Abstract
Neuropsychiatric disorders involve dysfunction of the prefrontal cortex (PFC), a highly evolved brain region that mediates executive functioning. The dorsolateral PFC is specialized for regulating attention and behavior, while the ventromedial PFC is specialized for regulating emotion. These abilities arise from PFC pyramidal cell networks that excite each other to maintain goals and rules `in mind'. Imaging studies have shown reduced PFC gray matter, weaker PFC connections and altered PFC function in patients with attention-deficit/hyperactivity disorder. Thus, medications that strengthen PFC network connections may be particularly useful for the treatment of attention-deficit/hyperactivity disorder and related disorders. Recent data show that compounds such as guanfacine can enhance PFC function by stimulating postsynaptic α-2A receptors on the dendritic spines of PFC pyramidal cells where networks interconnect. Stimulation of these receptors inhibits cAMP signaling, thus closing potassium channels and strengthening physiological connections. These actions may benefit patients with weak PFC function.
Keywords: α-2A adrenoceptors, attention-deficit/hyperactivity disorder, benzedrine, cAMP signaling, clonidine, dopamine, guanfacine, norepinephrine, prefrontal cortex, stimulants
The symptoms of attention-deficit/hyper-activity disorder (ADHD) are characterized by inattention and hyperactivity/impulsivity [1]. Patients can have either a combined type, or predominantly inattentive or predominantly hyperactive/impulsive types. Symptoms of inattention often include difficulties in sustaining attention, problems in ignoring distractions from either internal or external interference, difficulties in organization and planning and forgetfulness. Symptoms of hyperactivity/impulsivity often include impatience (e.g., difficulty waiting one's turn), poor impulse control and locomotor hyperactivity that can evolve into fidgety behavior as the patient grows older. These symptoms can disrupt relationships, impair performance in school and impede success in the workplace. They are particularly problematic in the `information age', when proper behavior must be steered through a sea of constant distractions. Thus, treating ADHD symptoms has become an increasing focus.
Neurobiological bases of ADHD
Neuroimaging and genetic studies have begun to provide a picture of the biological bases of ADHD (most of these studies have been performed on patients with the combined profile). ADHD is highly heritable [2], and likely can arise from a wide variety of insults that compromise the functioning of the prefrontal cortex (PFC) and/or its connections with the basal ganglia and cerebellum.
The symptoms of ADHD resemble those observed with lesions to the PFC, a highly evolved brain region that is critical for self-control [3–5]. In humans, the PFC in the right hemisphere is specialized for inhibition of inappropriate behavioral and emotional responses. Thus, patients with lesions to the right PFC have poor sustained attention [6], weaker ability to gate sensory input [7,8] and weak impulse control [9], similar to that seen in ADHD. PFC lesions also impair divided and focused attention [10], as well as the ability to shift attention from one sensory dimension (e.g., color) to another (e.g., shape) [11]. Lesions to the right orbital PFC produce disinhibited emotional responses and inappropriate social behaviors, including a blunted response to punishment, especially when they occur early in life [12].
Both functional and structural imaging studies have shown evidence of weaker PFC circuits in subjects with ADHD compared with age-matched control subjects. These changes have been observed in the PFC itself, and in cortical and subcortical regions that are tightly interconnected with the PFC. A variety of studies have shown weaker PFC functional activity in PFC circuits of ADHD subjects performing tasks that require regulation of attention and behavior [13], with hypoactivity particularly prominent in the right hemisphere [14–16]. Structural studies have shown reduced right PFC volume and cortical thickness, as well as reductions in the parieto-temporal regions, the cerebellum and sometimes the caudate [17–20]. Both functional and structural changes are beginning to be related to genotype (e.g., [21,22]). Diffusion tensor imaging and functional connectivity studies have demonstrated evidence of weaker connectivity between PFC and other brain regions [23–25]. Importantly, longitudinal imaging studies have demonstrated slowed maturation of the PFC and related regions [26]. Thus, genetic or environmental insults that slow or impair the development of PFC circuits may manifest in ADHD symptoms. ADHD symptoms may also arise from genetic insults in the neuromodulatory pathways that regulate PFC circuits [2].
Prefrontal cortex regulates attention, behavior & emotion using `top-down' control
The PFC thoughtfully guides attention, movement and emotion through extensive connections to the posterior cortices and to sub-cortical structures (summarized in Figure 1). The dorsal and lateral surfaces of the PFC use representational knowledge to guide overt responses (movement), as well as covert responses (attention), allowing us to inhibit inappropriate behaviors and to attenuate the processing of irrelevant stimuli (reviewed in [9,27–29]). This is accomplished through extensive interconnections with the sensory and motor association cortices, the basal ganglia and the cerebellum. By contrast, the ventromedial PFC performs these regulatory functions in the emotional domain, allowing flexible and appropriate emotional responses [12,30–32]. This is accomplished through connections with structures such as the amygdala, ventral striatum, hypothalamus and brainstem. The anterior cingulate can be considered part of the PFC, as these regions are intimately interconnected anatomically and functionally. The anterior cingulate detects errors and regulates movement, cognition and emotion, in a caudal to rostral organization [33].
Figure 1. The prefrontal cortex (PFC) is regionally specialized, with the dorsolateral PFC regulating attention, the inferior lateral PFC in the right hemisphere being specialized for inhibition of inappropriate actions, and the ventromedial PFC allowing flexible and appropriate emotional responding.
This `top-down' regulation is carried out by extensive PFC projections to posterior cortical sensory cortices and to subcortical regions. Parallel loops through the basal ganglia and cerebellum are key for the planning and execution of appropriate movements, thoughts and emotions.
Electrophysiological studies in monkeys have confirmed a key role for PFC in attention regulation and executive function. PFC neurons are able to hold modality-specific information `on-line' over a delay and use this information to guide behavior in the absence of environmental cues [34,35]. In contrast to inferior temporal neurons that stop firing when distracted by a new visual stimulus, PFC neurons can maintain delay-related activity in the presence of distracting stimuli [36]. Persistent neuronal firing also underlies behavioral inhibition, for example, when monkeys must look away from a remembered visual stimulus [37]. Inhibitory signals can also be recorded during the stop-signal task, similar to the task used in humans [38]. Electrophysiological studies have demonstrated that PFC neurons are essential for `top-down' attention, thoughtfully regulating stimulus processing in sensory cortices [39], and for high order decision-making [40] and abstraction [41]. By contrast, orbital PFC neurons fire in relationship to affective information regarding reward [42]. Thus, there is an excellent correspondence between single unit physiology in monkeys and findings from human imaging and lesion studies.
Anatomical and physiological studies in monkeys have revealed that PFC cognitive functions arise from networks of neurons that can process information, even in the absence of environmental stimulation. The PFC is able to represent information that is not currently in the environment through networks of pyramidal neurons that excite each other to maintain information `in mind'. This process has been referred to as representational knowledge, working memory or our `mental sketch-pad', and is thought to be a fundamental component of abstract thought [43]. Information such as a rule or goal is held temporarily in working memory and used to guide behavior, attention or emotions, dependent on the PFC region(s) involved. The circuitry underlying representational knowledge in PFC has been most intensively studied in the visuo-spatial realm. In primates, visuo-spatial information is processed by the parietal association cortices, and fed forward to the dorsolateral PFC, where pyramidal cells excite each other to maintain information briefly in memory. Network activity is `tuned' by inhibitory GABAergic interneurons so that the contents of working memory are specific and informative. As demonstrated in Figure 2, pyramidal cell networks interconnect on dendritic spines, exciting each other via postsynaptic NMDA receptors that pass both sodium and calcium [44]. As described below, these network connections depend on the proper neurochemical state.
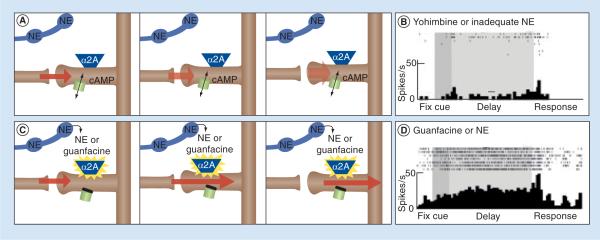
Figure 2. A working model of norepinephrine actions at postsynaptic α-2A receptors in the prefrontal cortex.
(A) Prefrontal cortex (PFC) pyramidal cell networks interconnect on dendritic spines, exciting each other to maintain information, such as goals and rules, in working memory. Recent data have shown that cAMP signaling can weaken these connections by opening potassium channels on spines, which `shunt' network inputs by hyperpolarizing the spine. (B) An example of PFC neuronal firing in a monkey performing a working memory task. The top shows action potentials on seven different trials as the monkey fixates to do the trial, sees a visuospatial cue, remembers the spatial position over a short delay period and then responds to the location where the cue had been. The bottom raster shows the summed response to all seven trials. This neuron shows very little firing during the delay period due to the application of the α-2 receptor antagonist, yohimbine. A similar reduction in neuronal firing during the delay period is observed with the application of other drugs that increase or mimic cAMP signaling, for example, agents that mimic cAMP or reduce cAMP catabolism. The work of Bao-Ming Li has shown that infusion of yohimbine into the monkey PFC impairs working memory, induces locomotor hyperactivity, and weakens response inhibition on a Go/No-Go task (see text). It is possible that individuals with attention-deficit/hyperactivity disorder symptoms arising from reduced norepinephrine production also have weaker PFC regulation of behavior due to reduced PFC network firing. (C) By contrast, α-2A agonists, such as guanfacine, can enhance the physiological strength of PFC network connections by stimulating postsynaptic α-2A receptors. This reduces cAMP signaling by activating G proteins (Gi), which inhibit adenylyl cyclase, the enzyme that generates cAMP. The reduction in cAMP closes the potassium channels, and depolarizes the spine. This strengthens network connectivity. (D) Stimulation of α-2A receptors in the monkey PFC increases neuronal firing during the delay period when the animal is using working memory to remember a spatial location. Guanfacine has been shown to enhance a variety of PFC functions in rodents, monkeys and humans.
NE: Norepinephrine Adapted from [49].
Prefrontal cortical networks require optimal catecholamine signaling
Neuroscience has made great leaps in the last 30 years, and we have begun to understand the molecular needs of the PFC and its cortical and subcortical targets (reviewed in [45]). Although we still know very little regarding the neurochemical needs of the posterior cortices, the landmark research of Goldman-Rakic et al. first revolutionized our understanding of how neurochemical state alters higher cortical function [46]. This work demonstrated that the catecholamines dopamine (DA) and norepinephrine (NE) were essential for PFC function, and that extensive depletion of DA and NE from the PFC was as detrimental as removing the PFC itself [46]. Although the first papers focused on DA, later work has demonstrated that NE also has critical beneficial actions through actions at postsynaptic α-2 receptors on PFC dendritic spines [47–49].
Norepinephrine acts at a variety of receptors; it has highest affinity for the α-2 receptor family, and lower affinity for α-1 and β receptors (reviewed in [50]). Three α-2 receptor subtypes have been cloned in humans: A, B and C [51]. The A and C subtypes are found in both PFC and on NE cell bodies in the locus coeruleus, as well as many other brain areas, whereas the B subtype has a more limited distribution in the brain [51]. However, there are high levels of α-2B receptors in the thalamus [52], a brain region where α-2 receptor stimulation can switch the brain into a sleep state [53]. The sedating effects of α-2 agonists also probably involve actions at presynaptic α-2A receptors, reducing NE cell firing and release, which in turn alter arousal mechanisms in the basal forebrain [54] and hypothalamus [55]. Although most research has focused on the negative feedback provided by presynaptic α-2 receptors, the majority of α-2 receptors in the brain are actually localized postsynaptic to NE cells [56], and it is these receptors that are key to PFC function.
Pharmacological profiles [57] and studies in genetically altered mice [58] have confirmed that the enhancing effects of NE on PFC working memory functions depend on the α-2A subtype. Thus, the beneficial effects of guanfacine disappear in mice with genetically altered –-2A receptors that cannot bind NE or the drug [58]. The enhancing effects of α-2 agonists involve postsynaptic receptors, as their effects are magnified in animals where the presynaptic element has been destroyed [47] or depleted [59]. Indeed, electron microscopy has identified α-2A receptors on the dendritic spines of pyramidal cells where PFC networks interconnect [49,60]. As described below, NE strengthens PFC network connectivity through actions at these receptors, closing nearby potassium channels and strengthening network `signals' [49]. These actions are engaged under optimal arousal conditions during alert interest, when the NE cell bodies fire in response to relevant environmental events, that is, appropriate, phasic firing [61]. Optimal levels of DA release provide a complementary role, suppressing irrelevant network inputs and, thus, decreasing `noise' [62]. This may occur when DA neurons fire to environmental stimuli predicting reward [63]. Thus, moderate levels of catecholamines enhance PFC function through complementary actions.
By contrast, very high levels of catecholamine release during stress exposure impair PFC function. The amygdala is key for activating NE and DA cell bodies during psychological stress [64], and new data suggest that a separate set of DA neurons fire to aversive events [65]. Under conditions of high NE release, NE engages lower affinity α-1 and β receptors, which suppresses PFC firing and impair cognition [66,67]. Similarly, excessive DA D1 receptor stimulation suppresses PFC neuronal firing via activation of cAMP signaling [62]. Interestingly, the emotional responses of the amygdala are modulated in an opposite fashion: the amygdala is strengthened by high levels of NE and DA engaging β, α-1 and D1 receptors [68,69], while it is weakened by α-2 receptor stimulation [69]. In this way, high levels of catecholamines can switch control of behavior from the thoughtful PFC to the automatic responses of the amygdala during stress exposure, for example, in response to danger [67]. By contrast, moderate levels of NE release during nonstressed arousal engage high-affinity α-2A receptors that weaken the amygdala, but strengthen PFC physiology and function.
Key role of noradrenergic α-2A receptor stimulation in prefrontal cortex
As described above, PFC microcircuits interconnect on dendritic spines, where they excite each other via glutamate release onto NMDA receptors [44]. These synaptic connections appear to be the target of many neuromodulatory systems, which can dynamically increase or decrease the strength of connections to coordinate cognitive capacity with arousal state [44]. For example, intracellular calcium and cAMP signaling can open potassium channels to rapidly weaken a network connection and reduce cell firing (Figure 2, top graphs) [49,70,71]. α-2A receptors are localized next to these potassium channels on spines, and α-2A agonists, such as guanfacine, inhibit cAMP signaling, close these potassium channels and strengthen PFC connectivity (Figure 2, bottom graphs) [49]. These enhancing effects have now been observed in rodents [72], monkeys [73,74] and humans [75], and have been observed as enhanced PFC activity in imaging studies of monkeys [76] and humans [77,78]. Importantly, the α-2A agonist guanfacine can enhance both dorsolateral PFC function (which regulates attention and action [79]) and ventromedial PFC function (which regulates emotion [80]). Thus, guanfacine can help animals or patients to overcome distractions and inhibit inappropriate behaviors and emotions (Table 1).
Table 1.
α-2A adrenergic influences on prefrontal cortical functions.
| Prefrontal cortex operation | Ref. |
|---|---|
| Improve working memory | [48,57,73,75,145,146] |
|
| |
| Attention regulation: | |
| • Reduce distractibility | [74,147] |
| • Improve sustained attention | [117] |
| Strengthen behavioral inhibition | |
| • Cognitive: | |
| – Stroop interference | [148] |
| – No-go performance | [82] |
| • Emotional: | |
| – Reward reversal | [80] |
|
| |
| High-order associative learning | [149] |
|
| |
| Planning and organization | [75] |
Conversely, blockade of α-2 receptors in the PFC markedly weakens PFC network firing and impairs PFC cognitive abilities. For example, the application of yohimbine directly onto PFC neurons in monkeys performing a working memory task causes a collapse in network firing [49,81]. PFC neuronal firing is restored by the concurrent blockade of potassium channels in PFC, consistent with endogenous NE inhibiting cAMP actions at these channels [49]. Importantly, infusions of yohimbine into the monkey PFC markedly impaired working memory performance [48], weakened impulse control [82] and induced locomotor hyperactivity [83]. Thus, many of the symptoms of ADHD can be recreated by blocking α-2 receptors in the monkey PFC.
Inadequate NE stimulation of α-2 receptors in humans also produces ADHD symptoms, for example, due to genetic insults in either dopamine β hydroxylase (DBH) – the synthetic enzyme for NE – or in the α-2A receptor itself. A polymorphism in the promoter region of the gene encoding for DBH leads to inadequate levels of the DBH enzyme and reduced levels of NE synthesis [84]. This genetic insult is associated with ADHD [85–87], including poor sustained attention [88], weaker impulse control [89] and impaired performance on PFC tests of executive function [90]. Genetic alterations in the α-2A adrenergic receptor have also been associated with ADHD [85,91–93] and impaired PFC executive function [94]. Thus, NE stimulation of α-2A receptors is needed for strong PFC regulation of attention and behavior in both animals and humans.
α-2A agonists in the treatment of ADHD & other PFC disorders
The use of α-2 agonists to treat ADHD began with clonidine [95], based on its success in treating the symptoms of Tourette's syndrome [96]. Clonidine has very potent presynaptic actions, reducing NE release and inhibiting the firing of NE locus coeruleus neurons [97]. For example, clonidine reduced excessive NE firing and release in hyperactive rats going through opiate withdrawal [98]. The similarly disinhibited behaviors of opiate-withdrawing rats and patients with uncontrolled tics led Aghajanian and Cohen to try clonidine in patients with Tourette's syndrome [99]. Given this background, it was presumed that clonidine had similar therapeutic actions in Tourette's and ADHD, quieting an overactive NE system through presynaptic actions, and thus calming behavior of `over-aroused' children [100].
It is now understood that the disinhibited behaviors of ADHD do not arise from NE overactivity, but from insults to PFC circuits and/or neuromodulators. Indeed, ADHD is associated with reduced NE synthesis (as described above, e.g., [88,90]). Current research is showing that both stimulant and nonstimulant ADHD medications can enhance the function of the PFC by increasing NE, as well as DA release in the PFC [101,102]. Both methyl phenidate and atomoxetine can improve PFC function in humans [103–105] and in animals [106], and these enhancing effects in animals can be blocked by either an NE α-2 or a DA D1 antagonist [107,108]. The importance of DA and D1 mechanisms in the stimulant response has been appreciated for many years [109], and recent studies are examining the relationship between stimulant response DA transporter genotype and surface expression (e.g., [110]). The recognition of important NE actions is more recent [102], and it is of interest that methylphenidate's therapeutic effects in ADHD are also influenced by the α-2A receptor genotype [111].
Guanfacine is an α-2 agonist with relatively higher affinity for the α-2A subtype than the B and C receptor subtypes [112–114]. Guanfacine has been marketed as an antihypertensive for more than 20 years [115]. However, it has weaker hypertensive and sedative actions than clonidine, but is more potent in enhancing PFC function [57].
Based on findings that guanfacine improved PFC functions and calmed behavior in monkeys with few side effects [4], guanfacine was tested in patients with ADHD first in open-label [116] and then in placebo-controlled [117] trials. Guanfacine is often used in combination with stimulants, as the therapeutic effects of these agents appear to be additive, while the side effects (e.g., sedation vs over-arousal) can cancel each other out [118]. An extended release formulation of guanfacine, Intuniv™ (Shire Plc, Dublin, Ireland), was recently approved by the US FDA in September 2009. When analyzed on a mg/kg basis, the higher doses had a large effect size (0.89–1.34), and improved symptom ratings on both the inattention and impulsivity/hyperactivity rating scales [119,120].
α-2A agonists, such as guanfacine, have a number of advantages for patients who cannot tolerate stimulant medications due to their worsening of comorbid symptoms (e.g., aggression or anxiety) or because of the potential for drug abuse [121]. For example, stimulants can exacerbate or induce tic disorders, while α-2A agonists reduce these symptoms [117]. Similarly, guanfacine can reverse stress-induced PFC deficits in animals [122], and thus may be more helpful in anxious patients or those with post-traumatic stress disorder. Stimulants can also aggravate symptoms of mania and psychosis; thus, guanfacine may be a safer treatment for children tentatively diagnosed with ADHD who actually have emerging bipolar disorder or schizophrenia. Given its beneficial effects on orbital PFC function, guanfacine may also help patients with oppositional defiant or conduct disorder who have underactive orbital PFC function [123]. Guanfacine may also be useful in helping patients overcome harmful, emotional habits, such as drug addiction [124], particularly in response to stress [125]. Guanfacine is also being tested in children with autism spectrum disorders for reduction of aberrant behaviors [126,127]. Guanfacine is also being tested in adult PFC cognitive disorders, and has been found to be helpful in patients recovering from parietal cortex strokes [128] or encephalitis [129], where strengthened PFC abilities are thought to facilitate attention and cognition. Guanfacine is also being tested in normal elderly subjects, as PFC cognitive abilities decline early in the aging process in both monkeys [130] and humans [131].
The side effects of α-2 agonists remain problematic for some patients. Although the sedative effects of guanfacine diminish with chronic use [119], there are some patients that appear to be particularly sensitive to these actions, (possibly those with cortical lesions or impaired cortical development? [126]). The therapeutic effects of these agents may be additive, but further work will be required to substantiate the preliminary finding of Spencer et al. [118]. Bradycardia can also be a concern with α-2 agonists [132,133], and syncope may be related to hypotensive drug actions [119,134]. Guanfacine has a lower affinity for imidazoline receptors than clonidine, which may contribute to a gentler cardiovascular profile [113,135].
Research is just beginning to look at the relationship between genotype and the efficacy of guanfacine actions. For example, McCracken and colleagues have recently found that guanfacine's effects in children with pervasive developmental disorder are influenced by variants of the P-glycoprotein gene (MDR1/ABCB1), a molecule that regulates drug entry into the brain [127]. As discussed below, correlating drug response and genotype will be an important area for future research.
In summary, α-2A adrenergic agonists, such as guanfacine, may be particularly helpful in patients with symptoms of PFC dysfunction, such as poor impulse control, poor attention regulation (e.g., easily distracted), impaired working memory and organization, and impaired regulation of emotion (e.g., impulsive aggression).
Expert commentary
Drug development in the past has often relied on chance observations, for example, the original discovery by Bradley in 1937 that the stimulant, Benzedrine, was helpful to children with behavioral problems [136]. Since then, most treatments for ADHD have built on this original discovery, trying to develop superior stimulant medications or formulations. However, neuroscientific information has now advanced sufficiently that we have entered a new era where drug development arises from a more rational approach: revealing the neurochemical needs of appropriate brain circuits, understanding their genetic and/or environmental alterations in illness and identifying compounds that may correct for these neurobiological insults. This approach requires a sophisticated, cross-disciplinary understanding of brain anatomy, neuro physiology, neuropharmacology, molecular biology and genetics.
As dysfunction of the PFC is central to most neuro psychiatric disorders (and highly relevant to normal aging), treatments that can improve PFC function are a focus of medication development. Impairments in PFC function are particularly disruptive to relationships, classroom experience and the ability to live independently in this `information age'. Thus, PFC dysfunction has a great cost to individuals, their families and society as a whole. However, drug development for PFC disorders is particularly challenging, as the PFC is often modulated differently from other brain areas (e.g., high levels of cAMP impairs PFC working memory function but improves hippocampal, amygdala and striatal function [137]). Thus, it can be difficult to design therapeutics that improve one cognitive domain without harming another. Furthermore, PFC functions are time-consuming to test in the laboratory, and one cannot do high-throughput screening of a large number of compounds using proper cognitive assays. On the same note, mice have a very small PFC, so it is difficult to detect clinical efficacy in this commonly used species.
Although the development of medications to treat PFC dysfunction is challenging, it is critical for real success in treating neuropsychiatric disorders. Treatment of childhood disorders is especially important, as there may be opportunities to rescue the developing brain and shape the vector of a productive life. As the PFC is the latest to mature (maturation continues into the third decade [138]), this critical time window continues into young adulthood.
Five-year view
We are at a revolutionary time in neuropsychiatry, where we are beginning to understand the genetics of neuro psychiatric symptoms, and how these arise from molecular insults in PFC circuits. Although this process is still in its early days, it is hoped that within the next 5 years we will be better able to match symptoms with afflicted circuit(s), and thus choose medications based on a more etiological basis. For example, the α-2A adrenergic receptor agonist guanfacine may be particularly helpful for patients with genetic insults to DBH, who have inadequate endogenous NE stimulation of α-2A receptors. Guanfacine might also help families with insults to Disrupted in Schizophrenia (DISC1), as both α-2A receptors and DISC1 may inhibit cAMP signaling in PFC dendritic spines [49,139–141]. It is hoped that an increased availability in genetic screening over the next 5 years will allow more widespread study of these types of targeted treatments.
Although medications for ADHD have heretofore focused on catecholamine compounds, it is likely that new knowledge of PFC neuromodulatory needs will lead to additional mechanisms that can strengthen PFC function. For example, the orexins and nicotine excite PFC physiology and may be helpful for some types of patients [142]. Nicotinic α7 agonists may have particular promise if they are able to strengthen PFC functions [44] without addictive, subcortical actions [143]. There is also emerging knowledge regarding serotonergic influences, which are particularly important for orbital PFC functions [144]. The large number of serotonin receptors has constrained advances in this field, but it is an important arena for future research.
It is hoped that the emerging knowledge of α-2 adrenoceptor influences on PFC networks may provide an example of how we can bridge basic neuroscience knowledge with informed treatment of neuropsychiatric symptoms.
Key issues
Attention-deficit/hyperactivity disorder (ADHD) symptoms involve weakened prefrontal cortex (PFC) circuitry.
Medications that strengthen PFC connections can be useful for the treatment of ADHD.
Stimulation of postsynaptic α-2A receptors on PFC dendritic spines strengthens PFC network connections by inhibiting cAMP and closing ion channels that hyperpolarize the spine and reduce network firing.
Guanfacine improves both dorsolateral and ventromedial PFC function in animals, and is thus able to enhance regulation of attention, behavior and emotion.
α-2A agonists are generally safe, but the sedative side effects can be problematic for some children.
Understanding important neuromodulatory influences on PFC may lead to new treatment strategies for ADHD and related disorders.
Acknowledgments
Amy Arnsten and Yale University receive royalties from Shire Pharmaceuticals from the sales of extended-release guanfacine (Intuniv™) for the treatment of ADHD and related disorders. Dr Arnsten consults and engages in teaching for Shire, and receives research funding from Shire for the study of catecholamine mechanisms in the prefrontal cortex.
Footnotes
Financial & competing interests disclosure The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
apers of special note have been highlighted as: • of interest •• of considerable interest
- 1.Barkley RA. ADHD And The Nature Of Self-Control. Guilford Press; NY, USA: 1997. [Google Scholar]
- 2.Faraone SV, Perlis RH, Doyle AE, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 3.Barkley RA, Grodzinsky G, DuPaul GJ. Frontal lobe functions in attention deficit disorder with and without hyperactivity: a review and research report. J. Abnorm. Child Psych. 1992;20:163–188. doi: 10.1007/BF00916547. [DOI] [PubMed] [Google Scholar]
- 4.Arnsten AF, Steere JC, Hunt RD. The contribution of α-2 noradrenergic mechanisms to prefrontal cortical cognitive function: potential significance to attention deficit hyperactivity disorder. Arch. Gen. Psychiatry. 1996;53:448–455. doi: 10.1001/archpsyc.1996.01830050084013. [DOI] [PubMed] [Google Scholar]
- 5.McLean A, Dowson J, Toone B, et al. Characteristic neurocognitive profile associated with adult attention-deficit/hyperactivity disorder. Psychol. Med. 2004;34:681–692. doi: 10.1017/S0033291703001296. [DOI] [PubMed] [Google Scholar]
- 6.Wilkins AJ, Shallice T, McCarthy R. Frontal lesions and sustained attention. Neuropsychologia. 1987;25:359–365. doi: 10.1016/0028-3932(87)90024-8. [DOI] [PubMed] [Google Scholar]
- 7.Woods DL, Knight RT. Electrophysiological evidence of increased distractability after dorsolateral prefrontal lesions. Neurology. 1986;36:212–216. doi: 10.1212/wnl.36.2.212. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi S, Knight RT. Gating of somatosensory input by human prefrontal cortex. Brain Res. 1990;521:281–288. doi: 10.1016/0006-8993(90)91553-s. [DOI] [PubMed] [Google Scholar]
- 9.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]; •• Excellent review on the neural basis of inhibitory control of behavior.
- 10.Godefroy O, Rousseaux M. Divided and focused attention in patients with lesion of the prefrontal cortex. Brain Cogn. 1996;30:155–174. doi: 10.1006/brcg.1996.0010. [DOI] [PubMed] [Google Scholar]
- 11.Manes F, Sahakian BJ, Clark L, et al. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- 12.Anderson SW, Bechara A, Damasio H, et al. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat. Neurosci. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- 13.Dickstein SG, Bannon K, Castellanos FX, et al. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J. Child Psychol. Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 14.Rubia K, Smith AB, Brammer MJ, et al. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am. J. Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- 15.Bush G, Valera EM, Seidman LJ. Functional neuroimaging of attention-deficit/hyperactivity disorder: a review and suggested future directions. Biol. Psychiatry. 2005;57:1273–1284. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 16.Smith AB, Taylor E, Brammer M, et al. Task-specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication-naive children and adolescents with attention deficit hyperactivity disorder. Am. J. Psychiatry. 2006;163:1044–1051. doi: 10.1176/ajp.2006.163.6.1044. [DOI] [PubMed] [Google Scholar]
- 17.Castellanos FX, Lee PP, Sharp W, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 18.Makris N, Biederman J, Valera EM, et al. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex. 2007;17:1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- 19.Seidman LJ, Valera EM, Makris N, et al. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol. Psychiatry. 2006;60:1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 20.Valera EM, Faraone SV, Murray KE, et al. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2007;15:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Brown AB, Biederman J, Valera EM, et al. Effect of dopamine transporter gene (SLC6A3) variation on dorsal anterior cingulate function in attention-deficit/hyperactivity disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2010;153B:365–375. doi: 10.1002/ajmg.b.31022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durston S, Fossella JA, Casey BJ, et al. Differential effects of DRD4 and DAT1 genotype on fronto-striatal gray matter volumes in a sample of subjects with attention deficit hyperactivity disorder, their unaffected siblings, and controls. Mol. Psychiatry. 2005;10:678–685. doi: 10.1038/sj.mp.4001649. [DOI] [PubMed] [Google Scholar]
- 23.Casey BJ, Epstein JN, Buhle J, et al. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. Am. J. Psychiatry. 2007;164:1729–1736. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- 24.Castellanos FX, Margulies DS, Kelly C, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makris N, Buka SL, Biederman J, et al. Attention and executive systems abnormalities in adults with childhood ADHD: a DT-MRI study of connections. Cereb. Cortex. 2008;18:1210. doi: 10.1093/cercor/bhm156. [DOI] [PubMed] [Google Scholar]
- 26.Shaw P, Eckstrand K, Sharp W, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl Acad. Sci. USA. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knight RT, Grabowecky MF, Scabini D. Role of human prefrontal cortex in attention control. Adv. Neurol. 1995;66:21–34. [PubMed] [Google Scholar]
- 28.Robbins TW. Dissociating executive functions of the prefrontal cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996;351:1463–1471. doi: 10.1098/rstb.1996.0131. [DOI] [PubMed] [Google Scholar]
- 29.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res. Brain Res. Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 30.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 31.Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from `on-line' processing. J. Neurosci. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rolls ET. The orbitofrontal cortex and reward. Cereb. Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- 33.van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol. Behav. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- 34.Fuster JM. Unit activity in prefrontal cortex during delayed response performance: Neuronal correlates of transient memory. J. Neurophysiol. 1973;36:61–78. doi: 10.1152/jn.1973.36.1.61. [DOI] [PubMed] [Google Scholar]; •• One of the first studies to discover persistent firing of prefrontal cortex (PFC) neurons during the delay period of a working memory task.
- 35.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J. Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 36.Miller EK, Erickson CA, Desimone R, et al. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J. Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature. 1993;365:753–756. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- 38.Boucher L, Palmeri TJ, Logan GD, et al. Inhibitory control in mind and brain: an interactive race model of countermanding saccades. Psychol. Rev. 2007;114:376–397. doi: 10.1037/0033-295X.114.2.376. [DOI] [PubMed] [Google Scholar]
- 39.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 40.Barraclough DJ, Conroy ML, Lee D. Prefrontal cortex and decision making in a mixed-strategy game. Nat. Neurosci. 2004;7:404–410. doi: 10.1038/nn1209. [DOI] [PubMed] [Google Scholar]
- 41.Wallis JD, Anderson KC, Miller EK. Single neurons in prefrontal cortex encode abstract rules. Nature. 2001;411:953–956. doi: 10.1038/35082081. [DOI] [PubMed] [Google Scholar]
- 42.Rolls ET, Critchley HD, Mason R, et al. Orbitofrontal cortex neurons: role in olfactory and visual association learning. J. Neurophysiol. 1996;75:1970–1981. doi: 10.1152/jn.1996.75.5.1970. [DOI] [PubMed] [Google Scholar]
- 43.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]; •• Excellent review of the neural basis of working memory, and the PFC microcircuits subserving our `mental sketch pad'.
- 44.Arnsten AF, Paspalas CD, Gamo NJ, et al. Dynamic Network Connectivity: a new form of neuroplasticity. Trends Cogn. Sci. 2010;14(8):365–375. doi: 10.1016/j.tics.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu. Rev. Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brozoski T, Brown RM, Rosvold HE, et al. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–931. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]; •• First paper to reveal that catecholamines are critical to PFC cognitive functioning.
- 47.Arnsten AF, Goldman-Rakic PS. α-2 adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science. 1985;230:1273–1276. doi: 10.1126/science.2999977. [DOI] [PubMed] [Google Scholar]
- 48.Li B-M, Mei Z-T. Delayed response deficit induced by local injection of the α-2 adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav. Neural Biol. 1994;62:134–139. doi: 10.1016/s0163-1047(05)80034-2. [DOI] [PubMed] [Google Scholar]
- 49.Wang M, Ramos B, Paspalas C, et al. α2A-adrenoceptor stimulation strengthens working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]; •• Revealed the ionic basis of guanfacine's actions.
- 50.Arnsten AF. Through the looking glass: differential noradrenergic modulation of prefrontal cortical function. Neural Plasticity. 2000;7:133–146. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.MacDonald E, Kobilka BK, Scheinin M. Gene targeting – homing in on α-2-adrenoceptor subtype function. Trends Pharmacol. Sci. 1997;18:211–219. doi: 10.1016/s0165-6147(97)01063-8. [DOI] [PubMed] [Google Scholar]
- 52.Winzer-Serhan UH, Leslie FM. α-2B adrenoceptor mRNA expression during rat brain development. Brain Res. Dev. Brain Res. 1997;100:90–100. doi: 10.1016/s0165-3806(97)00035-7. [DOI] [PubMed] [Google Scholar]
- 53.Buzsaki G, Kennedy B, Solt VB, et al. Noradrenergic control of thalamic oscillation: the role of α-2 receptors. Eur. J. Neurosci. 1991;3:222–229. doi: 10.1111/j.1460-9568.1991.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 54.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Brain Res. Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 55.Nelson LE, Lu J, Guo T, et al. The α2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 56.U'Prichard DC, Bechtel WD, Rouot BM, et al. Multiple apparent α-noradrenergic receptor binding sites in rat brain: effect of 6-hydroxydopamine. Mol. Pharmacol. 1979;16:47–60. [PubMed] [Google Scholar]; • Discovered that the majority of α-2 receptors in brain are postsynaptic to norepinephrine (NE) neurons.
- 57.Arnsten AF, Cai JX, Goldman-Rakic PS. The α-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects. J. Neurosci. 1988;8:4287–4298. doi: 10.1523/JNEUROSCI.08-11-04287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Franowicz JS, Kessler L, Dailey-Borja CM, et al. Mutation of the α2A-adrenoceptor impairs working memory performance and annuls cognitive enhancement by guanfacine. J. Neurosci. 2002;22:8771–8777. doi: 10.1523/JNEUROSCI.22-19-08771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cai JX, Ma Y, Xu L, et al. Reserpine impairs spatial working memory performance in monkeys: reversal by the α-2 adrenergic agonist clonidine. Brain Res. 1993;614:191–196. doi: 10.1016/0006-8993(93)91034-p. [DOI] [PubMed] [Google Scholar]; • Demonstrated that clonidine's beneficial effects on working memory were through actions at postsynaptic, not presynaptic, receptors.
- 60.Aoki C, Venkatesan C, Go C-G, et al. Cellular and subcellular sites for noradrenergic action in the monkey dorsolateral prefrontal cortex as revealed by the immunocytochemical localization of noradrenergic receptors and axons. Cereb. Cortex. 1998;8:269–277. doi: 10.1093/cercor/8.3.269. [DOI] [PubMed] [Google Scholar]
- 61.Aston-Jones G, Rajkowski J, Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Prog. Brain Res. 2000;126:165–182. doi: 10.1016/S0079-6123(00)26013-5. [DOI] [PubMed] [Google Scholar]
- 62.Vijayraghavan S, Wang M, Birnbaum SG, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 63.Schultz W. The phasic reward signal of primate dopamine neurons. Adv. Pharmacol. 1998;42:686–690. doi: 10.1016/s1054-3589(08)60841-8. [DOI] [PubMed] [Google Scholar]
- 64.Goldstein LE, Rasmusson AM, Bunney SB, et al. Role of the amygdala in the coordination of behavioral, neuroendocrine and prefrontal cortical monoamine responses to psychological stress in the rat. J. Neurosci. 1996;16:4787–4798. doi: 10.1523/JNEUROSCI.16-15-04787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brischoux F, Chakraborty S, Brierley DI, et al. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl Acad. Sci. USA. 2009;106(12):4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Birnbaum SB, Yuan P, Bloom A, et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- 67.Arnsten AF. Stress signaling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009;32:267–287. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ferry B, Roozendaal B, McGaugh JL. Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between β- and α-1-adrenoceptors. J. Neurosci. 1999;19:5119–5123. doi: 10.1523/JNEUROSCI.19-12-05119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nader K, LeDoux JE. Inhibition of the mesoamygdala dopaminergic pathway impairs the retrieval of conditioned fear associations. Behav. Neurosci. 1999;113:891–901. doi: 10.1037//0735-7044.113.5.891. [DOI] [PubMed] [Google Scholar]
- 70.Faber ES. Functional interplay between NMDA receptors, SK channels and voltage-gated Ca2+ channels regulates synaptic excitability in the medial prefrontal cortex. J. Physiol. 2010;588:1281–1292. doi: 10.1113/jphysiol.2009.185645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hagenston AM, Fitzpatrick JS, Yeckel MF. MGluR-mediated calcium waves that invade the soma regulate firing in layer V medial prefrontal cortical pyramidal neurons. Cereb. Cortex. 2008;18:407–423. doi: 10.1093/cercor/bhm075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramos B, Stark D, Verduzco L, et al. α-2A-adrenoceptor stimulation improves prefrontal cortical regulation of behavior through inhibition of cAMP signaling in aging animals. Learn. Mem. 2006;13:770–776. doi: 10.1101/lm.298006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rama P, Linnankoski I, Tanila H, et al. Medetomidine, atipamezole, and guanfacine in delayed response performance of aged monkeys. Pharmacol. Biochem. Behav. 1996;54:1–7. doi: 10.1016/s0091-3057(96)00111-6. [DOI] [PubMed] [Google Scholar]
- 74.O'Neill J, Fitten LJ, Siembieda DW, et al. Effects of guanfacine on three forms of distraction in the aging macaque. Life Sci. 2000;67:877–885. doi: 10.1016/s0024-3205(00)00681-0. [DOI] [PubMed] [Google Scholar]
- 75.Jakala P, Riekkinen M, Sirvio J, et al. Guanfacine, but not clonidine, improves planning and working memory performance in humans. Neuropsychopharmacology. 1999;20:460–470. doi: 10.1016/S0893-133X(98)00127-4. [DOI] [PubMed] [Google Scholar]
- 76.Avery RA, Franowicz JS, Studholme C, et al. The α-2A-adenoceptor agonist, guanfacine, increases regional cerebral blood flow in dorsolateral prefrontal cortex of monkeys performing a spatial working memory task. Neuropsychopharmacology. 2000;23:240–249. doi: 10.1016/S0893-133X(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 77.Swartz BE, Kovalik E, Thomas K, et al. The effects of an α-2 adrenergic agonist, guanfacine, on rCBF in human cortex in normal controls and subjects with focal epilepsy. Neuropsychopharmacology. 2000;23:263–275. doi: 10.1016/S0893-133X(00)00101-9. [DOI] [PubMed] [Google Scholar]
- 78.Clerkin SM, Schulz KP, Halperin JM, et al. Guanfacine potentiates the activation of prefrontal cortex evoked by warning signals. Biol. Psychiatry. 2009;66:307–312. doi: 10.1016/j.biopsych.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 79.Mao Z-M, Arnsten AFT, Li BM. Local infusion of α-1 adrenergic agonist into the prefrontal cortex impairs spatial working memory performance in monkeys. Biol. Psychiatry. 1999;46:1259–1265. doi: 10.1016/s0006-3223(99)00139-0. [DOI] [PubMed] [Google Scholar]
- 80.Steere JC, Arnsten AF. The α-2A noradrenergic agonist, guanfacine, improves visual object discrimination reversal performance in rhesus monkeys. Behav. Neurosci. 1997;111:1–9. doi: 10.1037//0735-7044.111.5.883. [DOI] [PubMed] [Google Scholar]
- 81.Li BM, Mao ZM, Wang M, et al. α-2 adrenergic modulation of prefrontal cortical neuronal activity related to spatial working memory in monkeys. Neuropsychopharmacology. 1999;21:601–610. doi: 10.1016/S0893-133X(99)00070-6. [DOI] [PubMed] [Google Scholar]
- 82.Ma CL, Qi XL, Peng JY, et al. Selective deficit in no-go performance induced by blockade of prefrontal cortical α2-adrenoceptors in monkeys. Neuroreport. 2003;14:1013–1016. doi: 10.1097/01.wnr.0000070831.57864.7b. [DOI] [PubMed] [Google Scholar]
- 83.Ma CL, Arnsten AF, Li BM. Locomotor hyperactivity induced by blockade of prefrontal cortical α-2-adrenoceptors in monkeys. Biol. Psychiatry. 2005;57:192–195. doi: 10.1016/j.biopsych.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 84.Kopecková M, Paclt I, Goetz P. Polymorphisms of dopamine-β-hydroxylase in ADHD children. Folia Biol. (Praha) 2006;52:194–210. [PubMed] [Google Scholar]
- 85.Comings DE, Gade-Andavolu R, Gonzalez N, et al. Additive effect of three noradrenergic genes (ADRA2A, ADRA2C, DBH) on attention-deficit hyperactivity disorder and learning disabilities in Tourette syndrome subjects. Clin. Genet. 1999;55:160–172. doi: 10.1034/j.1399-0004.1999.550304.x. [DOI] [PubMed] [Google Scholar]
- 86.Daly G, Hawi Z, Fitzgerald M, et al. Mapping susceptibility loci in attention deficit hyperactivity disorder: preferential transmission of parental alleles at DAT1, DBH and DRD5 to affected children. Mol. Psychiatry. 1999;4:192–196. doi: 10.1038/sj.mp.4000510. [DOI] [PubMed] [Google Scholar]
- 87.Roman T, Schmitz M, Polanczyk GV, et al. Further evidence for the association between attention-deficit/hyperactivity disorder and the dopamine-β-hydroxylase gene. Am. J. Med. Genet. 2002;114:154–158. doi: 10.1002/ajmg.10194. [DOI] [PubMed] [Google Scholar]
- 88.Greene CM, Bellgrove MA, Gill M, et al. Noradrenergic genotype predicts lapses in sustained attention. Neuropsychologia. 2009;47:591–594. doi: 10.1016/j.neuropsychologia.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 89.Hess C, Reif A, Strobel A, et al. A functional dopamine-b-hydroxylase gene promoter polymorphism is associated with impulsive personality styles, but not with affective disorders. J. Neural Transm. 2009;116:121–130. doi: 10.1007/s00702-008-0138-0. [DOI] [PubMed] [Google Scholar]
- 90.Kieling C, Genro JP, Hutz MH, et al. The -1021 C/T DBH polymorphism is associated with neuropsychological performance among children and adolescents with ADHD. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008;147B:485–490. doi: 10.1002/ajmg.b.30636. [DOI] [PubMed] [Google Scholar]
- 91.Roman T, Schmitz M, Polanczyk GV, et al. Is the α-2A adrenergic receptor gene (ADRA2A) associated with attention-deficit/hyperactivity disorder? Am. J. Med. Genet. B Neuropsychiatr. Genet. 2003;120:116–120. doi: 10.1002/ajmg.b.20018. [DOI] [PubMed] [Google Scholar]
- 92.Park L, Nigg JT, Waldman ID, et al. Association and linkage of α-2A adrenergic receptor gene polymorphisms with childhood ADHD. Mol. Psychiatry. 2005;10:572–580. doi: 10.1038/sj.mp.4001605. [DOI] [PubMed] [Google Scholar]
- 93.Deupree JD, Smith SD, Kratochvil CJ, et al. Possible involvement of α-2A adrenergic receptors in attention deficit hyperactivity disorder: radioligand binding and polymorphism studies. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006;141B:877–884. doi: 10.1002/ajmg.b.30371. [DOI] [PubMed] [Google Scholar]
- 94.Waldman ID, Nigg JT, Gizer IR, et al. The adrenergic receptor α-2A gene (ADRA2A) and neuropsychological executive functions as putative endophenotypes for childhood ADHD. Cogn. Affect. Behav. Neurosci. 2006;6:18–30. doi: 10.3758/cabn.6.1.18. [DOI] [PubMed] [Google Scholar]
- 95.Hunt RD, Mindera RB, Cohen DJ. Clonidine benefits children with attention deficit disorder and hyperactivity: reports of a double-blind placebo-crossover therapeutic trial. J. Am. Acad. Child Psychiatry. 1985;24:617–629. doi: 10.1016/s0002-7138(09)60065-0. [DOI] [PubMed] [Google Scholar]
- 96.Cohen DJ, Young JG, Nathanson JA, et al. Clonidine in Tourette's syndrome. Lancet. 1979;2:551–553. doi: 10.1016/s0140-6736(79)91614-3. [DOI] [PubMed] [Google Scholar]
- 97.Cedarbaum JM, Aghajanian GK. Catecholamine receptors on locus coeruleus neurons: pharmacological characterization. Eur. J. Pharmacol. 1977;44:375–385. doi: 10.1016/0014-2999(77)90312-0. [DOI] [PubMed] [Google Scholar]
- 98.Aghajanian GK. Central noradrenergic neurons: a locus for the functional interplay between α-2 adrenoceptors and opiate receptors. J. Clin. Psychiatry. 1982;43:20–24. [PubMed] [Google Scholar]
- 99.Cohen DJ. Sterling Lecture, February 27, 2001 “Into life: autism, Tourette's syndrome and the community of clinical research”. Isr. J. Psychiatry. 2001;38:226–234. [PubMed] [Google Scholar]
- 100.Hunt RD, Capper L, O'Connell P. Clonidine in child and adolescent psychiatry. J. Child Adolesc. Psychiatry. 1990;1:87–102. doi: 10.1089/cap.1990.1.87. [DOI] [PubMed] [Google Scholar]
- 101.Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 102.Berridge CW, Devilbiss DM, Andrzejewski ME, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol. Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]; • Demonstrates that methylphenidate has prominent NE actions in PFC, in addition to its well-known dopamine actions.
- 103.Mehta MA, Goodyer IM, Sahakian BJ. Methylphenidate improves working memory and set-shifting in AD/HD: relationships to baseline memory capacity. J. Child Psychol. Psychiatry. 2004;45:293–305. doi: 10.1111/j.1469-7610.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- 104.Mehta MA, Owen AM, Sahakian BJ, et al. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J. Neurosci. 2000;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates that methylphenidate improves performance and enhances PFC efficiency in normal subjects.
- 105.Chamberlain SR, Del Campo N, Dowson J, et al. Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol. Psychiatry. 2007;62:977–984. doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 106.Devilbiss DM, Berridge CW. Cognition-enhancing doses of methylphenidate preferentially increase prefrontal cortex neuronal responsiveness. Biol. Psychiatry. 2008;64:626–635. doi: 10.1016/j.biopsych.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through a2 adrenoceptor and dopamine D1 receptor actions: relevance to therapeutic effects in attention deficit hyperactivity disorder. Behav. Brain Funct. 2005;1:2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gamo NJ, Wang M, Arnsten AF. Methylphenidate and atomoxetine improve prefrontal cortical function via noradrenergic α-2 and dopaminergic D1 receptor stimulation. J. Am. Acad. Child Adolesc. Psychiatry. 2010 doi: 10.1016/j.jaac.2010.06.015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Volkow ND, Fowler JS, Wang GJ, et al. Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: results from imaging studies. Eur. Neuropsychopharmacol. 2002;12:557–566. doi: 10.1016/s0924-977x(02)00104-9. [DOI] [PubMed] [Google Scholar]
- 110.Zhu J, Reith ME. Role of the dopamine transporter in the action of psychostimulants, nicotine, and other drugs of abuse. CNS Neurol. Disord. Drug Targets. 2008;7:393–409. doi: 10.2174/187152708786927877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Polanczyk G, Zeni C, Genro JP, et al. Association of the adrenergic α2A receptor gene with methylphenidate improvement of inattentive symptoms in children and adolescents with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry. 2007;64:218–224. doi: 10.1001/archpsyc.64.2.218. [DOI] [PubMed] [Google Scholar]
- 112.Uhlen S, Wikberg JE. Delineation of rat kidney α 2A and α 2B-adrenoceptors with [3H]RX821002 radioligand binding: computer modeling reveals that guanfacine is an α-2A-selective compound. Eur. J. Pharmacol. 1991;202:235–243. doi: 10.1016/0014-2999(91)90299-6. [DOI] [PubMed] [Google Scholar]
- 113.Uhlén S, Muceniece R, Rangel N, et al. Comparison of the binding activities of some drugs on α 2A, α 2B and α 2C-adrenoceptors and non-adrenergic imidazoline sites in the guinea pig. Pharmacol. Toxicol. 1995;76:353–364. doi: 10.1111/j.1600-0773.1995.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 114.Jasper JR, Lesnick JD, Chang LK, et al. Ligand efficacy and potency at recombinant α2 adrenergic receptors: agonist-mediated [35S]GTPγS binding. Biochem. Pharmacol. 1998;55:1035–1043. doi: 10.1016/s0006-2952(97)00631-x. [DOI] [PubMed] [Google Scholar]
- 115.Sorkin EM, Heel RC. Guanfacine: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in the treatment of hypertension. Drugs. 1986;31:301–336. doi: 10.2165/00003495-198631040-00003. [DOI] [PubMed] [Google Scholar]
- 116.Hunt RD, Arnsten AF, Asbell MD. An open trial of guanfacine in the treatment of attention deficit hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 1995;34:50–54. doi: 10.1097/00004583-199501000-00013. [DOI] [PubMed] [Google Scholar]
- 117.Scahill L, Chappell PB, Kim YS, et al. Guanfacine in the treatment of children with tic disorders and ADHD: a placebo-controlled study. Am. J. Psychiatry. 2001;158:1067–1074. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]; • First placebo-controlled trial of guanfacine in patients with attention-deficit/hyperactivity disorder symptoms.
- 118.Spencer TJ, Greenbaum M, Ginsberg LD, et al. Safety and effectiveness of coadministration of guanfacine extended release and psychostimulants in children and adolescents with attention-deficit/hyperactivity disorder. J. Child Adolesc. Psychopharmacol. 2009;19:501–510. doi: 10.1089/cap.2008.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Biederman J, Melmed RD, Patel A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73–e84. doi: 10.1542/peds.2006-3695. [DOI] [PubMed] [Google Scholar]
- 120.Sallee FR, McGough JJ, Wigal T, et al. Guanfacine Extended Release in Children and Adolescents With Attention-Deficit/Hyperactivity Disorder: a placebo-controlled trial. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:155–165. doi: 10.1097/CHI.0b013e318191769e. [DOI] [PubMed] [Google Scholar]
- 121.Wigal SB. Efficacy and safety limitations of attention-deficit hyperactivity disorder pharmacotherapy in children and adults. CNS Drugs. 2009;23(Suppl. 1):21–31. doi: 10.2165/00023210-200923000-00004. [DOI] [PubMed] [Google Scholar]
- 122.Birnbaum SG, Arnsten AF. The α-2A noradrenergic agonist, guanfacine, reverses the working memory deficits induced by pharmacological stress (FG7142) Soc. Neurosci. Abstracts. 1996;22:1126. [Google Scholar]
- 123.Rubia K, Smith AB, Halari R, et al. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. Am. J. Psychiatry. 2009;166:83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- 124.Sofuoglu M. Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction. 2010;105:38–48. doi: 10.1111/j.1360-0443.2009.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sinha R. The role of stress in addiction relapse. Curr. Psychiatry Rep. 2007;9:388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- 126.Scahill L, Aman MG, McDougle CJ, et al. A prospective open trial of guanfacine in children with pervasive developmental disorders. J. Child Adolesc. Psychopharmacol. 2006;16:589–598. doi: 10.1089/cap.2006.16.589. [DOI] [PubMed] [Google Scholar]
- 127.McCracken JT, Aman MG, McDougle CJ, et al. Possible influence of variant of the P-glycoprotein gene (MDR1/ABCB1) on clinical response to guanfacine in children with pervasive developmental disorders and hyperactivity. J. Child Adolesc. Psychopharmacol. 2010;20:1–5. doi: 10.1089/cap.2009.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Malhotra PA, Parton AD, Greenwood R, et al. Noradrenergic modulation of space exploration in visual neglect. Ann. Neurol. 2006;59:186–190. doi: 10.1002/ana.20701. [DOI] [PubMed] [Google Scholar]
- 129.Singh-Curry V, Malhotra P, Farmer SF, et al. Attention deficits following ADEM ameliorated by guanfacine. J. Neurol. Neurosurg. Psychiatry. 2010 doi: 10.1136/jnnp.2009.195792. DOI: 10.1136/jnnp.2009.195792. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Moore TL, Killiany RJ, Herndon JG, et al. Executive system dysfunction occurs as early as middle-age in the rhesus monkey. Neurobiol. Aging. 2006;27:1484–1493. doi: 10.1016/j.neurobiolaging.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 131.West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol. Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- 132.Daviss WB, Patel NC, Robb AS, et al. Clonidine for attention-deficit/hyperactivity disorder: II. ECG changes and adverse events analysis. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47:189–198. doi: 10.1097/chi.0b013e31815d9ae4. [DOI] [PubMed] [Google Scholar]
- 133.Klein-Schwartz W. Trends and toxic effects from pediatric clonidine exposures. Arch. Pediatr. Adolesc. Med. 2002;156:392–396. doi: 10.1001/archpedi.156.4.392. [DOI] [PubMed] [Google Scholar]
- 134.Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J. Child Adolesc. Psychopharmacol. 2009;19:215–226. doi: 10.1089/cap.2008.0080. [DOI] [PubMed] [Google Scholar]
- 135.van Zwieten PA, Chalmers JP. Different types of centrally acting antihypertensives and their targets in the central nervous system. Cardiovasc. Drugs Ther. 1994;8:787–799. doi: 10.1007/BF00877397. [DOI] [PubMed] [Google Scholar]
- 136.Bradley C. The behavior of children receiving benzedrine. Am. J. Psychiatry. 1937;94:577–585. [Google Scholar]
- 137.Arnsten AF, Ramos B, Birnbaum SB, et al. Protein kinase A as a therapeutic target for memory disorders: rationale and challenges. Trends Mol. Med. 2005;11:121–128. doi: 10.1016/j.molmed.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 138.Giedd JN, Lalonde FM, Celano MJ, et al. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:465–470. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Millar JK, Pickard BS, Mackie S, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- 140.Kirkpatrick B, Xu L, Cascella N, et al. DISC1 immunoreactivity at the light and ultrastructural level in the human neocortex. J. Comp. Neurol. 2006;497:436–450. doi: 10.1002/cne.21007. [DOI] [PubMed] [Google Scholar]
- 141.Murdoch H, Mackie S, Collins DM, et al. Isoform-selective susceptibility of DISC1/phosphodiesterase-4 complexes to dissociation by elevated intracellular cAMP levels. J. Neurosci. 2007;27:9513–9524. doi: 10.1523/JNEUROSCI.1493-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lambe EK, Olausson P, Horst NK, et al. Hypocretin and nicotine excite the same thalamocortical synapses in prefrontal cortex: correlation with improved attention in rat. J. Neurosci. 2005;25:5225–5229. doi: 10.1523/JNEUROSCI.0719-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pons S, Fattore L, Cossu G, et al. Crucial role of α4 and α6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J. Neurosci. 2008;28:12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Clarke HF, Walker SC, Dalley JW, et al. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb. Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- 145.McClure MM, Barch DM, Romero MJ, et al. The effects of guanfacine on context processing abnormalities in schizotypal personality disorder. Biol. Psychiatry. 2007;61:1157–1160. doi: 10.1016/j.biopsych.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 146.Swartz BE, McDonald CR, Patel A, et al. The effects of guanfacine on working memory performance in patients with localization-related epilepsy and healthy controls. Clin. Neuropharmacol. 2008;31:251–260. doi: 10.1097/WNF.0b013e3181633461. [DOI] [PubMed] [Google Scholar]
- 147.Arnsten AF, Contant TA. α-2 adrenergic agonists decrease distractability in aged monkeys performing a delayed response task. Psychopharmacology. 1992;108:159–169. doi: 10.1007/BF02245302. [DOI] [PubMed] [Google Scholar]
- 148.Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention deficit-hyperactivity disorder. J. Clin. Psychopharm. 2001;21:223–228. doi: 10.1097/00004714-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 149.Wang M, Tang ZX, Li BM. Enhanced visuomotor associative learning following stimulation of α 2A-adrenoceptors in the ventral prefrontal cortex in monkeys. Brain Res. 2004;1024:176–182. doi: 10.1016/j.brainres.2004.07.062. [DOI] [PubMed] [Google Scholar]