Abstract
A simple and universal pathway to produce free multilayer synthetic nanoparticles is developed based on lithography, vapor phase deposition and a tri-layer resist lift off and release process. The fabrication method presented in this work is ideal for production of a broad range of nanoparticles, either free in solution or still attached to an intact release layer, with unique magnetic, optical, radioactive, electronic and catalytic properties. Multi-modal capabilities are implicit in the layered architecture. As an example, directly fabricated magnetic nanoparticles are evaluated to illustrate the structural integrity of thin internal multilayers and the nanoparticle stability in aggressive biological environments, which is highly desired for biomedical applications.
1. Introduction
Synthetic nanoparticles with unique magnetic, optical, radioactive or bio-functional properties have been widely exploited in biomedical applications [1–5]. These nanoparticles are usually fabricated by “bottom up” chemical routes that enable large scale production, but which often require careful control to obtain tolerable polydispersity and pose difficulties for precisely and independently controlling the particle shape, size, structure, and composition. To overcome these limitations, direct fabrication of synthetic nanoparticles by “top-down” physical routes is emerging as an alternative approach that has recently shown great potential [6–10]. For example, DeSimone and colleagues developed PRINT (Particle Replication in Non-Wetting Templates) technology for producing nanoparticles composed of polymeric biomaterials for engineered drug delivery [7,8]. To expand direct fabrication to inorganic nanoparticles, we recently developed a novel approach that uses template-assisted vacuum deposition, lift-off and release to produce water stable multifunctional magnetic nanoparticles [9, 10]. These nanoparticles, named synthetic antiferromagnetic nanoparticles (SAF), represent a new paradigm wherein two ferromagnetic layers sandwich a thin layer of non-magnetic material to produce high moment, monodisperse, disk-shape nanoparticles with zero remanence over a wide range of sizes. However, there are still several limitations associated with the original fabrication and release procedures which can be significantly improved. First, the Cu sacrificial layer in the original fabrication process allowed release of SAF from the substrate into aqueous solution, but required selective etching of Cu with a caustic (pH 12) and chemically reactive ammonia-CuSO4 solution, followed by careful neutralization, centrifugation, and disposal of the etching solution in which the released nanoparticles were initially suspended. Also, long vacuum cycles were required for deposition of the sacrificial Cu release layer and for ion milling of a Ta layer required to protect the Cu release layer. This Ta protection layer was necessary to prevent surface roughening (stress crazing) of the nanoimprint resist during a post-spincoat bake process. Fluctuations in Ta ion milling rates also occasionally lead to release failure (under mill) or serious damage of the nanoparticles (over mill). This ion milling step is also restrictive since some materials, such as Au or Ag, have much higher ion milling rates than Ta, and are thus difficult to preserve if they are deposited on the top surface. A simpler alternative to, or elimination of, these steps is obviously advantageous.
Here we report a simple and universal approach, which requires only that the materials are compatible with common resist developers and strippers, to fabricate synthetic multi-layer vapor deposited nanoparticles by using a novel tri-layer resist lift-off and release process. A spin-coated polymer resist, Durimide, is used to replace Cu as the sacrificial layer, and can be simply dissolved in an N-Methylpyrrolidone (NMP) based solvent. To demonstrate this process, thermally evaporated high moment Fe/Ti multilayer SAF nanoparticles were fabricated and characterized. Metallic Fe was chosen for its high saturation magnetization and reduced toxicity, as compared to other magnetic metals such as Co and Ni. Biocompatible Ti, whose surface can be functionalized for specific applications, is used for seed and cap layers.
2. Experiment
Figure 1 illustrates the fabrication procedure. To fabricate SAF nanoparticles, a 50 nm sacrificial film of fully imidized polyimide polymer, Durimide® 20 (Fujifilm Inc.), was spin-coated on a silicon substrate and baked on a 300°C hotplate for 10 minutes. A bilayer of resists, comprised of a polymethylglutarimide (PMGI, MicroChem) undercut layer and a polymethyl methacrylate (PMMA, MicroResist Technlogy, 75k MW) thermal imprinting layer were then sequentially spin-coated and baked at 200°C and 140°C, respectively, for 5 minutes. The thicknesses of the bilayer resists were selected to accommodate stamp dimensions and particle thickness. Thermal nanoimprint lithography was performed on the PMMA layer at 40 bar for 60 sec. at 180°C, using a 2×2 inch2 silicon stamp consisting of arrayed nanopillars, each 50 nm in diameter and 150 nm in height, that was fabricated by nanosphere lithography [11]. After a thin residue layer of PMMA was removed by O2 plasma (20sccm/50mTorr/50W/10sec), a standard tetramethylammonium hydroxide (TMAH) based developer, LDD-26W (Shipley), was used to create an undercut lift off profile in the PMGI underlayer and to form smooth, flat template bottoms at the PMGI/Durimide interface. Fe/Ti multilayer films with the structure of Ti (5 nm)/Fe (10 nm)/Ti (3 nm)/Fe (10 nm)/Ti (5 nm) were then thermally evaporated followed by lift-off, in acetone at 60 °C with sonication, of metal residues and PMMA. The PMGI layer abutting the nanoparticles was then removed in LDD-26W to produce samples comprised of ordered nanoparticle arrays still atop the release layer, which is very useful for characterization of nanoparticles with precise spacing and orientation. The final step was to release the particles from the substrate by dissolving the Durimide sacrificial layer in an NMP based solution, S-1165 (MicroChem Microposit). The particles were then collected by three cycles of centrifugation at 10,000×g (g = 9.8 m/sec2), solvent exchange and re-suspension in water.
Figure 1.
Procedures for synthetic nanoparticle fabrication: _(a)_spin-coat of trilayer polymer resists, (b) thermal NIL patterning of hole arrays on PMMA layer followed by residual layer removal, (c) undercut profile creation in the underlayer PMGI resist, (d) metal film deposition, (e) lift-off and (f) release nanoparticles into solution by dissolving the release layer.
Atomic force microscopy (Veeco, DI 3100) was used to monitor the surface topography of the polymeric sacrificial layer. Scanning (FEI, XL30) and transmission electron microscope (Philips, CM20) images were obtained to illustrate the morphology and structure of the synthesized nanoparticles. We also performed energy dispersive X-Ray Spectroscopy (EDS) analysis to verify the composition of these nanoparticles. The magnetic properties were measured at room temperature with an alternating gradient magnetometer from Princeton Measurements. The colloidal stability of the released particles was verified in acetone and in de-ionized water using Dynamic Light Scattering [12], and can be varied by changing solvent ionic strength or for surface functionalization desired for covalent attachment of biomolecules.
3. Results and Discussion
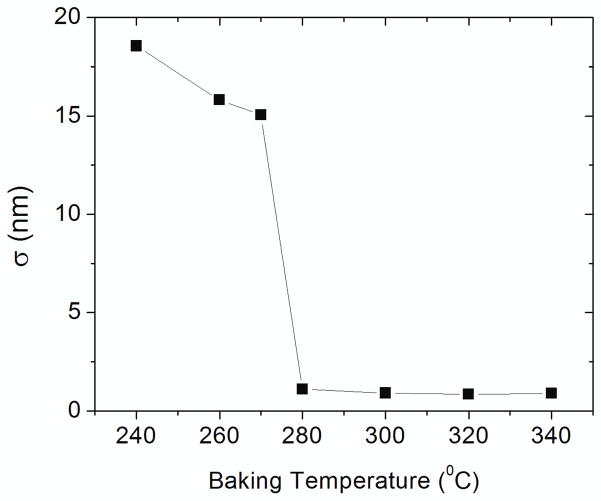
One critical requirement for the success of the new process is that the sacrificial polymer layer, Durimide, should not be affected by the PMGI or PMMA solvents, (cyclopentanone or anisole, respectively) during spin-coating, or by the developers used to remove PMMA and PMGI layers. We found this condition could be fulfilled by controlling the baking temperature of the spin-coated Durimide. Insufficient baking does allow the interaction of Durimide with cyclopentanone, resulting in visibly roughened PMGI/Durimide layers and in Durimide with very rough surface after the PMGI is dissolved. This is unacceptable since smooth, nominally flat template bases are required, preferably at the nm level or better, for vapor deposition of thin uniform multilayers. To determine the appropriate baking temperature, we performed AFM measurements of the surface roughness of exposed Durimide surfaces as a function of the baking temperature. Silicon substrates coated with 50 nm of Durimide were baked at different temperatures for 10 minutes on a hotplate, and then spin-coated with a layer of PMGI. The PMGI was dissolved in LDD-26W before AFM measurements were conducted. Figure 2 plots the root mean square surface roughness (σ) versus the baking temperature T. The high σ value (>15 nm) for T< 270 °C reveals the presence of strong interaction between Durimide and cyclopentanone. This effect is effectively suppressed for baking temperature at 280 °C or above, as indicated by the sharp decline of the surface roughness. The σ of the “developed” Durimide surface remains near 1 nm at temperatures above 300 °C. Further increase of the baking temperature above 340 °C causes difficulty for dissolving the sacrificial layer in the S-1165 release solution. Therefore, the Durimide sacrificial layer was preferably baked at 300 °C for 10 minutes in the present nanoparticle fabrication process.
Figure 2.
Root mean square surface roughness (σ) of Durimide, after the PMGI layer is stripped, as a function of the baking temperature. The sharp decline of σ indicates that the interaction between Durimide and cyclopentanone is effectively suppressed after baking at 280°C and above.
Similar resist/solvent incompatibilities could arise for other resist layers, but were not observed for our processing of PMMA (in anisole), PMGI (in cyclopentanone), and Durimide (in γ-butyrolactone). Similarly, the use of acetone and LDD-26W as removers for PMMA and PMGI, respectively, did not induce premature release of nanoparticles, so that lift off was readily achieved before the release of SAF nanoparticles, as release required thorough dissolution of Durimide by NMP.
Figure 3(a) shows the nanopillar arrays of the self-assembled stamp used for nanoparticle fabrication and Fig. 3(b) shows the corresponding imprinted holes on the PMMA resist layer. The nanopillars are in hexagonal arrays with uniform shape, size and height, but not in a perfect order. Fortunately, in our application, there is no demand for precise layout or order of the patterns. This permits the use of self-assembly techniques to produce inexpensive large area stamps for SAF nanoparticle fabrication. Figure 3(c) and (d) illustrate disk-shape SAF nanoparticles bound to the substrate (before release) and after drying from a dark gray solution (after release) containing ~1010 nanoparticles/mL (insert in Fig. 3d). Statistical analysis shows that these SAF nanoparticles have very uniform size of 65±7 nm in diameter (Fig. 4).
Figure 3.

SEM images of (a) self-assembled stamp with nanopillar arrays, (b) imprinted holes on the resist layer, (c) nanoparticle arrays bounded on the substrate (before release) and (d) dried nanoparticles after release into aqueous solution. The dark gray solution in the insert contains ~1010 nanoparticles/mL.
Figure 4.

Histogram of SAF nanoparticle size distribution. Solid line represents the corresponding normal fit.
To verify the structure and composition of the synthesized SAF nanoparticles, we performed TEM characterization and EDS analysis. Figure 5(a) shows a bright field TEM image of several randomly oriented SAF nanodisks on a carbon-coated Cu grid. The multilayer structure, which is Ti (5 nm)/Fe (10 nm)/Ti (3 nm)/Fe (10 nm)/Ti (5 nm), can sometimes be observed in TEM images of particles that bind in a perpendicular orientation, Fig. 5(b). The region of light contrast between two dark contrast areas, indicated by the arrows, is attributed to the 3 nm thick Ti layer (Z=22) sandwiched between the two 10 nm Fe layers (Z=26) although the contrast difference in the TEM image does not afford the great clarity of our previous work [9], presumably because the atomic number difference between the two materials is small and the particle normals may not be exactly orthogonal to the electron beam. It is worth noting that the sidewalls of these nanodisks are also covered by a thin layer of oxidized Ti, as observed by the region of lighter contrast surrounding the nanodisks from the TEM image. This may be due to the imperfection of the deposition process (such as stray or redeposited beam flux). This thin layer of Ti is important to the chemical and magnetic stability of SAF nanoparticles in an aqueous solution as we will discuss later. EDS measurement on a single particle, Fig. 5(c), confirms the existence of Fe and Ti. Additional peaks are from the Cu TEM grid.
Figure 5.
(a) Bright field TEM image of released SAF nanoparticles, (b) zoom-in image of the three particles with an edge on orientation, where red arrows point to the 3 nm Ti spacer layer (lighter region) sandwiched by two layers of 10 nm Fe (dark region). (c) The corresponding EDS spectrum of a single SAF nanoparticle.
As shown in Fig. 6, we obtained magnetic hysteresis loops for SAF nanoparticles bound to the substrate (before release, circular dots) and in aqueous solution (after release, triangular dots). The hysteresis loops for solution samples were obtained by attaching 10 μL volumes of dark gray aqueous suspensions, enclosed in paraffin containers, to an AGM probe. Both samples exhibit low remnant magnetization while nanoparticles in solution show an apparent reduction in the saturation field. At the concentrations used for AGM measurements (>1010/mL), these SAF nanoparticles are observed to form chain structures, induced by inter-particle interactions of magnetized nanoparticles, which are expected to have reduced saturation fields [13]. The saturation magnetization of these Fe/Ti multilayer SAF nanoparticles is much larger than for conventional superparamagnetic iron oxide nanoparticles, as summarized in Table 1.
Figure 6.
Magnetic hysteresis loops of SAF nanoparticles still bound on the substrate (circles) and in aqueous solution (triangles). The apparent reduction in saturation field in aqueous solution is due to magnetic chain formation. The insert illustrates the magnetization configuration and zero remanence at zero applied field, which attests to the integrity of the non-magnetic spacer layer.
Table 1.
Comparison of saturation magnetization between SAF nanoparticles and iron oxide nanoparticles.
| Particle | Saturation magnetization
|
||
|---|---|---|---|
| emu/g Fe | emu/g sample | emu/cm^3 | |
| Fe3O4 | 125 | 90 | 450 |
| SAF | 215 | 157 | 1050 |
emu=electromagnetic unit
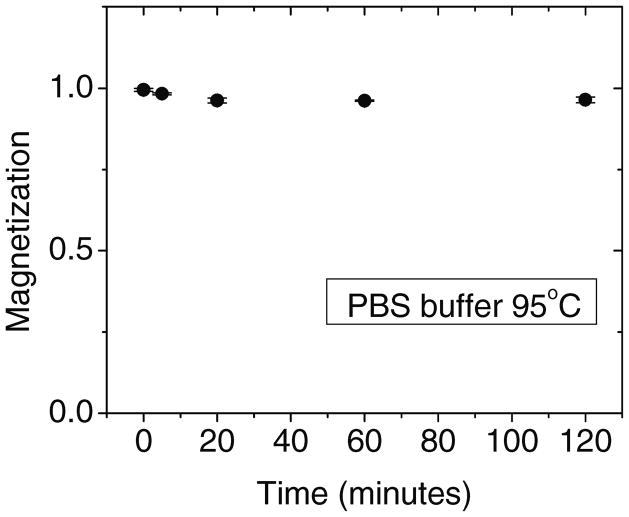
The high saturation magnetic moment derives from the use of metallic Fe, which could degrade in aqueous solutions. For biological applications of magnetic nanoparticles, a critical issue is to maintain high magnetic moments in buffered saline solutions over a range of temperatures, which could reduce the moment because of corrosion or oxidation. One prominent advantage of these SAF nanoparticles is that they show very good chemical stability in corrosive saline biological environments, as shown in Figure 7. Here, the particles were exposed to a standard phosphate buffered saline (PBS) at an elevated temperature of 95°C. Only a slight decrease in magnetic moment occurred over 2 hours and even smaller changes were observed at lower temperatures, both in saline environments and in pure water. This chemical robustness is attributed to the deposition of the Ti seed and cap, and the consequent thin side wall coverage in lift-off films, which oxidizes in air and passivates the reactive Fe component that can otherwise gradually degrade in aqueous solution.
Figure 7.
Stability test of SAF nanoparticles in phosphate buffered saline (PBS) at 95°C. Only small changes of the magnetic moment are observed.
Although originally developed for producing SAF nanoparticles, the method proposed in this article can be broadly applied to the design of any nanoparticle which is compatible with resist removers (acetone, TMAH and NMP), and in which unique properties may be conferred by precision layering of materials. In addition, by controlling the undercut profile of the PMGI layer or using the oblique angle metal deposition, we can incorporate a structural defect artificially on the top or the bottom of the nanoparticle as described in our previous report [14].
4. Conclusion
A simple and universal pathway was developed to fabricate synthetic nanoparticles with a novel tri-layer resist lift-off and release process. A spin-coated polymer resist, Durimide, is used as the sacrificial layer and enables release of SAF nanoparticles into aqueous solution. The novel approach described here has significant implications to nanofabrication of free multi-modal nanoparticles with unique magnetic, optical, radioactive, electronic and catalytic properties. With the advance of nanotechnology, especially low cost and high throughput lithography techniques, mass production of synthetic nanoparticles for biomedical applications is on the way.
Acknowledgments
This work was supported by grants from NIH (Grant No. 1U54CA119367-01), ARPA/Navy (Grant No. N00014-02-1-0807), and Stanford Graduate Fellowship (W.H.). We also acknowledge D. McKean and the use of Stanford Nanofabrication Facility and Stanford Nanocharacterization Laboratory, both partially supported by NSF, and the Stanford Nanomagnetics facility.
References
- 1.Lee JH, et al. Nat Med. 2007;13:95. doi: 10.1038/nm1467. [DOI] [PubMed] [Google Scholar]
- 2.Yavuz CT, et al. Science. 2006;314:964. doi: 10.1126/science.1131475. [DOI] [PubMed] [Google Scholar]
- 3.Li GX, Wang SX, Sun SH. IEEE Trans Magn. 2004;40:3000. [Google Scholar]
- 4.Wang C, Xu CJ, Zeng H, Sun SH, Salabas EL, Schuth F. Adv Mater. 2009;21:3045. doi: 10.1002/adma.200900320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanga BC, et al. Proc Nat Acad Sci USA. 2009;106:10268. [Google Scholar]
- 6.Elnathan R, Kantaev R, Patolsky F. Nano Lett. 2009;8:3964. doi: 10.1021/nl802467d. [DOI] [PubMed] [Google Scholar]
- 7.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. J Am Chem Soc. 2005;127:10096. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 8.Kelly JY, Desimone JM. J Am Chem Soc. 2008;130:5438. doi: 10.1021/ja8014428. [DOI] [PubMed] [Google Scholar]
- 9.Hu W, et al. Adv Mater 2008. 2008;20:1479. [Google Scholar]
- 10.Fu A, et al. Angewandte Chemie International Edition. 2009;48:1620. [Google Scholar]
- 11.Hu W, et al. J Appl Phys. 2009;105:07B508. doi: 10.1063/1.3072028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berne BJ, Pecora R. Biology, and Physics. Dover: 2000. Dynamic Light Scattering with Applications to Chemistry. [Google Scholar]
- 13.Wilson RJ, et al. J Magn Magn Mater. 2009;321:1452. doi: 10.1016/j.jmmm.2009.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu W, et al. J Vac Sci Tech A. 2007;25:1294. [Google Scholar]