Abstract
Background and Aims
While the climbing habit allows vines to reach well-lit canopy areas with a minimum investment in support biomass, many of them have to survive under the dim understorey light during certain stages of their life cycle. But, if the growth/survival trade-off widely reported for trees hold for climbing plants, they cannot maximize both light-interception efficiency and shade avoidance (i.e. escaping from the understorey). The seven most important woody climbers occurring in a Chilean temperate evergreen rainforest were studied with the hypothesis that light-capture efficiency of climbers would be positively associated with their abundance in the understorey.
Methods
Species abundance in the understorey was quantified from their relative frequency and density in field plots, the light environment was quantified by hemispherical photography, the photosynthetic response to light was measured with portable gas-exchange analyser, and the whole shoot light-interception efficiency and carbon gain was estimated with the 3-D computer model Y-plant.
Key Results
Species differed in specific leaf area, leaf mass fraction, above ground leaf area ratio, light-interception efficiency and potential carbon gain. Abundance of species in the understorey was related to whole shoot features but not to leaf level features such as specific leaf area. Potential carbon gain was inversely related to light-interception efficiency. Mutual shading among leaves within a shoot was very low (<20 %).
Conclusions
The abundance of climbing plants in this southern rainforest understorey was directly related to their capacity to intercept light efficiently but not to their potential carbon gain. The most abundant climbers in this ecosystem match well with a shade-tolerance syndrome in contrast to the pioneer-like nature of climbers observed in tropical studies. The climbers studied seem to sacrifice high-light searching for coping with the dim understorey light.
Keywords: Light-capture efficiency, 3-D canopy architecture modelling, climbing plants, temperate evergreen rainforest, shade tolerance, Mitraria coccinea, Cissus striata, Boquila trifoliolata, Hydrangea serratifolia, Elytropus chilensis, Luzuriaga radicans, Luzuriaga polyphylla
INTRODUCTION
Climbing plants are ubiquitous but their abundance and diversity in species and climbing mechanisms are remarkable in both tropical and temperate rainforests (Putz and Mooney, 1991; Schnitzer and Bongers, 2002). While the climbing habit allows these species to reach well-lit canopy areas with a minimum investment in support biomass (Gartner, 1991; Holbrook and Putz, 1996), many of them have to survive under the dim understorey light during certain stages of their life cycle (Peñalosa, 1982; Putz, 1984; Ray, 1992). Surviving these periods in the shade are thus crucial for the fitness of climbing plants, but only a few studies have addressed their performance in the understorey (Aide and Zimmerman, 1990; Nabe-Nielsen, 2002). Many climbers only reproduce under high light (Putz, 1984; Ray, 1992; Gianoli, 2003, 2004) and shading elicits architectural responses that enhance climbing probability in these species (Gianoli, 2001; Gonzalez-Teuber and Gianoli, 2008). Both climbing and searching for high light typically involve stem elongation at the cost of leaf surface area to escape the understorey (French, 1977; Peñalosa, 1983; Franklin, 2008; Isnard and Silk, 2009), which makes immediate light capture less efficient. In fact, a comparative study of plants co-occurring in a tropical rainforest revealed a relatively inefficient light capture in species with a climbing habit, which was explained by their limited capacity for optimal arrangement of the foliage for light interception and their small investment in support biomass (Valladares et al., 2002).
While several studies report that climbing plant species are preferentially distributed in open sites (Putz, 1984; Hegarty and Caballé, 1991; DeWalt et al., 2000; Schnitzer and Carson, 2001; Londré and Schnitzer, 2006), there is also substantial evidence that certain climbing plants can thrive along the entire light gradient in forests (Strong and Ray, 1975; Hegarty, 1991; Campbell and Newbery, 1993; Baars et al., 1998; Oberbauer and Noudali, 1998; Pérez-Salicrup et al., 2001; Gerwing, 2004; Mascaro et al., 2004; Carrasco-Urra and Gianoli, 2009; Gianoli et al., 2010). It is therefore important to understand the strategies displayed by climbing plants to either cope with or avoid the shaded forest understorey, particularly in the case of evergreen forests, where temporary windows of increased light availability due to seasonal leaf shedding do not take place.
The present study was carried out in an evergreen temperate rainforest in southern Chile, where climbing plants are fairly abundant in the deep shade of the forest understorey (Gianoli et al., 2010). The major aim was to investigate the functional ecology of climbing plants with regard to the light environment. Specifically addressed was the relationship between dominance in the shaded understorey at the species level and the expression of both leaf and crown morphological and light capture and carbon gain traits. In view of the abundance of climbing species in the understorey of these forests, we hypothesized that light-capture efficiency of climbers would be positively associated with their abundance or dominance in the understorey. Furthermore, because of the suggested inverse relationship between traits that improve light harvesting and those that enhance carbon gain in species of contrasting shade tolerance (Henry and Aarssen, 1997; Gilbert et al., 2006; Valladares and Niinemets, 2008), we also expected that carbon gain would not be maximized in the climber species that dominate the understorey.
Seven woody vines, differing in their phylogenetic history and climbing mechanism (Table 1 and Fig. 1), that are the most important climbers occurring in this temperate rainforest, were studied (Gianoli et al., 2010). Although light interception is a whole-crown issue, little quantitative information is available for architectural features associated with shade tolerance (Valladares and Niinemets, 2008). For this reason, light interception and potential carbon gain were explored with the 3-D architectural model Y-Plant (Pearcy and Yang, 1996). The model has been successfully used in a number of contrasting habitats and for numerous plant species spanning from annual herbs to trees (Valladares and Pugnaire, 1999; Falster and Westoby, 2003; Pearcy et al., 2005), including a few climbing species in a tropical rainforest as well (Valladares et al., 2002).
Table 1.
Scientific name, family, climbing mechanism and shoot types of the seven more common vine species found in the temperate rainforest selected for the study
| Species | Family | Climbing mechanism | Shoot type* |
|---|---|---|---|
| Boquila trifoliolata (DC.) Decne. | Lardizabalaceae | Twining stems | P – O |
| Cissus striata Ruiz et Pav. | Vitaceae | Adhesive tendrils | P – O |
| Elytropus chilensis (A.DC) Müll.Arg. | Apocynaceae | Twining stems | P – O |
| Hydrangea serratifolia (H. et A.) F.Phil. | Hydrangeaceae | Adhesive roots | P – O |
| Luzuriaga polyphylla (Hook) J.F. Macbr. | Luzuriagaceae | Adhesive roots | P |
| Luzuriaga radicans Ruiz et Pav. | Luzuriagaceae | Adhesive roots | P |
| Mitraria coccinea Cav | Gesneriaceae | Adhesive roots | P – O |
* O, orthotropic; P, plagiotropic.
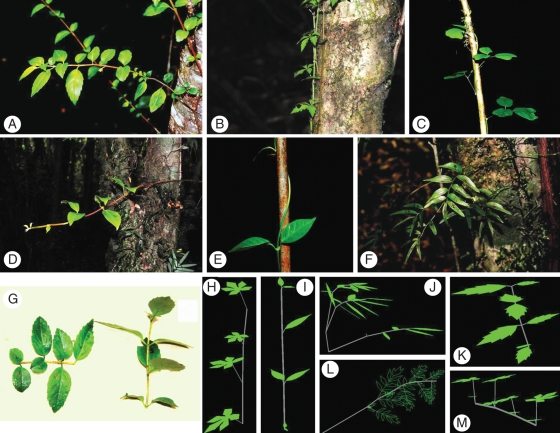
Fig. 1.
(A–F) Photographs of the climbing plants studied in the Chilean evergreen rainforest: Mitraria coccinea (A), Cissus striata (B), Boquila trifoliolata (C), Hydrangea serratifolia (D), Elytropus chilensis (E) and Luzuriaga radicans (F). (G) Excised horizontal (plagiotropic, left) and vertical (orthotropic, right) shoots of Mitraria coccinea. (H–M) Computer images reconstructed using the 3-D software Y-plant of Cissus striata (H, orthotropic; M, plagiotropic shoots), Elytropus chilensis (I), Luzuriaga radicans (J), Mitraria coccinea (K) and Luzuriaga polyphylla (L).
MATERIALS AND METHODS
Study site and species
The study took place in the lowland rainforest of Parque Nacional Puyehue (350–440 m a.s.l.; 40°39'S, 72°11'W), located in the western foothills of the Andean range in south-central Chile. The climate is maritime temperate, with an average annual precipitation of around 3500 mm and 13·8 °C and 5·4 °C mean maximum and minimum temperature, respectively. Both old-growth and secondary rainforest stands at this altitude are composed exclusively of broad-leaved evergreen tree species (Saldaña and Lusk, 2003; Lusk and Piper, 2007). The dominant canopy species in the mature lowland forest are Laureliopsis philippiana (Atherospermataceae), Aextoxicon punctatum (Aextoxicaceae), Nothofagus dombeyi (Fagaceae) and Eucryphia cordifolia (Cunoniaceae). Other, less abundant tree species in the canopy are Dasyphyllum diacanthoides (Asteraceae), Luma apiculata (Myrtaceae) and Weinmannia trichosperma (Cunioniaceae). Advanced regeneration in the understorey includes these tree species together with Amomyrtus luma (Myrtaceae), Azara serrata (Flacourtiaceae), Caldcluvia paniculata (Cunionaceae), Gevuina avellana (Proteaceae), Myrceugenia planipes (Myrtaceae) and Rhaphitamnus spinosus (Verbenaceae) (Saldaña and Lusk, 2003). Climbing plants – most of them woody vines – are very common in the mature lowland forest understorey (Gianoli et al., 2010). A total of 14 species belonging to ten families was reported for the study site, but the seven most abundant species account for 91 % of all vine individuals (Gianoli et al., 2010). These seven climbers were chosen as study species. They show contrasting climbing mechanisms, with adhesive organs being the most common climbing mechanism (Table 1).
Sampling and experimental design
Sampling of the climbing plants took place in 15 small plots (5 m × 5 m) located in closed canopy of an old-growth forest. Plots were located along transects laid out in random directions from the trails towards the forest interior, being at least 100 m apart from each other. Light availability was quantified in each of the 15 plots using hemispherical photographs (see details below). For each of the 15 plots, all climbing plant individuals rooted within the plot, excluding epiphytes, were recorded and identified to species level. Self-supporting seedlings and older plants trailing over the forest understorey or climbing onto trees or shrubs were counted. An attempt was made to count only independent stems not connected above ground to any other censused stem, i.e. apparent genets (Mascaro et al., 2004); this was verified by removing litter. In some cases, however, it was not possible to be absolutely sure that below-ground connections did not exist. Individuals were considered genets unless it was evident that they had connections with other vines. Five individuals of each species were measured.
The abundance value (IA) was calculated for each climbing plant species. This allowed a quantitative identification of the species prevailing in the shaded forest understorey. The IA of a plant species was originally defined as the summation of three values: the relative dominance, the relative frequency and the relative density (Curtis and McIntosh, 1951). However, depending on the ecological question, the IA may be restricted to only two components (e.g. DeWalt et al., 2000). In the present case, the dominance index, related to basal area, is not very meaningful because only one growth form, excluding trees and shrubs that were the dominant species within each plot, were sampled. Therefore, IA was determined as: (relative frequency + relative density)/2. Thus, IA ranged between 0 and 100. Relative frequency of species I = 100 × (frequency of species I)/Σ (frequencies of all species), where frequency of species I = number of plots where species I occurred/total number of plots. Relative density of species I = 100 × (density of species I)/Σ (densities of all species), where density of species I = total number of plants of species I/total area sampled.
Morphological and physiological measurements
Plants were randomly selected at each sampling plot, although senescent or heavily damaged or unhealthy looking individuals were left out as well as small juveniles (seedlings) or massive individuals surpassing the percentile 80 for stem diameter. In each individual plant, a representative section of the shoot avoiding both distal and basal parts and including three complete internodes was selected for 3-D reconstruction and for morphological and physiological measurements. Three contiguous metamers were considered. Each one included a stem, petiole and leaf blade or, in the case of opposite phyllotaxis, each included two leaves and two petioles in addition to the stem. There was a clear morphological distinction between orthotropic (vertically oriented) and plagiotropic (horizontally oriented) shoots in most species (Fig. 1); in these cases the two types of shoots were separately considered and measured in each individual plant (Table 1). The relative abundance of each shoot type was estimated from their contribution to the canopy of each individual. The abundance of each shoot type was then used to calculate the values of each response variable for each individual plant as weighed means.
After measuring the required parameters for a 3-D architectural reconstruction of the shoot on five individual plants per species and for each shoot pattern (i.e. orthotropic and plagiotropic shoots), plants were separated into leaf and stem fractions, dried for 48 h at 65 °C, and then weighed for determination of biomass parameters. Leaf area was measured from digital pictures and analysed with the software SigmaScan (Systat Software Inc., Chicago, IL, USA). Leaf mass fraction (LMF) was determined by dividing foliage dry mass by total plant dry mass. Leaf area ratio (LARabove) was determined by dividing total foliage area by total shoot dry mass.
Gas exchange parameters needed to run Y-plant simulations were obtained for the seven climbing plant species in the field (shaded understorey of mature forest). Area-based photosynthetic capacity (Amax) and area-based dark respiration rate (Rd) were measured in situ in 12 individuals of each climbing plant species. Amax and Rd measurements were made using a portable infrared gas analyser and a leaf chamber (PP Systems, Hitchin, UK). Photosynthetic capacity was measured at PAR 1000 µmol m−2 s−1 (checked to be a saturating level) and Rd was measured at PAR 0 µmol m−2 s−1, both at 20 °C. These measurements were carried out in mid-growing season (December) on two fully expanded leaves per plant. The average of these measurements was used as an individual plant value.
Crown architecture and light-capture efficiency
Light availability over each sapling was quantified by hemispherical photography. Comparisons of methods revealed a good accuracy of hemispherical photography for the description of understorey light availability (Bellow and Nair, 2003). The photographs were taken using a horizontally levelled digital camera (CoolPix 995; Nikon, Tokyo, Japan), mounted on a tripod and aimed at the zenith, using a fish-eye lens with a 180° field of view (FCE8; Nikon). Photographs were analysed for canopy openness using Hemiview canopy analysis software version 2·1 (1999; Delta-T Devices Ltd, Cambridge, UK). This software is based on the program CANOPY (Rich, 1990). Photographs were taken under homogenous sky conditions to minimize variations due to exposure and contrast. Hemispherical photographs were taken over the apex of each individual climbing plant or over a representative section of its crown when the climber was extended over a wide understorey zone; in all cases, the photograph was taken over the part of the crown reconstructed with Y-Plant. The direct site factor (DSF) and the indirect site factor (ISF) were computed by Hemiview, accounting for the geographic features of the site. These factors are estimates of the fraction of direct, and diffuse or indirect radiation, respectively, expected to reach the spot where the photograph was taken (Anderson, 1966). The hemispheric distribution of irradiance used for calculations of diffuse radiation was standard overcast sky conditions. A total of 160 sky sectors were considered resulting from 8 azimuth × 20 zenith divisions. A detailed description of the light environment in the understorey of this forest can be found in Valladares et al. (2011).
The 3-D crown architecture model Y-plant (Pearcy and Yang, 1996) was utilized to scale light capture and potential carbon gain measured at the single -leaf level to the crown level of individual plants (see photographs and screen images of the plants in Fig. 1). Y-plant calculates the efficiency of light capture as the ratio of the mean PFD absorbed by the crown to the PFD incident on a horizontal surface (of the same area as the total leaf area). Since a given light-capture efficiency can result from differential contribution of projection (orientation of leaf surfaces towards the light source, typically more important towards the zenith or towards big openings in the canopy) and display (projection minus mutual shading of the leaves of the shoot) efficiencies, the two were also calculated.
The crown architectural information required by Y-plant was obtained from measurements on five individual plants per species and shoot pattern. At each node of the three selected ones within each shoot, the internode and petiole angles and azimuths, the angle and azimuth of the leaf blade, and the azimuth of the midrib were recorded with a compass-protractor. Leaf, petiole and internode lengths were measured with a ruler, and petiole and internode diameters were measured with digital callipers. The nodes were numbered proceeding from the base to the top of the plant and along each branch. Indications of the connections among nodes within each shoot were annotated for a proper 3-D reconstruction of the shoot. Although most nodes had leaves, nodes without leaves were also considered when this was the case for the shoot sampled. Leaf shape was established from x- and y-co-ordinates of the leaf margins. Leaf size was then scaled in the crown reconstruction from the measured leaf length.
Leaves were assigned physiological characteristics including a maximum light-saturated assimilation rate (Amax) a dark respiration rate (Rd), leaf absorptance (a), a curvature factor (Θ) and quantum yield (ϕ) required for simulating the light response of CO2 assimilation with the rectangular hyperbola model of Johnson and Thornley (see Thornley, 2002). The equation for this model is:
| (1) |
where A is the assimilation rate and F is the PFD.
Simulations with Y-plant gave the diffuse and direct light interception and assimilation rate for each leaf in the crown and then for the whole shoot by integration of the corresponding values. Simulations for light-capture efficiency were estimated for the whole year, while potential carbon gain by the shoots was calculated for a given day under specific irradiance; the latter were carried at 30-min intervals between sunrise and sunset on a mid-summer day (15 January) at a latitude of 40° S. Clear sky conditions were simulated and a standard overcast sky distribution of solar radiation was assumed. These conditions were considered representative of the study system, where most carbon gain is achieved over the austral summer: the use of clear-sky conditions is done for the sake of comparisons with other studies because cloudy conditions are highly variable. It was checked that the absolute values did differ, but the relative differences across species did not vary significantly between clear sky and overcast conditions.
Data analysis
One- and two-way ANOVA were performed to test for species differences and for species × shoot pattern (orthotropic vs. plagiotropic) interactions for the morphological and functional variables estimated. Previous to the analysis, normality and homocedasticity of the dataset were checked. Linear regression was used to explore the relationships between the light-interception efficiency, potential carbon gain and the abundance value (IA) of each species in the understorey with the morphological variables studied.
RESULTS
Although there was some light heterogeneity in the forest understorey studied, neither the average direct light radiation (DSF) nor the diffuse light radiation (ISF) differed among the sites where the different climbing plant species were sampled (DSF: F6,53 = 1·01, P > 0·42; ISF: F6,53 = 0·88, P > 0·51; see Supplementary Data, available online). Thus, comparisons of traits among species are not confounded with the light environment. Pooling all the sites, the average DSF and ISF were 0·20 ± 0·01 and 0·18 ± 0·02, respectively.
The seven species studied exhibited significant differences for all morphological, allometric, light harvesting and carbon gain variables except for the degree of mutual shading among leaves (Table 2), which was similarly low for all of them (Fig. 2). The contrary was found for plagiotropic versus orthotropic shoots; despite their contrasting architecture (Fig. 1), they did not differ for the variables studied except for self-shading (Table 3), which was always higher in orthotropic shoots (data not shown). Species differences were not affected by the type of shoot, with the only exception of leaf mass fraction that exhibited a significant species × shoot type interaction (Table 3). There was a tendency for twining vines to show greater LAR values than adhesive climbers (Fig. 2), but this did not translate into greater light interception efficiencies.
Table 2.
One-way ANOVA for interspecific differences in leaf and crown morphological traits, and in light capture and carbon gain features
| Dependent variable | d.f. | SS | F | P |
|---|---|---|---|---|
| Specific leaf area | 6 | 1144076·7 | 98·28 | <0·001 |
| Leaf area ratio | 6 | 99904·986 | 52·64 | <0·001 |
| Leaf mass fraction | 6 | 1·094 | 63·25 | <0·001 |
| Self-shading | 6 | 0·006 | 0·29 | 0·936 |
| Light-interception efficiency | 6 | 0·184 | 54·02 | <0·001 |
| Projection efficiency (diffuse light) | 6 | 0·115 | 23·01 | 0·050 |
| Projection efficiency (direct light) | 6 | 0·110 | 33·01 | 0·010 |
| Potential carbon gain | 6 | 7470·52 | 2·41 | 0·039 |
For species with orthotropic and plagiotropic shoots, means were weighed according to the relative abundance of each shoot pattern.
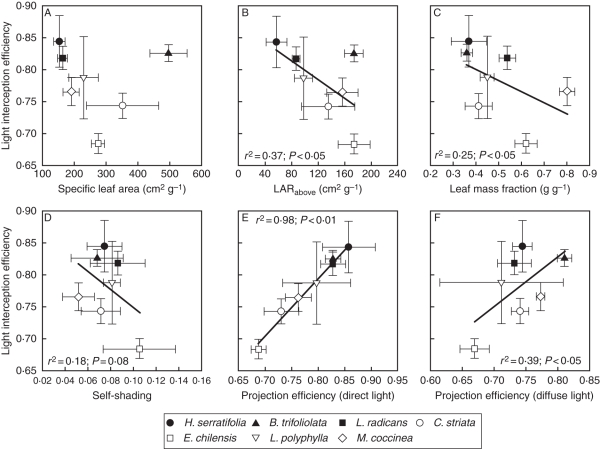
Fig. 2.
Light-interception efficiency as a function of (A) specific leaf area, (B) above-ground leaf area ratio, (C) leaf mass fraction, (D) self-shading, (E) projection efficiency for direct light and (F) for indirect light. Values of r2 and P are given for significant and borderline regressions. Data are the mean ± s.e. for each species. The means were calculated by weighing the relative contribution of orthotropic and plagiotropic shoots to the foliage of each species. The species are: Hydrangea serratifolia, Boquila trifoliolata, Luzuriaga radicans, Cissus striata, Elytropus chilensis, Luzuriaga polyphylla and Mitraria coccinea.
Table 3.
Two-way ANOVA (species × shoot pattern – orthotropic versus plagiotropic) for differences in leaf and crown morphological traits, and in light capture and carbon gain features
| Dependent variable | d.f. | SS | F | P |
|---|---|---|---|---|
| Specific leaf area | ||||
| Species | 4 | 699871·5 | 10·11 | <0·001 |
| Shoot pattern | 1 | 524·1 | 0·03 | 0·863 |
| Species × shoot pattern | 4 | 12865·8 | 0·19 | 0·944 |
| Leaf area ratio | ||||
| Species | 4 | 87667·7 | 6·75 | <0·001 |
| Shoot pattern | 1 | 601·4 | 0·18 | 0·669 |
| Species × shoot pattern | 4 | 33042·4 | 2·54 | 0·054 |
| Leaf mass fraction | ||||
| Species | 4 | 1·1 | 10·20 | <0·001 |
| Shoot pattern | 1 | 0·1 | 1·95 | 0·170 |
| Species × shoot pattern | 4 | 0·4 | 3·59 | 0·014 |
| Self-shading | ||||
| Species | 4 | <0·1 | 0·48 | 0·749 |
| Shoot pattern | 1 | 0·1 | 18·73 | <0·001 |
| Species × shoot pattern | 4 | <0·1 | 0·18 | 0·948 |
| Light-interception efficiency | ||||
| Species | 4 | 0·2 | 8·55 | <0·001 |
| Shoot pattern | 1 | 0·1 | 0·13 | 0·724 |
| Species × shoot pattern | 4 | 0·1 | 0·19 | 0·939 |
| Projection efficiency (diffuse light) | ||||
| Species | 4 | 0·1 | 7·03 | <0·001 |
| Shoot pattern | 1 | <0·1 | 3,52 | 0·678 |
| Species × shoot pattern | 4 | <0·1 | 0·73 | 0·575 |
| Projection efficiency (direct light) | ||||
| Species | 4 | 0·2 | 7·22 | <0·001 |
| Shoot pattern | 1 | <0·1 | 0·05 | 0·819 |
| Species × shoot pattern | 4 | <0·1 | 0·21 | 0·934 |
| Potential carbon gain | ||||
| Species | 4 | 6239·1 | 2·37 | 0·068 |
| Shoot pattern | 1 | 182·1 | 0·27 | 0·601 |
| Species × shoot pattern | 4 | 271·7 | 0·10 | 0·981 |
Only the five species with different shoot patterns are included in the analyses.
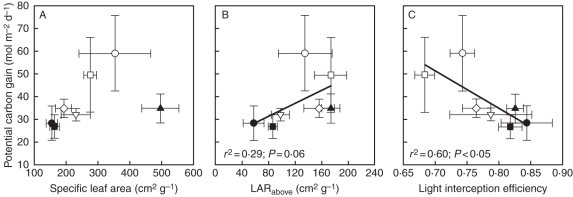
Light-interception efficiency was negatively related to above ground leaf area ratio (LARabove), leaf mass fraction (LMF) and self-shading, positively related to projection efficiency (in turn primarily influenced by the fraction of foliage arranged horizontally) to both direct and diffuse light, and not related to specific leaf area (SLA; Fig. 2). Projection efficiency to direct light had a more significant impact on light-interception efficiency than that of diffuse light, as revealed by a better linear fit of the regression (Fig. 2). Potential shoot carbon gain was positively related to LARabove, negatively related to light-interception efficiency and not related to SLA (Fig. 3). Finally, in support of our general hypothesis, the abundance value of climbing plant species in the understorey was positively related to light-interception efficiency, inversely related to both LARabove and potential carbon gain, and not associated with SLA (Fig. 4).
Fig. 3.
In situ potential carbon gain estimated by Y-plant as a function of specific leaf area (A), above-ground leaf area ratio (B) and light-interception efficiency (C). Values of r2 and P are given for significant and borderline regressions. Data are the mean ± s.e. for each species. The means were calculated by weighing the relative contribution of orthotropic and plagiotropic shoots to the foliage of each species. For species see Fig. 2D legend.
Fig. 4.
Abundance of each species in the understorey of the Chilean rainforest studied as a function of (A) specific leaf area, (B) above-ground leaf area ratio, (C) light-interception efficiency and (D) potential carbon gain. Values of r2 and P are given for significant and borderline regressions. Data are the mean ± s.e. for each species. The means were calculated by weighing the relative contribution of orthotropic and plagiotropic shoots to the foliage of each species. For species see Fig. 2D legend.
DISCUSSION
As it was hypothesized, light-interception efficiency significantly influenced the dominance of climbing plant species in the forest understorey. Moreover, potential carbon gain was inversely associated with climber abundance rather than unrelated to it, as was expected, based on earlier evidence for self-supporting plant species (Henry and Aarssen, 2001; Valladares and Niinemets, 2008). These results are in agreement with general expectations for a shade-tolerance syndrome since shade-tolerant species tend to maximize light-interception efficiency while gap and shade-intolerant species tend to maximize carbon gain capacities (Grime, 1966; Givnish, 1988; Valladares and Niinemets, 2008). Consequently, the most abundant climbers in the deep shade of this evergreen rainforest seem to prioritize endurance under low-light conditions over maximization of growth. A similar result was reported for congeneric lianas of contrasting shade tolerance in south-west China (Cai et al., 2007). The life history trade-off between high survival and rapid growth has been documented for both tropical (Kitajima, 1994; Poorter, 1999) and temperate (Kobe et al., 1995; Lusk and Del Pozo, 2002) tree species. A plausible hypothesis to explain this trade-off is that shade tolerance relies on the maintenance of a net carbon balance, which may be achieved through allocation to traits that enhance survival at the cost of allocation to traits that maximize growth (e.g. Kitajima and Mayer, 2008). Gilbert et al. (2006) showed that the survival/growth trade-off also holds for liana species in a tropical rainforest. Complementary evidence is provided here that climbing plant species behave in accordance with such a fundamental trade-off and hence confirm that life-history strategies in woody plants are rather conserved across growth forms and forest ecosystems.
Results also indicate that despite some observations and generalizations on the supposed pioneer-like nature of climbing plants, mostly arising from tropical studies (Schnitzer and Bongers, 2002), several of the climbers of the present study system can be considered as shade-tolerators. Comparable patterns have been found in two other studies conducted in the same southern temperate rainforest (Carrasco-Urra and Gianoli, 2009; Gianoli et al., 2010) and in two rainforests in New Zealand located at the same latitude (Baars et al., 1998). Although evidence is not yet sufficient to draw generalizations on light preferences of climbers in temperate versus tropical rainforests, we think that a preliminary hypothesis can be formulated. Thus, hypothetical differences could be related to the lower irradiance reaching the cloudy regions of southern evergreen temperate rainforests, which makes these understoreys darker and reduce both the light heterogeneity at the forest floor level and its contrast with the upper layers of the forest canopy. Interestingly, whereas only one of the 14 climbing plant species in this temperate rainforest reproduces in the upper canopy, namely Hydrangea serratifolia (A. Saldaña, pers. obs.), most of the species studied by Putz (1984) in a tropical rainforest attained reproduction in the canopy.
Selaya et al. (2007) showed that long-living pioneer trees and lianas intercept similar amount of light per unit mass, which contributes to their ability to persist in a 6-month-old regenerating tropical forest. However, as the forest succession progresses, the understorey becomes darker, and lianas become more scarce (Putz, 1984; Mascaro et al., 2004). In a comparative study of contrasting plants coexisting in the understorey of a tropical, old-growth rainforest, climbing plants had among the lowest values of light-interception efficiency (Valladares et al., 2002). The values of light-interception efficiency of the climbing plants studied here (0·66–0·84) are higher than most plants in this comparative study and among the highest of those obtained using the same method, i.e. reconstructing plant crowns with the computer model Y-plant (Zotz et al., 2002; Falster and Westoby, 2003; Pearcy et al., 2005). This fact reinforces the idea that the climbing plants studied here do differ in their light-capture efficiency from those in other forest ecosystems. Further support to this idea is given by the result on the negative relationship between abundance in the understorey and potential carbon gain of the plants studied here, which contrast with results from other ecosystems showing that carbon gain is limiting for lianas (Granados and Korner, 2002; Selaya and Anten, 2010). In a tropical, old-growth rainforest, lianas exhibited increased vigour when they were exposed to increased CO2 in the field (Granados and Korner, 2002). While photosynthesis in these lianas was limited by carbon availability, photosynthesis in the present study case seems to be limited by light availability. Interestingly, light-interception efficiency was negatively related with potential carbon gain. There was a slight trend for species with high potential carbon gain and low light-interception efficiency to have slightly higher photosynthetic rates (see Supplementary Data, available online), but the trend was not statistically significant, revealing that other factors like the respiration–maximum photosynthetic rate ratio and the distribution of direct and indirect light over the day are influencing the carbon balance of these species in the understorey studied.
Contrary to expectations, light capture by the climbing plants studied was very efficient in both orthotropic and plagiotropic shoots despite their contrasting architectures (Fig. 1 and Table 2). As expected, however, orthotropic shoots had higher self-shading, but this was compensated by longer internodes so the overall interception of light was not different between these two shoot patterns. Climbers and clonal herbs show a distribution of functions between horizontal (plagiotropic) and vertical (orthotropic) shoots, with different patterns of plasticity in response to light due to the contrasting predictability of the light environment over the vertical versus horizontal axes (Huber, 1996). In Glechoma hirsuta, growth of horizontal shoots was reduced by shading, whereas that of vertical shoots was unaffected by light availability (Huber and Hutchings, 1997). Plagiotropic shoots are typically well developed in shade-tolerant species and in individual plants acclimated to shade (Valladares and Niinemets, 2007). Plagiotropic shoots compensated for low light availability in the shade and significantly increased whole crown light capture and carbon gain in a very different system – the evergreen sclerophyll shrub Heteromeles arbutifolia growing in the understorey of a chaparral woodland (Valladares and Pearcy, 1998). This higher light-capture efficiency of plagiotropic versus orthotropic shoots is found in many temperate woody species (Valladares and Niinemets, 2007). However, it was not possible to find any relevant impact on light-interception efficiency and potential carbon gain of the shoot pattern in the case of the climbing species studied here.
The present study shows that leaf level traits like specific leaf area (SLA), which have a broad impact on plant performance in different light environments (Milla and Reich, 2007), were not explaining the abundance of climbers in the understorey. Values of SLA were, overall, lower than those of understorey trees in this forest (Lusk and Del Pozo, 2002), and LARabove was within the range found in a tropical rainforest (Valladares et al., 2002). Whole-crown features like leaf area ratio and leaf mass fraction did have a significant impact on light-interception efficiency, supporting the notion that light harvesting requires a co-ordinated response at different hierarchical scales within plants, with whole-plant allocation and architecture playing central roles in this (Pearcy et al., 2004; Poorter et al., 2005). Besides, whole shoot features were significant in explaining the abundance of plant species in the understorey studied.
In conclusion, the abundance of climbing plants in this southern rainforest understorey is directly related to their capacity to intercept light efficiently but not to their potential carbon gain (as influenced by LARabove and Amax) or to key leaf-level features such as SLA. We suggest that the dominant climbers in this ecosystem sacrifice high-light searching for coping with the dim understorey light.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
Constructive criticisms by Kaoru Kitajima and one anonymous referee significantly improved the clarity of the ideas. Financial support was provided by a collaborative grant between CSIC (Spain) and CONICYT (2007-168), FONDECYT grants 1070503 and 7080035, by the CYTED network ECONS (410RT0406) and by the grants Consolider Montes (CSD2008_00040), VULGLO (CGL2010-22180-C03-03) and REMEDINAL 2 (CM S2009/AMB-1783). The final stage of manuscript writing was promoted by the CONICYT–ACHA MEC–80090005 project.
LITERATURE CITED
- Aide TM, Zimmerman JK. Patterns of insect herbivory, growth and survivorship in juveniles of a neotropical liana. Ecology. 1990;71:1412–1421. [Google Scholar]
- Anderson MC. Some problems of simple characterization of the light climate in plant communities. In: Bainbridge R, Evans GC, Rackham O, editors. Light as an ecological factor (Symp. VI. Br. Ecol. Soc.) Oxford: Blackwell Scientific Publications; 1966. pp. 77–90. [Google Scholar]
- Baars R, Kelly D, Sparrow AD. Liane distribution within native forest remnants in two regions of the South Island New Zealand. New Zealand Journal of Ecology. 1998;22:71–85. [Google Scholar]
- Bellow JG, Nair PKR. Comparing common methods for assessing understory light availability in shaded-perennial agroforestry systems. Agricultural and Forest Meteorology. 2003;114:197–211. [Google Scholar]
- Cai Z-Q, Poorter L, Cao K-F, Bongers F. Seedling growth strategies in Bauhinia species: comparing lianas and trees. Annals of Botany. 2007;100:831–838. doi: 10.1093/aob/mcm179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EJF, Newbery DM. Ecological relationships between lianas and trees in lowland rain forest in Sabah, East Malaysia. Journal of Tropical Ecology. 1993;9:469–490. [Google Scholar]
- Carrasco-Urra F, Gianoli E. Abundance of climbing plants in a southern temperate rainforest: host-tree characteristics or light availability? Journal of Vegetation Science. 2009;20:1155–1162. [Google Scholar]
- Curtis JT, McIntosh RP. An upland forest continuum in the prairie-forest border region of Wisconsin. Ecology. 1951;32:476–496. [Google Scholar]
- DeWalt SJ, Schnitzer SA, Denslow JS. Density and diversity of lianas along a chronosequence in a central Panamanian lowland forest. Journal of Tropical Ecology. 2000;16:1–9. [Google Scholar]
- Falster DS, Westoby M. Leaf size and angle vary widely across species: what consequences for light interception? New Phytologist. 2003;158:509–525. doi: 10.1046/j.1469-8137.2003.00765.x. [DOI] [PubMed] [Google Scholar]
- Franklin KA. Shade avoidance. New Phytologist. 2008;179:930–944. doi: 10.1111/j.1469-8137.2008.02507.x. [DOI] [PubMed] [Google Scholar]
- French JC. Growth relationships of leaves and internodes in viny angiosperms with different modes of attachment. American Journal of Botany. 1977;64:292–304. [Google Scholar]
- Gartner BL. Relative growth rates of vines and shrubs of western poison oak, Toxicodendron diversilobum (Anacardiaceae) American Journal of Botany. 1991;78:1345–1353. [Google Scholar]
- Gerwing JJ. Life history diversity among six species of canopy lianas in an old-growth forest of the eastern Brazilian Amazon. Forest Ecology and Management. 2004;190:57–72. [Google Scholar]
- Gianoli E. Lack of differential plasticity to shading of internodes and petioles with growth habit in Convolvulus arvensis (Convolvulaceae) International Journal of Plant Sciences. 2001;162:1247–1252. [Google Scholar]
- Gianoli E. Phenotypic responses of the twining vine Ipomoea purpurea (Convolvulaceae) to physical support availability in sun and shade. Plant Ecology. 2003;165:21–26. [Google Scholar]
- Gianoli E. Plasticity of traits and correlations in two populations of Convolvulus arvensis (Convolvulaceae) differing in environmental heterogeneity. International Journal of Plant Sciences. 2004;165:825–832. [Google Scholar]
- Gianoli E, Saldaña A, Jiménez-Castillo M, Valladares F. Distribution and abundance of vines along the light gradient in a southern temperate rain forest. Journal of Vegetation Science. 2010;21:66–73. [Google Scholar]
- Gilbert B, Wright SJ, Muller-Landau HC, Kitajima K, Hernández A. Life history trade-offs in tropical trees and lianas. Ecology. 2006;87:1281–1288. doi: 10.1890/0012-9658(2006)87[1281:lhtitt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Givnish TJ. Adaptation to sun and shade: a whole-plant perspective. Australian Journal of Plant Physiolology. 1988;15:63–92. [Google Scholar]
- Gonzalez-Teuber M, Gianoli E. Damage and shade enhance climbing and promote associational resistance in a climbing plant. Journal of Ecology. 2008;96:122–126. [Google Scholar]
- Granados J, Korner C. In deep shade, elevated CO2 increases the vigor of tropical climbing plants. Global Change Biology. 2002;8:1109–1117. [Google Scholar]
- Grime PJ. Shade avoidance and shade tolerance in flowering plants. In: Bainbridge R, Evans GC, Rackham O, editors. Light as an ecological factor. Oxford: Blackwell Scientific Publications; 1966. pp. 187–207. [Google Scholar]
- Hegarty EE. Leaf litter production by lianas and trees in a subtropical Australian rainforest. Journal of Tropical Ecology. 1991;7:201–214. [Google Scholar]
- Hegarty EE, Caballé G. Distribution and abundance of vines in forest communities. In: Putz FE, Mooney HA, editors. The biology of vines. Cambridge: Cambridge University Press; 1991. pp. 313–336. [Google Scholar]
- Henry HAL, Aarssen LW. On the relationship between shade tolerance and shade avoidance strategies in woodland plants. Oikos. 1997;80:575–582. [Google Scholar]
- Henry HAL, Aarssen LW. Inter- and intraspecific relationships between shade tolerance and shade avoidance in temperate trees. Oikos. 2001;93:477–487. [Google Scholar]
- Holbrook N, Putz F. Physiology of tropical vines and hemiepiphytes: plants that climb up and plants that climb down. In: Mulkey SS, Chazdon RL, Smith AP, editors. Tropical forest plant ecophysiology. New York, NY: Chapman and Hall; 1996. pp. 363–394. [Google Scholar]
- Huber H. Plasticity of internodes and petioles in prostrate and erect Potentilla species. Functional Ecology. 1996;10:401–409. [Google Scholar]
- Huber H, Hutchings MJ. Differential response to shading in orthotropic and plagiotropic shoots of the clonal herb Glechoma hirsute. Oecologia. 1997;112:485–491. doi: 10.1007/s004420050336. [DOI] [PubMed] [Google Scholar]
- Isnard S, Silk WK. Moving with climbing plants from Charles Darwin' s time into the 21st century. American Journal of Botany. 2009;96:1205–1221. doi: 10.3732/ajb.0900045. [DOI] [PubMed] [Google Scholar]
- Kitajima K. Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia. 1994;98:419–428. doi: 10.1007/BF00324232. [DOI] [PubMed] [Google Scholar]
- Kitajima K, Myers JA. Seedling ecophysiology: strategies towards achievement of positive carbon balance. In: Leck MA, Parker VT, Simpson RL, editors. Seedling ecology and evolution. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- Kobe RK, Pacala SW, Silander JA, Canham CD. Juvenile tree survivorship as a component of shade tolerance. Ecological Applications. 1995;5:517–532. [Google Scholar]
- Londré RA, Schnitzer SA. The distribution of lianas and their change in abundance in temperate forests over the past 45 years. Ecology. 2006;87:2973–2978. doi: 10.1890/0012-9658(2006)87[2973:tdolat]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lusk CH, Del Pozo A. Survival and growth of seedlings of 12 Chilean rainforest trees in two light environments: gas exchange and biomass distribution correlates. Austral Ecology. 2002;27:173–182. [Google Scholar]
- Lusk CH, Piper FI. Seedling size influences relationships of shade tolerance with carbohydrate-storage patterns in a temperate rainforest. Functional Ecology. 2007;21:78–86. [Google Scholar]
- Mascaro J, Schnitzer SA, Carson WP. Liana diversity, abundance and mortality in a tropical wet forest in Costa Rica. Forest Ecology and Management. 2004;190:3–14. [Google Scholar]
- Milla R, Reich PB. The scaling of leaf area and mass: the cost of light interception increases with leaf size. Proceedings of the Royal Society Biology. 2007;274:2109–2114. doi: 10.1098/rspb.2007.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabe-Nielsen J. Growth and mortality rates of the liana Machaerium cuspidatum in relation to light and topographic position. Biotropica. 2002;34:319–322. [Google Scholar]
- Oberbauer SF, Noudali M. Potential carbon gain of single leaves in juveniles of the vine Monstera tenuis (Araceae) in Costa Rica. American Journal of Botany. 1998;85:850–854. [PubMed] [Google Scholar]
- Pearcy RW, Yang W. A three-dimensional shoot architecture model for assessment of light capture and carbon gain by understory plants. Oecologia. 1996;108:1–12. doi: 10.1007/BF00333208. [DOI] [PubMed] [Google Scholar]
- Pearcy RW, Valladares F, Wright SJ, Lasso de Paulis E. A functional analysis of the crown architecture of tropical forest Psychotria species: do species vary in light capture efficiency and consequently in carbon gain and growth? Oecologia. 2004;139:163–177. doi: 10.1007/s00442-004-1496-4. [DOI] [PubMed] [Google Scholar]
- Pearcy RW, Muraoka H, Valladares F. Crown architecture in sun and shade environments: assessing function and tradeoffs with a 3-D simulation model. New Phytologist. 2005;166:791–800. doi: 10.1111/j.1469-8137.2005.01328.x. [DOI] [PubMed] [Google Scholar]
- Peñalosa J. Morphological specialization and attachment success in two twining lianas. American Journal of Botany. 1982;69:1043–1045. [Google Scholar]
- Peñalosa J. Shoot dynamics and adaptive morphology of Ipomoea phillomega (Vell.) House (Convolvulaceae), a tropical rainforest liana. Annals of Botany. 1983;52:737–754. [Google Scholar]
- Pérez-Salicrup DR, Sork VL, Putz FE. Lianas and trees in a liana forest in Amazonian Bolivia. Biotropica. 2001;33:34–47. [Google Scholar]
- Poorter L. Growth responses of 15 rain-forest tree species to a light gradient: the relative importance of morphological and physiological traits. Functional Ecology. 1999;13:396–410. [Google Scholar]
- Poorter L, Bongers L, Bongers F. Architecture of 54 moist-forest tree species: traits, trade-offs, and functional groups. Ecology. 2005;87:1289–1301. doi: 10.1890/0012-9658(2006)87[1289:aomtst]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Putz FE. The natural history of lianas on Barro Colorado island, Panamá. Ecology. 1984;65:1713–1724. [Google Scholar]
- Putz FE, Mooney HA. The biology of vines. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- Ray TS. Foraging behaviour in tropical herbaceous climbers (Araceae) Journal of Ecology. 1992;80:189–203. [Google Scholar]
- Rich PM. Characterizing plant canopies with hemispherical photographs. Remote Sensing Reviews. 1990;5:13–29. [Google Scholar]
- Saldaña A, Lusk C. Influence of overstorey species identity on resource availability and variation in composition of advanced regeneration in a temperate rainforest in southern Chile. Revista Chilena de Historia Natural. 2003;76:639–650. [Google Scholar]
- Schnitzer SA, Bongers F. The ecology of lianas and their role in forests. Trends in Ecology and Evolution. 2002;17:223–230. [Google Scholar]
- Schnitzer SA, Carson WP. Treefall gaps and the maintenance of species diversity in a tropical forest. Ecology. 2001;82:913–919. [Google Scholar]
- Selaya NG, Anten NPR. Leaves of pioneer and later-successional trees have similar lifetime carbon gain in tropical secondary forest. Ecology. 2010;91:1102–1113. doi: 10.1890/08-2111.1. [DOI] [PubMed] [Google Scholar]
- Selaya NG, Anten NPR, Oomen RJ, Matthies M, Werger MJA. Above-ground biomass investments and light interception of tropical forest trees and lianas early in succession. Annals of Botany. 2007;99:141–151. doi: 10.1093/aob/mcl235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong DR, Ray TS. Host tree location behavior of a tropical vine (Monstera gigantea) by skototropism. Science. 1975;190:804–806. [Google Scholar]
- Thornley JH. Instantaneous canopy photosynthesis: analytical expressions for sun and shade leaves based on exponential light decay down the canopy and an acclimated non-rectangular hyperbola for leaf-photosynthesis. Annals of Botany. 2002;89:451–458. doi: 10.1093/aob/mcf071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladares F, Niinemets Ü. The architecture of plant crowns: from design rules to light capture and performance. In: Pugnaire F, Valladares F, editors. Functional plant ecology. Boca Raton, FL: CRC Press; 2007. pp. 101–149. [Google Scholar]
- Valladares F, Niinemets Ü. Shade tolerance, a key plant feature of complex nature and consequences. Annual Review of Ecology, Evolution and Systematics. 2008;39:237–257. [Google Scholar]
- Valladares F, Pearcy RW. The functional ecology of shoot architecture in sun and shade plants of Heteromeles arbutifolia M. Roem., a Californian chaparral shrub. Oecologia. 1998;114:1–10. doi: 10.1007/s004420050413. [DOI] [PubMed] [Google Scholar]
- Valladares F, Pugnaire FI. Tradeoffs between irradiance capture and avoidance in semiarid environments simulated with a crown architecture model. Annals of Botany. 1999;83:459–470. [Google Scholar]
- Valladares F, Skillman J, Pearcy RW. Convergence in light capture efficiencies among tropical forest understory plants with contrasting crown architectures: a case of morphological compensation. American Journal of Botany. 2002;89:1275–1284. doi: 10.3732/ajb.89.8.1275. [DOI] [PubMed] [Google Scholar]
- Valladares F, Saldaña A, Gianoli E. Costs versus risks: architectural changes with changing light quantity and quality in saplings of temperate rainforest trees of different shade tolerance. Austral Ecology. 2011;36 (in press). doi:10.1111/j.1442-9993.2011.02245.x. [Google Scholar]
- Zotz G, Reichling P, Valladares F. A simulation study on the importance of size-related changes in leaf morphology and physiology for carbon gain in an epiphytic bromeliad. Annals of Botany. 2002;90:437–443. doi: 10.1093/aob/mcf208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.