Abstract
During isometric contractions, slow twitch soleus muscles (SOL) from rats with chronic heart failure (chf) are more fatigable than those of sham animals. However, a muscle normally shortens during activity and fatigue development is highly task dependent. Therefore, we examined the development of skeletal muscle fatigue during shortening (isotonic) contractions in chf and sham-operated rats. Six weeks following coronary artery ligation, infarcted animals were classified as failing (chf) if left ventricle end diastolic pressure was >15mmHg. During isoflurane anaesthesia, SOL with intact blood supply was stimulated (1s on 1s off) at 30Hz for 15 min and allowed to shorten isotonically against a constant afterload. Muscle temperature was maintained at 37°C. In resting muscle, maximum isometric force (Fmax) and the concentrations of ATP and CrP were not different in the two groups. During stimulation, Fmax and the concentrations declined in parallel sham and chf. Fatigue, which was evident as reduced shortening during stimulation, was also not different in the two groups. The isometric force decline was fitted to a bi-exponential decay equation. Both time constants increased transiently and returned to initial values after approximately 200 s of the fatigue protocol. This resulted in a transient rise in baseline tension between stimulations, although this effect which was less prominent in chf than sham. Myosin light chain 2s phosphorylation declined in both groups after 100 s of isotonic contractions, and remained at this level throughout 15 min of stimulation. In spite of higher energy demand during isotonic than isometric contractions, both shortening capacity and rate of isometric force decline were as well or better preserved in fatigued SOL from chf rats than in sham. This observation is in striking contrast to previous reports which have employed isometric contractions to induce fatigue.
Introduction
Limited exercise capacity is a hallmark of chronic heart failure (chf). Central hemodynamic deterioration with low cardiac output, reduced perfusion of working skeletal muscle and low anaerobic threshold likely contribute to the fatigue development in these patients. However, there is a poor relationship between clinical symptoms and left ventricular function, and ejection fraction shows only a weak correlation to maximal oxygen uptake [1], [2]. It has become clear that the muscle itself demonstrates pathological features, independent of cardiac function [3]. During heart failure skeletal muscle function is found to be altered both in experimental models and in patients. One main experimental finding is reduced exercise tolerance to isometric contractions [4]. Increased fatigue in this setting seems not to be due to reduced muscle perfusion [5].
Importantly, the energy demand of the muscle during isometric contractions is small compared to the energy demand during shortening (isotonic) contractions [6]. In addition there are reports that aerobic energy metabolism and mitochondrial function in skeletal muscle are less well tuned to meet the requirement for ATP during exercise in the heart failure condition [7]. One would therefore expect that muscle performance in the heart failure condition would be more impaired during shortening contractions both compared to normal muscle and compared to isometric contractions.
Since skeletal muscle fatigue is highly task dependent, functional impairment in normal muscle might also be underestimated if maximum force is the only fatigue output measured [8], [9]. In line with this, some argue that fatigue will develop more rapidly when it is induced by shortening contractions [10], [11]. Furthermore, alterations in shortening properties can develop independently of changes in isometric tension [12]–[14]. For these reasons we designed an experimental protocol where muscles can shorten upon stimulation with maintained perfusion and physiological temperature, allowing assessment of muscle shortening capacity (Smax) as well as traditional fatigue parameters such as maximum isometric force (Fmax) [14].
The mechanisms of fatigue development can include altered electrolyte concentration, metabolite content, and calcium handling. Recently, posttranscriptional modifications of proteins associated with the contractile filaments have also proven important in regulating cardiac muscle function [14], [15]. Such modifications have been recently linked to heart failure [16]. How these protein modifications affect skeletal muscle function is less well known, but some reports have suggested that posttranslational modification of MLC2 is important in regulating skeletal muscle contraction [14], [17].
No experiments so far have been designed to evaluate how muscle function from animals with post infarction heart failure might change during shortening, isotonic contractions. Contrary to the hypothesis that shortening contractions would reveal an even more attenuated muscle function than isometric contractions, we found that skeletal muscle from heart failure rats tolerated shortening contractions very well, even better that the sham animals. MLC2 dephosphorylation could play an important role in fatigue development.
Methods
All animal experiments were conducted in accordance with current regulations and approved by the Norwegian Animal Research Authority, approval ID 2310. Adult male Wistar rats (Wistar Hannover, Taconic, Skensved, Denmark) were kept in a temperature-, humidity- and light-controlled (12∶12 hour light-dark cycle) environment. Animals had access to standard rat chow (B & K Universal, Oslo, Norway) and water ad libidum, and were caged for ≥1 week after arrival from the supplier prior to inclusion in the study. A total of 98 animals were used.
Induction of congestive heart failure
Rats were anesthetized with 1:3 O2-N2O with ∼2% isoflurane (Forene® Abbott no. 506949), intubated, ventilated, and subjected to ligation of the left coronary artery as described by Tønnessen et al [18]. Sham-operated animals, where the coronary artery was exposed but not ligated, served as controls (sham). Postoperatively, the rats were administered 0.2 mg·kg−1 buprenorphine (0.3 mg/ml) and kept under daily surveillance. With signs of severe discomfort animals were anaesthetized and sacrificed by neck dislocation.
Six weeks after induction of myocardial infarction, rats were once again anesthetized, intubated and placed on a heated (37°C) operating table. The right carotid artery was localized and a pressure sensitive catheter (SPR-407, Millar Instruments, Houston, TX, USA) was inserted and maneuvered retrogradely through the artery into the left ventricular cavity. Based on the conclusions from Sjaastad et al [19], only post infarction rats with left ventricle end diastolic pressure (EDP) >15 mmHg were included in the CHF group. In a subset consisting of 8 sham and 8 chf-animals, echocardiographic examinations were performed.
Surgical preparation
The right leg was skinned from the knee down. The Achilles tendon was cut and the soleus muscle (SOL) was dissected free from surrounding tissue except for the proximal connection to tibia and fibula. The blood supply was left intact. A combined force and length transducer (model 305B, Aurora Scientific, Ontario, Canada) was connected to the distal tendon and platinum electrodes were positioned at the proximal and distal end of the muscle. The ankle and the middle region of the tibia were clamped, leaving the knee immobile and stable. The muscle core temperature was maintained at 37°C by continuous flow of heated 0.9% NaCl across the epimysium of the muscle. During experiments, the pressure-sensitive catheter was positioned in the carotid artery, making it feasible to continuously measure blood pressure during the experiments. Because the anesthetics employed can be cardiodepressive [20], special attention was paid to any change in systolic blood pressure. At the end of the experiment, animals were killed by neck dislocationwhile still anaesthetized.
Stimulation protocol
The SOL was stimulated (Pulsar 6bp, FHC Brunswick, ME, USA) supramaximally (8 V) and muscle length was adjusted to obtain maximum isometric force at 1Hz and 100 Hz (Fmax). Increasing voltage did not increase force or shortening at any stage of the protocol and 100 Hz stimulation was tested to induce Fmax. During the entire stimulation protocol, the SOL was load-clamped at a load representing one third of Fmax (afterload) for that individual muscle; hence the muscle shortened after reaching that force during stimulation. At this load, muscles are able to produce maximum power, and fatigue most rapidly [8]. The muscle was stimulated intermittently (1 s on and 1 s off) at 30 Hz for 100 s or 15 min, with 1 ms pulse duration.
The SOL of Wistar rats consists of 94% slow twitch fibers [21], and it has been shown that 30 Hz is close to the physiological firing rate in SOL during activity [22]. Also, by stimulating intermittently perfusion is likely maintained in the same way as rotation between motor units can ensure perfusion in vivo. During the period (1 s) without electrical stimulation, the muscle was re-extended to its pre-shortening length by the force represented by the pre-clamped load, one third of Fmax. Force, muscle length, muscle surface temperature, aortic blood pressure and stimulation pulse were sampled at 2000 Hz throughout the protocol. At six specific time points (0 s, 100 s, 300 s, 450 s, 600 s and 900 s), the isometric relengthening curve was fitted to the biexponential decay equation, y = y0+a(−x/b)+c(−x/d), where x is time in ms and y is tension in N. The time constants obtained from this fit (b and d) where recorded as tau1 and tau2, respectively. See Table 1 for definitions of parameters calculated from the force and length recordings.
Table 1. Definitions of parameters calculated from the force and length recordings.
| Tbl | Baseline tension |
| Fmax | Maximum isometric force |
| V0 | Unloaded shortening velocity |
| Afterload | 1/3 of Fmax |
| L0 | Optimum resting muscle length |
| dF/dtmax | Rate of isometric force development (30 and 100 Hz) |
| −dF/dtmax | Isometric relaxation rate (rate of force decline) |
| dL/dtmax | Isotonic shortening velocity |
| −dL/dtmax | Isotonic relengthening velocity |
| Smax | Maximum shortening |
| tau1 | Time constant of the rapid component of force decline (the isometric relaxation rate) |
| tau2 | Time constant of the slow component of force decline (the isometric relaxation rate) |
Unloaded shortening velocity, isometric maximum force and contraction rate
Maximal isometric force (Fmax) and rate of isometric force development (100 Hz) were obtained before the stimulation protocol and after 100 s and 15 min of stimulation. The muscle was prepared as described above, and stimulated at the clamped optimal length at 100 Hz. A “slack test” was simultaneously performed to determine V0: The time until the muscle regained pull on the force transducer after a sudden, variable shortening (2–6 mm) from Fmax was plotted on the x-axis against the imposed shortening distance on the y-axis. V0 was defined as the slope of a linear fit to this plot. The rate of isometric force development (100 Hz) was determined by using TableCurve (TableCurve 2D v5.01, SYSTAT Software Inc., San Jose, CA, USA) to analyze the force recordings.
Metabolites
Working muscles were harvested after 100 s or 15 min of stimulation and plunged into liquid nitrogen as quickly as possible, normally within 5 s after the last stimulation. The non-working SOL of the contralateral leg served as control. ATP and CrP were determined by HPLC [23] and lactate by a fluorometric enzymatic coupled assay [24].
Myosin light chain (MLC) analysis
Myofibrillar proteins were separated by glycerol/SDS polyacrylamide gel electrophoresis. The gels were stained sequentially by ProQ Diamond and SYPRO Ruby (M33305, Invitrogen, Oslo, Norway) for phosphorylated and total protein, respectively. Bands were detected by a Typhoon laser scanner (9410, GE Healthcare, Oslo, Norway), and quantified by Imagequant (GE Healthcare, Oslo, Norway). Phosphorylation was quantified at 100 s and 15 min of isotonic stimulation by dividing the staining intensity reflecting phosphorylation level by the staining intensity of the MLC2s protein band. Values are presented as level of phosphorylation relative to the control muscle. MLC2s bands were identified by Western blotting (ALX-BC-1150-S, Clone F109.3E1, Alexis, AH Diagnostics, Oslo, Norway).
Statistics
Values are average ± SEM. Differences between groups (except parameters derived from the isotonic stimulation protocol) were tested using one-way ANOVA supplemented with post hoc Newman-Keuls test. A p-value <0.05 was considered significant. The parameters derived from the isotonic stimulation protocol were analyzed using natural splines [25] with six degrees of freedom, obtained using the ns function in the statistical programming language R (R Development CoreTeam, 2008). Natural splines is a statistical technique for analyzing time course data and allows detection of local group differences at one or a few time points. The values were considered different when p was below 0.05. Statistical analyses were performed with SigmaPlot 11.0 (Systat Software) and R: A language and environment for statistical computing (http://www.R-project.org).
Results
Animal characteristics
In comparison with sham, chf animals exhibited elevated EDP, lower systolic blood pressure, and dilated left ventricular dimensions during diastole (163% compared to sham, Table 2). Fractional shortening (defined as maximal left ventricular luminal diameter in systole relative to the diastolic diameter) was reduced in chf to one fourth of the level in sham, demonstrating severely depressed systolic left ventricular function. chf rats also exhibited clinical signs of pulmonary congestion such as tachypnoea and deeper respiration. This was confirmed by a doubling of left atrial diameter and lung weight in chf compared to sham (Table 2).
Table 2. Characteristics of the experimental groups.
| SHAM | CHF | |
| BW (g) | 398±7 (42) | 382±5 (33) |
| SystBP (mmHg) | 124±2 (40) | 108±2 (28)* |
| EDP (mmHg) | 2±0.2 (41) | 27±1.1 (32)* |
| LW (g) | 1.5±0.02 (31) | 3.6±0.12 (32)* |
| LAd (mm) | 3.5±0.1 (8) | 7.1±0.3 (8)* |
| LVDd (mm) | 6.0±0.2 (8) | 9.8±0.3 (8)* |
| LVDs (mm) | 2.9±0.2 (8) | 8.5±0.3 (8)* |
| FS (%) | 51±2 (8) | 13±1 (8)* |
BW: body weight; LW: lung weight; LAd: left atrial diameter; LVDd: left ventricular diastolic diameter; LVDs: left ventricular systolic diameter; FS: fractional shortening.
All values are average ± SEM (n)
*p<0.05 vs SHAM.
Isometric contractile properties of the SOL during the fatigue protocol
In resting muscles there were no significant differences in SOL force-frequency relationships between sham and chf (data not shown). During the stimulation protocol (see Figure 1A for representative tracings), the maximal rate of isometric force development (dF/dtmax) gradually decreased in both groups, without significant differences between them at any time point (Figure 2A). There was a rapid initial drop in dF/dtmax in the first 40 s followed by a modest reduction during the next 60 s (to 42% of the initial rate for sham and 46% for chf, p<0.001 vs initial rates for both groups). After 100 s of intermittent stimulation, there was no further change in dF/dtmax, and after 15 min of stimulation dF/dtmax was 45% the initial value for sham and 46% of the initial rate for chf.
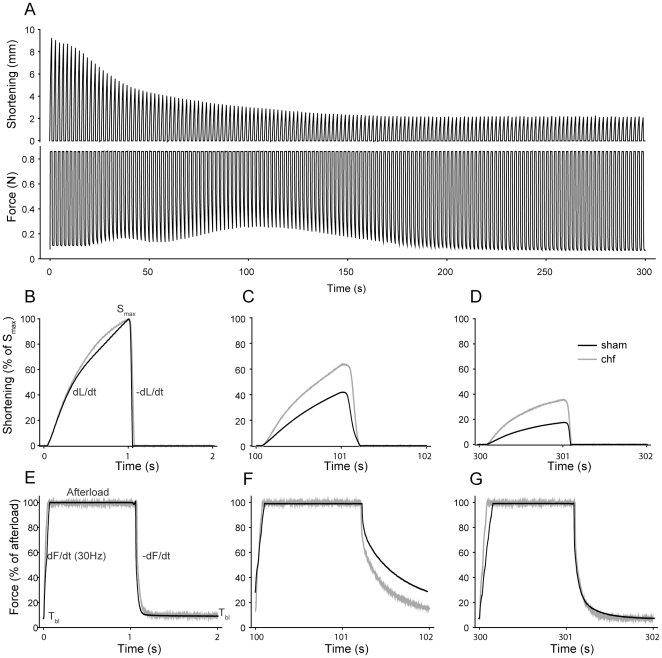
Figure 1. Dynamic exercise protocol.
A, Representative shortening (upper) and force (lower) tracings of the first 5 min of isotonic exercise in SOL from a sham animal. During the complete cycle, the muscle is load-clamped at an afterload representing 1/3 of maximal force (Fmax). B, Expanded view of the shortening tracings from the first exercise cycle (1 s stimulation, 1 s rest). When afterload is attained, the muscle shortens till Smax and stimulation ends after which the muscle is passively relengthened by the afterload to resting length. E, Expanded view of the force tracings from the first exercise cycle (1 s stimulation, 1 s rest). As stimulation starts from resting tension (Tbl) force rises and reaches the afterload. This force is maintained until stimulation ceases and the developed tension in the muscle decreases. C and F, shortening and force tracings after 100 s of exercise and D and G, tracings after 5 min of exercise. Representative sham and chf animal are shown in black and gray, respectively.
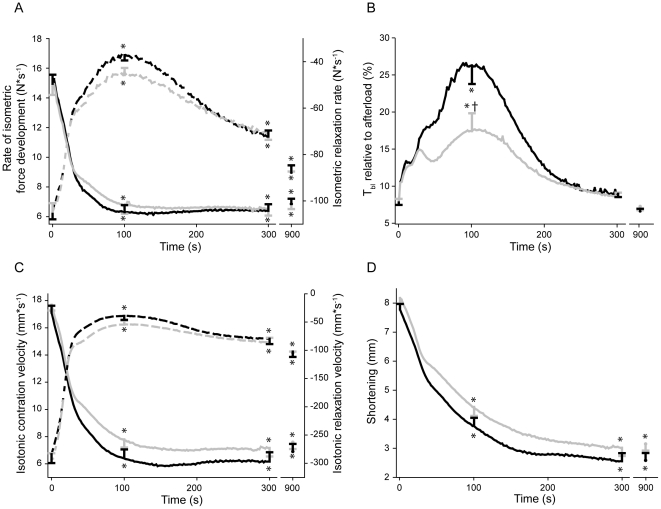
Figure 2. Isometric and isotonic properties during dynamic exercise.
The rates were calculated for every stimulation cycle. The complete tracing is shown for the initial 5 min, while the subsequent 10 min is not shown. The rates at 15 min are indicated. Black and gray lines are responses from SOL of sham (n = 22) and chf (n = 24) rats, respectively. A, Rate of isometric force development during the exercise protocol (solid lines: dF/dtmax (confer Figure 1E)) and rate isometric force decline (dashed lines: −dF/dtmax (confer Figure 1E)). Note the transient nature of −dF/dtmax in contrast to dF/dtmax. B, Baseline tension (Tbl, confer Figure 1E) before a new stimulation cycle starts. C, Isotonic shortening velocity (solid lines: dL/dtmax (confer Figure 1B)) and isotonic relengthening velocity (dashed lines: −dL/dtmax (confer Figure 1B)) during shortening exercise. Note that±dL/dtmax do not show the same transient behavior as −dF/dtmax in Panel A. D, Shortening, (Smax, confer Figure 1B). A–D, Bars at 0 s, 100 s, 300 s and 900 s time points are SEM. *p<0.05 vs. 0 s. †p<0.05 vs. sham.
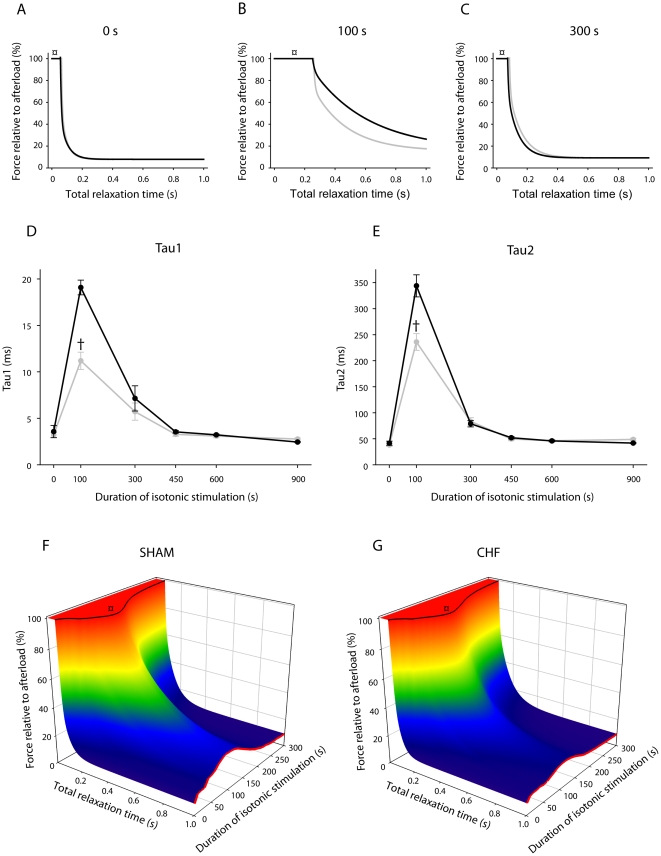
Throughout the stimulation protocol, rate of isometric force decline was maximal immediately following cessation of muscle relengthening (−dF/dtmax). There was no difference between the groups, with an initial rapid decline in −dF/dtmax the first 40 s (Figure 2A) followed by a slower progression during the subsequent 60 s (to 36% and 43% the initial value for sham and chf, respectively. p<0.001 vs initial rate for both groups). Unlike dF/dtmax, the −dF/dtmax was partially restored after 15 min to 85% and 81% of the initial value for sham and chf, respectively. However, the −dF/dtmax does not adequately describe the complete relaxation phase. Therefore, we fitted biexponential decay equations to isometric force recordings. The overall fit was excellent (r2>0.99) and the difference between sham and chf was striking (Figure 3D and E). The initial time constants were not different between the groups. The time constant for the first component (tau1) was initially 3.6 ms in sham and increased to 19.1 ms at 100 s. For the chf group tau1 increased from 3.2 to 11.2 ms, p<0.05 vs sham at 100 s. The time constant of the second slow component (tau2) also increased considerably. Initially, tau2 was 41.5 and 38.1 ms in sham and chf, respectively, and increased to 343.9 and 236.0 ms at 100 s (p<0.05), indicating more rapid isometric relaxation in chf. Both tau1 and 2 decreased in the course of the subsequent 200 s, to 7.1 and 5.7 ms (tau1) and 78.9 and 83.4 ms (tau2) for sham and chf, respectively. Both time constants stabilized at values close to initial values after 450 s of stimulation. The other parameters from the biexponential decay curve (y0, a and c) varied throughout the protocol without significant differences between the groups.
Figure 3. Isometric force decline.
A, B and C: Representative tracings of the isometric relaxation curves after 0 s (A), 100 s (B) and 300 s (C) of the exercise protocol. Note the time lag before force decline starts (marked with ¤) due to time required for relengthening. The transient slowing of isotonic relengthening peaked after 100 s of stimulation (confer Figure 2C and 3F and G). Sham in black and chf in gray. D and E: Time constants (tau1 (D) and tau2 (E), average values for sham (n = 22) and chf (n = 24)) from the isometric force decline at 0, 100, 300, 450, 600 and 900 s of stimulation calculated from a fitted biexponential decay curve. Error bars are ± SEM. Sham in black and chf in gray. †p<0.05 vs. sham. F and G: Averaged force recordings (y-axis) during the period of relaxation (isotonic and isometric phases, x-axis), assembled for the first 5 min of exercise (z-axis) for sham (F, n = 22) and chf (G, n = 24). The time period for relengthening before force decline starts is indicated (black line marked with ¤, confer same symbol in panel A, B and C). Tbl is indicated with a red line. Note the transient rise after 100 s in both groups (confer Figure 2B and 3A, B and C). Since there is no difference between the two groups regarding the isotonic relengthening (black line, F and G (¤)), the attenuated increase in Tbl in chf compared to sham after 100 s (red line, F and G) is due to differences in the isometric force decline.
The baseline tension (Tbl, Figure 2B) reflects the tension before the next stimulation and is dependent on the rate of force decline (−dF/dt) and available time for isometric relaxation. The isometric relaxation commences after the muscle is re-extended to its pre-shortening length (confer Figure 1). Since the isotonic relengthening velocity (−dL/dt) decreased during the initial 100 s the time available for isometric relaxation was reduced by 21% during the initial 100 s for sham and for chf. However, this was not enough to affect Tbl. Thus, the most important determinant of Tbl was the two time constants of the isometric force decline (Figure 3). Tbl showed a three-bumped transient rise during the initial 5 min of the protocol before stabilizing at near-initial tension. The shape of this curve was similar for both groups, with Tbl “peaking” after approximately 8, 40 and 100 s (Figure 2B). Tbl increased significantly less in the chf group as compared to sham (from 8% of the afterload to 26% and 18% in sham and chf, respectively; p<0.001for sham vs chf and vs initial tension for both groups). After 100 s Tbl was gradually restored in accordance with the decrease of tau1 and tau2 of the isometric relaxation and, after 160 s of the fatigue protocol, there were no significant differences between the groups. Tbl stabilized after 300 s at 9% of the afterload in both groups.
Shortening properties of the SOL during the fatigue protocol
The maximal isotonic shortening velocity (dL/dtmax) declined during the protocol, with a rapid decline during the initial 40 s (Figure 2C). The next 60 s was followed by a more moderate decrease (to 37% of the initial velocity for sham and 44% for chf, p<0.001 vs initial velocity in both groups). After 100 s, dL/dtmax remained stable, and after 15 min the velocity was 39% and 43% of the initial velocity for sham and chf, respectively. There were no significant differences between the groups at any time point.
The isotonic relengthening velocity (−dL/dtmax) also decreased rapidly during the initial 40 s of the protocol, and then declined less markedly in the next 60 s (at 100 s, −dL/dtmax = 14% (sham) and 18% (chf) of the initial velocity, p<0.001). After this time point −dL/dtmax increased modestly. After 5 min the rate was 28% (both groups), and after 15 min the −dL/dtmax was 35% (sham) and 37% (chf) of the initial velocity. There were no significant differences between the two groups.
During the first 40 s, muscle shortening (Smax) dropped rapidly in both groups (to 67% and 72% the initial length for sham and chf, respectively (p<0.001); Figure 2D). The decline of Smax was nearly mono-exponential during the protocol, and flattened out after 5 min at 33% (sham) and 37% (chf) of the initial length. At the end of the protocol, shortening was 32% and 39% of the initial Smax. Although not significantly different at any time point, SOL from chf animals tended to shorten more than sham throughout the entire stimulation period. During the initial 300 s accumulated shortening (proportional to total work) of SOL in sham animals was 555±35 mm compared to 633±38 mm in SOL from chf (p = 0.14).
Slack test and isometric properties under tetanic stimulation
Fmax, V0 and dF/dt (100Hz) were measured before and after 100 s and 15 min of isotonic stimulation. Initially there were no differences between the groups in any of these parameters (Table 3). All parameters dropped significantly by a maximum of roughly 25% during the first 100 s of the protocol. Following 15 min of isotonic stimulation, Fmax and dF/dtmax tended to be restored in sham compared to the level at 100 s whereas V0 was not restored. In chf, Fmax was further reduced, whereas V0 and dF/dt (100 Hz) increased compared to the level at 100 s.
Table 3. Isometric properties of SOL from CHF and SHAM rats stimulated at 100 Hz at rest and after 100 s and 15 min of the isotonic contractions.
| Fmax (N) | V0 (mm·s−1) | dF/dt (100 Hz) (N·s−1) | ||
| CTR | 2.4±0.05 (46) | 174±5 (9) | 39.4±3.1 (8) | |
| SHAM | EX – 100 s | 1.9±0.09 (12)* | 160±4 (12)* | 29.7±2.4 (12)* |
| EX – 15 min | 2.1±0.07 (9)* | 155±5 (8)* | 34.5±1.3 (9) | |
| CTR | 2.3±0.04 (32) | 183±3 (7) | 42.2±2.0 (9) | |
| CHF | EX – 100 s | 2.0±0.10 (7)* | 150±6 (7)* | 26.7±2.2 (6)* |
| EX – 15 min | 1.9±0.11 (8)* | 158±7 (8)* | 28.8±2.6 (5)* |
CTR – contralateral, resting SOL. EX – exercising SOL.
All values are average ± SEM (n)
*p<0.05 vs CTR.
Metabolites
Table 4 shows that during the first 100 s of stimulation there was a significant drop in CrP and ATP levels in working SOL from both sham and chf. Also, there was a significant three- to fourfold increase in lactate level in exercising muscles, which was not significantly different between chf and sham. The metabolite concentrations at 100 s of isotonic stimulation remained steady through 15 min, with the exception of a significant decrease in lactate concentration in chf. Phosphate (Pi) was not measured, but judging from the fall in CrP and ATP, Pi levels probably increased to the same extent in the two groups. Levels of ATP, CrP and lactate were not significantly changed in contralateral resting muscles harvested after 100 s and 15 min of the protocol.
Table 4. Metabolites in resting SOL and SOL from CHF and SHAM rats after 100 s and 15 min of isotonic contractions.
| ATP | CrP | Lactate | ||
| CTR | 5.0±0.2 (18) | 15.7±0.8 (18) | 3.3±0.5 (14) | |
| SHAM | EX – 100 s | 2.5±0.4 (10)* | 3.3±1.0 (10)* | 10.3±1.9 (8)* |
| EX – 15 min | 1.9±0.6 (7)* | 2.6±0.5 (7)* | 9.3±1.3 (6)* | |
| CTR | 5.4±0.2 (32) | 15.2±0.9 (32) | 2.8±0.3 (29) | |
| CHF | EX – 100 s | 2.3±0.3 (19)* | 4.0±0.5 (19)* | 11.6±1.2 (19)* |
| EX – 15 min | 2.2±0.4 (16) * | 5.0±1.3 (16)* | 4.6±0.9 (13)* † ¤ |
CTR – contralateral, resting SOL. EX – exercising SOL.
All values are in mmol·(kg wet weight)−1, average ± SEM (n).
*p<0.05 vs CTR,
p<0.05 vs EX – 100 s,
p<0.05 vs SHAM EX – 15 min.
MLC phosphorylation
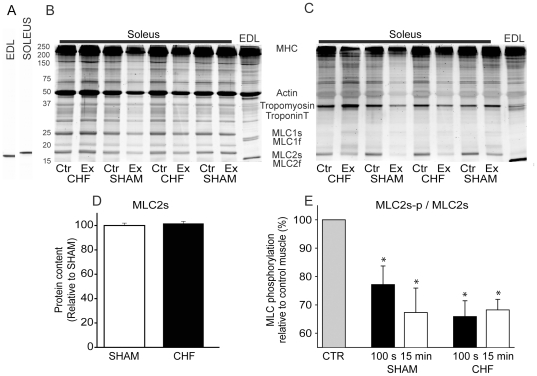
After 100 s of the protocol, MLC2s was significantly dephosphorylated in the exercised SOL compared to the control leg, by 23% and 34% for sham and chf, respectively (Figure 4). After 15 min MLC phosphorylation was reduced by 33% and 32% of control leg values in the sham and chf group, respectively. Thus, there was no significant change in phosphorylation level compared to the 100 s time point for either group, and no significant differences between groups at any time point.
Figure 4. MLC phosphorylation.
A, Representative immunoblot of extensor digitorum longus (EDL) and Soleus probed with monoclonal MLC2 antibody. B, Total protein, stained with SYPRO Ruby for total protein content. C, Same gel as in panel B stained with Pro Q Diamond for phosphorylated proteins. D, Spot density in MLC2s in sham and chf animals related to spot density in sham animals. E, MLC2 phosphorylation in resting (CTR) and exercising SOL normalized to the resting control muscle. Two time points during exercise (100 s and 15 min) were investigated. Average data, sham (100 s and 15 min, n = 5) and chf (100 s and 15 min, n = 15). Bars are + SEM. *p<0.05 vs. CTR.
Discussion
This is the first experimental study to examine the in situ shortening performance of SOL of animals with chf at physiological temperatures. Previous animal studies have focused solely on fatigue development during isometric contractions and we have previously reported reduced fatigue resistance in SOL from chf rats using such a protocol [4]. However, fatigue is typically task dependent, so important aspects of the development of fatigue that occur during shortening contractions may have escaped detection. Surprisingly, we did not observe any significant difference of initial performance for any contractility parameter between the sham and the chf group (Table 5). The main finding in this study was that during a fatiguing exercise protocol, the time constants describing the isometric relaxation phase (force decline after the muscle was relengthened) increased significantly less in chf animals compared to sham, resulting in an attenuated increase in Tbl in chf at 100 s compared to sham. Further, during the exercise protocol the changes in Fmax and V0 were remarkably similar between the two groups, as were the alterations in ATP and CrP. Hence, performance of the soleus muscle in chf rats was in fact superior to that of sham animals.
Table 5. Contractile properties of SOL from CHF and SHAM rats during the first stimulation cycle of the dynamic fatigue protocol.
| SHAM | CHF | |
| Animals | 22 | 24 |
| dF/dtmax (N·s−1) | 15.0±0.6 | 14.8±0.6 |
| −dF/dtmax (N·s−1) | −104.4±3.6 | −102.2±4.8 |
| dL/dtmax (mm·s−1) | 17.1±0.5 | 17.8±0.4 |
| −dL/dtmax (mm·s−1) | −283.9±15.6 | −298.2±12.0 |
| Smax (mm) | 7.8±0.2 | 8.2±0.2 |
| Tbl (% of afterload) | 7.9±0.3 | 8.1±0.4 |
| Tau1 (ms) | 3.6±0.6 | 3.2±0.3 |
| Tau2 (ms) | 41.5±4.0 | 38.1±2.8 |
See Figure 1B and E. All values are average ± SEM.
The muscle's internal energy demand is at least 3 times larger during shortening (working) than during static (non-working) contractions, and our protocol was designed to achieve maximum power and hence maximum energy demand [6], [26]. The changes in ATP, CrP and lactate during the first 100 s of the exercise protocol were notably similar in the two groups, contradicting the finding that muscle from chf rats has a reduced glycolytic capacity [27]. Subsequently, muscle metabolite levels were maintained during further exercise, with the exception of lactate in the chf group which fell considerably. This finding strongly indicates that aerobic metabolism and ATP supply to the SOL were not impaired in chf. Thus, it can be speculated that reported changes in mitochondrial function and metabolism in skeletal muscle from experimental animals and patients with heart failure [7] are not of sufficient severity to compromise energy delivery to ATP requiring processes during exercise. In fact in the SOL of chf animals lactate may have been oxidized during continued exercise. This agrees with studies that have reported a lower increase in muscle lactate in patients with heart failure compared to controls [28], [29]. Maintained aerobic capacity in skeletal muscle is also in line with recent findings from an exercise study in heart failure patients from our laboratory [30].
The isometric force decline is particularly sensitive to exercise, and a decreased rate of force decline is a characteristic sign of a fatigued muscle [31]. Therefore, it is interesting that during shortening contractions, the largest difference between sham and chf animals was observed during the isometric phase. Also of note is that in rats the slowing of force decline is a transient phenomenon, as we have previously reported using the same protocol with isotonic or isometric contractions [32]–[34]. Interestingly, in a human exercise model where isometric contractions at various intensities were carried out by the vastus lateralis muscle, decline of force became faster during exercise in line with the restitution we presently observed from the 100 s time point [35]. We can conclude that the increased energy demand introduced with shortening contractions did not aggravate the initial slowing or the subsequent acceleration of force decline. The cause of slowing could involve alterations in metabolites or signals that are transiently altered but show restitution during continued exercise. In normal rats partial restitution of CrP and lactate was observed during the exercise protocol [14]. In the sham group no such associations could be identified. In the chf group muscle lactate was transiently elevated, but on the other hand the slowing of force decline was much less pronounced than in sham. Taken together these observations indicate that no single factor can explain the transient slowing of force decline.
The rate of decline of force is partly dependent on cross-bridge kinetics and partly on the rate of decline of Ca2+ in the cytosol [31]. Rate of Ca2+ reuptake into the SR is not regarded to be of great importance for velocities and rates of relaxation except for the slower phase of force decline (tau2) [36]. Thus the major focus in the literature has been on changes in metabolites and their effects on cross-bridge kinetics. In line with the literature, we did not observe differences between groups in the resting concentration of ATP and CrP in SOL [37]. Slower relaxation rate is reported to be correlated to reduced levels of the high energy phosphates, and experiments indicate that increased ADP due to breakdown of ATP (Table 4) could delay both early (tau1) and late (tau2) force decline kinetics [38], [39]. Also, decrease in CrP with concomitant rise of Pi can slow relaxation [40]. Finally, lactate accumulation has been reported to reduce the myosin-actin detachment rate, thereby reducing the rate of force decline [41]. Thus, the initial slowing of force decline can very likely be related to one or several of the observed changes in metabolites. However, why is slowing of force decline transient and why is it less pronounced in the chf group when metabolites are not different?
Before returning to the question of why rate of force decline shows restitution, a few comments on the results of the slack test are required. Fmax and dF/dt (100 Hz) were moderately reduced at 100s in line with what is commonly reported with fatigue [42]. This could result from accumulation of Pi due to the breakdown of CrP. It has been shown that high Pi will cause a rightward shift of the force-pCa curve [43]. The slightly depressed V0 was probably related to elevated levels of ADP [38], [39]. Lactacidosis and H+ ions can depress both Fmax and V0 [44], [45], even at physiological temperatures [46]. Despite a partial restitution of the lactate concentration in SOL from chf-animals there was no restoration of either Fmax or V0. This indicates that lactate is not a major contributor to the sustained reduction in these parameters following 15 min of stimulation, and supports the notion that cytosolic lactate accumulation might not be a major factor in the development of fatigue [47]. However, lower peak cytosolic [Ca2+] is likely related to the small reduction in Fmax [44], [48] and a concomitant slower decline of the Ca2+ transient could have several explanations. First, this could be due to the inhibitory effect of elevated Pi on the SERCA pump in combination with acidosis after 100 s [49]. However, the rate of decline of cytosolic Ca2+ is also influenced by the size of Ca2+ leak from the SR through the ryanodine receptor. In fact a Ca2+ leak via the SERCA pump has also been reported under these conditions [50]. An increased leak of Ca2+ from the SR will counteract the pumping of Ca2+ by SERCA into the SR and slow the rate of decline of cytosolic Ca2+ in SOL during activity. Although this SERCA leak appears to be most prominent in fast twitch fibers, the mechanism could contribute to the increase in tau2 after 100 s. The amount of SERCA of slow twitch muscle has been reported to be upregulated in experimental models of heart failure [51], which could at least partly explain the differences between sham and chf in relaxation rate and tau2 after 100 s. Interestingly, we have shown that Ca2+ leak from the SR in biopsies from the vastus lateralis muscle is actually lower in chf patients than in control subjects which could fit with a less pronounced slowing of force decline in the SOL of chf rats [52]. One could speculate that a decline in the Ca2+ leak from the SR would cause a more rapid decline of the Ca2+ transient and that this could explain why rate of decline of force was gradually restored during continued exercise after 100 s. The proposal that the balance between Ca2+ leak and reuptake determines the rate of isometric relaxation (the slow component, tau2) needs further investigation.
MLC2 phosphorylation is commonly reported to be increased during exercise and is linked to the post tetanic twitch potentiation in fast twitch skeletal muscle [17]. We were therefore surprised to find a reduction in MLC2s phosphorylation already after 100 s of shortening contractions in both groups. This suggests a physiological role for altered MLC2 phosphorylation level also in slow twitch muscle. However, most analysis of MLC2 phosphorylation has been undertaken in fast twitch muscle fibers contracting isometrically where phosphorylation seems to lower contraction velocity in concert with low pH and high Pi [53]. During shortening contractions it seems that MLC2s is regulated differently since it was dephosphorylated [14]. Also, the enzyme systems engaged in MLC2 phosphorylation and dephosphorylation show fiber type specific differences [14], [54], [55], and thus could partly explain why our results in slow muscle fibers differ from what is reported in fast muscle fibers. MLC2s phosphorylation moves the myosin head closer to actin [56]. If dephosphorylation does the opposite, the likelihood of myosin and actin interaction during stimulation will be reduced. This could explain the parallel reduction of the rate of isometric force development at 30 Hz, Smax, and the velocity of isotonic shortening during the stimulation protocol. There were no statistical differences in the phosphorylation level in exercised SOL for the groups, which fits with the lack of significant differences for these three parameters.
Several publications have shown decreased fatigue resistance to isometric contractions in skeletal muscle of heart failure animals [4], [51]. The present study is the first to include results from shortening in situ contractions in chf rats at physiological temperatures. In conclusion, the performance of SOL from rats with chf is remarkably similar to sham. The slowing of isometric relaxation was the only mechanical parameter which differed between the two groups, with an attenuated reduction in the failing group. It is possible that different fatigue mechanisms are at work depending on mode of contraction. We speculate that patients with chf may tolerate cycling on an ergometer bike (isotonic, shortening exercise) better than climbing stairs, which to a greater extent is dependent on isometric force development. The functional importance of a maintained isometric relaxation rate during exercise is probably even underestimated in the present study, as the duty cycle employed here (0.5 Hz) is lower than limb frequency during human running (2 Hz) [57]. These results could have important implications for the evaluation of skeletal muscle function and the design of training programs for the heart failure patient.
Acknowledgments
The authors acknowledge Marianne Lunde Sneve and Tævje Strømme for technical assistance, Ståle Nygård for statistical support and Morten Eriksen for animal care.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The study was funded by grants from the Norwegian Foundation for Health and Rehabilitation, University of Oslo, Oslo University Hospital, Ullevål and Anders Jahre's fund for the Promotion of Science, Oslo, Norway. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Harrington D, Coats AJ. Skeletal muscle abnormalities and evidence for their role in symptom generation in chronic heart failure. Eur Heart J. 1997;18:1865–1872. doi: 10.1093/oxfordjournals.eurheartj.a015194. [DOI] [PubMed] [Google Scholar]
- 2.Minotti JR, Christoph I, Massie BM. Skeletal muscle function, morphology, and metabolism in patients with congestive heart failure. Chest. 1992;101:s333–s339. doi: 10.1378/chest.101.5_supplement.333s. [DOI] [PubMed] [Google Scholar]
- 3.Kemp GJ, Thompson CH, Stratton JR, Brunotte F, Conway M, et al. Abnormalities in exercising skeletal muscle in congestive heart failure can be explained in terms of decreased mitochondrial ATP synthesis, reduced metabolic efficiency, and increased glycogenolysis. Heart. 1996;76:35–41. doi: 10.1136/hrt.76.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lunde PK, Verburg E, Eriksen M, Sejersted OM. Contractile properties of in situ perfused skeletal muscles from rats with congestive heart failure. J Physiol. 2002;540:571–580. doi: 10.1113/jphysiol.2001.013324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiotz Thorud HM, Lunde PK, Nicolaysen G, Nicolaysen A, Helge JW, et al. Muscle dysfunction during exercise of a single skeletal muscle in rats with congestive heart failure is not associated with reduced muscle blood supply. Acta Physiol Scand. 2004;181:173–181. doi: 10.1111/j.1365-201X.2004.01285.x. [DOI] [PubMed] [Google Scholar]
- 6.Potma EJ, Stienen GJ. Increase in ATP consumption during shortening in skinned fibres from rabbit psoas muscle: effects of inorganic phosphate. J Physiol. 1996;496:1–12. doi: 10.1113/jphysiol.1996.sp021660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ventura-Clapier R, Garnier A, Veksler V. Energy metabolism in heart failure. J Physiol. 2004;555:1–13. doi: 10.1113/jphysiol.2003.055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vedsted P, Larsen AH, Madsen K, Sjogaard G. Muscle performance following fatigue induced by isotonic and quasi-isometric contractions of rat extensor digitorum longus and soleus muscles in vitro. Acta Physiol Scand. 2003;178:175–186. doi: 10.1046/j.1365-201X.2003.01123.x. [DOI] [PubMed] [Google Scholar]
- 9.Mancini DM, Walter G, Reichek N, Lenkinski R, McCully KK, et al. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85:1364–1373. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- 10.Beltman JGM, van der Vliet MR, Sargeant AJ, De Haan A. Metabolic cost of lengthening, isometric and shortening contractions in maximally stimulated rat skeletal muscle. Acta Physiol Scand. 2004;182:179–187. doi: 10.1111/j.1365-201X.2004.01338.x. [DOI] [PubMed] [Google Scholar]
- 11.Roots H, Ball G, Talbot-Ponsonby J, King M, McBeath K, et al. Muscle fatigue examined at different temperatures in experiments on intact mammalian (rat) muscle fibers. J Appl Physiol. 2009;106:378–384. doi: 10.1152/japplphysiol.90883.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ameredes BT, Brechue WF, Andrew GM, Stainsby WN. Force-velocity shifts with repetitive isometric and isotonic contractions of canine gastrocnemius in situ. J Appl Physiol. 1992;73:2105–2111. doi: 10.1152/jappl.1992.73.5.2105. [DOI] [PubMed] [Google Scholar]
- 13.Cheng AJ, Rice CL. Fatigue-Induced Reductions of Torque and Shortening Velocity Are Muscle-Dependent. Med Sci Sports Exerc. 2010;42:1651–1659. doi: 10.1249/MSS.0b013e3181d6c5b5. [DOI] [PubMed] [Google Scholar]
- 14.Munkvik M, Lunde PK, Sejersted OM. Causes of fatigue in slow-twitch rat skeletal muscle during dynamic activity. Am J Physiol Regul Integr Comp Physiol. 2009;297:R900–R910. doi: 10.1152/ajpregu.91043.2008. [DOI] [PubMed] [Google Scholar]
- 15.Solaro RJ, de Tombe PP. Review focus series: sarcomeric proteins as key elements in integrated control of cardiac function. Cardiovasc Res. 2008;77:616–618. doi: 10.1093/cvr/cvn004. [DOI] [PubMed] [Google Scholar]
- 16.Hamdani N, Kooij V, van Dijk S, Merkus D, Paulus WJ, et al. Sarcomeric dysfunction in heart failure. Cardiovasc Res. 2008;77:649–658. doi: 10.1093/cvr/cvm079. [DOI] [PubMed] [Google Scholar]
- 17.Zhi G, Ryder JW, Huang J, Ding P, Chen Y, et al. Myosin light chain kinase and myosin phosphorylation effect frequency-dependent potentiation of skeletal muscle contraction. Proc Natl Acad Sci U S A. 2005;102:17519–17524. doi: 10.1073/pnas.0506846102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tønnessen T, Christensen G, Oie E, Holt E, Kjekshus H, et al. Increased cardiac expression of endothelin-1 mRNA in ischemic heart failure in rats. Cardiovasc Res. 1997;33:601–610. doi: 10.1016/s0008-6363(96)00266-0. [DOI] [PubMed] [Google Scholar]
- 19.Sjaastad I, Sejersted OM, Ilebekk A, Bjornerheim R. Echocardiographic criteria for detection of postinfarction congestive heart failure in rats. J Appl Physiol. 2000;89:1445–1454. doi: 10.1152/jappl.2000.89.4.1445. [DOI] [PubMed] [Google Scholar]
- 20.Hettrick DA, Pagel PS, Warltier DC. Desflurane, sevoflurane, and isoflurane impair canine left ventricular-arterial coupling and mechanical efficiency. Anesthesiology. 1996;85:403–413. doi: 10.1097/00000542-199608000-00023. [DOI] [PubMed] [Google Scholar]
- 21.Soukup T, Zacharova G, Smerdu V. Fibre type composition of soleus and extensor digitorum longus muscles in normal female inbred Lewis rats. Acta Histochem. 2002;104:399–405. doi: 10.1078/0065-1281-00660. [DOI] [PubMed] [Google Scholar]
- 22.Hennig R, Lømo T. Firing patterns of motor units in normal rats. Nature. 1985;314:164–166. doi: 10.1038/314164a0. [DOI] [PubMed] [Google Scholar]
- 23.Sellevold OF, Jynge P, Aarstad K. High performance liquid chromatography: a rapid isocratic method for determination of creatine compounds and adenine nucleotides in myocardial tissue. J Mol Cell Cardiol. 1986;18:517–527. doi: 10.1016/s0022-2828(86)80917-8. [DOI] [PubMed] [Google Scholar]
- 24.Lowry OH, Passonneau JV. A collection of metabolite assays. In: Lowry OH, Passonneau JV, editors. A Flexible System of Enzymatic Analysis. New York, NY,, USA: Academic Press; 1972. pp. 146–218. [Google Scholar]
- 25.Hastie, T, Tibshirani, R, Friedman, J . Springer Series in Statistics; 2009. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. [Google Scholar]
- 26.Vøllestad NK, Wesche J, Sejersted OM. Gradual increase in leg oxygen uptake during repeated submaximal contractions in humans. J Appl Physiol. 1990;68:1150–1156. doi: 10.1152/jappl.1990.68.3.1150. [DOI] [PubMed] [Google Scholar]
- 27.Simonini A, Massie BM, Long CS, Qi M, Samarel AM. Alterations in Skeletal Muscle Gene Expression in the Rat with Chronic Congestive Heart Failure. J Mol Cell Cardiol. 1996;28:1683–1691. doi: 10.1006/jmcc.1996.0158. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan MJ, Green HJ, Cobb FR. Altered skeletal muscle metabolic response to exercise in chronic heart failure. Relation to skeletal muscle aerobic enzyme activity. Circulation. 1991;84:1597–1607. doi: 10.1161/01.cir.84.4.1597. [DOI] [PubMed] [Google Scholar]
- 29.Schaufelberger M, Eriksson BO, Held P, Swedberg K. Skeletal muscle metabolism during exercise in patients with chronic heart failure. Heart. 1996;76:29–34. doi: 10.1136/hrt.76.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slettaløkken G, Rehn TA, Munkvik M, Rud B, Søkjer-Petersen M, et al. Preserved metabolic reserve capacity in skeletal muscle of post-infarction heart failure patients. noScandinavian Journal of Medicine & Science in Sports. 2010;21 doi: 10.1111/j.1600-0838.2010.01226.x. doi: 10.1111/j.1600-0838.2010.01226.x. [DOI] [PubMed] [Google Scholar]
- 31.Westerblad H, Lannergren J. Slowing of relaxation during fatigue in single mouse muscle fibres. J Physiol. 1991;434:323–336. doi: 10.1113/jphysiol.1991.sp018472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, et al. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 33.Sjaastad I, Schiander I, Sjetnan A, Qvigstad E, Bøkenes J, et al. Increased contribution of 1-vs. -adrenoceptor-mediated inotropic response in rats with congestive heart failure. Acta Physiol Scand. 2003;177:449–458. doi: 10.1046/j.1365-201X.2003.01063.x. [DOI] [PubMed] [Google Scholar]
- 34.Mark AL. Sympathetic dysregulation in heart failure: mechanisms and therapy. Clin Cardiol. 1995;18:I3–I8. doi: 10.1002/clc.4960181303. [DOI] [PubMed] [Google Scholar]
- 35.Vøllestad NK, Sejersted I, Saugen E. Mechanical behavior of skeletal muscle during intermittent voluntary isometric contractions in humans. J Appl Physiol. 1997;83:1557–1565. doi: 10.1152/jappl.1997.83.5.1557. [DOI] [PubMed] [Google Scholar]
- 36.de Tombe PP, Belus A, Piroddi N, Scellini B, Walker JS, et al. Myofilament calcium sensitivity does not affect cross-bridge activation-relaxation kinetics. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2007;292:R1129–R1136. doi: 10.1152/ajpregu.00630.2006. [DOI] [PubMed] [Google Scholar]
- 37.Thompson CH, Kemp GJ, Rajagopalan B, Radda GK. Metabolic abnormalities in skeletal muscle after myocardial infarction in the rat. Clin Sci. 1994;87:403–406. doi: 10.1042/cs0870403. [DOI] [PubMed] [Google Scholar]
- 38.Dantzig JA, Goldman YE, Millar NC, Lacktis J, Homsher E. Reversal of the cross-bridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J Physiol. 1992;451:247–278. doi: 10.1113/jphysiol.1992.sp019163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooke R, Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985;48:789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mulligan IP, Palmer RE, Lipscomb S, Hoskins B, Ashley CC. The effect of phosphate on the relaxation of frog skeletal muscle. Pfl3gers Archiv European Journal of Physiology. 1999;437:393–399. doi: 10.1007/s004240050793. [DOI] [PubMed] [Google Scholar]
- 41.Pedersen TH, Nielsen OB, Lamb GD, Stephenson DG. Intracellular acidosis enhances the excitability of working muscle. Science. 2004;305:1144–1147. doi: 10.1126/science.1101141. [DOI] [PubMed] [Google Scholar]
- 42.Westerblad H, Allen DG, Bruton JD, Andrade FH, Lennergren J. Mechanisms underlying the reduction of isometric force in skeletal muscle fatigue. Acta Physiol Scand. 1998;162:253–260. doi: 10.1046/j.1365-201X.1998.0301f.x. [DOI] [PubMed] [Google Scholar]
- 43.Fitts RH. The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol. 2008;104:551–558. doi: 10.1152/japplphysiol.01200.2007. [DOI] [PubMed] [Google Scholar]
- 44.Westerblad H, Allen DG. The influence of intracellular pH on contraction, relaxation and [Ca2+]i in intact single fibres from mouse muscle. J Physiol. 1993;466:611–628. [PMC free article] [PubMed] [Google Scholar]
- 45.Chase PB, Kushmerick MJ. Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophys J. 1988;53:935–946. doi: 10.1016/S0006-3495(88)83174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knuth ST, Dave H, Peters JR, Fitts RH. Low cell pH depresses peak power in rat skeletal muscle fibres at both 30°C and 15°C: implications for muscle fatigue. J Physiol. 2006;575:887–899. doi: 10.1113/jphysiol.2006.106732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allen DG, Lamb GD, Westerblad H. Skeletal Muscle Fatigue: Cellular Mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 48.Westerblad H, Allen DG. The contribution of [Ca2+]i to the slowing of relaxation in fatigued single fibres from mouse skeletal muscle. J Physiol. 1993;468:729–740. doi: 10.1113/jphysiol.1993.sp019797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lamb GD, Recupero E, Stephenson DG. Effect of myoplasmic pH on excitation-contraction coupling in skeletal muscle fibres of the toad. J Physiol. 1992;448:211–224. doi: 10.1113/jphysiol.1992.sp019037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macdonald WA, Stephenson DG. Effects of ADP on sarcoplasmic reticulum function in mechanically skinned skeletal muscle fibres of the rat. J Physiol. 2001;532:499–508. doi: 10.1111/j.1469-7793.2001.0499f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lunde PK, Sejersted OM, Thorud HM, Tønnessen T, Henriksen UL, et al. Effects of congestive heart failure on Ca2+ handling in skeletal muscle during fatigue. Circ Res. 2006;98:1514–1519. doi: 10.1161/01.RES.0000226529.66545.e5. [DOI] [PubMed] [Google Scholar]
- 52.Munkvik M, Rehn TA, Slettaløkken G, Hasic A, Hallén J, et al. Training Effects on Skeletal Muscle Calcium Handling In Human Chronic Heart Failure. Med Sci Sports Exerc. 2009;42:847–855. doi: 10.1249/MSS.0b013e3181c29ec1. [DOI] [PubMed] [Google Scholar]
- 53.Karatzaferi C, Franks-Skiba K, Cooke R. Inhibition of shortening velocity of skinned skeletal muscle fibers in conditions that mimic fatigue. Am J Physiol Regul Integr Comp Physiol. 2008;294:R948–R955. doi: 10.1152/ajpregu.00541.2007. [DOI] [PubMed] [Google Scholar]
- 54.Sweeney HL, Houdusse A. The motor mechanism of myosin V: insights for muscle contraction. Philos Trans R Soc Lond B Biol Sci. 2004;359:1829–1842. doi: 10.1098/rstb.2004.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore RL, Stull JT. Myosin light chain phosphorylation in fast and slow skeletal muscles in situ. Am J Physiol Cell Physiol. 1984;247:462–471. doi: 10.1152/ajpcell.1984.247.5.C462. [DOI] [PubMed] [Google Scholar]
- 56.Sweeney HL, Bowman BF, Stull JT. Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am J Physiol Cell Physiol. 1993;264:C1085–C1095. doi: 10.1152/ajpcell.1993.264.5.C1085. [DOI] [PubMed] [Google Scholar]
- 57.McMahon, A T. Princeton: Princeton University Press; 1984. Muscles, reflexes, and locomotion. [Google Scholar]