SUMMARY
Purpose
Current treatments for epilepsy may control seizures, but have no known effects on the underlying disease. We sought to determine whether early treatment in a model of genetic epilepsy would reduce the severity of the epilepsy phenotype in adulthood.
Methods
We used Wistar albino Glaxo rats of Rijswijk (WAG/Rij) rats, an established model of human absence epilepsy. Oral ethosuximide was given from age p21 to 5 months, covering the usual period in which seizures develop in this model (age ~3 months). Two experiments were performed: (1) cortical expression of ion channels Nav1.1, Nav1.6, and HCN1 (previously shown to be dysregulated in WAG/Rij) measured by immunocytochemistry in adult treated rats; and (2) electroencephalogram (EEG) recordings to measure seizure severity at serial time points after stopping the treatment.
Results
Early treatment with ethosuximide blocked changes in the expression of ion channels Nav1.1, Nav1.6, and HCN1 normally associated with epilepsy in this model. In addition, the treatment led to a persistent suppression of seizures, even after therapy was discontinued. Thus, animals treated with ethosuximide from age p21 to 5 months still had a marked suppression of seizures at age 8 months.
Discussion
These findings suggest that early treatment during development may provide a new strategy for preventing epilepsy in susceptible individuals. If confirmed with other drugs and epilepsy paradigms, the availability of a model in which epileptogenesis can be controlled has important implications both for future basic studies, and human therapeutic trials.
Keywords: Epileptogenesis, Activity-dependent, Prevention, Idiopathic generalized epilepsy, Sodium channels, HCN1
Epilepsy is a chronic disease characterized by recurrent unprovoked seizures. Present treatments are aimed at control of seizures, but do not cure the underlying disease (Duncan et al., 2006). Genetic susceptibility plays an important role in many forms of epilepsy (Berkovic et al., 2006). With improved understanding of the human genome, the potential to predict epilepsy makes primary prevention a realistic goal (Collins, 1999). However, it is not known whether intervening at a critical stage of development can alter the course of epileptogenesis. As a proof-of-principle study, we sought to determine whether early treatment, prior to the development of epilepsy, would alter the course of epilepsy development in an animal model. This goal is in keeping with recently proposed “Benchmarks for epilepsy research” (http://www.ninds.nih.gov/).
Idiopathic generalized epilepsy is a relatively common form of genetic epilepsy, and includes subtypes with predominantly tonic–clonic, myoclonic, or absence seizures (Avoli et al., 2001; Jallon & Latour, 2005). Absence seizures are brief episodes of staring and behavioral unresponsiveness, accompanied by spike-wave discharges (SWD) on electroencephalogram (EEG) (Blumenfeld, 2005a, 2005b). Treatment of absence epilepsy is usually initiated in childhood shortly after the detection of seizures, typically at age 5–6 years, although the onset time of more subtle seizures is not known. Fortunately, most children outgrow the seizures in adolescence, but a substantial minority continue to have seizures into adulthood, and have significant long-term psychosocial sequelae (Wirrell et al., 1996, 1997; Camfield & Camfield, 2002). Wistar albino Glaxo rats of Rijswijk (WAG/Rij) are an established animal model of human absence epilepsy (Van Luijtelaar & Coenen, 1986; Coenen & Van Luijtelaar, 2003). At age 3 months, WAG/Rij rats develop similar episodes of behavioral unresponsiveness accompanied by SWD; however, unlike in children, the rat SWD always continue into adulthood (Coenen & Van Luijtelaar, 2003; Klein et al., 2004; Blumenfeld, 2005b). Ethosuximide, and several other medications, effectively block absence seizures in both humans, and in the WAG/Rij model (Peeters et al., 1988; Coenen et al., 1992).
We initiated antiepileptic therapy in WAG/Rij rats at an early age (p21), prior to the onset of SWD, by providing continuous ethosuximide in their drinking water. Treatment was continued until age 5 months, when SWD are typically well established. We found that this treatment had two interesting effects. First, the treatment blocked changes in the expression of sodium channels and hyperpolarization-activated cation channels (HCN1) seen in epileptic WAG/Rij rats (Klein et al., 2004; Strauss et al., 2004). Second, the treatment markedly suppressed SWD, an effect which persisted for the full 3 months of sequential recordings performed after stopping the medication. This treatment model may for the first time allow the investigation of primary prevention in epileptogenesis, with implications both for future basic studies, and human therapeutic trials.
Materials And Methods
Animals and ethosuximide treatment
All procedures were in full compliance with approved institutional animal care and use protocols. We used female WAG/Rij rats bred at our institution, which originated from the Radiobiological Institute, TNO, in Rijswijk (Reinhold, 1966), and age-matched nonepileptic control female Wistar rats from Charles River Laboratories (Wilmington, MA). These two strains were chosen for the experiments since they are genetically similar, both being Wistar substrains, but the WAG/Rij rats have a much higher rate of SWD than ordinary Wistars. Because even ordinary (unselected) Wistar rats can occasionally show a SWD phenotype (Coenen et al., 1992; Coenen & Van Luijtelaar, 2003), the nonepileptic control rats were screened by EEG and if significant SWD occurred, they were eliminated from the experiment. No selection procedure was used for the WAG/Rij rats. Animals were housed in groups of 2 or 3, and kept on a 12-h light/dark cycle (lights on at 07:00 h) with unlimited access to food and water. Following implants of recording electrodes, animals were single housed to prevent injuries.
Ethosuximide (ESX) was administered orally at a dose of 300 mg/kg/day by adding it to the drinking water beginning at the time of weaning (p21–p23). This dosage and route were selected based on prior work, and on initial pilot experiments. In previous rat studies, EEG recordings showed suppression of SWD shortly after single oral or IP doses of ethosuximide ranging from 12.5 to 200 mg/kg (Micheletti et al., 1985; Vergnes et al., 1985; Peeters et al., 1988; Wahle & Frey, 1990; van Rijn et al., 2004). However, prior work showed that SWD returned within a few hours after single doses (Micheletti et al., 1985; Peeters et al., 1988), and the half-life of ethosuximide is considerably shorter in rats than in humans (Faingold & Browning, 1987; Mifsud et al., 2001). Therefore, we added ethosuximide to the drinking water, which was freely available to the rats, in the hope that this would produce a more stable or nearly continuous delivery of the medication. Based on pilot measurements, we determined that the rats drink approximately 120 cc/kg/day, and drink slightly more (~200 cc/kg/day) in the first 1.5 months. Therefore, ethosuximide 300 mg/kg/day was given using 250 mg/5 ml syrup (Pharmaceutical Associates, Inc. Greenville, SC) by adding 3 cc of syrup per 100 cc H2O for p21 through p45, and 5 cc per 100 cc H2O for p45 onward. In initial pilot experiments, we tried lower doses of ethosuximide (200 mg/kg/day in three animals) but this did not consistently block SWD. Higher doses (400 mg/kg/day in three animals) caused significant lethargy, hair loss and gait ataxia. The dose of 300 mg/kg/day was chosen because it was effective in completely blocking SWD, and was also well tolerated without any side effects of lethargy, ataxia, hair loss, or reduced food intake. All animals were evaluated for these adverse effects three times weekly throughout the experiments, and none were detected with the 300 mg/kg/day dose.
Water bottles were coated on the outside with black paint since the medication is light-sensitive. Medication in the bottles was replaced at least weekly to ensure that therapeutic doses were continuously available.
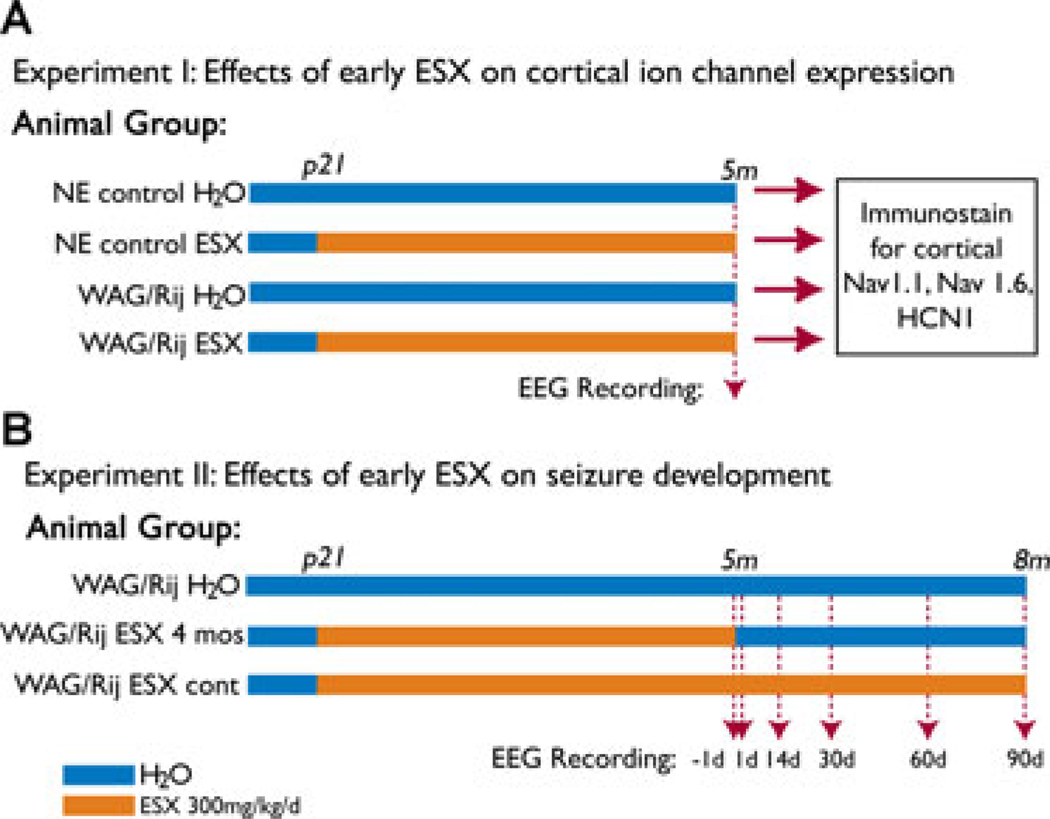
There were four groups of animals in the first experiment, and three groups of animals in the second experiment (Fig. 1). The four groups in Experiment 1 (Fig. 1A) were: nonepileptic (NE) control rats on water (n = 8), NE control rats on ethosuximide 300 mg/kg/day (n = 8), WAG/Rij rats on water (n = 7), and WAG/Rij rats on ethosuximide 300 mg/kg/day (n = 8). In Experiment 1, animals on ethosuximide were treated from weaning (p21) until they were sacrificed at 5 months. The three groups in Experiment 2 (Fig. 1B) were: WAG/Rij rats on water (n = 13), WAG/Rij rats on ethosuximide 300 mg/kg/day for p21 through age 5 months (ESX 4 months group) (n = 11), and WAG/Rij rats on ethosuximide for p21 through age 8 months (ESX continuous group) (n = 13).
Figure 1.
Experimental design for measuring effects of early ethosuximide treatment on ion channel expression (A) and epilepsy (B). (A) Experiment 1: Epileptic WAG/Rij rats and nonepileptic Wistar control rats (NE) were given either normal drinking water (H2O), or ethosuximide (ESX) 300 mg/kg/day from age p21 through age 5 months. EEG was recorded at age 5 months to determine amount of SWD. Animals were then sacrificed for immunocytochemistry to determine expression levels of Nav1.1, Nav1.6, and HCN1 in layer II–III cortical somatosensory neurons, shown previously to have abnormal protein expression levels in epileptic WAG/Rij rats (Klein et al., 2004; Strauss et al., 2004). Number of rats in each group were: NE control H2O (n = 8), NE control ESX (n = 8), WAG/Rij H2O (n = 7), and WAG/Rij ESX (n = 8). (B) Experiment 2: Epileptic WAG/Rij rats were given either normal drinking water (H2O group, n = 13); ethosuximide 300 mg/kg/day from age p21 through age 5 months and then normal drinking water from age 5 to 8 months (ESX 4 month group, n = 11); or ethosuximide 300 mg/kg/day continuously from age p21 through 8 months (ESX continuous group, n = 13). EEG was recorded in the ESX 4 month group, and in age-matched animals from the other two groups 1 day before stopping ethosuximide, and 1, 14, 30, 60, and 90 days after stopping ethosuximide.
Epilepsia © ILAE
Serum ethosuximide levels (National Medical Services; Willow Grove, PA) were measured at the time that animals were sacrificed. Oral ethosuximide 300 mg/kg/day produced therapeutic blood levels (88 ± 10 µg/dl; mean ± S.E.M.), and effective seizure blockade without toxic side effects (see above).
Surgery and recordings
At age 4.75 months all animals were implanted with electrodes for EEG recordings. Under ketamine (100 mg/kg), xylazine (10 mg/kg), and acepromazine (1 mg/kg) anesthesia, we implanted tripolar electrodes (Part # MS333/3-A, Tripolar electrode uncut untwisted 0.005, Pedestal Height: 8 mm, Internal control # 8LMS3333XXXE; Plastics One, Inc., Roanoke, VA, U.S.A.) using a stereotactic frame (David Kopf Instruments, Tujunga, CA, U.S.A.). To provide good electrical contact before wrapping around skull screws, the ends of the recording electrodes were prepared by scraping off all the polyimide insulation and exposing stainless steel wire up to 10 mm from the tip, leaving insulation intact proximally, as verified under the microscope. Level of anesthesia was monitored by respiration, heart rate, glabrous skin perfusion, and response to foot pinch. Small burr holes (using Micro Drill Steel Burrs, 2.3 mm shaft diameter, 44 mm overall length; Item # 19007-14, Fine Science Tools, Foster City, CA, U.S.A.) were made in the skull without disturbing the dura and electrodes were secured to the skull using stainless steel screws (Part # 0-80X1/16, Internal control # 8L010121201F, with shaft length = 1.60 mm, head diameter = 2.50 mm, shaft diameter = 1.57 mm; Plastics One, Inc.). EEG recording electrodes were placed at frontal cortex (AP +2.0, ML +2.0 mm), and parietal cortex (AP −6.0, ML +2.0 mm) and a ground electrode was placed in the midline over the cerebellum. An additional anchoring screw, without electrode, was placed at ML −2.5 mm, equal distance between the coronal suture and lamdoidal suture. Dental acrylic (Cat # 1255710; Henry Schein, Inc., Indianapolis, IN, U.S.A.; Lang Jet Denture Repair Acylic) was used to fix the electrode pedestal in place.
Animals were given a 1 week recovery period after surgery. EEG signals were recorded via commutator (Plastics One, Inc.) using a Grass CP 511 amplifier (Grass-Telefactor, Astro Med, Inc., West Warwick, RI). Band pass frequency filter settings were 1–300 Hz. Signals were digitized at a sampling rate of 1 kHz with an NI USB-6008 A/D converter and LabView 7.1 software (National Instruments, Austin, TX), and analyzed using Spike 2 (Cambridge Electronic Design, Cambridge, UK).
Continuous EEG data were recorded from awake-behaving rats between 10:00 a.m. and 4:00 p.m.. For Experiment 1 (Fig. 1A), recordings were obtained from each animal for 2 h per day over a 3-day period (6 h total per animal), and animals were then sacrificed for histology. For Experiment 2 (Fig. 1B), recordings were obtained from each animal for 3 h per day at the following time intervals in the ESX 4 months group, and age-matched animals in the other two groups: 1 day before stopping ESX; and 1, 14, 30, 60, and 90 days after stopping ESX.
Immunocytochemistry
Rats were anesthetized with ketamine/xylazine (80/5 mg/kg i.p.) and then underwent intracardiac perfusion with 0.01 M phosphate buffer solution (PBS) followed by a 4% solution of cold-buffered paraformaldehyde. Brains were postfixed and cryoprotected in 30% sucrose in PBS, and coronal sections (10 µm) of the cerebral hemispheres were cut. Separate serially consecutive slices with identical preparations were used for Nav1.1, Nav1.6, and HCN1 immunostaining. Slices were mounted onto slides and incubated in blocking solution (5% normal goat serum and 1% bovine serum albumin in PBS) containing 0.1% Triton X-100 and 0.02% sodium azide at room temperature for 30 min. Slides were then incubated with subtype-specific antibodies to either sodium channel α-subunits Nav1.1 (residues 465–481, 1:100 dilution, Alomone, Jerusalem, Israel), Nav1.6 (residues 1,042–1,061, 1:100, Alomone), or HCN1 antibody (rabbit anti-rat, 1:100 dilution, Chemicon, Temecula, CA, U.S.A.) overnight at 4 °C. Slides were washed in PBS and then incubated with goat anti-rabbit IgG-Cy3 (1:2,000, Amersham, Piscataway, NJ, U.S.A.). Immunofluorescence signal was detected using fluorescein illumination (emission wavelength 516–565 nm).
Analysis of immunocytochemistry data
Semiquantitative densitometry of immunostaining signals was performed with a Nikon Eclipse TE300 microscope using IPLab v3.0 Image Processing software (Scanalytics, Inc., Fairfax, VA, U.S.A.). The quantification process was done with the experimenter blinded to the identity of the experimental group. Signal intensities were determined by outlining individual cortical pyramidal neurons, and IPLab integrated densitometry functions were used to calculate mean signal intensities for each cell. Results from identical regions and layers of cortex in WAG/Rij (epileptic) rats were compared to nonepileptic Wistar (control) rats processed in parallel. Immunopositivity was quantified by averaging multiple counts within a defined area (1.9 × 104 µm2) within layers II–III of the S1BF somatosensory cortex, where changes in channel expression have been demonstrated previously in epileptic WAG/Rij rats (Klein et al., 2004). Only neurons with distinct borders whose nuclei fell within the plane of section were analyzed. Approximately 50 neurons were analyzed for each antibody (Nav1.1, Nav1.6, and HCN1) per animal (150 cells total per animal). Analysis of neurons from left versus right somatosensory cortex showed no significant difference in level of expression within each group of rats, so these data were combined. Mean immunofluorescence of neurons with each antibody from each set of animals was compared using one-way ANOVA with post hoc Bonferroni adjustment for multiple comparisons. All statistical analyses were run in SPSS (v. 14, SPSS, Inc., Chicago, IL, U.S.A.). An alpha level of 0.05 was used as a threshold for statistical significance. All data are presented as mean ± S.E.M.
Analysis of EEG data
SWDs were defined as large-amplitude (>2× the background EEG peak-to-peak amplitude) rhythmic 7–8 Hz discharges with typical spike-wave morphology lasting >1.0 s. Intervals containing artifact or slow wave sleep were excluded from the analysis. Start and end times for all SWDs were marked. Number of seizures, and seizure durations were then calculated. Percent time in SWD was determined as (sum of SWD interval durations/ total usable recording time) × 100%. The readers who analyzed the EEG files were blinded to the type of rat and treatment (water, ESX 4 months, or ESX continuous).
Power spectral analysis was performed for marked SWD intervals, as well as for all EEG data included in the above analysis. Power spectra were calculated for all animals and groups using Spike2 software, with scripts provided by Cambridge Electronic Design (Cambridge, U.K.), as well as in house programming in MATLAB 7.3 (MathWorks, Natick, MA, U.S.A.). Bin size for the FFT was 1.024 s.
Statistical analyses were run in SPSS (v. 14, SPSS, Inc.) using one-way ANOVA or MANOVA for repeated measures with Wilks’ Lambda multivariate analysis, followed by post hoc analyses if appropriate. As with the immunocytochemistry data, an alpha level of 0.05 was used as a threshold for statistical significance.
Results
In our first experiment (Fig. 1A), we were interested in determining whether seizure activity may potentiate more seizures through activity-dependent changes in specific molecules. Prior work in the WAG/Rij model has shown several molecular changes in the somatosensory cortex, where seizure activity is most intense (Meeren et al., 2002; Nersesyan et al., 2004), including increased cortical expression of voltage-gated sodium channels (Nav1.1 and 1.6), and reduced expression of HCN1 (Klein et al., 2004; Strauss et al., 2004). To test whether these changes are primary, or secondary to chronic epilepsy, we treated animals with continuous oral ethosuximide 300 mg/kg/day in the drinking water from weaning (p21) through age 5 months, and then stained for sodium channels and HCN1 expression in the somatosensory cortex.
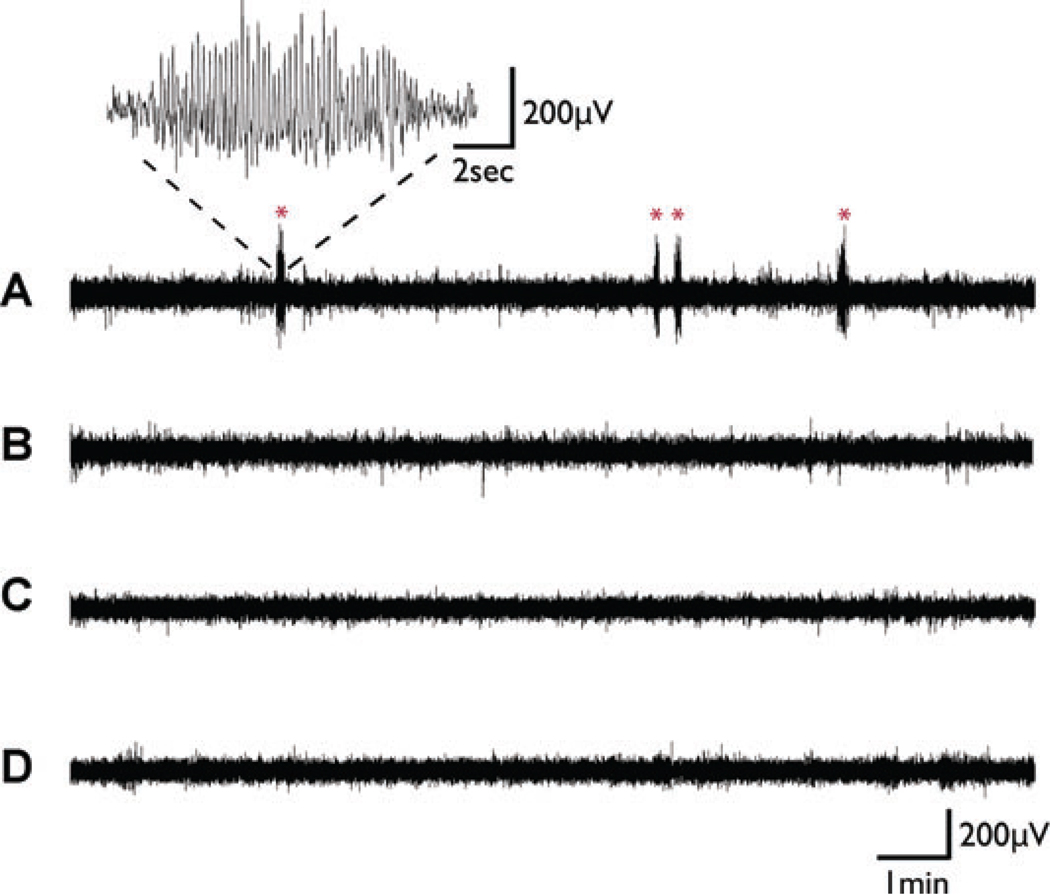
Oral ethosuximide was effective at blocking seizures (Fig. 2). Group data for Experiment 1 demonstrated that in WAG/Rij rats on normal drinking water, percent time in SWD was 1.75 ± 0.29% (mean ± S.E.M.), while on ethosuximide it was 0.28 ± 0.04% (p = 0.0001, two-tailed t-test). In nonepileptic Wistar control rats, percent time in SWD was 0.05 ± 0.04% on normal drinking water, and 0.00 ± 0.00% on ethosuximide.
Figure 2.
Examples of EEG recordings from Experiment 1 (Fig. 1A). Oral ethosuximide blocks SWD in WAG/Rij rats. (A) Recording from WAG/Rij rat on normal drinking water at age 5 months. Several episodes of typical SWD were observed (marked by *), with rhythmic large amplitude 7–8 Hz spike and wave lasting for a few seconds. Inset shows time expansion of one episode. (B) Recording from age-matched WAG/Rij rat on ethosuximide 300 mg/kg/day shows complete blockade of SWD episodes. Recordings from age-matched nonepileptic (NE) control rats on normal drinking water (C) or on ethosuximide 300 mg/kg/day (D) likewise showed a lack of SWD episodes.
Epilepsia © ILAE
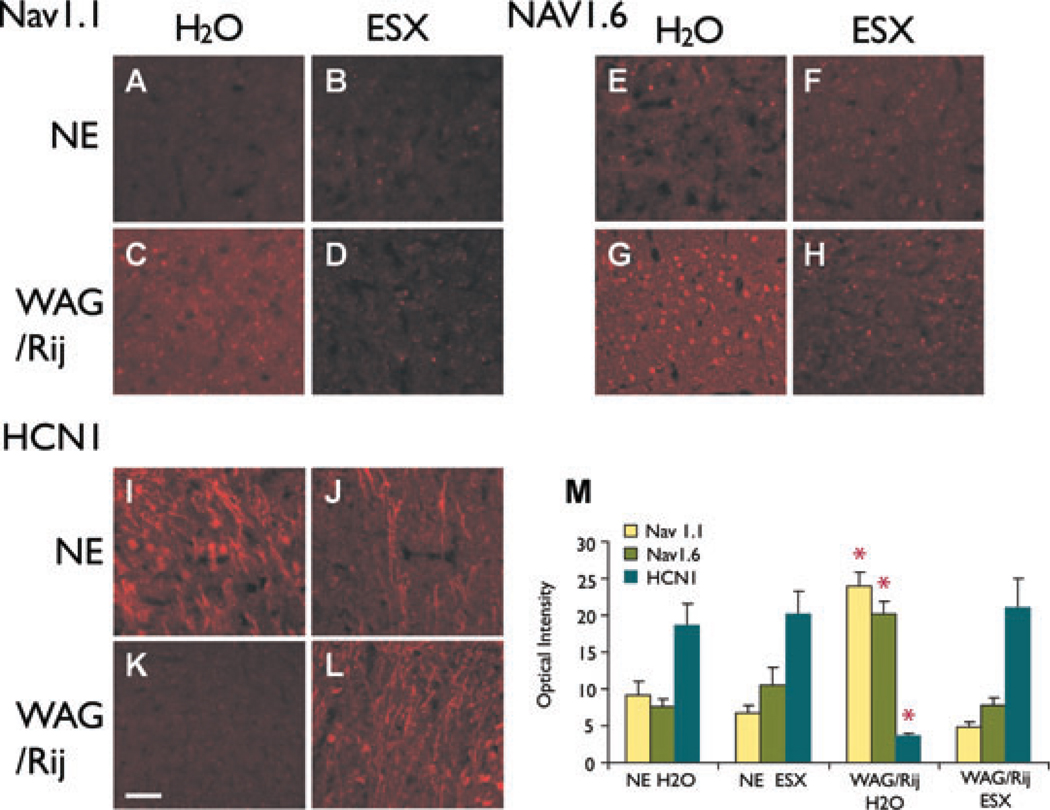
WAG/Rij rats that received no treatment, displayed abnormally increased levels of Nav1.1 and Nav1.6 sodium channel protein and decreased HCN1 at age 5 months (Fig. 3C, G, K, M) compared to controls (Fig. 3A, B, E, F, I, J, M), as described previously (Klein et al., 2004; Strauss et al., 2004). To determine if early treatment would prevent these molecular changes, we administered ethosuximide from p21 through adulthood (age 5 months; Fig. 1A). In WAG/Rij rats treated with early ethosuximide, cortical protein expression levels of Nav1.1, Nav1.6, and HCN1 at age 5 months (Fig. 3D, H, L, M) were indistinguishable from control nonepileptic rats of the same age. Thus, early treatment of epileptic WAG/Rij rats with ethosuximide completely blocked abnormal expression of Nav1.1, Nav1.6, and HCN1. The effects of ethosuximide were not simply a nonspecific reduction in ion channel expression, since changes were seen in opposite directions for sodium channels and for HCN1. In addition, chronic ethosuximide had no effects on ion channel expression when given to nonepileptic control rats compared to normal drinking water (Fig. 3A, B, E, F, I, J).
Figure 3.
Early ethosuximide treatment blocks changes in ion channel expression (Experiment 1). (A–L) Examples of immunocytochemistry for Nav1.1 (A–D), Nav1.6 (E–H), and HCN1 (I–L) in somatosensory cortex layers II–III. (A–D) Nav1.1 is increased in WAG/Rij rats on normal drinking water (H2O) (C). The increase in Nav1.1 is blocked in WAG/Rij rats treated with early ethosuximide (ESX) (D). Nav1.1 expression in treated animals (D) resembles nonepileptic (NE) control animals (A, B). (E–H) Nav1.6 is increased in WAG/Rij rats on H2O (G). The increase in Nav1.6 is blocked by early ESX treatment (H), producing levels that resemble NE controls (E, F). (I–L) HCN1 is decreased in WAG/Rij rats on H2O (K). The decrease in HCN1 is blocked by early ESX treatment (L), producing levels that resemble NE controls (I, J). (M) Quantitative optical density changes in immunocytochemistry for Nav1.1, Nav1.6, and HCN1 in somatosensory cortical layer II–III neurons. Epileptic WAG/Rij rats on H2O have increased expression of Nav1.1 and Nav1.6, and decreased expression of HCN1. Expression levels for these channels were significantly different in the WAG/Rij H2O group compared to all other groups (WAG/Rij H2O group vs. NE H2O, NE ESX and WAG/Rij ESX, respectively, F = 36.75, 38.62, 44.32; p = 1.07 × 10−9, 6.36 × 10−10, 9.09 × 10−11, d.f. = 2, between groups ANOVA for all three channels; *p < 0.01, post hoc between-group comparisons for each individual channel, Bonferroni corrected), and the other three groups did not differ significantly from each other (F = 0.24, p = 0.79, d.f. = 2). Images shown in A–L were equivalently enhanced (i.e., the identical brightness and contrast enhancements were made to each picture) to help demonstrate the differences in a way that would be clear in a printed format. For quantification (M), raw unenhanced images were used. Scale bar (K, applies for all panels) = 50 µm.
Epilepsia © ILAE
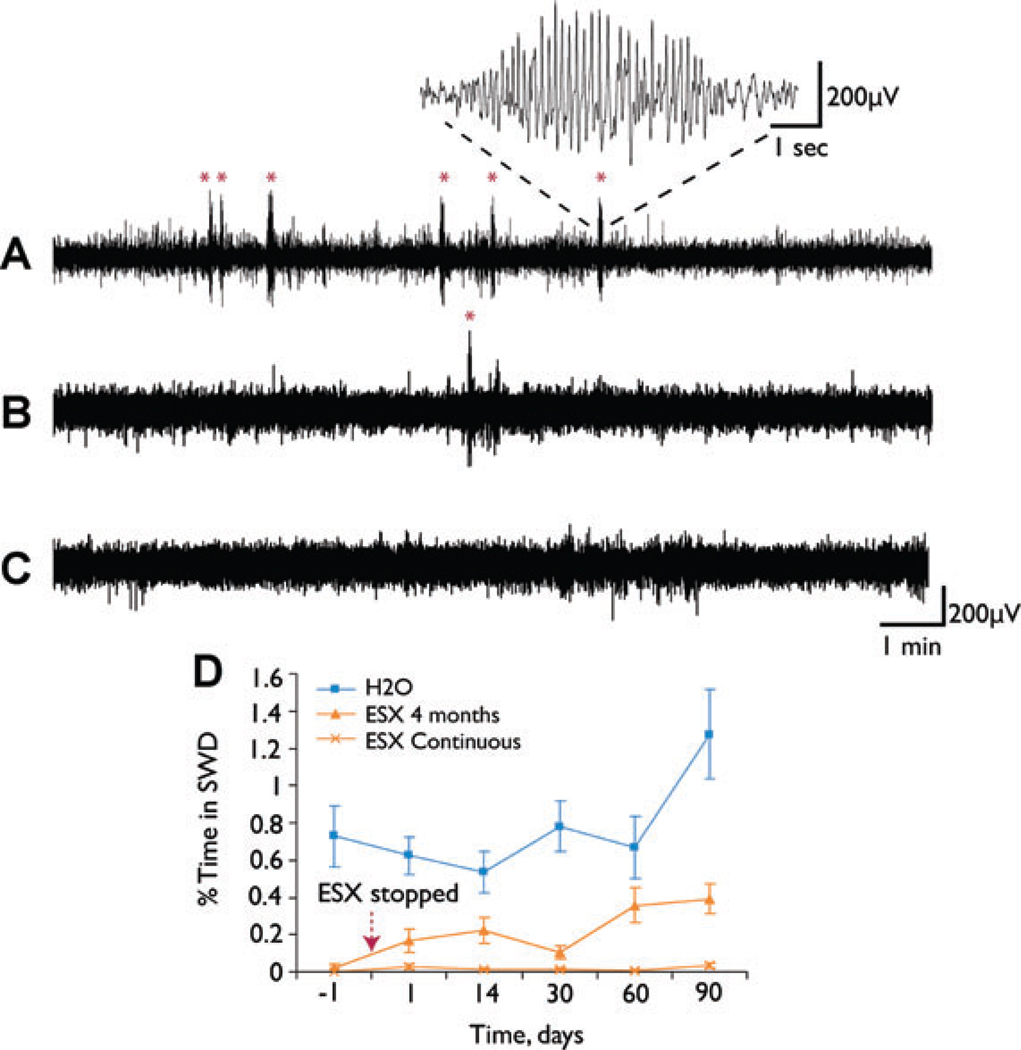
These findings suggest that early intervention could prevent long-term changes in gene expression responsible for epilepsy. The next question was whether early intervention would suppress the epileptic phenotype. To test this, in a second experiment, ethosuximide was again administered from p21 through age 5 months, but then stopped (Fig. 1B). Seizure frequency was measured by EEG at serial time points for 3 months after stopping the medication. We observed a marked and persistent suppression of seizures for the full 3 posttreatment months (Fig. 4). At age 8 months, WAG/Rij rats normally have a robust epileptic phenotype (Fig. 4A). However, when animals were treated early with ethosuximide, for p21 through age 5 months, seizures remained markedly suppressed at age 8 months, even though this was 90 days after stopping the medication (Fig. 4B). At age 8 months, these animals had significantly less SWD than untreated animals, and were not significantly different from animals remaining on continuous ethosuximide (Fig. 4B, C, D) (F = 18.02, p < 0.001, d.f. = 2, between groups ANOVA; p = 0.001, post hoc Bonferroni at 90 days for H2O vs. ESX 4 months groups, p = 0.35 for ESX 4 months vs. ESX continuous groups). The suppression of seizures in the animals treated p21 through 5 months (ESX 4 months group) was also evident when comparing all time points after stopping treatment (days 1 through 90) to the untreated (H2O) animals (Fig. 4D).
Figure 4.
Early ethosuximide (ESX) treatment persistently suppresses the development of SWD (Experiment 2). (A–C) Examples of EEG recordings from WAG/Rij rats. (A) WAG/Rij rat at age 8 months, on normal drinking water throughout development, has frequent SWD (marked by *). Inset shows typical SWD at higher gain. (B) WAG/Rij rat at age 8 months, treated with 300 mg/kg/day ESX in drinking water from p21 through age 5 months, and normal water from age 5 through 8 months (ESX 4 month group). SWD remain suppressed 90 days after stopping treatment (age 8 months). (C) WAG/Rij rat at age 8 months, treated with ESX 300 mg/kg/day from p21 onward (ESX continuous group), shows complete blockade of SWD. (D) Quantification of effects of early ESX treatment on percent time in SWD = (total SWD time/total recording time) × 100%. WAG/Rij rats were given either normal drinking water (■), ethosuximide 300 mg/kg/day from p21 through age 5 months (ESX 4 month group) (▲), or ethosuximide 300 mg/kg/day from p21 onward (ESX continuous group) (X). Recordings were done just before, and at serial time points after the ethosuximide was stopped in the ESX 4 month group, and in age-matched controls (see Fig. 1B). Even after stopping ESX, percent time in SWD remained markedly reduced in the treated WAG/Rij rats (ESX 4 months group) when comparing all time points for days 1 through 90 to WAG/Rij rats on normal H2O (F = 38.18, p = 4.32 × 10−9, d.f. = 2, between groups MANOVA; p < 0.0002, post hoc Games–Howell test for ESX 4 months vs. H2O groups).
Epilepsia © ILAE
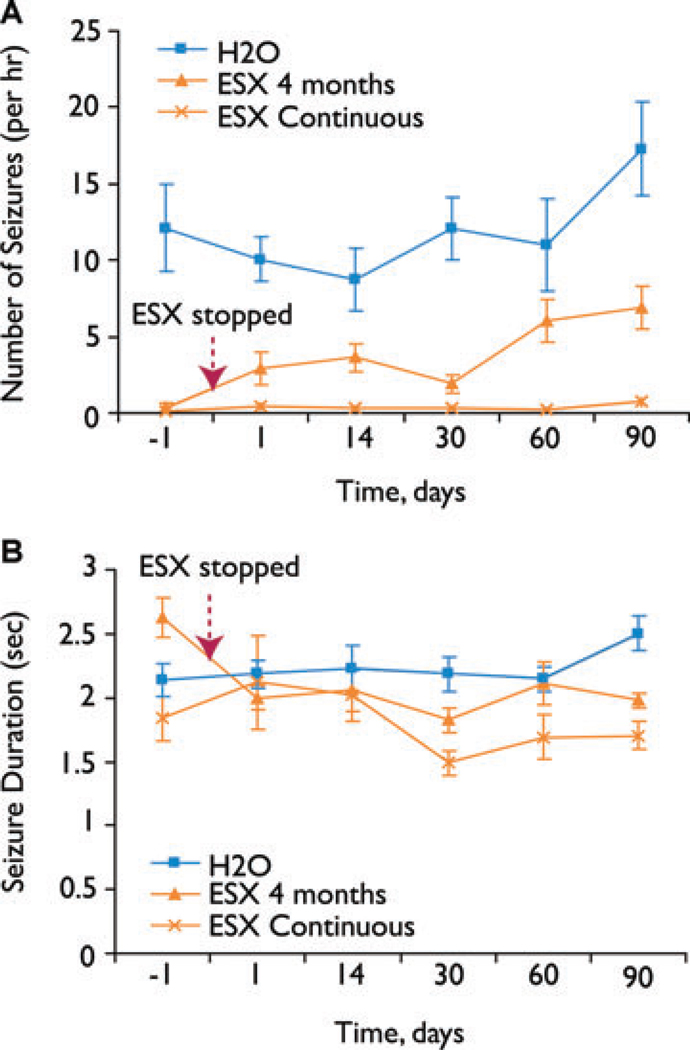
The suppression of seizures was due to a reduction in the number, not in the duration of individual seizures (Fig. 5). Thus, like the suppression of total percent time in SWD (Fig. 4D), the number of seizures was suppressed after early treatment (ESX 4 months group) compared to untreated (H2O) animals (Fig. 5A). SWD duration, on the other hand, was not significantly altered by treatment (Fig. 5B). As an additional check on the results, we also performed a power spectral analysis on the EEG data, which confirmed that SWD frequencies are suppressed in animals treated with early ethosuximide, even after the treatment is stopped (see Supplemental Figure 1, online).
Figure 5.
Early ethosuximide treatment persistently suppresses the number but not the duration of SWD episodes. (A) Effects of early ESX treatment on number of SWD episodes per hour. WAG/Rij rats were given either normal drinking water (■), ethosuximide 300 mg/kg/day from p21 through age 5 months (ESX 4 month group) (▲), or ethosuximide 300 mg/kg/day from p21 onward (ESX continuous group) (X). Recordings were done just before, and at serial time points after the ethosuximide was stopped in the ESX 4 month group, and in age-matched controls (see Fig. 1B). Even after stopping ESX, the number of SWD per hour remained markedly reduced in the treated WAG/Rij rats (ESX 4 months group) when comparing all time points for days 1 through 90 to WAG/Rij rats on normal H2O (F = 42.82, p = 1.20 × 10−9, d.f. = 2, between groups MANOVA; p = 0.0001, post hoc Games–Howell test for ESX 4 months vs. H2O groups). (B) Effects of early ESX treatment on SWD duration. Experimental groups and time points were the same as in (A). Treatment had no significant effect on SWD duration (F = 2.47, p = 0.14, MANOVA). Animals and EEG data for (A) and (B) were the same as in Fig. 4D.
Epilepsia © ILAE
Discussion
Taken together, these results suggest that early treatment can alter the molecular and electrophysiological phenotype in a genetic form of epilepsy. This supports a model in which seizures must occur during a critical stage of development in order for the full epileptic phenotype to emerge. The balance between excitation and inhibition in the brain is normally finely tuned to optimize information flow (Haider et al., 2006). We can speculate that in genetically susceptible individuals, subtle alterations in this balance at an early stage may initiate a self-reinforcing cycle of abnormal activity-dependent plasticity. In nongenetic forms of epilepsy, it has long been appreciated that “seizures beget seizures” (Chen et al., 1999; Morimoto et al., 2004; Ben-Ari & Holmes, 2006). For example, in the kindling model, a small electrical stimulus initially produces no behavioral seizures, but with repeated daily administration, the same small stimulus eventually produces major convulsions (Goddard, 1967; Morimoto et al., 2004). In chronic focal epilepsy, an initial injury can cause repeated epileptiform activity, leading to multiple structural and molecular changes that tend to increase excitability and further contribute to epileptogenesis (Chen et al., 1999; Morimoto et al., 2004; Ben-Ari & Holmes, 2006; Ransom & Blumenfeld, 2007). The current findings imply that “seizures beget seizures” holds for genetic as well as for secondary forms of epilepsy, and demonstrate for the first time the potential to interrupt this cycle through early treatment.
Like in secondary forms of epilepsy, patients with primary generalized epilepsy have chronic changes in the structure and function of the brain. These changes include ultrastructural and neurochemical changes (Woermann et al., 1998; Bernasconi et al., 2003; Duncan, 2005; Fojtikova et al., 2005; Chan et al., 2006; Fojtikova et al., 2006), impaired cognitive performance even in the interictal period (Mirsky & Van Buren, 1965; Fedio & Mirsky, 1969; Duncan, 1988; Levav et al., 2002), and other psychosocial problems (Olsson & Campenhausen, 1993; Wirrell et al., 1997; Pavone et al., 2001; Camfield & Camfield, 2002). It is not known whether these chronic changes are a result of seizures, part of the underlying genetic program in these individuals, or some of both. The availability of models in which the course of generalized epilepsy can be modified by treatment may eventually make it possible to answer this question.
Prior efforts to alter the course of generalized epilepsy by treatment have been limited, mainly because of the long time period needed for these studies, even in animal models. Investigation of a different rat strain and medication showed transiently reduced SWD following treatment, but therapy was briefer, and was stopped before adulthood (Dedeurwaerdere et al., 2005). Similarly, studies in mouse models have not been carried out for sufficient time to determine if there are persistent effects on epileptogenesis (Nahm & Noebels, 1998). Environmental manipulations such as maternal deprivation or neonatal handing can influence the development of SWD, but are not feasible for therapeutic intervention (Schridde et al., 2006). Studies with focal epilepsy have also been limited, perhaps in part because most anticonvulsants do not block focal interictal epileptiform activity (Staley et al., 2005).
The underlying cause of epilepsy in the present genetic rat model remains unknown. We can speculate that since treatment with ethosuximide prevented altered expression of Nav1.1, Nav1.6, and HCN1, changes in expression of these channels are likely secondary to activity-dependent plasticity, and are not the primary cause of epilepsy in WAG/Rij rats. However, once these changes are initiated, they could contribute to enhanced excitability. Previous studies have suggested that activity-dependent increases in voltage-gated sodium channels, and decreases in HCN1 may contribute to enhanced excitability in epilepsy (Lombardo et al., 1996; Bartolomei et al., 1997; Waxman, 2000; Brewster et al., 2002; Bender et al., 2003; Bertram, 2003; Rogawski & Loscher, 2004; Strauss et al., 2004; Yue et al., 2005). It is also possible that in treated animals, reduced expression of Nav1.1 and Nav1.6, and increased HCN1 may produce decreased excitability, and help prevent the emergence of seizures. However, many other molecules and mechanisms almost certainly also play a role in the development of epilepsy and in its prevention.
Caution is appropriate in interpreting the present results, and several limitations should be addressed in future studies. For example, we did not investigate possible effects of the treatment on circadian rhythms or on SWD at different times of day. Continuous 24-h recordings would be necessary to investigate these possible effects. In addition, it will be important to determine the optimum timing and minimum duration of treatment needed, and how long seizure suppression will persist (even beyond age 8 months, as we observed). The WAG/Rij rats differ from typical human absence, since the rats develop epilepsy in early adulthood (age 3 months) and the seizures in rats normally persist throughout adult life. Studies with additional models, and with other medications will be crucial to determine how widely applicable these results are. Since ethosuximide is known to block calcium as well as sodium channels (Coulter et al., 1989; Crunelli & Leresche, 2002), and the observed effects may at least in part be related to activity-dependent changes in channel expression, it will be important to test drugs with other mechanisms of action as well.
Further work is clearly needed to better understand the inhibition of epileptogenesis. The availability of a model in which epileptogenesis can be controlled, provides an opportunity to investigate cellular signaling pathways and physiological mechanisms underlying epilepsy development and its prevention. Ultimately, if this early treatment model is verified with other drugs and other epilepsy paradigms, it may help guide the design of human therapeutic trials to alter the course of epileptogenesis. For treatment to be contemplated before the onset of seizures, increased knowledge of epilepsy genetics will be critical. In addition, these findings heighten the urgency to find improved treatments, which can be administered safely for prolonged periods during early development. The potential for altering the course of epileptogenesis through early treatment described here will hopefully represent an important initial step towards not just treating the symptoms of epilepsy, but curing the disease.
Supplementary Material
Acknowledgments
We thank Dr. Robert Soufer and Dr. Amy Arnsten for helpful comments on the manuscript, and Steven Clifford of CED for Spike2 scripts used in the EEG analysis. This work was supported by NIH R01 NS049307, and by Betsy and Jonathan Blattmachr. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Avoli M, Rogawski MA, Avanzini G. Generalized epileptic disorders: an update. Epilepsia. 2001;42:445–457. doi: 10.1046/j.1528-1157.2001.39800.x. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Gastaldi M, Massacrier A, Planells R, Nicolas S, Cau P. Changes in the mRNAs encoding subtypes I, II and III sodium channel alpha subunits following kainate-induced seizures in rat brain. J Neurocytol. 1997;26:667–678. doi: 10.1023/a:1018549928277. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Holmes GL. Effects of seizures on developmental processes in the immature brain. Lancet Neurol. 2006;5:1055–1063. doi: 10.1016/S1474-4422(06)70626-3. [DOI] [PubMed] [Google Scholar]
- Bender RA, Soleymani SV, Brewster AL, Nguyen ST, Beck H, Mathern GW, Baram TZ. Enhanced expression of a specific hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN) in surviving dentate gyrus granule cells of human and experimental epileptic hippocampus. J Neurosci. 2003;23:6826–6836. doi: 10.1523/JNEUROSCI.23-17-06826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkovic SF, Mulley JC, Scheffer IE, Petrou S. Human epilepsies: interaction of genetic and acquired factors. Trends Neurosci. 2006;29:391–397. doi: 10.1016/j.tins.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Bernasconi A, Bernasconi N, Natsume J, Antel SB, Andermann F, Arnold DL. Magnetic resonance spectroscopy and imaging of the thalamus in idiopathic generalized epilepsy. Brain. 2003;126:2447–2454. doi: 10.1093/brain/awg249. [DOI] [PubMed] [Google Scholar]
- Bertram EH. How epilesy changes sodium channels. Epilepsy Curr. 2003;3:72–73. doi: 10.1046/j.1535-7597.2003.03215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H. Consciousness and epilepsy: why are patients with absence seizures absent? Prog Brain Res. 2005a;150:271–286. doi: 10.1016/S0079-6123(05)50020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H. Cellular and network mechanisms of spike-wave seizures. Epilepsia. 2005b;46 Suppl 9:21–33. doi: 10.1111/j.1528-1167.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- Brewster A, Bender RA, Chen Y, Dube C, Eghbal-Ahmadi M, Baram TZ. Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform- and cell-specific manner. J Neurosci. 2002;22:4591–4599. doi: 10.1523/JNEUROSCI.22-11-04591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camfield P, Camfield C. Epileptic syndromes in childhood: clinical features, outcomes, and treatment. Epilepsia. 2002;43 Suppl 3:27–32. doi: 10.1046/j.1528-1157.43.s.3.3.x. [DOI] [PubMed] [Google Scholar]
- Chan CH, Briellmann RS, Pell GS, Scheffer IE, Abbott DF, Jackson GD. Thalamic atrophy in childhood absence epilepsy. Epilepsia. 2006;47:399–405. doi: 10.1111/j.1528-1167.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- Chen K, Baram TZ, Soltesz I. Febrile seizures in the developing brain result in persistent modification of neuronal excitability in limbic circuits. Nat Med. 1999;5:888–894. doi: 10.1038/11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen AM, Van Luijtelaar EL. Genetic animal models for absence epilepsy: a review of the WAG/Rij strain of rats. Behav Genet. 2003;33:635–655. doi: 10.1023/a:1026179013847. [DOI] [PubMed] [Google Scholar]
- Coenen AM, Drinkenburg WH, Inoue M, van Luijtelaar EL. Genetic models of absence epilepsy, with emphasis on the WAG/Rij strain of rats. Epilepsy Res. 1992;12:75–86. doi: 10.1016/0920-1211(92)90029-s. [DOI] [PubMed] [Google Scholar]
- Collins FS. Shattuck lecture—medical and societal consequences of the Human Genome Project. New Engl J Med. 1999;341:28–37. doi: 10.1056/NEJM199907013410106. [DOI] [PubMed] [Google Scholar]
- Coulter DA, Huguenard JR, Prince DA. Characterization of ethosuximide reduction of low-threshold calcium current in thalamic neurons. Ann Neurol. 1989;25:582–593. doi: 10.1002/ana.410250610. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Leresche N. Block of thalamic T-Type Ca(2+) channels by ethosuximide is not the whole story. Epilepsy Curr. 2002;2:53–56. doi: 10.1046/j.1535-7597.2002.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedeurwaerdere S, Boon P, De Smedt T, Claeys P, Raedt R, Bosman T, Van Hese P, Van Maele G, Vonck K. Chronic levetiracetam treatment early in life decreases epileptiform events in young GAERS, but does not prevent the expression of spike and wave discharges during adulthood. Seizure. 2005;14:403–411. doi: 10.1016/j.seizure.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Duncan CC. Application of event-related brain potentials to the analysis of interictal attention in absence epilepsy. In: Mysoblodsky MS, Mirsky AF, editors. Elements of petit mal epilepsy. New York: Peter Lang; 1988. pp. 341–364. [Google Scholar]
- Duncan JS. Brain imaging in idiopathic generalized epilepsies. Epilepsia. 2005;46 Suppl 9:108–111. doi: 10.1111/j.1528-1167.2005.00321.x. [DOI] [PubMed] [Google Scholar]
- Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367:1087–1100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Browning RA. Mechanisms of anticonvulsant drug action. II. Drugs primarily used for absence epilepsy. Eur J Pediatr. 1987;146:8–14. doi: 10.1007/BF00647274. [DOI] [PubMed] [Google Scholar]
- Fedio P, Mirsky AF. Selective intellectual deficits in children with temporal lobe or centrencephalic epilepsy. Neuropsychologia. 1969;7:287–300. [Google Scholar]
- Fojtikova D, Brazdil M, Horky J, Mikl M, Kuba R, Krupa P, Rektor I. Magnetic resonance spectroscopy of the thalamus in patients with typical absence epilepsy. Epilepsia, ILAE Abstracts. 2005;46 Suppl 6:176. doi: 10.1016/j.seizure.2006.06.007. Abstr p469. [DOI] [PubMed] [Google Scholar]
- Fojtikova D, Brazdil M, Horky J, Mikl M, Kuba R, Krupa P, Rektor I. Magnetic resonance spectroscopy of the thalamus in patients with typical absence epilepsy. Seizure. 2006;15:533–540. doi: 10.1016/j.seizure.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Goddard GV. Development of epileptic seizures through brain stimulation at low intensity. Nature. 1967;214:1020–1021. doi: 10.1038/2141020a0. [DOI] [PubMed] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci. 2006;26:4535–4545. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jallon P, Latour P. Epidemiology of idiopathic generalized epilepsies. Epilepsia. 2005;46 Suppl 9:10–14. doi: 10.1111/j.1528-1167.2005.00309.x. [DOI] [PubMed] [Google Scholar]
- Klein JP, Khera DS, Nersesyan H, Kimchi EY, Waxman SG, Blumenfeld H. Dysregulation of sodium channel expression in cortical neurons in a rodent model of absence epilepsy. Brain Res. 2004;1000:102–109. doi: 10.1016/j.brainres.2003.11.051. [DOI] [PubMed] [Google Scholar]
- Levav M, Mirsky AF, Herault J, Xiong L, Amir N, Andermann E. Familial association of neuropsychological traits in patients with generalized and partial seizure disorders. J Clin Exp Neuropsychol. 2002;24:311–326. doi: 10.1076/jcen.24.3.311.985. [DOI] [PubMed] [Google Scholar]
- Lombardo AJ, Kuzniecky R, Powers RE, Brown GB. Altered brain sodium channel transcript levels in human epilepsy. Brain Res Mol Brain Res. 1996;35:84–90. doi: 10.1016/0169-328x(95)00194-w. [DOI] [PubMed] [Google Scholar]
- Meeren HK, Pijn JP, Van Luijtelaar EL, Coenen AM, Lopes da Silva FH. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci. 2002;22:1480–1495. doi: 10.1523/JNEUROSCI.22-04-01480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheletti G, Vergnes M, Marescaux C, Reis J, Depaulis A, Rumbach L, Warter JM. Antiepileptic drug evaluation in a new animal model: spontaneous petit mal epilepsy in the rat. Arzneimittel-Forschung. 1985;35:483–485. [PubMed] [Google Scholar]
- Mifsud J, Collier PS, Millership JS. The pharmacokinetics of ethosuximide enantiomers in the rat. Biopharm Drug Dispos. 2001;22:83–89. doi: 10.1002/bdd.266. [DOI] [PubMed] [Google Scholar]
- Mirsky AF, Van Buren JM. On the nature of the “absence” in centrencephalic epilepsy: a study of some behavioral, electroencephalographic, and autonomic factors. Electroencephalogr Clin Neurophysiol. 1965;18:334–348. doi: 10.1016/0013-4694(65)90053-2. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Nahm WK, Noebels JL. Nonobligate role of early or sustained expression of immediate-early gene proteins c-fos, c-jun, and Zif/268 in hippocampal mossy fiber sprouting. J Neurosci. 1998;18:9245–9255. doi: 10.1523/JNEUROSCI.18-22-09245.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nersesyan H, Herman P, Erdogan E, Hyder F, Blumenfeld H. Relative changes in cerebral blood flow and neuronal activity in local microdomains during generalized seizures. J Cereb Blood Flow Metab. 2004;24:1057–1068. doi: 10.1097/01.WCB.0000131669.02027.3E. [DOI] [PubMed] [Google Scholar]
- Olsson I, Campenhausen G. Social adjustment in young adults with absence epilepsies. Epilepsia. 1993;34:846–851. doi: 10.1111/j.1528-1157.1993.tb02101.x. [DOI] [PubMed] [Google Scholar]
- Pavone P, Bianchini R, Trifiletti RR, Incorpora G, Pavone A, Parano E. Neuropsychological assessment in children with absence epilepsy [see comment] Neurology. 2001;56:1047–1051. doi: 10.1212/wnl.56.8.1047. [DOI] [PubMed] [Google Scholar]
- Peeters BWMM, Spooren WPJM, van Luijtelaar ELJM, Coenen AML. The WAG/Rij rat model for absence epilepsy: anticonvulsant drug evaluation. Neurosci Res Commun. 1988;2:93–97. [Google Scholar]
- Ransom CB, Blumenfeld H. Acquired epilepsy: cellular and molecular mechanisms. In: Waxman SG, editor. Molecular neurology. Burlington, MA: Elsevier Academic Press; 2007. pp. 347–370. [Google Scholar]
- Reinhold HS. Quantitative evaluation of the radiosensitivity of cells of a transplantable rhabdomyosarcoma in the rat. Eur J Cancer. 1966;2:33–42. doi: 10.1016/0014-2964(66)90087-9. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5:553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- Schridde U, Strauss U, Brauer AU, van Luijtelaar G. Environmental manipulations early in development alter seizure activity, Ih and HCN1 protein expression later in life. Eur J Neurosci. 2006;23:3346–3358. doi: 10.1111/j.1460-9568.2006.04865.x. [DOI] [PubMed] [Google Scholar]
- Staley K, Hellier JL, Dudek FE. Do interictal spikes drive epileptogenesis? Neuroscientist. 2005;11:272–276. doi: 10.1177/1073858405278239. [DOI] [PubMed] [Google Scholar]
- Strauss U, Kole MH, Brauer AU, Pahnke J, Bajorat R, Rolfs A, Nitsch R, Deisz RA. An impaired neocortical Ih is associated with enhanced excitability and absence epilepsy. Eur J Neurosci. 2004;19:3048–3058. doi: 10.1111/j.0953-816X.2004.03392.x. [DOI] [PubMed] [Google Scholar]
- Van Luijtelaar EL, Coenen AM. Two types of electrocortical paroxysms in an inbred strain of rats. Neurosci Lett. 1986;70:393–397. doi: 10.1016/0304-3940(86)90586-0. [DOI] [PubMed] [Google Scholar]
- van Rijn CM, Sun MS, Deckers CL, Edelbroek PM, Keyser A, Renier W, Meinardi H. Effects of the combination of valproate and ethosuximide on spike wave discharges in WAG/Rij rats. Epilepsy Res. 2004;59:181–189. doi: 10.1016/j.eplepsyres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Vergnes M, Marescaux C, Micheletti G, Rumbach L, Warter JM. Blockade of “antiabsence” activity of sodium valproate by THIP in rats with petit mal-like seizures. comparison with ethosuximide. J Neural Transm—General Section. 1985;63:133–141. doi: 10.1007/BF01252613. [DOI] [PubMed] [Google Scholar]
- Wahle H, Frey HH. Development of tolerance to the anticonvulsant effect of valproate but not to ethosuximide in a rat model of absence epilepsy. Eur J Pharmacol. 1990;181:1–8. doi: 10.1016/0014-2999(90)90238-2. [DOI] [PubMed] [Google Scholar]
- Waxman SG. The neuron as a dynamic electrogenic machine: modulation of sodium-channel expression as a basis for functional plasticity in neurons. Philos Trans R Soc Lond—Ser B: Biol Sci. 2000;355:199–213. doi: 10.1098/rstb.2000.0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirrell EC, Camfield CS, Camfield PR, Gordon KE, Dooley JM. Long-term prognosis of typical childhood absence epilepsy: remission or progression to juvenile myoclonic epilepsy [see comment] Neurology. 1996;47:912–918. doi: 10.1212/wnl.47.4.912. [DOI] [PubMed] [Google Scholar]
- Wirrell EC, Camfield CS, Camfield PR, Dooley JM, Gordon KE, Smith B. Long-term psychosocial outcome in typical absence epilepsy. Sometimes a wolf in sheeps’ clothing. Arch Pediatr Adolesc Med. 1997;151:152–158. doi: 10.1001/archpedi.1997.02170390042008. [DOI] [PubMed] [Google Scholar]
- Woermann FG, Sisodiya SM, Free SL, Duncan JS. Quantitative MRI in patients with idiopathic generalized epilepsy. Evidence of widespread cerebral structural changes. Brain. 1998;121:1661–1667. doi: 10.1093/brain/121.9.1661. [DOI] [PubMed] [Google Scholar]
- Yue C, Remy S, Su H, Beck H, Yaari Y. Proximal persistent Na+ channels drive spike afterdepolarizations and associated bursting in adult CA1 pyramidal cells. J Neurosci. 2005;25:9704–9720. doi: 10.1523/JNEUROSCI.1621-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.