scholar]
- 16.Landry DW, Zhao K, Yang GX, Glickman M, Georgiadis TM. Antibody-catalyzed degradation of cocaine. Science. 1993;259:1899–1901. doi: 10.1126/science.8456315. [DOI] [PubMed] [Google Scholar]
- 17.Basmadjian GP, Singh S, Sastrodjojo B, et al. Generation of polyclonal catalytic antibodies against cocaine using transition state analogs of cocaine conjugated to diphtheria toxoid. Chem Pharm Bull (Tokyo) 1995;43:1902–1911. doi: 10.1248/cpb.43.1902. [DOI] [PubMed] [Google Scholar]
- 18.Yang GX, Chun J, Arakawa-Uramoto H, Wang X, Gawinowcz MA, Zhao K, Landry DW. Anti-cocaine catalytic antibodies: A synthetic approach to improved antibody diversity. J Am Chem Soc. 1996;118:5881–5890. [Google Scholar]
- 19.McKenzie KM, Mee JM, Rogers CJ, Hixon MS, Kaufmann GF, Janda KD. Identification and characterization of single chain anti-cocaine catalytic antibodies. J Mol Biol. 2007;365:722–731. doi: 10.1016/j.jmb.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrera MR, Ashley JA, Parsons LH, Wirsching P, Koob GF, Janda KD. Suppression of psychoactive effects of cocaine by active immunization. Nature. 1995;378:727–730. doi: 10.1038/378727a0. [DOI] [PubMed] [Google Scholar]
- 21.Carrera MR, Ashley JA, Zhou B, Wirsching P, Koob GF, Janda KD. Cocaine vaccines: antibody protection against relapse in a rat model. Proc Natl Acad Sci U S A. 2000;97:6202–6206. doi: 10.1073/pnas.97.11.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrera MR, Trigo JM, Wirsching P, Roberts AJ, Janda KD. Evaluation of the anticocaine monoclonal antibody GNC92H2 as an immunotherapy for cocaine overdose. Pharmacol Biochem Behav. 2005;81:709–714. doi: 10.1016/j.pbb.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Redwan el RM, Larsen NA, Zhou B, Wirsching P, Janda KD, Wilson IA. Expression and characterization of a humanized cocaine-binding antibody. Biotechnol Bioeng. 2003;82:612–618. doi: 10.1002/bit.10598. [DOI] [PubMed] [Google Scholar]
- 24.Larsen NA, Zhou B, Heine A, Wirsching P, Janda KD, Wilson IA. Crystal structure of a cocaine-binding antibody. J Mol Biol. 2001;311:9–15. doi: 10.1006/jmbi.2001.4839. [DOI] [PubMed] [Google Scholar]
- 25.Sakurai M, Wirsching P, Janda KD. Design and synthesis of a cocaine-diamide hapten for vaccine development. Tetrahedron Lett. 1996;37:5479–5482. [Google Scholar]
- 26.Carrera MR, Ashley JA, Wirsching P, Koob GF, Janda KD. A second-generation vaccine protects against the psychoactive effects of cocaine. Proc Natl Acad Sci U S A. 2001;98:1988–1992. doi: 10.1073/pnas.041610998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ino A, Dickerson TJ, Janda KD. Positional linker effects in haptens for cocaine immunopharmacotherapy. Bioorg Med Chem Lett. 2007;17:4280–4283. doi: 10.1016/j.bmcl.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 28.Ettinger RH, Ettinger WF, Harless WE. Active immunization with cocaine-protein conjugate attenuates cocaine effects. Pharmacol Biochem Behav. 1997;58:215–220. doi: 10.1016/s0091-3057(97)00005-1. [DOI] [PubMed] [Google Scholar]
- 29.Johnson MW, Ettinger RH. Active cocaine immunization attenuates the discriminative properties of cocaine. Exp Clin Psychopharmacol. 2000;8:163–167. doi: 10.1037//1064-1297.8.2.163. [DOI] [PubMed] [Google Scholar]
- 30.Orson FM, Kinsey BM, Singh RA, Wu Y, Kosten TR. Vaccines for cocaine abuse. Hum Vaccin. 2009:5. doi: 10.4161/hv.5.4.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hrafnkelsdottir K, Valgeirsson J, Gizurarson S. Induction of protective and specific antibodies against cocaine by intranasal immunisation using a glyceride adjuvant. Biol Pharm Bull. 2005;28:1038–1042. doi: 10.1248/bpb.28.1038. [DOI] [PubMed] [Google Scholar]
- 32.Danger Y, Devys A, Gadjou C, Galons H, Blanchard D, Follea G. Development of monoclonal antibodies directed against cocaine and cocaethylene: potential new tools for immunotherapy. Hybrid Hybridomics. 2004;23:212–218. doi: 10.1089/1536859041651286. [DOI] [PubMed] [Google Scholar]
- 33.Gadjou C, Danger Y, Sandouka P, Scherrmann J-M, Blanchard D, Follea G, Galons H. Design of cocaethylene and cocaine conjugates to produce highly selective polyclonal antibodies. Int J Biomed Sci. 2006;2:53–58. [PMC free article] [PubMed] [Google Scholar]
- 34.Paula S, Tabet MR, Farr CD, Norman AB, Ball WJ., Jr Three-dimensional quantitative structure-activity relationship modeling of cocaine binding by a novel human monoclonal antibody. J Med Chem. 2004;47:133–142. doi: 10.1021/jm030351z. [DOI] [PubMed] [Google Scholar]
- 35.Paula S, Tabet MR, Keenan SM, Welsh WJ, Ball WJ., Jr Three-dimensional structure-activity relationship modeling of cocaine binding to two monoclonal antibodies by comparative molecular field analysis. J Mol Biol. 2003;325:515–530. doi: 10.1016/s0022-2836(02)01235-4. [DOI] [PubMed] [Google Scholar]
- 36.Pozharski E, Moulin A, Hewagama A, Shanafelt AB, Petsko GA, Ringe D. Diversity in hapten recognition: structural study of an anti-cocaine antibody M82G2. J Mol Biol. 2005;349:570–582. doi: 10.1016/j.jmb.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 37.Fox BS, Kantak KM, Edwards MA, et al. Efficacy of a therapeutic cocaine vaccine in rodent models. Nat Med. 1996;2:1129–1132. doi: 10.1038/nm1096-1129. [DOI] [PubMed] [Google Scholar]
- 38.Kantak KM, Collins SL, Bond J, Fox BS. Time course of changes in cocaine self-administration behavior in rats during immunization with the cocaine vaccine IPC-1010. Psychopharmacology (Berl) 2001;153:334–340. doi: 10.1007/s002130000555. [DOI] [PubMed] [Google Scholar]
- 39.Kantak KM, Collins SL, Lipman EG, Bond J, Giovanoni K, Fox BS. Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology (Berl) 2000;148:251–262. doi: 10.1007/s002130050049. [DOI] [PubMed] [Google Scholar]
- 40.Kosten TR, Rosen M, Bond J, et al. Human therapeutic cocaine vaccine: safety and immunogenicity. Vaccine. 2002;20:1196–1204. doi: 10.1016/s0264-410x(01)00425-x. [DOI] [PubMed] [Google Scholar]
- 41.Martell BA, Mitchell E, Poling J, Gonsai K, Kosten TR. Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol Psychiatry. 2005;58:158–164. doi: 10.1016/j.biopsych.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 42.Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR. Cocaine Vaccine for the Treatment of Cocaine Dependence: A Randomized Double-Blind Placebo-Controlled Efficacy Trial. Arch Gen Psych. 2009;66:1116–1123. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenkins AJ, Keenan RM, Henningfield JE, Cone EJ. Correlation between pharmacological effects and plasma cocaine concentrations after smoked administration. J Anal Toxicol. 2002;26:382–392. doi: 10.1093/jat/26.7.382. [DOI] [PubMed] [Google Scholar]
- 44.Haney M, Gunderson EW, Jiang H, Collins ED, Foltin RW. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol Psychiatry. 2010;67:59–65. doi: 10.1016/j.biopsych.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith TW, Skubitz KM. Kinetics in interactions between antibodies and haptens. Biochemistry. 1975;14:1496–1502. doi: 10.1021/bi00678a023. [DOI] [PubMed] [Google Scholar]
- 47.Deliyannis G, Kedzierska K, Lau YF, Zeng W, Turner SJ, Jackson DC, Brown LE. Intranasal lipopeptide primes lung-resident memory CD8+ T cells for long-term pulmonary protection against influenza. Eur J Immunol. 2006;36:770–778. doi: 10.1002/eji.200535217. [DOI] [PubMed] [Google Scholar]
- 48.Gao Y, LaFleur D, Shah R, Zhao Q, Singh M, Brimijoin S. An albumin-butyrylcholinesterase for cocaine toxicity and addiction: catalytic and pharmacokinetic properties. Chem Biol Interact. 2008;175:83–87. doi: 10.1016/j.cbi.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao Y, Brimijoin S. An engineered cocaine hydrolase blunts and reverses cardiovascular responses to cocaine in rats. J Pharmacol Exp Ther. 2004;310:1046–1052. doi: 10.1124/jpet.104.068122. [DOI] [PubMed] [Google Scholar]
- 50.Gao Y, Orson FM, Kinsey BM, Kosten TR, Brimijoin S. The concept of pharmacologic cocaine interception as a treatment for drug abuse. Chem Biol Interact. 2010 doi: 10.1016/j.cbi.2010.02.036. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


