Abstract
Purpose
Enteric colonization with Oxalobacter formigenes, a bacterium whose main energy source is oxalate, has been demonstrated to decrease the risk of recurrent calcium oxalate kidney stone formation. We assessed the impact of diets controlled in calcium and oxalate contents on urinary and fecal analytes in healthy subjects who were naturally colonized with O. formigenes or not colonized with O. formigenes.
Materials and Methods
A total of 11 O. formigenes colonized and 11 noncolonized subjects were administered diets controlled in calcium and oxalate contents. We assayed 24-hour urine collections and stool samples obtained on the last 4 days of each 1-week diet for stone risk parameters and O. formigenes levels. Mixed model analysis was used to determine the effects of colonization status on these variables.
Results
Urinary calcium and oxalate excretion were significantly altered by the dietary changes in O. formigenes colonized and noncolonized individuals. Mixed model analysis showed significant interaction between colonization status and oxalate excretion on a low calcium (400 mg daily)/moderate oxalate (250 mg daily) diet (p = 0.026). Urinary oxalate excretion was 19.5% lower in O. formigenes colonized subjects than in noncolonized subjects on the low calcium/moderate oxalate diet (mean ± SE 34.9 ± 2.6 vs 43.6 ± 2.6 mg, p = 0.031).
Conclusions
Results suggest that O. formigenes colonization decreases oxalate excretion during periods of low calcium and moderate oxalate intake.
Keywords: kidney, kidney calculi, Oxalobacter formigenes, calcium oxalate, diet
A number of risk factors influence the development of calcium oxalate kidney stones, including urinary oxalate excretion. Curhan and Taylor observed in 3 large epidemiological cohorts that small increases in oxalate excretion, even in ranges considered to be normal, significantly enhance the risk of developing a kidney stone.1 Dietary oxalate consumption may significantly impact urinary oxalate excretion and, hence, stone risk.2 In addition, decreased calcium intake results in increased urinary oxalate excretion2 and stone risk.3,4 Taken together these results suggest that a low calcium, high oxalate diet may place an individual at risk for calcium oxalate kidney stones.
O. formigenes is a gram-negative, anaerobic bacterium whose main carbon and energy sources are derived from oxalate metabolism.5 Enteric colonization with Oxalobacter formigenes could theoretically decrease intestinal oxalate and decrease oxalate absorption and its urinary excretion, thereby, decreasing the risk of calcium oxalate stone formation. In a case-control study Kaufman et al observed that colonization was associated with a 70% decrease in the risk of stone recurrence.6
In this study subjects who were colonized and not colonized with O. formigenes were administered controlled diets in which calcium and oxalate consumption was varied to assess their effects on fecal composition and urinary excretion.
Materials and Methods
Subjects
Seven females and 4 males colonized with O. formigenes, and 5 females and 6 males who were noncolonized were recruited primarily from our medical center. They provided informed consent to participate in the study and were compensated for their time. They were nonstone forming adults in good health, as judged by their medical history and a normal profile on a complete metabolic serum panel. Subjects refrained from vigorous exercise and medications, including antibiotics and supplements, during the study course. Colonization was determined by liquid culture before assignment to diets and in the final stool collection.7 Colonization status did not change during the study. Subjects collected 2, 24-hour urine samples on self-selected diets to assess baseline urinary excretions. The study was approved by the Wake Forest University School of Medicine institutional review board.
Dietary Protocol
The study was divided into 2, 3-week dietary phases, separated by a washout period of at least 1 week and completed within 7 to 9 weeks. For the first phase dietary oxalate intake was varied, including 50 mg daily for week 1, 250 mg for week 2 and 750 mg for week 3. Calcium consumption was fixed at 1,000 mg daily. For the second phase dietary calcium intake was varied, including 400 mg daily for week 1, 1,000 mg for week 2 and 2,000 mg for week 3. Oxalate consumption was fixed at 250 mg daily. The ratio of calcium to oxalate was similar for all meals during each respective dietary sequence. Nonrandomized dietary sequences were used in an attempt to determine the impact of progressive increases in oxalate and calcium consumption on fecal O. formigenes population dynamics. All meals were prepared in the metabolic kitchen of the General Clinical Research Center. Caloric needs for subjects were determined by a software program and they were assigned to 1 of 3 diets containing 2000, 2,500 or 3,000 kcal. Table 1 lists the mean nutrient contents of the 2,500 kcal diets.
Table 1. Nutrient composition of oxalate and calcium varied 2,500 kcal diets.
| Diet (mg Ox/Ca) | |||||
|---|---|---|---|---|---|
| 50/1,000 | 250/1,000 | 750/1,000 | 250/400 | 250/2,000 | |
| Oxalate | 51 | 251 | 751 | 251 | 251 |
| Calcium | 1,011 | 1,011 | 1,010 | 410 | 2,008 |
| Magnesium | 235 | 331 | 417 | 299 | 345 |
| Phosphorus | 1,475 | 1,550 | 1,584 | 1,267 | 1,861 |
| Potassium | 2,554 | 2,750 | 3,220 | 2,868 | 3,286 |
| Sodium | 3,539 | 3,605 | 3,720 | 3,399 | 3,602 |
| Vitamin C | 109 | 104 | 142 | 134 | 126 |
After 3 days of equilibration to each diet 24-hour urine and stool specimens collected on the last 4 days of each diet were analyzed to determine excretory responses to the diets. Subjects received food daily at the General Clinical Research Center except on weekends and were allowed to consume it at the location of choice.
Food Oxalate Analysis
The oxalate content of foods was determined by IC, as previously described.8
Assays
Urinary
Creatinine, calcium, citrate, sodium, potassium, magnesium and phosphate were measured on a C5E Analyzer (Beckman Coulter®). Oxalate was measured using a commercially available kit (Trinity Biotech). The supersaturation of urine with calcium oxalate was measured using the index method described by Tiselius.9
Fecal
Fecal water was obtained by centrifuging 1 gm fecal samples for 1 hour at 21,000 × gravity in a bench top centrifuge at room temperature. For total fecal oxalate measurements a 1 gm fecal sample was vigorously mixed with 9 ml ultrapure 2 M HCl for 20 minutes at room temperature to ensure complete dissolution of all oxalate crystals. Fecal water oxalate and total fecal oxalate content were measured by IC, as described for food oxalate analysis. Fecal water calcium was determined by IC using a 2 × 250 mm CS12 ion exchange column and 18 mM methanesulfonic acid (Dionex, Sunnyvale, California) as the mobile phase, running at 0.25 ml per minute.
O. formigenes
Genotyping into Group I or II
DNA was extracted from stool samples using a mini DNA kit (Qiagen Inc). Genotyping primers were designed based on the sequences of oxc reported by Sidhu et al.10 For Group I the primers were 5′ ATGTAGAGTTGACTGATGGC 3′ and 5′ AAAACGCTGACCGTCATCTT 3′. For Group II the primers were 5′ ATGTAGAGTTGACTGATGGC 3′ and 5′ GAACTTCTGACCATCCTGTT 3′. The reaction was performed in a 25 μl volume containing 1 × PCR buffer, 0.2 mM dinucleoside triphosphate, 0.5 U Taq DNA polymerase, 100 ng genomic DNA, and 1.25 μM each of sense and antisense primers. The PCR reaction was done with an initial denaturing step at 94C for 10 minutes, followed by 30 cycles of 94C for 30 seconds, 60C for 30 seconds and 72C for 30 seconds, and a final 10-minute extension step at 72C.
Quantification in stool samples
Real-time PCR primers were designed based on the sequence alignment of the first 500 bp of oxc from O. formigenes colonized individuals and from pure O. formigenes strains. The specificity of these primers was confirmed by examining their homology with all other bacterial sequences deposited at the National Center for Biotechnology Information gene Basic Local Alignment Search Tool website (http://www.ncbi.nlm.nih.gov/BLAST). The real-time PCR primers used were 5′ ATGTAGAGTTGACTGATGGC 3′ as the sense primer, and 5′ GCCAGGTTCGTGATAGGAAT 3′ as the antisense primer based on sequence alignments. The real-time PCR reaction was done in triplicate using a QuantiTect® SYBR® Green PCR kit, 1.25 μM of each primer and 100 ng stool DNA. The assay was performed in a 96-well optical reaction plate with an optical adhesive film using an ABI Prism® 7000 real time PCR apparatus. The PCR reaction was done with incubation at 95C for 15 minutes to activate HotStarTaq® DNA polymerase and 45 following cycles, including 94C for 30 seconds, 53C for 30 seconds and 72C for 1 minute. Due to a 1 bp mismatch in group 2 strains, which resulted in slower amplification, group specific strains were used as DNA standards and also run in triplicate. HC-1 DNA served as the Group I standard and OxK DNA served as the Group II standard.
Statistical Analysis
All analyses were performed using SAS®, version 9.2. The mean of the last 4 days of 24-hour urine and fecal collections from the oxalate and calcium varied diets was used for 11 O. formigenes colonized and 11 noncolonized subjects per diet. Differences in baseline characteristics, including age, gender, BMI and self-selected diet urinary measures, between O. formigenes colonized and noncolonized individuals were compared using the t test. The tests of significance for bacteria group by diet interaction used in the multivariate mixed models were adjusted for gender, age, BMI, baseline urinary calcium and within individual correlation in PROC MIXED. Data are presented as the raw mean ± SE. To further investigate relationships between diet and colonization we examined models stratified by colonization status for some of the measures.
Results
The demographic characteristics of colonized and noncolonized subjects were similar, as was baseline urinary excretion on self-selected diets except calcium (table 2). Urinary calcium excretion was significantly different since several noncolonized subjects had calcium excretion at the higher end of normal and several in the colonized group had calcium excretion at the lower end of normal. This difference did not influence the results since including baseline urinary calcium as a covariate on mixed model analysis had no effect.
Table 2. Test of differences in O. formigenes groups at baseline.
| Mean ± SEM O. Formigenes | |||
|---|---|---|---|
| Colonized | Noncolonized | p Value | |
| Age (yrs) | 29.3 ± 5.3 | 29.6 ± 5.1 | 0.904 |
| BMI (kg/m2) | 23.2 ± 3.2 | 24.1 ± 4.3 | 0.580 |
| Urinary measures: | |||
| Vol (ml) | 1,340 ± 591 | 1,631 ± 385 | 0.187 |
| Creatinine (mg) | 1,366 ± 532 | 1,470 ± 366 | 0.601 |
| Oxalate (mg) | 26.4 ± 5.8 | 28.1 ± 7.2 | 0.550 |
| Calcium (mg) | 105 ± 43 | 200 ± 90 | 0.007 |
| Citrate (mg) | 383 ± 215 | 407 ± 200 | 0.791 |
| Potassium (mmol) | 58.2 ± 20.5 | 76.3 ± 28.3 | 0.100 |
| Magnesium (mg) | 72.3 ± 33.8 | 86.5 ± 33.8 | 0.335 |
| Sodium (mmol) | 131 ± 65 | 164 ± 53 | 0.197 |
| Phosphorus (mg) | 720 ± 184 | 907 ± 300 | 0.096 |
Urinary calcium and oxalate excretion were significantly altered by the dietary changes in O. formigenes colonized and noncolonized individuals (p <0.001). There were no significant effects of colonization status on urinary parameters when dietary oxalate was varied. Significant effects of colonization status were observed for calcium and oxalate excretion, and for calcium oxalate supersaturation when calcium intake was varied. Post hoc analysis with pairwise testing showed that significant differences in oxalate excretion occurred only on the 400 mg calcium diet (p = 0.031).
As might be expected, colonization with O. formigenes significantly altered fecal parameters due to oxalate degradation by O. formigenes. There were significant decreases in fecal water oxalate and total fecal oxalate in colonized subjects compared to those in noncolonized subjects on all diets. Mean O. formigenes levels increased 10-fold as dietary oxalate increased 15-fold (part A of figure). In contrast, there was a decrease in O. formigenes content with increasing calcium intake (part B of figure).
Figure.
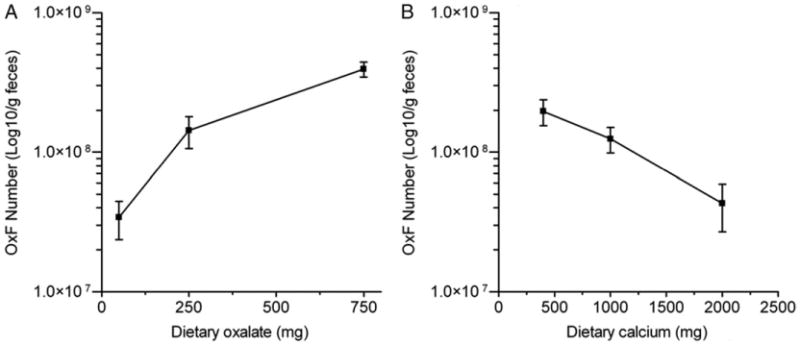
Changes in number of fecal O. formigenes (OxF) with changes in dietary oxalate (A) and dietary calcium (B). Dietary oxalate was 250 mg daily when dietary calcium was varied and dietary calcium was 1000 mg daily when dietary oxalate was varied.
Strains from Groups I and II differ in vitro in sensitivity to antibiotic and bile salts, and in tolerance to air and extremes of pH.11 Seven of the 11 colonized subjects harbored a Group I strain and 4 harbored a Group II strain. No difference in response to any of the diets was observed between the 2 O. formigenes groups. Any differences between the groups in growth and colonization of the intestine, and the clinical impact remain to be ascertained.
Discussion
The simplest hypothesis to explain the effect of O. formigenes colonization on stone disease is that the bacteria consume oxalate in the colon, decrease the amount of oxalate available for absorption and, consequently, decrease the amount excreted in urine. It may be further hypothesized that the concentration of the soluble oxalate anion in the intestinal lumen is a critical determinant of the amount of oxalate absorbed. However, the results of Kaufman et al suggest that colonization does not affect the amount of oxalate excreted in urine.6 Diet was not controlled during these collections, raising the possibility that differences in urinary oxalate excretion may have been masked by differences in dietary calcium or oxalate intake.
When colonized and noncolonized subjects consumed diets of varied oxalate but fixed calcium content, urinary oxalate increased and urinary calcium decreased as dietary oxalate intake increased. These results extend our previous studies of responses to controlled oxalate diets containing 0 to 250 mg oxalate daily, demonstrating the impact of dietary oxalate on urinary oxalate excretion.2 Average urinary oxalate excretion increased by 2 to 3 mg/100 mg oxalate consumed and such increases augment the kidney stone risk.1 The current study also shows more clearly than our previous investigation2 that increasing oxalate intake decreases urinary calcium excretion. This response is most likely due to calcium and oxalate binding in the intestine, resulting in decreased calcium absorption. Results on the calcium varied diets revealed a direct relationship between calcium intake and urinary calcium excretion, and an inverse relationship with oxalate excretion. The latter response was attributable to calcium binding to oxalate in the intestinal tract, decreasing the amount of oxalate available for absorption and subsequent urinary excretion. Our group previously noted this relationship in subjects on controlled diets containing 250 mg oxalate daily and 400 or 1,000 mg calcium.2 This has been confirmed by others.12 The use of a broader range of dietary calcium intake in our current study suggests that the ability of ingested calcium to suppress intestinal oxalate absorption extends to levels of 2,000 mg calcium intake daily. The decrease in urinary oxalate excretion was offset by an increase in urinary calcium, such that the supersaturation of urine with calcium oxalate did not change.
This study shows the impact of dietary oxalate and calcium on the quantity of O. formigenes in feces. Increasing dietary oxalate enabled the fecal population of O. formigenes to expand almost 12-fold as dietary oxalate increased 15-fold, while increasing dietary calcium 5-fold resulted in a 5-fold decrease. This differential response could be due to differences in the amount of oxalate in the crystalline form. Assuming that stool is 70% water, crystalline oxalate appears to be the major form of oxalate in feces since the amount of soluble oxalate in stool was less than 1.5% of the total stool oxalate.
Our findings suggest the setting in which O. formigenes colonization decreases urinary oxalate excretion. Significant interaction between O. formigenes colonization and oxalate excretion was observed when oxalate intake was held constant at 250 mg daily and calcium consumption varied. Pairwise post hoc analysis indicated that a significant difference only occurred in the oxalate excretion of subjects who consumed 400 mg calcium and 250 mg oxalate daily. The impact of colonization on this low calcium diet was consistent with O. formigenes degrading oxalate in the colon and limiting the amount of soluble oxalate available for absorption. Since a diet low in calcium can increase the risk of calcium oxalate stone formation, these data suggest that colonization with O. formigenes may be protective during periods of low calcium intake.
Fecal water oxalate, a possible surrogate for soluble oxalate in the colon, was much lower in colonized than in noncolonized individuals. Notably it was highest in noncolonized individuals on the 400 mg calcium diet, in whom colonization significantly affected urinary oxalate excretion.
This study has certain limitations. Only a few subjects participated due to the comprehensive nature of the study, its requirements for extensive urine and stool collections, its use of tightly controlled diets and its associated costs. The results of the fecal chemistry studies should only be considered inferential since they may not reflect what is happening in the colonic lumen. The different calcium excretion in these 2 cohorts may have influenced the urinary excretion of other analytes and perhaps contributed to the significant interaction of dietary calcium and O. formigenes colonization. This could only have been overcome by matching calcium excretion at study enrollment. In conclusion, our results suggest that O. formigenes colonization decreases urinary oxalate excretion during periods of low calcium and moderate oxalate intake.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health Grants RO1 DK62284 and MO1 RR07122.
Abbreviations and Acronyms
- BMI
body mass index
- IC
ion chromatography
- PCR
polymerase chain reaction
Footnotes
Supplementary material for this article can be obtained at http://www.wfubmc.edu/uploadedFiles/User_Content/Research/Departments/Urology/Stone_Disease/Supplementary%20Material%20(2).pdf.
References
- 1.Curhan GC, Taylor EN. 24-h uric acid excretion and the risk of kidney stones. Kidney Int. 2008;73:489. doi: 10.1038/sj.ki.5002708. [DOI] [PubMed] [Google Scholar]
- 2.Holmes RP, Goodman HO, Assimos DG. Contribution of dietary oxalate to urinary oxalate excretion. Kidney Int. 2001;59:270. doi: 10.1046/j.1523-1755.2001.00488.x. [DOI] [PubMed] [Google Scholar]
- 3.Curhan GC, Willett WC, Rimm EB, et al. Family history and risk of kidney stones. J Am Soc Nephrol. 1997;8:1568. doi: 10.1681/ASN.V8101568. [DOI] [PubMed] [Google Scholar]
- 4.Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol. 2004;15:3225. doi: 10.1097/01.ASN.0000146012.44570.20. [DOI] [PubMed] [Google Scholar]
- 5.Ruan ZS, Anantharam V, Crawford IT, et al. Identification, purification and reconstitution of OxlT, the oxalate: formate antiport protein of Oxalobacter formigenes. J Biol Chem. 1992;267:10537. [PubMed] [Google Scholar]
- 6.Kaufman DW, Kelly JP, Curhan GC, et al. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J Am Soc Nephrol. 2008;19:1197. doi: 10.1681/ASN.2007101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatch M, Cornelius J, Allison M, et al. Oxalobacter sp reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int. 2006;69:691. doi: 10.1038/sj.ki.5000162. [DOI] [PubMed] [Google Scholar]
- 8.Holmes RP, Kennedy M. Estimation of the oxalate content of foods and daily oxalate intake. Kid Intl. 2000;57:1662. doi: 10.1046/j.1523-1755.2000.00010.x. [DOI] [PubMed] [Google Scholar]
- 9.Tiselius HG. Aspects on estimation of the risk of calcium oxalate crystallization in urine. Urol Int. 1991;47:255. doi: 10.1159/000282232. [DOI] [PubMed] [Google Scholar]
- 10.Sidhu H, Yenatska L, Ogden SD, et al. Natural colonization of children in the Ukraine with the intestinal bacterium, Oxalobacter formigenes, using a PCR-based detection system. Mol Diagnosis. 1997;2:89. doi: 10.1054/MODI00200089. [DOI] [PubMed] [Google Scholar]
- 11.Duncan SH, Richardson AJ, Kaul P, et al. Oxalobacter formigenes and its potential role in human health. Appl Environ Microbiol. 2002;68:3841. doi: 10.1128/AEM.68.8.3841-3847.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto ED, Heller HJ, Adams-Huet B, et al. Effect of high and low calcium diets on stone forming risk during liberal oxalate intake. J Urol. 2006;176:132. doi: 10.1016/S0022-5347(06)00565-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


