Abstract
Background and Aims
Plant growth regulators play an important role in seed germination. However, much of the current knowledge about their function during seed germination was obtained using orthodox seeds as model systems, and there is a paucity of information about the role of plant growth regulators during germination of recalcitrant seeds. In the present work, two endangered woody species with recalcitrant seeds, Araucaria angustifolia (Gymnosperm) and Ocotea odorifera (Angiosperm), native to the Atlantic Rain Forest, Brazil, were used to study the mobilization of polyamines (PAs), indole-acetic acid (IAA) and abscisic acid (ABA) during seed germination.
Methods
Data were sampled from embryos of O. odorifera and embryos and megagametophytes of A. angustifolia throughout the germination process. Biochemical analyses were carried out in HPLC.
Key Results
During seed germination, an increase in the (Spd + Spm) : Put ratio was recorded in embryos in both species. An increase in IAA and PA levels was also observed during seed germination in both embryos, while ABA levels showed a decrease in O. odorifera and an increase in A. angustifolia embryos throughout the period studied.
Conclusions
The (Spd + Spm) : Put ratio could be used as a marker for germination completion. The increase in IAA levels, prior to germination, could be associated with variations in PA content. The ABA mobilization observed in the embryos could represent a greater resistance to this hormone in recalcitrant seeds, in comparison to orthodox seeds, opening a new perspective for studies on the effects of this regulator in recalcitrant seeds. The gymnosperm seed, though without a connective tissue between megagametophyte and embryo, seems to be able to maintain communication between the tissues, based on the likely transport of plant growth regulators.
Keywords: Ocotea odorifera, Araucaria angustifolia, endangered species, polyamines, ABA, IAA, recalcitrant seeds, germination, embryo, megagametophyte, Angiosperm, Gymnosperm
INTRODUCTION
Germination is a complex plant developmental process regulated by the balance between levels of plant growth regulators and the spatial and temporal expression of seed-specific gene networks (Barendse and Peeters, 1995). Morphologically, germination is completed when a part of the embryo, usually the radicle, has grown enough to penetrate the structures that surround it (Bewley, 1997). Although they operate at different levels, plant growth regulators play an important role in seed germination (Chen et al., 2004). Abscisic acid (ABA) regulates different aspects of seed development and germination. At the beginning of embryogenesis, ABA is produced by maternal tissue, suppressing viviparity and activating embryo growth and development (Cheng et al., 2002, Frey et al., 2004). During maturation, this hormone is produced by embryo tissues, allowing the synthesis of storage protein and lipids (Kermode, 1990; Gutierrez et al., 2007). Therefore, in many plant species endogenous ABA is involved in the induction, and perhaps in the maintenance, of the dormant state and germination delay (reviewed in Kucera et al., 2005). A decrease in ABA levels is usually detected at the start of the germination process, based on synthesis suppression as well as catabolism (Feurtado et al., 2004; Kushiro et al., 2004). ABA is known as an antagonist of gibberellins, a class of plant growth regulators that induce seed germination (Kermode, 2005).
Auxins are one of the most important classes of phytohormones, since they are key regulators of virtually every aspect of plant growth and development, regulating transcription by rapidly modulating levels of Aux/IAA proteins throughout development (reviewed in Mockaitis and Estelle, 2008). Local auxin biosynthesis plays essential roles in many different processes, including gametogenesis, embryogenesis, seedling growth, vascular patterning and flower development (reviewed by Zhao, 2010). At the cellular level, auxin controls division, elongation and differentiation, as well as plant cell polarity (Tromas and Perrot-Rechenmann, 2010).
Polyamines (PAs) are low molecular-weight organic polycations, displaying a broad biological activity. The main PAs present in higher plants are putrescine (Put), spermidine (Spd) and spermine (Spm) (Bouchereau et al., 1999; Kuznetsov et al., 2006; Takahashi and Kakehi, 2010). PAs exert a broad spectrum of biological activities such as the regulation of gene expression, signal modulation, cell proliferation and membrane stabilization (Tabor and Tabor, 1984; Cohen, 1998; Igarashi and Kashiwagi, 2000). These roles have been associated with the control of cell division, embryogenesis, root formation, fruit development and ripening, and responses to biotic and abiotic stresses (Kumar et al., 1997; Bouchereau et al., 1999; Minocha et al., 1999; Silveira et al., 2006; Santa-Catarina et al., 2006; Groppa et al., 2007). Besides the studies that have assessed the effect of single PAs alone, other studies associate physiological events in plants with the relationship between two or more PAs. It has been proposed that the Put : Spd ratio is an important biomarker of the regeneration capacity in plants (Shoeb et al., 2001). Studies on Nicotiana tabacum protoplast demonstrated that high levels of Put against Spd + Spm levels could be associated with totipotency (Papadakis et al., 2005). Other work has associated the Put : (Spm + Spd) ratio with embryo development and germination (Silveira et al., 2004; Dias et al., 2009). On the other hand, high Spd : Put ratios were recorded in Pinus radiata embryos capable of germinating and forming plantlets (Minocha et al., 1999).
Seed germination can also be compared according to the desiccation tolerance in the seed, directly related with their metabolic features. Orthodox seeds acquire desiccation tolerance during development; their water content can drop to a low level but they remain viable in the dry state for predictable periods. Upon imbibition, orthodox seeds switch from quiescence to a highly active metabolism. On the other hand, recalcitrant seeds have a high water content at the moment they are shed. These seeds are sensitive to desiccation and are metabolically active at shedding (Barbedo and Marcos Filho, 1998; Pammenter and Berjak, 2000). Additionally, recalcitrant seeds are able to germinate without the need to add exogenous water (Berjak and Pammenter, 2008).
Although there are several reports addressing the effects of plant hormones and PAs on growth and development, studies on the changes in endogenous levels of PAs during seed germination, as well as the relationship between these compounds, in recalcitrant seeds, are scarce.
Physiological aspects of germination can also be influenced by seed structure (Atwater, 1980) and the mobilization of growth regulators between the embryo and the surrounding tissues (Debeaujon, 2000; Leubner-Metzger, 2002; Hermann et al., 2007). Seed morphological features, in turn, may be related to the plant's evolutionary history. According to a review by Linkies et al. (2010), the main changes that occurred throughout the seed evolutionary process are concerned with megagametophyte specialization into endosperm and with the increase in embryo size in comparison with seeds, caused by its growth during embryogenesis.
The seeds used in this study were from two different woody species, Ocotea odorifera (Lauraceae, Angiosperm) and Araucaria angustifolia (Araucariaceae, Gymnosperm) which show distinct seed morphologies, due to the evolutionary distance between the species. In A. angustifolia only part of the storage reserves from megagametophytes is transported to the embryo during its development within the corrosion cavity (King and Gifford, 1997). This keeps the two structures physically apart during and after embryo development and germination and the two structures do not present a clear connection. On the other hand, in O. odorifera, the storage organs are the cotyledons, while the embryo and the seed reserves form a single, physically connected system (Kucera et al., 2005). The two seeds, produced by native trees of the Brazilian Atlantic Rainforest, have recalcitrant characteristics and cannot be stored for long periods. The two species have been extensively exploited in the past, have irregular phenology, and pollination and seed formation occur at long intervals. As a result, both species have been declared critically endangered, according to IUCN (2010).
In the present work, contents of indole-acetic acid (IAA), ABA and PAs during seed germination of O. odorifera and A. angustifolia were assessed. The results presented here will help increase the knowledge of the physiological changes that take place during seed germination, mainly in recalcitrant seeds, and allow a better understanding on the communication between the reserve tissue and embryo between the evolutionarily distant gymnosperm and angiosperm seeds. In addition, this information will be useful to monitor seed germinability upon storage, adding knowledge to efforts on somatic embryo research in conservation programmes for these species.
MATERIALS AND METHODS
Plant material and germination
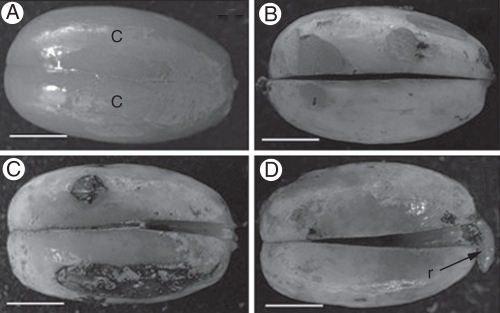
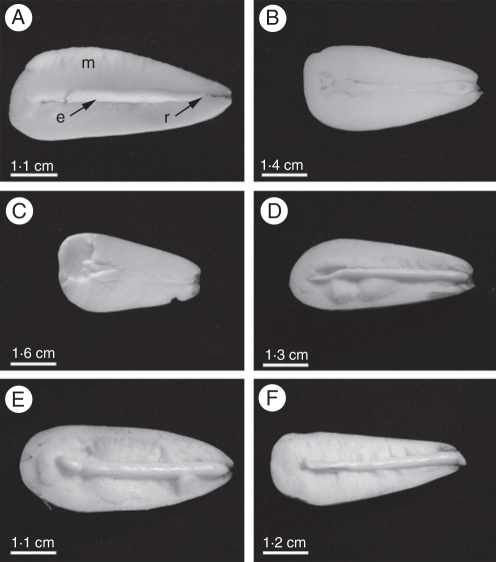
Mature seeds of Araucaria angustifolia (Araucariaceae, Gymnosperm) were harvested in Santa Catarina State, Brazil, in May 2008. Mature seeds of Ocotea odorifera (Lauraceae, Angiosperm) were harvested in São Paulo State, Brazil, in June 2008. Germination assays were carried out on three replicates of 15 seeds each. Before planting, seeds were scarified by cutting a small portion of the proximal pole (A. angustifolia) or excising part of the seed coat (O. odorifera). After that, seeds were placed in sterilized vermiculite : soil mixture (1 : 1), and irrigated every 2 d in a growth room at 27 °C under a 16-h photoperiod. Ocotea odorifera seeds were sampled 0, 15, 30 and 45 d after sowing, while A. angustifolia seeds were sampled 0, 2, 4, 8 and 10 d after sowing (Figs 1 and 2, respectively).
Fig. 1.
Morphological aspects of O. odorifera seed germination 0, 15, 30 and 45 d after sowing, with the radicle protruding in (D). Abbreviations: c, cotyledon; r, radicle. Scale bars = 0·6 cm.
Fig. 2.
Morphological aspects of A. angustifolia seed germination 0, 2, 4, 6, 8 and 10 d after sowing, with the radicle protruding in (F). Abbreviations: m, megagametophyte; e, embryo; r, radicle.
Germination was assessed based on the occurrence of radicle protrusion. Araucaria angustifolia seeds germinated within 10 d, while O. odorifera seeds germinated within 45 d. Because of morphological differences between these seeds, the analyses were conducted in different ways: A. angustifolia megagametophyte and embryos were isolated in every collection, while in O. odorifera embryos were isolated from the seed coat. All plant material was placed in liquid nitrogen and stored at –80 °C before HPLC analysis. Araucaria angustifolia megagametophytes and embryos were analysed separately, while O. odorifera embryos were analysed as one single structure.
Biochemical analysis by high-performance liquid chromatography (HPLC)
PAs
PA determination was performed according to Silveira et al. (2004). Samples (200 mg of fresh tissue) were ground in 1·6 mL of 5 % (v/v) perchloric acid. After 1 h, the samples were centrifuged for 20 min at 20 000 g at 4 °C. Free PAs were determined directly from the supernatant. Conjugated PAs were extracted by hydrolysing 200 µL of supernatant with 200 µL of 12 n HCl for 18 h at 110 °C. Samples were dried under nitrogen and then solubilized in 200 µL of 5 % perchloric acid. Free and conjugated PAs were derivatized by dansyl chloride and identified by HPLC, using a 5-μm C18 reverse-phase column (Shimadzu Shin-pack CLC ODS). The HPLC column gradient was created by adding incremental volumes of acetonitrile to an initial 10 % aqueous acetonitrile solution (pH 3·5). The gradient of acetonitrile was programmed to be 65 % over the first 11 min, ranging from 65 % to 100 % between 11 min and 25 min, and reaching 100 % in 35 min, at 1 mL min−1 flow and 40 °C. PA concentration was determined using a fluorescence detector at 340 nm (excitation) and 510 nm (emission). Peak areas and retention times were measured by comparison with standard PAs.
IAA and ABA
IAA and ABA concentrations were determined according to Santa-Catarina et al. (2006). Samples (1 g of fresh tissue) were ground in 5 mL of 80 % (v/v) ethanol and 1 % (w/v) polyvinylpyrrolidone-40. [3H]IAA and [3H]ABA were added as internal standards. After 90 min of incubation, samples were centrifuged for 15 min at 20 000 g at 4 °C. Supernatants were concentrated in a speed vac at 45 °C, upon reaching 20 % of the initial volume (1 mL). The volumes were adjusted with Milli'Q water to 3 mL, and the pH adjusted to 2·5 using HCl (1 n). Samples were partitioned twice with ethyl ether. The organic phases containing IAA and ABA were completely dried in a speed vac at 45 °C, dissolved in 300 µL of 100 % methanol, and stored at –70 °C until analysis. Aliquots of stored extracts were analysed by HPLC, using a 5-μm C18 reverse-phase column (Shimadzu Shin-pack CLC ODS). The gradient was created by adding increasing volumes of methanol to 10 % methanol plus 0·5 % acetic acid in water. The methanol gradient was programmed as: 20 % over the first 15 min; 20–45 % between 15 min and 22 min; 45–54 % between 22 min and 33 min; 54–100 % between 33 min and 34 min; and 100 % in 50 min, at a 1 mL min−1 flow rate at 40 °C. IAA concentration was determined using a fluorescence detector at 280 nm (excitation) and 350 nm (emission). ABA concentration was determined using a UV-VIS detector at 254 nm. Fractions containing IAA and ABA were collected and analysed in a Packard Tri-Carb liquid scintillation counter to estimate losses.
Data analysis
PAs, ABA and IAA contents were obtained in triplicate. Data are presented as mean and standard error.
RESULTS
PAs
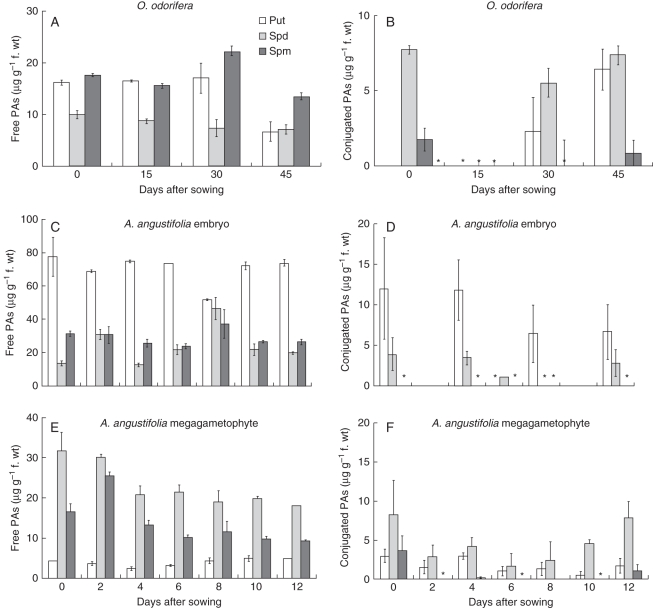
For O. odorifera, only Spm levels increased during radicle protusion (30 d after sowing), whereas the content of free Spd was constant and Put decreased after germination (Fig. 3A). The content of conjugated PAs followed a different pattern, when compared with free PAs. Conjugated PA levels had declined to zero 15 d after sowing, increasing continuously thereafter until the 45th day. The highest conjugated PA levels were observed for accumulated Put and Spd, while the lowest level was observed for Spm (Fig. 3B).
Fig. 3.
Free and conjugated PA levels in O. odorifera embryos (A, B) and A. angustifolia embryos (C, D) and megagametophytes (E, F) in mature seeds and throughout the germination process after sowing (mean ± s.e., n = 3). Asterisks represent the points where PAs content could not be detected.
For A. angustifolia embryos, free Put was the main PA observed, showing the highest levels throughout the experimental period, except for the 8th day, when levels were similar to those of the other PAs. At the same time, a peak was observed in the Spd level, while the Spm level did not vary considerably during the experimental period (Fig. 3C). Lower levels were observed for conjugated PAs, when compared with the variable levels measured for free PAs throughout seed germination. On the 10th day, when radicle protrusion was observed, levels of all conjugated PAs dropped to zero (Fig. 3D).
In A. angustifolia megagametophytes, the total free PA content was lower than in zygotic embryos. The highest levels were observed for Spd, followed by Spm and Put, in that order. Peaks of Spd and Spm were observed at the beginning of the experimental period (Fig. 3E). Comparatively, the levels of conjugated PAs in megagametophytes were lower than those observed for free PAs in embryos. In contrast to the A. angustifolia embryo, in megagametophytes the lowest PA level was observed for Put, while the highest levels were observed for Spd throughout the experimental period (Fig. 3F).
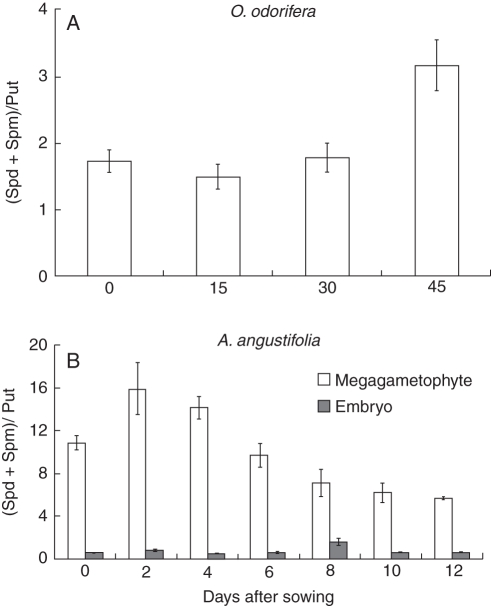
The (Spd + Spm) : Put ratio in O. odorifera seeds increased during the 45-d period after seed sowing, when seeds germinated (Fig. 4A) due to the increase in free Spm, in the same period (Fig. 3A). For A. angustifolia embryos, the (Spd + Spm) : Put ratio was <1 during seed germination, except on the 8th day (Fig. 4B), when an increase was observed. This peak resulted from the transient increase in free Spm and Spd levels and a reduced level of Put (Fig. 3C). In contrast to the pattern observed above, the (Spd + Spm) : Put ratio in megagametophytes was above 1 throughout the experimental period (Fig. 4C). The observed profile was probably caused by the higher levels of free Spm and Spd in comparison to free Put levels throughout seed germination, and by the decrease in both free Spm and Spd at the beginning of the process (Fig. 3E).
Fig. 4.
PAs ratio [(Spd + Spm)/Put] in (A) O. odorifera embryos and (B) A. angustifolia embryos and megagametophytes in mature seeds and throughout the germination process (mean ± s.e., n = 3).
IAA and ABA
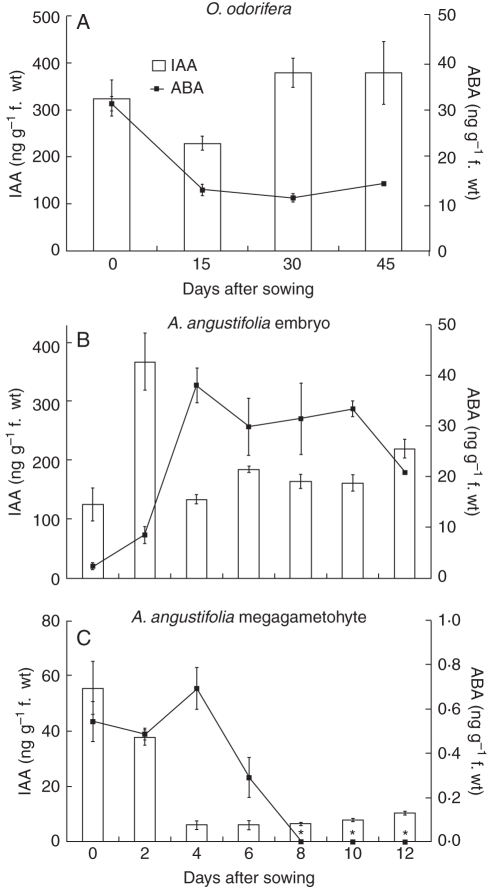
In all tissues analysed, IAA accumulated at higher levels when compared with ABA, for both species studied. However, the pattern of accumulation of both hormones during seed germination in O. odorifera and A. angustifolia displayed different profiles. In O. odorifera, IAA content decreased during the first 15 d after seed sowing, increasing and then returning to its initial content thereafter. ABA content also decreased during the beginning of seed germination, but then stabilized throughout the experimental period. Thirty days after sowing, IAA content was approx. 30 times higher than the ABA content (Fig. 5A).
Fig. 5.
IAA and ABA in (A) O. odorifera embryos, (B) A. angustifolia embryos and (C) A. angustifolia megagametophytes in mature seeds and throughout the germination process (mean ± s.e., n = 3). Asterisks represent the points where IAA or ABA content could not be detected.
Araucaria angustifolia embryos accumulated more IAA and ABA than megagametophytes during seed germination. Two days after seed sowing, the IAA content of embryos showed a major increase, and then decreased on the 4th day. A slight increase in IAA content was observed 12 d after seed sowing. ABA levels increased during the first 4 d after seed sowing, declining after that, throughout the experimental period (Fig. 5B). For megagametophytes, IAA content declined progressively until the 4th day and then remained stable 12 d after seed sowing. ABA levels were barely detected in megagametophytes, and, as in embryos, they started to decrease 4 d after seed sowing. Between the 8th and 12th day after seed sowing, the ABA content was undetectable (Fig. 5C).
DISCUSSION
In plants, intracellular levels of free PAs are tightly regulated at various steps, including de novo synthesis, degradation and transport (Kusano et al., 2008). This regulation plays an analogous role to the regulation required for plant growth regulators, and influences cell division and viability (Puga-Hermida et al., 2006; Handa and Mattoo, 2010). PAs can occur in the free form or conjugated with phenolic compounds and low molecular-weight molecules (Bouchereau et al., 1999; Kuznetsov et al., 2006), conjugation being a way to control intracellular concentration (Bagni and Pistocchi, 1990; Katerova and Todorova, 2009). On the other hand, other studies have provided evidence that both free and conjugated PAs play a role in floral initiation and flower development. They would act in important differentiation steps, or they may be part of the hormonal regulation of sex development in plants (reviewed in Bais and Ravishankar, 2002).
The increase in PAs in A. angustifolia and O. odorifera just prior to radicle protrusion were similar in both species analysed, and could be corroborated with observations reported for other systems. Seed dormancy in A. platanoides (Szczotka and Lewandowska, 1989) was broken by exogenous application of Spm. Total amounts of free and conjugated PAs increased during imbibition and decreased from radicle protrusion onwards in chick-pea seeds (Gallardo et al., 1992), as well as Brassica rapa (Puga-Hermida et al., 2006) and Picea abies (Gemperlová et al., 2009). A recent study demonstrated that inhibition of an intermediate compound at the methionine cycle alters PA levels, and impairs seedling growth and reproduction in arabidopsis (Bürstenbinder et al., 2010). In Arabidopsis seedlings, Put was the PA capable of restoring normal root growth after treatment with DFMO (difluoromethylornithine), a PA inhibitor. In the same species, another report showed that the addition of high Put concentrations, besides Spm and Spd treatment, inhibited seed germination (Mizra and Bagni, 1991). During apple seed germination, the effects of Put and Spd were stimulatory, while Spm was inhibitory (Sińska and Lewandowska, 2006).
Although the various studies cited above have shown variations in the content of PAs during germination, the type of PAs was different for each species mentioned. The results observed in the present work suggest that Spm and Spd could play an important role in the germination process of O. odorifera and A. angustifolia, respectively, in contrast to Put, whose profile did not change in either embryo (Fig. 3). In addition, as conjugation can be considered a way to control intracellular PA levels (Bagni and Pistocchi, 1990; Katerova and Todorova, 2009), the low content of conjugated Spm and Spd associated with the increase in conjugated Put (Fig. 3) reinforces the importance of free Spm and Spd in the germination process of both seeds analysed. Therefore, variation in PAs seems to be intrinsic to seed germination. Additionally, the different profiles observed here raise the question of whether a single PA can be used as a biomarker of the germination process or be more suitable to characterize this event considering the balance between the levels of PAs.
In the present work, an identical pattern of the (Spm + Spd) : Put ratio, which increased prior to radicle protrusion, could be observed, with the exception of the A. angustifolia megagametophyte. This result shows the importance of Spm and Spd in the period prior to radicle protrusion, in both species (Fig. 4). Similar results were observed in O. catharinensis seed germination (Dias et al., 2009). In Zea mays, endogenous Spd had a closer relationship with physiological changes of seeds during their development than that of Put and Spm (Cao et al., 2010). During embryogenesis, Put has been proposed to play a fundamental role in the beginning of this process, when the division rate is high, while higher levels of Spm and Spd would be essential at the end of embryo development, when growth largely depends on cellular elongation (Astarita et al., 2003; Santa-Catarina et al., 2006). These observations suggest the importance of the increase in Spm and Spd levels during cell elongation, a crucial event in the germination process.
On the other hand, the low levels of Spd and Spm in megagametophytes (Fig. 3E) (a haploid tissue of maternal origin) could be related to the low rates of cellular growth and division observed in this structure during germination. In addition, the low content of these PAs in the megagametophyte and the increase in their levels prior to radicle protrusion in the embryo (Fig. 3C) could be associated with possible transport of PAs from the megagametophyte to the embryo. The corrosion cavity, the space where the embryo develops, is filled with fluid that serves as a nutritional and hormonal interphase between the developing embryo and megagametophyte (Carman et al., 2005). In loblolly pine (Pinus taeda) seed, the megagametophyte has also been demonstrated to be a living tissue which interacts with the germinated embryo and young seedling (Brownfield et al., 2007). In Picea abies the accumulation of Spd in germinating embryos suggests that this PA is supplied by megagametophytes, since this tissue had high S-adenosylmethionine descarboxilase activity but low Spd levels (Gemperlová et al., 2009). Therefore, it may be that there is transport of PAs during germination of A. angustifolia seeds, in spite of the absence of the physical connection between the megagametophyte and embryo, as can be observed in more derived seeds like those of angiosperms. This would explain, at least in part, the increase in both free Spd and Spm in the embryo associated with the decrease in these PAs in megagametophytes (Fig. 3). Variation in PA levels in the different tissues and the communication between them in a seed has been reported for other species. In the seed of the angiosperm Cicer arietinum, where the reserve substance is concentrated in the cotyledons, the embryo axis attached to the cotyledons contains a high level of Put, Spm, Spd and Cad (cadaverine), as compared to cotyledons themselves, as well as a high level of Put, Spd and Spm, compared with the embryonic axis excised from the whole seed before germination. In contrast, embryotomized cotyledons showed a far higher PA content than whole seeds, implying that the cotyledons are at least a partial source of PAs (Gallardo et al., 1992).
IAA and ABA were differentially accumulated during O. odorifera and A. angustifolia seed germination. In O. odorifera, IAA and ABA variations (Fig. 5) were similar to the results observed in both dormant and non-dormant seeds of A. thaliana (Garciarrubio et al., 1997) and germinating seeds of O. catharinensis, a recalcitrant seed (Dias et al., 2009). In embryos of A. angustifolia, the IAA content (Fig. 5) was similar to those observed in Pinus sylvestris, in which the IAA levels were higher at the beginning of seed imbibition, decreasing during radicle elongation (Ljung et al., 2001). Interestingly, in megagametophytes the levels of both hormones decreased sharply during seed germination, similar to that observed for PA content, also suggesting a possible transport of hormones from this tissue to the embryo. An interaction between closely related tissues involving ABA mobilization has also been suggested. According to Farnsworth (2000), ABA can be detected in all seed components, and its regulatory action in seed dormancy may not be restricted to embryonic tissues alone, assuming a communication between embryo and endosperm. Another interesting mobilization of ABA between tissues was observed in Beta vulgaris seeds. Germination assays carried out in fruits of this species compared with their isolated seeds demonstrated that endogenous ABA content decreased upon imbibition, not only by degradation or absence of its biosynthesis but mainly by an embryo-mediated ABA extrusion system, in which the pericarp is involved to keep the ABA content in seeds low, supporting ABA extrusion (Hermann et al., 2007).
The development of somatic embryos, and their conversion to plants, is closely related to variations in endogenous phytohormone levels (Gemperlová et al., 2009). After imbibition during seed germination, the seeds set a specific metabolism, resulting in the decrease in ABA and gibberellin (GA) synthesis rate (Ali-Rachedi et al., 2004). The ABA/GA balance is crucial to the maturation and germination process, and ABA is responsible for promoting maturation and inhibiting cell-cycle progress, growth and germination (Curaba et al., 2003, Gazzarini et al., 2004), besides keeping imbibed seeds dormant (Finch-Savage et al., 2006). Like the relationship described for ABA and GA, a crosstalk between ABA and IAA was identified in different periods of seed development in A. thaliana. IAA activates the transcription factor FUS3, one of the factors responsible for the modulation of ABA synthesis at the beginning of seed maturation (Gutierrez et al., 2007). Hypersensitivity to ABA was reported to occur during sensu stricto germination and post-germination of these seeds, which were mutant to an auxin transcription factor (Liu et al., 2007). Therefore, in the present work, the decrease in ABA associated with increased IAA levels in O. odorifera embryos (Fig. 5) could be a result of crosstalk between these two phytohormones. In contrast, in a study that compared Beta vulgaris seed germination in the presence or absence of fruit, the authors reported that exogenous ABA did not affect the IAA content during the germination process, in any of the experimental situations (Hermann et al., 2007).
The repressor effect of ABA during seed germination could be explained by a restriction in energy and nitrogen availability, observed after exogenous application of this hormone in non-dormant arabidopsis seeds. However, this effect proved to be concentration dependent, though the seed is able to resume the germination process when amino acids and sugars are added to the culture medium (Garciarrubio et al., 1997). The ABA content in A. angustifolia embryos showed an unexpected increase (Fig. 5), compared with the drop in levels of this compound during the germination of orthodox seeds. However, this increase did not inhibit the germination process. Balbuena et al. (2011) observed an increase in ABA-induced proteins in embryos from germinated seeds of A. angustifolia. It is important to highlight the fact that this species did not show changes in its water content throughout germination (data not shown), and that in recalcitrant seeds the embryogenic and germination processes occur as a continuous process, without a metabolic interruption between the two events, as opposed to what occurs in orthodox seed. Therefore, it is possible that the germination process of A. angustifolia seeds had already begun when ABA levels increased, which made it more resistant to the effects of this compound. A greater tolerance to the effects of ABA has also been reported in desiccation-sensitive seeds, like Hopea odorata. In this species, even high levels of exogenously applied ABA could inhibit germination. The authors linked this fact to an active ABA metabolism, which showed high turnover and the existence of high levels of endogenous conjugates (Garello et al., 1995). In addition, despite the different behaviour of ABA between the embryos analysed, the absolute content at the end of the germination process in A. angustifolia and O. odorifera embryos was similar, around 10 ng g−1 fresh weight (f.wt). These levels are somewhat higher than that observed for the orthodox non-dormant arabidopsis seeds, which had levels around 3·6 ng g−1 f.wt at the end of germination (Ali-Rachedi et al., 2004).
The increase in IAA levels observed in both A. angustifolia embryos and O. odorifera embryos (Fig. 5) is probably related to radicle elongation. It is accepted that much of plant growth depends on auxin-induced cell division, expansion and differentiation. The auxin-mediated developmental process is a result of the differential distribution of auxin, established by spatio-temporally controlled synthesis, while the directional intercellular auxin transport is known to be essential to cell elongation (Cosgrove et al., 2002; Cosgrove, 2005; Vissenberg et al., 2005; Vanneste and Friml, 2009). Despite the participation of IAA in processes like cell division, expansion and differentiation, few studies have reported the correlation of this hormone to the germination process. Some works have shown that IAA increased PA levels and activities of enzymes involved in its biosynthesis (Rastogi and Davies, 1991; Park and Lee, 1994). In the present work, the highest values recorded for IAA accumulation, for both species, match the highest levels of Spm in O. odorifera embryos and the first peak of Spd in A. angustifolia embryos (Fig. 3). These results suggest a possible association between IAA and PAs during germination of this species. Auxin–polyamine interaction has been discussed in a study on the control of the rooting inductive phase of Poplar shoots in vitro (Hausman et al., 1995). In this study, the authors reported that exogenous Put caused an increase in endogenous levels of IAA as well as, to a limited extent, of its conjugated IAA aspartate. Quite similar results were obtained with application of aminoguanidine, an inhibitor of Spm enzyme synthesis. In addition, application of Spm and diamine oxidase, an enzyme that degrades Put, counteracted the effects induced by naphthaleneacetic acid. On the other hand, exogenous application of auxin in this root triggered a rise in endogenous Put level, which was reduced by applying Spd and aminoguanidine (Hausman et al., 1995).
Conclusions
The results reported here suggest that the interaction between the different seed tissues observed in angiosperms may also occur in gymnosperm seed tissues, despite the differences in seed structures caused by the evolutionary distance between these plants. In gymnosperms, transport of plant growth regulators may occur between megagametophyte and embryo, even though physical connections between these tissues are absent and the reserve tissue remains almost entirely outside the embryo, but inside the seed during embryogenesis, in contrast to angiosperms, in which endosperm is transported to the embryo. The present results also showed that PA accumulation in A. angustifolia differed from the values observed for O. odorifera during seed germination. Since both these seeds are recalcitrant, the difference observed could not be associated with the metabolic aspect of the seed (recalcitrant × orthodox), but probably with the evolutionary distance between the species. However, the PA balance in both embryos allowed us to propose the use of the (Spd + Spm) : Put ratio as a marker of the germination process. The increase in IAA levels, prior to germination, could be associated with variation in PA content. In addition, the ABA mobilization observed in the embryos could represent a greater resistance to this hormone in comparison to orthodox seeds, and could be explained by the recalcitrant behaviour of the seeds studied in this work. This pattern opens a new perspective for studies on the effects of this regulator on recalcitrant seeds. The results presented here shed new light on seed development and germination and may pave the way for further studies. In addition, this information will be useful for monitoring the ability of seeds to germinate after storage and it increases our knowledge in our efforts to conserve somatic embryos in these endangered native trees of the Atlantic Rainforest in Brazil.
ACKNOWLEDGEMENTS
This work was supported financially by FAPESP, CNPq and Petrobras.
LITERATURE CITED
- Ali-Rachedi S, Bouinot D, Wagner M-H, et al. Changes in endogenous abscisic acid levels during dormancy release and maintenance of mature seeds: studies with the Cape Verde Islands ecotype, the dormant model of Arabidopsis thaliana. Planta. 2004;219:479–488. doi: 10.1007/s00425-004-1251-4. [DOI] [PubMed] [Google Scholar]
- Astarita LV, Handro W, Floh EIS. Changes in polyamines content associated with zygotic embryogenesis in the Brazilian pine, Araucaria angustifolia (Bert.) O. Ktze. Revista Brasileira de Botânica. 2003;26:163–168. [Google Scholar]
- Atwater BR. Germination, dormancy and morphology of the seeds of herbaceous ornamental plants. Seed Science and Technology. 1980;8:523–573. [Google Scholar]
- Bagni N, Pistocchi R. Binding transport and subcellular compartmentation of polyamines. In: HE Flores, RN Arteca, Shannon JC., editors. Polyamines and ethylene: biochemistry, physiology and interactions. Rockville, MD: American Society of Plant Physiologists; 1990. pp. 62–72. [Google Scholar]
- Bagni N, Malucelli B, Torrigiani P. Polyamines, storage substances and abscisic acid-like inhibitors during dormancy and very early activation of Helianthus tuberosus tuber tissues. Physiologia Plantarum. 1980;49:341–345. [Google Scholar]
- Bais HP, Ravishankar GA. Role of polyamines in the ontogeny of plants and their biotechnological applications. Plant Cell, Tissue and Organ Culture. 2002;69:1–34. [Google Scholar]
- Balbuena TS, Jo L, Pieruzzi FP, et al. Differential proteome analysis of mature and germinated embryos of Araucaria angustifolia. Phytochemistry. 2011;72:302–311. doi: 10.1016/j.phytochem.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Barbedo CJ, Marcos Filho J. Tolerância à dessecação em sementes. Acta Botanica Brasilica. 1998;12:145–164. [Google Scholar]
- Barendse GW, Peeters TJM. Multiple hormonal control in plants. Acta Botanica Neerlandica. 1995;44:3–17. [Google Scholar]
- Berjak P, Pammenter NW. From Avicennia to Zizania: seed recalcitrance in perspective. Annals of Botany. 2008;101:213–228. doi: 10.1093/aob/mcm168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD. Seed germination and dormancy. The Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchereau A, Aziz A, Larher F, Martin-Tanguy J. Polyamines and environmental challenges: recent development. Plant Science. 1999;140:103–125. [Google Scholar]
- Brownfield DL, Todd CD, Stone SL, Deyholos MK, Gifford DJ. Patterns of storage protein and triacylglycerol accumulation during loblolly pine somatic embryo maturation. Plant Cell, Tissue and Organ Culture Volume. 2007;88:217–223. [Google Scholar]
- Bürstenbinder K, Waduwara I, Schoor S, et al. Inhibition of 5’-methylthioadenosine metabolism in the Yang cycle alters polyamine levels, and impairs seedling growth and reproduction in Arabidopsis. The Plant Journal. 2010;62:977–988. doi: 10.1111/j.1365-313X.2010.04211.x. [DOI] [PubMed] [Google Scholar]
- Cao DD, Hua J, Zhua SJ, Hua WM, Knapp A. Relationship between changes in endogenous polyamines and seed quality during development of sh2 sweet corn (Zea mays L.) seed. Scientia Horticulturae. 2010;123:301–307. [Google Scholar]
- Carman JG, Rodney GR, Fuller J, Ghermay T, Timmis R. Nutrient and hormone levels in Douglas-fir corrosion cavities, megagametophytes, and embryos during embryony. Canadian Journal of Forest Research. 2005;35:2447–2456. [Google Scholar]
- Chen M, Chory J, Fankhauser C. Light signal transduction in higher plants. Annual Review of Genetics. 2004;38:87–117. doi: 10.1146/annurev.genet.38.072902.092259. [DOI] [PubMed] [Google Scholar]
- Cheng WH, Endo A, Zhou L, et al. A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. The Plant Cell. 2002;14:2723–2743. doi: 10.1105/tpc.006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SS. A guide to the polyamines. Oxford: Oxford University Press; 1998. [Google Scholar]
- Cosgrove DJ, Li LC, Cho HT, Hoffmann-Benning S, Moore RC, Blecker D. The growing world of expansins. Plant Cell Physiology. 2002;43:1436–1444. doi: 10.1093/pcp/pcf180. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Growth of the plant cell wall. Nature Review Molecular Cell Biology. 2005;6:850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- Curaba J, Herzog M, Vachon G. GeBP, the first member of a new gene family in Arabidopsis, encodes a nuclear protein with DNA-binding activity and is regulated by KNAT1. The Plant Journal. 2003;33:305–317. doi: 10.1046/j.1365-313x.2003.01622.x. [DOI] [PubMed] [Google Scholar]
- Debeaujon I, Léon-Kloosterziel KM, Koornneef M. Influence of the testa on seed dormancy, germination, and longevity in arabidopsis. Plant Physiology. 2000;122:403–414. doi: 10.1104/pp.122.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias LLC, Santa-Catarina C, Silveira V, Pieruzzi FP, Floh EIS. Polyamines, amino acids, IAA and ABA contents during Ocotea catharinensis seed germination. Seed Science and Technology. 2009;37:42–51. [Google Scholar]
- Farnsworth E. The ecology and physiology of viviparous and recalcitrant seeds. Annual Review of Ecology and Systematics. 2000;31:107–138. [Google Scholar]
- Feurtado JA, Ambrose SJ, Cutler AJ, Ross ARS, Abrams SR, Kermode A. Dormancy termination of western white pine (Pinus monticola Dougl. Ex D. Don) seeds is associated with changes in abscisic acid metabolism. Planta. 2004;218:630–639. doi: 10.1007/s00425-003-1139-8. [DOI] [PubMed] [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. New Phytologist. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- Frey A, Godin B, Bonnet M, Sotta B, Marion-Poll A. Maternal synthesis of abscisic acid controls seed development and yield in Nicotiana plumbaginifolia. Planta. 2004;218:958–964. doi: 10.1007/s00425-003-1180-7. [DOI] [PubMed] [Google Scholar]
- Gallardo M, Bueno M, Angosto T, Gallardo E, Matilla AJ. Free polyamines in Cicer arietinum seeds during the onset of germination. Phytochemistry. 1992;31:2283–2287. [Google Scholar]
- Garciarrubio A, Legaria JP, Covarrubias AA. Abscisic acid inhibits germination of mature Arabidopsis seeds by limiting the availability of energy and nutrients. Planta. 1997;203:182–187. doi: 10.1007/s004250050180. [DOI] [PubMed] [Google Scholar]
- Garello G, LePaige-Degivry MT. Desiccation-sensitive Hopea odorata seeds: sensitivity to abscisic acid, water potential, and inhibitors of gibberellin biosynthesis. Physiologia Plantarum. 1995;95:45–50. [Google Scholar]
- Gazzarini S, Tsuchiya Y, Lumba S, Okamoto M, McCourt P. The transcription factor FUSCA3 controls developmental timing in Arabidopsis through the hormones gibberellin and abscisic acid. Developmental Cell. 2004;7:373–385. doi: 10.1016/j.devcel.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Gemperlová L, Fischerová L, Cvikrová M, et al. Polyamine profiles and biosynthesis in somatic embryo development and comparison of germinating somatic and zygotic embryos of Norway spruce. Tree Physiology. 2009;29:1287–1298. doi: 10.1093/treephys/tpp063. [DOI] [PubMed] [Google Scholar]
- Groppa MD, Zawoznik MS, Tomaro ML, MP Benavides. Inhibition of root growth and polyamine metabolism in sunflower (Helianthus annuus) seedlings under cadmium and copper stress. Biological Trace Element Research. 2007;126:1–3. doi: 10.1007/s12011-008-8191-y. [DOI] [PubMed] [Google Scholar]
- Gutierrez L, Wuytswinkel OV, Castelain M, Bellini C. Combined networks regulating seed maturation. Trends in Plant Science. 2007;12:294–300. doi: 10.1016/j.tplants.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Handa AK, Mattoo AK. Differential and functional interactions emphasize the multiple roles of polyamines in plants. Plant Physiology and Biochemistry. 2010;48:540–546. doi: 10.1016/j.plaphy.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Hausman JF, Keversa C, Gaspar T. Auxin-polyamine interaction in the control of the rooting inductive phase of poplar shoots in vitro. Plant Science. 1995;110:63–71. [Google Scholar]
- Hermann K, Meinhard J, Dobrev P, et al. 1-Aminocyclopropane -1-carboxylic acid and abscisic acid during the germination of sugar beet (Beta vulgaris L.): a comparative study of fruits and seeds. Journal of Experimental Botany. 2007;58:3047–3060. doi: 10.1093/jxb/erm162. [DOI] [PubMed] [Google Scholar]
- Igarashi K, Kashiwagi K. Polyamines: mysterious modulators of cellular functions. Biochemical and Biophysical Research Communications. 2000;271:559–564. doi: 10.1006/bbrc.2000.2601. [DOI] [PubMed] [Google Scholar]
- IUCN. IUCN Red List of Threatened Species. Version 2010·1. 2010 http://www.iucnredlist.org. (acessed September 2010) [Google Scholar]
- Katerova ZI, Todorova D. Endogenous polyamines lessen membrane damages in pea plants provoked by enhanced ultraviolet-C radiation. Plant Growth Regulation. 2009;57:145–152. [Google Scholar]
- Kermode AR. Regulatory mechanisms involved in the transition from seed development to germination. Critical Reviews in Plant Sciences. 1990;9:155–195. [Google Scholar]
- Kermode AR. Role of abscisic acid in seed dormancy. Journal Plant Growth Regulation. 2005;24:319–344. [Google Scholar]
- King JE, Gifford DJ. Amino acid utilization in seeds of loblolly pine during germination and early seedling growth. I. Arginine and arginase activity. Plant Physiology. 1997;113:1125–1135. doi: 10.1104/pp.113.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera B, Cohn A, Leubner-Metzger G. Plant hormone interactions during seed dormancy release and germination. Seed Science Research. 2005;15:281–307. [Google Scholar]
- Kumar A, Taylora M, Altabella T, Tiburcio AF. Recent advances in polyamine research. Trends in Plant Science. 1997;2:124–130. [Google Scholar]
- Kushiro T, Okamoto M, Nakabayashi K, et al. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: key enzymes in ABA catabolism. EMBO Journal. 2004;23:1647–1656. doi: 10.1038/sj.emboj.7600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzano T, Berberich T, Tateda C, Takahashi Y. Polyamines: essential factors for growth and survival. Planta. 2008;228:367–381. doi: 10.1007/s00425-008-0772-7. [DOI] [PubMed] [Google Scholar]
- Kuznetsov V, Radyukina NL, Shevyakova NI. Polyamines and stress: biological role, metabolism and regulation. Russian Journal Plant Physiology. 2006;53:583–604. [Google Scholar]
- Leubner-Metzger G. Seed after-ripening and over-expression of class I β-1,3-glucanase confer maternal effects on tobacco testa rupture and dormancy release. Planta. 2002;215:959–968. doi: 10.1007/s00425-002-0837-y. [DOI] [PubMed] [Google Scholar]
- Linkies A, Graeber K, Knight , Leubner-Metzger G. The evolution of seed. New Phytologist. 2010;186:817–831. doi: 10.1111/j.1469-8137.2010.03249.x. [DOI] [PubMed] [Google Scholar]
- Liu PP, Montgomery TA, Fahlgren N, Kasschau KD, Nonogaki H, Carrington JC. Repression of auxin response factor10 by microRNA160 is critical for seed germination and postgermination stages. The Plant Journal. 2007;52:133–146. doi: 10.1111/j.1365-313X.2007.03218.x. [DOI] [PubMed] [Google Scholar]
- Ljung K, Östin A, Lioussanne L, Sandberg G. Developmental regulation of indole-3-acetic acid turnover in Scots pine seedlings. Plant Physiology. 2001;125:464–475. doi: 10.1104/pp.125.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minocha R, Dale RS, Cathie R, Steele KD, Minocha SC. Polyamine levels during the development of zygotic and somatic embryos of Pinus radiata. Physiologia Plantarum. 1999;105:155–164. [Google Scholar]
- Mirza JI, Bagni N. Effects of exogenous polyamines and difluoromethylornithine on seed germination and root growth of Arabidopsis thaliana. Plant Growth Regulation. 1991;10:163–168. [Google Scholar]
- Mockaitis K, Estelle M. Auxin receptors and plant development: a new signaling paradigm. Annual Review of Cell Developmental Biology. 2008;24:55–80. doi: 10.1146/annurev.cellbio.23.090506.123214. [DOI] [PubMed] [Google Scholar]
- Pammenter NW, Berjak P. Evolutionary and ecological aspects of recalcitrant seed biology. Seed Science Research. 2000;10:301–306. [Google Scholar]
- Papadakis AK, Paschalidis KA, Roubelakis-Angelakis KA. Biosynthesis profile and endogenous titers of polyamines differ in totipotent and recalcitrant plant protoplasts. Physiologia Plantarum. 2005;125:10–20. [Google Scholar]
- Park KY, Lee SH. Effects of ethylene and auxin on polyamine levels in suspension-cultured tobacco cells. Physiologia Plantarum. 1994;90:382–390. [Google Scholar]
- Puga-Hermida MI, Gallardo M, Rodriguez-Gacio MC, Matilla AJ. Polyamine contents, ethylene synthesis, and BrACO2 expression during turnip germination. Biologia Plantarum. 2006;50:574–580. [Google Scholar]
- Rastogi R, Davies PJ. Effects of light and plant growth regulators on polyamine metabolism in higher plants. In: RD Slocum, Flores HE., editors. Biochemistry and physiology of polyamines in plants. Boca Raton, FL: CRC Press; 1991. pp. 187–198. [Google Scholar]
- Santa-Catarina C, Silveira V, Balbuena TS, Maranhão MEE, Handro W, Floh EIS. IAA, ABA, polyamines and free amino acids associated with zygotic embryo development of Ocotea catharinensis. Plant Growth Regulation. 2006;49:237–247. [Google Scholar]
- Shoeb F, Yadav JS, Bajaj S, Rajam MV. Polyamines as biomarkers for plant regeneration capacity: improvement of regeneration by modulation of polyamine metabolism indifferent genotypes of Indica rice. Plant Science. 2001;160:1229–1235. doi: 10.1016/s0168-9452(01)00375-2. [DOI] [PubMed] [Google Scholar]
- Silveira V, Balbuena TS, Santa-Catarina C, Floh EIS, Guerra MP, Handro W. Biochemical changes during zygotic embryogenesis in Pinus taeda L. Plant Growth Regulation. 2004;44:147–156. [Google Scholar]
- Silveira V, Santa-Catarina C, Tun NN, et al. Polyamine effects on the endogenous polyamine contents, nitric oxide release, growth and differentiation of embryogenic suspension cultures of Araucaria angustifolia (Bert.) O. Ktze. Plant Science. 2006;171:91–98. [Google Scholar]
- Sińska I, Lewandowska U. Polyamines and ethylene in the removal of embryonal dormancy in apple seeds. Physiologia Plantarum. 2006;81:59–64. [Google Scholar]
- Szczotka Z, Lewandowska U. Polyamines in dormancy breaking of tree seeds. Forest Tree Physiology. 1989;46:95–97. [Google Scholar]
- Tabor CW, Tabor H. Polyamines. Annual Review of Biochemistry. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kakehi JI. Polyamines: ubiquitous polycations with unique roles in growth and stress responses. Annals of Botany. 2010;105:1–6. doi: 10.1093/aob/mcp259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromas A, Perrot-Rechenmann C. Recent progress in auxin biology. Comptes Rendus Biologies. 2010;333:297–306. doi: 10.1016/j.crvi.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Vanneste S, Friml J. Auxin: a trigger for change in plant development. Cell. 2009;136:1005–1016. doi: 10.1016/j.cell.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Vissenberg K, Fry SC, Pauly M, Hofte H, Verbelen JP. XTH acts at the microfibril–matrix interface during cell elongation. Journal of Experimental Botany. 2005;56:673–683. doi: 10.1093/jxb/eri048. [DOI] [PubMed] [Google Scholar]
- Zhao Y. Auxin biosynthesis and its role in plant development. Annual Review of Plant Biology. 2010;61:49–64. doi: 10.1146/annurev-arplant-042809-112308. [DOI] [PMC free article] [PubMed] [Google Scholar]