Abstract
Background and Aims
High alpine environments are characterized by short growing seasons, stochastic climatic conditions and fluctuating pollinator visits. These conditions are rather unfavourable for sexual reproduction of flowering plants. Apomixis, asexual reproduction via seed, provides reproductive assurance without the need of pollinators and potentially accelerates seed development. Therefore, apomixis is expected to provide selective advantages in high-alpine biota. Indeed, apomictic species occur frequently in the subalpine to alpine grassland zone of the European Alps, but the mode of reproduction of the subnival to nival flora was largely unknown.
Methods
The mode of reproduction in 14 species belonging to seven families was investigated via flow cytometric seed screen. The sampling comprised 12 species typical for nival to subnival plant communities of the European Alps without any previous information on apomixis (Achillea atrata, Androsace alpina, Arabis caerulea, Erigeron uniflorus, Gnaphalium hoppeanum, Leucanthemopsis alpina, Oxyria digyna, Potentilla frigida, Ranunculus alpestris, R. glacialis, R. pygmaeus and Saxifraga bryoides), and two high-alpine species with apomixis reported from other geographical areas (Leontopodium alpinum and Potentilla crantzii).
Key Results
Flow cytometric data were clearly interpretable for all 46 population samples, confirming the utility of the method for broad screenings on non-model organisms. Formation of endosperm in all species of Asteraceae was documented. Ratios of endosperm : embryo showed pseudogamous apomixis for Potentilla crantzii (ratio approx. 3), but sexual reproduction for all other species (ratios approx. 1·5).
Conclusions
The occurrence of apomixis is not correlated to high altitudes, and cannot be readily explained by selective forces due to environmental conditions. The investigated species have probably other adaptations to high altitudes to maintain reproductive assurance via sexuality. We hypothesize that shifts to apomixis are rather connected to frequencies of polyploidization than to ecological conditions.
Keywords: Apomixis, European Alps, endosperm, flow cytometric seed screen, high-altitude plants, polyploidy, sexual reproduction
INTRODUCTION
Alpine biota often have a tendency to apomixis, the mode of reproduction via asexually formed seed (Asker and Jerling, 1992). Apomictic plants show a higher abundance of seed than their sexual relatives in higher altitudes and latitudes, in previously glaciated areas and in disturbed habitats (Bierzychudek, 1985; Hörandl, 2006; Hörandl et al., 2008). This distribution pattern is most pronounced in cases of gametophytic apomixis which involves the formation of an unreduced embryo sac and the development of an unreduced egg cell without fertilization. In contrast, the formation of embryos out of nucellar tissues (adventitious embryony) is more abundant in tropical plants (Richards, 1997).
The relationship of asexual reproduction to certain ecological conditions has often been referred to selective forces by the environment (Bell, 1982; Asker and Jerling, 1992). In the European Alps and in other comparable temperate mountain systems, flowering plants colonizing higher altitudes have to cope with conditions that are rather unfavourable for sexual reproduction. Colder climates, a short growing season and occasional frost during the reproductive period threaten a permanent risk of seed loss, especially for plants that need a long time for seed development (e.g. Ladinig and Wagner, 2007; Wagner et al., 2011). Apomixis, in contrast, is often associated with an acceleration of developmental pathways via maturation of embryo sacs before anthesis and pollination (e.g. Carman, 1997; Grimanelli et al., 2001; Carman et al., 2011). Such precocious development, known from alpine species of Alchemilla (Fröhner, 1990) and from Hieracium alpinum (Skawińska, 1963), would allow for rapid seed formation in a short growing season. Unfavourable weather conditions in high altitudes can reduce pollinator activities (e.g. Warren et al., 1988; McCall and Primack, 1992), which may limit seed set for outcrossing plants (e.g. Muñoz and Arroyo, 2006). Therefore, reproductive assurance that is independent of pollinators, as it is provided by apomixis or autogamy, would be advantageous in alpine habitats. Above the snowline, abiotic conditions become increasingly extreme in all these aspects.
Historical factors may have also promoted the occurrence of apomixis in the Alps. Glaciations of the Pleistocene have caused range fluctuations of species and may have brought previously isolated species together, thereby increasing frequencies of secondary contact hybridization and polyploidization (Hewitt, 1996, 2004). Hybridization and/or polyploidy have been thought to cause the epigenetic and genetic changes which would cause shifts to gametophytic apomixis (e.g. Carman, 1997; Grimanelli et al., 2001; Koltunow and Grossniklaus, 2003; Hörandl, 2009a, b). Moreover, uniparental reproduction provides the additional advantage of enhanced colonization abilities: a single individual can found a new population (Baker's law: Baker, 1967; Hörandl, 2006; Hörandl et al., 2008). In the European Alps, the postglacial retreat of glaciers offered opportunities for colonization by apomictic plants (Cosendai and Hörandl, 2010).
The European Alps comprise ecologically distinct altitudinal belts (Ozenda, 1988; Ellenberg and Leuschner, 2010): the forest zone (montane zone) reaches 1300 m/1500 m a.s.l. in the north/south, respectively, and is followed by the subalpine zone (upper limits 2000 m/2200 m) with a mosaic of patchy forest, shrub communities and pastures. The alpine zone (upper limits 2400 m/2600 m) is characterized by a cover of vegetation by small shrubs and grassland. This vegetation in the subnival zone (upper limits 2700/2900 m) breaks up into isolated patches and is increasingly replaced by screes, permanent snow fields and rocks. The nival zone (from 2700/2900 m up to 4500 m) is characterized by glaciers and a predominance of cryptogams in patchy remnants of vegetation, with a drastic reduction of species richness of both flowering plants and pollinators (Grabherr et al., 1995).
Both ecological and historical factors would suggest that asexuality becomes more frequent in high altitudes because of selective benefits. In fact, gametophytic apomixis has been documented in many common species of the subalpine and alpine zone of the European Alps (e.g. Alchemilla spp., Hieracium spp., Nardus stricta, Pilosella spp., Poa alpina, Taraxacum spp.; reviewed by Gustafsson, 1952, 1953; Asker and Jerling, 1992; Hörandl, 2011). Some of them even dominate widespread alpine grassland communities (e.g. Nardus stricta, Poa alpina, Alchemilla spp.). Therefore, apomixis has been supposed to be a general reproductive strategy of alpine plants (e.g. Körner, 2003). It would be expected that apomixis should be even more abundant in species inhabiting subnival and nival zones than in species restricted to the subalpine and alpine zone because of the increasingly extreme and stochastic climatic conditions. In arctic snowbeds, apomictic species become more frequent (Molau, 1993), which suggests a selective benefit to apomixis in habitats with a short vegetation period. However, the information on apomixis in high-alpine species of the European Alps was so far very scarce. From 12 species occurring over 4000 m a.s.l. as listed for example by Grabherr et al. (1995), the mode of reproduction has never been studied. Moreover, apomictic taxa often show a geographical differentiation of sexual and apomictic populations within and between mountain systems (reviewed by Hörandl et al., 2008; Hörandl, 2011), which infers that apomixis cannot be predicted for a certain region from studies on accessions in other mountain systems.
The reason for this lack of information on high-alpine plants is mostly due to methodological problems: experimental approaches (e.g. bagging and emasculation procedures) are difficult to monitor in the field under high-alpine conditions, and do not discrimate between selfing and pollen-dependent apomixis (pseudogamy; see Hörandl et al., 2008). This is critical because approx. 90 % of apomicts is in fact pseudogamous (Mogie, 1992). Reproductive tissues for studying embryo-sac development before anthesis (e.g. after Herr, 1971, 1992) are often not accessible in high-alpine plants. In the lowlands, high-alpine plants often do not flower in cultivation. Large-scale progeny tests using molecular markers are often hampered by low germination rates. For all these reasons, modes of reproduction in high-alpine plants have remained largely unknown.
The recently developed method of flow cytometric seed screen (FCSS; Matzk et al., 2000) can overcome these problems. FCSS measures DNA content in mature seeds and can therefore infer the DNA ploidy levels of embryo and endosperm, which allows the mode of apomixis to be determined (Krahulcová and Rotreklová, 2010). Sexual reproduction is characterized by double fertilization, the egg cell and the central cell of the embryo sac, and therefore the resulting ratio of embryo (n + n) to endosperm (2n + n) equals to 2 : 3. Gametophytic apomixis involves the formation of an unreduced embryo sac, and the development of the unreduced egg cell without fertili-zation. Seeds formed via apomixis, in contrast, have therefore 1 : 2, 1 : 2·5 or 1 : 3 ratios of embryo (2n) to endosperm (2n + 2n + 0, 2n + 2n + n or 2n + 2 n + n + n), depending on the contribution of sperm nuclei for endosperm fertilization. FCSS was so far mostly used for traditional apomictic model organisms (reviewed by Matzk, 2007), but has the potential to detect apomixis in plants without embryological information (e.g. Heenan et al., 2002, 2003).
The main goal of this study was to screen for the occurrence of apomixis in the high-alpine flora of the Alps and to test the utility of the FCSS methodology for non-model plants on seeds collected in the wild. Fourteen candidate species were selected from seven plant families that are characteristic of the subnival to the nival zone of the European Alps (Schroeter, 1908; Ozenda, 1988; Grabherr et al., 1995), and flow cytometric seed screening on seeds collected in the wild was conducted to test for the occurrence of apomixis.
MATERIALS AND METHODS
Materials
Seeds were collected in the wild during summer 2009 (for accessions see Table 1). Vouchers have been deposited in the herbarium WU. In a few cases, plants in postfloral, but premature seed stage were transferred to the Botanical Garden in Vienna, and seeds were collected there at maturity. Since embryo-sac formation and fertilization have happened before at the natural sites, this sampling strategy preserved the mode of reproduction in the wild. Material was selected from the most frequent species of the subnival to nival zone after Schroeter (1908), Ozenda (1988) and Grabherr et al. (1995). Because of fuzzy borderlines between the upper alpine, subnival and nival zones, ‘high alpine’ is used here as an umbrella term for species occurring in these zones of the European Alps. We focused on species with insect pollination to get insights into assumed selective effects of pollinator limitation (thus excluding Poaceae and Cyperaceae). Diagnostic (characteristic and/or dominant) species of high-alpine plant communities (screes, snowbeds, exposed ridges; after Grabherr and Mucina, 1993) were selected to understand selective effects of environmental conditions on modes of reproduction. Furthermore, an attempt was made to cover the main geographical zones of the European Alps that are characterized by different geology and siliceous/calcareous bedrock; see Table 1). The sampling comprises two groups.
Table 1.
Materials used for the study
| Taxon | Collection no. | Country* | Province | Locality, habitat type† | Altitude (m a.s.l.) | Co-ordinates | Date | Collector‡ |
|---|---|---|---|---|---|---|---|---|
| Asteraceae | ||||||||
| Achillea atrata | 9841 | A | Vorarlberg | St Anton am Arlberg, Ulmer Hütte; A, c | 2273 | 47°08′41·4′′N; 10°12′31·6′′E | 01·08·2009 | EH |
| Achillea atrata | 9862 | A | Tyrol | Großglockner, Lucknerhütte – Stüdlhütte; A, sc | 2357 | 47°02′42·0′′N; 12°41′21·0′′E | 11·08·2009 | EH |
| Achillea atrata | 9866 | A | Tyrol | Kals, Hohes Tor – Spöttling; A, sc | 2250 | 47°01′31·0′′N; 12°36′23·5′′E | 13·08·2009 | EH |
| Achillea atrata | s.n. | I | Friuli-Venezia | Julische Alps, Mt Kanin; A, c | approx. 2000 | 46°22′07′′N; 13°28′41′′E | 07·09·2009 | RN |
| Erigeron uniflorus | 9742 | CH | Valais | Furkapass, Furkastock; R, sc | 2638 | 46°34′31·1′′N; 8°24′48·5 ′′E | 26·07·2009 | EH |
| Erigeron uniflorus | 9749 | CH | Valais | Grimselpass, Sidelhorn; R, s | 2528 | 46°33′35·2′′N; 8°19′07·3′′E | 27·07·2009 | EH |
| Erigeron uniflorus | 9845 | A | Tyrol | Verwall, Kuchenjöchli – Scheibler; R, s | 2904 | 47°03′23·3′′N; 10°13′20·9′′E | 05·08·2009 | EH |
| Erigeron uniflorus | 9849 | A | Tyrol | Verwall, Kuchenjöchli – Scheibler; R, s | 2752 | 47°03′16·0′′N; 10°13′22·0′′E | 05·08·2009 | EH |
| Erigeron uniflorus | 9854 | A | Tyrol | Kals, Gorner; R, sc | 2704 | 46°59′09·7′′N; 12°36′08·4′′E | 08·08·2009 | EH |
| Erigeron uniflorus | 9859 | A | Tyrol | Großglockner, Stüdlhütte; R, sc | 2847 | 47°03′11·1′′N; 12°40′53·5′′E | 11·08·2009 | EH |
| Gnaphalium hoppeanum | 9838 | A | Tyrol | St Anton am Arlberg, Kapall; B, c | 2318 | 47°09′06·8′′N; 10°14′48·6′′E | 07·07·2009 | EH |
| Gnaphalium hoppeanum | 9840 | A | Tyrol | St Anton am Arlberg, Kapall; B, c | 2270 | 47°08′49·6′′N; 10°14′59·3′′E | 07·07·2009 | EH |
| Leontopodium alpinum | 9860 | A | Tyrol | Groβglockner, Stüdlhütte; R, sc | 2862 | 47°03′13·2′′N; 12°40′54·5′′E | 11·08·2009 | EH |
| Leontopodium alpinum | 9863 | A | Tyrol | Kals, Muntanitzschneid; R, c | 2288 | 47°03′45·9′′N; 12°36′43·9′′E | 08·08·2009 | EH |
| Leontopodium alpinum | 9865 | A | Tyrol | Kals, Dürrenfeldscharte; R, sc | 2814 | 47°02′32·4′′N; 12°35′15·1′′E | 08·08·2009 | EH |
| Leontopodium alpinum | 9877 | A | Lower Austria | Schneeberg, near Damböckhaus; G, c | 1865 | 47°45′46·5′′N; 15°49′49·7′′E | 30·08·2009 | EH |
| Leontopodium alpinum | 9878 | A | Steiermark | Raxalpe, near Raxkircherl; G, c | 1820 | 47°41′09·1′′N; 15°42′13·2′′E | 09·09·2009 | EH |
| Leucanthemopsis alpina | 9746 | CH | Valais | Furkapass, Kl. Furkahorn; A, s | 2739 | 46°34′50·3′′N; 8°24′45·0′′E | 26·07·2009 | EH |
| Leucanthemopsis alpina | 9847 | A | Tyrol | Verwall, Kuchenjöchli – Scheibler; A, s | 2873 | 47°03′21·7′′N; 10°13′21·9′′E | 05·08·2009 | EH |
| Leucanthemopsis alpina | 9861 | A | Tyrol | Großglockner, Lucknerhütte – Stüdlhütte; A, sc | 2475 | 47°02′52·2′′N; 12°41′25·1′′E | 11·08·2009 | EH |
| Brassicaceae | ||||||||
| Arabis caerulea | 9829 | CH | Valais | Furkapass, Blauberg; B, sc | 2709 | 46°34′01·7′′N; 8°25′13·1′′E | 29·07·2009 | EH |
| Arabis caerulea | 9839 | A | Tyrol | St Anton am Arlberg, Kapall: B, c | 2315 | 47°09′06·1′′N; 10°14′47·8′′E | 31·07·2009 | EH |
| Arabis caerulea | 9875 | A | Lower Austria | Schneeberg, Hackermulde; B, c | 2013 | 47°46′08·9′′N; 15°48′27·1′′E | 30·08·2009 | EH |
| Polygonaceae | ||||||||
| Oxyria digyna | 9850 | A | Tyrol | Verwall, Darmstädter Hütte – Saumspitze; A, s | 2576 | 47°02′48·7′′N; 10°15′23·3′′E | 06·08·2009 | EH |
| Oxyria digyna | s.n. | A | Tyrol | Ötztal Alps, Mittelbergferner, glacier foreland; A, s | 2850 | 46°55′36·5′′N; 10°52′55·0′′E | 22·09·2009 | JW |
| Primulaceae | ||||||||
| Androsace alpina | 9751 | CH | Valais | Grimselpass, Sidelhorn; A, s | 2516 | 46°33′35·5′′N; 8°19′08·9′′E | 27·07·2009 | EH |
| Androsace alpina | 9844 | A | Tyrol | Verwall, Kuchenjöchli – Scheibler; A, s | 2904 | 47°03′23·3′′N; 10°13′20·9′′E | 05·08·2009 | EH |
| Androsace alpina | 9852 | A | Tyrol | Kals, Gorner; A, s | 2506 | 46°59′24·4′′N; 12°35′57·7′′E | 08·08·2009 | EH |
| Androsace alpina | 9856 | A | Tyrol | Kals, Gorner; A, s | 2680 | 46°59′13·8′′N; 12°36′11·1′′E | 08·08·2009 | EH |
| Androsace alpina | 9858 | A | Tyrol | Großglockner, Stüdlhütte; A, s | 2847 | 47°03′11·1′′N; 12°40′53·5′′E | 11·08·2009 | EH |
| Ranunculaceae | ||||||||
| Ranunculus alpestris | 9835 | A | Tyrol | St Anton am Arlberg, Kapall; B, c | 2327 | 47°09′07·8′′N; 10°14′49·5′′E | 31·07·2009 | EH |
| Ranunculus alpestris | 9836 | A | Tyrol | St Anton am Arlberg, Kapall; B, c | 2401 | 47°09′19·0′′N; 10°15′19·3′′E | 31·07·2009 | EH |
| Ranunculus alpestris | 9876 | A | Lower Austria | Schneeberg, Hackermulde; A, c | 2030 | 47°46′09·2′′N; 15°48′25·1′′E | 08·08·2009 | EH |
| Ranunculus alpestris | s.n. | A | Tyrol | Innsbruck, Hafelekar; A, c | 2300 | 47°18′46·5′′N; 11°23′05·0′′E | Aug 2009 | UL |
| Ranunculus glacialis | 889 | F | Hautes-Alpes | Col de Galibier; not known | 2500 | 45°3′50·4′′N; 6°24′28·8′′E | 2009 | Unknown§ |
| Ranunculus glacialis | 9750 | CH | Valais | Goms, Sidelhorn; A, s | 2525 | 46°33′35·2′′N; 8°19′07·3′′E | 07·07·2009 | EH |
| Ranunculus glacialis | 9826 | CH | Valais | Furkapass, Blauberg; A, sc | 2643 | 46°34′05·3′′N; 8°25′15·2′′E | 29·07·2009 | EH |
| Ranunculus glacialis | 9831 | CH | Valais | Furkapass, Blauberg; A, sc | 2838 | 46°33′47·0′′N; 8°25′13·1′′E | 29·07·2009 | EH |
| Ranunculus glacialis | 9846 | A | Tyrol | Verwall, Kuchenjöchli -Scheibler; A, s | 2904 | 47°03′23·3′′N; 10°13′20·9′′E | 05·08·2009 | EH |
| Ranunculus glacialis | 9857 | A | Tyrol | Großglockner, Stüdlhütte; A, sc | 2847 | 47°03′11·1′′N; 12°40′53·5′′E | 11·08·2009 | EH |
| Ranunculus glacialis | s.n. | A | Tyrol | Stubai Alps, Schaufelferner, glacier foreland; A, s | 2850 | 46°59′15·8′′N. 11°06′58·0′′E | 28·07·2009 | UL |
| Ranunculus pygmaeus | 9855 | A | Tyrol | Kals, Gorner; B, sc | 2695 | 46°59′08·0′′N; 12°36′09·5′′E | 08·08·2009 | EH |
| Rosaceae | ||||||||
| Potentilla crantzii | 838 | F | Hautes-Alpes | Col de Lautaret; unknown | 2400 | 45°02′05′′N; 6°42′18′′E | 01·07·2009 | Unknown§ |
| Potentilla crantzii | 9873 | A | Salzburg | Großglockner, Mittertörl; R, sc | 2382 | 47°06′00·1′′N; 12°50′09·5′′E | 15·08·2009 | EH |
| Potentilla frigida | 9832 | CH | Valais | Furkapass, Blauberg; A, sc | 2838 | 46°33′47·0′′N; 8°25′13·1′′E | 29·07·2009 | EH |
| Saxifragaceae | ||||||||
| Saxifraga bryoides | s.n. | A | Tyrol | Stubai Alps, Schaufelferner, glacier foreland; A, s | 2850 | 46°59′15·8′′N. 11°06′58·0′′E | 22·09·2009 | UL |
* A, Austria; CH, Switzerland; F, France; I, Italy.
† A, screes, glacier moraines; B, snow beds; R, rocks and exposed ridges; G, grassland (patches); s, siliceous bedrock; c, calcareous bedrock; sc, intermediate types.
‡ EH, E. Hörandl, RN, Roland Nitsche; UL, Ursula Ladinig; JW, Johanna Wagner.
§ Materials from the Alpine Botanical Garden of Lautaret, France.
(1) Typical subnival to nival species without any previous information on apomixis: Androsace alpina, Leucanthemopsis alpina, Ranunculus glacialis, Oxyria digyna and Saxifraga bryoides characterize high-alpine to nival siliceous screes, R. pygmaeus siliceous snowbeds. Achillea atrata, Arabis caerulea, R. alpestris and Gnaphalium hoppeanum are diagnostic species of alpine to nival calcareous snowbeds; Erigeron uniflorus, Potentilla crantzii and P. frigida are typical of plant communities on exposed ridges. The mode of sexual versus apomictic reproduction has not yet been studied and so far apomixis is not even known in two of the respective families, Primulaceae and Saxifragaceae (Carman, 1997). However, apomixis occurs in other species of some of the respective genera [Achillea and Erigeron (Noyes, 2007), Ranunculus (Nogler, 1984; Cosendai and Hörandl, 2010) and Potentilla (Gustafsson, 1952, 1953)] and may be expected in the sampled species because of taxonomic predispositions (e.g. Van Dijk and Vijverberg, 2005).
(2) Species with apomixis reported from other areas: Potentilla crantzii and Leontopodium alpinum do have apomictic biotypes, as has been shown previously by cytological and embryological studies on materials outside the Alps (Sokolowska-Kulczycka, 1959; Czapik, 1961, 1962; Smith, 1963). Records of apomixis for L. alpinum in the Southern Alps (Maugini, 1962) need confirmation. Nevertheless, apomixis might be facultative in these species, and there is no information on the geographical distribution of modes of reproduction. Both species are not confined to the highest regions of the Alps but occur also in the alpine zone and are typical of plant communities on exposed ridges.
A total of 46 populations were investigated, with an average of 3·4 populations per taxon. Multiple populations were analysed for all but three species (Ranunculus pygmaeus, Potentilla frigida and Saxifraga bryoides). Apomixis is heritable, and usually results in apomictic clones with one predominant genotype and, if any, very low frequencies of deviating individuals within a population. Predominance of apomictic plants, even in the case of facultative recombination, was confirmed by numerous population genetic studies (e.g. Gornall, 1999; Houliston and Chapman, 2004; Nybom et al., 2006; Paun et al., 2006; Thompson and Ritland, 2007; Barcaccia et al., 2007) and by progeny tests (e.g. Bicknell et al., 2003). Comprehensive FCSS studies suggest that either sexuality or apomixis become rapidly fixed within a population (Aliyu et al., 2010). Therefore, the occurrence of apomixis can be assessed with high probability even on a single, randomly sampled individual of a population (e.g. Talent and Dickinson, 2007). [In contrast, the detection of rare recombinants within apomictic lineages requires large sample sizes (Bicknell et al., 2003; Barcaccia et al., 2007; Aliyu et al., 2010).] One individual for small populations and up to six for larger ones were collected. The number of seeds analysed per species ranged from one in Potentilla frigida to 248 in Erigeron uniflorus, totalling 1143 seeds (see Table 2).
Table 2.
Results of flow cytometric seed screens
| Species/family | Protocol used | Population no./locality | No. of individuals | No. of seeds | Mean endosperm;embryo peak ratio | Reproductive pathway |
|---|---|---|---|---|---|---|
| Asteraceae | ||||||
| Achillea atrata | 3 | 9841 | 1 | 127 | 1·48 | Sexual |
| 3 | 9862 | 2 | 20 (5 + 15) | 1·47 | Sexual | |
| 3 | 9866 | 1 | 20 | 1·48 | Sexual | |
| 3 | Julische Alps | 1 | 25 | 1·5 | Sexual | |
| Erigeron uniflorus | 3 | 9742 | 1 | 72 | 1·48 | Sexual |
| 3 | 9749 | 1 | 20 | 1·49 | Sexual | |
| 3 | 9845 | 2 | 40 (15 + 25) | 1·49 | Sexual | |
| 3 | 9849 | 1 | 35 | 1·47 | Sexual | |
| 3 | 9854 | 5 | 61 (6 + 15 + 10 + 20 + 10) | 1·48 | Sexual | |
| 3 | 9859 | 1 | 20 | 1·45 | Sexual | |
| Gnaphalium hoppeanum | 3 | 9838 | 2 | 31 (21 + 10) | 1·46 | Sexual |
| 3 | 9840 | 1 | 10 | 1·53 | Sexual | |
| Leontopodium alpinum | 3 | 9860 | 4 | 21 (6 + 6 + 5 + 4) | 1·47 | Sexual |
| 3 | 9863 | 1 | 10 | 1·48 | Sexual | |
| 3 | 9865 | 2 | 11 (6 + 5) | 1·46 | Sexual | |
| 3 | 9877 | 5 | 17 (7 + 6 + 2 + 1 + 1) | 1·46 | Sexual | |
| 3 | 9878 | 3 | 19 (12 + 5 + 2) | 1·47 | Sexual | |
| Leucanthemopsis alpina | 3 | 9746 | 2 | 20 (10 + 10) | 1·48 | Sexual |
| 3 | 9847 | 4 | 66 (12 + 11 + 27 + 16) | 1·47 | Sexual | |
| 3 | 9861 | 1 | 68 | 1·44 | Sexual | |
| Brassicaceae | ||||||
| Arabis caerulea | 1 | 9829 | 1 | 3 | 1·52 | Sexual |
| 1 | 9839 | 2 | 13 (5 + 8) | 1·55 | Sexual | |
| 1 | 9875 | 1 | 3 | 1·53 | Sexual | |
| Polygonaceae | ||||||
| Oxyria digyna | 2 | 9850 | 1 | 3 | approx. 1·5* | Sexual |
| 3 | Pitztal glacier | 4 | 23 (7 + 6 + 5 + 5) | 1·48 | Sexual | |
| Primulaceae | ||||||
| Androsace alpina | 1 | 9751 | 1 | 4 | 1·54 | Sexual |
| 1 | 9844 | 1 | 3 | 1·55 | Sexual | |
| 1 | 9852 | 1 | 3 | 1·56 | Sexual | |
| 1 | 9856 | 1 | 3 | 1·55 | Sexual | |
| 1 | 9858 | 1 | 3 | 1·56 | Sexual | |
| Ranunculaceae | ||||||
| Ranunculus alpestris | 2 | 9835 | 1 | 5 | 1·58 | Sexual |
| 3 | 9835 | 3 | 15 (each 5) | 1·59 | Sexual | |
| 2 | 9836 | 1 | 5 | 1·67 | Sexual | |
| 3 | 9836 | 3 | 15 (each 5) | 1·62 | Sexual | |
| 3 | 9876 | 2 | 10 (5 + 5) | 1·62 | Sexual | |
| 2 | Hafelekar | 9 | 45 (each 5) | 1·63 | Sexual | |
| 3 | Hafelekar | 3 | 15 (each 5) | 1·58 | Sexual | |
| Ranunculus glacialis | 2 | 889 | 2 | 8 (3 + 5) | 1·67 | Sexual |
| 3 | 889 | 1 | 9 | 1·64 | Sexual | |
| 3 | 9750 | 2 | 20 (15 + 5) | 1·72 | Sexual | |
| 2 | 9826 | 2 | 10 (5 + 5) | 1·82 | ??? | |
| 3 | 9826 | 2 | 17 (11 + 6) | 1·85 | ??? | |
| 2 | 9831 | 1 | 5 | 1·78 | Sexual | |
| 3 | 9831 | 3 | 26 (19 + 6 + 1) | 1·75 | Sexual | |
| 3 | 9846 | 6 | 37 (12 + 10 + 5 + 5 + 3 + 2) | 1·80 | ??? | |
| 2 | 9857 | 1 | 5 | 1·67 | Sexual | |
| 3 | 9857 | 3 | 11 (5 + 3 + 3) | 1·77 | Sexual | |
| 2 | Stubai glacier | 2 | 10 (5 + 5) | 1·64 | Sexual | |
| Ranunculus pygmaeus | 2 | 9855 | 1 | 5 | 1·69 | Sexual |
| Rosaceae | ||||||
| Potentilla crantzii | 1 | 838 | 1 | 6 | 2·96 | Pseudogamous apomictic |
| 1 | 9873 | 1 | 9 | 3·10 | Pseudogamous apomictic | |
| Potentilla frigida | 1 | 9832 | 1 | 1 | 1·50 | Sexual |
| Saxifragaceae | ||||||
| Saxifraga bryoides | 2 | Stubai glacier | 1 | 80 | 1·45 | Sexual |
For details on flow cytometric protocols, see Materials and methods.
* Calculated from G2 peaks of both embryo and endosperm in immature seeds.
Flow cytometric seed screen
The relative fluorescence intensity of nuclei from the embryo and endosperm were determined by flow cytometry from single and/or pooled seeds and fruitlets. Materials were chopped with a razor blade and then specifically analysed according to the following protocols (see Table 2).
(a) At the Department of Pharmacognosy, University of Vienna, samples were prepared following Matzk et al. (2000) using a one-step protocol using a slightly modified seed buffer [5 mm MgCl2.6H2O, 85 mm sodium chloride, 100 mm Tris, 0·09 % Triton X-100, 6·1 mm sodium citrate dihydrate, 1 µg mL−1 DAPI (4′-6-diamidino-2-phenylindole)] obtained from the Apomixis Working Group at the IPK, Gatersleben. The fluorescence intensity of 300–12 000 particles was recorded using the Partec ploidy analyser PA (Partec GmbH., Münster, Germany) equipped with a mercury arc lamp.
(b) At the Department of Systematic and Evolutionary Botany, University of Vienna, seeds were chopped in Otto I buffer (0·1 m citric acid, 0·5 % Tween 20). After filtration through a 30-μm mesh and incubation with RNase A (0·15 mg mL−1) at 37 °C for 30 min, propidium iodide (final concentration 50 µg mL−1; Greilhuber et al., 2007) containing Otto II buffer (0·4 m Na2HPO4.12H2O) was added. Staining was carried out at 7 °C from 1 h up to overnight. For measurement a Partec CyFlow ML flow cytometer equipped with a diode-pumped solid state green laser (532 nm, 100 mW, Cobolt Samba; Cobolt AB, Stockholm, Sweden) was used.
(c) At the Department of Botany, Faculty of Science, Charles University in Prague, seeds were analysed by DAPI (most samples) or propidium iodide (a few samples) flow cytometry following a simplified two-step protocol as described by Doležel et al. (2007). Whole seeds (filled achenes selected under the stereomicroscope) were chopped with or without a leaf tissue of internal reference standard (usually Pisum sativum) in 0·5 mL of ice-cold Otto I buffer. The crude suspension was filtered through a 42-μm nylon mesh and incubated at room temperature for 15 min. Nuclei were stained with 1 mL of Otto II buffer, supplemented with a fluorochrome and 2-mercaptoethanol (2 µL mL−1). As DNA-selective stains, either DAPI (final concentration of 4 µg mL−1) or propidium ioidide + RNase IIA (both at final concentrations of 50 µg mL−1) were used. After 10-min incubation at room temperature, fluorescence intensity of isolated nuclei was recorded on a Partec ML or CyFlow SL cytometer equipped with a power UV LED chip (365 nm) or a green solid-state laser (Cobolt Samba 532 nm, 100 mW), respectively, as an excitation source. Flow histograms were evaluated using the FloMax software. The following design of seed analysis was usually adopted: one analysis of a single seed, five analyses of seed pairs, followed by the analyses of five pooled seeds until the final seed number (see Table 2).
The embryo : endosperm ratios needed for the inference of the reproductive mode were calculated from the arithmetic means of the individual G0/G1 embryo and endosperm fluorescence peaks. The embryo/endosperm ratios of samples of identical reproductive mode were averaged within accessions for statistical presentation.
RESULTS
The FCSS analysis revealed clearly interpretable histograms with distinct embryo and endosperm peaks in 46 population samples (Figs 1 and 2, Table 2 and Supplementary Data Fig. S1, available online). In most cases, single seeds or pooling of two to five seeds provided sufficient amounts of tissue for revealing distinct peaks, except for Saxifraga bryoides, whose seeds are so small that extensive pooling of seeds (approx. 80) was necessary. Calculations of peak ratios usually show little variation among the samples of the same species. In all species except R. glacialis, intraspecific variation was below 6 % (Table 2).
Fig. 1.
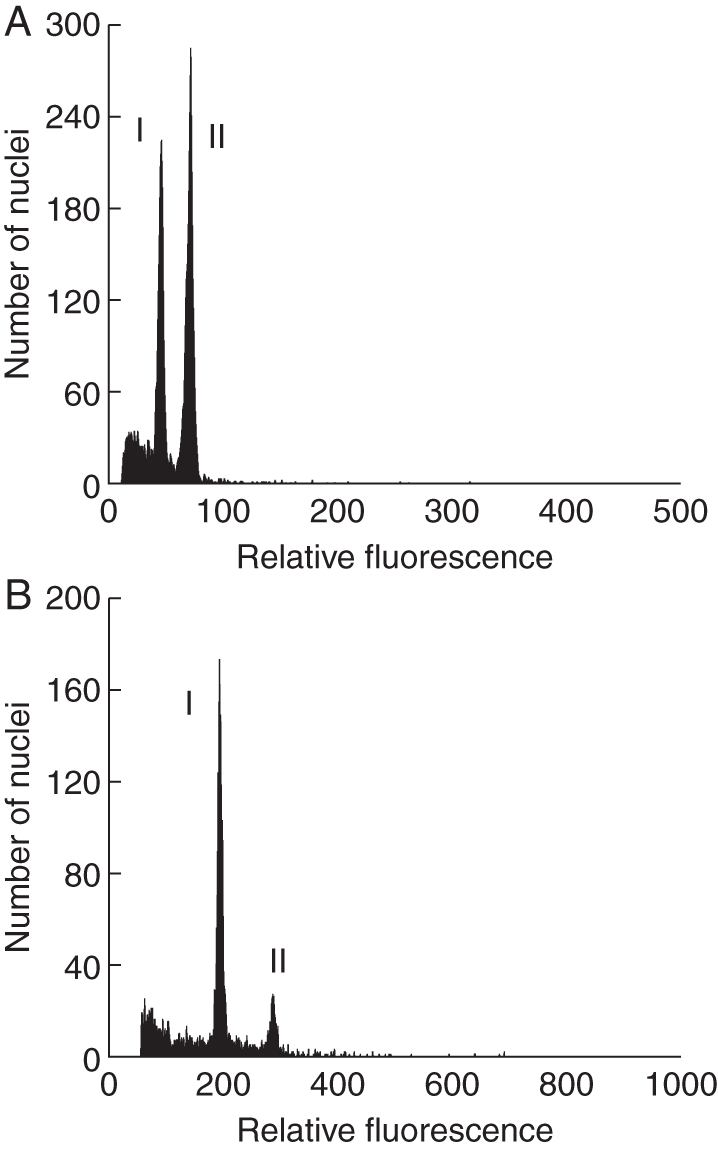
FCSS histogram from seeds formed by sexual reproduction. I, Embryo nuclei; II, endosperm nuclei, with a ratio of approx. 2 : 3. (A) Androsace alpina (no. 9844); (B) Leontopodium alpinum (no. 9878).
Fig. 2.
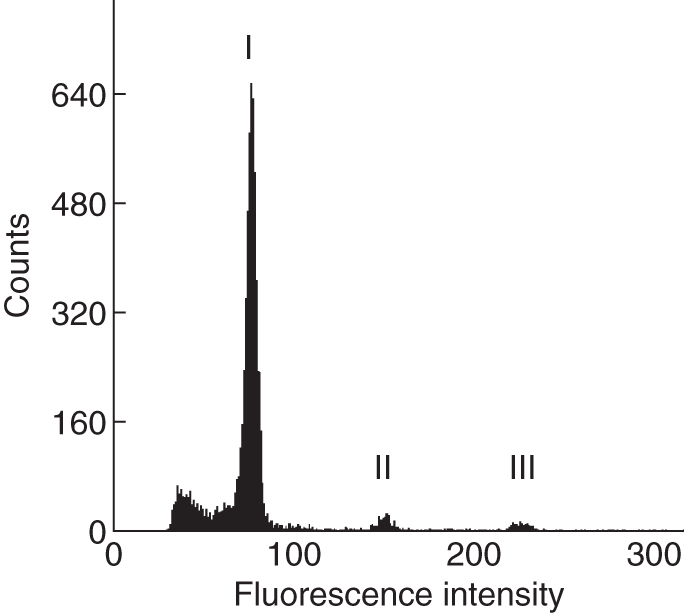
FCSS histogram from seeds of Potentilla crantzii (no. 9873) with apomictic pseudogamous reproduction. I, G1 embryo nuclei; II, G2 of the embryo; III, G1 endosperm nuclei.
All subnival to nival species with unknown mode of reproduction and no apomictic relatives (Androsace alpina, Arabis caerulea, Gnaphalium hoppeanum, Leucanthemopsis alpina, Oxyria digyna and Saxifraga bryoides) showed exclusively ratios of embryo to endosperm of 2 : 3 as is typical for double fertilization [a reduced egg cell fertilized by one sperm nucleus, n + n, and the two polar nuclei (or the central cell nucleus) fertilized by the other sperm nucleus, 2n + n; Fig. 1]. This result confirms sexual reproduction in these species. In Oxyria digyna, embryo tissue underwent endoreduplication, resulting in a high peak of nuclei with 4C DNA content.
Also the species with apomixis known in the same genus (Achillea atrata, Erigeron uniflorus, Potentilla frigida, Ranunculus alpestris, R. glacialis and R. pygmaeus) were all exclusively sexual (Table 2). In R. glacialis, peak ratios were rather variable (intraspecific variation nearly 13 %) and reached values from 1·64 up to 1·85, but always remained clearly below 2·0 which would be indicative of an unreduced embryo sac. Plants with peak ratios below 1·8 are considered as sexual and those with peak ratios above this arbitrary threshold (1·80–1·85) as having an uncertain mode of reproduction (populations 9826 and 9846; Table 2).
In the two alpine species with previously reported apomixis, only Potentilla crantzii showed apomictic seed formation with around three times the DNA content of the endosperm compared with the embryo (Fig. 2 and Table 2). This result indicates an unreduced embryo sac connected to pseudogamous apomixis, whereby either both sperm nuclei or one unreduced sperm nucleus must have fertilized the central cell (2n + 2n + n + n or 2n + 2n + 2n). In contrast, all five accessions of Leontopodium alpinum from the Alps clearly showed sexual reproduction.
DISCUSSION
This study confirms the broad applicability of FCSS for the determination of the mode of reproduction in high-alpine plant species. Further sampling and routine screening can establish geographical patterns of modes of reproduction, as has been shown by Cosendai and Hörandl (2010) in the alpine species R. kuepferi. Potential drawbacks are that FCSS cannot detect adventitious embryony and is further not applicable to rare forms of gametophytic development with four-nucleate embryo sacs, where only one polar nucleus is formed (e.g. Carman, 1997).
An essential prerequisite for FCSS is the presence of intact endosperm nuclei in mature seeds; this technique is thus not applicable if endosperm in seeds is never formed (e.g. many Orchidaceae, Podostemaceae or Trapa natans) or is completely resorbed at maturity (e.g. Ceratophyllaceae, most Fabaceae, some Fagaceae, Lythraceae and/or Asteraceae, including Helianthus annuus; Black et al., 2006). Seeds of the latter family usually have little endosperm (e.g. in Lactuca spp. this nutrition tissue is formed by only one to a few layers of cells; Black et al., 2006); nevertheless, this amount is often sufficient for FCSS to be successfully applied (e.g. Taraxacum, Mártonfiová, 2006; Hieracium, A. Krahulcová et al., Průhonice, unpubl. res.). Similarly, all Asteraceae species analysed in the present study had endospermous seeds as shown by a distinct peak corresponding to endosperm nuclei (see Fig. 1B and Supplementary Data Fig. S1). FCSS on other Asteraceae species is desirable to test the validity of the conventional assumption that mature seeds of this family generally lack endosperm. Another potential problem of FCSS may be the histogram interpretation, especially when pooled seeds are analysed. Mixed sexual and apomictic seed samples would reveal distinct endosperm peaks at the expected ratios to the embryo, but not an intermediate ratio (e.g. Barcaccia et al., 2007; Aliyu et al., 2010). Since just one endosperm peak was observed in R. glacialis, a mixed sexual–apomictic system can be excluded as interpretation. It may be difficult to set a reasonable threshold for discrimination of sexual versus apomictic seeds if pronounced, and more or less continuous, variation in peak ratios is observed, such as in Ranunculus glacialis (Table 2). In this species, endosperm : embryo peak ratios were always above 1·5 (i.e. the value typical for sexual reproduction) but below 2·0 (i.e. the value typical for autonomous apomixis). Because the peak ratios in other sexually reproducing buttercups are known to vary consistently between 1·5 and 1·8 (Cosendai and Hörandl, 2010; E. Hörandl and D. Hojsgaard, unpubl. res.; see also Table 2), ratios below 1·8 were regarded to be indicative of sexual reproduction. The mode of reproduction in samples with peak ratios exceeding this arbitrary threshold should be investigated using other methodological approaches such as dissecting techniques. Whether the observed variation in the endosperm : embryo peak ratios has technical backgrounds or reflects natural variation in the DNA content of gametes, remains to be studied. However, it should be noted that highly comparable peak ratios for a particular population of R. glacialis were obtained in two different laboratories and using different protocols (Table 2). Natural variation in DNA content within cytotypes, as it was observed in populations of diploid R. kuepferi (Cosendai and Hörandl, 2010), might influence gamete ratios. Another possibility is differential expression of secondary metabolites, or differential DNA degeneration due to drying of tissues in embryo and endosperm. Autonomous apomixis or haploid parthenogenesis were considered unlikely as no value reached the typical ratio of 2.
Despite theoretical evolutionary advantages, gametophytic apomixis turns out to be very rare in plants at extremely high elevations. This confirms the opinion of Gustafsson (1952, 1953) that frequencies of apomixis decline from high to very high altitudes. The selective benefits of uniparental reproduction for colonization, pollinator-independence and rapid development seem to be not strong enough to establish apomixis as a frequent trait. Facultative selfing might provide an alternative that is functionally easier to establish (Hörandl, 2006). From the species investigated here, selfing has been documented for Gnaphalium hoppeanum and Arabis caerulea, while Achillea atrata is predominantly outcrossing (Scheffknecht et al., 2007). The two selfing species occur in snow-beds, where extremely short vegetation periods may favour uniparental reproduction. Selfing and apomixis, however, are usually rather alternative strategies of uniparental reproduction (Hörandl, 2010). Predominant outcrossing appears to be a competitive strategy in subnival plants as well and has been confirmed, for example, on Ranunculus glacialis (Wagner et al., 2010) and on Saxifraga bryoides (Ladinig and Wagner, 2007). For the other species, detailed studies on breeding systems and reproductive success are largely missing. We hypothesize that these high-alpine specialists are so well adapted to the harsh and stochastic environmental conditions that a shift to apomixis would not provide a strong selective advantage.
No apomixis was found in those species where the trait has been observed in other species of the genus. However, the present results clearly confirm that gametophytic apomixis is connected to polyploidy. All the observed sexual species are diploid except for Androsace alpina (2n = 4x = 32; Dobeš and Vitek, 2000). Leucanthemopsis alpina has diploid and tetraploid cytotypes in the Alps (Watanabe, 2011). In contrast, species with recorded or here observed apomixis (Leontopodium alpinum and Potentilla crantzii, respectively) are all polyploid. Potentilla crantzii comprises several polyploid cytotypes with 2n = 28, 42 and 49 (Dobeš and Vitek, 2000). The present observation of apomixis in the plants from the Alps confirms previous studies on material from the British Isles, whereas the species is sexual in the Carpathians (Czapik, 1961, 1962; Smith, 1963). Leontopodium alpinum in the Tatry mountains is polyploid with 2n = 4x = 52 (Murín and Paclová, 1979). For edelweiss, apomixis has been reported in material from the Carpathians (Sokolowska-Kulczycka, 1959), while studies on material from the southern Alps suggested facultative apomixis (Maugini, 1962). The present data show sexual reproduction in five accessions of the eastern Alps. These cases may reflect a geographical differentiation of apomictic and sexual cytotypes between the Alps and the Tatry as also observed in Hieracium (Mráz et al., 2009).
Gametophytic apomixis has been observed as a rare trait in diploid, otherwise sexual plants (e.g. Boechera, Kantama et al., 2007; Paspalum, Siena et al., 2008). However, establishment of gametophytic apomixis is usually connected to polyploidy (Grimanelli et al., 2001; Koltunow and Grossniklaus, 2003). Polyploidization happens more frequently in colder climates, as cold temperatures can trigger the formation of unreduced gametes (Ramsey and Schemske, 1998). In this respect, frequencies of polyploidization in a certain taxon might be more important for the establishment of apomixis than selective forces of ecological conditions. In fact, apomixis in the Alps occurs in species of the more moderate subalpine and alpine grassland zone, sometimes extending to the forest zone (Alchemilla spp., Hieracium spp., Nardus stricta, Pilosella spp., Poa alpina, Taraxacum spp.; Hörandl, 2011). Plants from lower latitudes that are not adapted to cold temperatures may undergo more frequently polyploidization and shifts to apomixis during colonization of higher elevations. In contrast, the high-elevation plants studied here do not show a pronounced tendency towards polyploidy, which again may be explained by specific adaptations to cold temperatures. The present study on high-alpine plants confirms that apomixis is not increased in frequencies by selective forces of environmental conditions if functional requirements for shifts to apomixis are not met. This supports the hypothesis by Hörandl (2009b) that asexual reproduction is limited by functional constraints for shifts from sexuality to apomixis.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Jana Rauchová for her help with pilot flow cytometric analyses and Roland Nitsche (Vienna) for collection of a sample from the south-eastern Alps. This work was supported by the Austrian Academy of Sciences, Commission for Interdiscipli-nary Ecological Studies (KIÖS grant no. 2009/03) and the FWF Austrian Science Fund (grant number I310), both to E.H. Further support was provided by the Academy of Sciences of the Czech Republic (grant number AV0Z60050516) and the Ministry of Education, Youth and Sports of the Czech Republic (grant number MSM0021620828) to J.S.
LITERATURE CITED
- Aliyu OM, Schranz ME, Sharbel TF. Quantitative variation for apomictic reproduction in the genus Boechera (Brassicaceae) American Journal of Botany. 2010;97:1719–1731. doi: 10.3732/ajb.1000188. [DOI] [PubMed] [Google Scholar]
- Asker SE, Jerling L. Apomixis in plants. Boca Raton, FL: CRC Press; 1992. [Google Scholar]
- Baker HG. Support for Bakers law – as a rule. Evolution. 1967;21:853–856. doi: 10.1111/j.1558-5646.1967.tb03440.x. [DOI] [PubMed] [Google Scholar]
- Barcaccia G, Arzenton F, Sharbel T, Varotto S, Parrini P, Lucchin M. Genetic diversity and reproductive biology in ecotypes of the facultative apomict Hypericum perforatum L. Heredity. 2007;96:322–334. doi: 10.1038/sj.hdy.6800808. [DOI] [PubMed] [Google Scholar]
- Bell G. The masterpiece of nature: the evolution and genetics of sexuality. Berkeley, CA: California Press; 1982. [Google Scholar]
- Bicknell RA, Lambie SC, Butler RC. Quantification of progeny classes in two facultatively apomictic accessions of Hieracium. Hereditas. 2003;138:11–20. doi: 10.1034/j.1601-5223.2003.01624.x. [DOI] [PubMed] [Google Scholar]
- Bierzychudek P. Patterns in plant parthenogenesis. Experientia. 1985;41:1255–1264. doi: 10.1007/978-3-0348-6273-8_9. [DOI] [PubMed] [Google Scholar]
- Black M, Bewley JD, Halmer P. The encyclopedia of seeds. Wallingford, UK: CABI; 2006. [Google Scholar]
- Carman JG. Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biological Journal of the Linnean Society. 1997;61:51–94. [Google Scholar]
- Carman JG, Jamison M, Elliott E, Dwivedi KK, Naumova TN. Apospory appears to accelerate onset of meiosis and sexual embryo sac formation in sorghum ovules. BMC Plant Biology. 2011;11:9. doi: 10.1186/1471-2229-11-9. doi:10.1186/1471-2229-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosendai A-C, Hörandl E. Cytotype stability, facultative apomixis and geographical parthenogenesis in Ranunculus kuepferi (Ranunculaceae) Annals of Botany. 2010;105:457–470. doi: 10.1093/aob/mcp304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czapik R. Embryological studies in the genus Potentilla L. I. P. crantzii. Acta Biologica Cracoviensia, Ser. Botanica. 1961;4:97–119. [Google Scholar]
- Czapik R. Badania embriologiczne nad rodzajem Potentilla L. III. Mieszance miedzy P. crantzii i P. arenaria [Embryological studies in the genus Potentilla L. III. Hybrids between P. crantzii and P. arenaria]. Acta Biologica Cracoviensia, Ser. Botanica. 1962;5:43–61. [Google Scholar]
- Dobeš C, Vitek E. Documented chromosome number checklist of Austrian vascular plants. Vienna: Museum of Natural History; 2000. [Google Scholar]
- Doležel J, Greilhuber J, Suda J. Estimation of nuclear DNA content in plants using flow cytometry. Nature Protocols. 2007;2:2233–2244. doi: 10.1038/nprot.2007.310. [DOI] [PubMed] [Google Scholar]
- Ellenberg H, Leuschner C. Vegetation Mitteleuropas mit den Alpen. 6th edn. Stuttgart: Ulmer; 2010. [Google Scholar]
- Fröhner S. In: Alchemilla. edn 3. Conert HJ, et al., editors. IV 2B. Berlin: Parey; 1990. pp. 13–242. Illustrierte Flora von Mitteleuropa. [Google Scholar]
- Gornall RJ. Population genetic structure in agamospermous plants. In: Hollingsworth PM, Bateman RM, Gornall RJ, editors. Molecular systematics and plant evolution. London: Taylor & Francis; 1999. pp. 118–138. [Google Scholar]
- Grabherr G, Mucina L, editors. Die Pflanzengesellschaften Österreichs. Jena: G. Fischer; 1993. Teil II. Natürliche waldfreie Vegetation. [Google Scholar]
- Grabherr G, Gottfried M, Gruber A, Pauli H. Patterns and current changes in alpine plant diversity. In: Chapin FS III, Körner C, editors. Arctic and alpine biodiversity. Berlin: Springer; 1995. pp. 167–181. Ecological Studies 113. [Google Scholar]
- Greilhuber J, Temsch EM, Loureiro JCM. Nuclear DNA content measurement. In: Doležel J, Greilhuber J, Suda J, editors. Flow cytometry with plant cells: analysis of genes, chromosomes, and genomes. Weinheim: Wiley-VCH; 2007. pp. 67–101. [Google Scholar]
- Grimanelli D, Leblanc O, Perotti E, Grossniklaus U. Developmental genetics of gametophytic apomixis. Trends in Genetics. 2001;17:597–604. doi: 10.1016/s0168-9525(01)02454-4. [DOI] [PubMed] [Google Scholar]
- Gustafsson A. Apomixis in higher plants. Part I. The mechanisms of apomixis. Lunds Universitets Arsskrift. 1952;42:1–66. [Google Scholar]
- Gustafsson A. Apomixis in higher plants. Part II. The causal aspects of apomixis. Lunds Universitets Arsskrift. 1953;43:71–178. [Google Scholar]
- Heenan PB, Dawson MI, Bicknell RA. Evidence for apomictic seed formation in Coprosma waima (Rubiaceae) New Zealand Journal of Botany. 2002;40:347–355. [Google Scholar]
- Heenan PB, Molloy BPJ, Bicknell RA, Luo C. Levels of apomictic and amphimictic seed formation in a natural population of Coprosma robusta (Rubiaceae) in Riccarton Bush, Christchurch, New Zealand. New Zealand Journal of Botany. 2003;41:287–291. [Google Scholar]
- Herr JM. A new clearing-squash technique for the study of ovule development in angiosperms. American Journal of Botany. 1971;58:785–790. [Google Scholar]
- Herr JM. New York: Springer; 1992. Recent advances in clearing techniques for study of ovule and female gametophyte development. Angiosperm pollen and ovules; pp. 149–154. [Google Scholar]
- Hewitt GM. Some genetic consequences of ice ages, and their role in divergence and speciation. Biological Journal of the Linnean Society. 1996;58:247–276. [Google Scholar]
- Hewitt GM. Genetic consequences of climatic oscillations in the Quaternary. Philosophical Transactions of the Royal Society of London, Ser. B: Biological Sciences. 2004;359:183–195. doi: 10.1098/rstb.2003.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörandl E. The complex causality of geographical parthenogenesis. New Phytologist. 2006;171:525–538. doi: 10.1111/j.1469-8137.2006.01769.x. [DOI] [PubMed] [Google Scholar]
- Hörandl E. Geographical parthenogenesis: opportunities for asexuality. In: Schön I, Martens K, Van Dijk P, editors. Lost sex. Heidelberg: Springer Verlag; 2009a. pp. 161–186. [Google Scholar]
- Hörandl E. A combinational theory for maintenance of sex. Heredity. 2009b;103:445–457. doi: 10.1038/hdy.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörandl E. The evolution of self-fertility in apomictic plants. Sexual Plant Reproduction. 2010;23:73–86. doi: 10.1007/s00497-009-0122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörandl E. Evolution and biogeography of alpine apomictic plants. Taxon. 2011;60:390–402. [Google Scholar]
- Hörandl E, Cosendai A-C, Temsch E. Understanding the geographic distributions of apomictic plants: a case for a pluralistic approach. Plant Ecology and Diversity. 2008;2:309–320. doi: 10.1080/17550870802351175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houliston HJ, Chapman HM. Reproductive strategy and population genetic variability in the facultative apomict Hieracium pilosella (Asteraceae) American Journal of Botany. 2004;91:37–44. doi: 10.3732/ajb.91.1.37. [DOI] [PubMed] [Google Scholar]
- Kantama L, Sharbel TF, Schranz ME, Mitchell-Olds T, de Vries S, de Jong H. Diploid apomicts of the Boechera holboellii complex display large-scale chromosome substitutions and aberrant chromosomes. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14026–14031. doi: 10.1073/pnas.0706647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltunow A, Grossniklaus U. Apomixis, a developmental perspective. Annual Review of Plant Biology. 2003;54:547–574. doi: 10.1146/annurev.arplant.54.110901.160842. [DOI] [PubMed] [Google Scholar]
- Körner Ch. Alpine plant life: functional plant ecology of high mountain ecosystems. Berlin: Springer; 2003. [Google Scholar]
- Krahulcová A, Rotreklová O. Use of flow cytometry in research on apomictic plants. Preslia. 2010;82:23–39. [Google Scholar]
- Ladinig U, Wagner J. Timing of sexual reproduction and reproductive success in the high-mountain plant Saxifraga bryoides L. Plant Biology. 2007;9:683–693. doi: 10.1055/s-2007-965081. [DOI] [PubMed] [Google Scholar]
- McCall C, Primack RB. Influence of flower characteristics, weather, time of day, and season on insect visitation rates in three plant communities. American Journal of Botany. 1992;79:434–442. [Google Scholar]
- Mártonfiová L. Possible pathways of the gene flow in Taraxacum sect. Ruderalia. Folia Geobotanica. 2006;41:183–201. [Google Scholar]
- Matzk F. Reproduction mode screening. In: Doležel J, Greilhuber J, Suda J, editors. Flow cytometry with plant cells: analysis of genes, chromosomes, and genomes. Weinheim: Wiley–VCH; 2007. pp. 131–152. [Google Scholar]
- Matzk F, Meister A, Schubert I. An efficient screen for reproductive pathways using mature seeds of monocots and dicots. The Plant Journal. 2000;21:97–108. doi: 10.1046/j.1365-313x.2000.00647.x. [DOI] [PubMed] [Google Scholar]
- Maugini E. Morfologia fiorale, embriologia ed embryogenesis in Leontopodium alpinum Cass. var. typicum Fiori e Paoletti. Giornale Botanico Italiano. 1962;69:1–18. [Google Scholar]
- Mogie M. The evolution of asexual reproduction in plants. London: Chapman and Hall; 1992. [Google Scholar]
- Molau U. Realtionships between phenology and life history strategies in tundra plants. Arctic and Alpine Research. 1993;25:391–402. [Google Scholar]
- Mráz P, Chrtek J, Šingliarová B. Geographical parthenogenesis, genome size variation and pollen production in the arctic-alpine species Hieracium alpinum. Botanica Helvetica. 2009;119:41–51. [Google Scholar]
- Muñoz A, Arroyo MTK. Pollen limitation and spatial variation of reproductive success in the insect-pollinated shrub Chuquiraga oppositifolia (Asteraceae) in the Chilean Andes. Arctic, Antarctic and Alpine Research. 2006;38:608–613. [Google Scholar]
- Murín A, Paclová L. IOPB chromosome number reports LXIV. Taxon. 1979;28:403–405. [Google Scholar]
- Nogler GA. Genetics of apospory in apomictic Ranunculus auricomus. V. Conclusion. Botanica Helvetica. 1984;94:411–423. [Google Scholar]
- Noyes RD. Apomixis in the Asteraceae: diamonds in the rough. Functional Plant Science and Biotechnology. 2007;1:207–222. [Google Scholar]
- Nybom H, Esselink GD, Werlemark G, Leus L, Vosman B. Unique genomic configuration revealed by microsatellite DNA in polyploid dogroses, Rosa sect. Caninae. Journal of Evolutionary Biology. 2006;19:635–648. doi: 10.1111/j.1420-9101.2005.01010.x. [DOI] [PubMed] [Google Scholar]
- Ozenda P. Die Vegetation der Alpen im europäischen Gebirgsraum. Stuttgart: Gustav Fischer Verlag; 1988. [Google Scholar]
- Paun O, Greilhuber J, Temsch E, Hörandl E. Patterns, sources and ecological implications of clonal diversity in apomictic Ranunculus carpaticola (Ranunculus auricomus complex, Ranunculaceae) Molecular Ecology. 2006;15:897–910. doi: 10.1111/j.1365-294X.2006.02800.x. [DOI] [PubMed] [Google Scholar]
- Ramsey J, Schemske DW. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annual Review of Ecology and Systematics. 1998;29:467–501. [Google Scholar]
- Richards AJ. Plant breeding. London: Chapman & Hall; 1997. [Google Scholar]
- Scheffknecht S, Dullinger S, Grabherr G, Hülber K. Mating systems of snowbed plant species of the northeastern calcareous Alps of Austria. Acta Oecologica – International Journal of Ecology. 2007;31:203–209. [Google Scholar]
- Schroeter C. Das Pflanzenleben der Alpen. Zürich: A. Raustein; 1908. [Google Scholar]
- Siena LA, Sartor ME, Espinoza F, Quarin CL, Ortiz JPA. Genetic and embryological evidences of apomixis at the diploid level in Paspalum rufum support recurrent auto-polyploidization in the species. Sexual Plant Reproduction. 2008;21:205–215. [Google Scholar]
- Skawińska R. Apomixis in Hieracium alpinum L. Acta Biologica Cracoviensia, Ser. Botanica. 1963;5:7–14. [Google Scholar]
- Smith GL. Studies in Potentilla L. II. Cytological aspects of apomixis in P. crantzii (Cr.) Beck ex Fritsch. New Phytologist. 1963;62:283–300. [Google Scholar]
- Sokolowska-Kulczycka A. Apomiksja u Leontopodium alpinum CASS [Apomixis in Leontopodium alpinum CASS]. Acta Biologica Cracoviensia, Ser. Botanica. 1959;2:51–63. [Google Scholar]
- Talent N, Dickinson TA. Endosperm formation in aposporous Crataegus (Rosaceae, Spiraeoideae, tribe Pyreae): parallels to Ranunculaceae and Poaceae. New Phytologist. 2007;173:231–249. doi: 10.1111/j.1469-8137.2006.01918.x. [DOI] [PubMed] [Google Scholar]
- Thompson SL, Ritland K. A novel mating system analysis for modes of self-oriented mating applied to diploid and polyploid arctic Easter daisies (Townsendia hookeri) Heredity. 2007;97:119–126. doi: 10.1038/sj.hdy.6800844. [DOI] [PubMed] [Google Scholar]
- Van Dijk PJ, Vijverberg K. The significance of apomixis in the evolution of the angiosperms: a reappraisal. In: Bakker F, Chatrou L, Gravendeel B, Pelser PB, editors. Plant species-level systematics: new perspectives on pattern and process. Ruggell, Liechtenstein: Gantner Verlag; 2005. pp. 101–116. [Google Scholar]
- Wagner J, Steinacher G, Ladinig U. Ranunculus glacialis L.: successful reproduction at the altitudinal limits of higher plant life. Protoplasma. 2010;243:117–128. doi: 10.1007/s00709-009-0104-1. [DOI] [PubMed] [Google Scholar]
- Wagner J, Ladinig U, Steinacher G, Larl I. From the flower bud to the mature seed: timing and dynamics of flower and seed development in high-mountain plants. In: Lütz C, editor. Plants in alpine regions: cell physiology of adaption and survival strategies. 2011. New York: Springer. [Google Scholar]
- Warren SD, Harper KT, Booth GM. Elevational distribution of insect pollinators. American Midland Naturalist. 1988;120:325–330. [Google Scholar]
- Watanabe K. Index to chromosome numbers in Asteraceae. 2011 doi: 10.1007/978-1-0716-3389-2_12. Kobe University Library Digital Archive. http://www.lib.kobe-u.ac.jp/products/e-index.html. (accessed January 2011) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


