Abstract
BACKGROUND
Viral myocarditis is most frequently associated with infection by Coxsackievirus B3 (CVB3). Interferon (IFN)-ß therapy has been studied and may reduce virally induced tissue damage and improve heart function.
METHODS
In the present study we have investigated the role of translational suppression in the context of an IFN-α/ß mediated antiviral immune response to CVB3 infection. Specifically, we examine the effects of IFN-α/ß treatment of CVB3 infected mouse embryonic fibroblast (MEF) cells and splenocytes lacking 4E-BP1, a suppressor of 5′capped mRNA translation. Extending these in vitro studies, we examine the effects of CVB3 infection and IFN-ß treatment in 4E-BP1−/− mice.
RESULTS
Our data show that 4E-BP1−/− cells are more sensitive to the antiviral effects of IFN-α4 and IFN-ß treatment than 4E-BP1+/+ cells when infected with CVB3. Similarly, 4E-BP1−/− mice are more sensitive to treatment with IFN-ß, exhibiting lower viral titers in heart tissue than 4E-BP1+/+ mice during the course of infection. Additionally, we demonstrate that treatment with IFN-ß reduces inflammatory infiltrates into the hearts of infected mice.
CONCLUSION
Our data identify 4E-BP1 as a novel drug target to augment responsiveness to IFN-ß therapy in CVB3-induced myocarditis.
Keywords: Interferon, virus, myocarditis, inflammation, immunology
INTRODUCTION
Myocarditis is a major cause of heart failure in adults, described as an inflammation of the myocardium resulting in a loss of ventricular systolic function [1, 2]. Viral infection of the heart is the most common cause of myocarditis, and has thus been studied intensively in an effort to develop effective treatment strategies [1, 3, 4]. A number of anti-inflammatory therapies are currently being developed to reduce myocardial inflammation which often accompanies an acute infection and predicts subsequent development of dilated cardiomyopathy [1, 4]. Since myocytes are terminally differentiated and non-regenerating cells critical to heart function, effective antiviral therapeutics could limit the initial viral infection-induced damage, thereby reducing the severity of inflammatory sequealae. In a 2003 phase 2 clinical trial, recombinant interferon (IFN)-ß was proven effective in the treatment of viral myocarditis [5].
Belonging to the family of Picornaviridae, the positive sense ssRNA Coxsackievirus B3 (CVB3) is one of the most common pathogens associated with viral myocarditis [6-10]. Along with the closely related polioviruses, CVB3 has evolved strategies of subverting the innate immune response. In cardiac myocytes, where there is a particularly high basal expression of IFN-ß, CVB3 is nevertheless able to replicate efficiently [11, 12]. In vitro data suggest the CVB3-mediated ablation of IFN-ß transcription in poly I:C-treated human fibroblasts [13]. At the level of translation, the CVB3 2A protease rapidly and selectively reduces translation of cellular mRNAs by cleaving EIF4G and PABP while maintaining its own IRES-driven viral protein synthesis [14-18]. Accompanying this CVB3 inhibition of the host cell translational machinery, CVB3 activates the survival signaling cascade of phosphoinositide 3 kinase (PI3K)/Akt. Indeed, treatment of cells with the PI3K inhibitor, LY294002, reduces viral replication [19]. Mediated by the virus encoded non-structural proteins, 2B and 3A, CVB3 also inhibits cellular protein trafficking and secretion as another mechanism of immune evasion [20], including restriction of the surface expression of major histocompatability class I (MHC-I) molecules [21]. Notably, the homologous poliovirus 3A protein has been shown to inhibit the secretion of IFN-ß, IL-6 and IL-8 and may possibly play a similar role in CVB3-infected cells [22].
Since CVB3 so effectively represses 5′ capped mRNA translation in infected cells, thereby limiting a host antiviral response, we investigated the dynamics of an antiviral response in mice lacking the translational suppressor, eukaryotic initiation factor 4E-binding protein-1 (4E-BP1). Notably, CVB3 exhibits a very specific pattern of interference with the host cell machinery by activating the PI3K/Akt signaling pathway that is upstream of 4E-BP1, yet independently inhibiting 5′ capped mRNA translation which is governed by 4E-BP1. Previously, we have shown that mouse embryonic fibroblasts (MEFs) lacking this repressor of 5′ capped mRNA translation, 4E-BP1, induce greater expression of antiviral proteins upon IFN-α4 stimulation and are more sensitive to the effects of IFN-α4 treatment when challenged with encephalomyocarditis virus (EMCV) [23]. In the present study we demonstrate that 4E-BP1 plays a negative regulatory role in an IFN-α/ß mediated antiviral response to CVB3 infection, and that the absence of 4E-BP1 enhances the responsiveness to IFN-ß treatment.
MATERIALS AND METHODS
Animals, cells and virus
4E-BP1+/+ and 4E-BP1−/− mice (C57Bl/6 background) were maintained in a sterile, pathogen-free environment according to the Animal Care Committee guidelines of the Toronto General Research Institute. Splenocytes were isolated by mechanical dissociation of spleens harvested from 4E-BP1+/+ and 4E-BP1−/− mice, 8 weeks of age. Cells were stimulated in plates coated with anti-CD3 and anti-CD28 antibodies (BD Pharmingen, Mississauga) for 2 days followed by addition of 50 U/ml mIL-2 (R&D Systems, Minneapolis) in 10% FCS RPMI-1640 supplemented with 50 uM β-mercaptoethanol. Proliferating cells were harvested by Lympholyte® separation, according to the manufacturer’s instructions. Primary CD3+ T cells were seeded at a density of 106 cells/ml, MEFs derived from 4E-BP1+/+ and 4E-BP1−/− mice [23] were maintained in DMEM supplemented with 10% heat-inactivated fetal calf serum (FCS), 100U/ml penicillin, 100ug/ml streptomycin. CVB3 strain, Charles Gauntt, was propagated in HeLa cells as previously described [24].
IFN treatment and virus infection
In vitro
Cells were plated at a sub-confluent density in 2% medium prior to treatment with IFN and incubated at 37°C in 5% CO2. Following a 12-hour treatment with IFN-ß or IFN-α4, cells were infected with CVB3 at a multiplicity of infection (MOI) of 1.0 in 2% FCS DMEM and the virus was allowed to adsorb for 90 minutes. Cells were then washed three times with 2% FCS DMEM and incubated for a further 12 hours. Cells were lysed in PBS by 3 freeze thaw cycles at −80°C. Virus titers were then determined by standard plaque assay in HeLa cells.
In vivo
Mice, aged 6 -12 weeks were injected i.p. with 100 μl PBS carrier or mouse IFN-ß, followed 4hr later by i.p. inoculation with 103 plaque-forming units (pfu) of CVB3. At the indicated times, mice were euthanized, and hearts were aseptically removed and frozen in liquid nitrogen. After 3 freeze-thaw cycles, viral titers were determined by plaque assay in HeLa cells, and expressed as PFU per gram of tissue.
Histopathology
Heart tissue was harvested from CVB3-infected mice and processed for hematoxylin and eosin (H & E) staining of thin sections. Whole hearts were fixed in 10% (vol/vol) formalin (Sigma), embedded in paraffin, and sectioned at 5 μm. Cross-sectioned tissues were stained with H & E. Leukocyte infiltration was quantified using a blind scoring method (0-no infiltration, 1-sparse infiltration, 2-moderate infiltration, 3-severe infiltration).
RESULTS
IFN-α/ß invokes enhanced antiviral effects in cells lacking the translational suppressor 4E-BP1
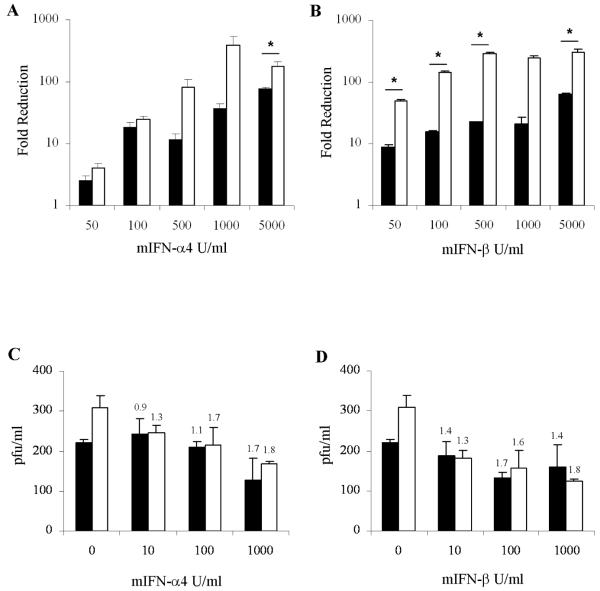
In a first series of experiments we examined the effects of IFN-α/ß treatment on the replication of CVB3 in murine embryonic fibroblasts (MEFs). Since the doubling time of 4E-BP1−/− cells is shorter than than their wildtype counterparts and, as a consequence, 4E-BP1−/− cells support increased CVB3 replication over a period of 12 hours, the effects of IFN-α4 and IFN-ß treatment are presented as fold-reduction in viral replication rather than absolute plaque forming units (Fig 1 A, B). Treatment with IFN-α4 or IFN-ß induced a strong antiviral response in 4E-BP1−/− MEFs, effectively reducing viral replication. IFN 90% inhibitory concentration (IC90) values revealed that 4E-BP1−/− cells were more sensitive to the effects of IFN treatment than 4E-BP1+/+ cells and showed that IFN-β exhibited stronger antiviral potency than IFN-α4: the IFN-α4 IC90 was 98 U/ml in 4E-BP1+/+ MEFs and 82 U/ml in 4E-BP1−/− MEFs; the IFN-β IC90 was 79 U/ml in 4E-BP1+/+ MEFs and 17 U/ml in 4E-BP1−/− MEFs. In subsequent experiments, we examined the antiviral effects of IFN-α/ß in primary cells derived from wildtype and 4E-BP1−/− mice, namely splenocytes. Notably, these primary cells exhibited no differences in their proliferative capacity whether derived from wildtype or 4E-BP1 null mice. As for the MEFs, the data demonstrate that the absence of 4E-BP1 enhances sensitivity to the antiviral effects of IFN (Figure 1 C,D).
Fig. 1. IFN-α4 and IFN-β elicit strong antiviral responses in cells lacking 4E-BP1.
4E-BP1 +/+ (■) or 4E-BP1−/− (□) MEFs (A, B) or primary splenocytes (C, D) were either left untreated, or treated with either mouse IFN-α4 (A, C) or IFN-β (B, D), at the doses indicated, 12 hrs prior to infection with CVB3 at an MOI of 1. 12 hrs post-infection, viral titers were measured by plaque assay in HeLa cells. Data for MEFs are expressed as fold reduction in viral titers relative to untreated cells Viral titers in untreated MEFs were 3.6×105 pfu/ml (4E-BP1 +/+) and 3.2×106 pfu/ml (4E-BP1−/−). IC90 values were calculated for MEFs treated with IFN-α4 (4E-BP1 +/+ (97.8 U/ml), 4E-BP1−/− (82.3 U/ml)) and IFN-β (4E-BP1 +/+ (79.2 U/ml), 4E-BP1−/− (16.8 U/ml)). Data for splenocytes are expressed as pfu/ml, and fold reduction relative to untreated cells is shown above histograms. Mean values of triplicates +/− SE are plotted, representative of three independent experiments. * P≤ 0.05 (Student’s t-test).
IFN-ß elicits a stronger antiviral effect in mice lacking the translational suppressor 4E-BP1
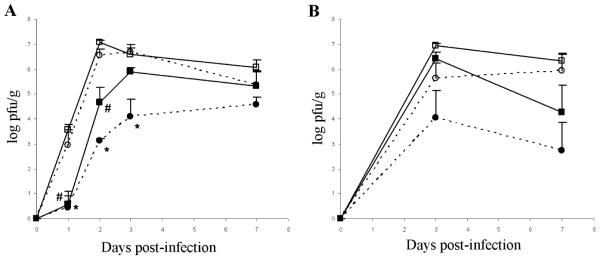
Previously, we demonstrated that mice lacking IFN-ß are more susceptible to CVB3 infection than wild-type mice, and other studies have consistently shown that IFN-α/ß treatment reduces the severity of CVB3-induced myocarditis [5, 24-27]. To investigate the role that translational regulation plays in an antiviral IFN-α/ß response in vivo, we treated 4E-BP1+/+ and 4E-BP1−/− mice with IFN-ß prior to infection with a sub-lethal dose of CVB3. All mice exhibited signs of disease, including reduced activity, ruffled fur and weight loss. In one series of experiments, three of the five control 4E-BP1+/+ mice (PBS as mock treatment) succumbed between days 3 and 5 post-infection. Heart viral titers indicate an acute viral infection with peak viral burden 3 days post-infection, followed by progressive clearance of the virus from the heart 7 days post-infection (Fig 2). As anticipated, mice treated with IFN-ß were more active and did not exhibit disease symptoms as severe as their mock-treated counterparts. Comparison between untreated (carrier alone) 4E-BP1+/+ and 4E-BP1−/− mice revealed only modest differences in viral titres during the course of infection. Consistent with previous reports, treatment with IFN-ß elicited a protective effect in the hearts of CVB3 infected mice, reducing the viral titers. Interestingly, 4E-BP1−/− mice showed an enhanced sensitivity to IFN-ß treatment, as indicated by an approximate 2 log-fold lower viral load in mice treated with 105 U IFN-ß than that measured in the 4E-BP1+/+ treated mice at the peak of infection, day 3 post-infection. A lesser, but still notable reduction in viral load in the hearts of 4E-BP1−/− mice was also observed at the lower treatment dose of 104 U IFN-ß.
Fig. 2. IFN-ß confers greater protection against CVB3 infection in 4E-BP1 null mice.
Mice were either treated with 105 U(A) or 104 U(B) mIFN-β (4E-BP1+/+ (■), 4E-BP1−/− (●)) or mock treated with 100 ul PBS carrier (4E-BP1+/+ (□), 4E-BP1−/− (○)), by ip injection. 4 hrs later all mice were infected ip with 103 pfu CVB3. At the indicated times, 5 mice from each group were sacrificed and their hearts harvested. Heart viral titers were measured by plaque assay in HeLa cells. Data for mock treated mice from (A) are representative of 3 independent experiments, and data for IFN-β treated mice represent 1 experiment. Values are the geometric means ± SE. *, # P≤0.05 (Student’s t-test).
IFN-ß treatment reduces inflammation in the hearts of CVB3 infected mice
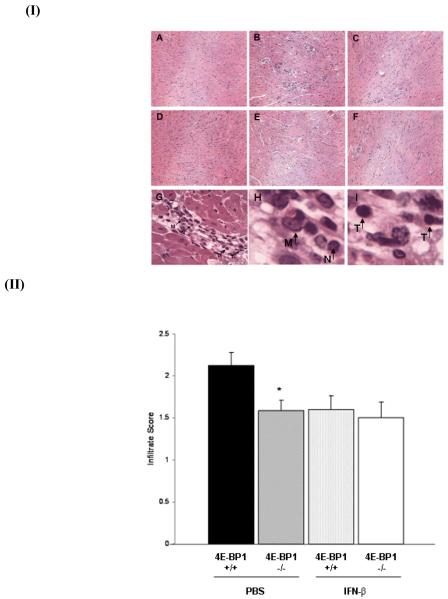
An important aspect of CVB3-induced myocarditis is the degree of infiltration of leukocytes into the infected myocardium. In an earlier study we showed that IFN-ß null mice are more susceptible to CVB3-induced myocarditis with increased infiltration of leukocytes into the myocardium [24]. Accordingly, we next examined the effects of IFN-ß treatment on leukocyte trafficking into the myocardium of CVB3-infected mice and whether or not translational suppression mediated by 4E-BP1 contributes to a reduction in this leukocyte infiltration. Scoring of H&E stained heart sections confirmed the previously described observation that IFN-ß treatment reduces myocardial inflammation (Fig 3). Our data reveal that there are less inflammatory infiltrates in the hearts of untreated 4E-BP1−/− mice than 4E-BP1+/+ mice. We were unable to detect a difference in the extent of myocardial leukocyte infiltration between untreated and IFN-ß treated 4E-BP1−/− mice. Close examination of infiltrating cells at high magnification (400x), reveals different immune cell types, including monocytes, neutrophils and T cells (Fig 3, panels G-I).
Fig. 3. CVB3 infected 4E-BP1−/− mice exhibit less severe pathology.
(I) Representative hematoxylin and eosin–stained sections of hearts from 4E-BP1+/+ (naïve (A), PBS treated (B) and IFN-β treated (C)) and 4E-BP1−/− (naïve (D), PBS treated (E) and IFN-β treated (F)) mice harvested on day 7 post-CVB3 infection. 40 x magnification. Leukocyte infiltration is indicated in the hearts of 4E-BP1+/+ mice 7 days post-CVB3 infection (G) (400x magnification). Monocyte (M), neutrophil (N) and T cell (T) enlarged images are shown in H and I.
(II) Heart sections were scored blind for degree of leukocyte infiltration (0-no infiltration, 1-sparse infiltration, 2-moderate infiltration, 3-severe infiltration) (4E-BP1+/+ + PBS(■), 4E-BP1−/− + PBS( ),4E-BP1+/+ + IFN-β (
),4E-BP1+/+ + IFN-β ( ), 4E-BP1−/− + IFN-β (□)). Data are expressed as the mean score ± SE. * P≤ 0.05 (Student’s t-test).
), 4E-BP1−/− + IFN-β (□)). Data are expressed as the mean score ± SE. * P≤ 0.05 (Student’s t-test).
DISCUSSION
IFNs exhibit pleiotropic effects in the context of innate and adaptive immunity. Accordingly, they have clinical application as therapeutics for viral, neurodegenerative and malignant diseases [28, 29]. Type I IFNs are rapidly produced in response to virus infection, inducing an antiviral state in neighbouring cells, thereby limiting the spread of virus. Following cell surface receptor activation in target cells, IFNs-α/ß invoke a series of intracellular signaling events that culminate in the expression of approximately 300 IFN sensitive genes (ISG). In addition to the well described transcriptional regulation exerted by IFNs-α/ß through the Jak/STAT pathway, we have identified a novel pathway whereby IFNs-α/ß coordinately regulate translation though the PI-3′K/mTOR pathway [23, 30, 31]. Recent studies have highlighted important roles for PI-3′K/mTOR signaling in the regulation of IFN-α/ß induction via TLR signaling [32-34] and there is evidence that the absence of translational suppressors 4E-BP1/2 enhances the production of virus-induced IFN-α/ß [35].
Distinct from these studies that investigated the importance of PI-3′K/mTOR signaling in the induction of IFNs-α/ß by viral or synthetic stimuli, we sought to examine the effects of IFN-α/ß treatment on the PI-3′K/mTOR pathway in the context of viral infection. Given the evidence supporting an important role for IFN-α/ß in viral myocarditis, and our earlier observation that cells lacking the translational suppressor 4E-BP1 are more sensitive to IFN-α treatment, we reasoned that treating 4E-BP1 null mice with IFN-ß would elicit a more robust innate immune response to CVB3 infection. Within the microarchitecture of the myocardium unique roles have been attributed to cardiac fibroblasts and cardiac myocytes in the context of a viral infection [11, 12]. A model has been proposed whereby cardiac myocytes express high basal levels of IFN-ß, thereby inducing high basal levels of IRF-7 in an autocrine fashion. This effectively pre-arms the myocyte innate immune response to rapidly produce IFN-α in response to viral infection and stimulate fibroblasts to produce antiviral proteins to further limit viral spread. Intriguingly, fibroblasts express high basal levels of the IFNAR1 receptor, and are thus highly sensitive to the IFN-α/ß produced by myocytes. We speculate that this cooperative interplay between cardiac myocytes and cardiac fibroblasts is affected in the absence of translational repression, in such a way as to enhance the innate immune response through the translation of antiviral proteins.
Data presented here demonstrate the importance of translational regulation in an IFN-α/ß antiviral response to infection. Consistent with previously published in vitro data using a related Picornavirus, EMCV, we show that MEFs and splenocytes lacking the translational suppressor 4E-BP1 are more sensitive to the effects of IFN-α4 and IFN-ß treatment than their wildtype counterparts when infected with CVB3. This is indicated by lower IC90 values for IFN-α4 and IFN-ß as calculated from titers in 4E-BP1−/− MEFs. While IFN-ß maintains a strong differential effect in treated MEFs through a range of doses, IFN-α4 treatment appears to lose this same effect at lower doses. This difference highlights the subtle differences between type I IFN subtypes. It is worth noting that although CVB3 is considerably more virulent, and antagonizes an IFN-α/ß response [13, 22], enhanced IFN-α/ß responsiveness is still observed in 4E-BP1−/− cells.
Viral myocarditis is a particularly insidious disease, since acute viral infection of the myocardium often leads to autoimmunity, where the host’s own inflammatory immune response damages the heart, ultimately leading to dilated cardiomyopathy. Several studies have shown that a reduction in T cell infiltration into the heart improves the outcome of viral myocarditis and that type I IFNs contribute toward limiting T cell infiltration [25, 36-38]. As anticipated, our data revealed less cell infiltration in the hearts of mice treated with IFN-ß. We speculate that this may be due to a reduced level of necrosis in the myocardium, resulting from an inhibition of viral replication by IFN-ß. It is also interesting to note that the degree of leukocyte infiltration in mock treated 4E-BP1−/− mice was comparable to the level of infiltration measured in IFN-treated mice. Although the viral titers do not reflect a difference between 4E-BP1+/+ and 4E-BP1−/− mice early in the infection, at 7 days post-infection the mock treated 4E-BP1−/− mice appear to be clearing virus as efficiently as the IFN-ß treated mice.
Data presented in this study provide evidence supporting the utility in targeting the PI3’K/mTOR signaling pathway, specifically the translational suppressor 4E-BP1, to augment the antiviral activity of IFN-ß.
Acknowledgements
These studies were supported by a Canadian Institutes of Health Research (CIHR) grant MOP 15094 to ENF and NIH grant CA77816 to LCP. ENF is a Tier 1 Canada Research Chair. JDB was supported by a CIHR Student Fellowship TGF-53877.
Footnotes
Disclosure Statement
The authors declare no conflicts of interest.
REFERENCES
- 1.Rose NR, Čiháková D, Barin JG. Mechanisms underlying Myocarditis. Drug Discovery Today: Disease Mechanisms. 2006;3:207–212. [Google Scholar]
- 2.Gupta S, Markham DW, Drazner MH, Mammen PPA. Fulminant myocarditis. Nature Clinical Practice Cardiovascular Medicine. 2008;5:693–706. doi: 10.1038/ncpcardio1331. [DOI] [PubMed] [Google Scholar]
- 3.Ellis CR, Di Salvo T. Myocarditis: Basic and clinical aspects. Cardiol Rev. 2007;15:170–177. doi: 10.1097/CRD.0b013e31806450c4. [DOI] [PubMed] [Google Scholar]
- 4.Liu PP, Mason JW. Advances in the understanding of myocarditis. Circulation. 2001;104:1076–1082. doi: 10.1161/hc3401.095198. [DOI] [PubMed] [Google Scholar]
- 5.Kühl U, Pauschinger M, Schwimmbeck PL, Seeberg B, Lober C, Noutsias M, Poller W, Schultheiss H. Interferon-β treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation. 2003;107:2793–2798. doi: 10.1161/01.CIR.0000072766.67150.51. [DOI] [PubMed] [Google Scholar]
- 6.Bowles NE, Ni J, Kearney DL, Pauschinger M, Schultheiss H, McCarthy R, Hare J, Bricker JT, Bowles KR, Towbin JA. Detection of viruses in myocardial tissues by polymerase chain reaction: Evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42:466–472. doi: 10.1016/s0735-1097(03)00648-x. [DOI] [PubMed] [Google Scholar]
- 7.Kim K, Hufnagel G, Chapman NM, Tracy S. The group B coxsackieviruses and myocarditis. Reviews in Medical Virology. 2001;11:355–368. doi: 10.1002/rmv.326. [DOI] [PubMed] [Google Scholar]
- 8.Tracy S, Chapman NM, McManus BM, Pallansch MA, Beck MA, Carstens J. A molecular and serologic evaluation of enteroviral involvement in human myocarditis. Journal of Molecular and Cellular Cardiology. 1990;22:403–414. doi: 10.1016/0022-2828(90)91476-n. [DOI] [PubMed] [Google Scholar]
- 9.Kühl U, Pauschinger M, Noutsias M, Seeberg B, Bock T, Lassner D, Poller W, Kandolf R, Schultheiss H. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation. 2005;111:887–893. doi: 10.1161/01.CIR.0000155616.07901.35. [DOI] [PubMed] [Google Scholar]
- 10.Kühl U, Pauschinger M, Seeberg B, Lassner D, Noutsias M, Poller W, Schultheiss H. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation. 2005;112:1965–1970. doi: 10.1161/CIRCULATIONAHA.105.548156. [DOI] [PubMed] [Google Scholar]
- 11.Stewart MJ, Smoak K, Blum MA, Sherry B. Basal and reovirus-induced beta interferon (IFN-β) and IFN-β-stimulated gene expression are cell type specific in the cardiac protective response. J Virol. 2005;79:2979–2987. doi: 10.1128/JVI.79.5.2979-2987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zurney J, Howard KE, Sherry B. Basal expression levels of IFNAR and Jak-STAT components are determinants of cell-type-specific differences in cardiac antiviral responses. J Virol. 2007;81:13668–13680. doi: 10.1128/JVI.01172-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richtsteiger R, Henke-Gendo C, Schmidtke M, Harste G, Heim A. Quantitative multiplex real-time PCR for the sensitive detection of interferon β gene induction and viral suppression of interferon β expression. Cytokine. 2003;24:190–200. doi: 10.1016/j.cyto.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Kerekatte V, Keiper BD, Badorff C, Cat A, Knowlton KU, Rhoads RE. Cleavage of poly(A)-binding protein by coxsackievirus 2A protease in vitro and in vivo: Another mechanism for host protein synthesis shutoff? Journal of Virology. 1999;73:709–717. doi: 10.1128/jvi.73.1.709-717.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joachims M, Van Breugel PC, Lloyd RE. Cleavage of poly(A)-binding protein by enterovirus proteases concurrent with inhibition of translation in vitro. Journal of Virology. 1999;73:718–727. doi: 10.1128/jvi.73.1.718-727.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sommergruber W, Ahorn H, Klump H, Seipelt J, Zoephel A, Fessl F, Krystek E, Blaas D, Kuechler E, Liebig H, Skern T. 2A proteinases of coxsackie- and rhinovirus cleave peptides derived from elF-4γ via a common recognition motif. Virology. 1994;198:741–745. doi: 10.1006/viro.1994.1089. [DOI] [PubMed] [Google Scholar]
- 17.Skern T. Purification of two picornaviral 2A proteinases: Interaction with eIF-4γ and influence on in vitro translation. Biochemistry. 1993;32:7581–7588. doi: 10.1021/bi00080a033. [DOI] [PubMed] [Google Scholar]
- 18.Lamphear BJ, Yan R, Yang F, Waters D, Liebig H, Klump H, Kuechler E, Skern T, Rhoads RE. Mapping the cleavage site in protein synthesis initiation factor eIF-4γ of the 2A proteases from human Coxsackievirus and rhinovirus. Journal of Biological Chemistry. 1993;268:19200–19203. [PubMed] [Google Scholar]
- 19.Esfandiarei M, Luo H, Yanagawa B, Suarez A, Dabiri D, Zhang J, McManus BM. Protein Kinase B/Akt Regulates Coxsackievirus B3 Replication through a Mechanism Which Is Not Caspase Dependent. J Virol. 2004;78:4289–4298. doi: 10.1128/JVI.78.8.4289-4298.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornell CT, Kiosses WB, Harkins S, Whitton JL. Inhibition of protein trafficking by coxsackievirus B3: Multiple viral proteins target a single organelle. J Virol. 2006;80:6637–6647. doi: 10.1128/JVI.02572-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornell CT, Kiosses WB, Harkins S, Whitton JL. Coxsackievirus B3 proteins directionally complement each other to downregulate surface major histocompatibility complex class I. J Virol. 2007;81:6785–6797. doi: 10.1128/JVI.00198-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dodd DA, Giddings THJ, Kirkegaard K. Poliovirus 3A protein limits interleukin-6 (IL-6), IL-8, and beta interferon secretion during viral infection. Journal of Virology. 2001;75:8158–8165. doi: 10.1128/JVI.75.17.8158-8165.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaur S, Lal L, Sassano A, Majchrzak-Kita B, Srikanth M, Baker DP, Petroulakis E, Hay N, Sonenberg N, Fish EN, Platanias LC. Regulatory effects of mammalian target of rapamycin-activated pathways in type I and II interferon signaling. J Biol Chem. 2007;282:1757–1768. doi: 10.1074/jbc.M607365200. [DOI] [PubMed] [Google Scholar]
- 24.Deonarain R, Cerullo D, Fuse K, Liu PP, Fish EN. Protective role for interferon-beta in coxsackievirus B3 infection. Circulation. 2004;110:3540–3543. doi: 10.1161/01.CIR.0000136824.73458.20. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Da Cunha V, Vincelette J, White K, Velichko S, Xu Y, Gross C, Fitch RM, Halks-Miller M, Larsen BR, Yajima T, Knowlton KU, Vergona R, Sullivan ME, Croze E. Antiviral and myocyte protective effects of murine interferon-β and -α2 in coxsackievirus B3-induced myocarditis and epicarditis in Balb/c mice. American Journal of Physiology - Heart and Circulatory Physiology. 2007;293:H69–H76. doi: 10.1152/ajpheart.00154.2007. [DOI] [PubMed] [Google Scholar]
- 26.Padalko E, Nuyens D, De Palma A, Verbeken E, Aerts JL, De Clercq E, Carmeliet P, Neyts J. The Interferon Inducer Ampligen [Poly(I)-Poly(C12U)] Markedly Protects Mice against Coxsackie B3 Virus-Induced Myocarditis. Antimicrobial Agents and Chemotherapy. 2004;48:267–274. doi: 10.1128/AAC.48.1.267-274.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumori A, Tomioka N, Kawai C. Protective effect of recombinant alpha interferon on coxsackievirus B3 myocarditis in mice. American Heart Journal. 1988;115:1229–1232. doi: 10.1016/0002-8703(88)90013-0. [DOI] [PubMed] [Google Scholar]
- 28.Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, Stark GR. Interferons at age 50: Past, current and future impact on biomedicine. Nature Reviews Drug Discovery. 2007;6:975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parmar S, Platanias LC. Interferons: Mechanisms of action and clinical applications. Curr Opin Oncol. 2003;15:431–439. doi: 10.1097/00001622-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Lekmine F, Sassano A, Uddin S, Smith J, Majchrzak B, Brachmann SM, Hay N, Fish EN, Platanias LC. Interferon-gamma engages the p70 S6 kinase to regulate phosphorylation of the 40S S6 ribosomal protein. Exp Cell Res. 2004;295:173–182. doi: 10.1016/j.yexcr.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 31.Lekmine F, Uddin S, Sassano A, Parmar S, Brachmann SM, Majchrzak B, Sonenberg N, Hay N, Fish EN, Platanias LC. Activation of the p70 S6 kinase and phosphorylation of the 4E-BP1 repressor of mRNA translation by type I interferons. J Biol Chem. 2003;278:27772–27780. doi: 10.1074/jbc.M301364200. [DOI] [PubMed] [Google Scholar]
- 32.Guiducci C, Ghirelli C, Marloie-Provost M, Matray T, Coffman RL, Liu Y, Barrat FJ, Soumelis V. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J Exp Med. 2008;205:315–322. doi: 10.1084/jem.20070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, Pulendran B. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nature Immunology. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmitz F, Heit A, Dreher S, Eisenächer K, Mages J, Haas T, Krug A, Janssen KP, Kirschning CJ, Wagner H. Mammalian target of rapamycin (mTOR) orchestrates the defense program of innate immune cells. European journal of immunology. 2008;38:2981–2992. doi: 10.1002/eji.200838761. [DOI] [PubMed] [Google Scholar]
- 35.Colina R, Costa-Mattioli M, Dowling RJ, Jaramillo M, Tai LH, Breitbach CJ, Martineau Y, Larsson O, Rong L, Svitkin YV, Makrigiannis AP, Bell JC, Sonenberg N. Translational control of the innate immune response through IRF-7. Nature. 2008;452:323–328. doi: 10.1038/nature06730. [DOI] [PubMed] [Google Scholar]
- 36.Bartlett EJ, Lenzo JC, Sivamoorthy S, Mansfield JP, Cull VS, James CM. Type I IFN-β gene therapy suppresses cardiac CD8+ T-cell infiltration during autoimmune myocarditis. Immunol Cell Biol. 2004;82:119–126. doi: 10.1046/j.0818-9641.2004.01234.x. [DOI] [PubMed] [Google Scholar]
- 37.Henke A, Huber S, Stelzner A, Whitton JL. The role of CD8+ T lymphocytes in coxsackievirus B3-induced myocarditis. Journal of Virology. 1995;69:6720–6728. doi: 10.1128/jvi.69.11.6720-6728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Opavsky MA, Penninger J, Aitken K, Wen W, Dawood F, Mak T, Liu P. Susceptibility to myocarditis is dependent on the response of αβ T lymphocytes to coxsackieviral infection. Circ Res. 1999;85:551–558. doi: 10.1161/01.res.85.6.551. [DOI] [PubMed] [Google Scholar]