Abstract
Intestinal and liver fatty acid binding proteins (IFABP and LFABP, respectively), are cytosolic soluble proteins with the capacity to bind and transport hydrophobic ligands between different sub-cellular compartments. Their functions are still not clear but they are supposed to be involved in lipid trafficking and metabolism, cell growth, and regulation of several other processes, like cell differentiation. Here we investigated the interaction of these proteins with different models of phospholipid membrane vesicles in order to achieve further insight into their specificity within the enterocyte. A combination of biophysical and biochemical techniques allowed us to determine affinities of these proteins to membranes, the way phospholipid composition and vesicle size and curvature modulate such interaction, as well as the effect of protein binding on the integrity of the membrane structure. We demonstrate here that, beside their apparently opposite ligand transfer mechanisms, both LFABP and IFABP are able to interact with phospholipid membranes, but the factors that modulate such interactions are different for each protein, further implying different roles for IFABP and LFABP in the intracellular context. These results contribute to the proposed central role of intestinal FABPs in the lipid traffic within enterocytes as well as in the regulation of more complex cellular processes.
Keywords: Fatty Acid Binding Proteins, Membrane Interaction, Intracellular Fatty Acid Traffic, Model Membranes
1. Introduction
The success of many important physiological processes relies on highly specific protein-protein and protein-membrane interactions. These interactions can lead to a vectorial delivery and uptake of the carrier proteins’ cargo, determining a one way trafficking pathway. The amphiphilic nature and low solubility of long-chain fatty acids (FAs), combined with their large metabolic flux in tissues such as the intestine, liver, heart, muscle, and adipose tissue, suggests a requirement for directed transport processes both between cells and intracellularly [1, 2]. Though the exact functions of fatty acid binding proteins (FABPs) have yet to be elucidated, they are in a favorable position to accomplish this transport function and facilitate lipid metabolism in different tissues and cell types [3-7]. The FABPs are also thought to participate in intracellular lipid homeostasis, providing a pool of non-esterified FAs which may then be specifically transported and targeted to intracellular sites of metabolism. Furthermore, FABPs could also modulate lipid-metabolism enzymatic activities or lipid-mediated signal transduction and, hence, have an impact on cell growth and differentiation. In the case of the intestinal epithelia, the FABPs may also protect against potential detergent-like effects of the high FA concentrations assimilated from the diet [6].
The small intestine is the initial site of dietary FA uptake and intestinal enterocytes coexpress two FABPs, namely liver FABP (LFABP or FABP1) and intestinal FABP (IFABP or FABP2). In humans, LFABP levels are greater than IFABP levels; in rodents, however, they are comparably expressed [8]. Though displaying almost superimposable backbone and secondary structure elements [7, 9], recent structural analyses have demonstrated some differences in the tertiary structure [10, 11] that, along with the different ligand binding and transfer properties [2, 4, 7], may help elucidate their specific functions. One of the most notorious differences between the intestinal FABPs is that LFABP contains two binding sites for FAs with differing affinities and has significant affinity for other acyl metabolites, such as acyl-coenzyme A, lysophospholipids, and monoacylglycerol. On the other hand, IFABP only binds a single long chain FA under physiological conditions, with somewhat lower affinity than LFABP [3].
The proposed role for FABPs as intracellular FAs transporters theoretically requires their direct interaction with ligand donor and acceptor membranes and/or proteins [12, 13]. Thus, FABPs may serve not only to deliver FAs to target organelles, but also to remove membrane bound FAs. In a first attempt to evaluate the potential for functional differences, the FA transfer mechanism from intestinal FABPs to small unilamellar vesicles (SUVs) was studied. These kinetic studies suggested that IFABP delivers fluorescent anthroyloxy-labeled FA (AOFA) to acceptor membranes via transient protein–membrane interactions; whereas delivery from LFABP is modulated solely by the rate of ligand dissociation from the protein [14], in accordance with an aqueous-diffusion mediated mechanism. The reverse reaction, whereby FAs in membranes are transferred to IFABP and LFABP, has also been examined at the mechanistic level with similar results for both proteins [15].
Many studies have provided substantial evidence demonstrating directly IFABP interaction with membranes, and have shown that electrostatic and hydrophobic forces modulate these physical interactions [14, 16-21]. In contrast, LFABP has been classically considered a “diffusional FABP,” based on FA transfer kinetic studies. Nevertheless, it has been demonstrated that LFABP can bind to small anionic phospholipid vesicles, albeit only under conditions of low ionic strength [22-24].
To directly compare the protein-membrane interaction characteristics of IFABP and LFABP, and determine whether their differences in FA binding properties and transfer mechanism are related to dissimilar protein-membrane interaction properties, as suggested by kinetics experiments, a series of biophysical approaches were employed, providing complementary information about protein-membrane interactions. The experiments employed could be grouped according to the kind of effect they are reporting. Tb leakage and hydrophobic photolabeling are a result of instant and irreversible (“short-term”) events and, after an incubation period, we see a sum of the individual, and possibly transient, contacts between the protein and the vesicles. On the other hand, the cytochrome c competition assay and sedimentation methods could be considered as techniques reporting “long-term” or averaged events since the results we see come from the equilibrium reached by the different components during the assay. The results obtained demonstrate that while both LFABP and IFABP are able to interact with phospholipid membranes, the factors that modulate this process are different for each protein, implying different roles for IFABP and LFABP in an intracellular context.
2. Matherial and methods
2.1 Materials
Dansyl-Phosphatidyl Ethanolamine (DPE), Egg Phosphatidyl Choline (EPC), Egg Phosphatidyl Ethanolamine (EPE), Phosphatidyl Serine (PS) and Cardiolipin (CL) were purchased from Avanti Polar Lipids. 3H-dipalmytoil Phosphatidyl Choline (3H-DPPC) and [125I] NaI were from Dupont NEN Products. Terbium (III) chloride, dipicolinic acid (DPA), cytochrome c, and BNPS-Skatole were purchased from Sigma. All other chemicals were reagent grade or better.
2.2 Protein expression and purification
The wild-type proteins and the chimeric variant were purified as detailed elsewhere [18]. The IFABP mutant lacking the α-helical domain (IFABP-HL) was overexpressed and purified as detailed in Córsico et al., 1998 [17].
2.3 Preparation of Photoactivable Reagents
The 125I-TID-PC was prepared by radioiodination of its nonradioactive tin-containing precursor according to Weber and Brunner [25] and our previous work [20, 21, 26]. The precursor was generously donated by Prof. J. Brunner from the Swiss Federal Institute of Technology (Zurich, Switzerland).
2.4 Preparation of Model Membranes
Large unilamellar vesicles (LUVs, ~100-140 nm) of EPC, EPC/PS (3:1, mol/mol), or EPC/CL (3:1, mol/mol) were prepared by extrusion through polycarbonate membranes of 100 nm pore diameter (Avestin Inc., Ottawa, Canada) as described previously [20, 21]. For hydrophobic photolabeling, 125I-TID-PC (200 μCi/mmol of phospholipids) was included in the lipid chloroform mixture. In the case of sucrose loaded LUVs; vesicles were prepared as described previously [27] and immediately used. Small Unilamellar Vesicles (SUVs, 20-40 nm) were prepared by sonication and ultracentrifugation as previously described [28, 29]. Vesicles for Tb Leakage assay were prepared according to the method of Wilschut et al. [30]. Vesicles for cytochrome c competition assay had a specific composition of EPC:EPE:CL/PS:DPE, 64:10:25:1 and were prepared in 20 mM Tris-HCl, 0.1 mM EDTA, pH 7.4. The rest of the vesicles were prepared in 30 mM Tris-HCl and 150 mM NaCl pH 7.4. When CL was present in the lipid mixture, buffer also contained 1 mM EDTA to minimize vesicles fusion. The final phospholipid concentration was determined by inorganic phosphorus assay [31].
2.5 Cytochrome c competition assays
The binding of cytochrome c to acidic membranes can be monitored using a FRET assay. The heme moiety of cytochrome c is a quencher of the dansyl fluorescence of DPE-labeled SUVs [32]. Competition of FABPs with cytochrome c for binding to SUVs was determined by the relief of cytochrome c-related quenching of the dansyl fluorescence [17, 33]. An inhibition of cytochrome c-dependent quenching is interpreted as evidence for FABPs interaction with the SUVs. Kd values for cytochrome c and FABPs were estimated from a single and independent binding site model for each protein (see Supplementary Material for details).
2.6 Sucrose loaded vesicle binding assay
Binding of FABPs to sucrose loaded LUVs was explored based on Smith & Storch (1999) [33] with some modifications. Briefly, reactions were prepared in 200 μl of binding buffer (5 mM MOPS, pH 7.4, 100 mM KCl, 2 mM CaCl2) with the desired concentration of sucrose-loaded vesicles and 5 μM protein. Tubes were centrifuged at 100,000 × g 21°C for 90 min, and the supernatant was immediately transferred to new tubes. The presence of LUVs in pellet and supernatant was monitored by including 1% 3H-DPPC. Portions of both supernatant and pellet were analyzed by SDS-PAGE and densitometry. Protein remaining in the supernatant was plotted versus the total phospholipids present in the binding reaction and KD values were obtained from a least-squares fit to experimental data to Eq 1 [34, 35]. The experiment was repeated at least 4 times and the calculated KD values were averaged and expressed as mean ± SEM.
| Eq.1 |
2.7 Terbium Leakage Assay
Stocks suspensions of FABP and SUVs loaded with the Tb-DPA complex in their internal aqueous space, were prepared in 20 mM Tris (pH 8.0), 155 mM NaCl 12.5 mM EDTA to eliminate osmotic effects. These working solutions were mixed and the fluorescence signal of Tb/DPA complex was monitored, with excitation at 250 nm and emission at 545 nm. Final conditions were 10 μM protein and 0.5 mM SUVs. Induced leakage by FABPs interaction with the membranes was monitor as the decrease in Tb fluorescence since EDTA replaces DPA in the complex. Data were expressed as percent of the total disruption of the vesicles with 0.05% Triton X-100 and calculated as follows:
| Eq. 2 |
where F and FB are, respectively, the fluorescence intensity registers for the sample and for a blank (vesicles mixed with the corresponding buffer (30)) and FT is the fluorescence intensity obtained for the addition of the detergent solution to the lipid vesicles.
2.8 Photo-Crosslinking Analysis of Membrane Interacting Proteins
Experiments were conducted as previously described [26]. In those experiments where oleate was included, we employed a 10:1 protein/ligand (mol/mol) ratio, and the protein-ligand complex was formed at least 5 min prior to the incubation with LUVs. After photoactivation, FABPs were precipitated from the aqueous phase and analyzed by SDS-PAGE and autoradiography. For the selective proteolysis on blotting membrane; IFABP photolabeled in the presence of CL-containing LUVs was separated in an SDS-PAGE and transferred by electroblotting to a PVDF membrane. The PVDF-bound- protein was subjected to selective proteolysis with BNPS-Skatole [36]. The proteolysis products were extracted with stripping buffer (Tris 50 mM, SDS 2.0 %, Triton X-100 1.0 %, pH 9.1) and analyzed in a Tricine-PAGE gel [36, 37] followed by autoradiography. Images from the proteolysis product were quantified using the program Image J (National Institutes of Health).
3. Results
Previous studies have suggested that IFABP may directly interact with membranes and thereby modulate the rate of FA transfer to phospholipid membranes. On the other hand, kinetic studies indicated that FA transfer from LFABP seems to be independent of the protein-membrane complex formation, and it is hypothesized that the rate limiting step is the liberation of the FA from the protein’s binding site into the aqueous media [14, 16-21]. To examine whether the difference in FA transfer mechanism showed by the two intestinal FABPs could be connected to the existence of different mechanisms of interaction of I- and LFABP with membranes, we undertook a series of experiments to analyze the factors that modulate the protein-membrane interaction process.
3.1 Competition for binding sites on anionic vesicles between cytochrome-c and FABPs
Cytochrome c is a well-known peripheral membrane protein that interacts with acidic membranes [38], we determined whether apo-IFABP and apo-LFABP could compete with cytochrome c for the binding sites on the membranes containing PS or CL, and the fluorophore dansyl-PE. Figure 1 show that preincubation of PS- or CL-containing vesicles (panels A and B, respectively) with either IFABP or LFABP were effective in preventing subsequent cytochrome c binding in a concentration-dependent manner. When IFABP (12 μM) was added to PS- and CL-containing SUVs, the dansyl fluorescence was approximately recovered in 1.9 and 2.5 times, respectively, in comparison to the presence of cytochrome c (1 μM) alone. Meanwhile, the effect of LFABP in the same conditions determined an increase of fluorescence intensity 2 and 3 times, respectively. Both proteins seem to interact more with CL- compared to PS-containing SUVs at the larger concentration tested (12 μM), 1.37 times for IFABP and 1.47 times LFABP. Furthermore, relief of dansyl quenching from CL-SUVs above 6 μM FABP are somewhat larger for LFABP than for IFABP (* indicates p< 0.05).
Figure 1. Intestinal FABPs Competition with Cytochrome c for Binding to Anionic Vesicles of Different Composition.
Binding of Cytochrome c (1 μM) to PS- (Panel A) and CL-containing SUVs (Panel B) preincubated with increasing concentrations (1-12 μM) of apo-LFABP (grey circles) and apo-IFABP (black circles). Results show that both intestinal apo-FABPs interact with similar affinities and compete with cytochrome c for anionic membranes. Results are the averages ± SD of at least 3 measurements. The line represents the fitted model (see supplementary material for details). Point to point differences were estimated by Student t-Test (*p<0.05).
Calculated KD values for cytochrome c binding to CL and PS containing SUV were 0.034±0.005 μM and 0.085±0.002 μM, respectively (see Supplementary Material). We estimated the number of phospholipid molecules involved in cytochrome c binding when CL is present to be approximately 168 (84 for the single outer layer of the SUVs) (see Supplementary Material). This represents an area much larger than that required for cytochrome c physical interaction, and is likely attributed to the FRET phenomenon which can report interaction distances that are larger than the actual physical contact of the components [39]. The results for IFABP showed a KD of 0.54±0.25 μM and 0.32±0.17 μM incubated with both CL- or PS-containing vesicles, respectively. On the other hand, LFABP showed KD values of 0.32±0.06 μM and 0.31±0.08 μM incubated with both CL- or PS-containing vesicles, respectively. Thus, no significant differences between either vesicle nor FABP type were found.
3.2 Sucrose loaded vesicle binding assay
We employed a differential centrifugation method to further examine interfacial membrane binding of FABPs to sucrose loaded LUVs of different compositions. Protein-membrane interaction is monitored by analyzing the modification of unbound protein as a function of increasing LUVs concentration. As exampled in Figure 2A, the concentration of both apo-FABPs in the supernatant decreased with increasing concentration of CL-containing LUVs. These results indicate a stable protein adsorption to the membrane interface during the ultracentrifugation. The protein fractions were quantified by densitometry and the dissociation constants for apo-IFABP and apo-LFABP were calculated as described in Material and Methods. The results for a representative experiment are shown in Figures 2B and 2C, with the fitted model. We found KD values of 23.5 ± 0.6 μM for IFABP and 50 ± 20 μM for LFABP, which are not statistically different (t-test, p>0.05). No stable complexes were observed when FABPs are incubated with zwitterionic LUVs (Figs. 2B and 2C). Furthermore, IFABP-HL, used as a control (a non-membrane interactive protein [17], showed no interaction with CL containing LUVs (Fig. 2B), in agreement with previous results [16].
Figure 2. Binding of intestinal FABPs to sucrose loaded LUVs.
The stable binding of apo-FABPs (5 μM) to LUVs (0-2 mM) of 100% EPC or 25% CL was analyzed by ultracentrifuge sedimentation. Panel A shows, as an example, the decrease of free IFABP remaining in the supernatant as the total phospholipid concentration increases. The same was observed for LFABP. The gels were quantified by densitometry and fitted individually. A representative experiment of four repetitions is shown in panel B: wild type IFABP (triangles) and IFABP-HL (square). Panel C presents the results for the parallel analysis of LFABP (circles).
3.3 Membrane structure destabilization
The effect of FABPs on membrane structure was assessed by analyzing the ability of FABPs to induce leakage of the Tb/DPA fluorescent complex from the internal aqueous space of SUVs. This methodology has proved to be efficient in protein-membrane interaction analysis [40]. Figure 3 shows the results for native intestinal FABPs and two structural variants in their apo-forms. A small, but significant, decrease in leakage was observed for LFABP compared to IFABP for 100% EPC vesicles (p<0.05). The IFABP-HL was again employed as a control which did not seem to induce destabilization of the membranes, but even seemed to slightly prevent it. On the other hand, the chimeric protein αLβIFABP (obtained by exchanging the Nt containing the α-helical region of IFABP for that of LFABP) seems to be as effective as wild-type IFABP inducing the complex leakage from zwitterionic vesicles. The comparison of the effects induced by IFABP and IFABP-HL indicate that the α-helical region of IFABP, but not its β-barrel is important for destabilizing the membrane structure. Furthermore, comparing the chimeric protein with wild-type LFABP, and considering that the chimeric protein contains β strand A as well as the whole α-helical region of LFABP, we conclude that key structural elements of this region could be important for the interaction of LFABP with zitterionic membranes.
Figure 3. Membrane destabilization by intestinal FABPs.
Induced Tb/DPA complex leakage from SUVs (0.5 mM) of different composition were analyzed upon mixing with FABPs (10 μM) (apo-forms). The final leakage, expressed as % of Reference (0.05% Triton X-100), for LFABP, IFABP, IFABP-HL and αLβIFABP are shown. Statistics was based on Student t-Test (p<0.05), * indicate significant difference between EPC 100% and CL25% for the same protein; while different letters indicate differences between proteins for the same SUV type.
When 25% of CL is present in the membrane composition, a higher energy barrier would prevent the Tb/DPA complex (Tb/DPA3 -3) to leak out. Therefore, all values of leakage with these SUVs were lower compared to 100% EPC vesicles. IFABP-HL tendency to protect SUVs from leaking the fluorescent complex seems to be still present, partially preventing the membrane from destabilization. It is also worth noting that IFABP and αIβLFABP showed a significant difference between 100% EPC and 25% CL, but due to the different energy barrier for the complex to leak out of the vesicles in each condition, this cannot be directly interpreted as lower destabilizing effect of these proteins on CL-containing SUVs than on its zwitterionic counterparts.
Altogether, these results on leakage induction from vesicles indicate that both IFABP and LFABP can destabilize phospholipid membranes. A relatively deep penetration of some FABP segments could be suggested by the leakage of the internal aqueous content. An interesting observation is that 100% leakage is not reached, indicating that no stable pore is formed in the vesicles. Since there is no reason to think that only a portion of the vesicles is able to leak, a more likely possibility is that the FABP–vesicle interaction would produce the leakage of only part of the trapped reagent. Thus, the FABP–vesicle interaction could be producing transient packing defects in the bilayer, which would be rapidly resealed before the equilibrium between the external and internal media can be reached, within a few minutes.
3.4 Photo-crosslinking Analysis of Membrane Interacting Proteins
We analyzed the membrane interaction of the wild-type proteins under different conditions using the photoactivable probe 125I-TID-PC. This probe allowed us to monitor the physical interaction of the proteins with the hydrophobic core of the phospholipid membranes. Figure 4 shows the results of representative experiments aimed to evaluate qualitatively the effect of changes in the lipid composition of the LUVs and the presence of the ligand (oleic acid) on the interaction. Both IFABP and LFABP showed marked interaction with membranes, but interestingly under different conditions (Fig. 4A and 4B). IFABP’s interaction with membranes seems to be more important when the protein is loaded with the FA than the apo-form (Fig. 4A). Furthermore, an increase in radiolabeling of apo- and holo-IFABP when anionic phospholipids are incorporated in the vesicles composition suggests a tighter interaction. Apo-IFABP shows a lower degree of radiolabeling except for CL-containing LUVs, where a similar degree of interaction is observed compared to holo-IFABP. On the other hand, the interaction of LFABP with membranes does not seem to be modulated by phospholipid composition. Nevertheless, a remarkable difference is observed between the apo and holo forms, being the apo-LFABP more interactive than the holo form, opposing to what is observed for IFABP. IFABP-HL was included as a negative control and, as previously observed, showed almost no radiolabeling with CL containing LUVs (Fig. 4B) in comparison with apo-IFABP and apo-LFABP, indicating the lack of interaction due to the missing helix-turn-helix motif [20]. This diminished interaction capacity was also observed with the other lipid compositions tested, EPC 100% and 25% PS (Fig. 4B). Controls for hydrolysis of the photoactivable probe were performed by TLC, to make sure that the protein was not extracting radiolabeled hydrolysis products.
Figure 4. Hydrophobic photolabeling of intestinal FABPs.
Physical interaction of native IFABP and LFABP was evidenced by radiolabeling with the photoactivable probe 125I-TID-PC employing LUVs (0.5 mM) of different compositions: 100% EPC, 25% PS and 25% CL. For each lipid composition, the upper panel shows the SDS-PAGE stained with Coomasie blue and the bottom panel its autoradiography. Results are shown here from a representative experiment. (A) shows the native proteins analyzed for the effect of the presence of oleic acid (apo- vs. holo-forms), (B) shows wild type intestinal apo-FABPs and IFABP-HL in the apo-form.
3.5 Structural determinants for membrane interaction
We have already presented here indirect evidence that support the idea that the α-helical region of IFABP is important for the interaction with membranes. With the aim to identify the structural determinants responsible for FABP-membrane interaction, a chemical proteolysis of IFABP was conducted using BNPS-Skatole after performing the photocrosslinking experiment by preincubation of IFABP with CL-containing SUVs. IFABP has two cleavage sites for this proteolytic agent, W6 and W82, producing two detectable fragments: peptides 7-82 (9.6 kDa) and 83-126 (5.5 kDa), that correspond to the α-helical region and the first half of the β-barrel, and the second half of the β-barrel, respectively. These fragments were resolved in a Tris-Glycine SDS-PAGE as shown in Figure 5. In the gel stained with Coomasie Blue, both resolved fragments have similar intensities showing a ratio of 1.1, but fragment 7-82 shows 2.6 times greater intensity of radiolabeling than fragment 83-126, indicating that the first half of the β-barrel and the α-helical region is much likely to get in contact with the hydrophobic core of the membrane than the last portion of the β-barrel. An equivalent analysis for wild-type LFABP was not possible due to the lack of Trp residues in the wild-type protein.
Figure 5. Selective proteolysis of radiolabeled wild type IFABP.
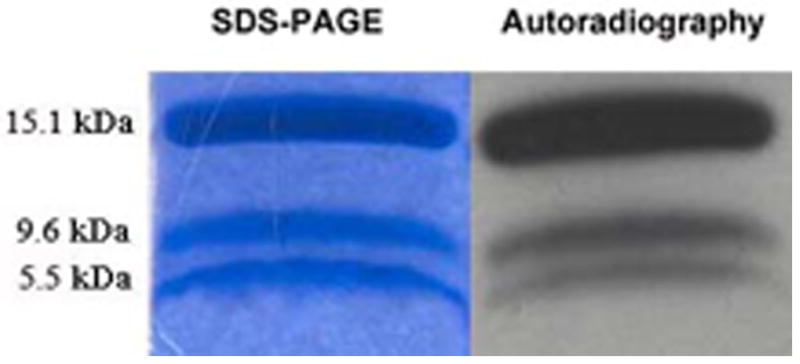
After hydrophobic photolabeling with 125I-TID-PC, apo-IFABP was blotted and subjected to selective proteolisys with BNPS-Skatole. The results showed a preferential radiolabeling (right panel) of the fragment containing the α-helical region (9.6 kDa) than the one corresponding to the second half of the β-barrel (5.5 kDa) compared to the coomasie blue staining (left panel).
4. Discussion
Protein-membrane interactions are thought to be responsible for dictating the functions of a particular protein, in coordination with the rest of the biomolecules that coexist with it at the cellular level. Since FABPs are proposed to function as cytosolic carriers of hydrophobic compounds through the aqueous media, it is necessary to analyze the interaction between FABPs and donor/acceptor membranes to achieve a better understanding of the lipid metabolism in the intestinal epithelium. The set of experiments presented here allowed us to analyze the binding of the intestinal FABPs to model phospholipid membranes from different perspectives, including time reference, phospholipid composition and presence of the ligand.
The “short-term” monitoring (Tb leakage and hydrophobic photolabeling assays) of the FABP-membrane complex indicate that IFABP and LFABP interact with the membrane, at least transiently, deep enough to come in contact with the hydrophobic layer and to produce irregularities in the membrane structure that may induce exchange of hydrophilic material between both sides of the vesicles. From another point of view, “long-term” (cytochrome c and sucrose loaded vesicles binding assay) monitoring experiments indicate that stable FABP-membrane complexes can primarily be formed only when PS or CL are present in the phospholipid composition of the vesicles. A KD was calculated for IFABP and LFABP when mixed with CL-containing LUVs from the sedimentation assay, and the affinity of these proteins is lower compared, to the sub-μM affinity calculated from the cytochrome c competition assay. This could be a consequence of the intrinsic differences of the methodologies employed. It is also worth noting that vesicles of different curvature were employed, which could affect the affinity of the protein for the membrane [41]. Previous studies of adipocyte FABP binding to LUVs employing a sedimentation assay showed one order of magnitude lower affinity for CL containing vesicles [33]. This may be a sign of different functional specialization between different members of the FABP family.
As a whole, these results support our previous kinetic observations that IFABP is a membrane interactive protein and that its α-helical domain is likely to be involved in the interaction with membranes [16, 17, 20]. The lower interaction of IFABP with PS compared to CL-containing LUVs also correlates with the results obtained in the kinetic studies [14]. Interestingly, LFABP also interacts with vesicles at physiological ionic strength. This was not expected considering our previous kinetic fatty acid transfer analysis from LFABP to membranes, which indicated that the formation of a LFABP-membrane complex does not seem to be involved in the transfer process. Nevertheless, in the interpretation of a “diffusional” transfer mechanism, as assessed for LFABP, protein-membrane interaction should not be excluded since the rate limiting step could be the liberation of the ligand, towards the aqueous media instead of the interaction with the membrane. It is important to note that, unlike the kinetic studies, in this work apo-and holo-FABPs were tested; and apo-LFBP is the one that shows a higher degree of interaction. Previous studies from another group have demonstrated that LFABP interacts with acidic but not with zwitterionic membranes, under conditions of low ionic strength [22, 24]. Additionally, apo-LFABP appears to be more interactive than apo-IFABP regardless of vesicle’s composition as indicated by the photocrosslinking experiments (Fig. 4B). This result correlates well with the higher capacity of interaction of LFABP compared to IFABP as seen in the cytochrome c experiments (Fig. 1A and 1B).
Regarding membrane structure, although apo-LFABP shows a higher labeling compared to apo-IFABP, its destabilizing effect seems to be lower as observed from the terbium leakage assay. This could be indicating a difference in the mechanism employed by each protein to attach itself to the phospholipid membrane. Molecular modeling studies of FABP-membrane interaction (Costabel et al. unpublished results) indicate that the α-helical region of IFABP is energetically favored for its interaction with anionic membranes. On the other hand, LFABP is predicted to interact through the bottom of the binding cavity. This may explain why the results from LFABP-membrane and transfer kinetic studies appear to be dissociated, and why the FA transfer step for LFABP may include passage through the aqueous phase [14, 15, 18]. Molecular dynamic simulations of the basic FABP from chicken liver (that shares the same in vitro diffusional mechanism) also demonstrated that this protein could establish contacts with membranes through the side of the barrel that is opposite to the portal region; and conformational changes in the latter occurred simultaneously with the binding to the membrane, possibly allowing the ligand to be released to the aqueous media [42].
Previous works already suggested that the α-helical region is critical for the mechanism of ligand transfer, defining the collisional or diffusional mechanisms for both intestinal FABPs, as well as for the physical interaction with phospholipid membranes [16-19, 21]. In the present work, the behavior of the helixless variant of IFABP seems to correlate well with the proposed function for IFABP’s α-helical region as the leading interactive motif in a sequential ligand transfer. Briefly, an initial step via electrostatic interaction between the α-helical region and the membrane would be followed by a conformational change that would then promote the exit of the ligand from the binding site towards the membrane [17, 20, 21]. In this sense, the selective proteolysis results suggest that, within this model mechanism, the α-helical region interaction should be further followed by the contact of the β-barrel with the membrane.
The different methodologies employed here indicate that IFABP and LFABP can both interact with model membranes. In general, the electrostatic interactions seem to be responsible for a tighter binding to membranes containing acidic phospholipids, with special behavior when CL is present. Cardiolipin is a unique phospholipid and an important component, almost exclusive, of mitochondrial inner membrane, where it is synthesized [43]. Cardiolipin also has active roles in mitochondrial-dependent apoptosis and in mitochondrial membrane dynamics and functionality [44-46]. Considering the affinity that IFABP and LFABP showed for CL in the different experiments, it is tempting to hypothesize that one of the FABP’s functions could be delivering FA specifically to mitochondria, where they would be used as energy source. Experiments using cultured cells [47] and LFABP ablation in knockout (KO) mice demonstrated that absence of LFABP seems to impair FA catabolism in the enterocytes’ mitochondria [48].
The different behavior of IFABP and LFABP in their apo- and holo-forms, suggests that these proteins must be carrying out specific functions and allows us to propose different roles in the FA transport and metabolism. While LFABP may interact strongly with membranes in its apo-form to upload the ligands; IFABP shows stronger interaction in its holo-form, probably indicating its capacity to selectively unload them. In this regard, recent molecular dynamic simulations addressed the question of how FAs exit from IFABP’s binding site [49] and the authors proposed two possible pathways for unloading its ligand in the membrane’s interface: 1) a direct, fast and energetically favorable transfer of ligand to the membrane, and 2) a slower and energetically less favorable exit to the aqueous media; both involving the portal region of IFABP. However, FABPs interactions with other proteins which would participate in lipid trafficking should also be considered. This is especially likely for LFABP, where the fatty acid transfer rates between LFABP and membranes are not dependent on protein-membrane interactions, in direct contrast to the membrane collisional mechanisms of fatty acid transfer between IFABP and phospholipid bilayers [14, 15]. Moreover, preliminary results from our laboratory suggest that IFABP and LFABP may physically interact, as assessed by fatty acid transfer experiments between them (Falomir Lockhart et al. unpublished data). Finally, recent reports account for significant differences in the metabolism of FAs and monoacyglycerides in the intestine of IFABP-/- and LFABP-/- KO mice, finally demonstrating that these proteins play unique roles in the assimilation of nutrients in the intestinal mucosa [50].
5. Conclusion
The present study demonstrates that, in spite of the different mechanisms employed for FA transfer, IFABP and LFABP are membrane interactive proteins responding to different modulators. The results presented here expand the knowledge about the properties of these proteins in vitro and allow us to propose a more detailed model for the role of intestinal FABPs in the metabolism of FA; where both, IFABP and LFABP, would be able to interact selectively with different subcellular membranous compartments, either to download or upload its cargo, responding to phospholipid composition and defining the fate of their ligands. IFABP shows a preferential binding and transfer of ligands to anionic phospholipid bilayers; the mitochondrial inner membrane, peroxisome membrane or plasma membrane cytosolic leaflet, are good candidates for such interaction. On the other hand, LFABP shows less specificity for membrane superficial charge, and it is more interactive in its apo-form. This may be related to its possible uploading function. Furthermore, FABPs might be in a central position to modulate several cellular processes dependent on lipid and energy availability; and interactions with other proteins must be considered to fully understand their specific roles. Transcription factors and lipid metabolizing enzymes are the most relevant candidates to coordinate their functions with those of FABPs for sensing FA concentrations as a substrate for energy, membrane synthesis and cell growth. A complete and comprehensive understanding of the role of these proteins represent a key step for developing new therapeutic strategies for pathologies such as diabetes, obesity, X syndrome, atherosclerosis or cancer.
Supplementary Material
Research Highligths.
We examine the capacity of intestinal and liver FABP to interact with model membranes> Both proteins interact with phospholipid membranes. > Such interactions are modulated by different factors.> The observed differences further imply different roles for intestinal and liver FABP in the intracellular context.
Acknowledgments
This work was supported by grants from the ANPCyT (PICT26218) to BC and the NIH (DK38389) to JS. LJFL is also grateful to CONICET, and GRF and MXG to ANPCyT for their fellowships. We wish to thank Dr. Horacio A. Falomir (Instituto de Física de La Plata, UNLP) for his help with the data analysis employing Mathematica software.
Abreviations
- FAs
long chain fatty acids
- FABPs
fatty acid-binding proteins
- IFABP
intestinal fatty acid-binding protein
- LFABP
liver fatty acid-binding protein
- IFABP-HL
helixless IFABP
- DPA
dipicolinic acid
- SUVs
small unilamellar vesicles
- LUVs
large unilamellar vesicles
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bass NM. Function and Regulation of Hepatic and Intestinal Fatty-Acid Binding Proteins. Chem Phys Lipids. 1985;38:95–114. doi: 10.1016/0009-3084(85)90060-x. [DOI] [PubMed] [Google Scholar]
- 2.Glatz JFC, vanderVusse GJ. Cellular fatty acid-binding proteins: Their function and physiological significance. Prog Lipid Res. 1996;35:243–282. doi: 10.1016/s0163-7827(96)00006-9. [DOI] [PubMed] [Google Scholar]
- 3.Storch J, Corsico B. The emerging functions and mechanisms of mammalian fatty acid-binding proteins. Annu Rev Nutr. 2008;28:73–95. doi: 10.1146/annurev.nutr.27.061406.093710. [DOI] [PubMed] [Google Scholar]
- 4.Storch J, Thumser AEA. The fatty acid transport function of fatty acid-binding proteins. Biochim Biophys Acta-Molecular and Cell Biology of Lipids. 2000;1486:28–44. doi: 10.1016/s1388-1981(00)00046-9. [DOI] [PubMed] [Google Scholar]
- 5.Storch J, Herr FM, Hsu KT, Kim HK, Liou HL, Smith ER. The role of membranes and intracellular binding proteins in cytoplasmic transport of hydrophobic molecules: Fatty acid-binding proteins. Comp Biochem Physiol Biochem Mol Biol. 1996;115:333–339. [Google Scholar]
- 6.Veerkamp JH, Maatman R. Cytoplasmatic Fatty-Acid Binding Proteins - Their Structure and Genes. Prog Lipid Res. 1995;34:17–52. doi: 10.1016/0163-7827(94)00005-7. [DOI] [PubMed] [Google Scholar]
- 7.Banaszak L, Winter N, Xu ZH, Bernlohr DA, Cowan S, Jones TA. Lipid-Binding Proteins - A Family of Fatty-Acid and Retinoid Transport Proteins. Adv Protein Chem. 1994;45:89–151. doi: 10.1016/s0065-3233(08)60639-7. [DOI] [PubMed] [Google Scholar]
- 8.Pelsers M, Namiot Z, Kisielewski W, Namiot A, Januszkiewicz M, Hermens WT, Glatz JFC. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem. 2003;36:529–535. doi: 10.1016/s0009-9120(03)00096-1. [DOI] [PubMed] [Google Scholar]
- 9.Sacchettini JC, Gordon JI. Rat Intertinal Fatty-Acid Binding Protein - A model System for Analyzing the Forces that Can Bind Fatty Acid to Proteins. J Biol Chem. 1993;268:18399–18402. [PubMed] [Google Scholar]
- 10.He Y, Yang X, Wang H, Estephan R, Francis F, Kodukula S, Storch J, Stark RE. Solution-state molecular structure of apo and oleate-liganded liver fatty acid-binding protein. Biochemistry. 2007;46:12543–12556. doi: 10.1021/bi701092r. [DOI] [PubMed] [Google Scholar]
- 11.Marcelino AMC, Smock RG, Gierasch LM. Evolutionary coupling of structural and functional sequence information in the intracellular lipid-binding protein family. Proteins. 2006;63:373–384. doi: 10.1002/prot.20860. [DOI] [PubMed] [Google Scholar]
- 12.Vork MM, Glatz JFC, VanderVusse GJ. Modelling intracellular fatty acid transport: possible mechanistic role of cytoplasmic fatty acid-binding protein. Prostaglandins Leukot Essent Fatty Acids. 1997;57:11–16. doi: 10.1016/s0952-3278(97)90486-5. [DOI] [PubMed] [Google Scholar]
- 13.Weisiger RA, Zucker SD. Transfer of fatty acids between intracellular membranes: roles of soluble binding proteins, distance, and time. Am J Physiol-Gastroint Liver Physiol. 2002;282:G105–G115. doi: 10.1152/ajpgi.00238.2001. [DOI] [PubMed] [Google Scholar]
- 14.Hsu KT, Storch J. Fatty acid transfer from liver and intestinal fatty acid-binding proteins to membranes occurs by different mechanisms. J Biol Chem. 1996;271:13317–13323. doi: 10.1074/jbc.271.23.13317. [DOI] [PubMed] [Google Scholar]
- 15.Thumser AEA, Storch J. Liver and intestinal fatty acid-binding proteins obtain fatty acids from phospholipid membranes by different mechanisms. J Lipid Res. 2000;41:647–656. [PubMed] [Google Scholar]
- 16.Wu F, Corsico B, Flach CR, Cistola DP, Storch J, Mendelsohn R. Deletion of the helical motif in the intestinal fatty acid-binding protein reduces its interactions with membrane monolayers: Brewster angle microscopy, IR reflection-absorption spectroscopy, and surface pressure studies. Biochemistry. 2001;40:1976–1983. doi: 10.1021/bi002252i. [DOI] [PubMed] [Google Scholar]
- 17.Corsico B, Cistola DP, Frieden C, Storch J. The helical domain of intestinal fatty acid binding protein is critical for collisional transfer of fatty acids to phospholipid membranes. Proc Natl Acad Sci U S A. 1998;95:12174–12178. doi: 10.1073/pnas.95.21.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corsico B, Liou HL, Storch J. The alpha-helical domain of liver fatty acid binding protein is responsible for the diffusion-mediated transfer of fatty acids to phospholipid membranes. Biochemistry. 2004;43:3600–3607. doi: 10.1021/bi0357356. [DOI] [PubMed] [Google Scholar]
- 19.Corsico B, Franchini GR, Hsu KT, Storch J. Fatty acid transfer from intestinal fatty acid binding protein to membranes: electrostatic and hydrophobic interactions. J Lipid Res. 2005;46:1765–1772. doi: 10.1194/jlr.M500140-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Lockhart LJ Falomir, Laborde L, Kahn PC, Storch J, Corsico B. Protein-membrane interaction and fatty acid transfer from intestinal fatty acid-binding protein to membranes - Support for a multistep process. J Biol Chem. 2006;281:13979–13989. doi: 10.1074/jbc.M511943200. [DOI] [PubMed] [Google Scholar]
- 21.Franchini GR, Storch J, Corsico B. The integrity of the alpha-helical domain of intestinal fatty acid binding protein is essential for the collision-mediated transfer of fatty acids to phospholipid membranes. Biochim Biophys Acta-Molecular and Cell Biology of Lipids. 2008;1781:192–199. doi: 10.1016/j.bbalip.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies JK, Thumser AEA, Wilton DC. Binding of recombinant rat liver fatty acid-binding protein to small anionic phospholipid vesicles results in ligand release: A model for interfacial binding and fatty acid targeting. Biochemistry. 1999;38:16932–16940. doi: 10.1021/bi991926q. [DOI] [PubMed] [Google Scholar]
- 23.Davies JK, Hagan RM, Wilton DC. Effect of charge reversal mutations on the ligand- and membrane-binding properties of liver fatty acid-binding protein. J Biol Chem. 2002;277:48395–48402. doi: 10.1074/jbc.M208141200. [DOI] [PubMed] [Google Scholar]
- 24.Hagan RM, Worner-Gibbs J, Wilton DC. The interaction of liver fatty-acid-binding protein (FABP) with anionic phospholipid vesicles: is there extended phospholipid anchorage under these conditions? Biochem J. 2008;410:123–129. doi: 10.1042/BJ20071109. [DOI] [PubMed] [Google Scholar]
- 25.Weber T, Brunner J. 2-(tributylstannyl)-4-[3-(trifluoromethyl)-3H-diazirin-3-yl]benzyl alcohol - A Building-Block for Photolabeling and Cross-Linking Reagents of Very High Specific Radioactivity. J Am Chem Soc. 1995;117:3084–3095. [Google Scholar]
- 26.Corsico B, Toledo JD, Garda HA. Evidence for a central apolipoprotein A-I domain loosely bound to lipids in discoidal lipoproteins that is capable of penetrating the bilayer of phospholipid vesicles. J Biol Chem. 2001;276:16978–16985. doi: 10.1074/jbc.M011533200. [DOI] [PubMed] [Google Scholar]
- 27.Hope MJ, Bally MB, Webb G, Cullis PR. Production of Large Unilamellar Vesicles by Rapid Extrusion Procedure - Characterization of Size Distribution, Trapped Volume and Ability to Maintain a Membrane-Potential. Biochim Biophys Acta. 1985;812:55–65. doi: 10.1016/0005-2736(85)90521-8. [DOI] [PubMed] [Google Scholar]
- 28.Huang C, Thompson TE. Preparation of homogeneous, single-walled phosphatidylcholine vesicles. Methods Enzymol. 1974;32:5. doi: 10.1016/0076-6879(74)32048-4. [DOI] [PubMed] [Google Scholar]
- 29.Storch J, Kleinfeld AM, Bass NM, Shields H. Interaction of Fatty-Acid Binding Protein (FABP) with Fluorescent Analogs of Free Fatty Acids. Biophys J. 1986;49:A106–A106. [Google Scholar]
- 30.Wilschut J, Duzgunes N, Fraley R, Papahadjopoulos D. Studies on the Mechanism of Membrane-Fusion - Kinetics of Calcium-Ion Induced Fusion of Phosphatidylserine Vesicles Followed by a New Assay for Mixing of Aqueous Vesicle Content. Biochemistry. 1980;19:6011–6021. doi: 10.1021/bi00567a011. [DOI] [PubMed] [Google Scholar]
- 31.Gomori G. A modification of the colorimetric phosphorus determination for use with the photoelectric colorimeter. J Lab Clin Med. 1942;27:955–960. [Google Scholar]
- 32.Mustonen P, Virtanen JA, Somerharju PJ, Kinnunen PKJ. Binding of Cytochrome-c to Liposomes as Revealed by the Quenching of Fluorescence from Pyrene-Labeled Phospholipids. Biochemistry. 1987;26:2991–2997. doi: 10.1021/bi00385a006. [DOI] [PubMed] [Google Scholar]
- 33.Smith ER, Storch J. The adipocyte fatty acid-binding protein binds to membranes by electrostatic interactions. J Biol Chem. 1999;274:35325–35330. doi: 10.1074/jbc.274.50.35325. [DOI] [PubMed] [Google Scholar]
- 34.Rebecchi M, Peterson A, McLaughlin S. Phosphoinositide-Specific Phospholipase C-delta-1 Binds with High Affinity to Phospholipid Vesicles Containing Phosphatidylinositol 4,5-biphosphate. Biochemistry. 1992;31:12742–12747. doi: 10.1021/bi00166a005. [DOI] [PubMed] [Google Scholar]
- 35.Buser CA, Sigal CT, Resh MD, McLaughlin S. Membrane-Binding of Myristylated Peptides Corresponding to the NH2-Terminus of Src. Biochemistry. 1994;33:13093–13101. doi: 10.1021/bi00248a019. [DOI] [PubMed] [Google Scholar]
- 36.Schagger H, Vonjagow G. Tricine Sodium Dodecyl-Sulfate Polyamide-Gel Electrophoresis for the Separation of Proteins in the Range from 1kDa to 100kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 37.Crimmins DL, McCourt DW, Thoma RS, Scott MG, Macke K, Schwartz BD. In Situ Chemichal Cleavage of Proteins Immobilized to Glass-Fiber and Polyvinylidendifluoride Membranes - Cleavage at Tryptophan Residues with 2-(2′-nitrophenylsulfenyl)-3-methyl-3′-bromoindolenine to Obtain Internal Amino-Acid Sequence. Anal Biochem. 1990;187:27–38. doi: 10.1016/0003-2697(90)90412-3. [DOI] [PubMed] [Google Scholar]
- 38.Rytomaa M, Kinnunen PKJ. Evidence for 2 Distinct Acidic Phospholipid-Binding Sites in Cytochrome-c. Journal of Biological Chemistry. 1994;269:1770–1774. [PubMed] [Google Scholar]
- 39.Lakowics J. Principles of Fluorescence Spectroscopy. 3. Springer; New York: 2006. [Google Scholar]
- 40.Tricerri A, Corsico B, Toledo JD, Garda HA, Brenner RR. Conformation of apolipoprotein AI in reconstituted lipoprotein particles and particle-membrane interaction: Effect of cholesterol. Biochim Biophys Acta -Lipids and Lipid Metabolism. 1998;1391:67–78. doi: 10.1016/s0005-2760(97)00187-2. [DOI] [PubMed] [Google Scholar]
- 41.Gerlach H, Laumann V, Martens S, Becker CFW, Goody RS, Geyer M. HIV-1 Nef membrane association depends on charge, curvature, composition and sequence. Nat Chem Biol. 2010;6:46–53. doi: 10.1038/nchembio.268. [DOI] [PubMed] [Google Scholar]
- 42.Villarreal MA, Perduca M, Monaco HL, Montich GG. Binding and interactions of L-BABP to lipid membranes studied by molecular dynamic simulations. Biochim Biophys Acta Biomembranes. 2008;1778:1390–1397. doi: 10.1016/j.bbamem.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 43.Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Prog Lipid Res. 2000;39:257–288. doi: 10.1016/s0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- 44.Houtkooper RH, Vaz FM. Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci. 2008;65:2493–2506. doi: 10.1007/s00018-008-8030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalvez F, Schug ZT, Houtkooper RH, MacKenzie ED, Brooks DG, Wanders RJA, Petit PX, Vaz FM, Gottlieb E. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J Cell Biol. 2008;183:681–696. doi: 10.1083/jcb.200803129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrosillo G, Moro N, Ruggiero FM, Paradies G. Melatonin inhibits cardiolipin peroxidation in mitochondria and prevents the mitochondrial permeability transition and cytochrome c release. Free Radic Biol Med. 2009;47:969–974. doi: 10.1016/j.freeradbiomed.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 47.Erol E, Cline GW, Kim JK, Taegtmeyer H, Binas B. Nonacute effects of H-FABP deficiency on skeletal muscle glucose uptake in vitro. Am J Physiol Endocrinol Metab. 2004;287:E977–E982. doi: 10.1152/ajpendo.00139.2004. [DOI] [PubMed] [Google Scholar]
- 48.Lagakos W, Zhou YX, Mandap B, Binas B, Storch J. Intestinal lipid metabolism is altered in liver fatty acid-binding protein-null mice (LFABP-/-) FASEB J. 2007;21:A109–A109. [Google Scholar]
- 49.Mihajlovic M, Lazaridis T. Modeling fatty acid delivery from intestinal fatty acid binding protein to a membrane. Protein Sci. 2007;16:2042–2055. doi: 10.1110/ps.072875307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lagakos WS, Gajda A, Agellon L, Binas B, Choi V, Mandap B, Russnak T, Zhou YX, Storch J. Different functions of the intestinal- and liver-type fatty acid-binding proteins in the intestine and in whole body energy homeostasis. Am J Physiol Gastrointest Liver Physiol. 2011 doi: 10.1152/ajpgi.00229.2010. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






