Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) causes various signs of toxicity in early life stages of vertebrates through activation of the aryl hydrocarbon receptor (AHR). We previously reported a sensitive and useful endpoint of TCDD developmental toxicity in zebrafish, namely a decrease in blood flow in the dorsal midbrain, but downstream genes involved in the effect are not known. The present study addressed the role of zebrafish cytochrome P450 1C (CYP1C) genes in association with a decrease in mesencephalic vein (MsV) blood flow. The CYP1C subfamily was recently discovered in fish and includes the paralogues CYP1C1 and CYP1C2, both of which are induced via AHR2 in zebrafish embryos. We used morpholino antisense oligonucleotides (MO or morpholino) to block initiation of translation of the target genes. TCDD-induced mRNA expression of CYP1Cs and a decrease in MsV blood flow were both blocked by gene knockdown of AHR2. Gene knockdown of CYP1C1 by two different morpholinos and CYP1C2 by two different morpholinos, but not by their 5 nucleotide-mismatch controls, was effective in blocking reduced MsV blood flow caused by TCDD. The same CYP1C-MOs prevented reduction of blood flow in the MsV caused by β-naphthoflavone (BNF), representing another class of AHR agonists. Whole mount in situ hybridization revealed that mRNA expression of CYP1C1 and CYP1C2 were induced by TCDD most strongly in branchiogenic primordia and pectoral fin buds. In situ hybridization using head transverse sections showed that TCDD increased the expression of both CYP1Cs in endothelial cells of blood vessels, including the MsV. These results indicate a potential role of CYP1C1 and CYP1C2 in the local circulation failure induced by AHR2 activation in the dorsal midbrain of the zebrafish embryo.
Keywords: aryl hydrocarbon; β-naphthoflavone (BNF); blood flow; cytochrome P450 (CYP); 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD); zebrafish embryo
Introduction
Planar halogenated aromatic hydrocarbons such as polychlorinated dibenzo-p-dioxins, dibenzofurans and polychlorinated biphenyls elicit a broad spectrum of toxic defects in most vertebrates including mammals, birds and fish. Fish embryos are particularly sensitive to the toxic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Peterson et al., 1993; Hahn, 2001). Exposure of developing fish to TCDD causes a reduction of peripheral circulation, edema, craniofacial malformations and growth retardation, culminating in mortality (Walker and Peterson, 1994; Henry et al., 1997; Teraoka et al., 2002).
Most of the toxic endpoints of TCDD exposure in vertebrates are thought to be mediated by a ligand-activated transcription factor, the aryl hydrocarbon receptor (AHR) and its dimerization partner, the aryl hydrocarbon receptor nuclear translocator (ARNT). Fish have several isoforms of AHR and ARNT, and AHR1a, AHR1b, AHR2, ARNT1 and ARNT2 have been cloned and characterized in zebrafish (Hahn et al., 1997; Karchner et al., 2005). Gene knockdown with morpholino antisense oligonucleotides (MO or morpholino) against AHR2 shows that toxic endpoints such as circulation failure and malformation of certain brain blood vessels seen in TCDD-exposed zebrafish larvae are mediated by AHR2 (Prasch et al., 2003; Teraoka et al., 2003a, 2010). It is reported that mutant zebrafish embryos that are null for ARNT2 fail to be rescued from TCDD-induced toxicity (Prasch et al., 2004), while knockdown of either AHR2 or ARNT1 significantly reduces the toxicity, suggesting the importance of ARNT1 rather than ARNT2 in TCDD embryo toxicity in zebrafish (Antkiewicz et al., 2006; Prasch et al., 2006). A recent study has indicated that the cyclooxygenase-2-thromboxane pathway is a potential target of TCDD toxicity to induce local circulation failure in the dorsal midbrain (Teraoka et al., 2009). Nevertheless, the signaling pathway following TCDD activation of AHR2/ARNT1 is only beginning to be understood.
Among the known AHR/ARNT target genes, cytochrome P4501A (CYP1A) is the best characterized and most strongly induced gene in developing zebrafish exposed to TCDD (Handley-Goldstone et al., 2005; Carney et al., 2006a). However, studies in the zebrafish embryo treated with morpholinos against CYP1A translation showed inconsistent results regarding the role of CYP1A in TCDD developmental toxicity (Teraoka et al., 2003a; Carney et al., 2004; Antkiewicz et al., 2006). Conflicting results seen in zebrafish CYP1A morphants imply that other genes participate in particular toxic endpoints of TCDD, possibly including other CYP1 genes. Recently, a novel CYP1 subfamily, the CYP1Cs, was discovered in fish, and CYP1C1 and CYP1C2 have been cloned and characterized in zebrafish (Godard et al., 2005; Jönsson et al., 2007a). Zebrafish embryos showed basal and TCDD- or 3,3'4,4',5-pentachlorobiphenyl (PCB126)-induced expression of both CYP1C transcripts (Jönsson et al., 2007b). Morpholino knockdown of AHR2 protein in the zebrafish embryo effectively inhibited the induction of CYP1Cs, showing regulation by AHR2 (Jönsson et al., 2007b). A recent study reported that vascular CYP1C1 and CYP1C2 were increased following treatment with TCDD in adult zebrafish, and the induced expression in mesenteric artery was greater than that in liver for both CYP1Cs, suggesting that vasculature is a target tissue for induction of CYP1C1 and CYP1C2 by AHR agonists (Bugiak and Weber, 2009). Whether CYP1C1 and CYP1C2 expression is induced in vasculature in early life stages of zebrafish remains to be determined. It is important to consider whether the vascular CYP1Cs may be involved in the AHR2/ARNT1 pathway to developmental toxicity, particularly vascular toxicity, in the zebrafish embryo.
We previously established a useful approach to study a particular effect of TCDD in development in zebrafish, namely a decrease in blood flow in the dorsal midbrain (Dong et al., 2002). TCDD elicited a significant reduction of blood flow in the mesencephalic vein (MsV) during 48 and 56 hours post fertilization (hpf) (Dong et al., 2002), which was blocked by antioxidants, general CYP inhibitors, and AHR2 knockdown (Dong et al., 2002, 2004). β-Naphthoflavone (BNF), another commonly studied AHR agonist, mimicked the inhibitory effect of TCDD on MsV blood flow (Dong et al., 2002). TCDD- and BNF-induced reduction of MsV blood flow is one of the earliest endpoints of toxicity, occurring before general circulation failure and pericardial edema (Teraoka et al., 2003b; Carney et al., 2006b). Therefore, the present study focuses on the potential involvement of the CYP1Cs in the mesencephalic circulation failure seen in the zebrafish embryo exposed to TCDD or BNF.
Materials and methods
Fish husbandry
The Tupfel/long fin wild-type strain of zebrafish were used for all experiments, and were maintained as previously described (Jönsson et al., 2007a; Teraoka et al., 2009). Fertilized eggs were collected from multiple group breedings of adult zebrafish. Embryos were reared as described previously (Jönsson et al., 2007b; Teraoka et al., 2009).
Chemical exposure
In the present study, TCDD (99.1% purity; Accustandard, Inc., New Haven, CT) and BNF (Sigma-Aldrich, St. Louis, MO) were used as model AHR agonists. Embryos were placed in polystyrene petri dishes with no more than 3 embryos per milliliter of either Zebrafish Ringer or 0.3× Danieau's, and then exposed to carrier (0.1% dimethyl sulfoxide [DMSO]) or an apparent concentration of waterborne TCDD or BNF (dissolved in 0.1% DMSO). Details of duration of chemical exposure are given below.
Knockdown with morpholinos
Morpholinos designed to block initiation of translation of AHR2, CYP1C1 and CYP1C2 were obtained from Gene Tools (Philomath, OR). Sequences of morpholinos used are shown in Table 1. The MO used for AHR2 was previously described (Dong et al., 2004). Two morpholinos that do not overlap each other were designed for CYP1C1 and CYP1C2. All morpholinos were designed around the translation start site, as both CYP1C genes are single exon genes which lack splice sites. CYP1C1-MO1 complements 25 base pairs of the 5'-UTR, starting from 27 base pairs upstream of the start site. CYP1C1-MO2 complements the first 25 base pairs of the coding region. CYP1C2-MO1 complements 25 base pairs of the 5'-UTR, starting from 68 base pairs upstream of the start site. CYP1C2-MO2 complements the first one residue upstream of the start codon and the first 24 residues of the coding region. The proximal 5'-UTR sequences of CYP1C1 and CYP1C2 targeted by the morpholinos used in these studies differ greatly from each other (Fig. 1A) and from those of CYP1A and CYP1B1. Negative control morpholinos with 5 nucleotides different from the original ones were synthesized for AHR2, CYP1C1 and CYP1C2. All morpholinos were diluted in deionized water to the concentrations indicated in Table 1. A Narishige IM-300 microinjector (Tokyo, Japan) with a fine glass needle was used to inject 2 nL of morpholinos into the yolk of 1- to 4-cell stage embryos.
Table 1.
Sequences of morpholino antisense oligonucleotides against translation of AHR2, CYP1C1 and CYP1C2.
| Target | Concentration | Sequence |
|---|---|---|
| AHR2-MO | 50 μM | 5'-TGTACCGATACCCGCCGACATGGTT-3' |
| 5mis-AHR2-MO | 50 μM | 5'-TcTAgCGATACCCcCCGAgATGcTT-3' |
| CYP1C1-MO1 | 50 μM | 5'-TTCTTCCCAAAGTGTATTCTTCTGA-3' |
| 5mis-CYP1C1-MO1 | 50 μM | 5'-TTCTTCCgAAAcTcTATTCTTgTcA-3' |
| CYP1C1-MO2 | 25 μM | 5'-TCTTCAGTCCAAACTCAGCCTCCAT-3' |
| 5mis-CYP1C1-MO2 | 25 μM | 5'-TCTTgAcTCgAAACTgAcCCTCCAT-3' |
| CYP1C2-MO1 | 50 μM | 5'-TAACTCCCCATCTTAAAAACGAGCC-3' |
| 5mis-CYP1C2-MO1 | 50 μM | 5'-ATtACTgCCCATgTTAAAAgGAcCC-3' |
| CYP1C2-MO2 | 25 μM | 5'-GAACTCTGAATCCGACTGCGCCATG-3' |
| 5mis-CYP1C2-MO2 | 25 μM | 5'-GAAgTCTcAATCCcACcCGCgATG-3' |
Modified nucleotides are indicated by small letters as for 5 nucleotide-mismatch negative controls.
Fig. 1.
Efficacy and specificity of morpholinos against CYP1C1 and CYP1C2 assessed by in vitro transcription/translation system. (A) Schematic of the proximal 5'-UTR of CYP1C1 and CYP1C2 highlighting the morpholino target sequences. The actual start site is shown in bold letters, while the out-of-frame ATGs (only seen in CYP1C2) are in italics. In CYP1C2, sequences between MO1 and MO2 are omitted except the out-of-frame ATG. (B) Proteins of zebrafish CYP1C1 and CYP1C2 were synthesized by in vitro transcription/translation system with or without respective target morpholinos or their 5 nucleotide-mismatch controls. The putative CYP1C1 and CYP1C2 bands were determined from the calculated molecular weight and the mobility of the in vitro synthesized proteins relative to the molecular weight marker proteins, and are indicated by the arrow in the left margin. Other bands are proteins that are non-specifically produced in the TNT in vitro translation system, as they are found in all samples including TNT reaction mixtures lacking added plasmids or morpholinos.
Confirmation of efficacy and specificity of morpholinos by in vitro protein synthesis
The efficacy and specificity of CYP1C-MOs used in the present study were determined by their ability to block in vitro translation of zebrafish CYP1C1 or CYP1C2 from a pGEM-T Easy or a pcDNA plasmid containing correctly oriented insert, using the Promega TNT® rabbit reticulocyte T7 Quick Coupled Translation system. Transcend™ biotinylated t-Lysyl-RNA was used to label the translated proteins. One to five μL of neat, or acetone precipitated, reaction mixture (per kit protocol) was resolved on 10% polyacrylamide gels, transferred to Hoefer 0.22 μm nitrocellulose, and membranes were then blocked with LiCor blocker and incubated with LiCor Streptavidin IRDye 680™ to fluorescently label biotinylated proteins. Detection was done with the Licor Odyssey™ near-IR laser fluorescence detection system using the 700 nm excitation laser to visualize labelled proteins and co-resolved BioRad Precision Plus™ All Blue prestained molecular weight standards.
Measurement of mesencephalic vein blood flow
Blood flow in MsV was evaluated using a recorder system, LRH1601 line recorder system (DigiMo, Japan) equipped with a high-speed video camera, VCC-H1600 (DigiMo, Japan), or Handycam HDR-UX7 (Sony, Japan), as described previously with a slight modification (Teraoka et al., 2002). Embryos were exposed to 0.1% DMSO, 0.5 ppb TCDD or 0.2 μM BNF (0.054 ppm) for 24 hr, starting at 24 hpf. We used TCDD and BNF at particular concentrations indicated above, based upon our preliminary studies and previous results showing dose-response relationships (Dong et al., 2002). At 48 hpf embryos were rinsed, and then dechorionated for measurement of blood flow in the MsV between 50 and 55 hpf. Zebrafish embryos were orientated for lateral observation in the flow studies (see Fig. 5J). The number of red blood cells passing through the MsV per 15 sec (RBC/15 sec) was determined as an index of blood flow. The MsV blood flow in each treatment group was expressed as a percentage of rate in the non-injected controls, to normalize the blood flow in each experiment.
Fig. 5.
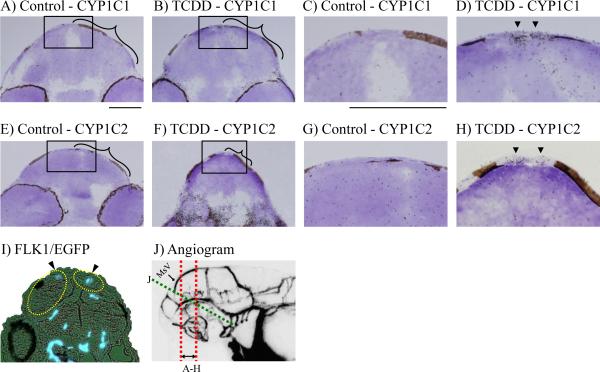
Expression of CYP1C1 (A–D) and CYP1C2 (E–H) in transverse sections of the heads of TCDD treated and control zebrafish embryos at 50 hpf assayed by in situ hybridization, and GFP (I) expression in vascular endothelium in an oblique section of a flk1-egfp transgenic zebrafish. For CYP1C1 and CYP1C2 mRNA expression, embryos were exposed to either vehicle (A, E) or 0.5 ppb TCDD (B, F), beginning at 24 hpf. The zebrafish embryo head was transverse-sectioned for in situ hybridization within the area indicated by the red dashed lines in J, an angiogram of blood vessels in the head region of an approximately 60 hpf embryo, kindly provided by Isogai et al. (2001). C, D, G and H are higher magnifications of the rectangular areas indicated in A, B, E and F, respectively. In I, an oblique section through the head of a 50 hpf flk1-egfp transgenic zebrafish, cut as indicated by the dashed green line in J, was fluorescently illuminated in dark field. Fluorescence is seen in endothelium of blood vessels. The mesencephalon is bracketed in A, B, E and F, and circled with a dashed yellow oval in I. Arrowheads in panels D, H and I identify the mesencephalic vein. Length of the bar in panel A = 200 μm; the same level of magnification was used in B, E, F and I. Length of the bar in panel C = 200 μm; the same level of magnification used in D, G and H. The two MsVs connect to the mesencephalic arteries by 2 dpf, extend dorsally and ventrally from the anterior rostrum, and join at the dorsal midline junction, and are distinct and well separated from other vessels in early development, as seen in J. The position of the MsVs is consistent throughout early development, including at 50 and 60 hpf as presented here. In I, the MsVs are deeper and farther apart, consistent with a more rostral oblique section, and the MsVs in sections A–H are more dorsal and closer together, consistent with the proximity of these transverse sections to the dorsal midline junction.
Real time PCR
Real time PCR analysis was carried out to measure expression levels of CYP1C1 and CYP1C2 during zebrafish development from 6 to 96 hpf, by the method described elsewhere (Teraoka et al., 2009), using the Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen, Carlsbad, CA) in a Chromo4 Real-Time PCR Detection System (Bio-Rad, Hercules, CA). Embryos were exposed continuously for up to 24 hr to DMSO (0.1%), 0.5 ppb TCDD or 0.2 μM BNF either beginning at 2 hpf for 6, 12 and 24 hpf collections or beginning at 24 hpf for 36, 48, 72 and 96 hpf collections. In the latter case, larvae collected at 72 and 96 hpf were rinsed at 48 hpf and then placed in fresh Zebrafish Ringer for the duration of the experiments. Total RNA was extracted with Trizol (Invitrogen), and purified with DNase treatment using RNeasy (Qiagen, Germany). The cDNA templates were synthesized from total RNA with Superscript III reverse transcriptase (Invitrogen) and oligo (dT) primer. Elongation factor 1-alpha (EF1α) was used as an internal control. The gene-specific primes used for real time PCR were as follows: CYP1C1 sense primer 5'-GCGTAATTGAGCACGGGA-3', antisense primer 5'-CATCCATTGCATTGCTGTT-3'; CYP1C2 sense primer 5'-TGGTGTAATGGATCATGCAG-3', antisense primer 5'-GCAGAAGCATCCAATTAAGC-3'; EF1α sense primer 5'-GATGCACCACGAGTCTCTGA-3', antisense primer 5'-TGATGACCTGAGCGTTGAAG-3'. Preliminary studies showed that each primer set produced a single peak in melting curve as well as a single band in agarose gel electrophoresis.
Whole-mount in situ hybridization
Whole-mount in situ hybridization analysis was carried out as described previously (Teraoka et al., 2002). We exposed embryos to TCDD only and increased the concentration to elicit a more robust signal of the CYP1Cs in tissues. Embryos were exposed to either 0.1% DMSO or 2 ppb TCDD for 24 hr, from 24–48 hpf. At 50 hpf, embryos were fixed in 4% paraformaldehyde (PFA), and then transferred to methanol until used for in situ hybridization. The antisense RNA probes were prepared from the cDNAs that were cloned by conventional PCR, and consisted of 610 base pairs (DQ007044, 347–956) for CYP1C1 and 599 base pairs (BC159255, 516–1114) for CYP1C2. The targeted CYP1C1 and CYP1C2 sequences for whole mount in situ hybridization do not share high identity with each other and are very different from CYP1A and CYP1B1. The color reaction was carried out using BM-purple substrate. After PFA fixation, the stained embryos were cleared in 70% glycerol and observed using inverted microscopy (IX71, Olympus, Japan).
In situ hybridization using transverse sections of zebrafish embryos
In situ hybridization analysis using head transverse sections for CYP1C1 and CYP1C2 was performed with radioactive antisense oligo DNA probes following the methods described elsewhere (Mowa and Iwanaga, 2000), using 50 hpf embryos exposed to 0.1% DMSO or 0.5 ppb TCDD as described above. Under tricaine anaesthesia, zebrafish embryos in O.C.T. compound (Sakura, Tokyo, Japan) were rapidly frozen in liquid nitrogen. Cryostat sections, 10–20 μm in thickness, were prepared and mounted on glass slides pre-coated with 3-amino-propyltriethoxysilane. We used two different DNA probes specific for each of CYP1C1 and CYP1C2 to check the specificity as well as to obtain a robust signal by using mixtures of the two probes. The sequences of DNAs used were GCGAACTCGGTGCTGTGTTGAACGAGTGCCTTCCTTATCGCCGCA and CGACGGTGGCTTCATGTGCAGGGTTGAAGTGTCGTCCATCAGCAC for CYP1C1, and GCAAATTCAGTGCTGTGTTGAAGCAATGCGCTGCGTATGGCAGAC and GCTTGCTATAACTGGCAAACGTCATGCTGGTCCCGCCGGAAACGT for CYP1C2. The probes were labeled with 33P-dATP using terminal deoxyribonucleotidyl transferase. The hybridized sections were dipped in Kodak NTB2 nuclear track emulsion and exposed for 10 weeks. After being developed, they were stained with hematoxylin for orientation and then observed using inverted microscopy (IX71, Olympus, Japan).
Flk1-egfp transgenic zebrafish embryos
Flk1-egfp transgenic zebrafish that express green fluorescent protein (GFP) in endothelial cells in blood vessels were the generous gift of Len Zon of Children's Hospital, Boston, MA. The transgenic fish were used to compare the expression profile of GFP in endothelium with CYP1C1 and CYP1C2 in head transverse sections of embryos at 50 hpf treated with vehicle or TCDD. At 50 hpf, embryos were fixed in 4% PFA and the head was removed and placed into a depression in 1% agarose created with the Adaptive Science Tools PT-1 mold. Then, melted agarose gel (1%) was added to fill the depression and immobilize the head. Gel immobilized samples were embedded in paraffin. Sections (5 μm) were mounted on slides (Superfrost/Plus: Fisher Scientific, Pittsburgh, PA), dried at room temperature for 2 days, and deparaffinized with Citrisolv (Fisher Scientific). GFP signal was imaged by fluorescent microscopy. GFP intensity was not greatly diminished by fixation and was sufficient to detect the signal in blood vessels.
Statistics
Data are presented as mean ± SEM or mean ± SD. Significant differences in the MsV blood flow index for each morpholino treatment group (AHR2-MO, CYP1C1-MOs, CYP1C2-MOs and their 5 nucleotide-mismatch controls, 5mis-MOs) were determined either by one-way ANOVA which if significant was followed by Tukey-Kramer test, or by Kruskal-Wallis test which if significant was followed by Dunn test. Significance of differences in the mRNA expression levels between control and treatment groups (i.e., TCDD or BNF) in each developmental stage was determined by one-way ANOVA which if significant was followed by Dunnett test. The significant level was set at 0.05. All statistical analyses were performed with GraphPad Prism 5.0 (GraphPad Software, San Diego, CA).
Results
Confirmation of morpholino target specificity by inhibition of in vitro translation
Our approach to discovering the roles of CYP1C1 and CYP1C2 in TCDD developmental toxicity was to block their translation with morpholinos. To increase the reliability of our observations, we used two different morpholinos for CYP1C1 and for CYP1C2, respectively (Table 1, Fig. 1A), with 5 nucleotide-mismatch controls for each gene. We first tested the efficacy and specificity of the morpholinos by assessing their inhibitory effect on the in vitro translation of the CYP1C1 and CYP1C2 genes. When a pGEM-T Easy vector was used for the TNT in vitro translation system, a plasmid containing CYP1C1 insert produced a specific protein band in the expected molecular weight. Both CYP1C1-MO1 and CYP1C1-MO2 efficiently inhibited the translation of CYP1C1, while the 5 nucleotide-mismatch controls, 5mis-CYP1C1-MO1 and 5mis-CYP1C1-MO2, did not inhibit the translation of CYP1C1. However, a CYP1C2 plasmid did not yield protein in the TNT with this vector, and thus we repeated the analysis using a pcDNA vector. Prominent protein bands of the expected molecular weights were produced in the TNT expression system using pcDNA plasmids containing CYP1C1 or CYP1C2 inserts. As before, CYP1C1-MO2, but not a 5mis-CYP1C1-MO2, efficiently blocked the translation of CYP1C1, confirming again the morpholino efficacy and specificity (Fig. 1B). Likewise, the CYP1C2-MO2 blocked translation of CYP1C2, while its 5 nucleotide-mismatch control, 5mis-CYP1C2-MO2, did not (Fig 1B). (The CYP1C2-MO1 was not tested, as there are out-of-frame ATG codons between that morpholino site and the actual start site (Fig. 1A), which could act as translation start sites in the TNT reaction.)
Effects of morpholino knockdown of CYP1Cs expression on the reduced mesencephalic vein blood flow caused by TCDD
The mesencephalic arteries branch towards the rostrum from the internal carotid arteries just before the cerebral arterial branch and join the mesencephalic veins by 2 days post fertilization (dpf) which flow through the dorsal surface of the mesencephalon. The two mesencephalic veins traverse a distance roughly equal to the width of the eye and join at the dorsal midline junction. They are easily observed in the developing embryo at 50 and 60 hpf as the primary vessels in the dorsal region of the mesencephalon (Isogai et al., 2001; Dong et al., 2002). Blood flow in the MsV at 50 hpf was markedly reduced by TCDD, and this inhibitory effect of TCDD on MsV blood flow continued significantly from 48 to 56 hpf (Dong et al., 2002). We reported also that gene knockdown of AHR2 by translational inhibition with a morpholino, AHR2-MO, was effective in blocking a TCDD-induced decrease in MsV blood flow (Dong et al., 2004). In the present study we confirmed again the inhibitory effect of the same AHR2-MO on TCDD-induced reduction of blood flow in the MsV (results not shown).
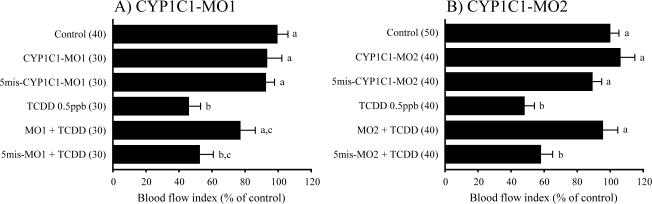
We sought next to determine if knockdown of expression of the CYP1Cs, which are regulated via AHR2 (Jönsson et al., 2007b), would influence the mesencephalic circulation failure caused by TCDD. As shown in Fig. 2, MsV blood flow was similar in untreated embryos and in embryos treated only with CYP1C1-MO1, CYP1C1-MO2, and 5mis-CYP1C1-MOs. TCDD markedly reduced MsV blood flow. Injection of CYP1C1-MO1 and CYP1C1-MO2 provided significant protection against the mesencephalic circulation failure caused by TCDD. The 5 nucleotide-mismatch control 5mis-CYP1C1-MOs were ineffective in blocking the TCDD-induced reduction of MsV blood flow.
Fig. 2.
Protection from the TCDD-induced decrease in mesencephalic vein blood flow by gene knockdown of CYP1C1. After injection of morpholino antisense oligonucleotides against CYP1C1 (A; CYP1C1-MO1, B; CYP1C1-MO2) or their 5 nucleotide-mismatch controls (5mis-MOs), embryos were exposed to vehicle (DMSO) or 0.5 ppb TCDD, starting at 24 hpf. During 50–55 hpf, the number of red blood cells passing through the mesencephalic vein (MsV) per 15 s was determined as an index of blood flow. The MsV blood flow in each treatment group was expressed as a percentage of the non-injected control. Thirty to 50 embryos were subjected to MsV blood flow measurement for each group, as indicated by numerical values in parentheses. Results are expressed as mean ± SEM. Significant differences in blood flow between groups were determined either by Tukey-Kramer test along with one-way ANOVA, or by Dunn test along with Kruskal-Wallis test. Values with different letters are significantly (p < 0.05) different.
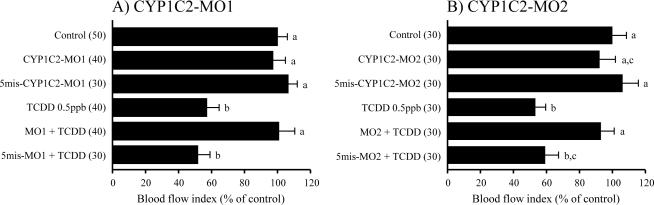
Results similar to those with CYP1C1-MOs were observed in fish treated with CYP1C2-MOs (Fig. 3). MsV blood flow was similar in untreated embryos and embryos injected with CYP1C2-MO1, CYP1C2-MO2, and 5mis-CYP1C2-MOs. Knockdown of CYP1C2 expression by CYP1C2-MO1 and CYP1C2-MO2 prevented TCDD-induced reduction of MsV blood flow. Injection of 5mis-CYP1C2-MOs failed to rescue the reduced blood flow in the MsV caused by TCDD.
Fig. 3.
Protection from TCDD-induced decrease in mesencephalic vein blood flow by gene knockdown of CYP1C2. After injection of morpholino antisense oligonucleotides against CYP1C2 (A; CYP1C2-MO1, B; CYP1C2-MO2) or their 5 nucleotide-mismatch controls (5mis-MOs), embryos were exposed to vehicle (DMSO) or 0.5 ppb TCDD, starting at 24 hpf. Other conditions are the same as given in the Fig. 2 legend.
MsV blood flow indices ranged from 9 – 221 for control embryos, from 6 – 247 for MO-injected DMSO embryos, and from 0 – 261 for non-injected and MO-injected embryos treated with TCDD.
Role of CYP1C genes in the reduced mesencephalic vein blood flow caused by β-naphthoflavone
We measured MsV blood flow in the zebrafish embryo treated with BNF, another class of AHR agonist. We used BNF to assess the role of AHR2/CYP1Cs signaling in the reduced blood flow response, since it was previously shown to inhibit blood flow in the MsV of the zebrafish embryo at 50 hpf (Dong et al., 2002). As shown in Table 2, embryos exposed to 0.2 μM BNF displayed a significantly lower blood flow in the MsV. Injection of AHR2-MO prevented the BNF-induced reduction of MsV blood flow. The 5 nucleotide-mismatch negative control MO (5mis-AHR2-MO) failed to block the reduction of MsV blood flow caused by BNF. Furthermore, knockdown of CYP1C1 and CYP1C2 by two different morpholinos each, that were all effective in blocking the TCDD-induced reduction of MsV blood flow, also significantly protected embryos against the decrease in MsV blood flow caused by BNF. The respective 5 nucleotide-mismatch controls, 5mis-CYP1C1-MOs or 5mis-CYP1C2-MOs, did not protect against the BNF effect. Similar to the TCDD treated embryos, MsV blood flow indices ranged from 0 – 240 for non-injected and MO-injected embryos treated with BNF.
Table 2.
Summary of effects of AHR2, CYP1C1 or CYP1C2 gene knockdown on the reduction of mesencephalic vein blood flow caused by BNF in zebrafish embryos.
| Control | MOs | BNF | MOs + BNF | |
|---|---|---|---|---|
| AHR2-MO | 100 ± 9.6 (20)a | 109 ± 10 (20)a | 51 ± 8.2 (20)b | 97 ± 10 (20)a |
| 5mis-AHR2-MO | - | 104 ± 9.0 (20)a | - | 53 ± 7.0 (20)b |
| CYP1C1-MO1 | 100 ± 5.6 (50)a | 94 ± 7.5 (30)a | 54 ± 5.2 (50)b | 110± 8.5 (30)a |
| 5mis-CYP1C1-MO1 | - | 82 ± 6.1 (30)a | 44 ± 8.1 (29)b | |
| CYP1C1-MO2 | 100 ± 6.8 (40)a | 96 ± 6.6 (30)a | 45 ± 4.8 (40)b | 83 ± 8.0 (30)a |
| 5mis-CYP1C1-MO2 | - | 88 ± 6.2 (30)a | 53 ± 8.1 (30)b | |
| CYP1C2-MO1 | 100 ± 5.0 (30)a | 89 ± 6.7 (30)a,c | 59 ± 7.4 (30)b,c | 100 ± 9.0 (29)a |
| 5mis-CYP1C2-MO1 | - | 90 ± 5.8 (30)a,c | 51 ± 7.3 (30)b | |
| CYP1C2-MO2 | 100 ± 6.8 (38)a | 90 ± 6.3 (36)a | 36 ± 5.1 (39)b | 60 ± 4.7 (36)c |
| 5mis-CYP1C2-MO2 | - | 86 ± 7.1 (29)a,c | 35 ± 3.7 (29)b |
Embryos were injected with morpholino antisense oligos (MOs) against AHR2, CYP1C1 or CYP1C2 at one to four cell stages. The embryos were exposed to vehicle only (DMSO: Control) or 0.2 μM BNF from 24 hpf to 48 hpf. During 50–55 hpf, the number of red blood cells passing through the mesencephalic vein (MsV) per 15 s was determined as an index of the blood flow. The MsV blood flow in each treatment group was expressed as a percentage of the non-injected control. Twenty to 50 embryos were subjected to the measurement for each group, as indicated by numerical values in parentheses. Results are expressed as mean ± SEM. Significance of differences in the blood flow was determined either by Tukey-Kramer test along with one-way ANOVA, or by Dunn test along with Kruskal-Wallis test. Values with different letters are significantly (p < 0.05) different.
Developmental expression of CYP1C transcripts
Jönsson et al. (2007b) reported temporal patterns for basal and PCB126-induced expression of CYP1C1 and CYP1C2 transcripts by quantitative real time PCR in developing zebrafish. We determined here the temporal expression patterns for these transcripts following exposure to TCDD and BNF, using quantitative real time PCR. Expression levels of transcripts of CYP1C1 and CYP1C2 were normalized to EF1α, a house keeping gene. As shown in Tables 3 and 4, sharp up-regulation by TCDD was first observed for both genes in embryos collected at 36 hpf, after which TCDD-induced expression of CYP1C1 and CYP1C2 continued to rise, reaching maximum levels of expression at 72 hpf. Significant increases in CYP1C1 expression were seen also in BNF-exposed embryos at 48 hpf or earlier stages (Table 3). The transcript levels of CYP1C1 in BNF-exposed embryos at the later developmental stages were much lower than those in the TCDD-exposed embryos. In BNF-exposed embryos there also were significant increases in CYP1C2 transcript levels at 6 and 36 hpf (Table 4), and at 48 hpf there was a trend towards induction of CYP1C2 transcript levels, with expression 5.7-fold greater than control embryos. Overall, both CYP1C1 and CYP1C2 mRNAs were induced by 0.5 ppb TCDD, and were induced or showed trends toward induction with 0.2 μM BNF, at those times when these chemicals caused a decrease in MsV blood flow.
Table 3.
Relative mRNA abundance of CYP1C1 in developing zebrafish exposed to TCDD and BNF.
| Hours post fertilization | Control | TCDD | BNF |
|---|---|---|---|
| 6 | 24 ± 8.1 | 120 ± 40* | 190 ± 74* |
| 12 | 90 ± 43 | 200 ± 62 | 230 ± 120* |
| 24 | 150 ± 52 | 270 ± 94 | 490 ± 280* |
| 36 | 160 ± 56 | 3000 ± 1200* | 4700 ± 1300* |
| 48 | 320 ± 170 | 8300 ± 2200* | 3400 ± 760* |
| 72 | 430 ± 130 | 13000 ± 14000* | 1100 ± 610 |
| 96 | 430 ± 43 | 5700 ± 3500* | 1200 ± 340 |
Embryos were exposed to vehicle (DMSO), 0.5 ppb TCDD or 0.2 μM BNF, and euthanized at each developmental stage for total RNA isolation. cDNAs converted were used for quantitative real-time PCR. Elongation factor 1α (EF1α) was used as a reference gene. Units are CYP1C1 transcript/EF1α × 1,000. Results are expressed as mean ± SD. N= 5 per group. Significance of differences in the mean mRNA expression levels between control and treatment groups (i.e., TCDD or BNF) in each developmental stage was determined by one-way ANOVA followed by Dunnett test. Asterisks indicate significant (p < 0.05) difference as compared to respective controls.
Table 4.
Relative mRNA abundance of CYP1C2 in developing zebrafish exposed to TCDD and BNF.
| Hours post fertilization | Control | TCDD | BNF |
|---|---|---|---|
| 6 | 88 ± 43 | 350 ± 120* | 310 ± 170* |
| 12 | 140 ± 67 | 270 ± 76 | 280 ± 210 |
| 24 | 320 ± 140 | 660 ± 210 | 300 ± 280 |
| 36 | 350 ± 120 | 2500 ± 1000* | 2100 ± 950* |
| 48 | 460 ± 220 | 8200 ± 3600* | 2600 ± 880 |
| 72 | 640 ± 280 | 14000 ± 5900* | 360 ± 100 |
| 96 | 670 ± 120 | 7700 ± 4800* | 960 ± 710 |
Embryos were exposed to vehicle (DMSO), 0.5 ppb TCDD or 0.2 μM BNF, and euthanized at each developmental stage for total RNA isolation. cDNAs converted were used for quantitative real-time PCR. Elongation factor 1α (EF1α) was used as a reference gene. Units are CYP1C2 transcript/EF1α × 1,000. Other conditions are the same as siven in the Table 3 footnote.
Spatial expression of CYP1C transcripts at 50 hpf
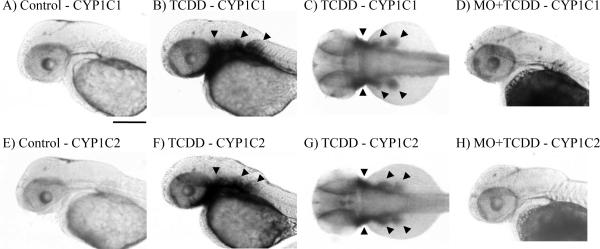
To investigate spatial expression patterns of CYP1C1 and CYP1C2, we performed whole-mount in situ hybridization using 50 hpf embryos treated with either DMSO or TCDD (Fig. 4). We increased the concentration of TCDD to elicit a more robust signal in tissues potentially having lower levels of inducible expression of the CYP1Cs. Neither CYP1C1 nor CYP1C2 was detected in control embryos at 50 hpf (Fig. 4A and 4E). Exposure to 2 ppb TCDD led to a striking increase in both CYP1C1 and CYP1C2 mRNA levels, especially in branchiogenic primordia and pectoral fin buds (Fig. 4B, 4C, 4F and 4G). Knockdown of AHR2 expression with AHR2-MO almost abolished the TCDD-induced expression of CYP1C1 and CYP1C2 in these embryonic tissues (Fig. 4D and 4H), confirming the regulation by AHR2 (Jönsson et al., 2007b). Despite the positive result in certain organs, we were unable to clearly distinguish the brain blood vessels in whole mount in situ hybridization (Fig. 4B, 4C, 4F and 4G).
Fig. 4.
Effect of TCDD treatment on mRNA expression of CYP1C1 (A–C) and CYP1C2 (E–G), and of AHR2-MO treatment on the TCDD-induced expression of CYP1C1 (D) and CYP1C2 (H) in 50 hpf zebrafish embryos assessed by whole-mount in situ hybridization. The representative embryos shown were exposed to either vehicle (A and E; lateral view) or 2 ppb TCDD (B, D, F and H; lateral view, and C and G; dorsal view). Arrowheads in panels B, C, F and G identify the staining regions. Length of the bar in panel A = 400 μm; the same level of magnification was used in B–H.
To further address whether or not TCDD induces CYP1C expression in the MsV, we conducted in situ hybridization with transverse sections of the heads only, from 50 hpf embryos treated with either DMSO or TCDD, using radioactive DNA probes for CYP1C1 and CYP1C2, respectively (Fig. 5). Exposure to 0.5 ppb TCDD led to an increase in expression of both CYP1Cs in the head region (Fig. 5B, 5D, 5F and 5H). The two MsVs are easily distinguished from other vessels in transverse sections including the mesencephalon region of the head. The increased signal in the two prominent vessels in the dorsal midbrain (mesencephalon) resulting from TCDD treatment (Fig. 5D and 5H) correspond to the vascular endothelium in the MsV. No other blood vessels exist in the dorsal midbrain at this stage of development (Fig. 5J). We were not able to discern a concentration dependent induction of CYP1Cs in the MsV at the doses used: expression patterns were similar in embryos exposed to 2 ppb TCDD and 0.5 ppb TCDD (results not shown).
We further compared using fluorescence microscopy, transverse sections of blood vessels in the heads of embryos exposed to TCDD with head oblique sections of flk1-egfp transgenic zebrafish that express GFP in the vascular endothelium. Although the flk1 transgenics are on a different genetic background than the zebrafish strain we used, the structure and morphology of the blood vessels, including the MsV, is conserved among these strains. The two MsVs in transverse sections (Fig. 5A–H) are closer to the dorsal surface and to each other than the same vessels in the oblique section (Fig. 5I) because they were taken nearer to the dorsal midline junction (see Fig. 5J). The co-localization of CYP1C mRNAs and flk1 regulated GFP protein expression confirms the vascular endothelial expression of these proteins.
Discussion
TCDD causes a variety of endpoints of toxicity in the developing zebrafish embryo, including pericardial edema, yolk sac edema, craniofacial malformation, and cardiac abnormalities, as well as a reduction of peripheral circulation. The present study focused on the reduction of MsV blood flow, one of the most sensitive endpoints caused by TCDD (Dong et al., 2002). TCDD-induced mesencephalic circulation failure can be rescued by antioxidants, general CYP inhibitors, and AHR2 knockdown (Dong et al., 2002, 2004), making it useful as a model for a decrease in regional blood flow caused by AHR agonists.
The role of particular genes in developmental toxicities triggered by activation of AHR still is unclear. Our results, using zebrafish embryos combined with knockdown by morpholino antisense oligonucleotides, suggest the involvement of an AHR2-CYP1C signaling pathway in the reduction of MsV blood flow caused by AHR agonists. Both TCDD and BNF caused a decrease in blood flow in the dorsal midbrain, which was prevented by two different morpholinos for CYP1C1 and for CYP1C2 as well as a morpholino for AHR2, but not by their 5 nucleotide-mismatch controls (5mis-MOs). The effectiveness of both CYP1C1-MOs and of CYP1C2-MO2 was shown by their ability to block in vitro translation of CYP1C1 and CYP1C2, respectively. While we were unable to test the efficacy of the CYP1C2-MO1 in the same way, the close similarity of the knockdown observations with the two different morpholinos, together with the lack of effect of their 5mis-MOs, supports the effectiveness of the specific morpholino for CYP1C2. It should also be emphasized that induced CYP1C1 and CYP1C2 transcripts were detected in the MsV of 50 hpf zebrafish embryos treated with TCDD using in situ hybridization with transverse sections of the heads.
Zebrafish and other fish have four CYP1 subfamilies and five genes, CYP1A, CYP1B1, CYP1C1, CYP1C2, and CYP1D1 (Jönsson et al, 2007a; Goldstone et al., 2009; Zanette et al., 2009). It is well known that CYP1A is highly induced in endothelium of the cardiovascular tissues of fish (Stegeman et al, 1989), including heart and vasculature in early life stages of zebrafish (Andreasen et al., 2002; Yamazaki et al., 2002; Dong et al., 2004). However, the role of CYP1A in TCDD-induced developmental toxicities is controversial, as knockdown of CYP1A expression prevented circulation failure and pericardial edema in one study (Teraoka et al., 2003a), but not in another (Carney et al., 2004). Interestingly, treatment of adult zebrafish with TCDD resulted in CYP1A induction equivalent between mesenteric artery and liver, while the induction of CYP1C1 and CYP1C2 was more prominent in mesenteric artery than in liver (Bugiak and Weber, 2009). In 48 hpf zebrafish embryos, the induction response of CYP1B1 gene to TCDD was considerably lower than that of CYP1C1 and CYP1C2 (Jönsson et al., 2007b). A new CYP1, termed CYP1D1, does not appear to be transcriptionally activated by TCDD (Goldstone et al., 2009), and it may contribute less to the developmental toxicities caused by TCDD. Thus, in the present study we sought to examine the possible roles of CYP1C1 and CYP1C2 in the AHR2-mediated vascular toxicity.
It has been reported for zebrafish and killifish (Fundulus heteroclitus) that CYP1C1 is induced by exposure to AHR agonists both in embryos and in various tissues of adults (Wang et al., 2006; Jönsson et al., 2007a,b; Timme-Laragy et al., 2007; Zanette et al., 2009). On the other hand, while zebrafish embryos show induction of CYP1C2 by TCDD in this study and in another (Jönsson et al., 2007b), only a minor response was seen in adult zebrafish (Jönsson et al., 2007a). Minor induction of CYP1C2 also was seen in killifish (Zanette et al., 2009), leading the authors to suggest that CYP1C2 regulation differs from that of CYP1C1, and from CYP1A and CYP1B1, and that there could be a silencing of the induction response of CYP1C2 in some organs of adult fish (Jönsson et al., 2007a; Zanette et al., 2009). As described above, in adult zebrafish TCDD exposure elicited differential expression patterns of CYP1Cs between mesenteric artery and liver, resulting in greater inducible expression of CYP1C1 and CYP1C2 in vasculature than in hepatocytes (Bugiak and Weber, 2009). That result in adults, together with our findings in embryos, suggests the vasculature is an important target for AHR agonists which induce CYP1C1 and CYP1C2, and that induced CYP1Cs might be involved in eliciting decreased blood flow in brain blood vessels, at least in specific veins of early zebrafish embryos.
Our previous study revealed that a reduction of MsV blood flow caused by TCDD was rescued by several CYP inhibitors (Dong et al., 2002). At present, inhibitors specific for either CYP1C1 or CYP1C2 have not been identified. However, rescue from the inhibitory effect of TCDD on MsV blood flow in CYP1C1 and CYP1C2 morphants suggests that CYP1C1 and CYP1C2 could be, in part, targets of those CYP inhibitors in the previous study.
We find it intriguing that knockdown of either CYP1C1 or CYP1C2 was able to prevent almost completely the reduction in blood flow in the MsV caused by TCDD. This suggests that while both CYP1C1 and CYP1C2 are important, they may function differently in the abnormal phenotype. Previously we reported that inhibitors for cyclooxygenase-2 (COX-2) and antagonists for the thromboxane receptor also provide protection against the TCDD-induced MsV circulation failure (Teraoka et al., 2009). Whether COX-2 and activators of the thromboxane receptor, as well as CYP1Cs act through different, possibly convergent pathways leading to the defect is unknown.
At present, we do not have a confirmed explanation for how CYP1Cs contribute to the reduction of MsV blood flow induced by AHR agonists. The etiology of a decrease in blood flow in the dorsal midbrain is not known, but it might involve increases in vascular permeability resulting from TCDD-induced oxidative stress. We previously showed that TCDD-induced mesencephalic circulation failure was associated with an increase in vascular permeability, and that these two endpoints were rescued by either antioxidants or general CYP inhibitors (Dong et al., 2002). It is reported that fish CYP1A is a source of reactive oxygen species when bound with halogenated AHR agonists (Schlezinger et al., 1999) and TCDD increases oxidative stress partly through CYP1A1 induction in primary human aortic endothelial cells (Kopf and Walker, 2010). The possibility that CYP1Cs have the potential to stimulate oxidative stress in the presence of TCDD resulting in decreased blood flow in a particular vein in the dorsal midbrain warrants further investigation. On the other hand, our results show that BNF also causes a decrease in MsV blood flow, and its inhibitory effect on MsV blood flow was blocked by knockdown of either AHR2 or CYP1Cs. Since BNF is known to be easily metabolized by induced CYP1s (Stegeman, J. J., unpublished findings), it likely does not produce oxidative stress via uncoupling of CYP1A (or CYP1Cs), but might through other mechanisms.
Several other CYP enzymes localized in vascular smooth muscle and endothelium participate in the regulation of vascular tone and homeostasis (Fleming, 2001, 2008), and could be involved in toxic effects of chemicals. Among these are some prostanoid synthesizing enzymes, including CYP2C and CYP2J subfamilies that produce epoxyeicosatrienoic acids, and CYP8A1 that synthesizes prostacyclin (PGI2) (Schildknecht et al., 2004). We recently identified and annotated the full suite of zebrafish CYP genes, and found 11 zebrafish genes (i.e., CYP2N13, 6 CYP2Ps, CYP2V1 and 3 CYP2ADs) that show a fine-scale synteny with human CYP2J2 (Goldstone et al., 2010). Our recent study showed also that thromboxane A2 synthase (CYP5A) could be a mediator of TCDD-induced regional mesencephalic circulation failure, as knockdown of CYP5A rescued zebrafish embryos from the decrement of MsV blood flow caused by TCDD (Teraoka et al., 2009). However, TCDD did not cause any increase in CYP5A transcript levels when whole embryos were assayed by quantitative real time PCR (Teraoka et al., 2009). Knockdown of COX-2a also provided protection against the inhibitory effects of TCDD on MsV blood flow (Teraoka et al., 2009). In medaka larvae, TCDD significantly increased COX-2 mRNA expression and elicited pericardial edema, and knockdown of COX-2 expression, as well as a COX-2 inhibitor, efficiently blocked TCDD-induced pericardial edema (Dong et al., 2010). The tissue distribution patterns and regulation of these CYPs and COX-2, and their link to vascular toxicities, as compared to the CYP1Cs, need to be examined during zebrafish development.
In summary, we provide evidence for a functional role of both CYP1C1 and CYP1C2 in the decrease in MsV blood flow caused by AHR agonists in zebrafish embryos. We show further that TCDD induced CYP1C gene expression in brain vasculature, including the MsV. CYP1C genes occur in amphibians and birds, as well as fish, but have been lost in mammals. Thus, identifying the catalytic functions and role of CYP1Cs in AHR-dependent toxicity becomes important for understanding similarities and differences in the mechanisms of AHR agonist toxicity between non-mammalian and mammalian vertebrate species.
Highlights.
> We examine role of zebrafish CYP1C1 and CYP1C2 in TCDD developmental toxicity. > TCDD induces mRNA expression of both CYP1Cs in the mesencephalic vein. > Knockdown of each CYP1C prevents mesencephalic circulation failure by TCDD. > Induced CYP1Cs are involved in reduction of mesencephalic vein blood flow by TCDD.
Acknowledgments
We thank Dr. Mark E. Hahn, Woods Hole Oceanographic Institution (WHOI), for critical reading of this manuscript. We also thank Dr. Victoria R. Starczak, WHOI, for advice on the statistical analysis. This study was supported by Grants-in-Aid for Scientific Research (C) (18580298) and (B) (21310044) to H.T. from the Japan Society for the Promotion of Science (JSPS), Grant-in-Aid for JSPS Fellows to A.K. (4313), Akiyama Foundation to H.T., the Promotion and Mutual Aid Corporation for Private Schools of Japan to H.T., Grant-in-Aid for the High Technological Research Center (Rakuno Gakuen University) from the Ministry of Education to H.T., National Institute of Health Grant (R01ES015912) to J.J.S., and the Superfund Research Program (5P42ES007381) to J.J.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement None of the authors has any conflict of interest regarding the research described in this article.
References
- Andreasen EA, Spitsbergen JM, Tanguay RL, Stegeman JJ, Heideman W, Peterson RE. Tissue-specific expression of AHR2, ARNT2, and CYP1A in zebrafish embryos and larvae: effects of developmental stage and 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure. Toxicol. Sci. 2002;68:403–419. doi: 10.1093/toxsci/68.2.403. [DOI] [PubMed] [Google Scholar]
- Antkiewicz DS, Peterson RE, Heideman W. Blocking expression of AHR2 and ARNT1 in zebrafish larvae protects against cardiac toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2006;94:175–182. doi: 10.1093/toxsci/kfl093. [DOI] [PubMed] [Google Scholar]
- Bugiak B, Weber LP. Hepatic and vascular mRNA expression in adult zebrafish (Danio rerio) following exposure to benzo-a-pyrene and 2,3,7,8-tetrachlorodibenzo-p-dioxin. Aquat. Toxicol. 2009;95:299–306. doi: 10.1016/j.aquatox.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Carney SA, Peterson RE, Heideman W. 2,3,7,8-Tetrachlorodibenzo-p-dioxin activation of the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator pathway causes developmental toxicity through a CYP1A-independent mechanism in zebrafish. Mol. Pharmacol. 2004;66:512–521. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Carney SA, Chen J, Burns CG, Xiong KM, Peterson RE, Heideman W. Aryl hydrocarbon receptor activation produces heart-specific transcriptional and toxic responses in developing zebrafish. Mol. Pharmacol. 2006a;70:549–561. doi: 10.1124/mol.106.025304. [DOI] [PubMed] [Google Scholar]
- Carney SA, Prasch AL, Heideman W, Peterson RE. Understanding dioxin developmental toxicity using the zebrafish model. Birth Defects Res. A Clin. Mol. Teratol. 2006b;76:7–18. doi: 10.1002/bdra.20216. [DOI] [PubMed] [Google Scholar]
- Dong W, Teraoka H, Yamazaki K, Tsukiyama S, Imani S, Imagawa T, Stegeman JJ, Peterson RE, Hiraga T. 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: local circulation failure in the dorsal midbrain is associated with increased apoptosis. Toxicol. Sci. 2002;69:191–201. doi: 10.1093/toxsci/69.1.191. [DOI] [PubMed] [Google Scholar]
- Dong W, Teraoka H, Tsujimoto Y, Stegeman JJ, Hiraga T. Role of aryl hydrocarbon receptor in mesencephalic circulation failure and apoptosis in zebrafish embryos exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2004;77:109–116. doi: 10.1093/toxsci/kfh023. [DOI] [PubMed] [Google Scholar]
- Dong W, Matsumura F, Kullman SW. TCDD induced pericardial edema and relative COX-2 expression in medaka (Oryzias Latipes) embryos. Toxicol. Sci. 2010;118:213–223. doi: 10.1093/toxsci/kfq254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I. Cytochrome p450 and vascular homeostasis. Circ. Res. 2001;89:753–762. doi: 10.1161/hh2101.099268. [DOI] [PubMed] [Google Scholar]
- Fleming I. Vascular cytochrome p450 enzymes: physiology and pathophysiology. Trends Cardiovasc. Med. 2008;18:20–25. doi: 10.1016/j.tcm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Godard CA, Goldstone JV, Said MR, Dickerson RL, Woodin BR, Stegeman JJ. The new vertebrate CYP1C family: cloning of new subfamily members and phylogenetic analysis. Biochem. Biophys. Res. Commun. 2005;331:1016–1024. doi: 10.1016/j.bbrc.2005.03.231. [DOI] [PubMed] [Google Scholar]
- Goldstone JV, Jonsson ME, Behrendt L, Woodin BR, Jenny MJ, Nelson DR, Stegeman JJ. Cytochrome P450 1D1: a novel CYP1A-related gene that is not transcriptionally activated by PCB126 or TCDD. Arch. Biochem. Biophys. 2009;482:7–16. doi: 10.1016/j.abb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone JV, McArthur AG, Kubota A, Zanette J, Parente T, Jönsson ME, Nelson DR, Stegeman JJ. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genomics. 2010;11:643. doi: 10.1186/1471-2164-11-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn ME, Karchner SI, Shapiro MA, Perera SA. Molecular evolution of two vertebrate aryl hydrocarbon (dioxin) receptors (AHR1 and AHR2) and the PAS family. Proc. Natl. Acad. Sci. U.S.A. 1997;94:13743–13748. doi: 10.1073/pnas.94.25.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn ME. Dioxin toxicology and the aryl hydrocarbon receptor: insights from fish and other non-traditional models. Mar. Biotechnol. 2001;3:S224–S238. doi: 10.1007/s10126-001-0045-y. [DOI] [PubMed] [Google Scholar]
- Handley-Goldstone HM, Grow MW, Stegeman JJ. Cardiovascular gene expression profiles of dioxin exposure in zebrafish embryos. Toxicol. Sci. 2005;85:683–693. doi: 10.1093/toxsci/kfi116. [DOI] [PubMed] [Google Scholar]
- Henry TR, Spitsbergen JM, Hornung MW, Abnet CC, Peterson RE. Early life stage toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish (Danio rerio) Toxicol. Appl. Pharmacol. 1997;142:56–68. doi: 10.1006/taap.1996.8024. [DOI] [PubMed] [Google Scholar]
- Isogai S, Horiguchi M, Weinstein BM. The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Dev. Biol. 2001;230:278–301. doi: 10.1006/dbio.2000.9995. [DOI] [PubMed] [Google Scholar]
- Jönsson ME, Orrego R, Woodin BR, Goldstone JV, Stegeman JJ. Basal and 3,3',4,4',5-pentachlorobiphenyl-induced expression of cytochrome P450 1A, 1B and 1C genes in zebrafish. Toxicol. Appl. Pharmacol. 2007a;221:29–41. doi: 10.1016/j.taap.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson ME, Jenny MJ, Woodin BR, Hahn ME, Stegeman JJ. Role of AHR2 in the expression of novel cytochrome P450 1 family genes, cell cycle genes, and morphological defects in developing zebra fish exposed to 3,3',4,4',5-pentachlorobiphenyl or 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2007b;100:180–193. doi: 10.1093/toxsci/kfm207. [DOI] [PubMed] [Google Scholar]
- Karchner SI, Franks DG, Hahn ME. AHR1B, a new functional aryl hydrocarbon receptor in zebrafish: tandem arrangement of ahr1b and ahr2 genes. Biochem. J. 2005;392:153–161. doi: 10.1042/BJ20050713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf PG, Walker MK. 2,3,7,8-tetrachlorodibenzo-p-dioxin increases reactive oxygen species production in human endothelial cells via induction of cytochrome P4501A1. Toxicol. Appl. Pharmacol. 2010;245:91–99. doi: 10.1016/j.taap.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowa CN, Iwanaga T. Differential distribution of oestrogen receptor-alpha and -beta mRNAs in the female reproductive organ of rats as revealed by in situ hybridization. J. Endocrinol. 2000;165:59–66. doi: 10.1677/joe.0.1650059. [DOI] [PubMed] [Google Scholar]
- Peterson RE, Theobald HM, Kimmel GL. Developmental and reproductive toxicity of dioxins and related compounds: cross-species comparisons. Crit. Rev. Toxicol. 1993;23:283–335. doi: 10.3109/10408449309105013. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Teraoka H, Carney SA, Dong W, Hiraga T, Stegeman JJ, Heideman W, Peterson RE. Aryl hydrocarbon receptor 2 mediates 2,3,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol. Sci. 2003;76:138–150. doi: 10.1093/toxsci/kfg202. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Heideman W, Peterson RE. ARNT2 is not required for TCDD developmental toxicity in zebrafish. Toxicol. Sci. 2004;82:250–258. doi: 10.1093/toxsci/kfh235. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Tanguay RL, Mehta V, Heideman W, Peterson RE. Identification of zebrafish ARNT1 homologs: 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in the developing zebrafish requires ARNT1. Mol. Pharmacol. 2006;69:776–787. doi: 10.1124/mol.105.016873. [DOI] [PubMed] [Google Scholar]
- Schildknecht S, Bachschmid M, Baumann A, Ullrich V. COX-2 inhibitors selectively block prostacyclin synthesis in endotoxin-exposed vascular smooth muscle cells. FASEB J. 2004;18:757–759. doi: 10.1096/fj.03-0609fje. [DOI] [PubMed] [Google Scholar]
- Schlezinger JJ, White RD, Stegeman JJ. Oxidative inactivation of cytochrome P-450 1A (CYP1A) stimulated by 3,3',4,4'-tetrachlorobiphenyl: production of reactive oxygen by vertebrate CYP1As. Mol. Pharmacol. 1999;56:588–597. doi: 10.1124/mol.56.3.588. [DOI] [PubMed] [Google Scholar]
- Stegeman JJ, Miller MR, Hinton DE. Cytochrome P450IA1 induction and localization in endothelium of vertebrate (teleost) heart. Mol. Pharmacol. 1989;36:723–729. [PubMed] [Google Scholar]
- Teraoka H, Dong W, Ogawa S, Tsukiyama S, Okuhara Y, Niiyama M, Ueno N, Peterson RE, Hiraga T. 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: altered regional blood flow and impaired lower jaw development. Toxicol. Sci. 2002;65:192–199. doi: 10.1093/toxsci/65.2.192. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Tsujimoto Y, Iwasa H, Endoh D, Ueno N, Stegeman JJ, Peterson RE, Hiraga T. Induction of cytochrome P450 1A is required for circulation failure and edema by 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish. Biochem. Biophys. Res. Commun. 2003a;304:223–228. doi: 10.1016/s0006-291x(03)00576-x. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Hiraga T. Zebrafish as a novel experimental model for developmental toxicology. Congenit. Anom. 2003b;43:123–132. doi: 10.1111/j.1741-4520.2003.tb01036.x. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Kubota A, Dong W, Kawai Y, Yamazaki K, Mori C, Harada Y, Peterson RE, Hiraga T. Role of the cyclooxygenase 2-thromboxane pathway in 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced decrease in mesencephalic vein blood flow in the zebrafish embryo. Toxicol. Appl. Pharmacol. 2009;234:33–40. doi: 10.1016/j.taap.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Ogawa A, Kubota A, Stegeman JJ, Peterson RE, Hiraga T. Malformation of certain brain blood vessels caused by TCDD activation of Ahr2/Arnt1 signaling in developing zebrafish. Aquat. Toxicol. 2010;99:241–247. doi: 10.1016/j.aquatox.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timme-Laragy AR, Cockman CJ, Matson CW, Di Giulio RT. Synergistic induction of AHR regulated genes in developmental toxicity from co-exposure to two model PAHs in zebrafish. Aquat. Toxicol. 2007;85:241–250. doi: 10.1016/j.aquatox.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MK, Peterson RE. Aquatic toxicity of dioxins and related chemicals. In: Schecter A, editor. Dioxins and Health. Plenum Press; New York: 1994. pp. 347–387. [Google Scholar]
- Wang L, Scheffler BE, Willett KL. CYP1C1 messenger RNA expression is inducible by benzo[a]pyrene in Fundulus heteroclitus embryos and adults. Toxicol. Sci. 2006;93:331–340. doi: 10.1093/toxsci/kfl072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Teraoka H, Dong W, Stegeman JJ, Hiraga T. cDNA cloning and expressions of cytochrome P450 1A in zebrafish embryos. J. Vet. Med. Sci. 2002;64:829–833. doi: 10.1292/jvms.64.829. [DOI] [PubMed] [Google Scholar]
- Zanette J, Jenny MJ, Goldstone JV, Woodin BR, Watka LA, Bainy AC, Stegeman JJ. New cytochrome P450 1B1, 1C2 and 1D1 genes in the killifish Fundulus heteroclitus: Basal expression and response of five killifish CYP1s to the AHR agonist PCB126. Aquat. Toxicol. 2009;93:234–243. doi: 10.1016/j.aquatox.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]