Abstract
Backgrounds and Aims
Lecanora conizaeoides was until recently western and central Europe's most abundant epiphytic lichen species or at least one of the most common epiphytes. The species is adapted to very acidic conditions at pH values around 3 and high concentrations of SO2 and its derivatives formed in aqueous solution, and thus spread with increasing SO2 deposition during the 19th and 20th centuries. With the recent decrease of SO2 emissions to nearly pre-industrial levels within 20 years, L. conizaeoides declined from most of its former range. If still present, the species is no longer the dominant epiphyte, but is occurring in small densities only. The rapid spread of the L. conizaeoides in Europe from an extremely rare species to the probably most frequent epiphytic lichen and the subsequent rapid dieback are unprecedented by any other organism. The present study aimed at identifying the magnitude of deacidification needed to cause the dieback of the lichen.
Methods
The epiphytic lichen diversity and bark chemistry of montane spruce forests in the Harz Mountains, northern Germany, were studied and the results were compared with data recorded with the same methods 13–15 years ago.
Key Results
Lecanora conizaeoides, which was the dominant epiphyte of the study area until 15 years ago, is still found on most trees, but only with small cover values of ≤1 %. The bark pH increased by only 0·4 pH units.
Conclusions
The data suggest that only slight deacidification of the substratum causes the breakdown of the L. conizaeoides populations. Neither competitors nor parasites of L. conizaeoides that may have profited from reduced SO2 concentrations are likely causes of the rapid dieback of the species.
Keywords: Acidity, air pollution, bark chemistry, epiphytes, Lecanora conizaeoides, lichen-forming fungi, sub-stratum pH, sulfur dioxide
INTRODUCTION
Though species of lichen-forming fungi may have large ranges, they are rarely as frequent within their distribution limits as are ‘frequent’ species of vascular plants. This is at least true for the density of localities, whereas the density of individuals is hardly comparable between organisms that differ so much in their size and biology as lichens and vascular plants. One of the rare exceptions of a lichen-forming fungus with very high densities of both inhabited locations and individuals was, until recently, the ascomycete Lecanora conizaeoides, which forms a lichen symbiosis together with the green alga Trebouxia simplex (Hauck et al., 2007).
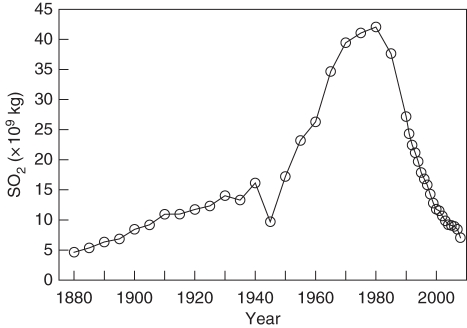
The range of L. conizaeoides includes the oceanic to sub-continental parts of Europe with a few occurrences in coastal areas of North America (LaGreca and Stutzman, 2006). In areas of western and central Europe, which were exposed to high acidic air pollution during the 20th century (Fig. 1), L. conizaeoides was, if not the most common, at least among the most common epiphytic lichen species (Wirth, 1993). In large parts of its range, L. conizaeoides was found in almost every tree stand and on nearly every tree. The abundance of the lichen was clearly correlated with the deposition of acidic sulfurous air pollutants (Hawksworth and Rose, 1970; Bates et al., 1996). Therefore, L. conizaeoides experienced an enormous spread during the 19th and 20th centuries with increasing SO2 emissions. The extent of this spread depended on the pollutant load and thus varied between regions. In England, the initial point of global industrialization, L. conizaeoides was first noticed in the mid-19th century (Hawksworth et al., 1973). Near London, L. conizaeoides already formed a universal cover of tree trunks and branches (Paulson and Thompson, 1913). In northern Germany, L. conizaeoides was widespread and locally abundant in the early 20th century, but was not found by Erichsen (1933) in the higher elevations of the Harz Mountains, the study area of the present work, in 1930, where the species became probably abundant in the 1950s (H. Ullrich, pers. comm.). In the 1950s, L. conizaeoides was already the most frequent epiphytic lichen of northern Germany (Erichsen, 1957; Klement, 1958). At that time, it was still rare in less polluted parts of southern Germany, but spread a decade later (Wirth, 1985). With the reduction of SO2 emissions in the late 20th century (Vestreng et al., 2007), L. conizaeoides declined from most areas of Europe as rapidly as it had spread before. For England and southern Germany, such a dieback of L. conizaeoides was documented for the 1980s and 1990s (Wirth, 1993; Bates et al., 2001). In England, Bates et al. (2001) established a decrease of the covered sub-stratum area by the species from up to 80 to 0 % within 10 years.
Fig. 1.
Sulfur dioxide emissions in Europe (excluding Russia) from 1880 to 2008 (after Mylona, 1996; European Environment Agency, unpubl. res.).
The rapid changes of the abundance of L. conizaeoides in Europe from a very rare species to the most common epiphytic lichen and afterwards from an extremely common species to a rare one demand an explanation. There is no doubt that the high tolerance to acidic sulfurous solutions with pH values as low as 3·0, which is far beyond the acidity tolerance of most other lichens, explains the spread of L. conizaeoides with increasing air pollution (Hauck et al., 2001a; LaGreca and Stutzman, 2006). The superhydrophobic thallus surface, which inhibits much of the acidic solution from soaking the thallus, is thought to be the main cause of the tolerance (Shirtcliffe et al., 2006; Hauck et al., 2008). Moreover, the production of the extracellular depsidone fumarprotocetraric acid is apparently a prerequisite for the high acidity tolerance of L. conizaeoides (Hauck et al., 2009a, b). The apparently outstanding vagility of L. conizaeoides and its photobiont also seems to be significant for the rapid spread of this lichen symbiosis (Hauck et al., 2007). However, while the mechanisms causing acidity tolerance are largely known, no mechanistic explanation for the recent dieback of L. conizaeoides with decreasing acidic pollutant load exists. Two research questions arise in this context. (1) How strong was the change in the environmental pH conditions to cause the dramatic dieback of L. conizaeoides? (2) What kind of physiological mechanism makes L. conizaeoides not only tolerant of, but even dependent on, acidity?
In the present work, we focused on the first of these questions, as the extent of the pH change has to be known to search for ecophysiological explanations. Since the decline of L. conizaeoides from a dominant species to a very rare one, or the extinction at a given place, can happen within short time scales (e.g. ≤5 years in southern Germany; Wirth, 1993), we tested the hypothesis that only a slight decrease of acidity may cause the decline of L. conizaeoides. Components which are relevant to the chemical environment of a lichen include the sub-stratum, precipitation, fog and gases (Farmer et al., 1991). Our study is based on the measurement of pH values of aqueous suspensions of tree bark, as the chemical traits of the sub-stratum underlie a lower short-term variation than that of precipitation (Hauck, 2000). Furthermore, bark samples were analysed for element concentrations to check whether chemical parameters other than the pH itself may have been altered due to the reduced pollutant load and thereby potentially affected the abundance of L. conizaeoides. The measurements, which were recorded from montane spruce forests of the Harz Mountains, northern Germany are compared with 13–15 year old data from the same study area. Lecanora conizaeoides was the dominant epiphyte on spruce in the Harz Mountains from the 1960s to the 1990s. In the 1990s, virtually all spruce trees were inhabited by L. conizaeoides. In a study including >200 spruce trees, all of them were colonized by L. conizaeoides and the species reached a cover of up to 80 % on individual tree trunks (Hauck, 2000).
MATERIALS AND METHODS
Study area
The study was conducted in montane forests of Norway spruce (Picea abies) in the Harz Mountains, northern Germany. Recent data were collected in 2010 on Mt Brocken (51 °47′N, 10 °38'E) at 1000 m and compared with data from the same forest of 1997 (published in Hauck et al., 2002) and from the Acker-Bruchberg ridge (51 °45' to 51 °46'N, 10 °27' to 10 °28'E) at 800 m from 1995 (Hauck et al., 2001a; Hauck and Runge, 2002). The Harz Mountains are the highest mountain range in northern Germany with elevations up to 1142 m (Mt Brocken) and are primarily formed of acidic Paleozoic rocks. At elevations between 800 and 1142 m, annual mean temperature varies between 3 and 6 °C and precipitation ranges from 1500 to 1600 mm. The prevailing winds come from the southwest and west; fog events and long-lasting snow cover are common. Norway spruce is native to the Harz Mountains at the studied altitudes.
Soil pH
The pH of aqueous soil suspensions of 10 g dry weight (d. wt) of soil in 20 mL of deionized water was determined in the upper soil (at 5 to 10 cm) from 25 randomly selected sample points in 2010 and compared with data from 12 sample points of 1997 (Hauck et al., 2002). The mean pH values in 2010 amounted to 3·6 ± 0·0 in H2O and 3·0 ± 0·0 in KCl, which exceeds the pH values published by Hauck et al. (2002) by approx. 0·4 pH units. Those pH values amounted to 3·2 ± 0·1 in H2O and 2·6 ± 0·1 in KCl.
Recording epiphytic lichen data, bark sampling and chemical analysis
A total of 90 mature spruce trees was sampled in 2010 in an area of 20 ha. All living spruce trees with a minimum diameter at breast height of 15 cm and all snags with at least some bark left at the trunk around the breast height level were sampled on 26 plots of a size 10 m × 10 m. These plots were available from a forest inventory and biodiversity study and covered different stages of the natural dynamics cycle in the spruce forest ecosystem. Therefore, the sample trees can be assumed to represent an unbiased collective of the forest trees in terms of the cover of L. conizaeoides Nyl. ex Crombie and bark acidity. The number of sample trees on the 10 m × 10 m plots amounted to 3·9 ± 0·3, with a maximum of seven trees per plot. Since living and dead conifers are known to differ in their bark chemistry and in the cover of L. conizaeoides (Hauck, 2005), the vitality status of the trees was recorded to enable a separate data evaluation for living and dead trees. In total, 53 living trees and 37 snags were sampled. Epiphytic lichen vegetation was recorded from the trunk of every sample tree at a height of 0–2 m above the ground. Cover of the individual epiphytic lichen species, including L. conizaeoides, was estimated as a percentage. The nomenclature of lichen species is based on Wirth et al. (2011).
Bark was sampled from each sample tree from a standard exposure (i.e. western exposure at a height of 100–200 cm above the ground) to be consistent with former studies of our group (Hauck et al., 2001a, 2002). Epiphytes were carefully removed from the bark surface with a wire brush before sampling. Bark samples were dried at 105 °C and homogenized in a swing mill using an insert that is free from metal abrasion. The bark powder was used to determine the C and N concentrations using a C/N analyser (Vario EL III, Elementar Analysensysteme, Hanau, Germany). Bark pH was measured with an MP 120 pH meter with electrode InLab 413 (Mettler-Toledo, Greifensee, Switzerland) in suspensions with deionized water (25 mL per g d. wt) shaken for 24 h before the measurements (pH [H2O]). After acid digestion with 65 % HNO3, the total concentrations of potassium (K), calcium (Ca), magnesium (Mg), iron (Fe), aluminium (Al), manganese (Mn), zinc (Zn), copper (Cu), phosphorus (P) and sulfur (S) were analysed with ICP-OES using an Optima 5300DV (Perkin Elmer, Waltham, MA, USA).
Reference data of lichen cover from 1995 to 1997 were collected from 14 living trees and six snags from Mt Brocken (Hauck et al., 2002) and 115 living trees and 25 snags from the Acker-Bruchberg ridge (Hauck et al., 2001a; Hauck and Runge, 2002). Data of bark pH and element content were available for 100 living trees and 20 dead trees from the Acker-Bruchberg ridge, but not from Mt Brocken. Epiphytic lichen data and bark chemistry characteristics were principally studied with the same methods as in the present work, though metal concentrations were studied with AAS (SpectrAA 30, Varian, Mulgrave, Victoria, Australia) instead of ICP-OES.
Statistics
Arithmetic means ± s.e. are presented throughout the paper. All data were tested for normal distribution with the Shapiro–Wilk test. Significance of differences in vegetation and bark chemistry data, which were not normally distributed, was tested with the Kruskal–Wallis test. These analyses were calculated with SAS 6·04 software (SAS Institute Inc., Cary, NC, USA). Detrended correspondence analysis (DCA) was applied to study differences between different tree collectives in variation of epiphytic lichen abundance. Canonical correspondence analysis (CCA) was used to relate site factors to the variation of epiphytic lichen cover on the tree trunks. The significance of correlations between epiphytic lichen data and environmental parameters was tested with a Monte Carlo permutation test with 999 iterations. Species occurring on <10 % of the sample trees were excluded from the CCA. DCA and CCA were calculated with the program PC-Ord 4·01 (MjM Software, Gleneden Beach, OR, USA).
RESULTS
Bark chemistry
The pH of aqueous bark suspensions increased by 0·4 units on living trees and by 0·25 on dead trees from the 1990s to 2010 (Table 1). The bark of dead trees was less acidic than that of living trees in both sampling periods. However, the pH difference between the bark of dead and living trees became smaller; it diminished from 0·3 to 0·1 pH units. The tree bark was richer in N, K and Mg in 2010 than in the 1990s, whereas the S and Fe concentrations of bark and even more the Fe/Mn ratio were lower in 2010 than 15 years earlier.
Table 1.
Element concentrations (in μmol g−1 d wt; in the case of C, mmol g−1 d. wt) and pH of spruce bark collected from trunks at 1·5 m height in the Harz Mountains in 2010 and 1997 (Mt Brocken; B2010, B1997) as well as 1995 (Acker-Bruchberg ridge; A1995)
| B2010 |
B1997 |
A1995 |
||||
|---|---|---|---|---|---|---|
| Living trees | Snags | Living trees | Snags | Living trees | Snags | |
| pH | 3·60 ± 0·02 | 3·71 ± 0·06 | – | – | 3·18 ± 0·01 | 3·46 ± 0·06 |
| N | 306 ± 6 | 333 ± 17 | – | – | 164 ± 17 | 496 ± 37 |
| P | 5·59 ± 0 | 7·93 ± 0·89 | – | – | 6·92 ± 0·22 | 12·3 ± 0·9 |
| S | 14·7 ± 1·3 | 14·2 ± 0·9 | – | – | 17·1 ± 0·4 | 21·0 ± 1·9 |
| C | 41·9 ± 0·1 | 42·0 ± 0·2 | – | – | 44·1 ± 0·1 | 43·9 ± 0·2 |
| K | 10·8 ± 1·9 | 18·8 ± 3·2 | 9·91 ± 1·18 | 13·5 ± 2·3 | 6·67 ± 0·43 | 8·04 ± 1·18 |
| Ca | 143 ± 6 | 147 ± 12 | 164 ± 16 | 201 ± 30 | 124 ± 5 | 163 ± 12 |
| Mg | 6·29 ± 0·33 | 11·3 ± 1·2 | 5·26 ± 0·29 | 9·26 ± 1·46 | 5·67 ± 0·27 | 10·3 ± 1·1 |
| Fe | 1·76 ± 0·13 | 1·15 ± 0·11 | 6·29 ± 1·12 | 6·24 ± 1·10 | 8·23 ± 0·34 | 4·98 ± 0·69 |
| Mn | 1·30 ± 0·08 | 1·75 ± 0·17 | 1·44 ± 0·11 | 1·79 ± 0·13 | 1·67 ± 0·07 | 2·06 ± 0·26 |
| Fe/Mn | 1·58 ± 0·14 | 1·17 ± 0·35 | 4·90 ± 0·89 | 3·53 ± 0·67 | 6·25 ± 0·51 | 4·79 ± 1·51 |
| Al | 4·74 ± 0·30 | 4·30 ± 0·34 | – | – | 6·74 ± 0·29 | 4·90 ± 0·81 |
| Zn | 1·15 ± 0·06 | 1·56 ± 0·14 | 1·71 ± 0·25 | 2·51 ± 0·51 | 1·66 ± 0·09 | 3·58 ± 0·20 |
| Cu | 0·09 ± 0·01 | 0·08 ± 0·01 | 0·16 ± 0·01 | 0·14 ± 0·01 | 0·19 ± 0·01 | 0·10 ± 0·01 |
Kruskal–Wallis test result (referring to the data of 1995 and 2010 where all parameters were measured) for all parameters P < 0·001. Number of sample trees (alive/dead): A1995, 100/20; B1997, 14/6; B2010, 59/31.
Epiphytic lichen diversity
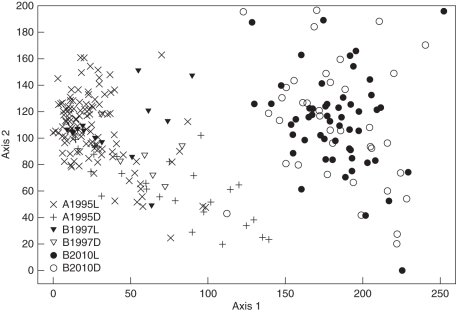
Trees clearly differ in their epiphytic lichen vegetation between 2010 and the mid-1990s. In the DCA, relevés from today and the 1990s form two distinct groups along the first axis (Fig. 2). This matches with the decline of L. conizaeoides (the most frequent epiphyte in the relevés of the 1990s) from the 1990s to 2010 (Fig. 3A). On living trees, the mean cover of L. conizaeoides on the lower 2 m of the trunks decreased from, respectively, 36 ± 1 % (Acker-Bruchberg ridge, 1995) or 26 ± 3 % (Mt Brocken, 1997) to 1 ± 0 % (Mt Brocken, 2010). On dead trees, L. conizaeoides covered a lower proportion of the trunk surface already in the 1990s (Acker-Bruchberg, 20 ± 2 %; Mt Brocken, 17 ± 4 %) and decreased to 0·5 ± 0·1 % in 2010. Despite the low cover values, L. conizaeoides was still present on 94 % of the living trees and 60 % of the dead trees in 2010. In the 1990s, the species occurred on all sample trees.
Fig. 2.
DCA ordination of Picea abies from the Harz Mountains (n = 250) depending on epiphytic lichen cover on the lower trunk at 0–2 m above the ground (34 species). Trees from different points in time (1995, 1997, 2010), sites (A, Acker-Bruchberg ridge; B, Mt Brocken) and status (L, alive; D, dead) are plotted with different symbols. Total variance in species data: 1·56. Eigenvalues: 0·57 (axis 1), 0·16 (axis 2). Length of gradient: 2·52 (axis 1), 1·97 (axis 2).
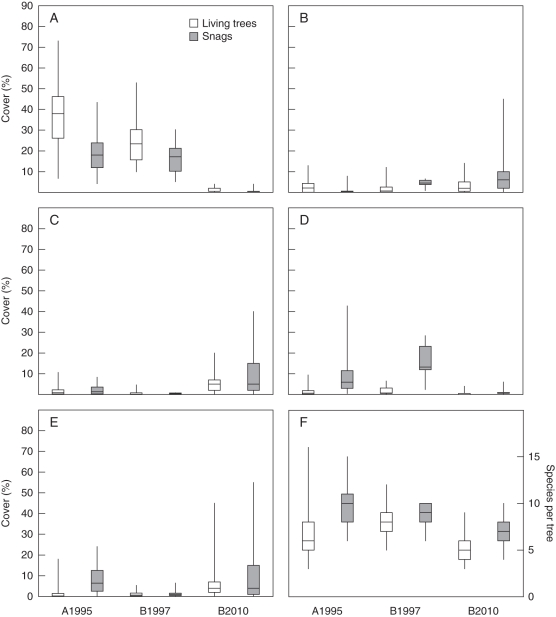
Fig. 3.
(A–E) Cover of epiphytic lichen species on the lower 2 m of Picea abies trunks including all species which exceed cover values of 5 % at one or more sites: (A) Lecanora conizaeoides, (B) Cladonia digitata, (C) C. polydactyla, (D) Hypogymnia physodes, (E) Lepraria jackii. (F) Number of lichen species per tree. Sites: A, Acker-Bruchberg sampled in 1995; B, Mt Brocken sampled in 1997 and 2010. Data from living trees and snags are displayed separately, as indicated. Columns show medians as wells as 25 % and 75 % quartiles, whereas bars indicate absolute minima and maxima. Kruskal–Wallis test results for differences between means of data represented by different columns (all P < 0·001): (A) χ2 = 188·2, (B) χ2 = 33·8, (C) χ2 = 81·7, (D) χ2 = 72·9, (E) χ2 = 91·9, (F) χ2 = 69·2. Number of sample trees (alive/dead): A1995, 115/25; B1997, 14/6; B2010, 53/37.
Only four lichen species besides L. conizaeoides exceeded a mean cover of 5 % in at least one site (Fig. 3). Hypogymnia physodes decreased in a similar way to L. conizaeoides, but started from much lower cover values in 1990s than the latter. Characteristic of H. physodes is the higher cover on dead trees than on living trees, which is in contrast to the behaviour of L. conizaeoides. The mean cover of three of these species, namely Cladonia digitata, C. polydactyla and Lepraria jackii, increased from the 1990s to 2010. In contrast to L. conizaeoides, which prefers the middle stem, those three species usually colonize the trunk from its base upwards. The total of lichen species per sample trees was always higher on dead trees than on living ones, but remained largely stable between the years (Fig. 3F). While differences in the epiphytic lichen vegetation caused the separation of living trees and snags into two groups along the second axis of the ordination diagram in the 1990s, living and dead trees clustered in a single scatter plot in 2010 (Fig. 2). In the 1990s, the influence of tree vitality was higher than that of the site, since living trees from either site clustered in one scatter plot, as did the dead trees in another scatter plot.
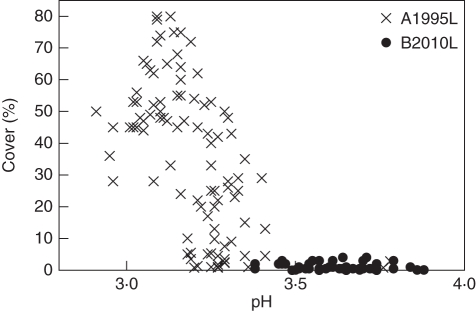
Results of the CCA show that the decrease in cover of L. conizaeoides and the increase of C. digitata, C. polydactyla and L. jackii is correlated with increasing pH and Ca, Mg and K concentrations, but decreasing Fe of the bark (Table 2). This result is supported by the results of bivariate correlation analyses as exemplarily shown for the cover of L. conizaeoides plotted against the pH (Fig. 4). The results of the CCA, which includes all species growing on >10 % of the sample trees irrespective of their cover, also show that Hypocenomyce caradocensis and Lecanora filamentosa declined together with L. conizaeoides, whereas the cover of Hypocenomyce scalaris and Platismatia glauca increased similarly to the more dominant species C. digitata, C. polydactyla and L. jackii. The observed decline of H. physodes (Fig. 3D) was correlated neither with the change of pH nor with the mentioned metal concentrations (Table 2).
Table 2.
Axis 1 scores in CCA of all species (n = 13) growing on >10 % of sample trees (n = 210)
| Species | CCA score |
|---|---|
| Hypocenomyce caradocensis | –1·040 |
| Lecanora conizaeoides | –0·820 |
| Lecanora filamentosa | –0·801 |
| Hypogymnia physodes | –0·066 |
| Parmeliopsis ambigua | –0·042 |
| Mycoblastus fucatus | 0·224 |
| Cladonia pyxidata s.l. | 0·027 |
| Pseudevernia furfuracea | 0·219 |
| Cladonia digitata | 0·954 |
| Platismatia glauca | 1·109 |
| Lepraria jackii | 1·285 |
| Cladonia polydactyla | 1·375 |
| Hypocenomyce scalaris | 2·115 |
Positive scores are positively correlated with the pH (r = 0·58) and concentrations of Ca (r = 0·75), S (r = 0·67),Mg (r = 0·65) and K (r = 0·53), and are negatively correlated with the concentration of Fe (r = –0·63) in bark (P ≤ 0·001 for correlations between species and bark chemistry parameters in a Monte Carlo test with 999 permutations).
Fig. 4.
Cover of Lecanora conizaeoides vs. the pH of aqueous bark suspensions (pH [H2O]) at the lower 2 m of living Picea abies trunks at the Acker-Bruchberg ridge in 1995 (A1995L; 100 sample trees) and Mt Brocken in 2010 (B2010L; 53 sample trees).
DISCUSSION
Change in bark chemistry
In a period of only 15 years, the spruce bark has responded with decreased acidity to the recently reduced deposition of acidic sulfurous air pollutants in central Europe. The increase of the pH by 0·4 units matches well with the increase of the pH in the upper soil and suggests that bark pH might be used as a surrogate for assessing the progress of soil acidification and deacidification. The recently reduced SO2 deposition already results in decreased S content of the bark. The concentrations of Mg and K typically increase along with the pH in biotic and abiotic surfaces with cation adsorption, including bark, living plant tissues lacking an intact cuticle, and soil (Finzi et al., 1998; Schmull and Hauck, 2003a). The lower concentrations of Fe and Mn in the bark in 2010 than in the 1990s is probably due to reduced availability from the presently less acidic soil (Römheld, 1987). The increased N content of bark indicates the accumulation of airborne N (Böhlmann et al., 2005).
Changes in epiphytic lichen cover and relationships to bark chemistry
Though L. conizaeoides was by far the most dominant epiphyte in the Harz Mountains during the 1990s, it is a rare species today, which, however, still occurs on most spruce trees but in very low cover values. The rapid dieback of L. conizaeoides agrees with recent observations obtained from other areas in western and central Europe (Wirth, 1993; Bates et al., 2001). Since the earlier spread of L. conizaeoides was repeatedly shown to be the result of the acidification of precipitation and the sub-stratum and perhaps elevated S concentrations (Wirth, 1985; Bates et al., 1996; Hauck et al., 2001b), it is plausible to assume that the continent-wide dieback of L. conizaeoides is caused either by the reduction in the deposition of acids or by that of SO2 to nearly to pre-industrial levels, which coincide with the dieback or precede it (Fig. 1). The high tolerance of L. conizaeoides to a broad range of microclimatic conditions (Wirth, 1985) supports this assumption and makes a detrimental effect of global warming on the species unlikely. While this reasoning is well established (Bates et al., 2001), the magnitude of pH increase sufficient to cause the decline of L. conizaeoides was unknown so far. The present results suggest that an increase in the sub-stratum pH by only 0·4 units was sufficient to cause the breakdown of the L. conizaeoides populations in the study area.
The putative inhibitory effect of a small increase in the pH from 3·2 to 3·6 is more difficult to explain than damage due to increasing acidity. A large decrease in acidity (i.e. an increase of several pH units) can cause Fe and P deficiency in lichens (Paul et al., 2009). That Fe or P deficiency has resulted from the slight pH increase observed in the present study is more than doubtful. The reduced total Fe content of bark should be more significant to the Fe nutrition of the lichen than the direct pH effect on the efficacy of L. conizaeoides for Fe uptake. Field data of Bates et al. (2001) and Hauck et al. (2001b), who independently described optimum curves for the dependence of L. conizaeoides cover on the supply with inorganic S, suggest that not only acidity itself, but also acidity combined with high S concentrations controls the abundance of this species. Thus the reduced total S content of the spruce bark could exert an additional unfavourable effect on L. conizaeoides. Massara et al. (2009) concluded from spraying experiments with different concentrations of bisulfite that the pH rather than the S supply controls the abundance of L. conizaeoides; however, as the pH was not manipulated independently of the bisulfite concentration, this conclusion is not completely convincing. The results of LaGreca and Stutzman (2006), who found L. conizaeoides on conifer bark of low pH in an area of eastern North America which was never exposed to SO2 pollution comparable with that in Europe, are more supportive of the hypothesis that SO2 and its derivatives formed in aqueous solution themselves are less significant for the vitality of L. conizaeoides than the pH itself.
Factors other than bark chemistry might also be responsible for the dramatic dieback of the former common and widespread species L. conizaeoides. Competition by other epiphytes was obviously of sub-ordinate significance for the dieback of L. conizaeoides, as most lichen species, which spread between the 1990s and 2010, including Cladonia spp., L. jackii and H. scalaris prefer the trunk base. Lecanora conizaeoides, a characteristic inhabitant of the middle stem, mostly leaves empty bark surfaces after its decline which are largely devoid of epiphytes. This observation from the Harz Mountains parallels results of Wirth (1993) from southern Germany. An interaction of atmospheric chemistry and the population dynamics of the lichenicolous basidiomycete Athelia arachnoidea, which is a widespread parasite of L. conizaeoides (Gilbert, 1988), was probably not the cause of the decline of this lichen in the Harz Mountains, as A. arachnoidea was never common in the upper elevations of this area. Thus, our field study provides evidence that the population dieback of L. conizaeoides was indeed caused by changes in the physicochemical site conditions and was not due to competing lichens.
It is remarkable that the lichen H. physodes decreased simultaneously with L. conizaeoides, as H. physodes preferred the spruce trees with the highest pH values and lowest S content in bark and stemflow during the 1990s (Hauck, 2003). The susceptibility of H. physodes to acidic sulfurous solutions is the reason why this species was formerly more frequent on dead or dying trees than on healthy trees, as the dense foliage of the latter intercepts more protons and S compounds from the atmosphere (Hauck and Runge, 2002; Hauck, 2003). The known sensitivity of H. physodes to acidity suggests that other factors are responsible for the present decline. Among these factors are the increased Mn/Fe ratio in the bark and the increased N deposition. Both high Mn/Fe ratios and high NO3− concentrations were experimentally shown to damage H. physodes at ambient concentration ranges (Schmull et al., 2002; Hauck et al., 2003). Interestingly, Schmull et al. (2002) and Schmull and Hauck (2003b) found the cover of H. physodes and other species to decrease with increasing Mn/Fe ratio in spruce bark and with increasing NO3− concentration in stemflow water in a spruce–fir forest of eastern North America. This dependency was found when the SO2 deposition was already largely reduced and the pH (H2O) values of the bark ranged at moderate values between 3·4 and 4·0. In the Harz Mountains, high correlation coefficients were found in multiple regression analyses for models explaining the variation in the cover of H. physodes with the concentrations of S and NO3− in stemflow using the data of the 1990s (Hauck and Runge, 2002; Hauck et al., 2002). At that time, however, the influence of S concentrations on lichen abundance clearly exceeded that of NO3− concentrations. Decreasing abundance of H. physodes along with the decline of L. conizaeoides was also found in England (Bates et al., 2001). Platismatia glauca, a species which resembles H. physodes in terms of acidity tolerance, is fairly tolerant to moderate N pollution (Palmqvist and Dahlman, 2006). In contrast to H. physodes, P. glauca became more abundant between the 1990s and 2010 (Table 2).
With decreasing acidic pollutant load of the atmosphere, the chemical composition of the bark becomes increasingly similar in trees with large or small amounts of foliage, or dead trees. In the 1990s, the contrasting epiphytic lichen diversity on living and dead or dying trees was attributed to the reduced interception of substances from the atmosphere in the canopy with declining needle mass (Hauck and Runge, 2002; Hauck, 2003). This explains the existence of the separate clusters of living and dead trees in the DCA (Fig. 2). Hauck (2005) already showed that this separation diminished when areas with low atmospheric pollutant load were studied. Today, living trees and snags form a single cluster in the DCA in the Harz Mountains, suggesting that the epiphytic lichen vegetation of living and dead trees became more alike due to the decreased acidic sulfurous deposition. This higher similarity of the epiphytic lichen vegetation between living and dead trees today than in the 1990s is a post-hoc confirmation of the hypothesis by Hauck and Runge (1999) and Hauck (2003) explaining the high epiphytic lichen diversity in forests affected by acidic air pollution with differences in stemflow chemistry and not with microclimate.
Conclusions
The highly acidophytic lichen L. conizaeoides showed a dramatic decrease in abundance in the Harz Mountains from the most dominant epiphyte to a rare species within only 15 years. Our analysis suggests that this decline is attributable to a slight increase of sub-stratum pH by only 0·4 pH units. Competition due to the expansion of less acidity-tolerant epiphytes or increased infestation with lichenicolous fungi can be ruled out as playing a crucial role in the dieback of L. conizaeoides in the area. Other lichen species increased with decreasing acidic sulfurous air deposition, but these species (e.g. Cladonia, Lepraria spp.) prefer the lower parts of the trunk which were never the main habitat for L. conizaeoides. Increased Mn/Fe ratios and the continuing atmospheric deposition of N seem to be the key chemical site factors in these spruce forests after the reduction of atmospheric SO2 levels in the 1990s. Due to the dieback of L. conizaeoides, the epiphytic lichen vegetation of living and dead spruce trees is today more similar to each other than it was in the 1990s, when this species dominated the bark of the living trees.
ACKNOWLEDGEMENTS
The study was supported by a grant from the Stemmler Foundation, a member of the Stifterverband für die Deutsche Wissenschaft, to M.H. and C.L. The grant was approved within the scope of the Stifterverband programme ‘Biodiversity and Ecology in National Parks (BEN)’. We are thankful to the Harz National Park, and to Dr H.-U. Kison and A. Rommerskirchen, in particular, for permission for field work and manifold support of our work.
LITERATURE CITED
- Bates JW, McNee PJ, McLeod AR. Effects of sulphur dioxide and ozone on lichen colonization of conifers in the Liphook Forest fumigation project. New Phytologist. 1996;132:653–660. doi: 10.1111/j.1469-8137.1996.tb01883.x. [DOI] [PubMed] [Google Scholar]
- Bates JW, Bell JNB, Massara AC. Loss of Lecanora conizaeoides and other fluctuations of epiphytes on oak in S.E. England over 21 years with declining SO2 concentrations. Atmospheric Environment. 2001;35:2557–2568. [Google Scholar]
- Böhlmann N, Meissner R, Bernsdorf S, Böhme F, Russow R, Wegener U. Studies of atmospheric nitrogen deposition in a mire of the German national park Hochharz Mountains using two different methods. Water, Air and Soil Pollution. 2005;168:17–32. [Google Scholar]
- Erichsen CFE. Neue und bemerkenswerte atlantische Flechten im deutschen Küstengebiet. Hedwigia. 1933;73:1–24. [Google Scholar]
- Erichsen CFE. Flechtenflora von Nordwestdeutschland. Stuttgart: G. Fischer; 1957. [Google Scholar]
- Farmer AM, Bates JW, Bell JNB. Seasonal variations in acidic pollutant inputs and their effects on the chemistry of stemflow, bark and epiphyte tissues in three oak woodlands in N.W. Britain. New Phytologist. 1991;118:441–451. [Google Scholar]
- Finzi AC, Canham CD, van Breemen N. Canopy tree–soil interactions within temperate forests: species effects on pH and cations. Ecological Applications. 1998;8:447–454. [Google Scholar]
- Gilbert OL. Studies on the destruction of Lecanora conizaeoides by the lichenicolous fungus Athelia arachnoidea. Lichenologist. 1988;20:183–190. [Google Scholar]
- Hauck M. Ecology of epiphytic lichens in a montane spruce forest: influence of forest dieback and forest management on chemical habitat conditions. Dissertationes Botanicae. 2000;327:1–232. [Google Scholar]
- Hauck M. Epiphytic lichen diversity and forest dieback: the role of chemical site factors. Bryologist. 2003;106:257–269. [Google Scholar]
- Hauck M. Epiphytic lichen diversity on dead and dying conifers under different levels of atmospheric pollution. Environmental Pollution. 2005;135:111–119. doi: 10.1016/j.envpol.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Hauck M, Runge M. Occurrence of pollution-sensitive epiphytic lichens in woodlands affected by forest decline: a new hypothesis. Flora. 1999;194:159–168. [Google Scholar]
- Hauck M, Runge M. Stemflow chemistry and epiphytic lichen diversity in dieback-affected spruce forest of the Harz Mountains, Germany. Flora. 2002;197:250–261. [Google Scholar]
- Hauck M, Jung R, Runge M. Relevance of element content of bark for the distribution of epiphytic lichens in a montane spruce forest affected by forest dieback. Environmental Pollution. 2001a;112:221–227. doi: 10.1016/s0269-7491(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Hauck M, Hesse V, Jung R, Zöller T, Runge M. Long-distance transported sulphur as a limiting factor for the abundance of Lecanora conizaeoides in montane spruce forests. Lichenologist. 2001b;33:267–269. [Google Scholar]
- Hauck M, Hesse V, Runge M. The significance of stemflow chemistry for epiphytic lichen diversity in a dieback-affected spruce forest on Mt. Brocken, northern Germany. Lichenologist. 2002;34:415–427. [Google Scholar]
- Hauck M, Paul A, Gross S, Raubuch M. Manganese toxicity in epiphytic lichens: chlorophyll degradation and interaction with iron and phosphorus. Environmental and Experimental Botany. 2003;49:181–191. [Google Scholar]
- Hauck M, Helms G, Friedl T. Photobiont selectivity in the epiphytic lichens Hypogymnia physodes and Lecanora conizaeoides. Lichenologist. 2007;39:195–204. [Google Scholar]
- Hauck M, Jürgens S-R, Brinkmann M, Herminghaus S. Surface hydrophobicity causes SO2 tolerance in lichens. Annals of Botany. 2008;101:531–539. doi: 10.1093/aob/mcm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck M, Jürgens S-R, Huneck S, Leuschner C. High acidity tolerance in lichens with fumarprotocetraric, perlatolic or thamnolic acids is correlated with low pKa1 values of these lichen substances. Environmental Pollution. 2009a;157:2776–2780. doi: 10.1016/j.envpol.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Hauck M, Jürgens S-R, Willenbruch K, Huneck S, Leuschner C. Dissociation and metal-binding characteristics of yellow lichen substances suggest a relationship with site preferences of lichens. Annals of Botany. 2009b;103:13–22. doi: 10.1093/aob/mcn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth DL, Rose F. Qualitative scale for estimating sulphur dioxide air pollution in England and Wales using epiphytic lichens. Nature. 1970;227:145–148. doi: 10.1038/227145a0. [DOI] [PubMed] [Google Scholar]
- Hawksworth DL, Rose F, Coppins BJ. Changes in the lichen flora of England and Wales attributable to pollution of the air by sulphur dioxide. In: Ferry BW, Baddeley MS, Hawksworth DL, editors. Air pollution and lichens. London: Athlone; 1973. pp. 330–367. [Google Scholar]
- Klement O. Die Flechtenvegetation der Stadt Hannover. Beiträge zur Naturkunde Niedersachsens. 1958;11:56–60. [Google Scholar]
- LaGreca S, Stutzman BW. Distribution and ecology of Lecanora conizaeoides. Lecanoraceae. in eastern Massachusetts. Bryologist. 2006;109:335–347. [Google Scholar]
- Massara AC, Bates JW, Bell JNB. Exploring causes of the decline of the lichen Lecanora conizaeoides in Britain: effects of experimental N and S applications. Lichenologist. 2009;41:673–681. [Google Scholar]
- Mylona S. Sulphur dioxide emissions in Europe 1880–1991 and their effect on sulphur concentrations and depositions. Tellus B. 1996;48:662–689. [Google Scholar]
- Palmqvist K, Dahlman L. Responses of the green algal foliose lichen Platismatia glauca to increased nitrogen supply. New Phytologist. 2006;171:343–356. doi: 10.1111/j.1469-8137.2006.01754.x. [DOI] [PubMed] [Google Scholar]
- Paul A, Hauck M, Leuschner C. Iron and phosphate uptake in epiphytic and saxicolous lichens differing in their pH requirements. Environmental and Experimental Botany. 2009;67:133–138. [Google Scholar]
- Paulson R, Thompson PG. Report on lichens of Epping Forest (second paper) Essex Naturalist. 1913;17:90–105. [Google Scholar]
- Römheld V. Different strategies for iron acquisition in higher plants. Physiologia Plantarum. 1987;70:231–234. [Google Scholar]
- Schmull M, Hauck M. Extraction methods for assessing the availability of cations for epiphytic lichens from bark. Environmental and Experimental Botany. 2003a;49:273–283. [Google Scholar]
- Schmull M, Hauck M. Element microdistribution in the bark of Abies balsamea and Picea rubens and its impact on epiphytic lichen abundance on Whiteface Mountain, New York. Flora. 2003b;198:293–303. [Google Scholar]
- Schmull M, Hauck M, Vann DR, Johnson AH, Runge M. Site factors determining epiphytic lichen distribution in a dieback-affected spruce–fir forest on Whiteface Mountain, New York: stemflow chemistry. Canadian Journal of Botany. 2002;80:1131–1140. [Google Scholar]
- Shirtcliffe NJ, Pyatt FB, Newton MI, McHale G. A lichen protected by a super-hydrophobic and breathable structure. Journal of Plant Physiology. 2006;163:1193–1197. doi: 10.1016/j.jplph.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Vestreng V, Myhre G, Fagerli H, Reis S, Tarrasón L. Twenty-five years of continuous sulphur dioxide emission reduction in Europe. Atmospheric Chemistry and Physics Discussions. 2007;7:5099–5143. [Google Scholar]
- Wirth V. Zur Ausbreitung, Herkunft und Ökologie anthropogen geförderter Rinden- und Holzflechten. Tuexenia. 1985;5:523–535. [Google Scholar]
- Wirth V. Trendwende bei der Auswertung der anthropogen geförderten Flechte Lecanora conizaeoides? Phytocoenologia. 1993;23:625–636. [Google Scholar]
- Wirth V, Hauck M, von Brackel W, et al. Checklist of lichens and lichenicolous fungi in Germany. 2011 version 2. Georg August University of Göttingen: http://www.gwdg.de/~mhauck , Göttingen, Germany. (Accessed 19 January 2011.) [Google Scholar]