Abstract
Background and Aims
The geographic distribution of the genus Plectocephalus comprises a single species in Ethiopia, two in North America and possibly four more in South America, in a striking disjunction that is exceptional for genera of the tribe Cardueae. The enormity of this disjunction cast doubts on the precise taxonomic delineation of the genus, which is not unanimously recognized as a natural entity. The aims of this study were to define the generic boundaries of Plectocephalus and to formulate a hypothesis that would explain its natural range.
Methods
A combined molecular approach, using nuclear internal transcribed spacers (ITS) and external transcribed spacers (ETS), and plastid trnL-trnL-F, rpl32-trnLUAG and ndhF markers, was chosen for phylogenetic reconstruction by maximum parsimony and Bayesian inference.
Key Results
Phylogenetic analysis shows that Plectocephalus is a natural genus that includes the African species P. varians, together with all the native South American species, currently classified as Centaurea, C. cachinalensis, C. floccosa and C. tweediei. The recognition of Centaurodendron as an independent genus, which we consider appropriate, would make Plectocephalus paraphyletic. Affinities of Plectocephalus should lie with eastern representatives of Centaureinae. Geographic disjunction is explained as a consequence of dispersal via the Bering Land Bridge during the Miocene–Pliocene. The phylogeny of the basal grade of Centaureinae differs from previous phylogenies, and artefacts resulting from differences in mutation rates of annual and perennial taxa are confirmed. Sensitivity of ITS to these differences was the highest observed for all DNA regions used in this study.
Conclusions
The natural status of the genus Plectocephalus is confirmed and several nomenclatural combinations are proposed. New evidence contributes to the debate concerning problems posed by the use of ITS in the phylogenetic reconstruction of groups that differ in terms of their life cycles. Dispersal from Caucasus and Anatolia along the Siberian route and then across the Bering Land Bridge follows a route previously proposed for other taxonomic groups.
Keywords: Plectocephalus, Compositae, Cardueae, Centaureinae, Centaurea, Bering Land Bridge, ETS, ITS, migration, ndhF, phylogeny, rpl32-trnLUAG, trnL-trnL-F
INTRODUCTION
It is not possible to discuss Plectocephalus D. Don without first discussing the generic concept of Centaurea L. (Compositae, Cardueae-Centaureinae). From the first description of Centaurea by Linnaeus (1753), it was clear that this genus was an artificial assemblage. Cassini (1817) declared: ‘Linné a reuni, sous ce nom, en un seul et même genre, une multitude d'espèces, qui ont en effet beaucoup de caractères communs, mais qui cependant peuvent et doivent être distribuées dans plusieurs genres, ne fût-il que pour rendre leur étude plus facile et plus commode. […] Il est vrai que Linnaeus a divisé ses centaurées en plusieurs sections; mais cet expédient ne suffit pas pour prevenir la confusion qui résulte surtout du même nom générique appliqué à un trop grand nombre d'espèces’. [Linnaeus has united, under this name, in a single genus, a multitude of species that indeed have many characters in common, but which should of necessity be distributed amongst several genera, if only to make it easier and more convenient to study. […] Linnaeus certainly assigned his centaureas to many sections, but this was not sufficient to prevent the confusion that resulted, especially as he used the same generic name for too many species]. Evidence for the artificial nature of Centaurea was accumulating, but it was a pollen study by Wagenitz (1955), in which he described eight different pollen types in a single genus, that sounded the death knell for the broad Linnean concept. Molecular tools were to provide the final evidence (Susanna et al., 1995; Garcia-Jacas et al., 2001, 2002, 2006) and, as late as 2001, a new type species was chosen for the genus, putting an end to the old problem of the delineation of Centaurea (Greuter et al., 2001). The latest revisions of subtribe Centaureinae recognized the new circumscription of Centaurea as a natural group comprising only 250 species (Susanna and Garcia-Jacas, 2007, 2009), an important reduction when compared with the broad concept of the genus in Dittrich (1977) who recognized 400 species. Currently accepted genera are Cheirolophus Cass., Crupina (Pers.) DC., Mantisalca Cass., Phalacrachena Iljin, Plectocephalus, Psephellus Cass., Rhaponticoides Vaill., Rhaponticum Vaill. and Stizolophus Cass., all of them formerly included in the broad Linnean concept of Centaurea.
Taxonomy and phylogenetic relationships
Plectocephalus was one of the earliest segregates of Centaurea and contained a single species, Centaurea americana Nutt. from Texas that was eventually renamed Plectocephalus americanus (Nutt.) D. Don (Sweet, 1830). It was distinguished from the rest of the heterogeneous Centaurea assemblage by its very large and showy peripheral florets, the scariose bracts with unarmed silvery appendages, and by its arcuate and faintly ribbed achenes. Don (cited in Sweet, 1830) described the pappus as triseriate, a misinterpretation of the obscurely multiseriate, easily deciduous pappus that is typical of Plectocephalus. This is in contrast to the neatly double and persistent pappus of Centaurea sensu stricto (Susanna and Garcia-Jacas, 2007). Another botanist with the same name, G. Don (cited in Loudon, 1855), described a second species for the genus, namely Plectocephalus chilensis G. Don ex Loudon from central Chile.
Boissier (1856) was the first to accept the new genus and he added a further two new species, Plectocephalus abyssinicus Boiss. and Plectocephalus cyanoides Boiss., though these are currently considered to be conspecific and assigned the name Plectocephalus varians (A. Rich.) C. Jeffrey ex Cufod. from Ethiopia. Nevertheless, despite obvious differences between Centaurea and Plectocephalus, the latter genus was soon forgotten and, consequently, the next closest relative of Plectocephalus americanus to be discovered was named Centaurea rothrockii Greenm. (from New Mexico). In fact, most works dealing with the flora of North America still tend to use the older names Centaurea americana Nutt. and C. rothrockii (e.g. Correl and Johnston, 1970; Martin and Hutchins, 1981), whereas only the latest flora of the USA recognizes Plectocephalus (Keil and Ochsmann, 2006). Indeed, major works generally consider Plectocephalus to be merely a section of a widely circumscribed Centaurea (Bentham, 1853; Hoffmann, 1894; Dittrich, 1977; Bremer, 1994). Besides C. americana, C. chilensis and C. rothrockii, sect. Plectocephalus included another three Centaurea species from South America, namely Centaurea cachinalensis Phil., C. floccosa Hook. & Arn. from Chile, and C. tweediei Hook. & Arn. from Argentina, Brazil and Uruguay.
Jeffrey (1968) was the next to recognize Plectocephalus as a genus distinct from Centaurea, soon to be followed by Nordenstam and El-Ghazaly (1977). However, the exact limits of Plectocephalus were not unanimously accepted. Even though Hind (1996) recognized the genus, he excluded the African and South American species and suggested that Plectocephalus should include only the two North American species P. americanus and P. rothrockii (Greenm.) D.J.N. Hind. Susanna and Garcia-Jacas (2007, 2009) considered Plectocephalus in its broader sense (including the species from Africa and the Americas), but this delineation was only tentative and they did not propose any formal nomenclatural combinations owing to lack of molecular evidence. None of the major molecular surveys of Cardueae to date has included many representatives of Plectocephalus.
The only other genus of Centaureinae native to America is Centaurodendron Johow (including Yunquea Skottsb.), with three species occurring on the Juan Fernández Islands. On both morphological and biogeographical grounds, Centaurodendron is considered a close relative of Plectocephalus, as suggested by Carlquist (1958) and Nordenstam and El-Ghazaly (1977). Indeed, despite anatomical (Carlquist, 1958) and palynological (Parra, 1969–70) differences, Hellwig (2004) even proposed incorporating Centaurodendron in Plectocephalus. As with Plectocephalus, Centaurodendron has not been included in any previous molecular survey. As well as problems in delineating the genus, the positions of Plectocephalus and Centaurodendron within Centaureinae remain obscure. Both genera have Serratula type pollen (Wagenitz, 1955; Parra, 1969–70). Species having this kind of pollen form an unresolved polytomy at the base of the subtribe (Garcia-Jacas et al., 2001), and all the molecular surveys have failed to elucidate the phylogeny of the basal branches of Centaureinae (Hidalgo et al., 2006).
Biogeography
The geographical distribution of Plectocephalus and Centaurodendron is one of the most interesting features of the group, as it exhibits a striking disjunction. Were the placing of P. varians within Plectocephalus confirmed, the genus would comprise one species in East Africa, two in North America and four in South America, together with the diversification of Centaurodendron (three species) on the Juan Fernández Islands. As well as displaying a remarkable Afro-American disjunction, Plectocephalus species grow at the margins of regions considered to be the natural range of Centaureinae. This subtribe originated at the edges of the Mediterranean and Irano-Turanian regions, close to the Caucasus (Wagenitz and Hellwig, 1996; Susanna and Garcia-Jacas, 2009). Its present distribution is mainly Mediterranean and, to a lesser extent, Eurasian, with some rare species extending as far as East Africa whilst Rhaponticum australe (Gaudich.) Soskov occurs in Australia. A few genera (Plagiobasis Schrenk, Russowia C. Winkl. and Schischkinia Iljin) reach as far as Middle Asia, but only the genera Goniocaulon Cass. and Tricholepis DC. extend beyond the natural geographical barrier formed by the high peaks of Central Asia (Tien San, Himalaya and Hindu Kush), reaching India and even Burma. Thus, Plectocephalus varians has the distinction of being one of the southernmost species of the whole subtribe in East Africa, with Plectocephalus and Centaurodendron being the only native representatives of the Centaureinae on the American continent (Susanna and Garcia-Jacas, 2009).
Disjunctions between African and South American taxa are known to occur among the basal Compositae. This is contrary to what is found here, in that South American genera from the basal groups of the family have some African-derived representatives (Ortiz et al., 2009). However, none of these Afro-South American distributions includes North America, where the basal branches of the Compositae are represented by a single, monotypic genus, Hecastocleis A. Gray (Funk and Hind, 2009). Other disjunctions that parallel the Afro-North American disjunction of Plectocephalus are the genera Datisca L. and Plantago L. (Stebbins and Day, 1967), Styrax L. (Fritsch, 1996, 2001), and the tribe Betoideae of the Chenopodiaceae (Hohmann et al., 2006). Should the relationships of Plectocephalus varians and the American species be confirmed, there are two possible ways of explaining this disjunction: (1) migration by continuous range expansion through East Asia and the Bering Land Bridge (BLB) as postulated for Datisca and Plantago (Stebbins and Day, 1967); or (2) long-distance dispersal, as proposed by Raven (1972) for some Mediterranean–Californian disjunctions and by Fritsch (1996, 2001) for Styrax. If one takes into account the relatively young age of the genus, estimated to be approx. 12 million years (cf. Barres et al., Botanic Institute of Barcelona, in prep.), the hypothesis that a North-Atlantic migration took place, as suggested for older floristic elements in the Paleogene, can be disregarded (see Hohmann et al., 2006).
Defining the limits of the genus and identifying the ancestors of Plectocephalus is a critical and necessary step in determining the route by which this group migrated. In view of the low resolution of basal branches of the subtribe achieved in previous analyses (Garcia-Jacas et al., 2001; Hidalgo et al., 2006), the only possible approach is that of combined molecular analysis, including plastid and nuclear markers. Consequently, information was collected from five different regions [internal transcribed spacers (ITS) and external transcribed spacers (ETS) as nuclear markers, and trnL-trnL-F, rpl32-trnLUAG and ndhF as plastid markers) with the following aims: (a) reaching a precise delineation of Plectocephalus and determining the systematic position of the African P. varians, and the South American P. chilensis, Centaurea cachinalensis, C. floccosa and C. tweediei; (b) verifying the relationships of Centaurodendron and Plectocephalus; (c) exploring the affinities of Plectocephalus within the basal genera of the Centaureinae; (d) using this new molecular evidence to improve our knowledge regarding the relationships of the ‘Basal Grade’ of the subtribe Centaureinae; and (e) determining the putative routes of geographic expansion and the largest area occupied by any native Cardueae.
MATERIALS AND METHODS
Plant material
The data set consisted of 178 accessions, including all the basal genera in subtribe Centaureinae, with the only exceptions of Karvandarina Rech. f. and Ochrocephala Dittrich (Susanna and Garcia-Jacas, 2007). The sampling was primarily aimed so as to represent the taxonomic and geographical diversity of the Plectocephalus group, for which the majority of the genomic regions were sequenced for the first time. We were able to sample only one of the three species of Centaurodendron. However, the general consensus is that all three species attributed to this genus constitute a natural group that includes monospecific Yunquea Skottsb., with the species Y. tenzii Skottsb. now considered a synonym of Centaurodendron tenzii (Skottsb.) Skottsb (Dittrich, 1977; Susanna and Garcia-Jacas, 2007).
For the plastid data set, we targeted three regions: ndhF, rpl32-trnLUAG and trnL-trnL-F, which were sequenced for 40 species of Centaureinae. Some of the sequences of the trnL-trnL-F region have previously been published, but the protein-encoding ndhF region and rpl32-trnLUAG (including a portion of the rpl32 gene and the complete rpl32-trnL intergenic spacer) data are new. Nuclear regions were newly sequenced for 12 (29 %, ITS) and 23 (65 %, ETS) of the species studied.
The outgroup was chosen from representatives of the sister clade of subtribe Centaureinae (Garcia-Jacas et al., 2002; Susanna et al., 2006). It included one species from the genus Cousinia and one from Saussurea. Voucher data, source of material and GenBank sequence accession numbers are given in Table 1.
Table 1.
Species, origin of the materials, herbaria and GenBank accession numbers
| Taxon | Voucher | Country | trnL-trnF | rpl32-trnL | ndhF | ITS | ETS |
|---|---|---|---|---|---|---|---|
| Acroptilon repens (L.) DC. | Susanna 2046 (BC) | UZB | AY772268 | JF754869 | JF754831 | AY826223 | DQ310989 |
| Amberboa turanica Iljin | Susanna 1643 (BC) | IRN | JF754753 | JF754870 | JF754832 | AY012311, AY012275 | JF754783 |
| Callicephalus nitens (M. Bieb. ex Willd.) C. A. Mey. | Susanna 1578 (BC) | ARM | AY772281 | JF754871 | JF754833 | AY826237 | DQ310972 |
| Centaurea cachinalensis Phil. | Belov s.n. (BC) | CHL | JF754755 | JF754872 | JF754834 | JF754804 | JF754784 |
| Centaurea floccosa Hook. & Arn. | Belov s.n. (BC) | CHL | JF754756 | JF754873 | JF754835 | JF754805 | JF754785 |
| Centaurea tweediei Hook. & Arn. | Dematteis 43 & Gutiérrez (BC) | ARG | JF775384 | JF775400 | JF775396 | JF775392 | JF775388 |
| Centaurodendron palmiforme Skottsb. | Tobar s.n. & Arredondo (BC) | CHL | JF754757 | JF754874 | JF754836 | JF754806 | JF754786 |
| Centaurothamnus maximus (Forssk.) Wagenitz & Dittrich | Molero s.n. (BC) | YEM | AY772301 | JF754875 | JF754837 | AY826259 | DQ310971 |
| Cheirolophus mauritanicus (Font Quer) Susanna | Romo 4617 (BC) | MAR | AY772303 | JF754876 | JF754838 | AY826261 | DQ131087 |
| Cheirolophus teydis (C.Sm.) G.López | Susanna 1429 (BC) | ESP | AY772304 | JF754877 | JF754839 | AY826262 | DQ131092 |
| Cousinia microcarpa Boiss. | Susanna 2160 et al. (BC) | KAZ | AY772312 | JF754878 | JF754840 | AY826270 | JF754787 |
| Crupina crupinastrum Vis. | Vilatersana s.n. (BC) | ITA | JF754762 | JF754879 | JF754841 | JF754829, JF754830 | JF754788 |
| Crupina vulgaris Cass. | Susanna 1813 (BC) | ESP | AY772320 | JF754880 | JF754842 | AY826280 | JF754789 |
| Goniocaulon indicum C.B.Clarke | Fris s.n. et al. (K) | ETH | JF775385 | JF775401 | JF775397 | JF775393 | JF775389 |
| Klasea coriacea (DC.) J.Holub | Susanna 1558 (BC) | ARM | DQ310892 | JF754881 | JF754843 | DQ310926 | DQ310965 |
| Klasea serratuloides (DC.) Greuter & Wagenitz | Susanna 1569 (BC) | ARM | AY772334 | JF754882 | JF754844 | AY826295 | DQ310962 |
| Leuzea conifera (L.) DC. | Font s.n. (BC) | ESP | AY772337 | JF754883 | JF754845 | AY826298 | JF754790 |
| Mantisalca salmantica (L.) Briq. & Cavill. | Susanna 1457 (BC) | ESP | JF754765 | JF754884 | JF754846 | AY012328, AY012292 | JF754791 |
| Myopordon aucheri Boiss. | Carls s.n. (BC) | IRN | AY772338 | JF754885 | JF754847 | AY826299 | DQ310977 |
| Myopordon persicum Boiss. | Remandieri s.n. (BC) | IRN | DQ310898 | JF754886 | JF754848 | AY826301 | DQ310976 |
| Oligochaeta divaricata K. Koch | Susanna 1583 (BC) | ARM | AY772344 | JF754887 | JF754849 | AY826306 | DQ310973 |
| Phalacrachena calva (Ledeb.) Iljin | Tipsgko s.n. (LE) | KAZ | JF754767 | JF754888 | JF754850 | JF754815 | JF754792 |
| Phalacrachena inuloides (Fisch.) Iljin | Romaschenko 402 & Didukh (BC) | UKR | JF754768 | JF754889 | JF754851 | JF754816 | JF754793 |
| Plagiobasis centauroides Schrenk | Susanna 2130 (BC) | KAZ | DQ310887 | JF754890 | JF754852 | AY826312 | DQ310956 |
| Plectocephalus americanus D.Don | Quayle 765 (TEX) | USA | JF754769 | JF754891 | JF754853 | JF754817 | JF754794 |
| Plectocephalus chilensis G.Don ex Loudon | Jardí Botànic de Barcelona s.n. (BC) | CHL | JF775386 | JF775402 | JF775398 | JF775394 | JF775390 |
| Plectocephalus rothrockii (Greenm.) D.J.N.Hind | Hiett s.n. (TEX) | USA | JF754770 | JF754892 | JF754854 | JF754818 | JF754795 |
| Plectocephalus varians (A.Rich.) C.Jeffrey in Cufod. | Ortiz s.n. & Vivero (BC) | ETH | JF775387 | JF775403 | JF775399 | JF775395 | JF775391 |
| Psephellus persicus (DC.) Wagenitz | Susanna 1716 et al. (BC) | IRN | AY772352 | JF754893 | JF754855 | AY826316 | DQ310957 |
| Psephellus pulcherrimus (Willd.) Wagenitz | Susanna 1492 et al. (BC) | ARM | AY772353 | JF754894 | JF754856 | AY826317 | DQ310958 |
| Rhaponticoides hajastana (Tzvelev) M.V.Agab. & Greuter | Susanna 1587 et al. (BC) | ARM | AY772279 | JF754895 | JF754857 | AY826235 | DQ310959 |
| Rhaponticoides iconiensis (Hub.-Mor.) M.V.Agab. & Greuter | Ertuğrul 1761 (BC) | TUR | DQ310889 | JF754896 | JF754858 | DQ310923 | DQ310960 |
| Rhaponticum acaule DC. | Montserrat 2331 et al. (BC) | DZA | AY772369 | JF754897 | JF754859 | AY826334 | DQ310995 |
| Russowia sogdiana B.Fedtsch | Kamelin s.n. (LE) | TJK | JF754775 | JF754898 | JF754860 | AY826320 | JF754796 |
| Saussurea maximowiczii Herder | Kanagawa Prefect. Ofuna Bot. Gard. s.n. (BC) | JPN | AY772359 | JF754899 | JF754861 | AY826324 | JF754797 |
| Schischkinia albispina (Bunge) Iljin | Botschantzev 827 (LE) | TKM | AY772360 | JF754900 | JF754862 | AY826325 | JF754798 |
| Serratula coronata L. | Vienna Univ. Bot. Gard. s.n. (BC) | AUT | AY772362 | JF754901 | JF754863 | AY826327 | DQ310961 |
| Stizolophus balsamita (Lam.) Cass. ex Takht. | Susanna 1547 et al. (BC) | ARM | AY772371 | JF754902 | JF754864 | AY826336 | JF754799 |
| Stizolophus coronopifolius Cass. | Ilarslan 4303 (ANK) | TUR | AY772372 | JF754903 | JF754865 | AY826337 | DQ310955 |
| Tricholepis tibetica Hook.f. & Thomson ex C.B.Clarke | Nüsser 1055 (B) | PAK | JF754780 | JF754904 | JF754866 | AY826341 | JF754800 |
| Volutaria crupinoides (Desf.) Maire | Vogt 11075 & Oberprieler (B) | MAR | AY772379 | JF754905 | JF754867 | AY826344 | – |
| Zoegea leptaurea L. | Susanna 1704 et al. (BC) | IRN | AY772383 | JF754906 | JF754868 | AY826349 | JF754801 |
Country codes follow ISO A3 standard. An dash indicates a region that was not sequenced.
DNA extraction, amplification and sequencing
Total genomic DNA was extracted following the cetyltrimethylammonium bromide (CTAB) method of Doyle and Doyle (1987) and Cullings (1992) from silica-gel-dried leaves collected in the field. In some cases, herbarium material was used.
Nuclear ribosomal DNA (nrDNA) ETS and ITS region amplification strategies
Double-stranded DNA of the ITS region was amplified using ITS1 as the forward primer and ITS4 as the reverse primer (White et al., 1990). The profile used for PCR amplification followed the protocol described by Susanna et al. (2006). The ETS region was amplified with ETS1F as the forward primer (Linder et al., 2000) and 18SETS as the reverse primer (Baldwin and Markos, 1998). In some cases, AST-1 and AST-2 were also used as internal primers (Markos and Baldwin, 2001). The profile used for PCR amplification was as described by Galbany-Casals et al. (2009). For both regions, reactions were performed in 25 µL volumes with 10 % of 10× AmpliTaq buffer, 10 % of 25 mm MgCl2, 10 % of 2 mm dNTP mix, 4 % of each primer at 5 µm, 0·5 µL of DMSO (dimethylsulfoxide; Sigma-Aldrich, St Louis, MO, USA), 1 U of AmpliTaq DNA polymerase (Applied Biosystems, Foster City, CA, USA) and 2 µL of template DNA of an unknown concentration. This was made up to 25 µL using sterile, distilled water.
Chloroplast DNA (cpDNA) amplification strategies
The trnL-F region was amplified using the trnL-c, forward, and trnL-f, reverse, primers (Taberlet et al., 1991). In some cases, trnL-d, reverse, and trnL-e, forward, were also used (Taberlet et al., 1991). The profile used for amplification of this region was as described by Susanna et al. (2006). The rpl32-trnLUAG intergenic spacer was amplified with the primers rpL32F as forward and trnL-UAG as reverse (Shaw et al., 2007) with the following termocycler settings: 4 min denaturing at 94 °C, followed by 35 cycles of 60 s denaturing at 95 °C, 90 s annealing at 54 °C and 2 min extension at 72 °C, with an additional final step of 10 min at 72 °C. The major part of the ndhF encoding region was amplified using a set of four primers. The 5′ end portion of the gene was not used in analysis because of its low substitution level (Kim and Jansen, 1995). Overlapping sequence fragments were obtained by amplifying the 3′ end portion of the gene in two pieces. For the 5′ quarter, we used 3′F as forward primer (Eldenas et al., 1999) and 1783R (R. Vilatersana, Botanic Institute of Barcelona, pers. comm.) as reverse primer. For the 3′ quarter, we used 1626F (R. Vilatersana, Botanic Institute of Barcelona, pers. comm.) as forward primer and +607 (Kim and Jansen, 1995) as reverse primer. The profile for amplifications was used as in Kim et al. 2002. The PCRs were performed following the protocol used for the nuclear regions, but with the addition of 2·5 µL of 400 ng μL−1 BSA (bovine serum albumin; New England Biolabs, NE, USA).
nrDNA and cpDNA sequencing strategies
Plastid and nuclear PCR products were purified using a QIAquick PCR Purification Kit (Qiagen Inc., Valencia, CA, USA). Direct sequencing of the amplified DNA segments was performed using a BigDye Terminator Cycle Sequencing v3·1 kit (Applied Biosystems) in accordance with the manufacturer's recommended protocol. Nucleotide sequencing was performed at the ‘Serveis Científico-Tècnics’ of the University of Barcelona on an ABI PRISM 3100 DNA Analyzer (Applied Biosystems).
Phylogenetic analyses
Sequences were aligned visually by sequential pairwise comparison (Swofford and Olsen, 1990). The data matrices are available on request from the corresponding author. A graphical representation of the changes in sequence divergence for all the data sets was constructed using the Jukes–Cantor value, as implemented in PAUP. Eight data sets were prepared and analysed: ITS, ETS, combined ITS–ETS, ndhF, rpl32-trnLUAG, trnL-F, combined plastid and combined ETS–plastid regions.
Maximum parsimony and Bayesian analysis were used to infer phylogeny. The maximum parsimony analysis was conducted in PAUP* 4·0b10 (Swofford, 2002). A heuristic search was performed employing the tree-bisection and reconnection (TBR) branch-swapping algorithm. The bootstrap statistical support was calculated using 1000 replicates and ten random addition sequences per replicate. The majority rule consensus tree of the 1000 resulting best trees found for each bootstrap reweighed data set was constructed. Bootstrap support (BS) values of 90 − 100 % were interpreted as strong support.
Bayesian posterior probabilities (PPs) were estimated using MrBayes v3·1·2 (Huelsenbeck and Ronquist, 2001; Ronquist and Huelsenbeck, 2003). The evolutionary models for separate regions and combined data sets were selected using jModeltest (Posada, 2008). The maximum likelihood parameters were specified according to the Akaike Information Criterion (AIC). The plastid data set and combined ETS–plastid data sets were partitioned according to the number of regions in use, and the relevant substitution models were applied for each partition (Table 2). The extent of rate variation across sites for individual data partitions and for the combined data set was estimated by the shape parameter of the gamma distribution (σ). The results of partitioned runs were tested against the Bayesian analyses of the combined sets according to the single model of substitution. The ITS data set was analysed separately.
Table 2.
Phylogenetic characteristics of the four plastid regions, ITS, ETS and combined regions used in the study
| ETS | ITS | ndhF | rpl32-trnL | trnL-F | Plastid | Plastid + ETS | |
|---|---|---|---|---|---|---|---|
| Total characters | 522 | 650 | 1277 | 1086 | 816 | 3026 | 3531 |
| Number of parsimony-informative characters | 202 | 181 | 65 | 78 | 32 | 172 | 361 |
| Tree length | 806 | 759 | 213 | 273 | 102 | 578 | 1349 |
| Consistency index (CI) | 0·5298 | 0·5059 | 0·8216 | 0·8278 | 0·9314 | 0·8408 | 0·6597 |
| Homoplasy index (HI) | 0·4702 | 0·4941 | 0·1784 | 0·1722 | 0·0686 | 0·1592 | 0·3403 |
| CI excluding uninformative characters | 0·4515 | 0·415 | 0·6696 | 0·6781 | 0·8444 | 0·6954 | 0·5219 |
| HI excluding uninformative characters | 0·5485 | 0·585 | 0·3304 | 0·3219 | 0·1556 | 0·3046 | 0·4781 |
| Retention index (RI) | 0·6199 | 0·5318 | 0·819 | 0·8105 | 0·9255 | 0·821 | 0·6781 |
| Rescaled consistency index (RC) | 0·3284 | 0·2691 | 0·6729 | 0·671 | 0·862 | 0·6903 | 0·4474 |
| Akaike Information Criterion (AIC) | TIM2 + G | SYM + G | TVM + G | TVM + G | TPM1 | TVM + G | TPM1uf + G |
| Gamma shape parameter (σ) | 0·684 | 0·438 | 0·176 | 0·176 | – | 0·27 | 8·169 |
Each Bayesian analysis was initiated with random starting trees and was run for 1 million generations with sampling frequency of trees set at the 100th iteration. For all analyses, the variance of split sequences was <0·01, which indicated convergence of chains (Huelsenbeck and Ronquist, 2001). The fraction of sampled values discarded as burn-in was set at 0·25. Posterior probabilities of 0·95 − 1·00 were considered statistically significant.
A partition homogeneity test [incongruence length difference (ILD); Farris et al. (1995a, b)] implemented in PAUP v. 4·0b10 (Swofford, 2002) was carried out to verify the congruence of plastid and nuclear data sets. The P-value (adjusted at <0·01) was scored after 1000 replications run for two established partitions excluding uninformative characters and using heuristic search and random addition of sequences. In addition to the ILD test, the topologies obtained from the separate analyses were examined in order to detect any contradictory placement of taxa.
RESULTS
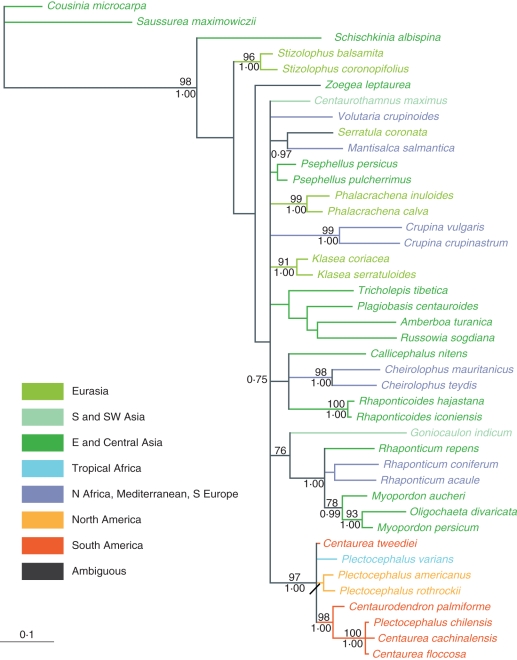
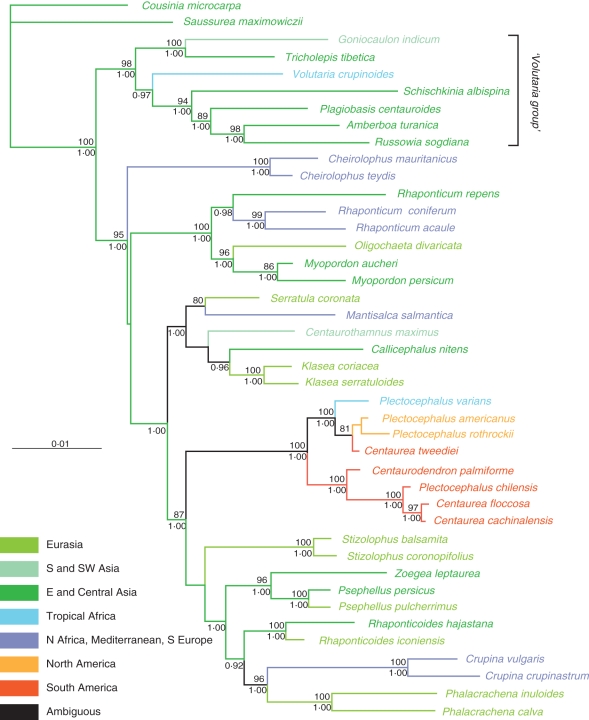
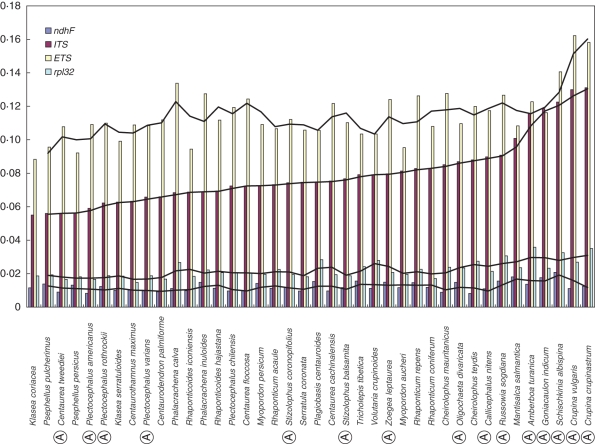
The topology of the trees obtained for separate regions by maximum parsimony and Bayesian approaches, respectively, was consistent. Therefore, only Bayesian majority-rule consensus trees are shown, with Bayesian PPs and parsimony BS added to the branches. With the exception of the ITS data, the phylogenetic analysis of the remaining regions revealed similar relationships for major groups. The ITS strongly supported a different placement for several annual species of interest belonging to the genera Schischkinia, Stizolophus and Zoegea L. compared with that shown by plastid and ETS data. The ILD test also indicated significant incongruence between the data sets of ITS and the remaining regions. Therefore, only plastid and ETS regions were used in combination, while the ITS data were processed separately. The value of the gamma shape parameter varied from 0·17 to 0·68 for the separate regions, which indicated substantial rate variation among sites in our data. The partitioned runs of the combined data sets under specified substitution models for each DNA region strengthened support for several crown nodes, as compared with searches made using the single model of substitution. The summary of the phylogenetic analyses of ITS, ETS, rpl32-trnLUAG, trnL-F, ndhF and combined data sets is given in Table 2. Two trees are shown: ITS alone (Fig. 1) and combined ETS–plastid regions (Fig. 2). Sequence divergence rates among the species studied are detailed in Fig. 3.
Fig. 1.
Majority-rule consensus tree based on Bayesian MCMC analysis of the ITS region. Numbers above branches are parsimony bootstrap percentages (BS); numbers below branches are Bayesian posterior probability values (PP). Colour codes of geographical distribution according to Funk et al. (2009).
Fig. 2.
Majority-rule consensus tree based on Bayesian MCMC analysis of the combined 3′ ETS and plastid regions. Numbers above branches are parsimony bootstrap percentages (BS); numbers below branches are Bayesian posterior probability values (PP). Colour codes are according to Funk et al. (2009).
Fig. 3.
Graphic of the sequence divergence (Jukes–Cantor coefficient) calculated for all the sequenced regions. ‘A’ indicates an annual species.
In the combined ETS–plastid analysis (Fig. 2), the Volutaria Cass. group includes Schischkinia with strong parsimony BS and the highest PP value (BS = 98 %; PP = 1·00). This clade is sister to the remaining taxa representing basal Centaureinae. Within the Volutaria group, there are two supported clades. The first includes Goniocaulon and Tricholepis (BS = 100 %; PP = 1·00), whereas the second comprises Amberboa Vaill., Plagiobasis, Russowia, Schischkinia and Volutaria (BS = 60 %; PP = 0·97).
The crown node for the clade of basal Centaureinae received good support (BS = 95 %; PP = 1·00). However, relationships among the main groups in this clade remain poorly resolved, forming a general trichotomy. It includes a strongly supported clade of two species of Cheirolophus (BS = 100 %; PP = 1·00), another equally supported clade (BS = 100 %; PP = 1·00) which encompasses Rhaponticum (including former Acroptilon Cass. and Leuzea DC.), Myopordon Boiss. and Oligochaeta (DC.) K. Koch., and a third clade containing the remaining taxa which has a high PP value (1·00), but no statistically significant BS. Consequently, the latter clade encompasses four subsumed clades which separate in the following order. (1) The clade with a high PP value (1·00) but no BS support which includes Callicephalus, Centaurothamnus Wagenitz & Dittrich, Klasea Cass., Mantisalca and Serratula L. as sister to the joint clade (BS = 87 %; PP = 1·00) of the remaining Centaureinae. (2) The well-supported clade (BS = 100 %; PP = 1·00) of several species of the genera Centaurodendron, Plectocephalus and three South American species hitherto included in the genus Centaurea (C. cachinalensis, C. floccosa and C. tweediei). In this clade, the representatives of the Plectocephalus group are placed in two equally strongly supported clades (BS = 100 %; PP = 1·00). In the first, P. varians is sister to a moderately supported clade (BS = 81 %; PP = 0·54) that contains P. americanus, P. rothrockii and C. tweediei, while the second clade includes Centaurodendron palmiforme Skottsb. as sister to a strongly supported clade (BS = 100 %; PP = 1·00) comprising P. chilensis, C. cachinalensis and C. floccosa. (3) The unsupported joint clade (BS = 61 %; PP = 0·69) includes a strongly supported clade (BS = 100 %; PP = 1·00) of two species of Stizolophus, as well as a moderately to strongly supported clade (BS = 71 %; PP = 1·00) that contains the five remaining genera: Crupina, Phalacrachena, Psephellus, Rhaponticoides and Zoegea. Each of the four genera, Crupina, Phalacrachena, Psephellus and Rhaponticoides, is represented by two species placed in separate well-supported clades (BS = 100 %; PP = 1·00). On the whole, the taxa are arranged in three cognate, phylogenetic groups: the Zoegea–Psephellus group with strong support for the joint clade (BS = 96 %; PP = 1·00), the Rhaponticoides group and the Crupina–Phalacrachena group (BS = 96 %; PP = 1·00). Of these groups, Rhaponticoides is rendered sister to the Crupina–Phalacrachena group, with virtually no statistical support (BS = 62 %; PP = 0·92).
The topology resulting from ITS analysis (Fig. 1) mainly differs from that obtained from combined plastid and combined ETS–plastid analyses in the placement of the annuals Schischkinia, Stizolophus and Zoegea, which in the ITS tree are, in turn, sisters to the entire clade of the basal Centaureinae.
DISCUSSION
Taxonomic implications
Delineation of Plectocephalus
Monophyly of Plectocephalus, including African P. varians, North American P. americanus and P. rothrockii, together with South American Centaurea cachinalensis, C. chilensis, C. floccosa and C. tweediei, could be confirmed, were it not for the position of Centaurodendron, deeply nested in the clade formed by the perennial species of the group (Figs 1 and 2). If Centaurodendron is recognized as a separate genus on molecular grounds, then Plectocephalus remains paraphyletic. Nevertheless, there are some arguments against incorporating Centaurodendron in Plectocephalus. First, the position of Centaurodendron within the clade of perennial species from Chile might reflect the peculiarities of genome evolution that are associated with perennial vs. annual life cycles, as will be discussed below. Secondly, the morphological differences that exist between Centaurodendron and Plectocephalus are substantial and are not limited solely to the habit (Parra, 1969–70). There are also differences in geographic distribution between Chilean Plectocephalus and Centaurodendron, in that the latter is a small tree characteristic of the Fernandezian Floristic Region, while species of Plectocephalus from Chile belong to the Northern and Middle Chilean Provinces (Takhtajan, 1986). Finally, the case of Centaurodendron is a timely example of the unwanted consequences of a strict cladistic interpretation of paraphyly in endemics from oceanic archipelagos as discussed by Hörandl and Stuessy (2010). When dealing with genera that are downgraded to species because of paraphyly, they pointed out: ‘The loss of endemic generic […] status, however, greatly lowers their conservation importance. When one multiplies this result in oceanic islands worldwide, the decrease in island endemism at the generic level declines substantially. One has to question the advisability of this approach, particularly because the reason the taxa have been treated as good genera in the first place is because they are highly morphologically divergent from their continental relatives.’ We must conclude that it is preferable to keep Centaurodendron as an independent genus.
All analyses confirm that Plectocephalus should also include African and South American species formerly assigned to Centaurea. Consequently, adequate nomenclatural combinations are proposed (see Appendix 1). However, relationships within the genus are difficult to explain. The combined molecular phylogeny defines two clades: the first, containing the annual species P. americanus, P. rothrockii, P. tweediei and P. varians (Fig. 2); and the second, the shrubby perennials Centaurea cachinalensis, C. floccosa, and Plectocephalus chilensis, as well as the arborescent Centaurodendron. If one accepts this result as reflecting true phylogenetic relationships, then one must also accept that two different dispersal events from North to South America must have occurred, the first giving rise to P. tweediei, and the second to the remainder of the species found in Chile and Juan Fernández. However, the most-parsimonious explanation is the occurrence of a single colonization event from North America. Based on our results, it is difficult to decide between these two hypotheses, but the character of the groups defined within the clade Plectocephalus–Centaurodendron (see above) makes us suspect its artefactual nature. In this case, a single introduction would be the most plausible explanation.
The affinities of Plectocephalus
Our results show Plectocephalus to be part of an unsupported polytomy, together with two other clades (Fig. 2): the first contains the genus Stizolophus, whereas the other is a moderately supported clade, containing the genera Crupina, Phalacrachena, Psephellus, Rhaponticoides and Zoegea. Based on strict morphological grounds, the only genera to share some similarities with Plectocephalus are Phalacrachena (especially the achenes) and Psephellus (in particular the sterile florets). Further studies are necessary to confirm which genus is the most closely related, but our results indicate that Plectocephalus belongs to an assemblage of genera that share an Eastern–Mediterranean and Irano-Turanian distribution (Fig. 2). The only exceptions are the circum-Mediterranean colonizing species of Crupina. However, Crupina is sister to the Siberian Phalacrachena with high statistical support, suggesting that it too could have had an eastern origin, as previously proposed by Garnatje et al. (2002).
The sister group of the Centaureinae: do annuals frustrate ITS analyses?
The results presented here show topological modification of the previous phylogeny with regard to the basal clade of Centaureinae, and this deserves further discussion.
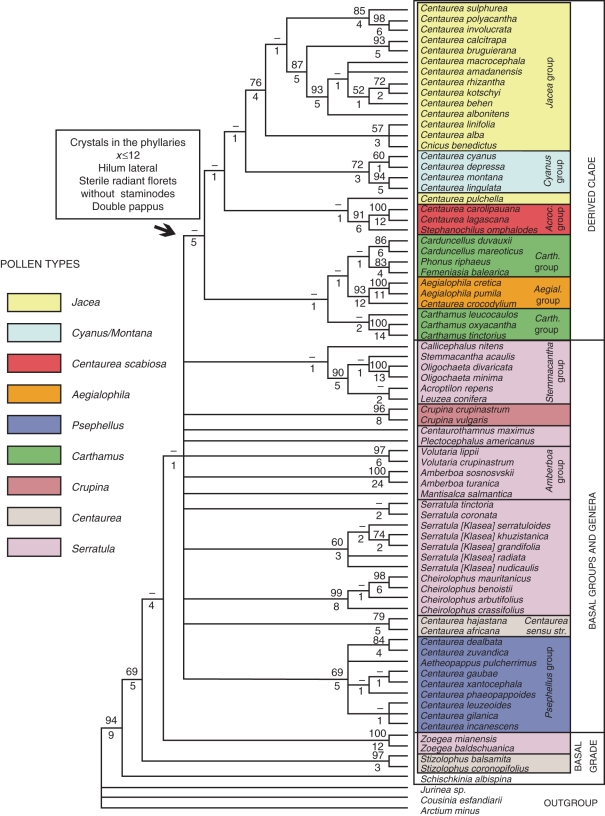
A previous survey of Centaureinae (Garcia-Jacas et al., 2001) identified the monotypic genus Schischkinia as sister to the rest of the subtribe (Fig. 4). Other successive sisters included two small genera, namely Zoegea (four species) and Stizolophus (two species). ITS analysis presented here yielded a similar phylogeny (Fig. 1). The only character shared by these three genera is their annual habit, and, therefore, it is possible that their presumed position is, in fact, incorrect (Garcia-Jacas et al., 2001; Susanna et al., 2006; Susanna and Garcia-Jacas, 2007, 2009). On morphological grounds, there are no plesiomorphic characters to support this position. Other cases of annual species being placed in a basal position in the tribe Cardueae are discussed in López-Vinyallonga et al. (2009).
Fig. 4.
Strict consensus tree resulting from the ITS analysis of the subtribe Centaureinae by Garcia-Jacas et al. (2001).
The addition of plastid regions (ndhF, rpl32-trnLUAG and trnL-F) strongly suggests that a difference in life cycle is the cause of the basal position of Schischkinia, Zoegea and Stizolophus. Schischkinia is nested within the Volutaria group, a complex of seven genera (Susanna and Garcia-Jacas, 2007, 2009) now defined as the basalmost group in the Centaureinae. Zoegea is currently considered sister to Psephellus Cass., a genus found in Anatolia, the Caucasus and northern Iran. Stizolophus appears to be unrelated to any genus, but is well nested within the Centaureinae, and distant from the basal branches (Fig. 2). These relationships and other unexpected associations, such as the link that exists between Crupina and Phalacrachena (Fig. 2), need to be reviewed on morphological and palinological grounds. However, this lies beyond the scope of the present paper. Even so, the present results confirm the aberrant position of some annuals in phylogenetic reconstructions, and this phenomenon has been much discussed in recent years. Aïnouche and Bayer (1999), Andreasen and Baldwin (2001), Smith and Donoghue (2008) and Smith and Beaulieu (2009), as well as many others, speculated that plant lineages with longer generation times, as a general rule, have lower molecular evolution rates than lineages with shorter generation times. This is surely true for the three genera whose position has been so difficult to explain in previous studies, no doubt because of the high sensitivity of the ITS region to differences in the life cycle (Aïnouche and Bayer, 1999; Andreasen and Baldwin, 2001).
The most sensitive region is the ITS, with a divergence rate ranging from 0·055 to 0·13 (Fig. 3). The ETS is also sensitive to the annual habit and shows a steep divergence peak for some annuals, the difference between maximum and minimum divergence being less marked. However, sensitivity to the different life cycles is not limited to raw divergence: Two of the misplaced genera, Stizolophus and Zoegea, are placed in the middle range of divergence (Fig. 3), and annual species of Plectocephalus that form a group, here suspected to be artefactual (see above), occur interspersed among perennial species. This suggests that difficulties relating to the placement of these annuals on the basis of ITS sequences cannot be attributed to raw divergence, but that other processes must be involved in the molecular evolution of annuals. Long-branch attraction, a frequent problem in parsimony analysis and less evident in Bayesian approaches (Swofford et al., 2001), could also be responsible for some of the unwanted results of ITS analyses. It is significant that the retention index for the ITS analysis is the lowest of all data sets, while the homoplasy index is the highest (Table 2).
This deficiency of ITS lends support to other criticisms relating to the widespread and automatic use of this marker (sensitivity to reticulation and proclivity to lineage sorting following biased concerted evolution; see Nieto Feliner and Rosselló, 2007). As suggested by Álvarez and Wendel (2003), ITS should always be used in combination with other markers, and workers should be fully aware of the limitations and deficiencies of this particular region. Based on our results, we recommend that the use of ITS should be carefully weighted for phylogenetic reconstruction when working with plants having different types of life cycle (annuals vs. perennials).
Evolution of life cycles within the group is also of interest. Species of the Plectocephalus group are either annual (P. americanus, P. rothrockii, C. tweediei and P. varians) or perennial (Centaurea cachinalensis, C. floccosa, Centaurodendron sp. pl. and Plectocephalus chilensis). Ancestral taxa for the group are likely to be perennial, as this is the ancestral state for all genera of Cardueae, a tribe derived from shrubby African ancestors of subfamily Carduoideae (Ortiz et al., 2009). Significantly enough, the most likely ancestors for subtribe Centaureinae (Cousinia, Jurinea and Saussurea) include mainly perennial species: there are only four annuals in total for these three genera that together sum >1000 species (Susanna et al., 2006; Susanna and Garcia-Jacas, 2007). Evidence that annual species developed only recently in the Plectocephalus group is based on low divergence rates (Fig. 3). While most annual species of other genera are grouped together at the right end of the graphic and show high levels of divergence, in contrast all the annual species of the Plectocephalus group are found at the other end of the range. It is thus interesting to note the affirmation made by Hind (1996) that the two North American species of Plectocephalus can be facultatively annual, biennial or perennial, a striking case of phenotypic plasticity that reinforces the hypothesis that the annual life cycle was recently adopted by species of this genus. The development of the arborescent habit in Centaurodendron is yet another example of a very rapid response to island habitats (Crawford et al., 1992). In fact, the Juan Fernández Islands are very young, the oldest being only 4 million years old (Stuessy et al., 1984).
In brief, perennial ancestors of Plectocephalus developed an annual life cycle, probably in response to climate change; but, in more stable conditions, they reverted to the perennial life cycle.
The migration of Plectocephalus
Susanna and Garcia-Jacas (2007, 2009) have suggested that Centaureinae originated at the boundaries of the Mediterranean and Irano-Turanian regions (Caucasus and Northern Iran). Since the affinities of Plectocephalus lie with Eastern–Mediterranean and Irano-Turanian groups, the occurrence of Plectocephalus varians in Ethiopia is likely to be due to the migration of more boreal ancestors. This is also known to occur in another rare species of the subtribe that grows in East Africa, namely Ochrocephala imatongenis (Philipson) Dittrich, which belongs to the mostly Eurosiberian Rhaponticum group (Hidalgo et al., 2006). Taking into account the Caucasian or north-Irano-Turanian origin of Plectocephalus, its disjunction parallels that of two of the best studied examples to date, namely subtribe Betoideae of the Chenopodiaceae (Hohmann et al., 2006) and the genus Styrax (Fritsch, 1996). A discussion of the two possible hypotheses for the origin of American Plectocephalus, namely long-distance dispersal or migration (in the sense of continuous range expansion), now follows.
Long-distance dispersal was the preferred hypothesis for Styrax (Fritsch, 1996), not just based partly on Axelrod's (1975) strong opposition to migration, but also because only a frost- and drought-tolerant ancestor could travel through the cold latitudes between Caucasus and Beringia. If the migration of this ancestor was as hypothesized, then it is also possible to speculate about its extinction. Despite the objections of Fritsch (1996), extinction of intermediate populations is supported by other disjunctions between North America and the Mediterranean. The Filago group (Compositae, Gnaphalieae) is a case in point. According to Ward et al. (2009) and Galbany-Casals et al. (2010), species of this group are mostly annuals that are adapted to xeric or high-mountain habitats. The Filago group has a Mediterranean distribution extending eastwards to Central Asia, with another centre of speciation in the south of the USA (California, Texas and Arkansas) and north of Mexico (Stebbins and Day, 1967). Thus, the most plausible explanation for this distribution is a continuous range expansion from Central Asia to North America via the BLB, followed by the extinction of landmark species in Far East Asia. This could also be the case for Datisca (Liston et al., 1992) distributed throughout the East Mediterranean, Central Asia and North America, as well as the tribe Betoideae (Hohmann et al., 2006).
Ever since Stebbins and Day (1967) suggested the existence of such a corridor, the possibility that species from xeric habitats were able to cross Central and East Asia on their way to the BLB has been much debated. The presence of two species of the genus Phalacrachena (assigned to subtribe Centaureinae) in Siberia indicates that this expansion was, at least to some extent, possible. Distribution of Phalacrachena cannot be considered direct evidence for migration by way of Siberia to North America because, despite some morphological similarities (especially the achenes), a close relationship between Phalacrachena and Plectocephalus is not supported by molecular analyses. Nevertheless, Phalacrachena does still provide indirect evidence. Close examination of the leaves of Phalacrachena and Plectocephalus reveals the presence of many sessile glands. These are frequent in many North American desert plants, and occur as critical adaptations to xeric habitats, since they greatly increase the reflectance capacity of leaves (Ehleringer, 1984). This same role has also been suggested for sessile glands in Mediterranean taxa such as Origanum L. (Kokkini et al., 1994). The presence of glands in Phalacrachena, together with its somewhat incrassate leaves, demonstrates that this genus belongs to a stratum of xeromorphic plants that has had the opportunity to migrate in a north-easterly direction (Fig. 5). Other examples of xeric taxa that migrated in the same way are cited by Yurtsev (2001) and, as previously stated, the case for the Filago group is very convincing (Galbany-Casals et al., 2010). The hypothesis for a corridor that allowed the migration of xerophytes via East Asia is increasingly supported, despite being on a very different time scale from the Palaeogene suggested by Stebbins and Day (1967).
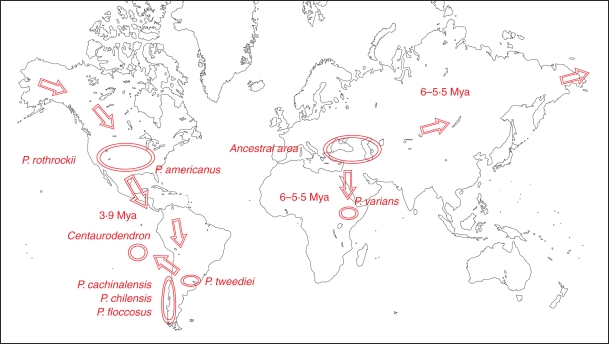
Fig. 5.
Geographic distribution of the genera Centaurodendron and Plectocephalus. Arrows indicate the hypothesized migration route with possible dates. Mya = million years ago.
The hypothesis that migration occurred via the BLB is also supported by the time framework. As for Betoideae (Hohman et al., 2006), separation of Plectocephalus from the basal Centaureinae occurred no later than the middle Miocene [approx. 12 million years ago (Mya), cf. Barres et al., Botanic Institute of Barcelona, in prep.], and this agrees with the time scale proposed for the period when the BLB was most used as a migration corridor (Marincovich and Gladenkov, 1999).
Within this time scale, the event that triggered the processes of extinction, transformation and migration of Plectocephalus may have been the Messinian Salinity Crisis (MSC), which reached its zenith at about 5·5 Mya (Rögl, 1998, 1999; Suc and Popescu, 2005). The date of 5·5 Mya is also generally regarded as the period when the BLB closed, and it is possible that the migration of Plectocephalus also took place at about this time. The BLB then reopened approx. 2·4 Mya (Ogasawara, 1998) and, thus, it is equally possible that colonization could have happened then. However, there is an argument that favours the date of approx. 6–5 Mya. Environmental transformations triggered by the MSC (and emanating outwards from the Mediterranean region) may have resulted in the front-line migration of some plant species. These followed the edges of the drastically changing and expanding climatic zones, and it is assumed that the latter provided conditions that favour annual forms of Plectocephalus. Had a later migration taken place during the intermittent reopening of the BLB, then it would have been necessary for representatives of this genus to survive in some of the East Asian Tertiary refuges for a period of approx. 3 million years. If this is so, then one would expect some species of the group still to grow in Far East Asia, much like three other genera of tribe Cardueae: Atractylodes DC. and Synurus Iljin in Korea and Japan, and Tricholepis in Burma (Susanna and Garcia-Jacas, 2009).
The emergence of the Panamanian Land Bridge (Isthmus of Panama) 3–3·5 Mya (Webb, 1991; Graham, 1992; Coates and Obando, 1996) dramatically changed the climatic condition of both Americas. The Pliocene climate was much cooler than that of the Late Miocene, with more conspicuous seasonal changes and ecological zonality (Pascual et al., 1996). Additionally, the final phase of the Andean orogenesis produced a rain-shadow effect that resulted in the establishment of extremely xeric conditions (Pascual et al., 1996). At the time when the two Americas were connected, the savannah–grassland environments were predominant on both sides of the Isthmus of Panama, and this facilitated the first phase of the Great American Biotic Interchange (Pascual et al., 1996; Webb and Rancy, 1996; Koepfli et al., 2007). It is assumed that this was the time when the annual ancestors of the modern branch of the South American Plectocephalus and Centaurodendron migrated via the Isthmus of Panama. The alternative explanation (an earlier migration involving long-distance dispersal) is less plausible for the following reasons: (1) the probability that species became established in a totally new environment through long-distance dispersal is very low (Nathan, 2006); and (2) in the late Miocene, most of South America was covered by flooded plains, while elevated territories were occupied mostly by tropical forests. The first appearance of C4 grasses in the diet of grazing animals as indicators of xerophytization in response to the climate occurred no earlier than 6·5 Mya and became widespread by 3·9 Mya, i.e. by the time the Isthmus of Panama closed (McFadden et al., 1996). The xerothermic habit of Plectocephalus would be inconsistent with the supposed new environment, even if seed were successfully transported by birds.
Therefore, the expansion of Plectocephalus to South America can be dated to the time when the latter was still connected to North America and the subsequent establishment of an arid environment throughout the territory of migration. However, the increasing zonality and complexity of the developing Pliocene climates presented new challenges to the adaptive capacity of Plectocephalus. This time, it resulted in new transformations in response to, and as adaptations to new environments. These included the perennial habit and finally, the arborescent habit.
An outline of the speculated pathway and dates of migration of Plectocephalus, from its place of origin to East Africa, East Asia, North America and South America, is presented in Fig. 5.
Concluding remarks
The study of Plectocephalus confirms that it is a natural genus comprising African (P. varians), North American (P. americanus and P. rothrockii) and South American (P. cachinalensis, P. chilensis, P. floccosus and P. tweediei) species. We have confirmed that the genus Centaurodendron from Juan Fernández Islands derived from the genus Plectocephalus from continental Chile in a fine example of budding. The group made an astonishing journey, from the Caucasus to Africa and to North America, South America and finally to the Juan Fernández Islands. The results presented here support the existence of a migration route for Mediterranean xerophilous taxa via Beringia. This is supported by other cases that together indicate the late Miocene as the most plausible time for the opening of this pathway. Finally, this study stresses the importance of using plastid and nuclear DNA regions in combination for inferring the phylogenies of groups with different life cycles. In the case of the basal Centaureinae, a study using the nuclear marker ITS in combination with two plastid markers resulted in an incorrect phylogeny. This is most likely because molecular evolution in annuals differs from that of perennials and because ITS are extremely sensitive to such differences.
ACKNOWLEDGEMENTS
This work was supported by the Ministry of Science and Innovation of Spain (projects CGL2006-01765/BOS and CGL2009-13322-C03-03) and the Generalitat de Catalunya (‘Ajuts a Grups de Recerca Consolidats’ 2009-SGR-439). The authors thank M. Dematteis (Corrientes) and D. Gutiérrez (La Plata) for providing samples of P. tweediei; T. F. Stuessy (Vienna), and D Arredondo and M. Tobar (Juan Fernández Islands), for providing samples of Centaurodendron; S. Ortiz (Santiago de Compostela) for providing samples of Plectocephalus varians; Carles and Carme Puche (Campins) for providing samples of P. americanus; and T. Wendt (University of Texas) for providing samples of P. americanus and P. rothrockii. Two anonymous reviewers and the editor A. Brysting made many valuable suggestions that greatly improved this article.
APPENDIX
Plectocephalus cachinalensis (Phil.) N. Garcia & Susanna, comb. nov.; basionym: Centaurea cachinalensis Phil., Flora Atacamensis: 34. 1860.
Plectocephalus floccosus (Hook. & Arn.) N. Garcia & Susanna, comb. nov.; basionym: Centaurea floccosa Hook. & Arn., Companion to the Botanical Magazine 1: 110. 1835.
Plectocephalus tweediei (Hook. & Arn.) N. Garcia & Susanna, comb. nov.; basionym: Centaurea tweedieii Hook. & Arn., Companion to the Botanical Magazine 1: 110. 1835.
LITERATURE CITED
- Aïnouche AK, Bayer RJ. Phylogenetic relationships in Lupinus (Fabaceae: Papilionoideae) based on internal transcribed spacer sequences (ITS) of nuclear ribosomal DNA. American Journal of Botany. 1999;86:590–607. [PubMed] [Google Scholar]
- Álvarez I, Wendel JF. Ribosomal ITS sequences and plant phylogenetic inference. Molecular Phylogenetics and Evolution. 2003;29:417–434. doi: 10.1016/s1055-7903(03)00208-2. [DOI] [PubMed] [Google Scholar]
- Andreasen K, Baldwin BG. Unequal evolutionary rates between annual and perennial lineages of checker mallows (Sidalcea, Malvaceae): evidence from 18S–26S rDNA internal and external transcribed spacers. Molecular Biology and Evolution. 2001;18:936–944. doi: 10.1093/oxfordjournals.molbev.a003894. [DOI] [PubMed] [Google Scholar]
- Axelrod DI. Evolution and biogeography of Madrean–Tethyan sclerophyll vegetation. Annals of the Missouri Botanical Garden. 1975;62:280–334. [Google Scholar]
- Baldwin BG, Markos S. Phylogenetic utility of the external transcribed spacer (ETS) of 18S–26S rDNA: congruence of ETS and ITS trees of Calycadenia (Compositae) Molecular Phylogenetics and Evolution. 1998;10:449–463. doi: 10.1006/mpev.1998.0545. [DOI] [PubMed] [Google Scholar]
- Bentham G. Compositae. In: Bentham G, Hooker JD, editors. Genera plantarum 2 (1) London: Reeve & Co; 1873. pp. 162–533. [Google Scholar]
- Boissier PE. Diagnoses plantarum orientalium novarum, ser. 2, 3. Leipzig: B. Herrmann; 1856. [Google Scholar]
- Bremer K. Asteraceae. Cladistics and classification. Portland, OR: Timber Press; 1994. [Google Scholar]
- Carlquist S. Anatomy and systematic position of Centaurodendron and Yunquea (Compositae) Britonnia. 1958;10:78–93. [Google Scholar]
- Cassini H. In: Centaurea. Cuvier G, editor. Paris: Le Normant, 373; 1817. Dictionnaire de sciences naturelles, 18. [Google Scholar]
- Coates AG, Obando JA. The geologic evolution of the Central American Isthmus. In: Jackson JBC, Budd AF, Coates AG, editors. Evolution of environments in Tropical America. Chicago and London: University of Chicago Press; 1996. pp. 21–56. [Google Scholar]
- Correl DS, Johnston MC. Manual of the vascular flora of Texas. Renner: Texas Research Foundation; 1970. [Google Scholar]
- Crawford DJ, Stuessy TF, Haines DW, Cosner MB, Silva M, López P. Allozyme diversity within and divergence among four species of Robinsonia (Asteraceae: Senecioneae), a genus endemic to the Juan Fernandez Islands, Chile. American Journal of Botany. 1992;79:962–966. [Google Scholar]
- Cullings KW. Design and testing of a plant-specific PCR primer for ecological and evolutionary studies. Molecular Ecology. 1992;1:233–240. [Google Scholar]
- Dittrich M. Cynareae – systematic review. In: Heywood VH, Harborne JB, Turner BL, editors. The biology and chemistry of the Compositae. Vol. 2. London: Academic Press; 1977. pp. 999–1015. [Google Scholar]
- Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin, Botanical Society of America. 1987;19:11–15. [Google Scholar]
- Ehleringer J. Ecology and ecophysiology of leaf pubescence in North American desert plants. In: Rodríguez E, Healey PL, Mehta I, editors. Biology and chemistry of plant trichomes. New York: Plenum Press; 1984. pp. 113–132. [Google Scholar]
- Eldenas P, Källersjö M, Anderberg AA. Phylogenetic placement and circumscription of tribes Inuleae s. str. and Plucheeae (Asteraceae): evidence from sequences of plastid gene ndhF. Molecular Phylogenetics and Evolution. 1999;13:50–58. doi: 10.1006/mpev.1999.0635. [DOI] [PubMed] [Google Scholar]
- Farris JS, Källersjö M, Kluge AG, Bult C. Testing significance of incongruence. Cladistics. 1995a;10:315–319. [Google Scholar]
- Farris JS, Källersjö M, Kluge AG, Bult C. Constructing a significance test for incongruence. Systematic Biology. 1995b;44:570–572. [Google Scholar]
- Fritsch P. Isozyme analysis of intercontinental disjuncts within Styrax (Styracaceae): implications for the Madrean–Tethyan Hypothesis. American Journal of Botany. 1996;83:342–355. [Google Scholar]
- Fritsch P. Phylogeny and biogeography of the flowering plant genus Styrax (Styracaceae) based on plastid DNA restriction sites and DNA sequences of the Internal Transcribed Spacer region. Molecular Phylogenetics and Evolution. 2001;19:387–408. doi: 10.1006/mpev.2001.0933. [DOI] [PubMed] [Google Scholar]
- Funk VA, Hind DJN. Hecastocleideae (Hecastocleidoideae) In: Funk VA, Susanna A, Stuessy TF, Bayer RJ, editors. Systematics, evolution, and biogeography of Compositae. Vienna: IAPT; 2009. pp. 261–265. [Google Scholar]
- Galbany-Casals M, Garcia-Jacas N, Sáez L, Benedí C, Susanna A. Phylogeny, biogeography and character evolution in Mediterranean, Asiatic and Macaronesian Helichrysum (Asteraceae, Gnaphalieae) inferred from nuclear phylogenetic analyses. Intenational Journal of Plant Sciences. 2009;170:365–380. [Google Scholar]
- Galbany-Casals M, Andrés-Sánchez S, Garcia-Jacas N, Susanna A, Rico E, Martínez-Ortega MM. How many of Cassini's anagrams should there be? Molecular systematics and phylogenetic relationships in the ‘Filago group’ (Asteraceae, Gnaphalieae), with special focus on the genus Filago. Taxon. 2010;59:1671–1689. [Google Scholar]
- Garcia-Jacas N, Susanna A, Garnatje T, Vilatersana R. Generic delimitation and phylogeny of the subtribe Centaureinae (Asteraceae): a combined nuclear and chloroplast DNA analysis. Annals of Botany. 2001;87:503–515. [Google Scholar]
- Garcia-Jacas N, Garnatje T, Susanna A, Vilatersana R. Tribal and subtribal delimitation and phylogeny of the Cardueae (Asteraceae): a combined nuclear and plastid DNA analysis. Molecular Phylogenetics and Evolution. 2002;22:51–64. doi: 10.1006/mpev.2001.1038. [DOI] [PubMed] [Google Scholar]
- Garcia-Jacas N, Uysal T, Romashchenko K, Suárez-Santiago VN, Ertuğrul K, Susanna A. Centaurea revisited: a molecular survey of the Jacea group. Annals of Botany. 2006;98:741–753. doi: 10.1093/aob/mcl157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnatje T, Vilatersana R, Roché CT, Garcia-Jacas N, Susanna A, Thill DC. Multiple introductions from the Iberian Peninsula responsible for invasion of Crupina vulgaris Cass. in western North America. New Phytologist. 2002;154:419–428. doi: 10.1046/j.1469-8137.2002.00382.x. [DOI] [PubMed] [Google Scholar]
- Graham A. Utilization of the isthmian land bridge during the Cenozoic: paleobotanical evidence for timing, and the selective influence of altitudes and climate. Review of Paleobotany and Palynology. 1992;72:119–128. [Google Scholar]
- Greuter W, Wagenitz G, Aghababian M, Hellwig FH. Proposal to conserve the name Centaurea (Compositae) with a conserved type. Taxon. 2001;50:1201–1205. [Google Scholar]
- Hellwig FH. Centaureinae (Asteraceae) in the Mediterranean – history of ecogeographical radiation. Plant Systematics and Evolution. 2004;246:137–162. [Google Scholar]
- Hidalgo O, Garcia-Jacas N, Garnatje T, Susanna A. Phylogeny of Rhaponticum (Asteraceae, Cardueae-Centaureinae) and related genera inferred from nuclear and chloroplast DNA sequence data: taxonomic and biogeographic implications. Annals of Botany. 2006;97:705–714. doi: 10.1093/aob/mcl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hind DJN. Plant portraits: 283. Plectocephalus rothrockii. Compositae. Curtis's Botanical Magazine. 1996;13:3–7. [Google Scholar]
- Hoffmann O. Compositae. In: Engler A, Prantl AEK, editors. Die natürlichen Pflanzenfamilien IV. Leipzig: Wilhelm Engelmann; 1894. pp. 87–391. [Google Scholar]
- Hohmann S, Kadereit J, Kadereit G. Mediterranean–Californian disjunctions: molecular evidence from Chenopodiaceae–Betoideae. Taxon. 2006;55:67–78. [Google Scholar]
- Hörandl E, Stuessy TF. Paraphyletic groups as natural units of biological classification. Taxon. 2010;59:1641–1653. [Google Scholar]
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Jeffrey C. Notes on Compositae: III – the Cynareae in East Tropical Africa. Kew Bulletin. 1968;22:107–140. [Google Scholar]
- Keil DJ, Ochsmann J Flora of North America Editorial Committee. Flora of North America North of Mexico. New York: Oxford University Press; 2006. Centaurea; pp. 181–194. [Google Scholar]
- Kim HG, Loockerman DJ, Jansen RK. Systematic implications of ndhF sequences in the Mutisieae (Asteraceae) Systematic Botany. 2002;27:598–609. [Google Scholar]
- Kim KJ, Jansen RK. ndhF sequence evolution and the major clades in the sunflower family. Proceedings of the National Academy of Sciences, USA. 1995;92:10379–10383. doi: 10.1073/pnas.92.22.10379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepfli KP, Gompper ME, Eizirik E, Ho CC, Linden L, Maldonado JE, Wayne RK. Phylogeny of the Procyonidae (Mammalia: Carnivora): molecules, morphology and the Great American Interchange. Molecular Phylogenetics and Evolution. 2007;43:1076–1095. doi: 10.1016/j.ympev.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Kokkini S, Karousou R, Vokou D. Pattern of geographic variations of Origanum vulgare trichomes and essential oil content in Greece. Biochemical Systematics and Ecology. 1994;22:517–528. [Google Scholar]
- Linder CR, Goertzen LR, Vanden Heuvel B, Francisco-Ortega J, Jansen RK. The external transcribed spacer of the rDNA repeat: a new nuclear region for low-level taxonomic analysis of the Asteraceae and closely allied families. Molecular Phylogenetics and Evolution. 2000;14:285–303. doi: 10.1006/mpev.1999.0706. [DOI] [PubMed] [Google Scholar]
- Linnaeus C. Species Plantarum. Holmiae: Impensis Laurentii Salvii; 1753. [Google Scholar]
- Liston A, Rieseberg LH, Hanson MA. Geographic partitioning of plastid DNA variation in the genus Datisca (Datiscaceae) Plant Systematics and Evolution. 1992;181:121–132. [Google Scholar]
- López-Vinyallonga S, Mehregan I, Garcia-Jacas N, Tscherneva O, Susanna A, Kadereit JW. Phylogeny and evolution of the Arctium–Cousinia complex (Compositae, Cardueae–Carduinae) Taxon. 2009;58:153–171. [Google Scholar]
- Loudon JG. Loudon's encyclopaedia of plants. New edition. London: Longman, Brown, Green & Longmans; 1855. [Google Scholar]
- Marincovich L, Gladenkov AY. Evidence for an early opening of the Bering Strait. Nature. 1999;397:149–151. [Google Scholar]
- Markos S, Baldwin BG. Higher-level relationships and major lineages of Lessingia (Compositae, Astereae) based on nuclear rDNA internal and external transcribed spacers (ITS and ETS) sequences. Systematic Botany. 2001;26:168–183. [Google Scholar]
- Martin WC, Hutchins CR. A flora of New Mexico. Vaduz: J. Cramer; 1981. [Google Scholar]
- McFadden BJ, Cerling TE, Prado JL. Cenozoic terrestrial ecosystem evolution in Argentina: evidence from carbon isotopes of fossil mammal teeth. Palaios. 1996;11:319–327. [Google Scholar]
- Nathan R. Long-distance dispersal of plants. Science. 2006;313:786–788. doi: 10.1126/science.1124975. [DOI] [PubMed] [Google Scholar]
- Nieto Feliner G, Rosselló JA. Better the devil you know? Guidelines for insightful utilization of nrDNA ITS in species-level evolutionary studies in plants. Molecular Phylogenetics and Evolution. 2007;44:911–919. doi: 10.1016/j.ympev.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Nordenstam B, El-Ghazaly G. Floral micromorphology and pollen ultrastructure in some Centaureinae (Compositae) mainly from Egypt. Publications from Cairo University Herbarium. 1977;7–8:143–155. [Google Scholar]
- Ogasawara K. Review and comments on late Neogene climatic fluctuations and the intermittence of the Bering Land Bridge. Journal of Asian Earth Sciences. 1998;16:45–48. [Google Scholar]
- Ortiz S, Bonifacino JM, Crisci JV, et al. The basal grade of Compositae: Mutiseae (sensu Cabrera) and Carduoideae. In: Funk VA, Susanna A, Stuessy TF, Bayer RJ, editors. Systematics, evolution, and biogeography of Compositae. Vienna: IAPT; 2009. pp. 193–213. [Google Scholar]
- Parra O. Morfología de los granos de polen de las compuestas cynareas chilenas. Boletín de la Sociedad de Biología de Concepción. 1969–70;42:89–96. [Google Scholar]
- Pascual R, Ortiz-Jaureguizar E, Prado JL. Arratia G, editor. Land mammals: paradigm of Cenozoic South American geobiotic evolution. Contribution of Southern South America to vertebrate paleontology. Müncher Geowissenschaftliche Abhandlungen (A) 1996;30:265–319. [Google Scholar]
- Posada D. jModeltest: phylogenetic model averaging. Molecular Biology and Evolution. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Raven PH. Plant species disjunctions: a summary. Annals of the Missouri Botanical Garden. 1972;59:234–246. [Google Scholar]
- Rögl F. Palaeogeographic considerations for Mediterranean and Paratethys seaways (Oligocene to Miocene) Annalen des Naturhistorischen Museums in Wien. 1998;99:279–310. [Google Scholar]
- Rögl F. Mediterranean and Paratethys: facts and hypotheses of an Oligocene to Miocene paleogeography (short overviews) Geologica Carpathica (Bratislava) 1999;50:339–349. [Google Scholar]
- Ronquist F, Huelsenbeck JP. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Shaw J, Lickey EB, Schilling EE, Small RL. Comparison of whole plastid genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: the tortoise and the hare III. American Journal of Botany. 2007;94:275–288. doi: 10.3732/ajb.94.3.275. [DOI] [PubMed] [Google Scholar]
- Smith SA, Beaulieu JM. Life history influences rates of climatic niche evolution in flowering plants. Proceedings of the Royal Society B: Biological Sciences. 2009;276:4345–4352. doi: 10.1098/rspb.2009.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Donoghue MJ. Rates of molecular evolution are linked to life history in flowering plants. Science. 2008;322:86–89. doi: 10.1126/science.1163197. [DOI] [PubMed] [Google Scholar]
- Stebbins GL, Day A. Cytogenetic evidence for long continued stability in the genus Plantago. Evolution. 1967;21:409–428. doi: 10.1111/j.1558-5646.1967.tb03399.x. [DOI] [PubMed] [Google Scholar]
- Stuessy TF, Foland KA, Sutter JF, Sanders RW, Mario SO. Botanical and geological significance of potassium–argon dates from the Juan Fernández Islands. Science. 1984;225:49–51. doi: 10.1126/science.225.4657.49. [DOI] [PubMed] [Google Scholar]
- Suc JP, Popescu SM. Pollen records and climatic cycles in the North Mediterranean region since 2·7 Ma. In: Head MJ, Gibbard PL, editors. Early–Middle Pleistocene transitions: the land–ocean evidence. London: Geological Society of London, Special Publication; 2005. pp. 147–158. [Google Scholar]
- Susanna A, Garcia-Jacas N. Tribe Cardueae. In: Kadereit JW, Jeffrey C, editors. Flowering plants. Eudicots. Asterales. VIII. Berlin: Springer Verlag; 2007. pp. 123–146. In: Kubitzki K. ed. The families and genera of vascular plants. [Google Scholar]
- Susanna A, Garcia-Jacas N. Cardueae (Carduoideae) In: Funk VA, Susanna A, Stuessy TF, Bayer RJ, editors. Systematics, evolution, and biogeography of Compositae. Vienna: IAPT; 2009. pp. 293–313. [Google Scholar]
- Susanna A, Garcia-Jacas N, Soltis DE, Soltis PS. Phylogenetic relationships in tribe Cardueae (Asteraceae) based on ITS sequences. American Journal of Botany. 1995;82:1056–1068. [Google Scholar]
- Susanna A, Garcia-Jacas N, Hidalgo O, Vilatersana R, Garnatje T. The Cardueae (Compositae) revisited: insights from ITS, trnL-trnF, and matK nuclear and plastid DNA analysis. Annals of the Missouri Botanical Garden. 2006;93:150–171. [Google Scholar]
- Sweet R. Hortus Britannicus. London: James Ridgway; 1830. [Google Scholar]
- Swofford DL. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods) Sunderland, MA: Sinauer; 2002. ver. 4·0b10. [Google Scholar]
- Swofford DL, Olsen GJ. Phylogeny reconstruction. In: Hillis D, Moritz C, editors. Molecular systematics. Sunderland, MA: Sinauer; 1990. pp. 411–501. [Google Scholar]
- Swofford DL, Waddell PJ, Huelsenbeck JP, Foster PG, Lewis PO, Rogers JS. Bias in phylogenetic estimation and its relevance to the choice between parsimony and likelihood methods. Systematic Biology. 2001;50:525–539. [PubMed] [Google Scholar]
- Taberlet P, Gielly L, Pautou G, Bouvet J. Universal primers for amplification of three non-coding regions of plastid DNA. Plant Molecular Biology. 1991;17:1105–1109. doi: 10.1007/BF00037152. [DOI] [PubMed] [Google Scholar]
- Takhtajan A. Floristic regions of the world. Berkeley: University of California Press; 1986. [Google Scholar]
- Wagenitz G. Pollenmorphologie und Systematik in der Gattung Centaurea L. s. l. Flora. 1955;142:213–279. [Google Scholar]
- Wagenitz G, Hellwig FH. Evolution of characters and phylogeny of the Centaureinae. In: Hind DJN, Beentje HG, editors. Compositae: systematics. Proceedings of the International Compositae Conference, Kew, 1994. Kew: Royal Botanic Gardens; 1996. pp. 491–510. [Google Scholar]
- Ward JM, Bayer RJ, Breitwieser I, Smissen RD, Galbany-Casals M, Unwin M. Gnaphalieae – systematic and phylogenetic review. In: Funk VA, Susanna A, Stuessy TF, Bayer RJ, editors. Systematics, evolution, and biogeography of Compositae. Vienna: IAPT; 2009. pp. 537–585. [Google Scholar]
- Webb SD. Ecography and the Great American Interchange. Paleobiology. 1991;17:266–280. [Google Scholar]
- Webb SD, Rancy A. Late Cenozoic evolution of the Neotropical mammal fauna. In: Jackson JBC, Budd AF, Coates AG, editors. Evolution and environments in tropical Americas. Chicago: The University of Chicago Press; 1996. pp. 335–358. [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego: Academic Press; 1990. pp. 315–322. [Google Scholar]
- Yurtsev BA. The Pleistocene ‘Tundra-Steppe’ and the productivity paradox: the landscape approach. Quaternary Science Reviews. 2001;20:165–174. [Google Scholar]