Abstract
Peroxisome proliferator–activated receptors (PPARs) are members of the nuclear hormone receptor superfamily and have a dominant regulatory role in adipocyte and monocyte differentiation. PPAR-γ agonists are also negative regulators of macrophage activation and have modulatory effects on tumorigenesis. In this study we demonstrate that synovial tissue localized expression of PPAR-γ in patients with rheumatoid arthritis (RA). We detected markedly enhanced expression of PPAR-γ in macrophages, as well as modestly enhanced expression in the synovial lining layer, fibroblasts, and endothelial cells. Activation of the PPAR-γ by 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) and the synthetic PPAR-γ ligand (troglitazone) induced RA synoviocyte apoptosis in vitro. Moreover, intraperitoneal administration of these PPAR-γ ligands ameliorated adjuvant-induced arthritis with suppression of pannus formation and mononuclear cell infiltration in female Lewis rats. Anti-inflammatory effects of 15d-PGJ2 were more potent than troglitazone. These findings suggest that PPAR-γ may be an important immunoinflammatory mediator and its ligands, especially 15d-PGJ2, may be useful in the treatment of RA.
Introduction
Rheumatoid arthritis (RA) is a chronic, destructive inflammatory polyarticular joint disease, characterized by massive synovial proliferation and subintimal infiltration of inflammatory cells, along with angiogenesis (1, 2). The inflammation of RA results in the production of prostaglandins (PGs) E2, cytokines, and nitric oxide (1, 3). In particular, macrophage-derived cytokines such as TNF-α (4) and IL-1 (5), which are abundant in synovial tissues and fluid from patients with RA, are mitogens for synovial fibroblasts, as well as osteoclasts. Blocking of these cytokines inhibits the release of synovial production of other proinflammatory cytokines. This suggests that TNF-α and IL-1 are key proinflammatory molecules in cytokine cascade in RA. In fact, TNF-α receptor antagonists, neutralizing antibodies against TNF-α, and IL-1 receptor antagonist can control joint inflammation in most RA patients (6–8). Moreover, the formation of active inflamed pannus is thought to be critical for erosive disease and results in irreversible destruction of the cartilage and bone in affected joints. The mechanism responsible for synovial hyperplasia leading to pannus formation may be due to reduced apoptosis of inflammatory cells and tumor-like synoviocytes (9, 10). Therefore, suppression of the production of inflammatory molecules and formation of hyperplastic synovium may be important targets of therapy for RA.
Peroxisome proliferator–activated receptors (PPARs) are members of the nuclear hormone receptor superfamily of ligand-activated transcriptional factors that include receptors for steroids, thyroid hormone, vitamin D, and retinoic acid (11). PPAR binds to peroxisome proliferator responsive element (PPRE) as a heterodimer with the retinoic receptor (RXR), implying a role of 9-cis retinoic acid in the regulation of PPAR target genes (12, 13). To date, three mammalian PPAR subtypes have been isolated and termed PPAR-α, NUC1 (also known as PPAR-δ), and PPAR-γ (11, 14, 15). PPAR-α is highly expressed in the liver, heart, kidney, muscle, brown adipose tissue, and gut, which exhibit high carbolic rates of fatty acids (14, 16). PPAR-δ may be expressed ubiquitously, and its function is relatively unknown (17). Recent studies suggest that PPAR-δ may be a target for nonsteroidal anti-inflammatory drug–induced (NSAID-induced) tumor suppression in colorectal tumors (18). PPAR-γ is expressed at high levels in adipose tissue and is a critical regulator of adipocyte differentiation (19).
In addition, PPAR-α and PPAR-γ have been suggested to be important immunomodulatory factors (20). PPAR-α–knockout mice exhibit exacerbated inflammatory responses, and leukotriene B4, a chemotactic mediator, seems to regulate the clearance of itself as an agonist of PPAR-α (21). PPAR-γ is also expressed in the immune system, such as the spleen (16), monocytes, bone-marrow precursors (22), and helper T-cell clones (23). Recent data have shown that the natural PG, 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2), and synthetic anti-diabetic thiazolidinedione, which are PPAR-γ ligands, lead to inhibition of phorbol ester–induced nitric oxide and macrophage-derived cytokines, i.e., TNF-α, IL-1, and IL-6. These PPAR-γ ligands inhibit gene expression in part by antagonizing the activities of the transcription factors such as AP-1 and NF-κB (24, 25). Moreover, PPAR-γ ligands, including troglitazone (anti-diabetic thiazolidinedione) and 15d-PGJ2, have potent tumor modulatory effects against colorectal, prostate, and breast cancer (26–28). They also induce apoptosis in macrophages, fibroblasts, and endothelial cells (29–31).
The dietary supplementation of fish oil, including highly polyunsaturated fatty acids that can bind directly to PPAR-α and PPAR-γ (32), results in significant improvement in tender joints and other clinical parameters of disease activity in RA (33, 34). NSAIDs inhibit synthesis of PGs and are widely used for treatment of RA. PPAR-α and -γ are also activated by indomethacin and other NSAIDs at high doses (35). Because NSAIDs work better at higher doses than those required for inhibition of cyclooxygenase (COX) in RA (36), some of the actions of NSAIDs are thought to be mediated not only through inhibition of COX, but also through activation of PPARs. Moreover, IL-4, which is one of the Th2 cytokines with anti-inflammatory effects in RA (37), induces PPAR-γ expression in macrophages (38). These findings suggest that PPAR-γ may negatively regulate the inflammatory processes in RA.
In this study, we determined the expression of PPAR-γ on synovial tissues and cultured synoviocytes in patients with RA. We also demonstrated the effect of growth-inhibitory effects of troglitazone and 15d-PGJ2 and whether these PPAR-γ ligands have potency in suppressing chronic inflammation and pannus formation of adjuvant-induced arthritis (AIA) in female Lewis rats.
Methods
Materials.
Troglitazone was obtained from Sankyo Co., Ltd. (Tokyo, Japan) and 15d-PGJ2 was purchased from Cayman Chemical (Ann Arbor, Michigan, USA). PGE2 and PGF2α were from Sigma Chemical Co. (St. Louis, Missouri, USA). Antibody against human PPAR-γ was from Santa Cruz Biotechnology Inc. (Santa Cruz, California, USA), anti-macrophage (CD68) antibody was from DAKO Japan (Kyoto, Japan). Control normal goat serum was from Vector Laboratories (Burlingame, California, USA), and mouse immunoglobulin G3 κ was from Biogenesis (Poole, United Kingdom).
Tissue specimens and synovial cell preparation.
Synovial tissues were obtained during replacement surgery from patients with RA (n = 20) and osteoarthritis (OA) (n = 10), according to criteria of the American College of Rheumatology. Synovial samples were obtained from the knee, elbow, and hip joint. Synovial tissues from RA were minced and stirred with 1 mg/mL collagenase (Sigma Chemical Co.) in serum-free RPMI-1640 (Nissui, Tokyo, Japan) for 3 hours, filtered through a nylon mesh, and washed extensively. Cells were cultured in RPMI-1640 supplemented with 10% FCS (BioWhittaker, Walkersville, Maryland, USA) and 1% antibiotics (BioWhittaker) in a humidified incubator at 37°C in the presence of 5% CO2. The synovial cells used were from passage 3–9, when they showed fibroblastoid morphology. Adipose tissues surrounding joints were used as positive controls for RT-PCR and Western blot analysis and were obtained from joint replacement surgery of RA patients, as well.
Immunostaining.
Synovial tissue specimens were preserved in 10% formalin and embedded in paraffin, serially sectioned onto microscope slides at a thickness of 4 μm, and then deparaffinized. Cultured synoviocytes were fixed with acetone for 30 seconds. Immunohistochemical staining was performed with the VectoStain avidin-biotin peroxidase complex kit (Vector) as described previously (39). Primary antibodies against human PPAR-γ (1:100 dilution, in PBS) and control normal goat serum was used. In double-antibody immunostaining, tissue specimens were stained with anti–PPAR-γ antibody (1:100 dilution, in PBS) using the alkaline phosphatase method and with anti-macrophage antibody (50 μg/mL) using the peroxidase method (39). Positive staining was indicated by brownish-black deposits for the peroxidase method, red deposits for the alkaline-phosphatase method, and black-red deposits for the double antibody–staining method. The sections were counterstained with 0.5% light-green SF yellowish (Sigma Chemical Co.).
Immunohistochemical analysis.
For each of the tissue specimens from RA and OA, the extent and intensity of staining with anti–PPAR-γ antibody in synovial lining cells, macrophages, endothelial cells, and fibroblasts were graded on a scale of 0–4+ by two blinded observers on two separate occasions using coded slides as previously described (39). A 4+ grade implies maximally intense staining, whereas 0 implies no staining.
RNA preparation and analysis.
Total cellular RNA was isolated from synoviocytes and fatty tissues in patients with RA (n = 5) by the acid guanidinium thiocyanate-phenol-chloroform method. RT-PCR reactions were performed as described previously (40). The primers used were: (a) human PPAR-γ (41), sense 5′-CAATCGAGCTGTCAAGAGAGC-3′ and antisense 5′-GGAAGAAAT CCAAGGGCTGAG-3′; (b) G3PDH (40), sense 5′-CCACCCATGGCAAATTCCATGGCA-3′ and antisense 5′-TCTAGAGGGCAGGTCAGGTCCACC-3′. The conditions for thermal cycling were as follows: 94°C for 45 seconds, 52°C for 45 seconds, and 72°C for 2 minutes for 40 cycles. This program was preceded by 5 minutes at the given denaturation temperature and followed by 7 minutes at 72°C. The amplification reactions were analyzed on 2.0% agarose gels.
Western blot analysis of PPAR-γ.
Western blot analysis was performed to determine PPAR-γ protein expression as described previously (42). Total protein extract from patients with RA (n = 5) and fat tissue was analyzed on blots incubated for 1 hour at room temperature with 1:200 dilution of goat anti–PPAR-γ antibody (Santa Cruz Biotechnology Inc.), washed, and subsequently incubated for 1 hour at room temperature with horseradish peroxidase–linked rabbit anti-goat IgG (1:1500 dilution; EY Laboratories Inc., San Mateo, California, USA). After being washed again, blots were analyzed using an Amersham enhanced-chemiluminescence (ECL) system (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom), and exposed to Hyperfilm (Amersham Pharmacia Biotech) for 30 seconds to 5 minutes, resulting in adequate exposure to visualize the bands.
Cell-proliferative studies.
RA synoviocytes (5 × 103 in media, n = 5) placed onto 8 × 8-mm multichamber slides (Nunc, Copenhagen, Denmark) were treated with troglitazone (1–50 μM), 15d-PGJ2 (1–20 μM), PGE2 (20 μM), and PGF2α (20 μM). Cell viability was measured at day 1 by a microplate reader using a modified 3-[4,5-dimethylthiazol-2-thiazolyl]-2,5-diphenyltetrazolium bromide (MTT) assay (40) (WST-1 assay; Dojindo, Kumamoto, Japan), and presented as the percentage of control-culture conditions.
Detection of synovial apoptosis.
DNA chromatin morphology was assessed using HOECHST staining (40). Synoviocytes were incubated with 30 μM of troglitazone or 20 μM of 15d-PGJ2 for 24 hours. Cells were washed by RPMI-1640 and labeled with 8 mg/mL of HOECHST 33342 (Sigma Chemical Co.) for 10 minutes; propidium iodide (Sigma Chemical Co.) was added (10 mg/mL final concentration), and the cells were examined by fluorescence microscopy as described previously (40).
Adjuvant-induced arthritis in rats.
Nine-week-old female Lewis rats were obtained from Charles River Japan (Yokohama, Japan). CFA was prepared by suspending heat-killed Mycobacterium butyricum (Difco Laboratories, Detroit, Michigan, USA) in liquid paraffin at 12 mg/mL. CFA-induced arthritis was stimulated by injection of 50 μL of the CFA emulsion intradermally at the base of the tail, as described previously (39, 43). Treatment commenced at the onset of the disease; 15d-PGJ2 (2 times a day) and troglitazone (once a day) were intraperitoneally administered at the specified dose, respectively, until 10 days after onset of arthritis. 15d-PGJ2 was diluted in PBS and troglitazone was freshly suspended in 0.3 % carboxymethyl cellulose (CMC) PBS. In each experiment, a group of rats was injected with PBS or 0.3 % CMC PBS, which served as a control. From day 8 after immunization (onset of arthritis), rats were examined daily for AIA using two clinical parameters, paw swelling and clinical score. The footpad volume was measured with a water replacement plethysmometer (Unicom Japan, Tokyo, Japan).
For clinical evaluation of AIA, we used a scoring system as follows: mid-forepaw, wrist, joints of finger, midfoot, ankle, and joints of digits, were scored 0 to 4: 0, normal; 1, minimal swelling; 2, medium swelling; 3, severe swelling; 4, severe and non–weight-bearing arthritis. Each limb was graded, resulting in a maximal clinical score of 48 per animal.
For histological evaluation, we performed hematoxylin and eosin (H&E) staining of tissue specimens of ankle, liver, and kidney. Two blinded observers evaluated cartilage and bone destruction by pannus formation and mononuclear cell infiltration in synovial tissues on two separate occasions, using the following scoring system (44): cartilage and bone destruction by pannus formation: 0, no change; 1, mild change (pannus formation within cartilage); 2, moderate change (pannus invasion into cartilage/subchondral bone); 3, severe change (pannus invasion into the subchondral bone); mononuclear cell infiltration: 0, no infiltration; 1, mild infiltration; 2, moderate infiltration; 3, severe infiltration.
Statistical analysis.
The Mann-Whitney U test to compare nonparametric data for statistical significance was applied on the analysis of histological examination, paw volume of hindlimbs, and clinical score of arthritis.
Results
PPAR expression of synovial tissues and cultured synoviocytes.
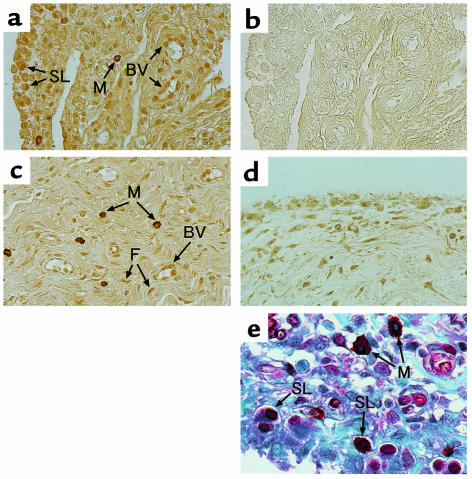
To examine the expression and localization of PPAR-γ in synovial tissues of RA and OA, immunohistochemistry was performed. In all subjects from patients with RA (20 of 20) and OA (10 of 10), PPAR-γ was expressed in synovial tissues. In RA we found markedly enhanced expression of PPAR-γ in macrophages (immunohistochemical score, mean ± SD: 3.1 ± 0.7) and moderate expression in synovial cells (2.9 ± 0.8), endothelial cells (2.1 ± 0.7), and fibroblasts (2.6 ± 0.7) (Figure 1, a and c). PPAR-γ in macrophage-like cells was strongly localized in the perinuclear and cytoplasmic regions, but not in the nucleus. In OA the localization of PPAR-γ in synovial tissues is almost the same as that in RA (Figure 1d). However, immunohistochemical score of PPAR-γ in synovial lining cells (1.0 ± 0.7), macrophages (0.7 ± 0.6), endothelial cells (1.3 ± 0.7), and fibroblasts (1.3 ± 0.7) in OA was significantly smaller than those in RA (P < 0.01). Immunostaining with normal goat serum was completely negative in all subjects from RA (Figure 1b) and OA (data not shown). Immunostaining with anti–PPAR-γ antibody absorbed with synthetic PPAR-γ polypeptide was also negative (data not shown).
Figure 1.
Immunostaining for PPAR-γ in synovial tissues from RA and OA. (a–c, e) Synovial tissues from RA. (d) Synovial tissues from OA. Synovial tissue sections were stained with goat anti-PPAR-γ (a, c, d) antibodies and a normal goat IgG (b). Tissues seen in a, b, and c are derived from different patients. Positive immunostaining was indicated by brownish deposits. In double immunostaining for macrophage and PPAR-γ (e), sections were stained with anti–PPAR-γ antibody using alkaline phosphatase method (red deposits) and anti-human macrophage using antibody peroxidase method (brownish-black deposits). Double-positive staining cells were indicated by black-red deposits. Counter staining was done with 0.5% light-green SF yellowish. ×100 (a–d); ×200 (e). SL, synovial lining cell; BV, blood vessel; M, macrophage; F, fibroblast.
To confirm the expression of PPAR-γ in macrophages, we immunostained the same sections from the synovial tissue specimens of RA by a double antibody–staining method with anti-macrophage antibody and anti–PPAR-γ antibody. Intense black-red deposits (double-positive cells) were detected in macrophages (Figure 1e). Control staining with normal goat IgG or mouse IgG3κ was uniformly negative (data not shown). These observations suggest that PPAR-γ is localized in macrophages in synovial tissues.
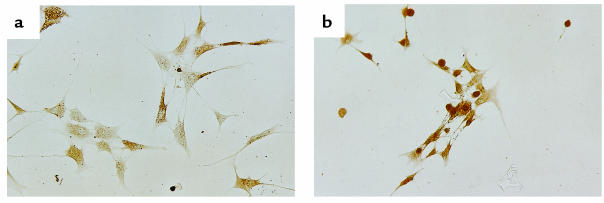
We also detected the expression of PPAR-γ in cultured synoviocytes. The intensity and cellular localization of PPAR-γ in cultured synoviocytes were almost the same as those of synovial lining cells in tissues. In culture, immunostaining of PPAR-γ varied in that some synoviocytes had a predominantly cytoplasmic expression, whereas others had both cytoplasmic and nuclear expression (Figure 2a). However, treatment with 10 μM of 15d-PGJ2 for 12 hours resulted in nuclear translocation of PPAR-γ (Figure 2b).
Figure 2.
The expression of PPAR-γ in cultured synoviocytes. (a) Untreated cultured synoviocytes. Synoviocytes expressed fibroblastoid morphology. Immunoreactive PPAR-γ was localized in cytoplasmic region and nucleus, but predominantly in the cytoplasmic region in some synoviocytes. (b) Synoviocytes treated with 15d-PGJ2 (10 μM, 12 hours). Synoviocytes appeared less active and had an irregular or round shape, with nuclear translocation of PPAR-γ. ×100.
PPAR-γ mRNA and protein expression in synoviocytes.
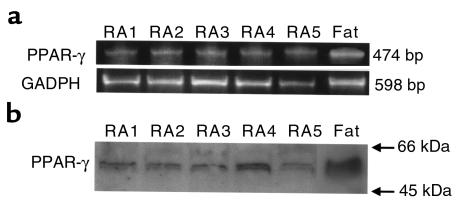
We next determined the expression of PPAR-γ mRNA and protein in cultured synoviocytes from RA patients (n = 5) using RT–PCR and Western blot analysis. Amplification of cDNA with PPAR-γ primers used in this study predicted a fragment of 474 bp in length. The predicted band size was detected in cultured synoviocytes after amplification of reverse-transcribed mRNA in all subjects (5 of 5) and was also detected in adipose tissue (Figure 3a). Negative controls, performed with no RNA or no reverse transcriptase in the reverse transcription reaction yielded no detectable bands (data not shown). Primers specific for human G3DPH generated the expected 598 bp in all synovial tissues and cultured synoviocytes (Figure 3a). Western blot analysis was performed using PPAR-γ–specific antibody. A specific band corresponding to PPAR-γ at a molecular size of 55 kDa was detected in all subjects (5 of 5) and in adipose tissue (Figure 3b). Negative control with no PPAR-γ antibody or antibody absorbed with synthetic PPAR-γ polypeptide did not yield a detectable signal.
Figure 3.
The expression of PPAR-γ mRNA and protein in cultured synoviocytes. (a) Primers for PPAR-γ generated the expected 474 nucleotide band after 40 cycles of PCR. Primers for human GAPDH generated the expected 598 bp in all subjects and fat tissue. (b) PPAR-γ protein (55 kDa) expressed in cultured synoviocytes and fat tissue by Western blot analysis. RA 1–5, synovial tissue from patients with RA.
Inhibition of synoviocyte proliferation by PPAR-γ ligands.
To investigate the effects of PPAR-γ ligands on RA synoviocyte proliferation (n = 5), we analyzed cell viability in vitro by a modified MTT assay. Synoviocytes were treated with troglitazone, 15d-PGJ2, PGE2, and PGF2α at concentrations of 20 μM. Cell counting at day 1 clearly showed a marked inhibition of synoviocyte proliferation with both troglitazone and 15d-PGJ2. In contrast, a 10–15% increase in cell proliferation was observed in synoviocytes with other PGs (Figure 4a). Suppression of synoviocyte proliferation with troglitazone and 15d-PGJ2 was induced in a concentration-dependent manner (Figure 4, b and c). The half-maximal concentration of synoviocyte inhibition (IC50) was approximately 5–8 μM for 15d-PGJ2 and 20 μM for troglitazone. In addition, the morphologic change of cultured synoviocytes was confirmed by a microscopic analysis. Cells treated with 15d-PGJ2 (10 μM, 12 hours) appeared less active and had an irregular or round shape, with the nuclear translocation of PPAR-γ (Figure 2b). But untreated synoviocytes maintained the characteristics of adherent fibroblasts (Figure 2a).
Figure 4.
The expression of PPAR-γ ligands on synoviocyte viability. Synoviocytes treated with various prostanoids (20 μM), troglitazone (20 μM), and 15d-PGJ2 (20 μM) for 24 hours were measured by an MTT assay (n = 5) and expressed as percentage of control conditions (a). The dose-response analysis of synoviocytes treated with 15d-PGJ2 (b) or troglitazone (c) was also measured by an MTT assay (n = 5). PPAR ligands suppressed the proliferation of synoviocyte concentration dependently. Data represent mean ± SD.
Induction of synovial apoptosis with PPAR-γ ligands.
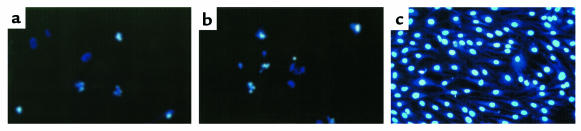
To delineate whether the cell death induced with PPAR-γ ligands was the result of apoptosis or not, we evaluated the cells by assessing chromatin morphology using HOECHST staining. Synoviocytes treated with troglitazone (40 μM) and 15d-PGJ2 (20 μM) showed chromatin condensation, cellular shrinkage, small membrane-bound bodies (apoptotic bodies), and cytoplasmic condensation (Figure 5, a and b). These cellular changes were typical characteristic of apoptosis. In contrast, untreated cells maintained normal chromatin patterns and cell size (Figure 5c).
Figure 5.
Fluorescence microscopic appearance of apoptotic cells by PPAR-γ ligands. HOECHST staining was used to stain apoptotic synoviocytes treated with PPAR-γ ligands. Synoviocytes treated with troglitazone (40 μM, 24 hours) (a) and 15d-PGJ2 (20 μM, 24 hours) (b) demonstrated typical findings of apoptosis, marked chromatin condensations, small membrane-bound bodies (apoptotic bodies), cytoplasmic condensations, and cellular shrinkage. Untreated synovial cells (c) kept a normal chromatin pattern and showed no change in a nuclear morphology. ×200.
Intraperitoneal administration of 15d-PGJ2 ameliorated adjuvant-induced arthritis.
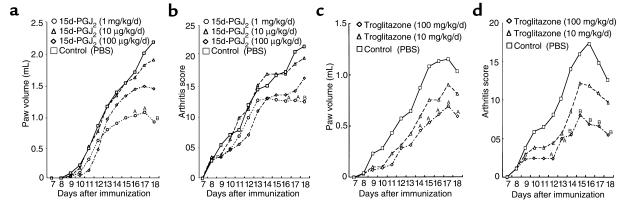
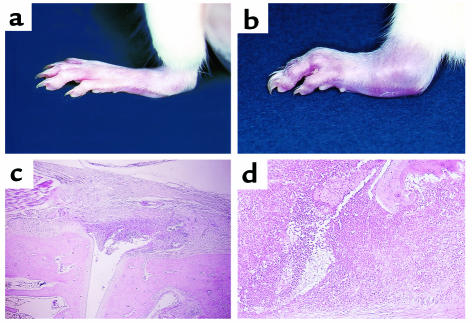
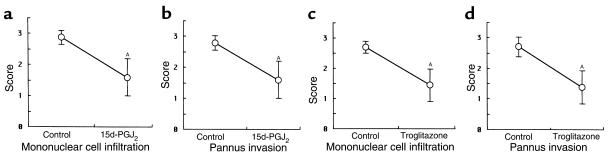
We demonstrated the effect of 15d-PGJ2 and troglitazone on AIA in female Lewis rats. After onset of arthritis at day 7, 15d-PGJ2 and troglitazone were administered intraperitoneally for several doses. Troglitazone and 15d-PGJ2 suppressed the progression of clinical arthritis dose dependently when compared with control rats treated with PBS, as demonstrated by both paw volume (Figure 6, a and c) and arthritis score (Figure 6, b and d). In 15d-PGJ2–treated rats, significant effects were obtained with the dose of 1 mg/kg/day at day 16–18. Especially at day 18, joint swelling was suppressed with a dose of 1 mg/kg/day of 15d-PGJ2 (Figure 7, a and b), and both paw volume and arthritis score were significantly decreased (P < 0.01). In troglitazone-treated rats, significant effects were also obtained with a dose of 100 mg/kg/day (P < 0.01; Figure 6, c and d). In addition, at day 18 histological findings of the foot joint in both 15d-PGJ2 –treated rats (1 mg/kg/day) and troglitazone-treated (100 mg/kg/day) rats, revealed that the infiltration of mononuclear cells and the formation of pannus in synovial tissues were suppressed significantly (P < 0.01; Figures 7, c and d, and Figure 8). These data suggest that PPAR-γ ligands have anti-inflammatory effects and growth inhibition of synovial cells in AIA.
Figure 6.
Suppression of clinical arthritis by 15d-PGJ2 and troglitazone treatment in AIA. 15d-PGJ2 (2 times a day) and troglitazone (once a day) were intraperitoneally administered daily in female Lewis rats after onset of arthritis, at day 7. (a and b) 15d-PGJ2–treated rats, 10 μg/kg/d (n = 8), 100 μg/kg/d (n = 8), 1 mg/kg/d (n = 8); PBS (n = 8). (c and d) Troglitazone-treated rats, 10 mg/kg/d (n = 7), 100 mg/kg/d (n = 7); PBS (n = 7). Paw volume (a and c) and arthritis score (b and d) were assessed daily after onset of arthritis. AP < 0.05; BP < 0.01.
Figure 7.
Photographic and histological features of the hindlimb in AIA treated with 15d-PGJ2 or PBS. (a and c) Samples from a representative rat treated with 15d-PGJ2 in AIA. (b and d) Samples from a PBS-treated rat in AIA. Joint swelling of the foot in AIA was clearly reduced with 15d-PGJ2 administration at a dose of 1 mg/kg/d, at day 18 (a), compared with control (b). H&E staining of the foot joint also revealed a marked decrease of synovial inflammatory cell infiltrate and synovial lining hyperplasia (c). In contrast, hindlimbs treated with PBS revealed typical features of arthritis such as massive inflammatory cell infiltrate, pannus, and bone destruction (d). Similar findings of photographic and histological features were observed in all rats of each group. (c) ×25; (d) ×50.
Figure 8.
Histopathological scores of the hindlimb in AIA treated with 15d-PGJ2 and troglitazone. Mononuclear cell infiltration and pannus invasion into the cartilage and bone were measured by microscopic examination of the sections. (a and b) 15d-PGJ2–treated rats at day 18 (n = 8). (c and d) Troglitazone-treated rats at day 18 (n = 7). Data represent mean ± SD of scores in the subsets. The differences between the subsets were statistically significant. AP < 0.01.
Side effects of intraperitoneal administration of PPAR-γ ligands.
There was no mortality in either 15d-PGJ2- and troglitazone-treated rats. Troglitazone treatment significantly increased adipose tissue mass in the abdomen and dose dependently, though not to a statistically significant level, increased body weight. The liver and kidney histological examination showed no abnormalities. In 15d-PGJ2–treated rats, we could not detect any abnormal changes compared with controls.
Discussion
In the present study, we found immunoreactive PPAR-γ expressed strongly in macrophages and moderately in synovial cells, fibroblasts, and endothelial cells in synovial tissues from RA. The extent and intensity of immunoreactive PPAR-γ of these cells in RA was greater than those in OA. We also demonstrated that cultured synoviocytes from RA expressed PPAR-γ mRNA and protein, and that PPAR-γ ligands inhibited the growth of synoviocytes through apoptosis in vitro. Moreover, 15d-PGJ2 and troglitazone were potent in suppressing chronic inflammation and pannus formation in AIA.
Macrophages initiate immune/inflammatory response, and macrophage-derived cytokines mediate the synovial cell proliferation and bone destruction in RA. PPAR-γ promotes monocyte/macrophage differentiation (45) and has anti-inflammatory properties. PPAR-γ expression is undetectable in circulation in human monocytes, but is strongly induced in macrophage upon differentiation (29). PPAR-γ ligands inhibit the induction of NO synthase, gelatinase B, and scavenger receptor A (24). They also can reduce the secretion of IL-1β, IL-6, and TNF-α, which are key proinflammatory cytokines in RA (25). Treatment of differentiated macrophages with PPAR-γ agonist induces apoptosis, which is more pronounced in macrophages activated with IFN-α and TNF-α (29). Recent data have shown that PPAR-γ agonists also have immunosuppressive effects in helper T lymphocytes (23). Moreover, PPAR-γ agonists induce apoptosis in the fibroblast (30) and endothelial cell (31), and inhibit VEGF-induced angiogenesis in rats (46). Angiogenesis is also important for pannus formation in RA (2). We found 15d-PGJ2 and troglitazone inhibited the growth of the synovial cell in vitro and induced its apoptosis. These findings suggest that PPAR-γ agonists inhibit the function of macrophages and suppress pannus formation through apoptosis of macrophages, synovial cells, and endothelial cells in RA.
15d-PGJ2 is thought to be derived from PGD2, which is a major COX product in a variety of tissues and cells (47), and especially from the COX-1 pathway in peritoneal macrophages (48). It is likely that J2 cyclopentenone can be synthesized in vivo (49, 50) and that PGD2 can be converted readily to PGJ2 in the presence of plasma (51). Members of the PGJ series have been reported to have their own unique spectrum of biological effects, including the inhibition of cell cycle progression and cytokine production in macrophages, the suppression of viral replication, the induction of heat shock protein expression, and the stimulation of osteogenesis (24, 25, 52). But, the mechanism of these effects is not well understood, and the natural site and the extent of 15d-PGJ2 production in vivo remain unclear. In our study, it is of great interest that intraperitoneal administration of 15d-PGJ2, which is an intrinsic natural PPAR-γ ligand, suppressed the chronic inflammation in AIA in rats; however, anti-inflammatory effects of PPAR-γ ligands have not been established in vivo. In these synovial tissues, infiltration of inflammatory cells and pannus formation were markedly decreased. In addition, immunoreactive PPAR-γ was localized predominantly in the perinuclear legion and cytoplasm in both macrophages and cultured synoviocytes. Treatment of cultured synoviocytes with 15d-PGJ2 caused the nuclear translocation of PPAR-γ and the induction of synoviocytes apoptosis. In fact, Δ12 PGJ2 can be actively incorporated into the nucleus of cells in 10 minutes (53). Because PPAR-γ is considered to work at the level of transcription, 15d-PGJ2–induced nuclear localization of PPAR-γ may be an initial step in its activation; however, it is still unclear, especially in vivo, whether PPAR-γ is activated in these cells before treatment of 15d-PGJ2. Taken together, these results suggest that the mechanism of the anti-inflammatory effects of 15d-PGJ2 in AIA can be explained, at least in part, by the activation of PPAR-γ, which leads to the apoptosis of several cells such as synovial cells and inhibition of macrophage function.
Troglitazone, which is a specific PPAR-γ ligand and synthetic anti-diabetic thiazolidinedione, also suppressed the chronic inflammation and pannus formation in AIA. However, with intraperitoneal administration, a troglitazone dose of 100 mg/kg/day is required to suppress AIA to the same degree as 1 mg/kg/day of 15d-PGJ2. These results are, however, in overall agreement with in vitro data that indicate 15d-PGJ2 is 5–30 times more potent than various thiazolidinediones, including troglitazone, at inducing synoviocyte and endothelial cell apoptosis (31) and inhibiting macrophage-derived cytokine (24, 25). Furthermore, 15d-PGJ2 is a direct inhibitor of IκB kinase independent of PPAR-γ (54, 55), which suggests additional anti-inflammatory effects independent of PPAR–γ. Oral administration of 500 mg/kg/day, but not 100 mg/kg/day, troglitazone, if started at the same time as immunization with adjuvant, also significantly suppresses AIA in rats (data not shown). In contrast, 5 mg/kg/day of troglitazone (administered orally) is required for a significant increase in insulin sensitivity, indicating at least a 100-fold–higher dose of troglitazone is required for anti-inflammatory effects. Although we cannot detect any side effects of this dose (100 mg/kg/day, intraperitoneally) of troglitazone on AIA in rats, liver toxicity has been reported in a small percentage of patients taking troglitazone (56). We should therefore carefully evaluate potential usefulness and any other side effects of new thiazolidinediones such as rosiglitazone and pioglitazone for RA treatment.
In conclusion, we demonstrate the expression of immunoreactive PPAR-γ in macrophages and synoviocytes of the RA synovium. Furthermore, PPAR-γ ligands inhibit the growth of synoviocytes in vitro through apoptosis. Naturally occurring PPAR-γ ligands, especially 15d-PGJ2, had high potency in suppressing the chronic inflammation and pannus formation of AIA in rats, compared with troglitazone. These findings suggest that 15d-PGJ2 is a novel therapeutic reagent for RA, and new PPAR-γ ligands may be potentially useful for the treatment of RA.
Acknowledgments
We thank Yoshiaki Kusaka and Toshikazu Kubo for providing the synovial samples of RA and OA and also thank Shigehiko Mukai for advice. T. Hla acknowledges the support of NIH grant HL-54710 and an established investigator award from the American Heart Association.
Footnotes
David Bishop-Bailey’s present address is: Department of Cardiovascular and Inflammation Research, William Harvey Research Institute, St. Bartholomew’s and the Royal London School of Medicine and Dentistry, London, United Kingdom.
References
- 1.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 2.Koch AE. Angiogenesis: implications of rheumatoid arthritis. Arthritis Rheum. 1998;41:951–962. doi: 10.1002/1529-0131(199806)41:6<951::AID-ART2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 3.Clancy RM, Amin AR, Abramson SB. The role of nitric oxide in inflammation and immunity. Arthritis Rheum. 1998;41:1141–1151. doi: 10.1002/1529-0131(199807)41:7<1141::AID-ART2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Hopkins SJ, Meager A. Cytokines in synovial fluid: II. The presence of tumour necrosis factor and interferon. Clin Exp Immunol. 1988;73:88–92. [PMC free article] [PubMed] [Google Scholar]
- 5.Fontana A, et al. Interleukin-1 activity in the synovial fluid of patients with rheumatoid arthritis. Rheumatol Int. 1982;2:49–53. doi: 10.1007/BF00541245. [DOI] [PubMed] [Google Scholar]
- 6.Moreland LW, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor (p75)-Fc fusion protein. N Engl J Med. 1997;337:141–147. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- 7.Elliott MJ, et al. Randomized double-blind comparison of chimeric monoclonal antibody to tumor necrosis factor α (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344:1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 8.Campion GV, et al. Dose-range and dose-frequency study of recombinant human interleukin-1 receptor antagonist in patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1092–1101. doi: 10.1002/art.1780390704. [DOI] [PubMed] [Google Scholar]
- 9.Vaishnaw AK, Mcnally JD, Elkon KB. Apoptosis in the rheumatic diseases. Arthritis Rheum. 1997;40:1917–1927. doi: 10.1002/art.1780401102. [DOI] [PubMed] [Google Scholar]
- 10.Fassbender HG, Gay S. Synovial processes in rheumatoid arthritis. Scand J Rheumatol. 1988;76:1–7. doi: 10.3109/03009748809102945. [DOI] [PubMed] [Google Scholar]
- 11.Mangelsdorf DJ, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Issemann I, Grenn S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 13.Gearing KL, Gottlicher M, Teboul M, Widmark E, Gustafsson J-Å. Interaction of the peroxisome-proliferator-activated receptor and retinoid X receptor. Proc Natl Acad Sci USA. 1993;90:1440–1444. doi: 10.1073/pnas.90.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kliewer SA, et al. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci USA. 1994;91:7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sher T, Yi H-F, McBride OW, Gonzalez FJ. cDNA cloning, chromosomal mapping, and functional characterization of the human peroxisome proliferator activated receptor. Biochemistry. 1993;32:5598–5604. doi: 10.1021/bi00072a015. [DOI] [PubMed] [Google Scholar]
- 16.Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-α, -β, and -γ in the adult rat. Endocrinol. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt A, et al. Identification of a new member of the steroid hormone receptor superfamily that is activated by peroxisome proliferator and fatty acid. Mol Endocrinol. 1992;6:1634–1641. doi: 10.1210/mend.6.10.1333051. [DOI] [PubMed] [Google Scholar]
- 18.He TC, Chan TA, Vogelstein B, Kinzler KW. PPARδ is an APC-regulated target of nosteroidal anti-inflammatory drugs. Cell. 1999;99:335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 20.Serhan CN. Inflammation. Signalling the fat controller. Nature. 1996;384:23–24. doi: 10.1038/384023a0. [DOI] [PubMed] [Google Scholar]
- 21.Devchand PR, et al. The PPARα-leukotriene B4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- 22.Greene ME, et al. Isolation of the human peroxisome proliferator-activated receptor γ cDNA: expression in hematopoietic cells and chromosomal mapping. Gene Expr. 1995;4:281–299. [PMC free article] [PubMed] [Google Scholar]
- 23.Clark RB, et al. The nuclear receptor PPARγ and immunoregulation: PPARγ mediates inhibition of helper T cell responses. J Immunol. 2000;164:1364–1371. doi: 10.4049/jimmunol.164.3.1364. [DOI] [PubMed] [Google Scholar]
- 24.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 25.Jiang C, Ting AT, Seed B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 26.Sarraf P, et al. Differentiation and reversal of malignant changes in colon cancer through PPARγ. Nat Med. 1998;4:1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 27.Kubota T, et al. Ligand for peroxisome-proliferator activated receptor γ (Troglitazone) has potent antitumor effect against human prostate cancer in vitro and in vivo. Cancer Res. 1998;58:3344–3352. [PubMed] [Google Scholar]
- 28.Elstner E, et al. Ligands for peroxisome-proliferator activated receptor γ and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc Natl Acad Sci USA. 1998;95:8806–8811. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chinetti G, et al. Activation of peroxisome-activated receptor α and γ induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998;273:25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- 30.Altoik S, Xu M, Spiegelman BM. PPARγ induces cell cycle withdrawal: inhibition of E2F/DP DNA binding activity via down-regulation of PP2A. Genes Dev. 1997;11:1987–1998. doi: 10.1101/gad.11.15.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bishop-Bailey D, Hla T. Endothelial cell apoptosis induced by the peroxisome proliferator-activated receptor (PPAR) ligand 15-deoxy-δ12,14-prostaglandin J2. J Biol Chem. 1998;274:17042–17048. doi: 10.1074/jbc.274.24.17042. [DOI] [PubMed] [Google Scholar]
- 32.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. Proc Natl Acad Sci USA. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kremer JM, et al. Effects of manipulation of dietary fatty acids on clinical manifestations of rheumatoid arthritis. Lancet. 1985;1:184–187. doi: 10.1016/s0140-6736(85)92024-0. [DOI] [PubMed] [Google Scholar]
- 34.Kremer JM, et al. Effects of high-dose fish oil on rheumatoid arthritis after stopping nonsteroidal antiinflammatory drugs. Arthritis Rheum. 1995;38:1107–1114. doi: 10.1002/art.1780380813. [DOI] [PubMed] [Google Scholar]
- 35.Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors α and γ are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1997;272:3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- 36.Meade EA, Smith WL, DeWitt DL. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993;268:6610–6614. [PubMed] [Google Scholar]
- 37.Miossec P, van den Berg W. Th1/Th2 cytokine balance in arthritis. Arthritis Rheum. 1997;40:2105–2115. doi: 10.1002/art.1780401203. [DOI] [PubMed] [Google Scholar]
- 38.Huang JT, et al. Interleukin-4-dependent production of PPAR-γ ligands in macrophage by 12/15-lipoxygenase. Nature. 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 39.Sano H, et al. In vivo cyclooxygenase expression in synovial tissues of patients with rheumatoid arthritis and osteoarthritis and rats with adjuvant and streptococcal cell wall arthritis. J Clin Invest. 1992;89:97–108. doi: 10.1172/JCI115591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashiramoto A, et al. C-myc antisense deoxyoligonucleotide can induce apoptosis and Fas downregulation in rheumatoid synoviocyte. Arthritis Rheum. 1999;42:954–962. doi: 10.1002/1529-0131(199905)42:5<954::AID-ANR14>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 41.Auboeuf D, et al. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-α in humans: no alteration in adipose tissue of obese and NIDDM patients. Diabetes. 1997;46:1319–1327. doi: 10.2337/diab.46.8.1319. [DOI] [PubMed] [Google Scholar]
- 42.Yamada R, et al. Auranofin inhibits interleukin-1β-induced transcript of cyclooxygenase-2 on cultured human synoviocytes. Eur J Pharmacol. 1999;385:71–79. doi: 10.1016/s0014-2999(99)00707-4. [DOI] [PubMed] [Google Scholar]
- 43.Kawahito Y, et al. Localization of quantitative trait loci regulating adjuvant induced arthritis: evidence for genetic factors common to multiple autoimmune diseases. J Immunol. 1998;161:4411–4419. [PubMed] [Google Scholar]
- 44.Taniguchi K, et al. Induction of the p16INK4a senescence gene as a new therapeutic strategy for the treatment of rheumatoid arthritis. Nat Med. 1999;5:760–767. doi: 10.1038/10480. [DOI] [PubMed] [Google Scholar]
- 45.Tontonoz P, Nagy L, Alvarez JGA, Thomazy VA, Evans RM. PPARγ promote monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 46.Xin X, Yang S, Kowalski J, Gerritsen ME. Peroxisome proliferator-activated receptor γ ligands are potent inhibitors of angiogenesis in vitro and in vivo. J Biol Chem. 1999;274:9116–9121. doi: 10.1074/jbc.274.13.9116. [DOI] [PubMed] [Google Scholar]
- 47.Giles H, Leff P. The biology and pharmacology of PGD2. Prostaglandins. 1988;35:277–300. doi: 10.1016/0090-6980(88)90093-7. [DOI] [PubMed] [Google Scholar]
- 48.Brock TG, McNish RW, Peters-Golden M. Arachidonic acid is preferentially metabolized by cyclooxygenase-2 to prostacyclin and prostaglandin E2. J Biol Chem. 1999;274:11660–11666. doi: 10.1074/jbc.274.17.11660. [DOI] [PubMed] [Google Scholar]
- 49.Hirata Y, et al. Occurrence of 9-deoxy-Δ9,Δ12-13,14-dihydro-prostaglandin D2 in human urine. J Biol Chem. 1988;263:16619–16625. [PubMed] [Google Scholar]
- 50.Gilroy DW, et al. Inducible cyclooxygense may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 51.Kikawa Y, Narumitya S, Fukusgima M, Wakatsuka H, Hayaishi O. 9-deoxy-Δ9,Δ12-13,14-dihydroprostaglandin D2, a metabolite of prostaglandin D2 formed in human plasma. Proc Natl Acad Sci USA. 1984;81:1317–1321. doi: 10.1073/pnas.81.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fukushima M. Biological activities and mechanisms of action of PGJ2 and related compounds: an update. Prostaglandins Leukot Essent Fatty Acids. 1992;47:1–12. doi: 10.1016/0952-3278(92)90178-l. [DOI] [PubMed] [Google Scholar]
- 53.Narumiya S, Ohno K, Fijiwara M, Fukushima M. Site and mechanism of growth inhibition by prostaglandins. II. Temperature-dependent transfer of a cyclopentenone prostaglandin to nuclei. J Phamacol Exp Ther. 1986;239:506–511. [PubMed] [Google Scholar]
- 54.Rossi A, et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature. 2000;403:103–118. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 55.Straus DS, et al. 15-Deoxy-δ12,14-prostaglandin J2 inhibits multiple steps in the NF-kB signaling pathway. Proc Natl Acad Sci USA. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Imura H. A novel antidiabetic drug, troglitazone: reason for hope and concern. N Engl J Med. 1998;338:908–909. doi: 10.1056/NEJM199803263381311. [DOI] [PubMed] [Google Scholar]