Abstract
We present a method called muscle synergy analysis, which can offer clinicians insight into both underlying neural strategies for movement and functional outcomes of muscle activity. Although neural dysfunction is central to many motor deficits, neural activity during movements is not directly measurable. Consequently, the majority of clinical tests focus on evaluating motor outputs at the behavioral and kinematic levels. However, altered behavioral or kinematic outcomes could be the result of multiple distinct neural abnormalities with very different muscle coordination patterns. Because muscle activity reflects motoneuron activity and generates the forces that produce behavioral outcomes, an analysis of muscle activity may provide a better understanding of the functional neural deficits in the impaired nervous system. Unfortunately electromyographic datasets can be large, highly variable, and difficult to interpret, precluding their clinical utility. Computational analyses can be used to extract muscle synergies from such datasets, revealing underlying patterns that may reflect different levels of neural function. These muscle synergies are hypothesized to represent motor modules recruited by the nervous system to flexibly perform biomechanical subtasks necessary for movement. For example, hemiparetic stroke patients exhibit differences in the number of muscle synergies, which may reflect disruptions in descending neural pathways and are correlated to deficits in motor function. Muscle synergy analysis may thus offer the clinician a better view of the neural structure underlying motor behaviors and how they change in motor deficits and rehabilitation. Such information could inform diagnostic tools and evidence-based interventions specifically targeted to a patient’s deficits.
Keywords: diagnosis, electromyography (EMG), muscle synergies, rehabilitation, stroke
INTRODUCTION
Although neural dysfunction is central to many motor deficits, in diagnosis and rehabilitation of movement, clinical tests mainly focus on behavioral or kinematic outcomes.1 Even though such tests are descriptive of the overall motor behavior, they provide little information about the underlying differences between the healthy and impaired nervous system (Figure 1). Moreover, an altered behavioral or kinematic outcome (eg, walking slowly) could be the result of multiple distinct neural abnormalities, and thus it is not a clear indication of what is altered at the neural level. Behavioral tests like sit-to-stand, Timed Up and Go, and the Berg Balance Scale can only offer global and/or descriptive information about the behavior, such as the time for a person to complete or maintain a task.2–4 Kinematic measures from gait analysis and posturography5–8 provide a more detailed and quantitative description of behaviors, but it is difficult to dissociate differences in kinematics due to neural versus musculoskeletal deficits. Furthermore, neural deficits may be masked at the kinematic level by compensatory strategies, as similar movements may be produced through different neuromuscular mechanisms. Forces due to the neural activation of muscles can be evaluated through muscle tone and force production tests during isometric tasks,9,10 however such measures may have limited relevance to dynamic production of movement due to the activation of different neural pathways. Additionally, the same endpoint force and resulting movement can be achieved by many different muscle coordination patterns.11 This concept is illustrated in split-belt treadmill walking, an experimental paradigm in which subjects walk with each leg on a belt moving at a different velocity, inducing gait asymmetries. Both poststroke patients and healthy adults are able to adapt their gait to reestablish the global measure of gait symmetry in which the limbs move in alternation, despite differences in belt speed.12,13 However, gait symmetry in itself is an insufficient measure to distinguish the different kinematic strategies that underlie the adaptation.14 Therefore, the behavioral outcome (ie, gait symmetry) alone is not informative of the range of adaptation strategies, which are likely mediated by different muscle activation patterns – especially when comparing the impaired versus healthy subjects. Because muscle activity represents the output of the nervous system, the examination of muscle activation may reflect differences in the flexibility and adaptability of neural mechanisms in patients with motor disorders, leading to differences in kinetic and kinematic strategies for movement.
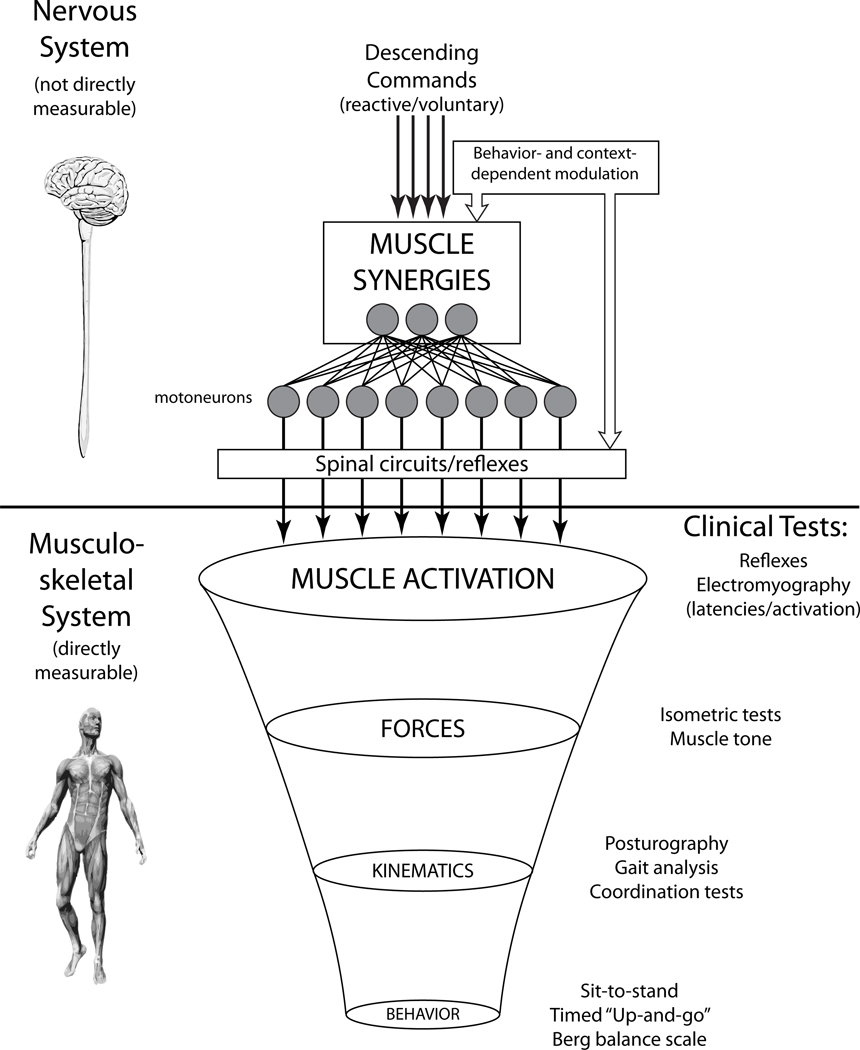
Figure 1.
Conceptual model of motor coordination. Although there are many competing influences and complex circuits in the nervous system, motoneurons represent the motor output of the nervous system and activate the musculature. The existing muscle activation patterns give rise to a smaller set of producible forces, which in turn produces a smaller set of kinematics and, ultimately, an even smaller set of motor behaviors. Clinical motor tests mainly focus on these musculoskeletal outcomes, as the nervous system is not directly measurable. We propose that muscle synergies reflect the underlying neural structure of muscle activation and can be accessed through a variety of neural circuits. The resultant EMG patterns reflect a superposition of muscle synergy activation and local circuits, both of which are subject to behavior and context dependent modulation. Using the muscle synergy hypothesis as a framework, it is possible to identify the functional significance of individual muscle activity and muscle coordination.
Although it is possible to measure muscle activity during movements, it is difficult to interpret the functional implications of electromyographic (EMG) signals during motor tasks, and this difficulty is compounded by the variability of EMG signals, particularly in patient populations. The same motor behavior can be executed by a variety of muscular patterns due to the overabundant musculature of the body (Figure 1). This can result in highly variable EMG patterns between repeated measurements, even in single muscle recordings.15 This variability does not necessarily indicate dysfunction: for example, in postural control, EMG patterns are normally variable due to a range of factors such as attention,16 body configuration,17 and emotional state.18 Furthermore, functional motor behaviors require coordination between different joints, and motor pathologies generally feature abnormal patterns of multi-segmental coordination. Although it may be prudent to assess EMG of multiple muscles spanning body segments, multi-muscle EMG recordings result in very large datasets with even more variability. Thus, simply using expensive and sophisticated equipment to simultaneously record multiple EMG signals may not be sufficient to interpret EMG data in terms of neural function or biomechanical outcomes.
Here we present a method called muscle synergy analysis, which can offer clinicians insight into both underlying neural strategies for movement as well as functional outcomes of muscle activation. The technique reveals the underlying coordination patterns within a highly variable set of muscle activation patterns. Traditionally, the term “synergy” has been used clinically to describe the pathologic co-activation of muscles as seen in stroke19 leading to dysfunctional coordination across joints. However in recent years, the concept of a muscle synergy has reemerged in neuroscience as a proposed mechanism for neural control of normal movement.20–23 Specifically, it has been suggested that muscle synergies in healthy subjects represent functional muscle coordination patterns used to reliably produce motor functions during natural motor behaviors.23,24 This suggests that the existence of muscle synergies in the production of movements may not be restricted to pathology, but may reflect a general principle of neural control. For example, it has been shown that the number of muscle synergies of stroke patients during walking is reduced in a manner that predicts the degree of impairment25 (see below). Muscle synergy analysis may thus allow us to better characterize a patient’s motor deficits and/or compensations and assess the degree of flexibility and adaptability of their motor patterns. Understanding the degree of plasticity in muscle coordination could allow more targeted interventions to maximize a patient’s rehabilitation potential.
MUSCLE SYNERGY CONCEPT
We hypothesize that muscle synergies represent a library of motor subtasks, which the nervous system can flexibly combine to produce complex and natural movements.23,26,27 In our formulation, a muscle synergy defines a consistent ratio of muscle co-activation necessary to coordinate body segments to perform a motor subtask15,28 (Figure 2A). Thus, a single neural command can recruit a muscle synergy to reliably produce the motor subtask. In contrast to the more traditional concept of synergies in stroke,19 in normal function we hypothesize that muscle synergies can be comprised of any number of muscles and individual muscles can belong to multiple muscle synergies. Moreover, multiple synergies can be simultaneously recruited in different proportions, giving rise to a wide range of possible movements. Using component analysis algorithms on EMG data, muscle synergies have been identified in a range of movements and in many different tasks.26,29,30 Thus, muscle synergy analysis is robust enough to reveal an underlying neural organization even when there are competing influences on muscle activity, such as local circuits (cf. Figure 1). Moreover, a muscle synergy organization has been proposed to exist in a variety of contexts and behaviors with different neural control schemes, including central pattern generators (CPGs),31 long-loop reflexes,23 and descending cortical commands,27 suggesting a common neural substrate for producing movement.
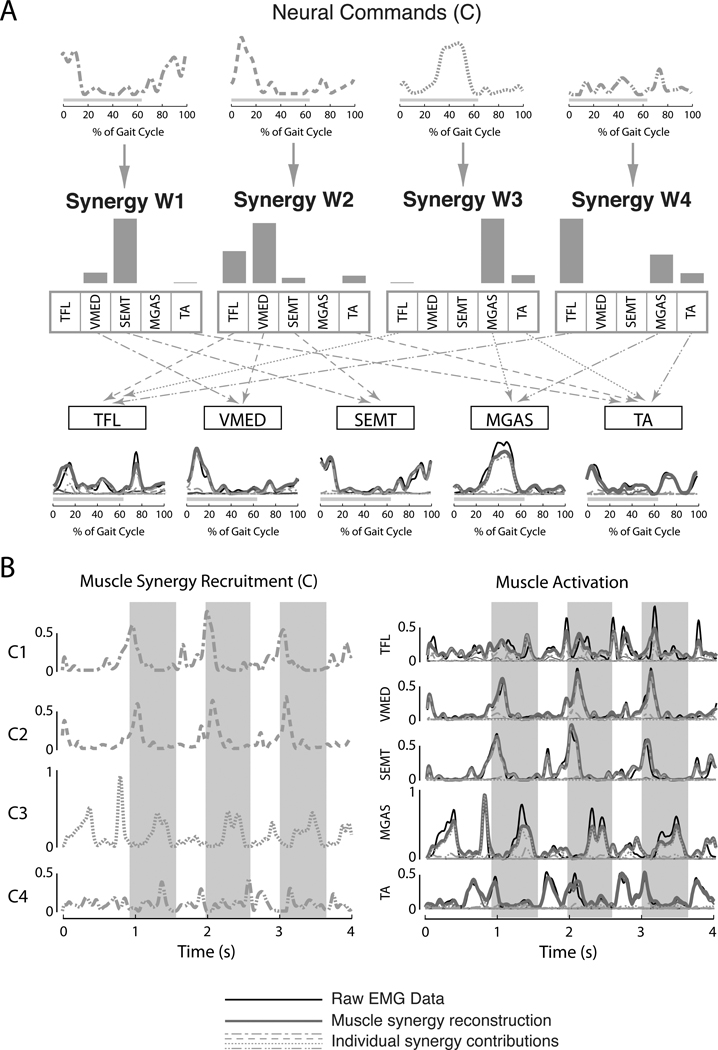
Figure 2.
Modulation of muscle synergies during walking. (A) In our formulation, descending neural commands (C) that vary temporally across the gait cycle recruit muscle synergies (W) that define spatial patterns of muscle activation across multiple muscles, represented by specific ratios of activity. Note that any muscle can belong to multiple muscle synergies. The resulting patterns of muscle activity are due to the net activation of each muscle by all of the muscle synergies recruited. (B) Although muscle synergies tend to be recruited at specific times in the gait cycle related to specific biomechanical functions, the specific modulation of a muscle synergy may vary from step to step during walking (left). This variability in the neural commands to muscle synergies accounts for the observed variability in EMG patterns across step cycles (right). Black lines indicate actual EMG data; grey lines indicate EMG reconstruction with muscle synergies; dotted and dashed lines represent the individual contributions made by each muscle synergy. Grey bars indicate stance phase. TFL = tensor fascia lata; VMED = vastus medialis; SEMT = semitendonosus; MGAS = medial gastrocnemius; TA = tibialis anterior.
For example, during forward walking in healthy subjects, the variability in muscle activation patterns from step to step can be explained by the recruitment of 4 muscle synergies (Figure 2). Each muscle synergy is activated in a particular phase of gait (Figure 2A, top row), and activates multiple muscles (Figure 2A, middle row). Our analysis reveals multi-muscle coordination patterns across the leg and trunk,23,32 but only 5 muscles are shown for simplicity. The resulting muscle activation patterns may be due to the summed effects of simultaneous recruitment of multiple muscle synergies activating a single muscle, as in the tensor fascia lata (Figure 2A, W2-dashed and W4-dot-dashed. Moreover, the variability in the duration, amplitude, and phasing of muscle activation patterns observed from one step to the next can be explained as modulation of the neural commands to the muscle synergies, thus maintaining the relative level of activation across the muscles within a muscle synergy (Figure 2B). This suggests that the variations in muscle activity during walking are not independent but are coupled in muscle synergy patterns. Similar results have been demonstrated during postural responses to perturbations during standing balance control where a fixed set of muscle synergies can be variably recruited to reconstruct widely different muscular responses,33 even across a variety of postural configurations,32,34 producing a continuum of kinematic strategies for balance control.35,36
A muscle synergy organization allows the nervous system to produce consistent biomechanical functions that are shared across motor tasks. For example, walking muscle synergies are recruited at specific times in the gait cycle related to functional variables37,38 such as standing leg stabilization (Figure 2, W2), forward propulsion (W3 and W4), swing initiation (W4), and leg deceleration during swing to stance transitions (W1). Similar relationships to a particular phase of movement requiring different biomechanical functions have been identified in muscle synergies for reaching.39,40 More precise relationships to biomechanical outputs have been found between muscle synergies used during standing balance control, in which recruitment of each muscle synergy is proportional to the production of a particular force vector at the foot.28,41 Moreover, the relationships between muscle synergy activation and endpoint force are preserved across stance widths,23,32 suggesting that the muscle synergies represent a consistent mapping between a desired functional outcome and a low-level muscle activation pattern. Muscle synergy analysis may thus provide important clinical information about the ability of a subject to produce a desired functional outcome.
CLINICAL IMPLICATIONS OF MUSCLE SYNERGY ANALYSIS
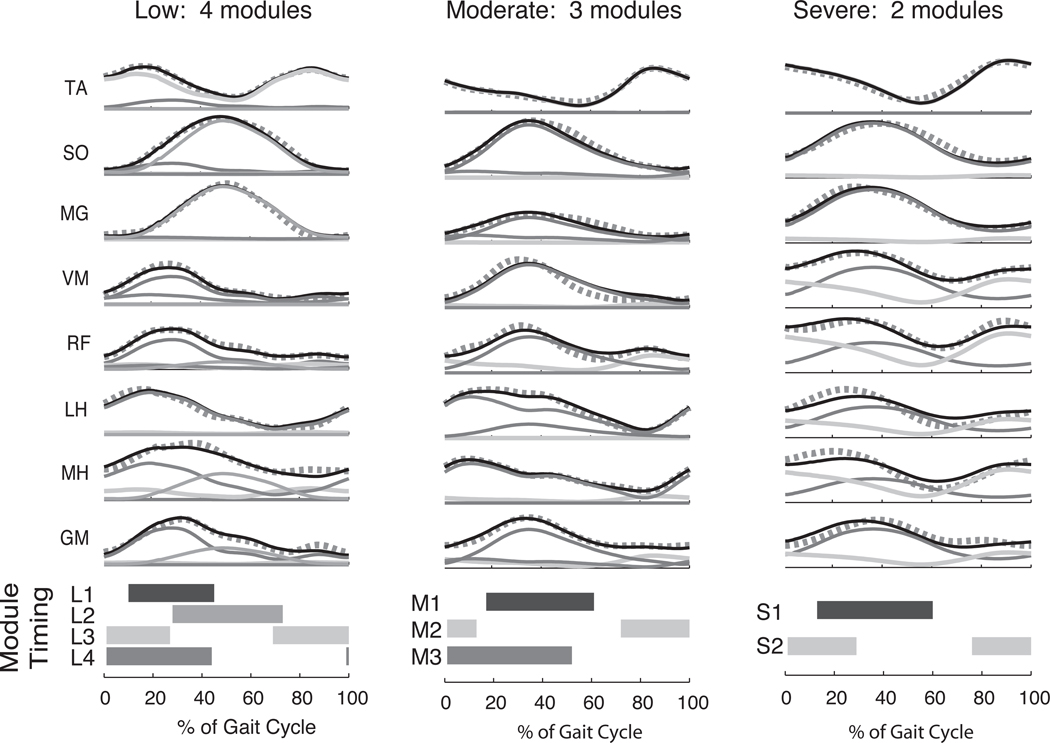
When applied to muscle activation patterns in hemiparetic stroke patients, muscle synergy analysis reveals differences in the number of muscle synergies, which reflect disruptions in descending neural pathways and are correlated to motor deficits in motor function.25 In both healthy subjects and the nonparetic limb of stroke subjects, an average of 4 muscle synergies were recruited during forward walking across a range of speeds. In contrast, the number of muscle synergies identified in the paretic limb varied between 2 and 4 across subjects (n = 55) depending on the degree of impairment, as indicated by their walking speed and gait asymmetry (Figure 3). The structure of muscle synergies was remarkably consistent across both healthy and impaired limbs; when fewer muscle synergies were observed, the resulting muscle synergies resembled merged versions of muscle synergies found in the nonparetic limbs. The resulting muscle activation patterns were thus progressively less complex as the number of muscle synergies decreased and the degree of impairment increased (Figure 3). Thus, the muscle synergies used in more impaired subjects were recruited more broadly through the gait cycle. The inability to independently recruit muscle synergies with distinct biomechanical functions resulted in slower walking speeds, reduced propulsion, and increased gait asymmetry as the number of muscle synergies decreased. These findings are consistent with the idea that descending neural commands to the spinal cord are impaired in stroke, resulting in decreased muscular independence42 and co-contraction of large muscle groups.10,43,44 The muscle synergy analysis suggests that the observed changes in muscle activation patterns are a result of recruiting a reduced number of muscle synergies compared to healthy subjects.45,46
Figure 3.
Decreased complexity of locomotor muscle coordination patterns in hemiparetic stroke subjects. As the level of gait impairment worsens (as determined by walking speed and gait asymmetry), muscle activation patterns in the affected limb become more abnormal (black lines). Muscle synergy analysis reveals that fewer muscle synergies (modules) are needed to reconstruct the muscle activity during locomotion. Thin solid lines indicate individual contributions of muscle synergies, and thick dotted lines indicate muscle synergy reconstructions. Horizontal bars indicate where in the gait cycle each muscle synergy is most actively recruited (defined as >50% of mean activity). When 4 muscle synergies are available, they are activated in a phasic pattern typical of normal walking (left column, shaded bars). However, as the number of muscle synergies decreases, they become active for a greater proportion of the gait cycle (middle and right column). TA = tibialis anterior; SO = soleus; MG = medial gastrocnemius; VM = vastus medialis; RF = rectus femoris; MH = medial hamstrings; LH = lateral hamstrings; GM = gluteus medius. Adapted, with permission, from Clark DJ, et al. Merging of healthy motor modules predicts reduced locomotor performance and muscle J Neurophysiol. 2010;103(2):844–857. Copyright 2010 by the American Physiological Society.
Muscle synergy analysis may thus be a useful metric for motor assessment, as changes in the number, structure, and recruitment of muscle synergies may be able to discriminate among a variety of pathological changes in the nervous system. Changes in muscle synergy number would affect the number of independent motor subtasks that can be independently recruited. For example, the merged muscle synergies seen in stroke patients25 may be the result of reduced corticospinal drive, compromising the ability of the nervous system to recruit spinal locomotor muscle synergies in the paretic limb.47 Consequently, stroke patients may rely more heavily on alternative pathways (eg, reticulospinal, bulbospinal) that could recruit the same spinal muscle synergies but with less individuation, causing abnormal joint and torque patterns.43,48 Depending on the task, muscle synergies have been hypothesized to be encoded at different levels of the central nervous system, including motor cortex for grasping,27,40 brainstem for postural control,23,34 and spinal cord for locomotion.31 Lesions to neural structures encoding the muscle synergies could result in a reduced number of muscle synergies available for a given task or changes in muscle synergy structure. Changes in muscle synergy structure would affect the muscle coordination patterns themselves and could reflect changes in neural connectivity or excitability,49,50 such as in stroke, spinal injury, multiple sclerosis, or traumatic brain injury. Even if the number and structure of muscle synergies remains intact, changes in muscle synergy recruitment could result in abnormal muscle patterns by affecting the timing and strength of normal motor subtasks. It is possible that changes in muscle synergy recruitment could be seen in motor disorders such as writer’s cramp, a task-specific focal hand dystonia. These patients can produce normal and complex hand postures in nonwriting tasks, suggesting that they have access to a full library of muscle synergies with normal structure; however, abnormal contractions during writing may result from decreased surround inhibition at the level of motor cortex,51,52 - which would cause abnormal recruitment of muscle synergies that are typically silent during writing. In general, the number, structure, and recruitment of muscle synergies can indicate whether motor subtasks are accessible, functional, or able to be appropriately modulated, respectively. This information may be used for classifying differences across patients within and across pathologies that would more precisely describe the nature of impairment and better inform rehabilitation or treatment decisions.
We further propose that the number of muscle synergies may more generally reflect motor skill level in healthy subjects, in which differences in the number and structure of muscle synergies have been identified in both walking and balance tasks.23,25 Across healthy subjects, many of the identified muscle synergies have similar structures for performing similar functions necessary for balance and locomotion. However, in some cases, muscle synergies with different muscular patterns but similar functional outcomes can be identified in a subpopulation. During balance control, some subjects use a knee-bending strategy, recruiting a muscle synergy specific to that strategy that is not identified in other subjects.23 It is possible that new muscle synergies are formed during motor skill acquisition. For example, musicians have great muscular independence,53 and patterns of joint coordination elicited through transcranial magnetic stimulation (TMS) of the motor cortex of musicians cannot be reproduced in patterns of joint coordination elicited by TMS stimulation of motor cortex in nonmusicians.54 These differences in muscle coordination are consistent with white matter changes seen in musicians with extensive training.55 Other skilled populations, such as dancers56 and tai chi practitioners,57 also exhibit fine motor control that improves with practice. This motor independence is likely to represent an increase of muscle synergies available to these groups. As motor training for as little as 6 weeks can induce changes in white matter,58 motor training may encourage the development of new muscle synergies for new tasks, change the structure of existing muscle synergies, or change the manner in which existing muscle synergies are recruited.
Examining whether muscle synergies change following rehabilitation may provide a novel assessment of interventions that improve patients’ motor function by inducing plasticity at the neural level. Changes in muscle synergy number, structure, and recruitment may help elucidate why different interventions work for certain patients and not for others. For example, 50% of fallers significantly reduce their fall risk after balance training,59 but there is currently no metric to determine which subjects benefit from the intervention. Muscle synergy analysis could be useful in comparing different therapies and evaluating whether subjects have gained motor functions that generalize to activities of daily living. By regularly assessing a patient’s muscle synergy profile, it may be possible to identify a patient’s functional deficit, track rehabilitation results, and adjust treatments. In conclusion, much like current approaches in genetics and molecular biology, a patient’s muscle synergy profile could possibly allow clinicians to more effectively treat motor dysfunctions by organizing patients into subclasses and tailoring the treatment to the specific patient’s deficit.
REFERENCES
- 1.Horak F. Clinical Assessment of Balance Disorders. Gait & Posture. 1997;6:76–84. [Google Scholar]
- 2.Berg K, Wood-Dauphinee S, Williams JI. The Balance Scale: reliability assessment with elderly residents and patients with an acute stroke. Scand J Rehabil Med. 1995 Mar;27(1):27–36. [PubMed] [Google Scholar]
- 3.Horak FB, Henry SM, Shumway-Cook A. Postural perturbations: new insights for treatment of balance disorders. Phys Ther. 1997 May;77(5):517–533. doi: 10.1093/ptj/77.5.517. [DOI] [PubMed] [Google Scholar]
- 4.Tinetti ME. Clinical practice. Preventing falls in elderly persons. N Engl J Med. 2003 Jan 2;348(1):42–49. doi: 10.1056/NEJMcp020719. [DOI] [PubMed] [Google Scholar]
- 5.Norris JA, Marsh AP, Smith IJ, Kohut RI, Miller ME. Ability of static and statistical mechanics posturographic measures to distinguish between age and fall risk. J Biomech. 2005 Jun;38(6):1263–1272. doi: 10.1016/j.jbiomech.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Berg K, Norman KE. Functional assessment of balance and gait. Clin Geriatr Med. 1996 Nov;12(4):705–723. [PubMed] [Google Scholar]
- 7.Camicioli R, Panzer VP, Kaye J. Balance in the healthy elderly: posturography and clinical assessment. Arch Neurol. 1997 Aug;54(8):976–981. doi: 10.1001/archneur.1997.00550200040008. [DOI] [PubMed] [Google Scholar]
- 8.Gill J, Allum JH, Carpenter MG, et al. Trunk sway measures of postural stability during clinical balance tests: effects of age. J Gerontol A Biol Sci Med Sci. 2001 Jul;56(7):M438–M447. doi: 10.1093/gerona/56.7.m438. [DOI] [PubMed] [Google Scholar]
- 9.Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002 Sep;16(3):232–240. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- 10.Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995 Apr;118(Pt 2):495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein N. The Coordination and Regulation of Movements. New York: Pergamon Press; 1967. [Google Scholar]
- 12.Reisman DS, McLean H, Bastian AJ. Split-belt treadmill training poststroke: a case study. J Neurol Phys Ther. 2010 Dec;34(4):202–207. doi: 10.1097/NPT.0b013e3181fd5eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain. 2007 Jul;130(Pt 7):1861–1872. doi: 10.1093/brain/awm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malone LA, Bastian AJ. Thinking about walking: effects of conscious correction versus distraction on locomotor adaptation. J Neurophysiol. 2010 Apr;103(4):1954–1962. doi: 10.1152/jn.00832.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ting LH. Dimensional reduction in sensorimotor systems: a framework for understanding muscle coordination of posture. Prog Brain Res. 2007;165:299–321. doi: 10.1016/S0079-6123(06)65019-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002 Aug;16(1):1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- 17.Horak F, Moore SP. The effect of prior leaning on human postural responses. Gait Posture. 1993;1:203–210. [Google Scholar]
- 18.Hillman CH, Rosengren KS, Smith DP. Emotion and motivated behavior: postural adjustments to affective picture viewing. Biol Psychol. 2004 Mar;66(1):51–62. doi: 10.1016/j.biopsycho.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Bourbonnais D, Vandennoven S, Carey KM, Rymer WZ. Abnormal Spatial Patterns of Elbow Muscle Activation in Hemiparetic Human-Subjects. Brain. 1989 FEB;112:85–102. doi: 10.1093/brain/112.1.85. [DOI] [PubMed] [Google Scholar]
- 20.Tresch MC, Saltiel P, Bizzi E. The construction of movement by the spinal cord. Nat Neurosci. 1999 FEB;2(2):162–167. doi: 10.1038/5721. [DOI] [PubMed] [Google Scholar]
- 21.Ting LH, McKay JL. Neuromechanics of muscle synergies for posture and movement. Curr Opin Neurobiol. 2007 Dec;17(6):622–628. doi: 10.1016/j.conb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saltiel P, Wyler-Duda K, D'Avella A, Tresch MC, Bizzi E. Muscle synergies encoded within the spinal cord: evidence from focal intraspinal NMDA iontophoresis in the frog. J Neurophysiol. 2001 Feb;85(2):605–619. doi: 10.1152/jn.2001.85.2.605. [DOI] [PubMed] [Google Scholar]
- 23.Torres-Oviedo G, Ting LH. Muscle synergies characterizing human postural responses. J Neurophysiol. 2007 Oct;98(4):2144–2156. doi: 10.1152/jn.01360.2006. [DOI] [PubMed] [Google Scholar]
- 24.Ivanenko YP, Cappellini G, Dominici N, Poppele RE, Lacquaniti F. Coordination of locomotion with voluntary movements in humans. J Neurosci. 2005 Aug 3;25(31):7238–7253. doi: 10.1523/JNEUROSCI.1327-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol. 2010 Feb;103(2):844–857. doi: 10.1152/jn.00825.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.d'Avella A, Bizzi E. Shared and specific muscle synergies in natural motor behaviors. Proc Natl Acad Sci U S A. 2005 Feb 22;102(8):3076–3081. doi: 10.1073/pnas.0500199102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Overduin SA, d'Avella A, Roh J, Bizzi E. Modulation of Muscle Synergy Recruitment in Primate Grasping. J. Neurosci. 2008 January 23;28(4):880–892. doi: 10.1523/JNEUROSCI.2869-07.2008. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ting LH, Macpherson JM. A limited set of muscle synergies for force control during a postural task. J Neurophysiol. 2005 Jan;93(1):609–613. doi: 10.1152/jn.00681.2004. [DOI] [PubMed] [Google Scholar]
- 29.Hart CB, Giszter SF. Modular Premotor Drives and Unit Bursts as Primitives for Frog Motor Behaviors. J. Neurosci. 2004 June 2;24(22):5269–5282. doi: 10.1523/JNEUROSCI.5626-03.2004. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheung VC, d'Avella A, Tresch MC, Bizzi E. Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviors. J Neurosci. 2005 Jul 6;25(27):6419–6434. doi: 10.1523/JNEUROSCI.4904-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drew T, Kalaska J, Krouchev N. Muscle synergies during locomotion in the cat: a model for motor cortex control. J Physiol. 2008 Mar 1;586(5):1239–1245. doi: 10.1113/jphysiol.2007.146605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres-Oviedo G, Ting LH. Subject-specific muscle synergies in human balance control are consistent across different biomechanical contexts. J Neurophysiol. 2010 Jun;103(6):3084–3098. doi: 10.1152/jn.00960.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nashner LM. Adapting reflexes controlling the human posture. Exp Brain Res. 1976 Aug 27;26(1):59–72. doi: 10.1007/BF00235249. [DOI] [PubMed] [Google Scholar]
- 34.Torres-Oviedo G, Macpherson JM, Ting LH. Muscle synergy organization is robust across a variety of postural perturbations. J Neurophysiol. 2006 Sep;96(3):1530–1546. doi: 10.1152/jn.00810.2005. [DOI] [PubMed] [Google Scholar]
- 35.Creath R, Kiemel T, Horak F, Peterka R, Jeka J. A unified view of quiet and perturbed stance: simultaneous co-existing excitable modes. Neurosci Lett. 2005 Mar 29;377(2):75–80. doi: 10.1016/j.neulet.2004.11.071. [DOI] [PubMed] [Google Scholar]
- 36.Runge CF, Shupert CL, Horak FB, Zajac FE. Ankle and hip postural strategies defined by joint torques. Gait Posture. 1999 Oct;10(2):161–170. doi: 10.1016/s0966-6362(99)00032-6. [DOI] [PubMed] [Google Scholar]
- 37.Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F. Motor patterns in human walking and running. Journal of Neurophysiology. 2006 Jun;95(6):3426–3437. doi: 10.1152/jn.00081.2006. [DOI] [PubMed] [Google Scholar]
- 38.Ivanenko YP, Poppele RE, Lacquaniti E. Five basic muscle activation patterns account for muscle activity during human locomotion. Journal of Physiology-London. 2004 APR 1;556(1):267–282. doi: 10.1113/jphysiol.2003.057174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yakovenko S, Krouchev N, Drew T. Sequential activation of motor cortical neurons contributes to intralimb coordination during reaching in the cat by modulating muscle synergies. J Neurophysiol. 2011 Jan;105(1):388–409. doi: 10.1152/jn.00469.2010. [DOI] [PubMed] [Google Scholar]
- 40.d'Avella A, Fernandez L, Portone A, Lacquaniti F. Modulation of phasic and tonic muscle synergies with reaching direction and speed. J Neurophysiol. 2008 Sep;100(3):1433–1454. doi: 10.1152/jn.01377.2007. [DOI] [PubMed] [Google Scholar]
- 41.McKay JL, Ting LH. Functional muscle synergies constrain force production during postural tasks. J Biomech. 2008;41(2):299–306. doi: 10.1016/j.jbiomech.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brunnstrom S. Movement Therapy in Hemiplegia: A Neurophysiological Approach. Hagerstown: Harper and Row; 1970. [Google Scholar]
- 43.Dewald JP, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve. 2001 Feb;24(2):273–283. doi: 10.1002/1097-4598(200102)24:2<273::aid-mus130>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 44.Gowland C, deBruin H, Basmajian JV, Plews N, Burcea I. Agonist and antagonist activity during voluntary upper-limb movement in patients with stroke. Physical Therapy. 1992 Sep 1;72(9):624–633. doi: 10.1093/ptj/72.9.624. [DOI] [PubMed] [Google Scholar]
- 45.Raasch CC, Zajac FE. Locomotor strategy for pedaling: Muscle groups and biomechanical functions. Journal of Neurophysiology. 1999 AUG;82(2):515–525. doi: 10.1152/jn.1999.82.2.515. [DOI] [PubMed] [Google Scholar]
- 46.Kautz SA, Brown DA. Relationships between timing of muscle excitation and impaired motor performance during cyclical lower extremity movement in post-stroke hemiplegia. Brain. 1998 Mar;121(Pt 3):515–526. doi: 10.1093/brain/121.3.515. [DOI] [PubMed] [Google Scholar]
- 47.Nielsen JB, Brittain JS, Halliday DM, Marchand-Pauvert V, Mazevet D, Conway BA. Reduction of common motoneuronal drive on the affected side during walking in hemiplegic stroke patients. Clin Neurophysiol. 2008 Dec;119(12):2813–2818. doi: 10.1016/j.clinph.2008.07.283. [DOI] [PubMed] [Google Scholar]
- 48.Lum P, Burgar CG, Shor PC. Evidence for strength imbalances as a significant contributor to abnormal synergies in hemiparetic subjects. Muscle & Nerve. 2003;27(2):211–221. doi: 10.1002/mus.10305. [DOI] [PubMed] [Google Scholar]
- 49.Dietz V, Berger W. Interlimb coordination of posture in patients with spastic paresis. Impaired function of spinal reflexes. Brain. 1984 Sep;107(Pt 3):965–978. doi: 10.1093/brain/107.3.965. [DOI] [PubMed] [Google Scholar]
- 50.Dietz V, Sinkjaer T. Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol. 2007 Aug;6(8):725–733. doi: 10.1016/S1474-4422(07)70193-X. [DOI] [PubMed] [Google Scholar]
- 51.Sohn YH, Hallett M. Disturbed surround inhibition in focal hand dystonia. Ann Neurol. 2004 Oct;56(4):595–599. doi: 10.1002/ana.20270. [DOI] [PubMed] [Google Scholar]
- 52.Sitburana O, Jankovic J. Focal hand dystonia, mirror dystonia and motor overflow. J Neurol Sci. 2008 Mar 15;266(1–2):31–33. doi: 10.1016/j.jns.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 53.Chen J, Woollacott M, Pologe S. Accuracy and underlying mechanisms of shifting movements in cellists. Exp Brain Res. 2006 Oct;174(3):467–476. doi: 10.1007/s00221-006-0483-x. [DOI] [PubMed] [Google Scholar]
- 54.Gentner R, Gorges S, Weise D, aufm Kampe K, Buttmann M, Classen J. Encoding of motor skill in the corticomuscular system of musicians. Curr Biol. 2010 Oct 26;20(20):1869–1874. doi: 10.1016/j.cub.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 55.Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005 Sep;8(9):1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- 56.Gerbino PG, Griffin ED, Zurakowski D. Comparison of standing balance between female collegiate dancers and soccer players. Gait Posture. 2007 Oct;26(4):501–507. doi: 10.1016/j.gaitpost.2006.11.205. [DOI] [PubMed] [Google Scholar]
- 57.Tsang WW, Hui-Chan CW. Comparison of muscle torque, balance, and confidence in older tai chi and healthy adults. Med Sci Sports Exerc. 2005 Feb;37(2):280–289. doi: 10.1249/01.mss.0000152735.06282.58. [DOI] [PubMed] [Google Scholar]
- 58.Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009 Nov;12(11):1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolf SL, Barnhart HX, Kutner NG, McNeely E, Coogler C, Xu T. Reducing frailty and falls in older persons: an investigation of Tai Chi and computerized balance training. Atlanta FICSIT Group. Frailty and Injuries: Cooperative Studies of Intervention Techniques. J Am Geriatr Soc. 1996 May;44(5):489–497. doi: 10.1111/j.1532-5415.1996.tb01432.x. [DOI] [PubMed] [Google Scholar]