Abstract
Background
Obesity is increasing among American women, especially as they age. The influence of obesity on the accuracy of screening mammography has not been studied extensively.
Methods
We analyzed 100622 screening mammography examinations performed on members of a nonprofit health plan. The relationship between body mass index (weight in kilograms divided by the square of height in meters) and measures of screening accuracy was assessed. Body mass index was categorized as underweight or normal weight (<25), overweight (25–29), obesity class I (30–34), and obesity classes II to III (≥35).
Results
Compared with underweight or normal weight women, overweight and obese women were more likely to be recalled for additional tests after adjusting for important covariates, including age and breast density (overweight odds ratio [OR], 1.17; 95% confidence interval [CI], 1.11–1.23); obesity class I OR, 1.27; 95% CI, 1.19–1.35; obesity classes II–III OR, 1.31; 95% CI, 1.22–1.41). As body mass index increased, women were more likely to have lower specificity (overweight OR, 0.86; 95% CI, 0.81–0.90; obesity class I OR, 0.79; 95% CI, 0.74–0.84; and obesity classes II–III OR, 0.77; 95% CI, 0.71–0.82). No statistically significant differences were noted in sensitivity. Adjusted receiver operating characteristic analysis showed statistically significant improvement in the area under the curve (AUC) for underweight or normal weight women (AUC=0.941) vs overweight women (AUC=0.916, P=.02) and underweight or normal weight women vs obesity classes II and III women (AUC=0.904, P=.02).
Conclusions
Obese women had more than a 20% increased risk of having false-positive mammography results compared with underweight and normal weight women, although sensitivity was unchanged. Achieving a normal weight may improve screening mammography performance.
Obese, postmenopausal women have an increased risk of breast cancer compared with postmenopausal women of normal weight.1 Obese women are also diagnosed as having a later stage of breast cancer compared with nonobese women.2 It has been hypothesized that women with larger breasts may be less able to feel small breast lumps, thereby increasing the likelihood of progression in disease stage.2 This possibility is supported by at least 1 study,3 which found that women with larger breasts were likely to have larger tumors at diagnosis. It is unclear from these studies what role mammography plays in cancer detection, but the findings at least raise the question of whether mammography performs differently among obese women compared with normal weight women.
In fact, the influence of obesity on the accuracy of screening mammography has not been studied well. Such analysis might provide greater understanding of the quality of available modes of breast cancer screening in obese women. We conducted an analysis of 100622 screening mammography examinations performed on patients in a nonprofit health plan to explore the relationship between obesity and mammographic accuracy.
METHODS
PATIENT POPULATION AND DATA COLLECTION
Data came from Group Health Cooperative, a nonprofit health plan that serves more than 600000 individuals in the Northwestern United States. A breast cancer screening program, initiated in 1986, is offered to the approximately 80 000 women who are 40 years or older. Women are recruited on an ongoing basis through a mailed survey, to which 86% respond.4,5 Recruitment involves automatic invitations for screening to women 50 years and older and to women aged 40 to 49 years with breast cancer risk factors. At the time of this study, the screening visit included a clinical breast examination, instruction in breast self-examination, and mammography. Women are invited for screening mammography at varying times, depending on their age and other breast cancer risk factors. Baseline demographic data, health history (including height and weight), screening history, and breast cancer risk factor information are ascertained by a 4-page, self-administered survey mailed to women 40 years and older.
Self-reported height and weight were collected in the baseline survey and at each screening visit. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. If height data were present on one screening visit but missing on another, the value was imputed from the available data. Data were excluded for the following reasons: missing weight, reported height less than 0.91 m or more than 2.13 m, or reported weight less than 22.68 kg.
Between January 1, 1996, and September 30, 1999, 76472 breast screening program participants 40 years or older underwent 122800 mammography examinations identified as routine screening by the radiologist. Unilateral mammography was excluded (n=7399), since this often indicated that either the woman had a previous unilateral mastectomy or that the examination was performed for diagnostic purposes. Additional exclusions, applied in a stepwise manner, included a history of breast cancer (n=4810); any radiologic breast examination performed within the prior 9 months, which would likely make this a follow-up examination (n=2850); history of breast implants (n=944); missing Breast Imaging Reporting and Data System (BI-RADS) assessment (n=123); or missing or improbable BMI data (n = 6052). Specific reasons for excluding mammography examinations due to BMI data include missing weight (n=5454), missing height (n=386), weight less than 22.68 kg (n=171), height less than 0.91 m (n=33), and height greater than 2.13 m (n=8). The final cohort included 100622 screening mammography examinations on 67984 women.
Women who reported breast symptoms when they called to schedule an appointment after receiving a screening invitation were referred directly to diagnostic appointments. Despite this triage system, some women still reported breast symptoms when seen for screening mammography. We included these examinations if the visit was categorized as a screening examination by the radiologist, because women often report minor breast symptoms, such as breast tenderness, that vary with menstrual cycles or chronic conditions (eg, nipple inversion).
BREAST CANCER CASES
Breast cancer cases were identified through the health plan cancer registry or Surveillance, Epidemiology, and End Results data bank or both. Only invasive breast cancer and ductal carcinoma in situ were included. Cases known to be lobular carcinoma in situ only were excluded, because they are not mammographically detectable (they represent <1% of all breast cancers).
ANALYTIC DEFINITIONS
Standard definitions developed by the Breast Cancer Surveillance Consortium using BI-RADS categories6 were used.7 Screening mammography was considered positive if interpreted as BI-RADS category 0, 4, 5, or 3 with a recommendation for immediate workup (denoted as 3+). A mammogram result was considered negative if interpreted as BI-RADS category 1, 2, or 3 without any recommendations or with only a recommendation for normal or short-interval follow-up (ie, ≥6 months; denoted as 3−). Standard screening views repeated due to poor image quality were not included as a positive film finding. If additional nonscreening imaging was performed on the same day as the screening mammogram, the original BI-RADS category was recoded to 0 (needs additional assessment). If a woman had different assessments for each breast, the higher assessment level was used according to the following hierarchy: 1 < 2 < 3 < 0 < 4 < 5.
Examination results were false positive when the assessment was positive and a breast cancer diagnosis did not occur within the follow-up period (365 days following the index screening examination or until the next screening examination, whichever occurred first). Examinations were true positive when the assessment was positive and a breast cancer diagnosis followed within the specified follow-up period. A false-negative examination result was a negative assessment result with a diagnosis of breast cancer within the follow-up period. A true-negative examination result was a negative assessment result with no subsequent cancer diagnosis within the follow-up period. The recall rate was defined as the number of examination results interpreted as positive divided by the total number of examinations. Sensitivity was calculated as true positive/(true positive+false negative). Specificity was calculated as true negative/(true negative+false positive).
Menopausal or hormone therapy (HT) status was determined by self-report for women younger than 55 years, but women older than 55 years were assumed to be postmenopausal. Breast density was determined by the radiologist using the BI-RADS classification: (1) almost entirely fatty, (2) scattered fibroglandular tissue, (3) heterogeneously dense, and (4) extremely dense. Time since last mammogram was computed using self-reported data in addition to any evidence of a prior mammogram found in our database.
The BMI data were collapsed into 4 categories for analyses based on distribution frequency and national guidelines8: underweight or normal weight (BMI, <25; n=42350), overweight (BMI, 25.0–29.9; n=30897), obesity class I (BMI, 30.0–34.9; n=15803), and obesity classes II to III (BMI, ≥35.0; n=11572).
STATISTICAL ANALYSIS
We evaluated the relationships between BMI category and recall rate, sensitivity, and specificity of screening mammography. Factors potentially related to breast cancer risk and mammography accuracy included age (in 5-year categories), menopausal status or HT use, breast density, patient-reported breast symptoms, family history of breast cancer, history of breast biopsy or surgery, and time since last mammogram.
Unadjusted recall rates, sensitivities, and specificities were computed to assess the relationship between BMI and mammography accuracy. We also fit multivariable logistic regression models using the GENMOD procedure in SAS,9 adjusting for the variables mentioned herein. Possible interactions between BMI and breast density and between BMI and menopausal or HT status were also examined. Receiver operating characteristic (ROC) analyses examined the effect of BMI on sensitivity and specificity simultaneously.10 The ROC models were fit, adjusting for the same variables included in the multivariable logistic regression analyses. From the ROC models, we determined ROC curves and the areas under the curve (AUCs). The AUC can range from 0 to 1.0, with higher values indicating greater accuracy. The ROC models were compared using likelihood ratio tests with pairwise comparisons between ROC curves performed using t tests. Analyses were performed using 2-sided P<.05 as the critical value considered to be statistically significant.
RESULTS
Demographic and clinical characteristics of the women obtaining screening mammography examinations are given in Table 1. For most mammography examinations, the women were overweight or obese (58%) and postmenopausal (74%). The women ranged in age from 40 to 100 years. Because the data are presented by screening examination encounter, some women had more than 1 examination in the database. Among the 67984 women with mammography visits, there were 38 154 women with 1 examination, 27 360 with 2 examinations, 2133 with 3 examinations, and 337 with 4 or more screening examinations. Overweight and obese women were equally likely to have multiple examinations compared with underweight and normal weight women.
Table 1.
Demographic and Clinical Characteristics of Women Obtaining Screening* Mammography Examinations
| Characteristic | Screening Mammography Examination (N = 100 622) | Mammography Examination With Cancer Diagnosis During Follow-up (n = 702) |
|---|---|---|
| Body mass index category | ||
| Underweight or normal weight | 42 350 (42.1) | 279 (39.7) |
| Overweight | 30 897 (30.7) | 227 (32.3) |
| Obesity class I | 15 803 (15.7) | 116 (16.5) |
| Obesity classes II–III | 11 572 (11.5) | 80 (11.4) |
| Age, y | ||
| 40–44 | 13 306 (13.2) | 40 (5.7) |
| 45–49 | 15 368 (15.3) | 69 (9.8) |
| 50–54 | 18 786 (18.7) | 103 (14.7) |
| 55–59 | 12 371 (12.3) | 92 (13.1) |
| 60–64 | 9840 (9.8) | 92 (13.1) |
| 65–69 | 9381 (9.3) | 88 (12.5) |
| 70–74 | 9073 (9.0) | 94 (13.4) |
| ≥75 | 12 497 (12.4) | 124 (17.7) |
| Breast density (0.4% missing) | ||
| Almost entirely fat | 8601 (8.6) | 29 (4.2) |
| Scattered fibroglandular tissue | 40 731 (40.6) | 275 (39.4) |
| Heterogeneously dense | 39 462 (39.4) | 295 (42.3) |
| Extremely dense | 11 459 (11.4) | 99 (14.2) |
| Menopausal or HT status (0.002% missing) | ||
| Premenopausal | 25 754 (25.6) | 114 (16.2) |
| Postmenopausal (no HT) | 33 952 (33.7) | 256 (36.5) |
| Postmenopausal (HT) | 34 295 (34.1) | 269 (38.3) |
| Postmenopausal (HT status unknown) | 6619 (6.6) | 63 (9.0) |
| Breast symptoms (3.7% missing) | ||
| None | 91 885 (94.8) | 605 (89.9) |
| Lump, discharge, or other | 5001 (5.2) | 68 (10.1) |
| Family history of breast cancer (0.3% missing) | ||
| No | 82 980 (82.7) | 538 (76.9) |
| Yes | 17 324 (17.3) | 162 (23.1) |
| Breast biopsy or surgery (0.1% missing) | ||
| No | 80 946 (80.5) | 517 (73.6) |
| Yes | 19 555 (19.5) | 185 (26.4) |
| Time since last mammogram (0.3% missing) | ||
| No previous mammogram | 6916 (6.9) | 41 (5.9) |
| Within 2 y | 77 684 (77.5) | 550 (78.7) |
| 3–4 y | 9338 (9.3) | 66 (9.4) |
| ≥5 y | 6361 (6.3) | 42 (6.0) |
| Screen result | ||
| 1 (Negative) | 75 579 (75.1) | 71 (10.1) |
| 2 (Benign finding) | 10 778 (10.7) | 14 (2.0) |
| 3− (Probably benign finding) | 2272 (2.3) | 13 (1.9) |
| 3+ (Probably benign finding) | 70 (0.1) | 2 (0.3) |
| 0 (Additional imaging evaluation) | 11 828 (11.8) | 558 (79.5) |
| 4 (Suspicious abnormality) | 55 (0.1) | 10 (1.4) |
| 5 (Highly suggestive of malignancy) | 40 (<0.1) | 34 (4.8) |
Abbreviation: HT, hormone therapy.
Data are given as numbers (percentages). 3− Represents examinations where there was a Breast Imaging Reporting and Data System (BI-RADS) assessment of 3 with no recommendations for immediate follow-up; 3+ represents examinations where there was a BI-RADS assessment of 3 with 1 or more recommendations for immediate follow-up.
A BI-RADS interpretation of 0 (additional imaging evaluation) was given for 11828 examinations (11.8%) with a BI-RADS interpretation of 4 or 5 in 95 (0.1%). When looking at examinations that received an initial BI-RADS assessment of 0, women with a higher BMI were equally likely to go on to have additional imaging (ie, additional mammography views or ultrasound) on that same day as normal weight and underweight women (data not shown).
Overweight and obese women were slightly less likely to report a screening mammography examination within 2 years compared with underweight and normal weight women (77% vs 79%). Breast cancer was diagnosed in 702 women during the follow-up period (Table 1 and Table 2). Overweight and obese women were less likely to be diagnosed as having ductal carcinoma in situ compared with underweight or normal weight women (16% vs 22%, P=.046). Among women with invasive breast cancer, overweight and obese women were more likely to have tumors larger than 15 mm at time of diagnosis compared with underweight or normal weight women (33% vs 28%, P=.18).
Table 2.
Breast Cancer Outcomes by Body Mass Index Category (N = 702 Women)*
| Cancer Type | No. (%) of Women With Breast Cancer
|
P Value | |
|---|---|---|---|
| Underweight or Normal Weight (n = 279) | Overweight or Obesity Classes I–III (n = 423) | ||
| Ductal carcinoma in situ present | 60/279 (22) | 66/423 (16) | .046 |
| Invasive, stage I | 156/214 (73) | 248/346 (72) | .75 |
| Invasive, stage II | 41/214 (19) | 83/346 (24) | .18 |
| Invasive, stage III–IV | 17/214 (8) | 15/346 (4) | .07 |
| Invasive tumors >15 mm | 54/195 (28) | 101/302 (33) | .18 |
Seventy-nine invasive cancers (of 576) were missing tumor size; 16 invasive cancers (of 576) were missing stage.
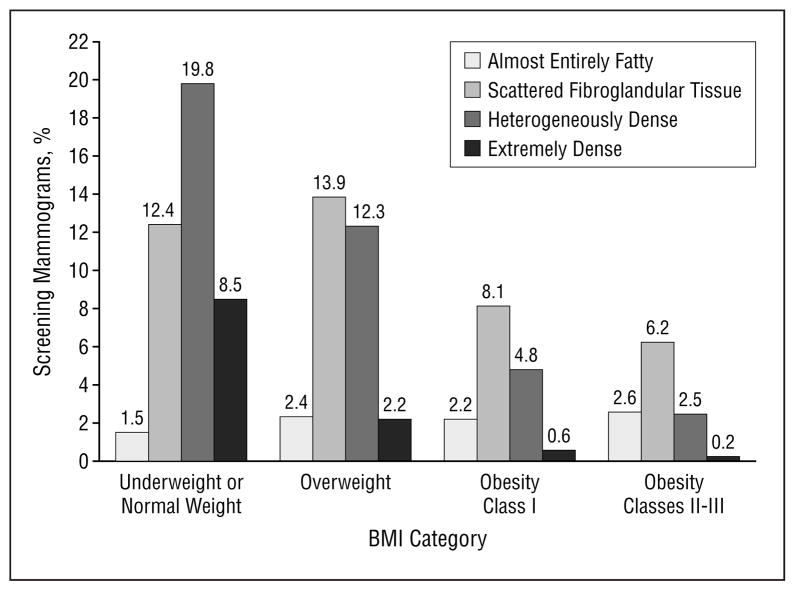
The distribution of breast density rating for the 100622 screening mammography examinations was almost entirely fat (8.6%), scattered fibroglandular tissue (40.6%), heterogeneously dense (39.4%), and extremely dense (11.4%). A significant association was noted between breast density and breast cancer diagnosis during follow-up. The distribution of breast density rating by BMI category is shown in Figure 1. Underweight or normal weight women were more likely to have dense breasts compared with overweight and obese women. Among the screening mammograms noted as extremely dense, most (74.2%) were from women in the underweight or normal weight BMI category, whereas 2.0% were from women in the obesity classes II to III category.
Figure 1.
Distribution of mammogram breast density by body mass index (BMI) category (n=100253).
The unadjusted performance measures of screening mammography are given in Table 3. Higher BMI was associated with higher recall rate, higher sensitivity, and lower specificity, but these differences were not all statistically significant. The recall rate was highest in young women and those with dense breasts. Table 4 provides the performance measurements of screening mammography after adjustment for important covariates, including age group, breast density, menopausal or HT status, breast symptoms, family history of breast cancer, history of breast biopsy or surgery, and time since last mammogram. Overweight and obese women had a significantly higher likelihood of recall compared with underweight or normal weight women. The odds ratio (OR) for recall increased as BMI increased, using the underweight or normal weight category as the reference (reference OR, 1.00; overweight OR, 1.17; 95% confidence interval [CI], 1.11–1.23; obesity class I OR, 1.27; 95% CI, 1.19–1.35; obesity classes II-III OR, 1.31; 95% CI, 1.22–1.41). No significant difference was noted in sensitivity by BMI categories.
Table 3.
Unadjusted Performance Measurements for Screening Mammography (N = 100 622)
| Characteristic | Rate (95% Confidence Interval)
|
||
|---|---|---|---|
| Recall* | Sensitivity (True-Positive Rate) | Specificity (1 - False-Positive Rate) | |
| Total | 11.9 (11.7–12.1) | 86.0 (83.3–88.5) | 88.6 (88.4–88.8) |
| Body mass index category | |||
| Underweight or normal weight | 11.5 (11.2–11.8) | 84.6 (79.8–88.6) | 89.0 (88.7–89.3) |
| Overweight | 12.2 (11.9–12.6) | 85.9 (80.7–90.2) | 88.3 (88.0–88.7) |
| Obesity class I | 12.3 (11.8–12.8) | 86.2 (78.6–91.9) | 88.3 (87.8–88.8) |
| Obesity classes II-III | 12.1 (11.5–12.7) | 91.3 (82.8–96.4) | 88.5 (87.9–89.0) |
| Age, y | |||
| 40–44 | 13.3 (12.7–13.9) | 87.5 (73.2–95.8) | 86.9 (86.4–87.5) |
| 45–49 | 13.4 (12.8–13.9) | 76.8 (65.1–86.1) | 86.9 (86.4–87.5) |
| 50–54 | 12.8 (12.3–13.3) | 83.5 (74.9–90.1) | 87.6 (87.1–88.1) |
| 55–59 | 12.0 (11.4–12.6) | 82.6 (73.3–89.7) | 88.6 (88.0–89.1) |
| 60–64 | 11.3 (10.7–11.9) | 90.2 (82.2–95.4) | 89.5 (88.8–90.1) |
| 65–69 | 11.2 (10.6–11.9) | 87.5 (78.7–93.6) | 89.5 (88.9–90.1) |
| 70–74 | 9.8 (9.2–10.4) | 89.4 (81.3–94.8) | 91.1 (90.5–91.7) |
| ≥75 | 9.9 (9.4–10.5) | 88.7 (81.8–93.7) | 90.9 (90.4–91.4) |
| Breast density | |||
| Almost entirely fat | 6.1 (5.6–6.6) | 96.6 (82.2–99.9) | 94.2 (93.7–94.7) |
| Scattered fibroglandular tissue | 10.5 (10.2–10.8) | 91.3 (87.3–94.3) | 90.0 (89.7–90.3) |
| Heterogeneously dense | 13.9 (13.5–14.2) | 83.4 (78.6–87.5) | 86.7 (86.3–87.0) |
| Extremely dense | 14.1 (13.5–14.7) | 75.8 (66.1–83.8) | 86.5 (85.8–87.1) |
Recall rate was defined as the number of examination results interpreted as positive divided by the total number of examinations.
Table 4.
Adjusted Performance Measurements of Screening Mammography*
| Characteristic | Odds Ratio (95% Confidence Interval)
|
||
|---|---|---|---|
| Recall* (n = 95 989) | Sensitivity (True-Positive Rate) (n = 667) | Specificity (1 - False-Positive Rate) (n = 95 322) | |
| Body mass index category | |||
| Underweight or normal weight (referent) | 1.00 | 1.00 | 1.00 |
| Overweight | 1.17 (1.11–1.23) | 0.89 (0.52–1.55) | 0.86 (0.81–0.90) |
| Obesity class I | 1.27 (1.19–1.35) | 0.88 (0.44–1.82) | 0.79 (0.74–0.84) |
| Obesity classes II-III | 1.31 (1.22–1.41) | 1.13 (0.47–3.02) | 0.77 (0.71–0.82) |
| Age, y | |||
| 40–44 (referent) | 1.00 | 1.00 | 1.00 |
| 45–49 | 1.20 (1.12–1.29) | 0.56 (0.14–1.86) | 0.84 (0.78–0.90) |
| 50–54 | 1.26 (1.16–1.36) | 0.80 (0.19–2.77) | 0.81 (0.75–0.88) |
| 55–59 | 1.30 (1.18–1.44) | 0.93 (0.20–3.75) | 0.80 (0.72–0.88) |
| 60–64 | 1.25 (1.13–1.39) | 1.63 (0.33–7.21) | 0.85 (0.77–0.95) |
| 65–69 | 1.32 (1.19–1.46) | 1.04 (0.22–4.32) | 0.81 (0.73–0.90) |
| 70–74 | 1.19 (1.07–1.33) | 1.35 (0.28–5.71) | 0.91 (0.82–1.02) |
| ≥75 | 1.25 (1.13–1.39) | 1.21 (0.26–4.88) | 0.86 (0.78–0.96) |
| Breast density | |||
| Almost entirely fat (referent) | 1.00 | 1.00 | 1.00 |
| Scattered fibroglandular tissue | 1.86 (1.69–2.05) | 0.41 (0.02–2.15) | 0.54 (0.49–0.60) |
| Heterogeneously dense | 2.59 (2.34–2.86) | 0.21 (0.01–1.07) | 0.39 (0.35–0.43) |
| Extremely dense | 2.71 (2.43–3.04) | 0.13 (0.01–0.71) | 0.37 (0.33–0.42) |
| Menopausal or HT status | |||
| Premenopausal (referent) | 1.00 | 1.00 | 1.00 |
| Postmenopausal (no HT) | 0.76 (0.71–0.82) | 0.61 (0.25–1.44) | 1.32 (1.23–1.41) |
| Postmenopausal (HT) | 0.96 (0.89–1.03) | 0.60 (0.24–1.46) | 1.04 (0.97–1.11) |
| Postmenopausal (HT status unknown) | 0.90 (0.82–1.00) | 0.46 (0.16–1.38) | 1.12 (1.01–1.23) |
| Breast symptoms | |||
| None (referent) | 1.00 | 1.00 | 1.00 |
| Lump, discharge, or other | 1.46 (1.35–1.58) | 0.99 (0.50–2.13) | 0.70 (0.65–0.76) |
| Family history of breast cancer | |||
| No (referent) | 1.00 | 1.00 | 1.00 |
| Yes | 1.06 (1.01–1.12) | 1.20 (0.69–2.14) | 0.96 (0.91–1.01) |
| Breast biopsy or surgery | |||
| No (referent) | 1.00 | 1.00 | 1.00 |
| Yes | 1.29 (1.23–1.35) | 1.35 (0.80–2.35) | 0.78 (0.74–0.82) |
| Time since last mammogram | |||
| No previous mammogram (referent) | 1.00 | 1.00 | 1.00 |
| Within 2 y | 0.50 (0.47–0.54) | 0.73 (0.21–2.04) | 1.99 (1.85–2.15) |
| 3–4 y | 0.69 (0.63–0.75) | 0.54 (0.14–1.80) | 1.44 (1.31–1.58) |
| ≥5 y | 0.84 (0.76–0.92) | 3.95 (0.53–80.57) | 1.19 (1.08–1.31) |
Abbreviation: HT, hormone therapy.
Values in boldface are statistically significant.
As BMI increased, the adjusted OR for specificity also decreased significantly (Table 4). In other words, overweight women had approximately a 14% increased risk, and obese women had a more than 20% increased risk of having a false-positive mammogram result compared with underweight and normal weight women. Overweight and obese women were equally likely to have a recommendation for surgical evaluation and/or biopsy compared with underweight and normal weight women. No statistically significant difference was noted between mammograms of women classified as underweight (n=1595) compared with those classified as normal weight (n=40755), when looking at recall rate, sensitivity, and specificity.
No interaction was noted between BMI and breast density or BMI and age, but a significant interaction was noted between BMI and menopausal or HT status (P =.003). Data was therefore reviewed for the subgroup of screening mammography examinations obtained on postmenopausal women restricted to age 55 to 89 years at the time of mammography (n=49863); an interaction between HT status and BMI was included in the model. Similar statistically significant trends were noted between BMI and recall rate and BMI and specificity, most noticeable for women not undergoing HT (data not shown). In this subgroup analysis, women not using HT had a slightly higher likelihood of recall when compared with HT users within the same BMI category. Sensitivity was not examined for this subgroup due to small sample size.
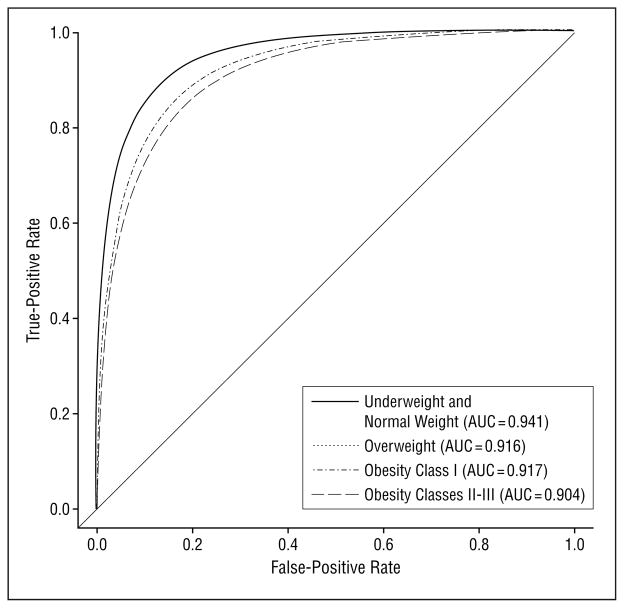
The ROC analysis was adjusted for the same covariates included in the multivariate analyses. The adjusted AUC was inversely associated with BMI and ranged from 0.941 for women in the underweight or normal weight category to 0.904 for women in obesity classes II to III (Figure 2). Statistically significant pairwise comparisons were noted between underweight or normal weight vs overweight and underweight or normal weight vs obesity classes II to III. Although mammograms from the same woman are not statistically independent, adjustment for this correlation using general estimating equation methods showed no impact on the standard errors. Similar results were also obtained if just 1 mammogram was randomly selected per woman (analyses not shown).
Figure 2.
Receiver operating characteristic curves for body mass index adjusted for clinical covariates, including age, menopausal or hormone therapy, breast density, time since last mammogram (N=100622 screening mammogram encounters; n=67989 women). AUC indicates area under the curve.
COMMENT
A woman’s weight may influence the accuracy of screening mammography in several important ways. Obese women had more than a 20% increased risk of having a false-positive mammogram result compared with underweight and normal weight women. We did not find statistically significant improvements in sensitivity in obese women to counter this increase in false-positive rates. Understanding the quality of mammography among obese women is important, especially since the American population is becoming more obese11,12 and obesity is a modifiable risk factor.13–15
Although the difference noted in false-positive rates between underweight or normal weight women and obese women was small, this difference is extremely important at a population level. When screening 10 million obese women, a false-positive rate increase of 2% (eg, from 10% to 12%) would lead to approximately 200000 additional women with false-positive mammography examination results. An additional $120 million would be required to evaluate the false-positive examination results, at an estimated cost of $600 per false-positive mammography examination result.16 We cannot put a quantitative value on the resultant anxiety these 200000 women would additionally experience.
Our findings that obese women were more likely to have fatty breast tissue than nonobese women are consistent with others.17 In general, mammograms of women with fatty breast tissue are easier to interpret than mammograms in women with dense breast tissue. Because of this, one would expect obese women to have more accurate mammograms than nonobese women; therefore, our finding that obese women have less accurate mammograms is surprising. The higher false-positive rates noted in obese women may be a result of poor image clarity. Obese women might have a thicker volume of breast tissue compressed between the mammography plates, which can lead to more scattered radiation and decreased image contrast and quality.18,19 Larger breast size in obese women may also increase the search area that radiologists have to review. Currently in the United States, the maximum size of plates is restricted20; therefore, additional images may be needed to adequately cover the entire breast in each projection for obese women.
Our definition of a positive mammogram result did not include technical recalls (ie, screening views repeated by radiologic technologists due to poor positioning, patient motion, or inadequate image quality). We suspect that technical recalls also may be more common in obese women.
Our findings are consistent with those reported by Hunt and Sickles,21 who found that women with higher adiposity had increased screening mammography recall rates and increased breast cancer size and stage. Elmore et al22 recently noted an increase in false-positive screening mammography rates among overweight and obese women who were screened between 1985 and 1993. In the current study, we provide additional information compared with these earlier results, because we included more recent mammogram films from a larger population-based setting and performed multivariable analyses, adjusting for prospectively gathered important covariates. We also performed ROC analyses to see how BMI affects sensitivity and specificity simultaneously.
Obese women in our study were less likely to have had recent screening mammography than nonobese women. Studies23–25 have shown that obese women delay or avoid breast cancer screening more so than non-obese women. The delay in obtaining preventive care may result in later stage disease at breast cancer diagnosis among women who detect their own cancer.2 Because clinical examination may be less accurate in women with large breasts and obese postmenopausal women are at increased risk of breast cancer, obese women should be encouraged to obtain screening mammograms even though they may have a higher risk of having a false-positive examination result.
Our study population should be considered when interpreting our findings. First, the study was conducted in the urban Northwest, where the population is mostly white, so our findings may not be generalizable to populations with different geographic or demographic backgrounds. In addition, the breast cancer screening program studied specifically encourages women aged 40 to 49 years who have elevated breast cancer risk to be screened, so high-risk women are likely overrepresented in this age group. However, our findings persisted in subgroup analysis of data on just premenopausal women. The self-reported measures of height and weight may not be as accurate as heights and weights taken from other sources, such as the medical record. Finally, even though our study included 67984 women, the small number of women diagnosed as having breast cancer (n=702) limits evaluation of changes in sensitivity for different BMI categories.
Despite these limitations, this population provides many unique strengths, including long-term, prospectively collected data from a large group of women. In addition, information on important covariates and complete ascertainment of subsequent breast cancer status for every woman are available. In this closed health care system, women are more likely to have films available for comparison, and there should be no differential in film availability by BMI category. Finally, additional screening views obtained by technologists to improve image quality are not included in this analysis but may also be more frequent in obese women.
From a screening program perspective, the distribution of BMI categories in our study is similar to patterns seen in the United States.26 Our study is also comparable to others on the proportion of ductal carcinoma in situ and invasive cancer detected. Eighteen percent of the cancers detected were ductal carcinoma in situ in our study, whereas US population–based screening studies27,28 report a range of 18% to 21%. The proportion of large (>15 mm) tumors detected in our study was slightly lower (31%) compared with similar studies,28–30 where the proportions ranged from 40% to 45%.
In conclusion, obese women had less accurate results on screening mammograms compared with non-obese women. Obese women had more than a 20% increased risk of having a false-positive mammogram result compared with underweight and normal weight women. No significant differences were noted in sensitivity (the true-positive rates in cancer detection). The current technique of screening mammography may not be optimized in obese women. The accuracy of newer imaging modalities (digital mammography, computer-assisted diagnostic methods, ultrasonography, and magnetic resonance imaging) should be assessed by BMI category. To reduce the potential for anxiety, obese women should be informed of the increased likelihood that they may need additional imaging or other diagnostic procedures following their screening mammograms to rule out possible breast cancer. Weight modification may influence the accuracy of mammography in detecting breast cancer and deserves study. In addition, the creation of plates that reduce the number of images that have to be taken for women with large size breasts should be investigated. Also, more research is needed to understand how obesity affects specificity, including the effect of adequate compression for women with large breasts and the effect of scattered radiation. All of these issues could be important to clinical image quality, which has also been shown to affect performance.31
Acknowledgments
This project was supported by Public Health Service grant HS-10591 (Dr Elmore) from the Agency for Health-care Research and Quality and the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and National Cancer Institute Surveillance grant UO1 CA63731 (Dr Taplin).
We appreciate the dedication of participating radiologists and project support staff and the helpful comments of Alan Gelfand, PhD.
Footnotes
The authors have no relevant financial interest in this article.
References
- 1.van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152:514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 2.Reeves MJ, Newcomb PA, Remington PL, Marcus PM, MacKenzie WR. Body mass and breast cancer: relationship between method of detection and stage of disease. Cancer. 1996;77:301–307. doi: 10.1002/(SICI)1097-0142(19960115)77:2<301::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Hoe AL, Mullee MA, Royle GT, Guyer PB, Taylor I. Breast size and prognosis in early breast cancer. Ann R Coll Surg Engl. 1993;75:18–22. [PMC free article] [PubMed] [Google Scholar]
- 4.Carter AP, Thompson RS, Bourdeau RV, Andenes J, Mustin H, Straley H. A clinically effective breast cancer screening program can be cost-effective, too. Prev Med. 1987;16:19–34. doi: 10.1016/0091-7435(87)90003-x. [DOI] [PubMed] [Google Scholar]
- 5.Taplin SH, Mandelson MT, Anderman C, et al. Mammography diffusion and trends in late-stage breast cancer: evaluating outcomes in a population. Cancer Epidemiol Biomarkers Prev. 1997;6:625–631. [PubMed] [Google Scholar]
- 6.Kopans DB, D’Orsi CJ, Adler DD, et al. Breast Imaging Reporting and Data System. 2. Reston, Va: American College of Radiology; 1995. [Google Scholar]
- 7.Ernster VL, Ballard-Barbash R, Barlow WE, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002;94:1546–1554. doi: 10.1093/jnci/94.20.1546. [DOI] [PubMed] [Google Scholar]
- 8.Clinical Guidelines on the Identification, Evaluation and Treatment of Overweight and Obesity in Adults [electronic resource] Bethesda, Md: National Institutes of Health, National Heart, Lung & Blood Institute & North American Association for the Study of Obesity; 2000. [Accessed February 12, 2004]. Available at: http://www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm. [Google Scholar]
- 9.SAS Institute Inc. SAS/STAT User’s Guide, Version 8. Cary, NC: SAS Institute Inc; 1999. [Google Scholar]
- 10.Tosteson AN, Begg CB. A general regression methodology for ROC curve estimation. Med Decis Making. 1988;8:204–215. doi: 10.1177/0272989X8800800309. [DOI] [PubMed] [Google Scholar]
- 11.Walsh JM, Dolan NC, Charney P, Gillock MR, Cramer DA, Kefalides PT. Update in women’s health. Ann Intern Med. 2000;133:808–814. doi: 10.7326/0003-4819-133-10-200011210-00014. [DOI] [PubMed] [Google Scholar]
- 12.Nelson DE, Bland S, Powell-Griner E, et al. State trends in health risk factors and receipt of clinical preventive services among US adults during the 1990s. JAMA. 2002;287:2659–2667. doi: 10.1001/jama.287.20.2659. [DOI] [PubMed] [Google Scholar]
- 13.Walker AR. Breast cancer: can risks really be lessened? Eur J Cancer Prev. 2000;9:223–229. doi: 10.1097/00008469-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Hofer TP, Katz SJ. Healthy behaviors among women in the United States and Ontario: the effect on use of preventive care. Am J Public Health. 1996;86:1755–1759. doi: 10.2105/ajph.86.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayward LM, Nixon C, Jasper MP, et al. The process of restructuring and the treatment of obesity in women. Health Care Women Int. 2000;21:615–630. doi: 10.1080/07399330050151851. [DOI] [PubMed] [Google Scholar]
- 16.Taplin S, Barlow W, Urban N, et al. Stage, age, comorbidity, and direct costs of colon, prostate, and breast cancer care. J Natl Cancer Inst. 1995;87:417–426. doi: 10.1093/jnci/87.6.417. [DOI] [PubMed] [Google Scholar]
- 17.Boyd NF, Lockwood GA, Byng JW, Little LE, Yaffe MJ, Tritchler DL. The relationship of anthropometric measures to radiological features of the breast in pre-menopausal women. Br J Cancer. 1998;78:1233–1238. doi: 10.1038/bjc.1998.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guest AR, Helvie MA, Chan HP, Hadjiiski LM, Bailey JE, Roubidoux MA. Adverse effects of increased body weight on quantitative measures of mammographic image quality. AJR Am J Roentgenol. 2000;175:805–810. doi: 10.2214/ajr.175.3.1750805. [DOI] [PubMed] [Google Scholar]
- 19.Berns EA, Hendrick RE, Cutter GR. Performance comparison of full-field digital mammography to screen-film mammography in a clinical setting. Med Phys. 2002;29:830–834. doi: 10.1118/1.1472497. [DOI] [PubMed] [Google Scholar]
- 20.US Quality Determinants of Mammography Guideline Panel. Quality Determinants of Mammography Guideline Panel. Rockville, Md: US Dept of Health & Human Services, Public Health Service, Agency for Health Care Policy & Research; 1994. [Google Scholar]
- 21.Hunt KA, Sickles EA. Effect of obesity on screening mammography: outcomes analysis of 88,346 consecutive examinations. AJR Am J Roentgenol. 2000;174:1251–1255. doi: 10.2214/ajr.174.5.1741251. [DOI] [PubMed] [Google Scholar]
- 22.Elmore JG, Miglioretti DL, Reisch LM, et al. Screening mammograms by community radiologists: variability in false-positive rates. J Natl Cancer Inst. 2002;94:1373–1380. doi: 10.1093/jnci/94.18.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fontaine KR, Heo M, Allison DB. Body weight and cancer screening among women. J Womens Health Gend Based Med. 2001;10:463–470. doi: 10.1089/152460901300233939. [DOI] [PubMed] [Google Scholar]
- 24.Wee CC, McCarthy EP, Davis RB, Phillips RS. Screening for cervical and breast cancer: is obesity an unrecognized barrier to preventive care? Ann Intern Med. 2000;132:697–704. doi: 10.7326/0003-4819-132-9-200005020-00003. [DOI] [PubMed] [Google Scholar]
- 25.Carney PA, Harwood BG, Weiss JE, Eliassen MS, Goodrich ME. Factors associated with interval adherence to mammography screening in a population-based sample of New Hampshire women. Cancer. 2002;95:219–227. doi: 10.1002/cncr.10681. [DOI] [PubMed] [Google Scholar]
- 26.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of US adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 27.Ernster VL, Ballard-Barbash R, Barlow WE, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002;94:1546–1554. doi: 10.1093/jnci/94.20.1546. [DOI] [PubMed] [Google Scholar]
- 28.Poplack SP, Tosteson AN, Grove MR, Wells WA, Carney PA. Mammography in 53,803 women from the New Hampshire mammography network. Radiology. 2000;217:832–840. doi: 10.1148/radiology.217.3.r00dc33832. [DOI] [PubMed] [Google Scholar]
- 29.Hall IJ, Newman B, Millikan RC, Moorman PG. Body size and breast cancer risk in black women and white women: the Carolina Breast Cancer Study. Am J Epidemiol. 2000;151:754–764. doi: 10.1093/oxfordjournals.aje.a010275. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg RD, Hunt WC, Williamson MR, et al. Effects of age, breast density, ethnicity, and estrogen replacement therapy on screening mammographic sensitivity and cancer stage at diagnosis: review of 183,134 screening mammograms in Albuquerque, New Mexico. Radiology. 1998;209:511–518. doi: 10.1148/radiology.209.2.9807581. [DOI] [PubMed] [Google Scholar]
- 31.Taplin SH, Rutter CM, Finder C, Mandelson MT, Houn F, White E. Screening mammography: clinical image quality and the risk of interval breast cancer. AJR Am J Roentgenol. 2002;178:797–803. doi: 10.2214/ajr.178.4.1780797. [DOI] [PubMed] [Google Scholar]