Abstract
We have recently established the socially monogamous prairie vole (Microtus ochrogaster) as an animal model with which to investigate the involvement of mesocorticolimbic dopamine (DA) in the amphetamine (AMPH)-induced impairment of social behavior. As the majority of our work, to date, has focused on males, and sex differences are commonly reported in the behavioral and neurobiological responses to AMPH, the current study was designed to examine the behavioral and neurobiological effects of AMPH treatment in female prairie voles. We used a conditioned place preference (CPP) paradigm to determine a dose–response curve for the behavioral effects of AMPH in female prairie voles, and found that conditioning with low to intermediate (0.2 and 1.0 mg/kg), but not very low (0.1 mg/kg), doses of AMPH induced a CPP. We also found that exposure to a behaviorally relevant dose of AMPH (1.0 mg/kg) induced an increase in DA concentration in the nucleus accumbens (NAcc) and caudate putamen but not the medial prefrontal cortex or ventral tegmental area (VTA). Finally, repeated AMPH exposure (1.0 mg/kg once per day for 3 consecutive days; an injection paradigm that has been recently shown to alter DA receptor expression and impair social bonding in male prairie voles) increased D1, but not D2, receptor mRNA in the NAcc, and decreased D2 receptor mRNA and D2-like receptor binding in the VTA. Together, these data indicate that AMPH alters mesocorticolimbic DA neurotransmission in a region- and receptor-specific manner, which, in turn, could have profound consequences on social behavior in female prairie voles.
Keywords: Psychostimulant, Nucleus accumbens, Ventral tegmental area, Autoreceptor, Pair bonding, Conditioned place preference
1. Introduction
Drugs of abuse are thought to exert their powerful control over behavior, in part, through their effects on the mesocorticolimbic dopamine (DA) system (Kelley and Berridge, 2002; Nesse and Berridge, 1997; Nestler, 2004, 2005; Panksepp et al., 2002), a neural circuit that consists of DA-producing cells that originate in the ventral tegmental area (VTA) and project to various forebrain regions, including the medial prefrontal cortex (PFC) and nucleus accumbens (NAcc). This highly conserved neural circuit, which plays an important role in the generation of adaptive goal-directed behaviors (Zahm, 2000) – including behaviors ubiquitous to all animals (e.g., feeding (Narayanan et al., 2010; Palmiter, 2007)) and those that are species-specific (e.g., pair bonding in monogamous species (Aragona and Wang, 2009; Curtis et al., 2006; Young et al., 2010)) – is significantly altered by exposure to drugs of abuse. For example, acute and/or repeated exposure to psychostimulant drugs of abuse, such as cocaine or amphetamine (AMPH), results in altered DA release, DA receptor expression and sensitivity, and neuronal morphology within mesocorticolimbic brain regions (Henry et al., 1989; Henry and White, 1995; Hu et al., 2002; Nestler, 2005; Pierce and Kalivas, 1997; Robinson et al., 2001, 1988; Robinson and Kolb, 1997; White and Kalivas, 1998). It is thought that these neuroadaptations may underlie drug-induced alterations in animal behavior (Robinson and Becker, 1986), including social behaviors (for review, see (Young et al., 2011)).
Recent work from our laboratory has established the prairie vole as an animal model to investigate the involvement of mesocorticolimbic DA in the effects of drugs of abuse on social behavior (Liu et al., 2010). Prairie voles are socially monogamous rodents that form preferences for a familiar partner (i.e., partner preferences) after extended cohabitation and/or mating (Insel et al., 1995; Williams et al., 1992; Winslow et al., 1993), and mesocorticolimbic DA – particularly DA neurotransmission in the NAcc – is essential for this process (Aragona et al., 2003, 2006; Aragona and Wang, 2009; Curtis et al., 2006; Gingrich et al., 2000; Liu and Wang, 2003; Wang et al., 1999; Young et al., 2010). Interestingly, exposure to AMPH significantly alters mesocorticolimbic DA activity and neurotransmission in male prairie voles. For example, a single AMPH injection significantly increased extracellular DA levels in the NAcc (Curtis and Wang, 2007). Additionally, three days of AMPH exposure, which induced the formation of a conditioned place preference (CPP) when paired with an environmental context, altered DA receptor expression in the NAcc in a receptor-specific manner (Liu et al., 2010). Importantly, this same drug treatment inhibited the formation of mating-induced partner preferences, indicating that AMPH-induced alterations in mesocorticolimbic DA neurotransmission may underlie the AMPH-induced impairment of pair bonding in this species (Liu et al., 2010).
Although the studies described above have established the prairie vole as an excellent model with which to examine the AMPH-induced impairment of social bonding and its underlying neural mechanisms, they were conducted exclusively in males. Consequently, we know very little about the behavioral and neurobiological effects of AMPH in female prairie voles. Evidence exists to suggest that female prairie voles are more sensitive to AMPH than male prairie voles (Aragona et al., 2007) and studies in other species commonly report sex differences in both the behavioral and neurobiological effects of AMPH and other psychostimulant drugs of abuse (Becker and Hu, 2008; Fattore et al., 2008; Lynch, 2006). For example, female rats show greater locomotor activity and a more rapid induction of behavioral sensitization in response to AMPH (Camp and Robinson, 1988), acquire cocaine and methamphetamine self-administration faster (Hu et al., 2004; Lynch, 2006; Lynch and Carroll, 1999; Roth and Carroll, 2004), and demonstrate a higher degree of motivation to obtain psychostimulants (Roberts et al., 1989; Roth and Carroll, 2004) than males. Additionally, sex differences have been noted in the neurobiological response to psychostimulants, including differences in AMPH-induced DA release (Becker, 1990; Becker and Ramirez, 1981), DA metabolism (Camp and Robinson, 1988), and immediate early gene expression (Castner and Becker, 1996). Investigating the neurobiological effects of AMPH in female prairie voles is therefore essential to fully establish the prairie vole model for studies examining the relationships between drugs of abuse, social behavior, and mesocorticolimbic DA.
The current study was designed to examine the behavioral and neurobiological effects of AMPH exposure in the female prairie vole. We used a CPP paradigm previously established in male prairie voles (Liu et al., 2010) to examine the behavioral relevance of various doses of AMPH in females. As females tend to show a greater behavioral sensitivity to AMPH than males (Aragona et al., 2007; Becker et al., 2001; Camp and Robinson, 1988), we hypothesized that female prairie voles would form a CPP at lower doses of AMPH than those reported for males. We also examined the effects of AMPH exposure on DA concentration and DA receptor gene expression and binding in various mesocorticolimbic brain regions. We hypothesized that AMPH exposure would alter DA concentration and DA receptor expression in a receptor- and region-specific manner. Results from the current study will provide useful insight for future work that examines the effects of AMPH on social behavior in females of this species.
2. Results
2.1. Experiment 1: AMPH conditioning induced CPP
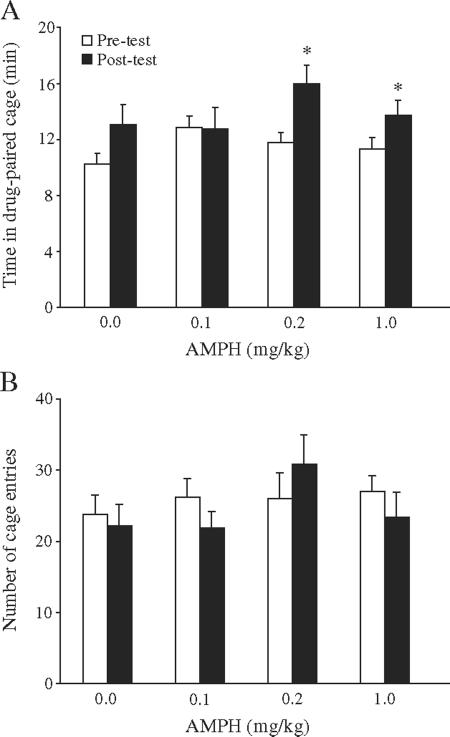
Experiment 1 established a dose–response curve for AMPH-induced CPP in female prairie voles. In order to ultimately compare the dose–response curve of females to males, we used a conditioning paradigm identical to that recently developed in male prairie voles (Liu et al., 2010). Subjects were randomly assigned into one of four experimental groups that were differentiated by the concentration of AMPH [0.0 (n=20), 0.1 (n=8), 0.2 (n=12), or 1.0 mg/kg (n=13)] they received during AMPH conditioning sessions (see Experimental procedures for details). All subjects were tested for the presence of a CPP in a drug-free state on the day following the final conditioning session. A CPP was defined by a significant increase in the time spent in the drug-paired cage during the post-test as compared to the pre-test.
Subjects treated with saline alone [0.0 mg/kg; t(19)=1.65; p<0.12] or saline containing the lowest [0.1 mg/kg; t(7)=1.89; p<0.90] concentration of AMPH spent statistically equal amounts of time in the drug-paired chamber before and after conditioning and, therefore, did not form a CPP (Fig. 1A). Instead, subjects treated with 0.2 [t(11)=2.77; p<0.02] or 1.0 mg/kg [t(12)=2.53; p<0.03] AMPH displayed a robust CPP, as they spent significantly more time in the drug-paired chamber during the post-test than the pre-test (Fig. 1A). No differences in locomotor activity were noted within or between groups either before or following drug treatment (Fig. 1B).
Fig. 1.
Amphetamine (AMPH)-induced conditioned place preference (CPP) and locomotor activity in female prairie voles. Females that received 0.0 (saline only) or 0.1 mg/kg AMPH during 3 days of conditioning did not form a CPP, as they spent equal amounts of time in the drug-paired cage during the 30 minute pre- and post-tests. However, females conditioned with 0.2 or 1.0 mg/kg AMPH formed a robust CPP, and spent significantly more time in the drug-paired cage in the post-test than the pre-test (A). No differences in the number of cage entries (an index of locomotor activity) were noted within or between groups either before or following drug treatment (B). *p<0.05.
2.2. Experiment 2: AMPH treatment altered mesocorticolimbic DA concentration
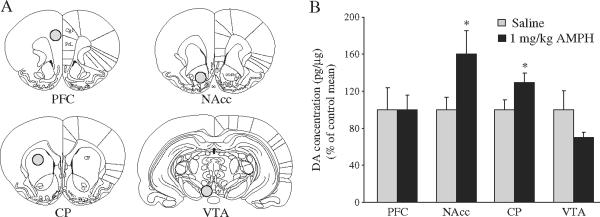
Experiment 2 examined the effect of a single AMPH treatment on DA concentration in select brain areas including the PFC, NAcc, caudate putamen (CP) and VTA (Fig. 2A). Subjects were randomly assigned into one of two experimental groups that received either a single i.p. injection of 0.9% saline (n=6) or 1.0 mg/kg AMPH dissolved in saline (n=6). This dose was chosen because it was sufficient to induce a CPP in female (Experiment 1) and male prairie voles (Aragona et al., 2007; Liu et al., 2010), indicating its behavioral relevance for both sexes. All subjects were sacrificed 30 min after injection, and the concentration of DA in their brain tissue was measured using high performance liquid chromatography with electrochemical detection (HPLC-ECD).
Fig. 2.
The effects of a single AMPH injection (1 mg/kg) on DA concentration in mesocorticolimbic brain regions. Schematic illustration of tissue-punch locations for the medial prefrontal cortex (PFC), nucleus accumbens (NAcc), caudate putamen (CP) and ventral tegmental area (VTA). Illustrations are adapted from the atlas of Paxinos and Watson (1998) (A). Thirty minutes post-injection, DA concentration was higher in the NAcc and CP of female prairie voles treated with AMPH than saline. No differences between treatment groups were seen in the PFC or VTA (B). Data are presented as the percent DA concentration of the saline control mean. *p<0.05.
A single AMPH treatment altered DA concentration in a region-specific manner within the mesocorticolimbic DA system (Fig. 2B). Subjects treated with AMPH had a significantly higher concentration of DA in the NAcc [t(10) = 2.06; p<0.03] and CP [t(10)=2.07, p<0.03] than did saline-injected controls. However, no group differences were found in the PFC [t(10)=0.03; p<0.49] or VTA [t(10)=1.41; p<0.09].
2.3. Experiments 3 and 4: repeated AMPH exposure alters DA receptor mRNA expression and binding
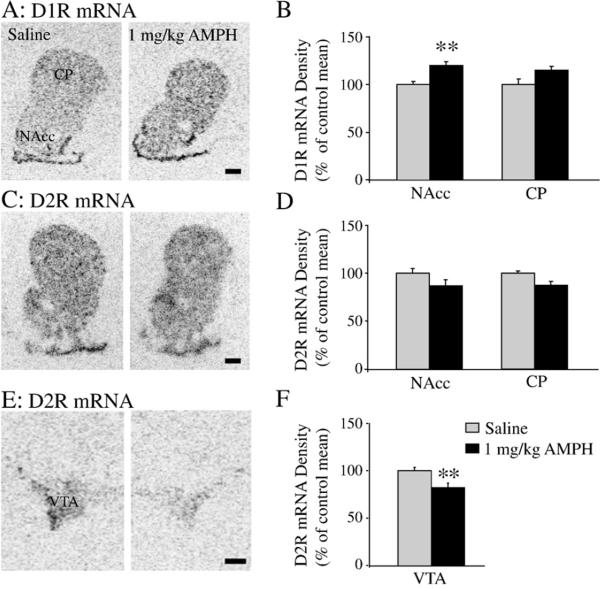
Experiments 3 and 4 examined the effects of repeated AMPH treatment on D1 receptor and D2 receptor mRNA expression and D1-like and D2-like receptor binding, respectively. Previous experiments in male prairie voles have demonstrated that repeated AMPH exposure (1.0 mg/kg once per day for 3 consecutive days) significantly alters DA receptor expression in the NAcc 24 h after the final injection and that this alteration may underlie the AMPH-induced impairment of social bonding (Liu et al., 2010). Therefore, we used this drug injection paradigm to investigate the neurobiological effects of repeated AMPH exposure in females. Subjects were randomly assigned into one of two groups that received i.p. injections of saline (control, n=6) or saline containing 1.0 mg/kg AMPH (n=8), once per day for three consecutive days. All subjects were sacrificed 24 h following the final injection. The densities of D1 receptor mRNA and D1-like receptor binding were measured in the NAcc and CP while D2 receptor mRNA and D2-like receptor binding were measured in the NAcc, CP and VTA. D1R mRNA and D1-like receptor binding were not measured in the VTA due to the lack of their presence in this brain region (Weiner et al., 1991).
Repeated AMPH exposure altered DA receptor mRNA expression in a receptor- and region-specific manner. Subjects that received repeated AMPH treatment showed a significantly higher level of D1 receptor mRNA labeling in the NAcc [t(12)=2.85; p < 0.01], but not the CP [t(12)=1.96; p < 0.07], than saline-injected controls (Figs. 3A and B). No group differences were found in D2 receptor mRNA labeling in either the NAcc [t(12)=1.56; p < 0.14] or CP [t(12)=1.79; p <0.10] (Figs. 3C and D). However, repeated AMPH treatment significantly decreased the level of D2 receptor mRNA in the VTA [t(12)=3.11; p < 0.01] (Figs. 3Eand F).
Fig. 3.
The effects of repeated AMPH administration (1 mg/kg/day for 3 consecutive days) on dopamine receptor mRNA labeling in the female prairie vole. Repeated AMPH treatment increased D1 receptor (D1R) mRNA labeling in the nucleus accumbens (NAcc), but not the caudate putamen (CP) (A and B). No group differences were found in the density of D2 receptor (D2R) mRNA labeling in either the NAcc or CP (C and D). However, AMPH-treated voles had significantly lower levels of D2R mRNA in the ventral tegmental area (VTA) than saline-treated controls (E and F). Data are presented as the percent mRNA optical density of the saline control mean. **p<0.01. (Scale bars=500 μm).
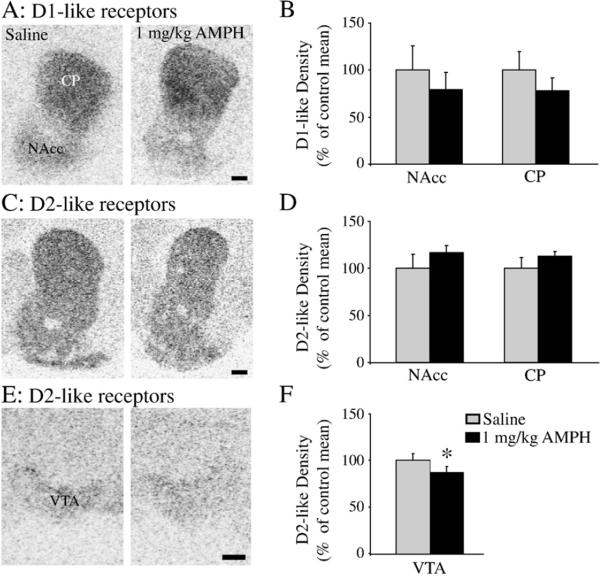
Repeated AMPH exposure had no effect on D1-like receptor (Figs. 4A and B) or D2-like receptor (Figs. 4C and D) binding levels in the NAcc [D1-like: t(12)=0.40; p < 0.35, D2-like: t(12)=0.77; p<0.23] or CP [D1-like: t(12)=0.63; p<0.27, D2-like: t(12)=0.91; p<0.19]. However, AMPH-treated subjects had a significantly lower level of D2-like receptor binding in the VTA than did saline-injected controls [t(12)=1.91; p<0.04] (Figs. 4E and F).
Fig. 4.
The effects of repeated AMPH administration (1 mg/kg/day for 3 consecutive days) on dopamine receptor binding levels in the female prairie vole. Repeated AMPH treatment did not alter D1-like (A and B) or D2-like receptor binding levels (C and D) in the nucleus accumbens (NAcc) or caudate putamen (CP). However, repeated AMPH treatment significantly decreased D2-like receptor binding levels in the ventral tegmental area (VTA) (E and F). Data are presented as the percent receptor binding density of the saline control mean. *p<0.05. (Scale bars=500 μm).
3. Discussion
The current study investigated the behavioral and neurobiological effects of AMPH exposure in female prairie voles. Collectively, our data demonstrate that AMPH has dose-dependent effects on behavior, increases DA concentration in the NAcc and CP, and alters DA receptor gene expression and binding in a receptor- and region-specific manner. These data may ultimately provide useful insight for future studies investigating the effects of AMPH on social behavior in females of this species.
A CPP reflects a preference for an environmental context that has been paired with a primary reinforcer (Bardo and Bevins, 2000) – in this case, AMPH – and is often used as a behaviorally relevant, albeit indirect, measure of drug reward. Our results demonstrate that female prairie voles form a CPP after treatment with low to intermediate doses of AMPH. When compared to our recent results in male prairie voles that were attained using the same CPP paradigm (Liu et al., 2010), these data, together, demonstrate a leftward shift in the dose–response curve for CPP in female prairie voles. Specifically, 0.2 mg/kg or higher doses of AMPH induced a CPP in females, whereas 1.0 mg/kg or higher doses of AMPH were required for the induction of a CPP in males (Liu et al., 2010). This leftward shift in the dose–response curve of females is consistent with a previous study in prairie voles that used a different conditioning paradigm (Aragona et al., 2007), and suggests that females are more sensitive to the behavioral effects, and perhaps more vulnerable to the rewarding effects, of AMPH than males—a finding that has been consistently demonstrated in other species (Camp and Robinson, 1988; Hu et al., 2004; Lynch, 2006; Lynch and Carroll, 1999; Roberts et al., 1989; Roth and Carroll, 2004) and that may have important implications for the effects of AMPH on social behavior in female prairie voles.
In the present study, we also found that AMPH administration – at a behaviorally relevant dose (1.0 mg/kg) for female prairie voles – increased DA concentration in the NAcc and CP but not the PFC or VTA. These results indicate a region-specific AMPH-induced enhancement of DA concentration. As previous studies in a number of species have demonstrated the induction of extracellular DA release in the NAcc and CP shortly after AMPH injection (Cho et al., 1999; Clausing and Bowyer, 1999; Curtis and Wang, 2007; Di Chiara et al., 1993; Drevets et al., 2001), the increased DA concentration in these regions in the present study may be due to an AMPH-induced enhancement of DA release. However, as DA concentration is also affected by DA synthesis and metabolism, this speculation needs to be tested in further experiments. Additionally, there was a noteworthy trend toward a decrease in DA concentration in the VTA following AMPH exposure in female prairie voles. Although this effect was not significant (p < 0.09), further experimentation is required to rule-in or rule-out an effect of AMPH on DA concentration in this brain region.
To further understand the neurobiological consequences of AMPH exposure in female prairie voles, we investigated the effects of repeated AMPH treatment on DA receptor mRNA expression and binding in various brain regions. We used an AMPH dose and injection paradigm that has recently been demonstrated to alter DA receptor expression and to impair social behavior in male prairie voles (Liu et al., 2010). Our data indicate that repeated AMPH exposure significantly increased the level of D1 receptor mRNA in the NAcc. A similar, but not significant (p < 0.07), effect was noted in the CP, indicating that AMPH may have effects on D1R mRNA expression in this region as well. Despite these changes in gene expression, AMPH exposure did not alter the level of D1-like receptor binding in the NAcc or CP. There are two types of D1-like receptors – D1 receptors and D5 receptors – both of which had the potential to be labeled by the D1-like ligand used in our receptor binding experiment. However, as D5 receptors are virtually nonexistent in the NAcc and CP (Missale et al., 1998; Tiberi et al., 1991), our data suggest a lack of change, specifically, in D1 receptor protein levels. Similarly, previous reports in other rodent species have indicated that repeated exposure to AMPH or other psychostimulants does not reliably alter D1 receptor affinity or density in these brain regions (for review see (Pierce and Kalivas, 1997; White and Kalivas, 1998)), despite enhancing the responsiveness of NAcc neurons to D1 receptor agonists for up to one month following drug treatment (Henry et al., 1989; Henry and White, 1991, 1995). We also report no changes in D2 receptor mRNA or D2-like receptor binding levels in the NAcc or CP in female prairie voles following AMPH treatment, a finding consistent with those in rats and mice (Richtand et al., 1997; Sora et al., 1992) and the suggestion that NAcc D1 receptors play a greater role in the response to repeated AMPH exposure (Berke and Hyman, 2000).
An interesting finding in the present study is that repeated AMPH treatment significantly decreased the levels of D2 receptor gene expression and D2-like receptor binding in the VTA of female prairie voles. D2 receptors in the VTA are located on somatodendritic regions of A10 DA neurons (DA projection neurons that originate in the VTA and project to mesocorticolimbic areas) (Aghajanian and Bunney, 1977; Mercuri et al., 1997; Oades and Halliday, 1987; White and Wang, 1984b). These receptors function as autoreceptors and their activation leads to hyperpolarization of the cell membrane and the inhibition of cell firing (Mercuri et al., 1997) (for review see (Mercuri et al., 1992)), diminishing the amount of DA released into target regions such as the NAcc (Usiello et al., 2000). Accordingly, D2 receptor blockade or gene deletion results in a lack of A10 cell inhibition and a subsequent overflow of DA into the NAcc in response to a variety of stimuli (Mercuri et al., 1997; Rouge-Pont et al., 2002). Therefore, the decrease in D2 receptors in the VTA noted in the present study may indicate an AMPH-induced down-regulation of somatodendritic autoreceptors in the female prairie vole. As auto-receptor density is inversely related to the rate of activity of A10 DA neurons (White and Wang, 1984a), this effect may lead to enhanced DA release and neurotransmission in the NAcc. Similarly, previous research has demonstrated a subsensitivity of somatodendritic autoreceptors on A10 DA neurons after repeated psychostimulant exposure, resulting in increased spontaneous activity and basal firing rate of A10 DA cells (Henry et al., 1989) that may persist for days following the end of drug treatment (Ackerman and White, 1990). It is important to note, however, that both D2 and D3 receptors are expressed in the VTA and localized presynaptically on dopaminergic neurons (Diaz et al., 1995; Mercuri et al., 1997), indicating that the current decrease in D2-like receptor binding could be attributed to changes in either or both receptor subtypes. Knowledge of the specific receptor subtype affected by AMPH exposure is important for our data interpretation, as D2, but not D3, receptors, are necessary for the autoreceptor inhibition of DA neurons (Mercuri et al., 1997; Rouge-Pont et al., 2002). Still, as D3 receptor expression is extremely low in the VTA relative to that of D2 receptors (Bouthenet et al., 1991), and spiperone shows a higher affinity for the D2 than the D3 receptor (Missale et al., 1998), it is likely that the current effects on D2-like receptor binding represent a specific decrease in the levels of D2, rather than D3 receptors.
While the neurobiological effects of repeated AMPH exposure in females show some similarities with those found previously in male prairie voles (Liu et al., 2010), two important differences are evident. First, although AMPH experience increased D1 receptor mRNA in the NAcc in both sexes, the functional consequencesofthisincreaseingene transcription was only retained in males (i.e., females did not show any changes in D1-like receptor binding levels whereas AMPH increased NAcc D1 receptor protein levels in males). These differences may be due to the use of different quantitative techniques to detect these functional consequences (i.e. receptor binding was used in females whereas Western blotting was used in males) or may indicate sex-specific effects of repeated AMPH treatment on D1 receptors within the NAcc of prairie voles. Secondly, AMPH treatment had no effects on D2 receptor mRNA expression in the VTA in male prairie voles (Liu et al., 2010), but significantly decreased it, as well as D2 receptor binding levels, in females—further suggesting that the neurobiological effects of AMPH are sex-specific. This idea is supported by findings in other species that indicate sex differences in gene expression following AMPH treatment (Castner and Becker, 1996).
AMPH-induced alterations in the mesocorticolimbic DA system may have important consequences on social behavior in prairie voles. As aforementioned, adult male and female prairie voles form enduring pair bonds after mating (Carter et al., 1995; Williams et al., 1992; Winslow et al., 1993) and NAcc DA regulates this behavior in both sexes in a receptor-specific manner: D2-like receptor activation facilitates and D1-like receptor activation inhibits partner preference formation (Aragona et al., 2003, 2006; Aragona and Wang, 2009; Gingrich et al., 2000; Liu and Wang, 2003; Wang et al., 1999). As such, AMPH-induced changes in mesocorticolimbic brain regions, including those reported here, could have profound consequences on pair bonding behavior in the prairie vole. In males, for example, AMPH-induced increases in D1-like receptors in the NAcc are thought to underlie the AMPH-induced impairment of partner preference formation (Liu et al., 2010), as NAcc D1 receptor activation inhibits mating-induced partner preferences (Aragona et al., 2006). Additionally, the pharmacological blockade of D1 receptors during AMPH treatment dose-dependently eliminated the AMPH-induced impairment of partner preference formation, further indicating that AMPH may impair pair bonding through a D1 receptor-mediated mechanism (Liu et al., 2010). In females instead, due to the lack of autoreceptor inhibition implied by the current findings (i.e., decreased D2 receptor expression in the VTA), mating-induced DA release in the NAcc would likely be enhanced in AMPH-treated voles. As robust elevations in DA concentration activate low affinity D1 receptors (Richfield et al., 1989), this neuroadaptation may have important behavioral consequences on social bonding in females.
In conclusion, the current study demonstrates that a behaviorally relevant dose of AMPH alters DA concentration and receptor expression within the mesocorticolimbic DA system of female prairie voles, a key circuit involved in the monogamous social behavior of this species. These results provide a foundation for future studies in female prairie voles to examine the effects of AMPH on pair bonding and the involved neurochemical mechanisms.
4. Experimental procedures
4.1. Animals
Captive-bred female prairie voles (Microtus ochrogaster) descended from populations in southern Illinois were weaned at 21 days of age and then housed in same-sex sibling pairs in plastic cages (29×18×13 cm) containing cedar chip bedding. They were maintained on a 14:10 light:dark cycle (lights on at 0700 h) with ad libitum access to food and water. Temperature was maintained at 21±1 °C. All animals used in this study were between 90 and 120 days of age. Experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee at Florida State University.
4.2. Conditioned place preference paradigm
The CPP apparatus was identical to that previously described and consisted of two plastic cages that were visually distinct (white vs. black) and connected to one another by a hollow tube (Aragona et al., 2007; Liu et al., 2010). We used a conditioning paradigm recently developed in male prairie voles (Liu et al., 2010). Briefly, all subjects were given a 30 min pre-test on day 1 and the amount of time spent in each cage was quantified. The cage in which an individual spent less time during the pre-test was designated as the drug-paired cage and the other was designated as the saline-paired cage. Conditioning occurred during two 40 minute sessions each day for the next three days (days 2–4). During the morning sessions (0900 h) subjects received intraperitoneal (i.p.) injections of 0.0, 0.1, 0.2, or 1.0 mg/kg d-AMPH sulfate (Sigma, St. Louis, MO, USA) dissolved in saline, immediately before being placed into the drug-paired cage. During the afternoon sessions (1500 h), subjects received an i.p. injection of saline immediately before being placed into the saline-paired cage. This two trial per day training schedule has been employed in rats (Campbell and Spear, 1999; Zhou et al., 2010) and was used in our previous study in male prairie voles (Liu et al., 2010). Further, this paradigm was chosen because our pilot data indicated no differences in behavior between subjects treated with counterbalanced and fixed injection/conditioning paradigms (unpublished data) and because standardized injection and tissue collection schedules were important for the measurement of DA marker expression in subsequent experiments as well as for direct comparisons with data from male prairie voles (Liu et al., 2010). On day 5, all subjects were tested for the presence of a CPP in a 30 min post-test. The number of times that animals crossed between cages was recorded during the pre- and post-test and used as an index of locomotor activity.
4.3. Tissue preparation
Subjects were rapidly decapitated 30 min after injection in Experiment 2 and 24 h following the final injection in Experiments 3 and 4. Their brains were quickly removed and immediately frozen on dry ice, before being stored at −80 °C. Brains from Experiment 2 were sectioned coronally at 300 μm and sections were thaw-mounted onto Superfrost/plus slides. The Paxinos and Watson rat brain atlas (Paxinos and Watson, 1998) was used to identify various brain regions, including the PFC (Plates 8–10), NAcc (Plates 9–11), CP (Plates 10–12) and VTA (Plates 40–43), from which bilateral tissue punches of a 1 mm diameter were taken (Fig. 2A) and stored at −80 °C until processed. Although not a mesocorticolimbic brain region, the CP was included in our analysis because it, like the NAcc and PFC, receives DAergic input from the VTA (Oades and Halliday, 1987) but does not appear to be involved in the DAergic regulation of prairie vole partner preference formation (Aragona et al., 2003, 2006; Liu and Wang, 2003). For Experiments 3 and 4, brains were cut coronally into 10 sets of 14 μm sections that were thaw-mounted onto Superfrost/plus slides.
4.4. DA extraction and HPLC-ECD analysis
DA extraction was performed as described previously (Aragona et al., 2002), except that tissue samples were sonicated in 50 μL of 0.1 M perchloric acid with 0.02% EDTA. DA concentration was assessed using high performance liquid chromatography with electrochemical detection (HPLC-ECD) as described previously (Curtis et al., 2003) with the following exceptions. The mobile phase consisted of 75 mM sodium dihydrogen phosphate monohydrate, 1.7 mM 1-octanesulfonic acid sodium salt, 0.01% triethylamine, 25 um EDTA, and 7% acetonitrile and the pH was adjusted to 3.0 with 85% phosphoric acid. The flow rate was 0.5 ml/min. The standard curve and peak area were calculated as described previously (Aragona et al., 2003). The detection limit was ~10 pg per sample.
4.5. In situ hybridization for D1 and D2 receptor mRNA
Alternate sets of brain sections from Experiment 3 were processed for in situ hybridization labeling of DA receptor mRNA. Antisense and sense riboprobes (generously provided by Dr. O. Civelli at the University of California, Irvine, CA), were used for D1 and D2 receptor mRNA labeling and prepared as previously described (Liu et al., 2010). Probes were labeled individually at 37 ° C for 1 h in a transcription-optimized buffer consisting of 0.5 μg/μl of the respective DNA template, [35S]-CTP, 4 mM of ATP, UTP, and GTP, 0.2 M dithiothreitol (DTT), RNasin (40 U/μl), and RNA polymerase (20 U/μl). The DNA template was then digested with 1 U/μl DNaseI. Probes were purified using Chromatography Columns (Bio-Rad, Hercules, CA) and then diluted in hybridization buffer comprised of 50% deionized formamide, 10% dextran sulfate, 3× SSC, 10 mM sodium phosphate buffer (PB, pH 7.4), 1× Denhardt's solution, 0.2 mg/ml yeast tRNA, and 10 mM DTT to yield 5×106 cpm/ml.
Brain sections were fixed in 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS) at 4 °C for 20 min, rinsed in PBS for 10 min, and treated with 0.25% acetic anhydride in triethanolamine (pH 8.0) for 15 min to reduce non-specific binding. Slides were then washed in 2× saline sodium citrate (SSC), dehydrated through increasing concentrations of ethanol (ETOH) (70, 95, and 100%), and air-dried.
Each slide received 100 μl hybridization solution containing the appropriate 35S-labeled probe, was cover-slipped and then incubated at 55 °C in a humidified chamber overnight. After incubation, cover-slips were removed in 2× SSC, slides were washed twice in 2× SSC for 5 min and then washed at 37 °C for 1 h in RNase buffer (8 mM Tris–HCl, 0.8 mM EDTA, and 0.4 M NaCl, pH 8.0) containing 25 mg/ml RNaseA. Next, slides were washed in decreasing concentrations of SSC (2× SSC, 1× SSC, and 0.5× SSC) for 5 min each and were incubated in 0.1× SSC at 65 °C for 60 min. Finally, slides were brought to room temperature, dehydrated through increasing concentrations of ETOH, and air-dried. Sections were apposed to BioMax MR film (Kodak, Rochester, NY) for different time periods, depending on the probe and region of interest, to generate optimal autoradiograms. For the NAcc and CP, D1R and D2R mRNA labeled sections were apposed to film for 14 and 60 h, respectively, while sections labeled for D1-like and D2-like receptor binding were apposed for 15 and 6.5 h, respectively. For the VTA, sections labeled for D2R mRNA were apposed for 60 h and those labeled for D2-like binding were apposed for 40 h. A sense RNA control was also tested for each probe and yielded no labeling, as expected.
4.6. DA receptor autoradiography
For Experiment 4, alternate sets of brain sections were processed for D1-like and D2-like receptor autoradiography. The D1-like ligand [125I] SCH23982 and the D2-like ligand [125I] 2′-iodospiperone were obtained from PerkinElmer (Waltham, MA). DA receptor autoradiography was performed as described previously (Aragona et al., 2006).
4.7. Data analysis
For Experiment 1, a CPP was defined by a significant increase in time spent in the drug-paired cage during the post-test as compared to the pre-test, as measured by a paired t-test. Locomotor activity was analyzed using a two-way repeated measures ANOVA comparing pre- vs. post-test locomotion (within-subject variable) and locomotion by treatment (between-subject variable). For Experiment 2, DA concentration of each sample was normalized using the total protein concentration of that sample to control for the amount of tissue collected. The normalized value of DA concentration (pg/μg tissue) was then converted to percent of the mean DA concentration of the saline control. For each brain region, the percent DA concentration between groups was compared with a t-test. In Experiments 3 and 4, autoradiograms were analyzed for the optical densities of mRNA labeling or receptor binding in the NAcc, CP and VTA using a computerized image program (NIH IMAGE 1.60) (the PFC was not included in analysis as this region showed no response to AMPH treatment in Experiment 2). The rostral/caudal extent of image analysis for the NAcc, CP and VTA was the same as described for Experiment 2. Neuroanatomical distinction between the NAcc and CP was made using the Paxinos and Watson rat brain atlas (Paxinos and Watson, 1998) as a guide, referencing both the shape of the labeling and the location of the anterior commissure. Sections for each brain area were anatomically matched between subjects, and individual means for each subject were obtained by measuring optical density bilaterally in three sections from each brain region per animal. The background density was subtracted from the measurement of each section. The final optical densities were converted to percent of the saline control mean. Group differences in mRNA or binding levels within brain region were analyzed for each DA receptor using a t-test. The significance level was set at p<0.05.
Acknowledgments
We thank Kevin Young and Adam Smith for their critical reading of the manuscript. This work was supported by the National Institutes of Health grants DAF31-25570 to KAY, MHF31-79600 to KLG, and DAR01-19627, DAK02-23048, and MHR01-58616 to ZXW.
Footnotes
Abbreviations: AMPH, amphetamine; ANOVA, analysis of variance; CP, caudate putamen; CPP, conditioned place preference; DTT, dithiothreitol; DA, dopamine; ETOH, ethanol; HPLC, high performance liquid chromatography; i.p., intraperitoneal; PCF, medial prefrontal cortex; NAcc, nucleus accumbens; PBS, phosphate buffered saline; SSC, saline sodium citrate; PB, sodium phosphate buffer; VTA, ventral tegmental area
REFERENCES
- Ackerman JM, White FJ. A10 somatodendritic dopamine autoreceptor sensitivity following withdrawal from repeated cocaine treatment. Neurosci. Lett. 1990;117:181–187. doi: 10.1016/0304-3940(90)90141-u. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Bunney BS. Dopamine “autoreceptors”: pharmacological characterization by microiontophoretic single cell recording studies. Naunyn Schmiedebergs Arch. Pharmacol. 1977;297:1–7. doi: 10.1007/BF00508803. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z. Dopamine regulation of social choice in a monogamous rodent species. Front. Behav. Neurosci. 2009;3:1–11. doi: 10.3389/neuro.08.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Curtis JT, Davidson AJ, Wang Z, Stephan FK. Behavioral and neurochemical investigation of circadian time-place learning in the rat. J. Biol. Rhythms. 2002;17:330–344. doi: 10.1177/074873002129002636. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J. Neurosci. 2003;23:3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat. Neurosci. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Detwiler JM, Wang Z. Amphetamine reward in the monogamous prairie vole. Neurosci. Lett. 2007;418:190–194. doi: 10.1016/j.neulet.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl.) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Becker JB. Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synapse. 1990;5:157–164. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front. Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Ramirez VD. Sex differences in the amphetamine stimulated release of catecholamines from rat striatal tissue in vitro. Brain Res. 1981;204:361–372. doi: 10.1016/0006-8993(81)90595-3. [DOI] [PubMed] [Google Scholar]
- Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann. N. Y. Acad. Sci. 2001;937:172–187. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- Camp DM, Robinson TE. Susceptibility to sensitization. I. Sex differences in the enduring effects of chronic D-amphetamine treatment on locomotion, stereotyped behavior and brain monoamines. Behav. Brain Res. 1988;30:55–68. doi: 10.1016/0166-4328(88)90008-3. [DOI] [PubMed] [Google Scholar]
- Campbell J, Spear LP. Effects of early handling on amphetamine-induced locomotor activation and conditioned place preference in the adult rat. Psychopharmacology (Berl.) 1999;143:183–189. doi: 10.1007/s002130050934. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci. Biobehav. Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Castner SA, Becker JB. Sex differences in the effect of amphetamine on immediate early gene expression in the rat dorsal striatum. Brain Res. 1996;712:245–257. doi: 10.1016/0006-8993(95)01429-2. [DOI] [PubMed] [Google Scholar]
- Cho AK, Melega WP, Kuczenski R, Segal DS, Schmitz DA. Caudate-putamen dopamine and stereotypy response profiles after intravenous and subcutaneous amphetamine. Synapse. 1999;31:125–133. doi: 10.1002/(SICI)1098-2396(199902)31:2<125::AID-SYN5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Clausing P, Bowyer JF. Time course of brain temperature and caudate/putamen microdialysate levels of amphetamine and dopamine in rats after multiple doses of D-amphetamine. Ann. N. Y. Acad. Sci. 1999;890:495–504. doi: 10.1111/j.1749-6632.1999.tb08031.x. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Wang Z. Amphetamine effects in microtine rodents: a comparative study using monogamous and promiscuous vole species. Neuroscience. 2007;148:857–866. doi: 10.1016/j.neuroscience.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis JT, Stowe JR, Wang Z. Differential effects of intraspecific interactions on the striatal dopamine system in social and non-social voles. Neuroscience. 2003;118:1165–1173. doi: 10.1016/s0306-4522(03)00032-0. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Liu Y, Aragona BJ, Wang Z. Dopamine and monogamy. Brain Res. 2006;1126:76–90. doi: 10.1016/j.brainres.2006.07.126. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Frau R, Carboni E. On the preferential release of dopamine in the nucleus accumbens by amphetamine: further evidence obtained by vertically implanted concentric dialysis probes. Psychopharmacology (Berl.) 1993;112:398–402. doi: 10.1007/BF02244939. [DOI] [PubMed] [Google Scholar]
- Diaz J, Levesque D, Lammers CH, Griffon N, Martres MP, Schwartz JC, Sokoloff P. Phenotypical characterization of neurons expressing the dopamine D3 receptor in the rat brain. Neuroscience. 1995;65:731–745. doi: 10.1016/0306-4522(94)00527-c. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol. Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Fattore L, Altea S, Fratta W. Sex differences in drug addiction: a review of animal and human studies. Womens Health (Lond. Engl.) 2008;4:51–65. doi: 10.2217/17455057.4.1.51. [DOI] [PubMed] [Google Scholar]
- Gingrich B, Liu Y, Cascio C, Wang Z, Insel TR. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster) Behav. Neurosci. 2000;114:173–183. doi: 10.1037//0735-7044.114.1.173. [DOI] [PubMed] [Google Scholar]
- Henry DJ, White FJ. Repeated cocaine administration causes persistent enhancement of D1 dopamine receptor sensitivity within the rat nucleus accumbens. J. Pharmacol. Exp. Ther. 1991;258:882–890. [PubMed] [Google Scholar]
- Henry DJ, White FJ. The persistence of behavioral sensitization to cocaine parallels enhanced inhibition of nucleus accumbens neurons. J. Neurosci. 1995;15:6287–6299. doi: 10.1523/JNEUROSCI.15-09-06287.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry DJ, Greene MA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: repeated administration. J. Pharmacol. Exp. Ther. 1989;251:833–839. [PubMed] [Google Scholar]
- Hu XT, Koeltzow TE, Cooper DC, Robertson GS, White FJ, Vezina P. Repeated ventral tegmental area amphetamine administration alters dopamine D1 receptor signaling in the nucleus accumbens. Synapse. 2002;45:159–170. doi: 10.1002/syn.10095. [DOI] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Insel TR, Preston S, Winslow JT. Mating in the monogamous male: behavioral consequences. Physiol. Behav. 1995;57:615–627. doi: 10.1016/0031-9384(94)00362-9. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J. Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Liu Y, Aragona BJ, Young KA, Dietz DM, Kabbaj M, Mazei-Robison M, Nestler EJ, Wang Z. Nucleus accumbens dopamine mediates amphetamine-induced impairment of social bonding in a monogamous rodent species. Proc.Natl. Acad.Sci.U.S.A. 2010;107:1217–1222. doi: 10.1073/pnas.0911998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ. Sex differences in vulnerability to drug self-administration. Exp. Clin. Psychopharmacol. 2006;14:34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl.) 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Mercuri NB, Calabresi P, Bernardi G. The electrophysiological actions of dopamine and dopaminergic drugs on neurons of the substantia nigra pars compacta and ventral tegmental area. Life Sci. 1992;51:711–718. doi: 10.1016/0024-3205(92)90479-9. [DOI] [PubMed] [Google Scholar]
- Mercuri NB, Saiardi A, Bonci A, Picetti R, Calabresi P, Bernardi G, Borrelli E. Loss of autoreceptor function in dopaminergic neurons from dopamine D2 receptor deficient mice. Neuroscience. 1997;79:323–327. doi: 10.1016/s0306-4522(97)00135-8. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol. Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Guarnieri DJ, DiLeone RJ. Metabolic hormones, dopamine circuits, and feeding. Front. Neuroendocrinol. 2010;31:104–112. doi: 10.1016/j.yfrne.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesse RM, Berridge KC. Psychoactive drug use in evolutionary perspective. Science. 1997;278:63–66. doi: 10.1126/science.278.5335.63. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47(Suppl 1):24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat. Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30:375–381. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Knutson B, Burgdorf J. The role of brain emotional systems in addictions: a neuro-evolutionary perspective and new `self-report' animal model. Addiction. 2002;97:459–469. doi: 10.1046/j.1360-0443.2002.00025.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res. Brain Res. Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Penney JB, Young AB. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience. 1989;30:767–777. doi: 10.1016/0306-4522(89)90168-1. [DOI] [PubMed] [Google Scholar]
- Richtand NM, Kelsoe JR, Kuczenski R, Segal DS. Quantification of dopamine D1 and D2 receptor mRNA levels associated with the development of behavioral sensitization in amphetamine treated rats. Neurochem. Int. 1997;31:131–137. doi: 10.1016/s0197-0186(96)00097-6. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl.) 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J. Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Jurson PA, Bennett JA, Bentgen KM. Persistent sensitization of dopamine neurotransmission in ventral striatum (nucleus accumbens) produced by prior experience with (+)-amphetamine: a microdialysis study in freely moving rats. Brain Res. 1988;462:211–222. doi: 10.1016/0006-8993(88)90549-5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl.) 2004;172:443–449. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Usiello A, Benoit-Marand M, Gonon F, Piazza PV, Borrelli E. Changes in extracellular dopamine induced by morphine and cocaine: crucial control by D2 receptors. J. Neurosci. 2002;22:3293–3301. doi: 10.1523/JNEUROSCI.22-08-03293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Fujiwara Y, Tomita H, Ishizu H, Akiyama K, Otsuki S, Yamamura HI. Lack of effect of haloperidol or methamphetamine treatment on the mRNA levels of two dopamine D2 receptor isoforms in rat brain. Jpn. J. Psychiatry Neurol. 1992;46:967–973. doi: 10.1111/j.1440-1819.1992.tb02868.x. [DOI] [PubMed] [Google Scholar]
- Tiberi M, Jarvie KR, Silvia C, Falardeau P, Gingrich JA, Godinot N, Bertrand L, Yang-Feng TL, Fremeau RT, Jr., Caron MG. Cloning, molecular characterization, and chromosomal assignment of a gene encoding a second D1 dopamine receptor subtype: differential expression pattern in rat brain compared with the D1A receptor. Proc. Natl. Acad. Sci. U. S. A. 1991;88:7491–7495. doi: 10.1073/pnas.88.17.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yu G, Cascio C, Liu Y, Gingrich B, Insel TR. Dopamine D2 receptor-mediated regulation of partner preferences in female prairie voles (Microtus ochrogaster): a mechanism for pair bonding? Behav. Neurosci. 1999;113:602–611. doi: 10.1037//0735-7044.113.3.602. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Sunahara RK, Niznik HB, O'Dowd BF, Seeman P, Brann MR. D1 and D2 dopamine receptor mRNA in rat brain. Proc. Natl. Acad. Sci. U. S. A. 1991;88:1859–1863. doi: 10.1073/pnas.88.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY. A10 dopamine neurons: role of autoreceptors in determining firing rate and sensitivity to dopamine agonists. Life Sci. 1984a;34:1161–1170. doi: 10.1016/0024-3205(84)90088-2. [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY. Pharmacological characterization of dopamine autoreceptors in the rat ventral tegmental area: microiontophoretic studies. J. Pharmacol. Exp. Ther. 1984b;231:275–280. [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm. Behav. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: insights from a socially monogamous rodent. Front. Neuroendocrinol. 2010 doi: 10.1016/j.yfrne.2010.07.006. doi:10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Wang ZX. The role of mesocorticolimbic dopamine in regulating interactions between drugs of abuse and social behavior. Neurosci. Biobehav. Rev. 2011;35:498–515. doi: 10.1016/j.neubiorev.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci. Biobehav. Rev. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Zhou JY, Mo ZX, Zhou SW. Effect of rhynchophylline on central neurotransmitter levels in amphetamine-induced conditioned place preference rat brain. Fitoterapia. 2010;81(7):844–848. doi: 10.1016/j.fitote.2010.05.007. [DOI] [PubMed] [Google Scholar]