Abstract
Malignant mesothelioma (MM) is a neoplasm arising from mesothelial cells lining the pleural, peritoneal, and pericardial cavities. Over 20 million people in the US are at risk of developing MM due to asbestos exposure. MM mortality rates are estimated to increase by 5-10% per year in most industrialized countries until about 2020. The incidence of MM in men has continued to rise during the past 50 years, while the incidence in women appears largely unchanged. It is estimated that about 50-80% of pleural MM in men and 20-30% in women developed in individuals whose history indicates asbestos exposure(s) above that expected from most background settings. While rare for women, about 30% of peritoneal mesothelioma in men has been associated with exposure to asbestos. Erionite is a potent carcinogenic mineral fiber capable of causing both pleural and peritoneal MM. Since erionite is considerably less widespread than asbestos, the number of MM cases associated with erionite exposure is smaller. Asbestos induces DNA alterations mostly by inducing mesothelial cells and reactive macrophages to secrete mutagenic oxygen and nitrogen species. In addition, asbestos carcinogenesis is linked to the chronic inflammatory process caused by the deposition of a sufficient number of asbestos fibers and the consequent release of pro-inflammatory molecules, especially HMGB-1, the master switch that starts the inflammatory process, and TNF-alpha by macrophages and mesothelial cells. Genetic predisposition, radiation exposure and viral infection are co-factors that can alone or together with asbestos and erionite cause MM.
Keywords: Mesothelioma, Genetics, Asbestos, Erionite, SV40
INTRODUCTION
Malignant mesothelioma (MM) is a tumor of the lining of the lung and chest cavity (pleura) or lining(s) of the abdomen (peritoneum) that is typically related to exposure to mineral fibers such as asbestos and erionite. The duration, quality and intensity of the exposure(s) are important variables in any mineral fiber related disease. Asbestos refers to a family of silicate minerals divided into two major groups: the serpentine form of asbestos- chrysotile and the amphibole forms (crocidolite, anthophyllite, actinolite amosite, and tremolite) (Carbone et al., 2002). Erionite is a naturally occurring fibrous mineral that belongs to a group of minerals called zeolites. Asbestos has been shown to cause asbestosis, pleural fibrosis/plaques, lung and laryngeal cancer, and particularly MM (Carbone et al., 2002). Erionite, on the other hand, appears to specifically cause MM and is more potent than asbestos in causing MM (Wagner et al., 1985, Carbone et al., 2007). In this review we examine the incidence of MM in men and women, the role of mineral fibers and co-factors in MM pathogenesis and the role of exposure history and diagnosis in research and disease management.
INCIDENCE
According to Surveillance, Epidemiology and End Results (SEER) Program data, the incidence of MM in the U.S. is estimated to be between 1-2/million in states with minimal exposure to mineral fibers and 10-15/million in states where large amounts of asbestos were used (Altekruse et al., 2010). Development of MM has primarily been associated with the widespread commercial use of asbestos in the early and mid 20th century. Prior to the 1950’s, MM tumors were very rare; however, presently, MM is responsible for approximately 3,000 deaths per year in the U.S. and an additional 5,000 deaths in Western Europe (Ismail-khan et al., 2006). The latency period, which is the interval between first exposure and the development of MM, ranges from about 25 to 71 years and appears to be influenced by the amount of exposure, because workers in trades with higher amounts of exposure may experience shorter latencies compared to those exposed to lower amounts of asbestos (Bianchi et al., 2007). However, latency periods for MM were similar among individuals who left Turkish villages that experience a very high incidence of MM when they were 6 years old and individuals who spent their whole lives in those villages, (Carbone et al., 2007). Accordingly, there does not appear to be a linear dose-response relationship between asbestos exposure and MM, i.e., the more the exposure the higher the risk (Carbone et al., 2002). Rather, some individuals are more susceptible to asbestos or erionite carcinogenicity than others, and once a sufficient amount of asbestos or erionite has been inhaled, such as in a 6 year old child growing up in a village contaminated with erionite, they will develop MM, which suggests that additional exposure(s) may not significantly increase the risk (Carbone et al., 2002 and 2007). The threshold limit above which asbestos and erionite will cause MM may vary among individuals due to genetics, exposure to cofactors, the type of mineral fiber inhaled, etc. In the US, the incidence of MM has dramatically increased since the late 50s until the beginning of this century and has remained stable at about 2,500-3,000/year since then (Moolgavkar et al., 2009). Since about 100% of those who develop MM die of it, more than 100,000 US citizens are expected to die of MM during the next 40 years. Due to its long latency period, it is estimated that MM mortality rates will continue to increase by 5-10% per year in most industrialized countries for the next 2-3 decades, despite asbestos abatement efforts (Britton, 2002; Goldberg et al., 2006).
MM: A PREVENTABLE TRAGEDY
MM is a particular and peculiar tragedy in the field of cancer, because most deaths are linked to exposure to carcinogenic mineral fibers, and thus most MM deaths should be preventable. We would like to note here that the US has invested billions of dollars to implement measures aimed at preventing death of US citizens caused by terrorist attacks, with the hypothesis that such attacks and consequent deaths can be prevented. Over the past 10 years, less than 5,000 people died in terrorist attacks in the US. During the same decade, about 25,000 to 30,000 US citizens died of MM, which are largely preventable deaths. Yet, the US invests less than 2-3 million dollars/year (total of federal grants/year) to support medical research aimed at developing strategies to prevent or cure MM. At the same time, the private sector spends/earns several billions of dollars/year in asbestos-related MM litigation and asbestos abatement. Some of the largest US companies have gone bankrupt because of this litigation and thousands of jobs have been lost (Lagnese 2005). Although several large companies have paid billions in litigation costs and eventually become bankrupt, to the best of our knowledge, none has made major investments to promote research to prevent and treat MM, research that would have likely decreased the incidence of MM and saved these companies from bankruptcy. The lack of funding for MM research has severely hampered its progress, because many researchers are reluctant to enter or pursue research in this field as limited availability of funding puts their careers in jeopardy. Due to insufficient funding, new researchers are reluctant to enter the field and existing ones have been forced to leave.
What is behind this? One influence is the myth — fueled by recent papers (Price and Ware, 2009; Tan et al., 2010) — that MM is disappearing. Believing this, an investor may rightly ask, “Why spend money on MM, when it will soon be gone?” In our view, however, this belief is mistaken.
Myth: MM will soon disappear
To support the myth that MM will soon disappear, four major assumptions are made. First, it is assumed that nearly all MM are caused by exposure to asbestos. Second, it is assumed that the higher rate in men is caused exclusively by a higher rate of asbestos exposure. Third, it is assumed that asbestos exposure has been nearly eliminated. Finally, it is assumed that asbestos exposure can be reduced to minimal by removing all asbestos from the environment. If these assumptions were true, then the MM rate should decline. However, the data do not support these assumptions. In contrast, the data show that the rate of MM in males is not declining. In fact, in the US, the rate of MM has remained constant since 1994, and is increasing in several countries. The rate of MM in women has remained unchanged for several decades (Hillerdal 1999; Moolgavkar 2009). In the US, asbestos use in some commercial products has not been banned (unlike the prohibition of the further use of asbestos containing products in many European countries). The continued import of asbestos containing products as well as potential exposure to asbestos in place will be risk factors for induction of MM for decades. In addition, asbestos and erionite exposures can occur from outcrops of asbestos and erionite containing soil in certain locations of the world including the US (Maher B., 2010). Such environmental exposures must be promptly identified to prevent the risk of “disturbing” these deposits and exposing the population, as it has occurred in Cappadocia and more recently in certain areas of the US (Carbone et al., 2007; Maher B., 2010). Finally, the presence of asbestos or “asbestos like” minerals may occur as natural components of some non-asbestos minerals exploited for various commercial applications (such as some deposits of vermiculite and talc) and in such cases it may be difficult to realize that a fibrous component exists.
The implementation of safety measures to reduce exposure in the workplace in the 30s and 40s led to the prediction that the incidence of asbestos-related malignancies would diminish significantly. Richard Doll, in his seminal paper proving the link between asbestos exposure and lung cancer, predicted that the risk of developing asbestos-related malignancies was significantly reduced past 1933 thanks to new regulations to limit exposure in the workplace (Doll, 1955). When these predictions were proven wrong, a new theory was developed that MM would soon disappear due to the diminished use of asbestos and the asbestos abatement efforts since the early 80s. According to this theory, starting in the 90s, MM would sharply decline in the Western world. These optimistic projections may have had the immediate effect of pleasing some, but ultimately they caused long-term harm because research was not funded due to the overly optimistic notion that MM would vanish spontaneously. Unfortunately, the incidence of MM has not shown any sign of vanishing anywhere in the world (Hillerdall 1999; Price and Ware 2009; Moolgavkar 2009; Altekruse et al., 2010; Tan et al., 2010). While asbestos exposure in the workplace has largely been eliminated in the US and Europe, environmental exposure that result from outdoor air pollution has not and may be increasing as natural deposits of asbestos and erionite are disturbed by human activities including land development (Baumann et al., 2007; Maher B 2010). Asbestos is widespread in the environment due to previous industrial use, because asbestos is extremely difficult to remove, and because houses are built near natural geological deposits of asbestos or erionite (Baumann et al., 2007). Moreover, as time passes, asbestos products degrade and more asbestos fibers are dispersed in the environment, for example from the asbestos roofs still present in many US homes. In other words, in the US and Europe, exposure to high levels of asbestos in the work place has largely been eliminated. However, the number of people exposed to “low” but “above background” levels of asbestos has increased. How these changes will influence the future incidence of MM is unknown. Data available in the literature do not allow an estimation of the risk of MM following “low” environmental exposure to asbestos, for example in an urban city (Bourdes et al., 2000). When environmental asbestos exposures are high, such as among the residents of Wittenoom, an Australian town near a crocidolite mine, or in Casale Monferrato, an Italian town next to an asbestos cement factory, or among individuals living near geological asbestos deposits in New Caledonia, then the risk of developing MM is several folds higher (Bauman et al., 2007; Magnani et al., 2001; Bourdes et al., 2000; Hillerdal 1999). A dramatic increase in MM incidence is expected in the third world, particularly in India, where the use of asbestos continues to increase exponentially and few, if any, precautions are taken to prevent exposure (Burki et al., 2010). The impact of erionite in the incidence of MM in the USA cannot be estimated yet as the extent of exposure remains to be determined.
Mesothelioma in women
In women, MM often develops in the absence of evidence for asbestos exposure. However, some studies have suggested that many female MM patients, like their male counterparts, have a known history of asbestos exposure(s). Dawson et al (1993) found that 74/93 (80%) female MM patients had a history of asbestos exposure in the United Kingdom. However, as noted by the authors, these cases were from referrals (possibly legal?) to the Environmental Lung Disease Research Group Department of Histopathology. Thus consideration of this fact must be taken into account when interpreting these results. Using data from his medical-legal files, Roggli et al (1997) reported a history of asbestos exposure in 45/59 (76%) of female MM cases. However, since these data were derived from collections of legal cases, there is inherent bias in favor of exposure. A note of caution should be introduced about the reliability of history of exposure. Histories are generally reliable when examining a cohort (for example of asbestos miners), but their reliability decreases at the individual level (i.e., for example employees of an asbestos mine may include accountants, lawyers, etc., who may actually not physically work in the mine). Moreover, exposure among employees will vary depending on the type of job performed. Many workers and their surviving family members do not have any idea if they have been exposed to asbestos containing products and it is often impossible to obtain reliable information because the human eye can only assess exposure to dust.
McDonald and McDonald (1980) examined all cases of fatal MM in North America through the end of 1972 and of the 557 cases of MM reported, 162 (29%) occurred in women. In a review of the literature through 1982, Hillerdal (1983) found that out of 2867 cases of MM, 619 (22%) occurred in women. In 2001, Murai (2001) analyzed all registered MM autopsy cases in Japan from 1958-1996 and found that of 1,846 MM cases, 558 (30%) were female. More recently, an article using an evidence-based medical approach reviewed the literature linking non-occupational asbestos exposure and MM and reported that 314 out of 1028 cases (31%) were female (Marchevsky et al., 2006).
Mortality data
In the US, the number of MM deaths is likely to exceed the estimated projection of 3000 year. This estimate is based on mortality data and death certificates. Percy et al, 1981 reviewed 48,836 cases of cancer and found that the underlying cause of cancer death was inaccurate in 35% of them. The International Classification of Diseases (ICD) captures cancer deaths: each cancer has its own code. MM is a special case in that only in 1999, with the publication of ICD-10, MM was given its own code: C45. Until 1999, under ICD-9, MM death was coded as ICD-163 (malignant neoplasm of the pleura) or ICD 162 (malignant tumor of the trachea bronchus and lung) or ICD 199 (malignant neoplasm without specification of site). Selikoff and collaborators found that 20% or more MM were incorrectly coded using ICD-9 and therefore were not captured by the SEER data (Ribak et al., 1991; Selikoff and Seidman, 1992). Even with the development of ICD-10, the percentage of MM that are incorrectly coded, and therefore not captured by statistics, remains at about 20-25% (Pinheiro et al., 2004, Camidge et al., 2006). The estimate for incorrect coding is probably even higher for peritoneal MM, a cancer that is often misdiagnosed as “carcinoma,” and vice-versa.
ASBESTOS AND MM
The role of serpentine form of asbestos (chrysotile) in causing MM is the subject of continued debate while the role of amphibole asbestos in MM pathogenesis is well established. Occupationally exposed individuals are commonly exposed to both serpentine and amphibole forms of asbestos in the work environment. Furthermore chrysotile veins of asbestos often contain amphibole forms of asbestos and thus products and resultant user exposures may involve both forms. Tissue analysis from occupationally exposed individuals often identifies mixed types of asbestos (Table 1). There seems to be a general agreement in the literature that amphibole asbestos, and especially crocidolite, is a much more potent carcinogen than serpentine asbestos such as chrysotile in causing MM (Sluis-Cremer 1991, and 1992; Boutin et al., 1996; Boffetta 2007). However, it is accepted that chrysotile can cause lung cancer in man and MM in rats, and some authors have proposed that even if chrysotile is less potent than amphiboles, it is a known carcinogen that accounts for about 95% of the asbestos used worldwide, and therefore chrysotile is the main cause of MM. At the opposite extreme are those who believe that there is no epidemiological evidence that chrysotile can cause MM (reviewed in Tweedale and McCulloch 2004, and in Britton 2002).
Table 1. Asbestos Fibers/gm Tissuea,b.
Lung content analysis in 38 human MM biopsies by ATEM as described (Berry et al., 1989; Mitha and Pooley, 1993). The data indicates chrysotile, commercial amphiboles (amosite and crocidolite), non-commercial amphiboles and total asbestos fiber burden.
| Sex | Age at Diagnosis |
History of Asbestos Exposure |
SV40 | Chrysotile | Amosite | Crocidolite | Tremolite | Anthophyllite | Total Amphibole |
Total Asbestos Fibers |
|---|---|---|---|---|---|---|---|---|---|---|
| F | 48 | N | − | 0.030 | 0.030 | 0.025 | 0.090 | 0.045 | 0.190 | 0.220 |
| M | 61 | N | − | 0.170 | 0.170 | 0.025 | 0.045 | 0.045 | 0.285 | 0.455 |
| M | 60 | N | + | 0.130 | 0.030 | 1.470 | 0.045 | 0.045 | 1.590 | 1.720 |
| M | 69 | Y | − | ND | 0.470 | 0.230 | 0.120 | 0.045 | 0.865 | 0.865 |
| M | ? | N | + | 0.030 | 0.060 | 0.110 | 0.110 | 0.045 | 0.325 | 0.355 |
| M | 62 | N | − | 0.110 | 0.110 | 0.025 | 0.230 | 0.045 | 0.410 | 0.520 |
| M | 56 | Y | + | 0.740 | 0.610 | 0.370 | 0.045 | 0.045 | 1.070 | 1.810 |
| M | 68 | N | − | 0.550 | 0.220 | 0.025 | 0.045 | 0.045 | 0.335 | 0.885 |
| F | 34 | Y | − | 0.090 | 0.090 | 0.170 | 0.090 | 0.045 | 0.395 | 0.485 |
| M | 58 | Y | + | 3.330 | 1.950 | 0.025 | 0.560 | 2.220 | 4.755 | 8.085 |
| M | 46 | Y | + | 0.030 | 5.250 | 0.025 | 0.320 | 0.045 | 5.640 | 5.670 |
| M | 52 | Y | − | 0.030 | 0.120 | 0.025 | 1.450 | 0.120 | 1.715 | 1.745 |
| M | 55 | N | − | 0.250 | 0.030 | 0.025 | 0.045 | 0.045 | 0.145 | 0.395 |
| M | 67 | N | + | 0.110 | 0.030 | 0.025 | 0.330 | 0.045 | 0.430 | 0.540 |
| M | 50 | Y | − | 0.030 | 2.600 | 0.025 | 0.045 | 0.045 | 2.715 | 2.745 |
| M | 69 | Y | − | 0.190 | 0.030 | 0.025 | 0.045 | 0.045 | 0.145 | 0.335 |
| F | 57 | N | + | 0.390 | 0.100 | 1.450 | 0.045 | 0.045 | 1.640 | 2.030 |
| F | 52 | Y | − | 0.270 | 0.030 | 1.610 | 0.045 | 0.045 | 1.730 | 2.000 |
| F | 41 | Y | − | 0.030 | 0.030 | 0.025 | 0.045 | 0.045 | 0.145 | 0.175 |
| M | 50 | Y | − | 0.620 | 0.270 | 0.025 | 0.045 | 0.090 | 0.430 | 1.050 |
| M | 68 | Y | + | 0.260 | 0.260 | 0.050 | 0.045 | 0.045 | 0.400 | 0.660 |
| M | 34 | N | + | 0.340 | 1.710 | 1.370 | 0.045 | 0.045 | 3.170 | 3.510 |
| F | 42 | N | + | 0.090 | 0.760 | 0.025 | 0.380 | 0.470 | 1.635 | 1.725 |
| M | 56 | N | + | 0.390 | 0.130 | 0.780 | 0.045 | 0.045 | 1.000 | 1.390 |
| M | 72 | Y | + | 0.030 | 0.030 | 0.025 | 0.045 | 0.045 | 0.145 | 0.175 |
| M | 59 | N | − | 0.530 | 0.030 | 0.025 | 0.260 | 0.045 | 0.360 | 0.890 |
| M | 54 | Y | − | 0.280 | 1.270 | 0.025 | 0.045 | 0.045 | 1.385 | 1.665 |
| M | 67 | Y | − | 9.520 | 109.480 | 0.025 | 4.760 | 0.045 | 114.310 | 123.830 |
| M | 30 | N | + | 0.030 | 0.150 | 0.025 | 0.045 | 0.045 | 0.265 | 0.295 |
| M | 52 | Y | − | 0.590 | 4.320 | 0.790 | 0.980 | 0.045 | 6.135 | 6.725 |
| M | 59 | N | + | 0.060 | 0.030 | 0.060 | 0.060 | 0.045 | 0.195 | 0.255 |
| M | 63 | N | + | 0.400 | 0.400 | 0.025 | 0.790 | 0.045 | 1.260 | 1.660 |
| F | 42 | N | − | 0.110 | 0.030 | 0.025 | 0.320 | 0.045 | 0.420 | 0.530 |
| F | 37 | Y | + | 0.170 | 0.030 | 0.080 | 0.045 | 0.045 | 0.200 | 0.370 |
| M | 48 | Y | + | 0.120 | 0.030 | 0.025 | 0.045 | 0.045 | 0.145 | 0.265 |
| M | 69 | Y | + | 0.980 | 11.700 | 0.025 | 0.980 | 0.045 | 12.750 | 13.730 |
| M | 64 | N | + | 0.030 | 0.030 | 0.025 | 0.200 | 0.045 | 0.300 | 0.330 |
| M | 68 | Y | + | 0.030 | 1.260 | 0.280 | 0.280 | 0.280 | 2.100 | 2.130 |
Fiber numbers expressed as millions/gm tissue
ND, not detected
Epidemiological and laboratory studies have demonstrated a clear connection between exposure to amphibole asbestos fibers and the subsequent development of MM (Wagner et al., 1960; Spirtas et al., 1994 Boffetta et al., 2007). The risk of asbestos exposure is underscored by the observation that up to 4.7% of deaths among workers with ten or more years of heavy asbestos exposure, such as those working in crocidolite and amosite asbestos mines in South Africa, were caused by MM (Sluis-Cremer, 1991 and 1992). Specifically, Sluis-Cremer et al studied a cohort of 7317 white asbestos miners (3212 amosite miners, 3430 crocidolite miners, 675 exposed to both, crocidolite and amosite) of which only 8% had been exposed for more than 10 years. There were 1225 deaths during the period of observation, an excess of 331 over controls. Thirty of those deaths were due to MM, 20 among crocidolite miners (423 total deaths, 4.7%), 4 among amosite miners (648 total deaths, 0.6%) and 6 among miners with mixed exposures (154 total deaths, 3.9%). Twenty-eight of those 30 MM occurred 10 or more years since first employment (latency) and only 1 occurred in a worker with “only” 8 years since exposure. The exposure time in the remaining case of MM could not be evaluated because this individual grew up in an area highly contaminated with asbestos. None of these 30 MM had period of exposure of less than 3 months (amosite and mixed exposure) or 12 months (crocidolite). Among the cases of crocidolite-associated MM, 6 developed in individuals exposed for a period ranging from 12 to 95 months, 6 in miners exposed for 96-191 months, and 8 in miners exposed for more than 192 months. Thus, crocidolite-induced MM developed only after long periods of continued heavy exposure, on average 15 years. Among the 10 cases of amosite or mixed-exposure MM, 4 developed after exposure of 3 to 11 months, 2 in miners exposed from 12 to 95 months, and 4 in miners exposed for more than 192 months. The mean latency of MM from exposure was 44.9 years for crocidolite miners, 51 years for amosite miners, and 12.3 years for miners exposed to both, crocidolite and amosite. These findings underscore the risk of MM in miners exposed to crocidolite and amosite. The risk of developing MM for amosite miners was 1 order of magnitude lower than for crocidolite miners. Thus, prolonged continuous exposures to asbestos of at least 3 months and usually 10 years were required to induce MM. At the same time, these findings revealed that most individuals exposed for prolonged time to extremely high levels of amosite and or crocidolite asbestos did not develop MM (Sluis-Cremer, 1991 and 1992). The finding that individuals with less than 3 months of continuous exposure to asbestos did not develop MM were independently supported by the observations that there were no MM among the Wittenoom crocidolite miners in Australia exposed for less than 3 months (De Klerk et al., 1989), none among US insulators exposed for less than 15 months (Selikoff et al., 1979), and only 1 rather than the 25 “expected’ among Rochdale textile workers exposed for less than 10 years (Peto et al., 1985; Iwatsubo et al., 2002). It is important to appreciate that only prolonged exposures increase the risk of MM to reassure individuals who may have had sporadic exposure and who are concerned about their own risk of developing MM.
Myth: MM are slow growing tumors that take several decades to produce clinical symptoms
We wish now to briefly discuss the difference between the time that elapses between first exposure to the diagnosis of the disease (latency period), and the period that elapses from the time of the initial growth of the MM to diagnosis. MM is a rapidly growing and very aggressive malignancy that most likely will produce clinical symptoms within a few years, not decades, from its initial growth. If MM was a “slow growing tumor” that takes decades to produce clinical symptoms, we would detect some of these early tumors and cure many patients, such as in the case of cervical and colon cancer. Instead, it is the latency from exposure to carcinogenic mineral fibers to the development of MM that is long and the actual growth of MM occurs within months to years, not decades. The distinction implies that a long period elapses during which the asbestos present in the pleural tissues promotes a chronic inflammatory process that with time may favor the emergence of a malignant clone in up to 5% of exposed individuals. This distinction is important because it implies that there is a long period of time during which it may be possible to arrest or delay the carcinogenic process.
MECHANISMS OF ASBESTOS PATHOGENESIS
It has been estimated that approximately 60-70% of pleural MM are associated with a history of asbestos exposure (Spirtas et al., 1994; Sebbag and Sugarbaker, 2001). Asbestos causes DNA damage directly, possibly by mechanically interfering with the segregation of chromosomes during mitosis (Olofsson and Mark, 1989), and indirectly by inducing mesothelial cells and macrophages to release mutagenic reactive oxygen (ROS) and nitrogen (iNOS) species (Xhao et al., 1996; Choe et al., 1997 and 1998; Quinlan et al., 1998; Xu et al., 1999 and 2002).
A key mechanism by which asbestos causes the transformation of mesothelial cells has recently been elucidated. Working with primary human mesothelial (HM) cells, Yang et al (2010) discovered that asbestos induces necrotic cell death with resultant release of HMGB-1 in the extra cellular space. HMGB-1 release causes a chronic inflammatory response, macrophage accumulation and the secretion of TNF-alpha, which in turn activates NF-kB, leading to the survival of HM that have accumulated genetic damage because of asbestos exposure (Figure 1, Yang et al., 2006 and 2010). These studies provided a mechanistic rationale to the observation by Liu et al (1998) that asbestos is not pathogenic in transgenic mice that do not express the TNF-alpha receptor. On one hand, TNF-alpha protects HM from asbestos-induced cell death and on the other promotes the growth of HM that have accumulated DNA damage following asbestos exposure. The role of chronic inflammation in favoring the growth of MM appears supported by the increased risk of peritoneal MM in patients with familial Mediterranean fever (Bani-Hani and Gharaibeh 2005; Herschovici et al 2006, Ishak et al. 2006), a condition characterized by recurrent polyserositis that mainly involves the peritoneum and the observation that MM may follow pneumothorax and/or trauma (Gorovenko GG, Slepukha IM, et al 1986; Hillerdal and Berg 1972). MM has also been reported in dogs with idiopathic hemorrhagic effusions that develop chronic reactive inflammation (Machida et al., 2004). Presently, we are using animal models to test chemo-preventive strategies such as inhibiting HMGB-1 and the inflammatory response associated with HMGB-1 release to prevent MM in high-risk individuals.
Figure 1.
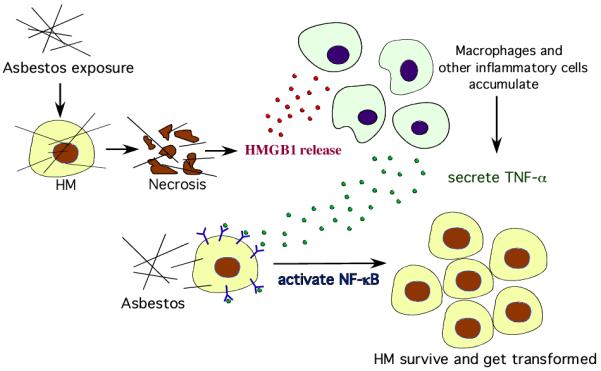
Asbestos causes necrotic cell death in HM, which leads to the release of HMGB1 into the extra cellular space. HMGB1 release causes macrophages accumulation, inflammatory response, and especially the secretion of TNF-alpha. TNF-alpha activates NF-kB pathway, which increases HM survival after asbestos exposure. This allows HM with asbestos-induced DNA damage to divide rather than die and, if key genetic alterations accumulate, to eventually develop into MM.
Previous studies have shown that asbestos can induce apoptosis in a small fraction, about 8-18% of cells, exposed in tissue culture (Broaddus et al., 1996; Jimenez et al., 1997). In contrast to necrosis, apoptosis does not cause the release of HMGB-1 into the extracellular space and therefore does not cause inflammation (Yang et al 2010). By eliminating mesothelial cells that have accumulated asbestos-induced mutations without promoting inflammation, apoptosis may be regarded as a protective mechanism against the development of MM.
Longer asbestos fibers (>8μm), especially amphibole asbestos, can become coated with iron rich proteins, such as ferritin and hemosiderin, which appear to favor the production of mutagenic ROS (reviewed in Heintz et al., 2010). Such structures are termed ferruginous bodies and when formed on asbestos cores can correctly be defined as asbestos bodies. It should be noted that definitive identification of an asbestos body requires EM analyses. A recent study from Japan proposed that ferruginous bodies that often form around amphibole asbestos contain high concentrations of radium, a finding that led the authors to suggest that ionizing radiation from these bodies may cause DNA damage and cancer, including MM (Nakamura et al., 2009). The possible significance of this study remains to be confirmed. At the molecular level, crocidolite-mediated induction of the AP-1 pathway and related genes has also been proposed as a factor in the development of MM in a series of papers by the research team directed by B. Mossman and these data have been independently confirmed by others (reviewed in Heintz et al., 2010).
In addition, asbestos may directly induce these pathways by activating the epidermal growth factor receptor and downstream pathways (Zanella C et al., 1999), which are often activated in MM (Destro A et al., 2006). The hepatocyte growth/scatter factor (HGF) and its receptor tyrosine kinase c-Met are highly expressed in most MM, especially those containing SV40; HGF activation is mediated through a PI3K/ERK5/Fra-1 feedback pathway (Cacciotti et al., 2001; Jagadeeswaran R et al. 2006; Ramos-Nino ME et al., 2008). Accordingly MAPK and phosphatidylinositol-3-kinase (PI3K)-AKT and the downstream mTOR (the target for rapamacyin) are often activated in MM (Altomare DA et al., 2005; Wilson SM et al., 2008).
FIBER CHARACTERISTICS IN LUNG AND PLEURA
Lung and pleural content analyses, in which the amount of asbestos present in the lung or pleura are measured, are a more objective and reliable measure of exposure, although asbestos is removed over time by physiological clearance mechanisms from the lungs and probably from the pleura. The half-life of crocidolite in the lung has been estimated to be 7-8 years, and that of chrysotile is much shorter (Hillerdal 1999). Berry and collaborators reviewed the literature and proposed that the elimination rate of amphibole asbestos from the lungs is about 15-20% year (Berry et al., 1989). Contrast phase light microscopy allows for the detection of fibers that are longer than 5 microns with a diameter larger than 0.2 microns. According to the Stanton’s hypothesis, which is based on experiments in rats, long fibers (> 8 microns) are more potent in causing MM than shorter or thicker fibers when compared on a one to one basis. Humans are exposed to a mixture of fibers of different sizes that include “medium size fibers” longer than 5 micron as well as shorter and thin fibers. The latter, include a large portion of amphibole fibers and the majority of chrysotile fibers, and are invisible by optical microscopy. Thus these so-called “ultra thin” and “ultra short” fibers must be evaluated using transmission electron microscopy (TEM). When using TEM, short chrysotile fibers outnumbered long fibers in most pleural content analyses (Paoletti et al., 1993; Dodson et al., 2003; Suzuki et al., 2005) with the exception of the study conducted by Boutin et al (1996) that almost exclusively detected amphibole asbestos in the lymphatic areas within the parietal pleura (designated as black spots). It should be noted that the authors also exclusively reported amphibole fibers within the lung samples of these individuals, but also noted a concern that sample loading may have interfered with detection of small chrysotile structures in the tissue preparations. The so-called black spots that Boutin et al (1996) sampled are the areas where lymphatics concentrate, forming relatively large openings (“stoma”) on the parietal pleura. These areas acquire a black pigment that is often seen on the pleura of smokers and of individuals living in industrialized towns. The lymphatic pathways influence dust and asbestos relocation (Dodson et al., 2007). Other authors have sampled various parts of the “whole” pleura (Paoletti et al., 1993; Suzuki et al., 2005) or pleural plaques (Dodson et al., 1990) in occupationally exposed individuals but did not specifically sample the “black spots”. Boutin et al., 1996 reported that in the “black spots” 22.5% of the fibers were 5μm or longer and 10% were 8 μm or longer. These observations compare with observations of LeBouffant et al. (1980) where 7% of the amosite and 5% of the chrysotile in the parietal pleura were 5μm or longer. Sebastien et al. (1980) reported 24% of the amphibole fibers and 14% of the chrysotile in the parietal pleura exceeded 4μm in length. In the study by Dodson and colleagues (1990) 10% of the amphiboles and 3.1% of the chrysotile were >5μm. Thus, the findings in the study by Boutin et al. (1996) are in line with previous studies showing that the vast majority of asbestos fibers in pleural tissue (in their study 77.5% and in their case amphiboles within “black spots”) are <5μm. Mitchev et al (2002) from the same research team continued the study of black spots and found “no relationship between the predominant locations of ‘black spots’ and hyaline pleural plaques”. Muller et al. (2001) evaluated “black spots” from the parietal pleura of twelve individuals who were former coal miners from the German Ruhr region. They stated that the “findings do not support the classification of black spots as an obligate early lesion in development of malignant mesothelioma”. Muller et al., 2001 found mixed dust within the “black spots” but did not find amphibole fibers directly located in the “black spots”. These findings have caused more controversy in this research field: in addition to the type of asbestos, what type of fibers causes MM? More specifically, are the smaller percentage of longer fibers that reach the pleura responsible for MM, or do both long as well as short fibers contribute to the process? As described by Dodson et al. 2003, the literature suggest that both types of fibers contribute to malignancy, although it is possible that fiber for fiber, longer fibers are more pathogenic, but also less likely to reach the pleura. The one thing all seem to agree with, is that ultrathin fibers, 0.3 micron or less in diameter, are those most frequently, if not exclusively, detected in the pleura (Paoletti et al., 1993; Boutin et al., 1996; Dodson et al., 2003; Suzuki et al., 2005). Although pleural and possibly peritoneal analyses may reflect the exposure associated with MM more accurately, such samples are rarely available. Therefore, lung samples are often used since asbestos has to transit through the lung in order to reach the pleura. In one of the few studies that have attempted to evaluate peritoneal tissue for the content of asbestos/ferruginous bodies, Dodson and colleagues (2000) reported a comparison of the asbestos burden in lung tissue with that found in omentum and mesentery samples from patients with MM. Their data confirmed that asbestos fibers reach the peritoneum. A companion study by Dodson et al. (2001) indicated that only an occasionally short uncoated asbestos fiber was found in similar samples from individuals defined as representing the general population “i.e., background exposure”.
Lung Content Analysis
Using analytical transmission electron microscopy (ATEM), we performed lung content analysis in 38 lung tissue biopsies surgically resected by H.I.P. from patients who were seen for analyses and treatment unrelated to litigation and who were diagnosed with pleural MM by M.C. Of these 38, 8 were women and 4 had reported a history of exposure to asbestos compared to 16/30 men with such history (Table 1). Of the 4 asbestos-exposed women, history suggested exposure through the household (father with occupational history of exposure) in three, and one might have been exposed through the use of contaminated talcum powder. Both exposure to asbestos and duration of exposure were documented with the use of the American Thoracic Division of Lung Disease Adult questionnaire. We tested reliability of exposure history by performing lung-biopsy content analyses from each of the 38 patients. The preparative technique and the asbestos count scheme followed the model described by Mitha and Pooley 1993 and Berry et al., 1989. The ATEM data included all fibers (>1μm) with an aspect ratio greater than 3:1 as evaluated at high magnification using an ATEM with the fiber type being determined by morphology, selected area diffraction and X-ray energy dispersive analysis. As expected, these studies revealed that all patients had some asbestos in their lungs. Asbestos fibers are commonly found in the lungs of individuals living in industrialized areas and there is no magic number that clearly separates amount of asbestos that is associated with an increased risk of MM (exposed individuals) from that found in the general population. Based on previous studies performed in the laboratory of F.D. Pooley who performed these studies, amounts of asbestos below 1 million per gram of dry lung were found in most of the “background control populations” studied from Australia, United Kingdom, and North America (Berry et al., 1989). These are the so called background amounts of asbestos that are found in the lungs of most people living in industrialized areas and that are not associated with a detectable increase in the incidence of MM. We arbitrarily decided to lower this threshold to 0.5 million/dry gram to increase the chance to capture those individuals in our cohort with levels of asbestos that might justify the definition of “exposed”. The findings showed a variation in total fiber burden as well as fiber type. It was common to find “commercial” types of asbestos (chrysotile, amosite, and/or crocidolite) and “non-commercial forms” (tremolite and anthophyllite) within most of the samples. The total amount of non-commercial amphiboles exceeded the total of the commercial forms in eight cases (Cases 1, 6, 12, 14,19,25,32 and 33). These observations are consistent with tissue burden characteristics found in other studies of lung tissue from individuals with MM (Dodson et al., 1997), (Dodson et al., 2003), (Dodson et al., 2005). The results showed that 25 (21 men and 4 women) of the 38 individuals had levels of asbestos (total particles = total amphiboles + chrysotile) above 0.5. Of interest, 11 of the patients with above 0.5 millions/dry gm tissue levels of asbestos in their lungs had reported a negative history of exposure (3 women and 8 men). Thus of 18 individuals reporting no history of asbestos exposure only 7 had been truly unexposed and 11 were unaware that they had been exposed. On the other hand, 6 patients (3 men and 3 women) who had reported a history of exposure had only background levels of asbestos in their lungs. Thus, of 20 individuals reporting exposure -16 men and 4 women - only 14 had been truly exposed. In other words we found a lack of association between actual asbestos levels in the lungs and exposure history: [χ2(1)=0.33, p=0.56]. These findings underscore the limitation of relying on histories to assess exposure at the individual level. Only analytical electron microscopic analyses of pleura, lymph node and lung samples can reveal if, how much and the type(s) of mineral fibers that an individual has been exposed to. Morphologically, many fibers in human tissue are short (<5 μm) or very thin (even if longer than 5 μm) and thus require the level of resolution afforded by ATEM for detection. Even these analyses have some limitations based on tissue burden determined by ATEM since the content of the lung tissue represents what is left at the time of sampling after the impact of clearance/translocation of inhaled dusts/fibers (Berry et al., 1989). Even less reliable are attempts to devise complicated formulas and questionnaires to attribute the amount of exposure to different categories of workers years and often decades after the exposure took place. Such studies are based on extrapolations that are error prone (Hillerdal 1999). For example, when more than one expert interpreted these questionnaires, they often provided discordant results (Rodelsperger 2001).
On average, the 8 women we studied were 14 years younger than men at diagnosis (p=0.001). The amounts of asbestos detected in these women did not exceed those found in men. This observation suggests that factors in addition to asbestos influenced the development of MM in these women. Genetics may play a role as, in genetically susceptible families in Cappadocia, we observed that MM develops at an earlier age compared to sporadic MM (Carbone et al., 2007). However, our results include too few women to reach a definitive conclusion about the sex differences in the pathogenesis of MM. Our study shows that even histories taken by trained personnel and according to established guidelines may be unreliable.
It should be noted that there is no real magic number to establish a firm threshold between exposed and not exposed. If one were to exclude chrysotile exposure that some do not believe contributes to MM, only 18/38 would qualify as exposed (total amphiboles >0.5). We used the total fiber count to encompass any type of asbestos exposure that might contribute to MM. In addition, if more strict criteria were to be implemented, i.e. a cut off of 1.0 as used in the laboratory that performed these analyses (Berry et al., 1989), then only 18 individuals out of 38 would qualify as exposed (total particles >1.0). In summary, individuals with amount of asbestos >1 have clearly been exposed to asbestos, as one would expect in individuals working in the asbestos industry, and those with amounts between 0.5 and 1.0 contain asbestos levels that are at the upper limits of background and the interpretation of these results as exposed at levels “above” or “at background levels” in these individuals is subjective. We decided to include them in the “exposed” category, as it appears possible that these relatively low levels of exposure in individuals susceptible to mineral fiber carcinogenesis due to genetic predisposition or exposure to co-factors may be sufficient to cause MM. The development of MM in individuals exposed to low levels of asbestos fibers argues against a classic dose response relationship (i.e., otherwise many more than 4.7% of asbestos miners should have died of MM). The absence of this dose response relationship and the relative infrequency of MM development in heavily asbestos exposed individuals suggest that additional factors, either alone or in conjunction with asbestos may play a role in the development of this malignancy (Carbone et al., 2002).
CO-FACTORS IN THE PATHOGENESIS OF MM
SV40
Simian Virus 40 (SV40), a DNA tumor virus endemic to the Asian macaque species of monkeys, may be one such etiological factor (Carbone et al., 2002). Between 1954-1978, millions of people were exposed to the virus through contaminated Salk and Sabin polio vaccines (Cutrone et al., 2005). Within 6-9 months, SV40 causes MM in 60% of hamsters injected intracardially. Moreover, intrapleural injections lead to the development of MM in 100% of the animals within 4-6 months (Cicala et al., 1993). The capacity of SV40 to cause tumors in humans is debated. A review by the Institute of Medicine concluded that there is no epidemiological evidence in favor or against the hypothesis that SV40-contaminated vaccines caused malignancy in humans (Stratton et al., 2003). However, an analysis of human MM biopsies revealed that SV40 sequences were present in about half of them (Carbone et al., 1994). In human MM biopsies, SV40 has been shown to bind and inactivate p53 and pRb, and to activate c-met, IGF-I and other oncogenes (Carbone et al., 2002; Gazdar et al., 2002; Bocchetta et al., 2008). These finding were substantiated by several other labs as well as by a multi-laboratory study sponsored by the International Mesothelioma Interest Group (Testa et al., 1998). Other studies found that only a low percentage, 5-6%, of MM contained SV40 and suggested that both the low incidence and the low amunt of SV40 present argued against a pathogenic role (Lopez-Rios et al., 2004; Aoe et al., 2006; Ziegler et al., 2007).
Among the 38 MM subjects reported in Table 1, 20 contained SV40 as determined by PCR and verified by DNA sequencing using the PYV and PYV reverse set of primers that amplify a 172 bp region of the early region of the SV40 DNA according to established procedures (Gazdar et al., 2002). Among the 8 women, 3 contained SV40. There was no significant correlation with asbestos exposure. HM are unusually susceptible to SV40 infection and transformation (Bocchetta et al., 2000). In contrast, other human cell types such as fibroblasts are lysed following infection (Bocchetta et al., 2000), or cannot be infected at all, such as kidney tubular epithelial cells (Low et al., 2004). Following infection, SV40 remains episomal in HM and viral replication is prevented by a unique antisense suppression mechanism (Carbone et al., 2008). Bocchetta et al (2000) reported that in tissue culture, HM exposed to both asbestos and SV40 become transformed at a higher rate than HM exposed to SV40 alone (i.e. asbestos alone does not cause malignant transformation of primary HM in vitro). Further evidence in support of co-carcinogenesis was provided when Kroczynska et al (2006) found that asbestos and SV40 had synergistic effects in causing MM in hamsters. Experiments in transgenic mice confirmed the co-carcinogeneic properties of SV40 with asbestos (Robinson et al., 2006; Pietruska and Kane, 2007). Although it is well accepted that SV40 causes MM in animals and data unequivocally show that SV40 acts as a co-factor for asbestos carcinogenesis, the role of SV40 in causing human MM is controversial (Carbone and Albelda et al, 2007; Rivera et al., 2008).
Genetics and Erionite
Multiple reports have suggested that certain individuals in familial cohorts may be genetically predisposed to MM. Risberg et al (1980) proposed that genetics, in addition to asbestos exposure, was responsible for the development of MM in a father, his three sons and one daughter. Krousel et al (1986) reported three MM occurrences in a mother and two of her five children despite only one of her children having occupational exposure to asbestos. Similarly, Ascoli et al (1998) reported an aggregation of MM in three sisters and one male cousin, which is suggestive of a hereditary predisposition. Other case reports in the literature correlate women and other family members with MM (Musti, 2002; Bianchi et al., 2004; Picklesimer et al., 2005).
Evidence supporting genetic predisposition to MM comes from the Cappadocian region of Turkey where MM causes up to 50% of deaths in the villages of Karain, Sarihidir, and Tuzköy as a result of the inhabitants building their homes from stones containing high levels of erionite, a very carcinogenic mineral fiber that specifically causes MM (Roushdy-Hammady et al., 2001; Dogan et al., 2006). In these villages the incidence or lung cancer is not significantly increased compared to other regions in Turkey, indicating that erionite carcinogenesis is not exactly comparable to asbestos carcinogenesis, since asbestos also causes lung cancer. In the Turkish villages, the Male/Female ratios of MM were: 76/74 (out of 305 total deaths in Karain), 54/51 (out of 432 total deaths in Tuzkoy), and 7/8 (out of 87 total deaths in Sarihidir) (Carbone et al., 2007). Thus, the incidence of MM in men and women is similar in Cappadocia. This is the only incidence of MM in which both sexes are equally affected; further indicating that erionite is very potent in causing MM. Moreover, it was observed that MM in Cappadocia was very prevalent in certain families and less in other families (Carbone et al., 2007). Quantitative mineralogical analysis of erionite samples from the villages in Turkey and from the United States revealed that the composition of erionite in the U.S. and in Turkey is identical (Dogan et al., 2006). Pedigree analysis of the affected families supports the interpretation of genetic predisposition to mineral fiber carcinogenesis. The data indicate that erionite is the major cause of the MM epidemic in Turkey and genetics influences the risk of developing MM following exposure to erionite in the villages of Cappadocia (Dogan et al., 2006).
It should be noted that erionite is a very potent carcinogen that specifically causes MM in humans as well as in animals. Erionite is much more potent than asbestos in causing MM; for example, 48% of animals injected with asbestos developed MM compared to almost 100% MM incidence in animals injected with erionite (Wagner et al., 1985). Sporadic cases of MM caused by erionite have been reported outside of Turkey (Ilgren et al., 2008; Kliment et al., 2009). The unprecedented very high incidence of MM in Cappadocia may be exacerbated by the unfortunate combination of erionite exposure and genetic predisposition to mineral fiber carcinogenesis. Incidences of MM similar to those observed in the Cappadocian families have also been detected in some U.S. and European families. In these cases, genetic predisposition and exposure to asbestos appears to be the most likely cause. In other words, there are some families who are very sensitive to mineral fiber carcinogenesis and whether the fiber is asbestos or erionite does not appear to make any difference. With support from the NCI, some of us, in collaboration with N. Cox (University of Chicago) and JR Testa (Fox Chase Cancer Center) are trying to identify the gene(s) that influence susceptibility to mineral fiber carcinogenesis. Identification of the putative MM-susceptibility gene(s) is crucial to enable us to devise novel specific mechanistic therapeutic approaches.
Radiation
Prior radiation therapy has been linked to the pathogenesis of MM. In a study by Roggli et al (1997), 5/62 female MM patients gave a history of prior radiation exposure. One woman received radiation therapy for the treatment of Wilm’s tumor as a child and analysis of her lung tissue showed asbestos levels no different from those found in the general population. Whether this MM case is also related to the absence of the Wilm’s tumor suppressor protein is unknown. The other four women were treated for various other malignancies, and despite the history of household exposure to asbestos provided by two of these four women, lung tissue analysis showed no pleural plaques or asbestosis in either of them, suggesting that previous radiation exposure may have been the causal factor. In addition to radiation treatment, other sources of radiation have also been described as risk factors to MM. A recent review by Goodman et al (2009) evaluating the impact of various ionizing radiation sources including atomic energy plants and Thorotrast concluded that there appears to be enough evidence to support a causal link between ionizing radiation and MM. Other reports have corroborated the link between radiation exposure and MM (Sugarbaker et al., 2008; Merler and Roberti, 2008).
The association between MM, asbestos and erionite exposure, SV40 infection, genetic predisposition, and radiation exposures suggests that a multi factorial etiology may be more common than presently appreciated. Probably any factor that leads to HM necrosis, proliferation, inflammation, and DNA damage may contribute to the development of MM, especially in genetically predisposed individuals and in the presence of a background of DNA damage caused by asbestos, erionite, radiation, SV40 and other carcinogens.
MESOTHELIOMA PATHOGENESIS
Pleural mesothelioma
Prior to 1950, the incidence of pleural MM in both men and women was similar, with an average of 1 or 2 cases per million (Ross and McDonald, 1995). However, following the dramatic increase in commercial use of asbestos during the first half of the 20th century, pleural MM rates rose dramatically in men, but not in women. Using the Surveillance, Epidemiology, and End Results (SEER) database, Connelly et al (1987) reported that the incidence of pleural MM among white women in the U.S. remained almost unchanged in approximately 2 cases per million persons in both 1973 and 1984 while corresponding rates among white men were 5.1 per million and 14.1 per million respectively. Analysis of the SEER database from 1990-1995 by Jemal et al (2000) revealed that the incidence of pleural MM was 16.3 cases per million among white males compared to 2.6 per million among white females. Most recently, analysis of the SEER database from 1973-2005 using extensions of the age-period-cohort (APC) model by Moolgavkar et al (2009) revealed that the age-adjusted rates for pleural MM in men appear to have stabilized since increasing from 7.5 per million person-years in 1973 to approximately 15 per million person-years in the early 1990s in states that used large amounts of asbestos. In contrast, corresponding data in women shows a relatively consistent incidence of 2.5 per million person-years over the same period. It is obvious that these numbers do not “add up” with some studies reporting that 1/3 of MM occur in women and others reporting that female MM accounts for only 1/8 of the total. However, all studies agree that there has been a steady rise in the incidence of MM in men but not in women over the past half century.
One obvious reason for these gender-related differences in the MM trend is that historically men have been exposed to asbestos while working in asbestos mines, shipyards, cement factories, etc. While this is certainly true, this cannot fully explain why the incidence of MM has not increased in women. For example, during the Second World War, many women took the place of men in asbestos exposed occupations. However, no appreciable increase of MM in women was noted. Moreover, asbestos exposure does not occur only in the workplace. For example, the development of MM in wives of asbestos workers demonstrates that sufficient amounts of asbestos were carried home by these men to cause MM in their wives (at least during the early years of asbestos exposure, i.e. during the 30’s and 40’s when levels of asbestos dust in the work place were high). Asbestos is also present in schools, public and private buildings, etc. There are natural deposits of asbestos and erionite in the U.S. to which local populations are increasingly exposed because these areas are being developed. Yet, there has not been a significant increase in the incidence of MM in women in the US. A hypothesis that may help account for the low incidence of MM in women comes from a recent report by Pinton et al (2009). The authors found that both normal pleura and a fraction of malignant pleural mesothelioma tissue express estrogen receptor (ER) beta. Multivariate analysis of 78 MM patients indicated that high levels of ER beta was an independent protective factor of all-cause mortality (HR= 0.2; 95% CI, 0.05-0.6), although only 4 out of 19 women (21.1%) and 8 out of 59 men (13.6%) were classified as having high levels. In addition, the authors confirmed that ER beta-mediated inhibition of MM cell proliferation, in vitro, was possible through treatment with 17beta-estradiol (E2). These data were supported by a recent study by Chua et al (2009a and b) who investigated the expression of sex steroid receptors in 20 peritoneal MM patient tumor specimens. They found a higher expression of estrogen receptors in females (M= 0.6%, F= 1.7%) in addition to higher expressions of progesterone receptors (M= 1.0%, F= 1.4%). Thus, there is some initial molecular evidence that may support a role for sex hormones and their receptors as a possible mechanism that might help to explain the lower incidence of MM in women. These results need to be validated and expanded upon by future studies; for example, by testing the hypothesis that ER beta expression may be linked to the epithelial MM phenotype.
Due to its aggressive nature and resistance to chemotherapy, development of pleural MM leads to a median survival of approximately 14 months from the time of diagnosis in patients undergoing surgery (Flores et al., 2008). However, MM patients who are candidates for surgery generally have less advanced disease. Total survival, including patients who are not candidates for surgical resection, is less than 1 year from diagnosis (Bertino et al., 2009). Survival is influenced by stage, and in 663 consecutive patients (538 men, 125 women) who underwent pleurectomy or extrapleural pneumectomy from 1990 until 2006, median survival was 38 months for stage I, 19 months for stage II, 11 months for stage III and 7 months for stage IV (Flores et al., 2008).
Peritoneal Mesothelioma
Pleural MM outnumbers peritoneal MM in both sexes (Boffetta, 2007; Moolgavkar et al., 2009). Griffiths et al (1980) reported that between 1965-1978, among 25 men and 10 women with MM at the Austin Hospital in Australia, 31 MM were in the pleura, 3 in the peritoneum (1 female) and 1 was in the pericardium. In Australia, between 1947-1980, 560 cases of MM arose in the pleura and 66 in the peritoneum (Musk et al., 1989). Murai (2001) examined 1,784 cases of MM in Japan and reported that pleural MM was predominant in both sexes compared to the frequency of peritoneal MM in females and males, which was 35.5% and 19.3% respectively.
The mortality of peritoneal MM used to be dismal but has significantly decreased due to novel therapeutic approaches (Yan et al., 2006). Early diagnosis is rare, partly due to the relative rarity of peritoneal MM. Initially, patients are often misdiagnosed with the more common benign and malignant gastrointestinal conditions (Chua et al., 2009b; Hesdorffer et al., 2008). Based on select European cancer registries and the U.S. SEER database, the incidence rate in industrialized countries range between approximately 0.5-3 cases per million in men and between 0.2-2 cases per million in women (Boffetta, 2007), a markedly less M:F discrepancy than for pleural MM. This finding supports the interpretation that most peritoneal MM is not asbestos-related. Sebbag and Sugarbaker (2001) observed that peritoneal MM has a much weaker association with asbestos than pleural MM, with rates of 15-30% and 60-70% respectively. These data did not distinguish between men and women.
Analysis of the SEER database from 1973-2005 showed no significant temporal trends for both men and women (Moolgavkar et al., 2009). These authors found that asbestos exposure was responsible for only a minor fraction of peritoneal MM in the SEER data from 1973-2005. Accordingly, peritoneal MM is often reported in men and women with no known asbestos exposure. The etiology in these cases is unclear, although some of the cases may be due to unknown asbestos/erionite exposure as well as exposure to other carcinogens. In a study of 7 peritoneal MM in women with no known asbestos exposure, 6 out of 7 cases had asbestos fibers burdens lower or similar to that found in normal ovarian tissue in the control group with no history of exposure (Heller et al., 1999). Other studies based on history of exposure, often taken decades later, suffer from the inherent imprecision of this methodology (Carbone and Bedrossian, 2006).
It has been suggested that higher asbestos exposure levels are needed to induce MM in the peritoneum compared to the pleura. Dawson et al (1993) reported that peritoneal MM in women was associated with an increased lung amphibole burden and high levels of fibrosis. Furthermore, lung tissue evaluated in patients with peritoneal MM has shown higher asbestos burdens than lung tissue from patients with pleural MM (Heller et al., 1999). It appears possible that in heavily exposed individuals, asbestos fibers migrate through the lymphatic system in sufficient amounts to the peritoneum to cause MM. The ability of asbestos fibers to migrate through the lymphatics was first suggested by studies in mice in which subcutaneously injected asbestos was found in lymph nodes, pleura and peritoneum (Roe et al., 1967). In the absence of heavy exposure, asbestos fibers mostly accumulate in the lungs where some of them may promote the occurrence of lung cancer while others have been shown to migrate to the lymph nodes/lymphatics as well as to the pleura and eventually to the peritoneum promoting MM development. It could be speculated that in individuals who have not been heavily exposed, the fibers do not reach the peritoneum in sufficient quantities to stimulate the occurrence of MM. Concerning the clinical presentation of peritoneal MM, a retrospective study by Eltabbakh (1999) of 15 women with peritoneal MM revealed that women most commonly presented with abdominal distension (73%), abdominal pain (40%), ascites (60%), abdominopelvic masses (93%), thrombocytosis (27%), and thromboembolic manifestations (20%). Median survival of these patients was 12.5 months. However, median survival was longer in those who underwent cytoreductive therapy versus biopsy alone (13.5 versus 6 months). Patients who received chemotherapy also survived significantly longer (29 versus 1 month), suggesting that cytoreductive surgery and/or chemotherapy is of significant benefit to women with malignant peritoneal MM. Recent advances in cytoreductive surgery and perioperative intraperitoneal chemotherapy protocols for peritoneal MM have increased median survival to 60-90 months (Yan et al., 2006). However, this is a subset of patients with early disease that is eligible for this type of therapy. Women with peritoneal MM have been observed to have better overall survival than men with peritoneal MM although the exact reasons are unclear (Yan et al., 2006). In the study by Yan et al (2006) at the Washington Cancer Institute, 34 men and 28 women underwent combined therapy. Reported survival rates in women at years 1, 3, and 5 were 96, 72, and 63% respectively, significantly better than corresponding survival rates in men that were 74, 48, and 42%. The authors concluded that among other factors, the observed survival benefit might be related to favorable clinical and histopathological (i.e. epithelial MM, tubulopapillary variant) features specific to the female cohort.
EXPOSURE HISTORY
Exposure can be classified as direct from asbestos-related occupations, indirect through family members, neighborhood conditions, or from environmental factors. In reality, it is often difficult to distinguish between these types as people have multiple types of exposures or may be unaware of any exposure at all. Vianna and Polan (1978) reported the results of 52 histologically confirmed MM cases among female residents of New York State who died between 1967 and 1977. Thirty-seven of these patients had no identifiable exposure history and among the patients with history of exposure, six worked in the asbestos industry, two were exposed through family members and one additional patient lived near an asbestos factory. The remaining eight patients may have had indirect exposure by washing the contaminated clothes of their husband or relatives who worked directly with asbestos. Dawson et al (1993) reported that 51/93 (55%) female MM patients reported an occupational history of asbestos exposure while 13/93 (14%) claimed indirect exposures through family contacts. In another study, Roggli et al (1997) examined the rates of exposure in 59 female MM cases and found that 19% of these women reported occupational asbestos exposure. Of the remaining women, household contact was the predominant method of asbestos exposure (61%). Since both studies include medical-legal cases, they are biased in favor of exposure (i.e., presumably patients who have not been exposed to asbestos are less likely to sue).
Indirect Exposure
Indirect exposure occurs in the wives and children of asbestos workers. The main source of indirect exposure is the husband’s work clothing. Washing or handling of the contaminated clothing can aerosolize substantial amounts of asbestos. It has been well established that family members of workers in asbestos industries are at risk for the development of MM. Probably, the largest study was that conducted by Magnani et al (1993). The authors studied mortality of wives married to workers in the asbestos cement industry in Casale Monferrato, Italy. Active from 1907-1986, this was the largest asbestos cement factory in Italy. Their analysis of 1,740 female household members monitored from 1965-1988 found that 4 of the women died from pleural tumors (3 confirmed MM) versus the expected number of 0.5. More recent results from the study with over 40 years of follow-up showed that 21 women died from pleural tumors compared to the 1.2 expected. In addition, the authors reported 11 incident cases of pleural MM from 1990-2001 (Ferrante et al., 2007). Likewise, reporting on 1,145 (123 women) histologically confirmed cases of MM from his medical-legal consultation files, Roggli et al (2002) observed women with MM predominated among household contacts of asbestos workers (79%). However, since these are medico-legal cases, they are biased in favor of exposure; and although they confirm that household contacts can develop MM, they do not contribute to our understanding of the true incidence of MM in household contacts. Many other studies have shown a connection between indirect asbestos exposure and MM (Lieben and Pistamka, 1967; Anderson et al., 1976; Vianna and Polan, 1978; Li et al., 1978; Epler et al., 1980; Nicholson, 1983; Krousel et al., 1986; Dodoli et al., 1992; Bianchi et al., 1993; Dawson et al., 1993; Magnani et al., 1993; Schneider and Woitowitz, 1995; Schneider et al., 1996; Roggli et al., 1997; Paoletti et al., 2000; Murai, 2001, Leigh et al., 2002; Roggli et al., 2002; Dodson et al., 2003). It is encouraging that the number of reports in recent years (Bianchi et al., 2004; Miller, 2005; Ampleford and Ohar, 2007; Ferrante et al., 2007; Patel et al., 2008) has seemingly decreased, which may be attributed to societal changes (i.e. the number of women who wash their husband’s clothes has declined during the past 30 years). While there are more case reports detailing MM in women, due to their anecdotal nature, not all of them are reported here. It should be noted that the levels of indirect exposure were particularly high during the first half of the 20th century, well before measures to reduce exposure and the risk that workers carried asbestos home were implemented in the workplace in the US and Europe. Such measures were implemented at different times in different countries, and in some countries such as India, appropriate measures are still either lacking or are inadequate (Burki 2010).
Neighborhood Exposure
Neighborhood exposure occurs in those who reside in an area near asbestos mines or factories using or producing asbestos based goods. Evidence of this association was reported in a retrospective study by Magnani et al (1995). In Casale Monferrato, Italy, 64 cases of MM were identified in which patients had no occupational or familial exposure to asbestos. The incidence of histologically confirmed MM among local residents (annual × 100,000; age adjusted) was 4.2 in men and 2.3 in women compared to rates from the Cancer Registry of Varese (a nearby town) and Italian Cancer Registries which was 1.0 and 1.8 in men and 0.3 and 0.6 in women respectively. Two more recent case-controlled epidemiological studies by the same authors provide further evidence linking the association between residential exposure and the risk of MM development (Magnani et al., 2000; Magnani et al., 2001). Kurumatani and Kumagai (2009) proposed that the distance of place of residence to an asbestos plant was related to the risk of MM. It should be noted that approaches based on proximity inevitably lead to exposure misclassification, which will attenuate or bias the exposure response association. It is difficult to make accurate predictions without detailed information on individual behavior within the concentrated area (Driece et al., 2010).
It has been estimated that in South Africa, home to many asbestos mines, neighborhood and environmental exposure account for about 26% of all MM (Abratt et al., 2005). Kielkowski et al (2000) conducted a birth cohort mortality study in Prieska, South Africa, a small remote town located near a crocidolite asbestos mining area that was active from the early 1890s to the late 1960s. In addition to the mines that were the main source of exposure, other possible sources of exposure cited included asbestos dumps (on which children played) and spillage of fibers during transport. Of the reported cases of cancer, 28/118 (24%) was due to MM, and of these 28 cases, 8 were in women (cause-specific mortality of 172 per million person-years) and 20 were in men (cause-specific mortality of 366 per million person-years). The study showed that although males were more likely to die from MM as expected, high mortality in the female group was likely indicative of very high environmental exposure as they were rarely employed in the asbestos industry in this area. Thus, although a comprehensive evaluation of exposure in women is sometimes difficult, especially among women living in close proximity to an asbestos factory where more than one source of exposure is likely, there is evidence to conclude that proximal neighborhood exposure to a crocidolite mine is risk factor for MM development.
Environmental Exposure
Environmental exposure occurs in individuals who live near natural asbestos and erionite deposits. Asbestos can be released into the air through construction practices or the normal process of erosion. Such exposure may have occurred in Biancavilla, a town in eastern Sicily. An epidemiological study by Paoletti et al (2000) showed a significantly increased mortality from MM among men and women in Biancavilla between 1980 and 1993, despite an absence of industrial activities involving asbestos in the area. Results showed a particularly evident increase in mortality from pleural MM in females (15 cases), people 65 years of age or younger, and during the years 1988-1993. The only possible suggested exposure to asbestos was through stone quarries located in Monte Calvario (northeast of Biancavilla), whose products contained intermediate phases between the fibrous amphiboles actinolite and tremolite, since stones from this quarry were used to build homes in Biancavilla. Similarly, residential proximity to naturally occurring asbestos in California was found to increase the incidence rate for MM. Pan et al (2005) conducted a cancer registry-based case control study and found 554 women with MM in California from 1988-1997. They found that people who lived closer to an asbestos source had a greater chance of developing MM and they estimated that the odds of developing MM decreased by 6.3% for every 10 km farther from the nearest asbestos source compared to controls. The interpretation of these results is biased by the same concerns raised under neighborhood exposure (see Driece et al., 2010).
A particularly interesting type of environmental exposure occurs in New Caledonia where villages in proximity of deposits of serpentine asbestos experience an increased rate of MM (Baumann et al., 2007). As discussed in the “genetics” section, environmental exposure to erionite is the main cause of the MM epidemic in Turkey (Carbone et al., 2007). Finally, as time passes asbestos “in place” degrades and millions of asbestos fibers are dispersed into the environment, such as from asbestos roofs still covering many US homes. It has been estimated that in Japan, the risk of environmental asbestos exposure will contribute to 13,000-30,000 cases of MM by the year 2030 (Azuma et al., 2009). Overall, enough evidence exists to state that environmental exposure to asbestos and erionite is a risk factor that causes MM in both men and women (Baumann et al., 2007; Carbone et al., 2007; Magnani et al., 2001; Bourdes et al., 2001).
PATHOLOGICAL FEATURES
Any type of malignancy can spread to the pleura and peritoneum and when they do, they often cause an effusion. Of course, MM patients also develop effusions. Thus, as for other cancers, often the first specimen that is analyzed in a MM patient is pleural or peritoneal fluid by cytology. Overall, about 1/3 of all effusions are malignant, and among them MM is rare, thus cytologists rarely see these tumors and misdiagnoses are relatively common. In men the most common causes of malignant pleural effusions are, in order, lung cancer, tumors of the GI tract and pancreatic cancer, and of malignant peritoneal effusions, carcinomas of the GI tract, followed by carcinoma of the pancreas and of the lung. In women, the most common causes of malignant pleural effusions are, in order, cancer of the breast, lung and ovary, and of malignant peritoneal effusions, carcinomas of the ovary, GI tract, and pancreas. Less than 1% of malignant effusions are caused by MM. Although cytology is very useful to suggest the diagnosis of MM, it is insufficient to confirm the diagnosis as other malignancies can show identical morphology. Therefore, the current standard of treatment requires that the diagnosis be confirmed by a biopsy followed by immunohistochemistry of the tumor specimen using a large panel of markers to distinguish MM from other tumors. Moreover, if the resources are available, and always when the results of immunohistochemistry are not clear-cut, EM studies should be conducted to confirm the diagnosis. Too many times these procedures are by-passed and the diagnosis is rendered on insufficient information. Due to these short-cuts, and at times because of pathologists who are not used to see this type of tumor, the diagnosis of MM is relatively frequently incorrect. Iwatsubo et al (2002) reported that 10% of the previously diagnosed MM they reviewed were in fact carcinomas and other malignancies that had been incorrectly diagnosed as MM. They suggested that the risk of misdiagnosis is higher when a patient has a documented history of asbestos exposure (Iwatsubo et al., 2002). In a large follow-up study that covered 25% of the French population, Goldberg et al (2006) reported that initial diagnosis of MM was confirmed in 67% of the cases, ruled out in 13% of the cases previously diagnosed as MM, and left uncertain in the remaining previously diagnosed MM with clinical evidence favoring MM in half of the uncertain cases. In our experience, the opposite mistake, incorrect diagnosis of a carcinoma or a sarcoma in a patient with MM, is also relatively common, particularly in small hospitals that rarely see MM patients (Carbone and Bedrossian 2006). Thus, too many patients continue to be misdiagnosed and consequently do not receive proper treatment for their pleural or peritoneal tumors. Probably, the most critical issue when dealing with a patient with a pleural or peritoneal tumor is to perform all the necessary tests to reach a reliable diagnosis (Husain 2009; Carbone and Bedrossian, 2006; Fresco, 2005).
The pathological features of MM in women are similar to those observed in men. The three most common histological subtypes are epithelial, biphasic, and sarcomatoid, of which epithelioid MM is the most common (Husain et al., 2009; Goldberg et al., 2006). Some studies have shown a greater proportion of the epithelial variant in women compared to men. In a study of 52 female MM patients, Roggli et al (1997) reported that 29/52 (56%) pleural MM were epithelial, 14/52 were biphasic (27%), and 9/52 (17%) were sarcomatoid variants. According to this study, the ratio of epithelial to biphasic to sarcomatoid pleural MM showed a greater proportion of epithelial variants in women (56:27:17) compared to men (41:35:23). In addition, the proportion of epithelial tumors was also increased in the 9 peritoneal female MM patients in the same series with the epithelial type accounting for 56% in women compared to 49% in men. This preponderance of the epithelial variant in women has also been reported in other series. For example, in Canada, Magner and McDonald (1972) showed a greater proportion of the epithelial subtype in women - 19/32 (59%) compared to men 26/67 (39%). The reasons for the different subtype proportions are unknown. However, because epithelial MM has a much better survival than other histological subtypes, women with MM have better survival rates than men.
Differential Diagnosis
It has been suggested that some studies may have mistakenly included tumors that resemble peritoneal MM, such as papillary carcinomas of the peritoneum and of the ovary and tumors originating from the extraovarian Mullerian system or endosalpingosis (Dawson et al., 1993; Husain et al., 2009). Special caution must be exercised when making the diagnosis of epithelial peritoneal MM in females because peritoneal MM can sometimes be mistaken for other tumor types that involve the peritoneum due to overlapping histological patterns (Ordonez, 2007). In particular, peritoneal MM must be distinguished from primary and secondary serous papillary carcinomas of the peritoneum and ovary, well-differentiated papillary mesotheliomas of the peritoneum. Similar to its pleural counterpart, immunohistochemistry, histology, electron microscopy, radiological imaging, and clinical experience are crucial in making an accurate diagnosis, which can have wide-ranging effects on subsequent treatment.
Serous carcinomas diffusely involving the peritoneum without an apparent origin in the ovaries (in women) can be difficult to differentiate from epithelioid peritoneal MM, although a number of distinct histological features commonly seen in serous carcinomas (e.g. cellular papillae, slit-like spaces and Psammoma bodies) that are less frequently observed in peritoneal MM makes the diagnosis solely by histological diagnosis possible (Baker et al., 2005), but in our opinion unreliable without further testing. Immunohistochemistry plays an important role in helping to differentiate between these two distinct pathological entities (Ordonez, 1998, 2006). Electron microscopy remains the gold standard for ultimate diagnosis, as the microvilli of serous papillary carcinomas tend to be sparse and short in contrast to the numerous long slender microvilli seen in MM (Fresco, 2005).
Well-differentiated papillary mesotheliomas (WDPMs) are unusual tumors that arise predominantly in the peritoneum of young to middle-aged women (Daya and McCaughey, 1990). WDPM may also occur in men and may rarely arise in the pericardium, pleura, and tunica vaginalis (Butnor et al., 2001). The majority of WDPMs behave in a clinically indolent fashion, in contrast to the highly aggressive course of MM. The prognostic significance of age, sex, and tumor location, as well as the role of chemotherapy, remains to be determined for WDPMs (Butnor et al., 2001). Asbestos exposure, however, does not appear to cause WDPM.
Deciduoid MM are rare in both women and men. The deciduoid appearance may predominate or represent a minor component within a more conventional appearing epithelioid MM. The immunohistochemical profile is similar to epithelial MM. In addition, positivity for neuronal markers is present in some cases (Shanks et al., 2000). Deciduoid MM is a part of the histopathologic spectrum encountered in epithelioid MM that can be associated with asbestos exposure (Shanks et al., 2000; Shia et al., 2002).
Finally, there are rare cases of epithelial peritoneal MM with proven invasion, which are found predominantly in women and behave in an indolent fashion. Michele Carbone (M.C.) has seen over 10 such patients over the past 25 years with survival ranging from 8 to more than 30 years. While most of these patients were young (28-50 years old), one patient was a 67 years old man who had had MM for 9 years and was the only male patient seen in this group. Most of these patients had received different forms of treatment in the past, had not received treatment for years and presented to the clinic because of recurrent accumulation of ascitic or pleural fluid. Some of these patients seem to develop a condition of diffuse mesothelial hyperplasia with multiple mesothelial lesions, at times located in both peritoneum and pleura, ranging from atypical hyperplasia, WDPM, multicystic mesothelioma, and with areas showing true tumor invasion. Imunohistochemistry and EM were used to confirm the diagnosis. However, these tumors behave indolently, creating a sub-category of pseudo-MM that cannot be histologically distinguished from an epithelial MM, but which clearly has a different biological behavior and is compatible with long and possibly indefinite survival. It should be noted that M.C. receives numerous non-legal referrals for MM diagnosis from different countries. It is likely that while very rare, several cases were seen because they are unique and therefore more likely to be sent to M.C. for a second opinion. The relevance of this rare finding is that a pathological diagnosis of MM does not necessarily equate to a death sentence and this message should be transmitted to the patient.
CONCLUSION
MM is a very aggressive malignancy that often develops in asbestos exposed individuals. There are significant differences in the strength of this association. Pleural MM in men is strongly associated with asbestos exposure and most of them occur in asbestos exposed individuals. In women, a lower percent, about 30%, of MM is associated with asbestos exposure. About 30% of peritoneal MM in men is associated with above background asbestos exposure. The association of peritoneal MM in women to asbestos exposure is epidemiologically weak to undetectable. Thus, factors other than asbestos appear to be the main cause of MM in women. The gender-based difference in MM caused by erionite is less striking, probably due to the higher potency of erionite in causing MM compared to asbestos.
Although MM has been considered to be untreatable for a long time, in recent years, medical intervention has been successful in increasing median survival of peritoneal mesothelioma from a dismal 9 months to 5 years or more. Early diagnosis is critical, especially for patients with peritoneal MM, because prolonged survival can be best achieved among patients who are candidates for surgery. Thus, it is expected that the development of serological markers for the early detection of MM will lead to a further increase of the median survival of peritoneal MM patients by increasing the percent of these patients who are candidates for surgical intervention. Moreover, it is hoped that early detection will also lead to improved survival of patients with pleural MM, similar to what has been achieved for peritoneal MM in recent years.
ACKNOWLEDGEMENTS
Supported by a generous donation from the Butitta Mesothelioma Foundation. Research performed by M.C. is also supported by grants from the NCI and AACR-Landon Innovator Award. H.Y. is supported by grants from MARF, by the Riviera Country Club, ARAMEC, and the Hawaii Community Foundation.
Contract grant sponsor: National Cancer Institute
Contract grant number: P01 CA114047 to M.C.
REFERENCES
- Abratt RP, White NW, Vorobiof DA. Epidemiology of mesothelioma--a South African perspective. Lung Cancer. 2005;49(Suppl 1):S13–15. doi: 10.1016/j.lungcan.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Altekruse S, Kosary C, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner M, Lewis D, Cronin K, Chen H, Feuer E, Stinchcomb D, Edwards B. In: SEER Cancer Statistics Review, 1975-2007. Altekruse S, Kosary C, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner M, Lewis D, Cronin K, Chen H, Feuer E, Stinchcomb D, Edwards B, editors. National Cancer Institute; Bethesda, MD: 2010. http://seer.cancer.gov/csr/1975_2007/ based on November 2009 SEER data submission, posted to the SEER web site. [Google Scholar]
- Altomare DA, You H, Xiao GH, Ramos-Nino ME, Skele KL, De Rienzo A, Jhanwar SC, Mossman BT, Kane AB, Testa JR. Human and mouse mesotheliomas exhibit elevated AKT/PKB activity, which can be targeted pharmacologically to inhibit tumor cell growth. Oncogene. 2005;24(40):6080–6089. doi: 10.1038/sj.onc.1208744. [DOI] [PubMed] [Google Scholar]
- Ampleford EJ, Ohar J. Mesothelioma: you do not have to work for it. Diagn Cytopathol. 2007;35(12):774–777. doi: 10.1002/dc.20766. [DOI] [PubMed] [Google Scholar]
- Anderson HA, Lilis R, Daum SM, Fischbein AS, Selikoff IJ. Household-contact asbestos neoplastic risk. Ann N Y Acad Sci. 1976;271:311–323. doi: 10.1111/j.1749-6632.1976.tb23127.x. [DOI] [PubMed] [Google Scholar]
- Aoe K, Hiraki A, Murakami T, Toyooka S, Shivapurkar N, Gazdar AF, Sueoka N, Taguchi K, Kamei T, Takeyama H, Sugi K, Kishimoto T. Infrequent existence of simian virus 40 large T antigen DNA in malignant mesothelioma in Japan. Cancer Sci. 2006;97(4):292–295. doi: 10.1111/j.1349-7006.2006.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli V, Scalzo CC, Bruno C, Facciolo F, Lopergolo M, Granone P, Nardi F. Familial pleural malignant mesothelioma: clustering in three sisters and one cousin. Cancer Lett. 1998;130(1-2):203–207. doi: 10.1016/s0304-3835(98)00142-6. [DOI] [PubMed] [Google Scholar]
- Azuma K, Uchiyama I, Chiba Y, Okumura J. Mesothelioma risk and environmental exposure to asbestos. Int J Occup health. 2009;15:166–172. doi: 10.1179/oeh.2009.15.2.166. [DOI] [PubMed] [Google Scholar]
- Bani-Hani KE, Gharaibeh KA. Malignant peritoneal mesothelioma. J Surg Oncol. 2005;91:17–25. doi: 10.1002/jso.20266. [DOI] [PubMed] [Google Scholar]
- Baker PM, Clement PB, Young RH. Malignant peritoneal mesothelioma in women: a study of 75 cases with emphasis on their morphologic spectrum and differential diagnosis. Am J Clin Pathol. 2005;123(5):724–737. doi: 10.1309/2h0n-vrer-pp2l-jdua. [DOI] [PubMed] [Google Scholar]
- Baumann F, Rougier Y, Ambrosi JP, Robineau BP. Pleural mesothelioma in New Caledonia: an acute environmental concern. Cancer Detection and Prev. 2007;31:70–76. doi: 10.1016/j.cdp.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Berry G, Roger J, Pooley FD. Mesotheliomas. Asbestos exposure and lung burden. In: Bignon J, Peto J, Saracci R, editors. Non occupational exposure to mineral fibers. Lyon, France: 1989. pp. 486–496. IARC scientific publication No 90. [PubMed] [Google Scholar]
- Bertino P, Carbone M, Pass H. Chemotherapy of malignant pleural mesothelioma. Expert Opin Pharmacother. 2009;10(1):99–107. doi: 10.1517/14656560802631285. [DOI] [PubMed] [Google Scholar]
- Bianchi C, Brollo A, Ramani L, Bianchi T, Giarelli L. Familial mesothelioma of the pleura--a report of 40 cases. Ind Health. 2004;42(2):235–239. doi: 10.2486/indhealth.42.235. [DOI] [PubMed] [Google Scholar]
- Bianchi C, Brollo A, Ramani L, Zuch C. Asbestos-related mesothelioma in Monfalcone, Italy. Am J Ind Med. 1993;24(2):149–160. doi: 10.1002/ajim.4700240203. [DOI] [PubMed] [Google Scholar]
- Bianchi C, Bianchi T, Tommasi M. Long latency periods in asbestos-related mesothelioma of the pleura. Eur J Oncol. 2007;12(3):189–195. [PubMed] [Google Scholar]
- Bocchetta M, Di Resta I, Powers A, Fresco R, Tosolini A, Testa JR, Pass HI, Rizzo P, Carbone M. Human mesothelial cells are unusually susceptible to simian virus 40-mediated transformation and asbestos cocarcinogenicity. Proc Natl Acad Sci U S A. 2000;97(18):10214–10219. doi: 10.1073/pnas.170207097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchetta M, Eliasz S, De Marco MA, Rudzinski J, Zhang L, Carbone M. The SV40 large T antigen-p53 complexes bind and activate the insulin-like growth factor-I promoter stimulating cell growth. Cancer Res. 2008;68(4):1022–1029. doi: 10.1158/0008-5472.CAN-07-5203. [DOI] [PubMed] [Google Scholar]
- Boffetta P. Epidemiology of peritoneal mesothelioma: a review. Ann Oncol. 2007;18(6):985–990. doi: 10.1093/annonc/mdl345. [DOI] [PubMed] [Google Scholar]
- Bourdes V, Boffetta P, Pisani P. Environmental exposure to asbestos and risk of pleural mesothelioma: review and meta analysis. European J Epidemiol. 2000;16:411–417. doi: 10.1023/a:1007691003600. [DOI] [PubMed] [Google Scholar]
- Boutin C, Dumortier P, Rey F, Villiat JR, De Vuyst P. Black Spots concentrate oncogenic asbestos fibers in the parietal pleura. Am J Respir Crit Care Med. 1996;153:444–449. doi: 10.1164/ajrccm.153.1.8542156. [DOI] [PubMed] [Google Scholar]
- Britton M. The epidemiology of mesothelioma. Semin Oncol. 2002;29(1):18–25. doi: 10.1053/sonc.2002.30237. [DOI] [PubMed] [Google Scholar]
- Broaddus VC, Yang L, Scavo LM, Ernst JD, Boylan AM. Asbestos induces apoptosis of human and rabbit pleural mesothelial cells via reactive oxygen species. J Clin Invest. 1996;98:2050–2059. doi: 10.1172/JCI119010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burki T. Health experts concerned over India’s asbestos industry. Lancet. 2010;375(9715):626–7. doi: 10.1016/s0140-6736(10)60251-6. [DOI] [PubMed] [Google Scholar]
- Butnor KJ, Sporn TA, Hammar SP, Roggli VL. Well-differentiated papillary mesothelioma. Am J Surg Pathol. 2001;25(10):1304–1309. doi: 10.1097/00000478-200110000-00012. [DOI] [PubMed] [Google Scholar]
- Cacciotti P, Libener R, Betta P, Martini F, Porta C, Procopio A, Strizzi L, Penengo L, Tognon M, Mutti L, Gaudino G. SV40 replication in human mesothelial cells induces HGF/Met receptor activation: a model for viral-related carcinogenesis of human malignant mesothelioma. Proc Natl Acad Sci U S A. 2001;98(21):12032–12037. doi: 10.1073/pnas.211026798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camidge DR, Stockton DL, Bain M. Factors affecting the mesothelioma detection rate within national and international epidemiological studies: insights from Scottish linked cancer registry-mortality data. Br J Cancer. 2006;95(5):649–652. doi: 10.1038/sj.bjc.6603293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone M, Bedrossian CW. The pathogenesis of mesothelioma. Semin Diagn Pathol. 2006;23(1):56–60. doi: 10.1053/j.semdp.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Carbone M, Emri S, Dogan AU, Steele I, Tuncer M, Pass HI, Baris YI. A mesothelioma epidemic in Cappadocia: scientific developments and unexpected social outcomes. Nat Rev Cancer. 2007;7(2):147–154. doi: 10.1038/nrc2068. [DOI] [PubMed] [Google Scholar]
- Carbone M, Albelda SM, Broaddus VC, Flores RM, Hillerdal G, Jaurand MC, Kjaerheim K, Pass HI, Robinson B, Tsao A. Eighth International Mesothelioma Interest Group. Oncogene. 2007;26(49):6959–6967. doi: 10.1038/sj.onc.1210515. [DOI] [PubMed] [Google Scholar]
- Carbone M, Kratzke RA, Testa JR. The pathogenesis of mesothelioma. Semin Oncol. 2002;29(1):2–17. doi: 10.1053/sonc.2002.30227. [DOI] [PubMed] [Google Scholar]
- Carbone M, Pannuti A, Zhang L, Testa JR, Bocchetta M. A novel mechanism of late gene silencing drives SV40 transformation of human mesothelial cells. Cancer Res. 2008;68(22):9488–9496. doi: 10.1158/0008-5472.CAN-08-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone M, Pass HI, Rizzo P, Marinetti M, Di Muzio M, Mew DJ, Levine AS, Procopio A. Simian virus 40-like DNA sequences in human pleural mesothelioma. Oncogene. 1994;9(6):1781–1790. [PubMed] [Google Scholar]
- Chao CC, Park SH, Aus E. Partecipation of nitric oxide and iron in the oxidation of DNA in asbestos-treated human ling epithelial cells. Arch Biochem Biophys. 1996;326:152–157. doi: 10.1006/abbi.1996.0059. [DOI] [PubMed] [Google Scholar]
- Choe N, Tanaka S, Xia W, Hemenway DR, Roggli VL, Kagan E. Pleural macrophage recruitment and activation in asbestos-induced pleural injury. Environ Health Perspect. 1997;105(Suppl 5):1257–1260. doi: 10.1289/ehp.97105s51257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe N, Tananaka S, Kagan E. Asbestos fibers and interleukin 1 upregulate the formation of reactive nitrogen species in rat pleural mesothelial cells. Am J respir Cell Mol Biol. 1998;19:226–236. doi: 10.1165/ajrcmb.19.2.3111. [DOI] [PubMed] [Google Scholar]
- Chua TC, Yan TD, Morris DL. Surgical biology for the clinician: peritoneal mesothelioma: current understanding and management. Can J Surg. 2009a;52(1):59–64. [PMC free article] [PubMed] [Google Scholar]
- Chua TC, Yao P, Akther J, Young L, Bao S, Samaraweera U, Yan TD, Morris DL. Differential Expression of Ki-67 and Sex Steroid Hormone Receptors Between Genders in Peritoneal Mesothelioma. Pathol Oncol Res. 2009b;15(4):671–678. doi: 10.1007/s12253-009-9170-0. [DOI] [PubMed] [Google Scholar]
- Cicala C, Pompetti F, Carbone M. SV40 induces mesotheliomas in hamsters. Am J Pathol. 1993;142(5):1524–1533. [PMC free article] [PubMed] [Google Scholar]
- Connelly RR, Spirtas R, Myers MH, Percy CL, Fraumeni JF., Jr. Demographic patterns for mesothelioma in the United States. J Natl Cancer Inst. 1987;78(6):1053–1060. [PubMed] [Google Scholar]
- Cutrone R, Lednicky J, Dunn G, Rizzo P, Bocchetta M, Chumakov K, Minor P, Carbone M. Some oral poliovirus vaccines were contaminated with infectious SV40 after 1961. Cancer Res. 2005;65(22):10273–10279. doi: 10.1158/0008-5472.CAN-05-2028. [DOI] [PubMed] [Google Scholar]
- Dawson A, Gibbs AR, Pooley FD, Griffiths DM, Hoy J. Malignant mesothelioma in women. Thorax. 1993;48(3):269–274. doi: 10.1136/thx.48.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daya D, McCaughey WT. Well-differentiated papillary mesothelioma of the peritoneum. A clinicopathologic study of 22 cases. Cancer. 1990;65(2):292–296. doi: 10.1002/1097-0142(19900115)65:2<292::aid-cncr2820650218>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- De Klerk NH, Armstrong BK, Musk AW, et al. Cancer mortality in relation to measures of occupational exposure to crocidolite at Wittenoom George in Western Australia. Br J Ind Med. 1989;46:529–536. doi: 10.1136/oem.46.8.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destro A, Ceresoli GL, Falleni M, Zucali PA, Morenghi E, Bianchi P, Pellegrini C, Cordani N, Vaira V, Alloisio M, Rizzi A, Bosari S, Roncalli M. EGFR overexpression in malignant pleural mesothelioma. An immunohistochemical and molecular study with clinico-pathological correlations. Lung Cancer. 2006;51(2):207–215. doi: 10.1016/j.lungcan.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Dodoli D, Del Nevo M, Fiumalbi C, Iaia TE, Cristaudo A, Comba P, Viti C, Battista G. Environmental household exposures to asbestos and occurrence of pleural mesothelioma. Am J Ind Med. 1992;21(5):681–687. doi: 10.1002/ajim.4700210508. [DOI] [PubMed] [Google Scholar]
- Dodson RF, Atkinson MAL, Levin JL. Asbestos fiber length as related to potential pathogenicity: a critical review. Am J Ind Med. 2003;44:291–297. doi: 10.1002/ajim.10263. [DOI] [PubMed] [Google Scholar]
- Dodson RF, Graef R, Shepherd S, O’Sullivan M, Levin JL. Asbestos burden in cases of mesothelioma from individuals from various regions of the United States. Ultrast Path. 2005;29:415–433. doi: 10.1080/019131290945682. [DOI] [PubMed] [Google Scholar]
- Dodson RF, O’Sullivan M, Corn C, McLarty JW, Hammar SP. Analysis of asbestos fiber burden in lung tissue from mesothelioma patients. Ultrastruct. Pathol. 1997;21:321–336. doi: 10.3109/01913129709021930. [DOI] [PubMed] [Google Scholar]
- Dodson RF, O’Sullivan M, Brooks DR, Hammar SP. Quantitative analysis of asbestos burden in women with mesothelioma. Am J Ind Med. 2003;43(2):188–195. doi: 10.1002/ajim.10164. [DOI] [PubMed] [Google Scholar]
- Dodson RF, O’Sullivan M, Brooks DR, Bruce JR. Asbestos content of omentum and mesentery in nonoccupationally exposed individuals, Toxicol. Ind. Health. 2001;17:138–143. doi: 10.1191/0748233701th101oa. [DOI] [PubMed] [Google Scholar]
- Dodson RF, O’Sullivan M, Huang J, Holiday DB, Hammar SP. Asbestos in extrapulmonary sites omentum and mesentery. Chest. 117:486–493. doi: 10.1378/chest.117.2.486. [DOI] [PubMed] [Google Scholar]
- Dodson RF, Shepherd S, Levin J, Hammar SP. Characteristics of the asbestos concentration in various levels of lymph nodes that collect drainage from the lung. Ultrastructural Pathology. 2007;31:95–153. doi: 10.1080/01913120701423907. [DOI] [PubMed] [Google Scholar]
- Dodson RF, Williams MG, Corn CJ, Brollo A, Bianchi C. Asbestos content of lung tissue, lymph nodes and pleural plaques from former shipyard workers. Am. Rev. Respir. Dis. 1990;142:843–847. doi: 10.1164/ajrccm/142.4.843. [DOI] [PubMed] [Google Scholar]
- Dogan AU, Baris YI, Dogan M, Emri S, Steele I, Elmishad AG, Carbone M. Genetic predisposition to fiber carcinogenesis causes a mesothelioma epidemic in Turkey. Cancer Res. 2006;66(10):5063–5068. doi: 10.1158/0008-5472.CAN-05-4642. [DOI] [PubMed] [Google Scholar]
- Doll R. Mortality from lung cancer in asbestos workers. Br J Ind Med. 1955;12(2):81–86. doi: 10.1136/oem.12.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driece HA, Siesling S, Swuste PH, Burdorf A. Assessment of cancer risks due to environmental exposure to asbestos. J Expo Sci Environ Epidemiol. 2010;(5):478–85. doi: 10.1038/jes.2009.56. [DOI] [PubMed] [Google Scholar]
- Eltabbakh GH, Piver MS, Hempling RE, Recio FO, Intengen ME. Clinical picture, response to therapy, and survival of women with diffuse malignant peritoneal mesothelioma. J Surg Oncol. 1999;70(1):6–12. doi: 10.1002/(sici)1096-9098(199901)70:1<6::aid-jso2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Epler GR, Gerald MX Fitz, Gaensler EA, Carrington CB. Asbestos-related disease from household exposure. Respiration. 1980;39(4):229–240. doi: 10.1159/000194221. [DOI] [PubMed] [Google Scholar]
- Ferrante D, Bertolotti M, Todesco A, Mirabelli D, Terracini B, Magnani C. Cancer mortality and incidence of mesothelioma in a cohort of wives of asbestos workers in Casale Monferrato, Italy. Environ Health Perspect. 2007;115(10):1401–1405. doi: 10.1289/ehp.10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores RM, Pass HI, Seshan VE, Dycoco J, Zakowski M, Carbone M, Bains MS, Rusch VW. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg. 2008;135(3):620–626. e621–623. doi: 10.1016/j.jtcvs.2007.10.054. 626. [DOI] [PubMed] [Google Scholar]
- Fresco R. Malignant mesothelioma electron microscopy. In: Pass HIVN, Carbone M, editors. Malignant Mesothelioma Advances in Pathogenesis, Diagnosis, and Translational Therapies. Springer Science+Business Media, Inc.; New York: 2005. pp. 508–516. [Google Scholar]
- Gazdar AF, Butel JS, Carbone M. SV40 and human tumours: myth, association or causality? Nat Rev Cancer. 2002;2(12):957–964. doi: 10.1038/nrc947. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Imbernon E, Rolland P, Ilg AGS, de Quillacq A, Frenay C, Chamming’s S, Arveux P, Boutin C, Pairon JC, Astoul P, Galateau-Salle F, Brochard P. The French National Mesothelioma Surveillance Program. Occup Env Med. 2006;63:390–395. doi: 10.1136/oem.2005.023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman JE, Nascarella MA, Valberg PA. Ionizing radiation: a risk factor for mesothelioma. Cancer Causes Control. 2009;20(8):1237–1254. doi: 10.1007/s10552-009-9357-4. [DOI] [PubMed] [Google Scholar]
- Gorovenko GG, Slepukha Im, et al. Pleural mesothelioma after therapeutic artificial pneumothorax. Vrach Delo. 1986;6:54–56. [PubMed] [Google Scholar]
- Griffiths MH, Riddell RJ, Xipell JM. Malignant mesothelioma: a review of 35 cases with diagnosis and prognosis. Pathology (Phila) 1980;12(4):591–603. doi: 10.3109/00313028009086812. [DOI] [PubMed] [Google Scholar]
- Heintz NH, Janssen-Heininger YM, Mossman BT. Asbestos, lung cancers, and mesotheliomas: from molecular approaches to targeting tumor survival pathways. Am J Respir Cell Mol Biol. 2010;42(2):133–139. doi: 10.1165/rcmb.2009-0206TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller DS, Gordon RE, Clement PB, Turnnir R, Katz N. Presence of asbestos in peritoneal malignant mesotheliomas in women. Int J Gynecol Cancer. 1999;9(6):452–455. doi: 10.1046/j.1525-1438.1999.99060.x. [DOI] [PubMed] [Google Scholar]
- Hershcovici T, Chajek-Shaul T, Hasin T, et al. Familial Mediterranean fever and peritoneal malignant mesothelioma: a possible association. Isr Med Assoc J. 2006;8:501–511. [PubMed] [Google Scholar]
- Hesdorffer ME, Chabot J, DeRosa C, Taub R. Peritoneal mesothelioma. Curr Treat Options Oncol. 2008;9(2-3):180–190. doi: 10.1007/s11864-008-0072-2. [DOI] [PubMed] [Google Scholar]
- Hillerdal G. Malignant mesothelioma 1982: review of 4710 published cases. Br J Dis Chest. 1983;77(4):321–343. [PubMed] [Google Scholar]
- Hillerdal G. Mesothelioma: cases associated with non-occupational and low dose exposures. Occup Environ Med. 1999;56(8):505–513. doi: 10.1136/oem.56.8.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillerdal T, Berg J. Malignant mesothelioma secondary to chronic inflammation and old scars. Cancer. 1985;55:1968–1972. doi: 10.1002/1097-0142(19850501)55:9<1968::aid-cncr2820550923>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Husain AN, Colby TV, Ordonez NG, Krausz T, Borczuk A, Cagle PT, Chirieac LR, Churg A, Galateau-Salle F, Gibbs AR, Gown AM, Hammar SP, Litzky LA, Roggli VL, Travis WD, Wick MR. Guidelines for pathologic diagnosis of malignant mesothelioma: a consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2009;133(8):1317–1331. doi: 10.5858/133.8.1317. [DOI] [PubMed] [Google Scholar]
- Ilgren EB, Pooley FD, Larragoitia JC, Talamantes M, Navarrete GL, Krauss E, Brena AF. First confirmed erionite related mesothelioma in North America. Indoor Built Environ. 2008;0:1–2. [Google Scholar]
- Ismail-Khan R, Robinson LA, Williams CC, Jr., Garrett CR, Bepler G, Simon GR. Malignant pleural mesothelioma: a comprehensive review. Cancer Control. 2006;13(4):255–263. doi: 10.1177/107327480601300402. [DOI] [PubMed] [Google Scholar]
- Iwatsubo Y, Matrat M, Michel E, Boutin C, Galateau-Salle F, Jougla E, Bignon J, Pairon JC, Brochard P. Estimation of the incidence of pleural mesothelioma according to death certificates in France. Am J Ind Med. 2002;42(3):188–99. doi: 10.1002/ajim.10098. [DOI] [PubMed] [Google Scholar]
- Jagadeeswaran R, Ma PC, Seiwert TY, Jagadeeswaran S, Zumba O, Nallasura V, Ahmed S, Filiberti R, Paganuzzi M, Puntoni R, Kratzke RA, Gordon GJ, Sugarbaker DJ, Bueno R, Janamanchi V, Bindokas VP, Kindler HL, Salgia R. Functional analysis of c-Met/hepatocyte growth factor pathway in malignant pleural mesothelioma. Cancer Res. 2006;66(1):352–361. doi: 10.1158/0008-5472.CAN-04-4567. [DOI] [PubMed] [Google Scholar]
- Jemal A, Grauman D, Devesa S. Recent geographic patterns of lung cancer and mesothelioma mortality rates in 49 shipyard counties in the United States, 1970-94. Am J Ind Med. 2000;37(5):512–521. doi: 10.1002/(sici)1097-0274(200005)37:5<512::aid-ajim7>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Jimenez LA, et al. Role of extra cellular signal regulated protein kinases in apoptosis by asbestos and H2O2. Am J Physiol. 1997;273:L1029–L1035. doi: 10.1152/ajplung.1997.273.5.L1029. [DOI] [PubMed] [Google Scholar]
- Kielkowski D, Nelson G, Rees D. Risk of mesothelioma from exposure to crocidolite asbestos: a 1995 update of a South African mortality study. Occup Environ Med. 2000;57(8):563–567. doi: 10.1136/oem.57.8.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliment CR, Clemens K, Oury TD. North american erionite-associated mesothelioma with pleural plaques and pulmonary fibrosis: a case report. Int J Clin Exp Pathol. 2009;2(4):407–410. [PMC free article] [PubMed] [Google Scholar]
- Kroczynska B, Cutrone R, Bocchetta M, Yang H, Elmishad AG, Vacek P, Ramos-Nino M, Mossman BT, Pass HI, Carbone M. Crocidolite asbestos and SV40 are cocarcinogens in human mesothelial cells and in causing mesothelioma in hamsters. Proc Natl Acad Sci U S A. 2006;103(38):14128–14133. doi: 10.1073/pnas.0604544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krousel T, Garcas N, Rothschild H. Familial clustering of mesothelioma: a report on three affected persons in one family. Am J Prev Med. 1986;2(4):186–188. [PubMed] [Google Scholar]
- Kurumatani N, Kumagai S. Mapping the risk of mesothelioma due to neighborhood asbestos exposure. Am J Respir Crit Care Med. 2008;178(6):624–629. doi: 10.1164/rccm.200801-063OC. [DOI] [PubMed] [Google Scholar]
- Lagnese JA. Economic Aspects of Meothelioma. In: Pass HI, Vogelzang N, Carbone M, editors. Malignant Mesothelioma, Advances in Pathogenesis, adiagnosis and Translational therapies. Springer Science+Business Media, Inc.; New York: 2002. pp. 821–832. [Google Scholar]
- LeBouffant L. Physics and chemistry of asbestos dust. In: Wagner JC, editor. Biological Effects of mineral fibres. IARC Scientific Publications; Lyon, France: 1980. pp. 15–33. [PubMed] [Google Scholar]
- Leigh J, Davidson P, Hendrie L, Berry D. Malignant mesothelioma in Australia, 1945-2000. Am J Ind Med. 2002;41(3):188–201. doi: 10.1002/ajim.10047. [DOI] [PubMed] [Google Scholar]
- Li FP, Lokich J, Lapey J, Neptune WB, Wilkins EW., Jr. Familial mesothelioma after intense asbestos exposure at home. JAMA. 1978;240(5):467. [PubMed] [Google Scholar]
- Lieben J, Pistawka H. Mesothelioma and asbestos exposure. Arch Environ Health. 1967;14(4):559–563. doi: 10.1080/00039896.1967.10664792. [DOI] [PubMed] [Google Scholar]
- Liu JY, Brass DM, Hoyle GW, Brody AR. TNF-alpha receptor knockout mice are protected from the fibroproliferative effects of inhaled asbestos fibers. Am J Pathol. 1998;153(6):1839–1847. doi: 10.1016/s0002-9440(10)65698-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rios F, Illei PB, Rusch V, Ladanyi M. Evidence against a role for SV40 infection in human mesotheliomas and high risk of false-positive PCR results owing to presence of SV40 sequences in common laboratory plasmids. Lancet. 2004;364(9440):1157–1166. doi: 10.1016/S0140-6736(04)17102-X. [DOI] [PubMed] [Google Scholar]
- Low J, Humes HD, Szczypka M, Imperiale M. BKV and SV40 infection of human kidney tubular epithelial cells in vitro. Virology. 2004;323(2):182–188. doi: 10.1016/j.virol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Machida N, Tanaka R, Takemura N, et al. Development of pericardial mesothelioma in golden retrievers with a long term history of idiopathic haemorrhagic pericardial effusion. J Comp Path. 2004;131:166–175. doi: 10.1016/j.jcpa.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Maher B. Fear in the dust. Nature. 2010;468:884–885. doi: 10.1038/468884a. [DOI] [PubMed] [Google Scholar]; Asbestos scandal. Nature. 2010;468:868. doi: 10.1038/468868a. Editorial. [DOI] [PubMed] [Google Scholar]
- Magnani C, Agudo A, Gonzalez CA, Andrion A, Calleja A, Chellini E, Dalmasso P, Escolar A, Hernandez S, Ivaldi C, Mirabelli D, Ramirez J, Turuguet D, Usel M, Terracini B. Multicentric study on malignant pleural mesothelioma and non-occupational exposure to asbestos. Br J Cancer. 2000;83(1):104–111. doi: 10.1054/bjoc.2000.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani C, Dalmasso P, Biggeri A, Ivaldi C, Mirabelli D, Terracini B. Increased risk of malignant mesothelioma of the pleura after residential or domestic exposure to asbestos: a case-control study in Casale Monferrato, Italy. Environ Health Perspect. 2001;109(9):915–919. doi: 10.1289/ehp.01109915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani C, Terracini B, Ivaldi C, Botta M, Budel P, Mancini A, Zanetti R. A cohort study on mortality among wives of workers in the asbestos cement industry in Casale Monferrato, Italy. Br J Ind Med. 1993;50(9):779–784. doi: 10.1136/oem.50.9.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani C, Terracini B, Ivaldi C, Botta M, Mancini A, Andrion A. Pleural malignant mesothelioma and non-occupational exposure to asbestos in Casale Monferrato, Italy. Occup Environ Med. 1995;52(6):362–367. doi: 10.1136/oem.52.6.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magner D, McDonald AD. Malignant mesothelial tumors - histologic type and asbestos exposure. N Engl J Med. 1972;287(11):570–571. [PubMed] [Google Scholar]
- Marchevsky AM, Harber P, Crawford L, Wick MR. Mesothelioma in patients with nonoccupational asbestos exposure. An evidence-based approach to causation assessment. Ann Diagn Pathol. 2006;10(4):241–250. doi: 10.1016/j.anndiagpath.2006.06.012. [DOI] [PubMed] [Google Scholar]
- McDonald AD, McDonald JC. Malignant mesothelioma in North America. Cancer. 1980;46(7):1650–1656. doi: 10.1002/1097-0142(19801001)46:7<1650::aid-cncr2820460726>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Merler E, Roberti S. A population-based study on Radiotherapy (RT) as a risk factor for Malignant Mesothelioma (MM). The 9th International Conference of the International Mesothelioma Interest Group.2008. p. 116. [Google Scholar]
- Miller A. Mesothelioma in household members of asbestos-exposed workers: 32 United States cases since 1990. Am J Ind Med. 2005;47(5):458–462. doi: 10.1002/ajim.20167. [DOI] [PubMed] [Google Scholar]
- Mitchev K, Dumortier P, DeVuyst P. “Black Spots” and Hyaline Pleural Plaques on the Parietal Pleura of 150 Urban Necropsy Cases. The Am J Surg Path. 2002;25:1198–1206. doi: 10.1097/00000478-200209000-00010. [DOI] [PubMed] [Google Scholar]
- Mitha R, Pooley FD. Determination of asbestos in lung tissue using transmission electron microscopy. IARC Sci Publ. 1993;(109):190–195. [PubMed] [Google Scholar]
- Moolgavkar SH, Meza R, Turim J. Pleural and peritoneal mesotheliomas in SEER: age effects and temporal trends, 1973-2005. Cancer Causes Control. 2009;20(6):935–944. doi: 10.1007/s10552-009-9328-9. [DOI] [PubMed] [Google Scholar]
- Muller K,-M, Schmitz I, Konstantinidis K. Black spots of the parietal pleura: Morphology and Formal Pathogenesis. Respiration. 2002;69:261–267. doi: 10.1159/000063630. [DOI] [PubMed] [Google Scholar]
- Murai Y. Malignant mesothelioma in Japan: analysis of registered autopsy cases. Arch Environ Health. 2001;56(1):84–88. doi: 10.1080/00039890109604058. [DOI] [PubMed] [Google Scholar]
- Musk AW, Dolin PJ, Armstrong BK, Ford JM, de Klerk NH, Hobbs MS. The incidence of malignant mesothelioma in Australia, 1947-1980. Med J Aust. 1989;150(5):242–243. 246. doi: 10.5694/j.1326-5377.1989.tb136455.x. [DOI] [PubMed] [Google Scholar]
- Musti M, Cavone D, Aalto Y, Scattone A, Serio G, Knuutila S. A cluster of familial malignant mesothelioma with del(9p) as the sole chromosomal anomaly. Cancer Genet Cytogenet. 2002;138(1):73–76. doi: 10.1016/s0165-4608(02)00575-7. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Makishima A, Hagino K, Okabe K. Accumulation of radium in ferruginous protein bodies formed in lung tissue: association of resulting radiation hotspots with malignant mesothelioma and other malignancies. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(7):229–239. doi: 10.2183/pjab.85.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson WJ. Tumour incidence after asbestos exposure in the USA: cancer risk of non-occupational population. 1983. pp. 161–177. Report nr VDI-Report No. 475.
- Olofsson K, Mark J. Specificity of asbestos-induced chromosomal aberrations in short-term cultured human mesothelial cells. Cancer Genet Cytogenet. 1989;41(1):33–39. doi: 10.1016/0165-4608(89)90105-2. [DOI] [PubMed] [Google Scholar]
- Ordonez NG. Role of immunohistochemistry in distinguishing epithelial peritoneal mesotheliomas from peritoneal and ovarian serous carcinomas. Am J Surg Pathol. 1998;22(10):1203–1214. doi: 10.1097/00000478-199810000-00005. [DOI] [PubMed] [Google Scholar]
- Ordonez NG. The diagnostic utility of immunohistochemistry and electron microscopy in distinguishing between peritoneal mesotheliomas and serous carcinomas: a comparative study. Mod Pathol. 2006;19(1):34–48. doi: 10.1038/modpathol.3800471. [DOI] [PubMed] [Google Scholar]
- Ordonez NG. Pathologic characterization and differential diagnosis of malignant peritoneal mesothelioma. Recent Results Cancer Res. 2007;169:123–136. doi: 10.1007/978-3-540-30760-0_12. [DOI] [PubMed] [Google Scholar]
- Pan XL, Day HW, Wang W, Beckett LA, Schenker MB. Residential proximity to naturally occurring asbestos and mesothelioma risk in California. Am J Respir Crit Care Med. 2005;172(8):1019–1025. doi: 10.1164/rccm.200412-1731OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti L, Falchi M, Batisti D, Zappa M, Chellini E, Biancalani M. Characterization of asbestos fibers in pleural tissue from 21 cases of mesothelioma. Med Lav. 1993;84:373–378. [PubMed] [Google Scholar]
- Paoletti L, Batisti D, Bruno C, Di Paola M, Gianfagna A, Mastrantonio M, Nesti M, Comba P. Unusually high incidence of malignant pleural mesothelioma in a town of eastern Sicily: an epidemiological and environmental study. Arch Environ Health. 2000;55(6):392–398. doi: 10.1080/00039890009604036. [DOI] [PubMed] [Google Scholar]
- Pass HI, Lott D, Lonardo F, Harbut M, Liu Z, Tang N, Carbone M, Webb C, Wali A. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N Engl J Med. 2005;353(15):1564–1573. doi: 10.1056/NEJMoa051185. [DOI] [PubMed] [Google Scholar]
- Patel AV, Bogner PN, Klippenstein D, Ramnath N. Malignant pleural mesothelioma after household exposure to asbestos. J Clin Oncol. 2008;26(33):5480–5483. doi: 10.1200/JCO.2008.18.8268. [DOI] [PubMed] [Google Scholar]
- Percy C, Stanek E, 3rd, Gloeckler L. Accuracy of cancer death certificates and its effect on cancer mortality statistics. Am J Public Health. 1981;71(3):242–250. doi: 10.2105/ajph.71.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto J, Doll R, Hermon C, et al. Relationship of mortality to measures of environmental asbestos pollution in an asbestos textile factory. Ann Occup Hyg. 1985;29:305–355. doi: 10.1093/annhyg/29.3.305. [DOI] [PubMed] [Google Scholar]
- Picklesimer AH, Zanagnolo V, Niemann TH, Eaton LA, Copeland LJ. Case report: malignant peritoneal mesothelioma in two siblings. Gynecol Oncol. 2005;99(2):512–516. doi: 10.1016/j.ygyno.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Pietruska JR, Kane AB. SV40 oncoproteins enhance asbestos-induced DNA double-strand breaks and abrogate senescence in murine mesothelial cells. Cancer Res. 2007;67(8):3637–3645. doi: 10.1158/0008-5472.CAN-05-3727. [DOI] [PubMed] [Google Scholar]
- Pinheiro GA, Antao VC, Bang KM, Attfield MD. Malignant mesothelioma surveillance: a comparison of ICD 10 mortality data with SEER incidence data in nine areas of the United States. Int J Occup Environ Health. 2004;10(3):251–255. doi: 10.1179/oeh.2004.10.3.251. [DOI] [PubMed] [Google Scholar]
- Pinton G, Brunelli E, Murer B, Puntoni R, Puntoni M, Fennell DA, Gaudino G, Mutti L, Moro L. Estrogen receptor-beta affects the prognosis of human malignant mesothelioma. Cancer Res. 2009;69(11):4598–4604. doi: 10.1158/0008-5472.CAN-08-4523. [DOI] [PubMed] [Google Scholar]
- Price B, Ware A. Time trend of mesothelioma incidence in the United States and projection of future cases: an update based on SEER data for 1973 through 2005. Crit Rev Toxicol. 2009;39(7):576–588. doi: 10.1080/10408440903044928. [DOI] [PubMed] [Google Scholar]
- Quinlan TR, BeruBe KA, Hacker MP, Taatjes DJ, Timblin CR, Goldberg J, Kimberley P, O’Shaughnessy O, He,enway D, Torino J, Jimenez LA, Mossman BT. Mechanisms of asbestos-induced nitric oxide production by rat alveolar macrophages in inhalation and in vitro models. Radic Biol Med. 1998;24:778–788. doi: 10.1016/s0891-5849(97)00357-2. [DOI] [PubMed] [Google Scholar]
- Ramos-Nino ME, Blumen SR, Sabo-Attwood T, Pass H, Carbone M, Testa JR, Altomare DA, Mossman BT. HGF mediates cell proliferation of human mesothelioma cells through a PI3K/MEK5/Fra-1 pathway. Am J Respir Cell Mol Biol. 2008;38(2):209–217. doi: 10.1165/rcmb.2007-0206OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak J, Lilis R, Suzuki Y, Penner L, Selikoff IJ. Death certificate categorization of malignant pleural and peritoneal mesothelioma in a cohort of asbestos insulation workers. J Soc Occup Med. 1991;41(3):137–139. doi: 10.1093/occmed/41.3.137. [DOI] [PubMed] [Google Scholar]
- Risberg B, Nickels J, Wagermark J. Familial clustering of malignant mesothelioma. Cancer. 1980;45(9):2422–2427. doi: 10.1002/1097-0142(19800501)45:9<2422::aid-cncr2820450930>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Rivera Z, Strianese O, Bertino P, Yang H, Pass H, Carbone M. The relationship between simian virus 40 and mesothelioma. Curr Opin Pulm Med. 2008;14(4):316–321. doi: 10.1097/MCP.0b013e3283018220. [DOI] [PubMed] [Google Scholar]
- Robinson C, van Bruggen I, Segal A, Dunham M, Sherwood A, Koentgen F, Robinson BW, Lake RA. A novel SV40 TAg transgenic model of asbestos-induced mesothelioma: malignant transformation is dose dependent. Cancer Res. 2006;66(22):10786–10794. doi: 10.1158/0008-5472.CAN-05-4668. [DOI] [PubMed] [Google Scholar]
- Rodelsperger K, Jockel KH, Pohlabeln H, Romer W, Woitowitz HJ. Asbestos and man made vitreous fibers as risk factors for diffuse malignant mesothelioma: Results from a german hospital based case control study. Am J Ind Med. 2001;39:262–275. doi: 10.1002/1097-0274(200103)39:3<262::aid-ajim1014>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Roe FJ, Carter RL, Walters MA, Harington JS. The pathological effects of subcutaneous injections of asbestos fibres in mice: migration of fibres to submesothelial tissues and induction of mesotheliomata. Int J Cancer. 1967;2(6):628–638. doi: 10.1002/ijc.2910020624. [DOI] [PubMed] [Google Scholar]
- Roggli VL, Oury TD, Moffatt EJ. Malignant mesothelioma in women. Anat Pathol. 1997;2:147–163. [PubMed] [Google Scholar]
- Roggli VL, Sharma A, Butnor KJ, Sporn T, Vollmer RT. Malignant mesothelioma and occupational exposure to asbestos: a clinicopathological correlation of 1445 cases. Ultrastruct Pathol. 2002;26(2):55–65. doi: 10.1080/01913120252959227. [DOI] [PubMed] [Google Scholar]
- Ross D, McDonald JC. Occupational and geographical factors in the epidemiology of malignant mesothelioma. Monaldi Archives for Chest isease. 1995;50(6):459–463. [PubMed] [Google Scholar]
- Roushdy-Hammady I, Siegel J, Emri S, Testa JR, Carbone M. Genetic-susceptibility factor and malignant mesothelioma in the Cappadocian region of Turkey. Lancet. 2001;357(9254):444–445. doi: 10.1016/S0140-6736(00)04013-7. [DOI] [PubMed] [Google Scholar]
- Schneider J, Straif K, Woitowitz HJ. Pleural mesothelioma and household asbestos exposure. Rev Environ Health. 1996;11(1-2):65–70. doi: 10.1515/reveh.1996.11.1-2.65. [DOI] [PubMed] [Google Scholar]
- Schneider J, Woitowitz HJ. Asbestos-related mesotheliomas in housewives from indoor air pollution. Zentralbl Hyg Umweltmed. 1995;196(6):495–503. [PubMed] [Google Scholar]
- Sebastien P, Jason X, Gaudichet A, Hirsch A, Bignon J. Asbestos retention in human respiratory tissues: Comparative measurements in lung parenchyma and in parietal pleura. In: Wagner JC, editor. Biological effects of mineral fibers. IARC; Lyon: 1980. pp. P237–246. [PubMed] [Google Scholar]
- Sebbag G, Sugarbaker PH. Peritoneal mesothelioma proposal for a staging system. Eur J Surg Oncol. 2001;27(3):223–224. doi: 10.1053/ejso.2001.1044. [DOI] [PubMed] [Google Scholar]
- Selikoff IJ, Hammond EC, Seidman H. Mortality experience of insulation workers in the United States and in Canada 1943-1976. Ann. NY Acad Sci. 1979;330:91–96. doi: 10.1111/j.1749-6632.1979.tb18711.x. [DOI] [PubMed] [Google Scholar]
- Selikoff IJ. Use of death certificates in epidemiological studies, including occupational hazards: discordance with clinical and autopsy findings. Am J Ind Med. 1992;22(4):469–480. doi: 10.1002/ajim.4700220402. [DOI] [PubMed] [Google Scholar]
- Shanks JH, Harris M, Banerjee SS, Eyden BP, Joglekar VM, Nicol A, Hasleton PS, Nicholson AG. Mesotheliomas with deciduoid morphology: a morphologic spectrum and a variant not confined to young females. Am J Surg Pathol. 2000;24(2):285–294. doi: 10.1097/00000478-200002000-00015. [DOI] [PubMed] [Google Scholar]
- Shia J, Erlandson RA, Klimstra DS. Deciduoid mesothelioma: a report of 5 cases and literature review. Ultrastruct Pathol. 2002;26(6):355–363. doi: 10.1080/0913120290104647. [DOI] [PubMed] [Google Scholar]
- Sluis-Cremer GK. Asbestos disease at low exposures after long residence times. Ann N Y Acad Sci. 1991;643:182–193. doi: 10.1111/j.1749-6632.1991.tb24461.x. [DOI] [PubMed] [Google Scholar]
- Sluis-Cremer GK, Liddell FDK, Logan WPD, Bezuidenhout BN. British J Ind Med. 1992;49:566–575. doi: 10.1136/oem.49.8.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirtas R, Heineman EF, Bernstein L, Beebe GW, Keehn RJ, Stark A, Harlow BL, Benichou J. Malignant mesothelioma: attributable risk of asbestos exposure. Occup Environ Med. 1994;51(12):804–811. doi: 10.1136/oem.51.12.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton K, Alamario DA, McCormick MC. Immunization safety review SV40 contamination of polio vaccine and cancer. The National Academies Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- Sugarbaker DJ. Pleural MM as a second primary cancer post therapeutic radiation for Hodgkin’s and non Hodgkin’s disease. The 9th international conference of the International Mesothelioma Interest Group.2008. p. 115. [Google Scholar]
- Suzuki Y, Yuen RS, Ashley R. Short, thin asbestos fibers contribute to the development of malignant mesothelioma: pathological evidence. Int J hyg Environ Health. 2005;208:201–210. doi: 10.1016/j.ijheh.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Tan E, Warren N, Darnton AJ, Hodgson JT. Projections of mesothelioma mortality in Britain using Bayesian methods. British J Cancer. 2010 doi: 10.1038/sj.bjc.6605781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa JR, Carbone M, Hirvonen A, Khalili K, Krynska B, Linnainmaa K, Pooley FD, Rizzo P, Rusch V, Xiao GH. A multi-institutional study confirms the presence and expression of simian virus 40 in human malignant mesotheliomas. Cancer Res. 1998;58(20):4505–4509. [PubMed] [Google Scholar]
- Tweedale G, McCulloch J. Chrysophiles versus chrysophobes: the white asbestos controversy, 1950s-2004. 2004. pp. 239–59. [DOI] [PubMed]
- Vianna NJ, Polan AK. Non-occupational exposure to asbestos and malignant mesothelioma in females. Lancet. 1978;1(8073):1061–1063. doi: 10.1016/s0140-6736(78)90911-x. [DOI] [PubMed] [Google Scholar]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, Niyikiza C, Paoletti P. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21(14):2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- Wagner JC, Skidmore JW, Hill RJ, Griffiths DM. Erionite exposure and mesotheliomas in rats. Br J Cancer. 1985;51(5):727–730. doi: 10.1038/bjc.1985.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JC, Sleggs CA, Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med. 1960;17:260–271. doi: 10.1136/oem.17.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Barbone D, Yang TM, Jablons DM, Bueno R, Sugarbaker DJ, Nishimura SL, Gordon GJ, Broaddus VC. mTOR mediates survival signals in malignant mesothelioma grown as tumor fragment spheroids. Am J Respir Cell Mol Biol. 2008;39(5):576–583. doi: 10.1165/rcmb.2007-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A, Wu LJ, Santella RM, Hei TK. Role of oxyradicals in mutagenicity and DNA damage induced by crocidolite asbestos in mammalian cells. Cancer Res. 1999;59(23):5922–5926. [PubMed] [Google Scholar]
- Xu A, Zhou H, Yu DZ, Hei TK. Mechanisms of the genotoxicity of crocidolite asbestos in mammalian cells: implication from mutation patterns induced by reactive oxygen species. Environ Health Perspect. 2002;110(10):1003–1008. doi: 10.1289/ehp.021101003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan TD, Popa E, Brun EA, Cerruto CA, Sugarbaker PH. Sex difference in diffuse malignant peritoneal mesothelioma. Br J Surg. 2006;93(12):1536–1542. doi: 10.1002/bjs.5377. [DOI] [PubMed] [Google Scholar]
- Yang H, Bocchetta M, Kroczynska B, Elmishad AG, Chen Y, Liu Z, Bubici C, Mossman BT, Pass HI, Testa JR, Franzoso G, Carbone M. TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc Natl Acad Sci U S A. 2006;103(27):10397–10402. doi: 10.1073/pnas.0604008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Rivera Z, Jube S, Nasu M, Bertino P, Goparaju C, Franzoso G, Lotze MT, Krausz T, Pass HI, Bianchi ME, Carbone M. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc Natl Acad Sci U S A. 2010;107(28):12611–12616. doi: 10.1073/pnas.1006542107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanella CL, Timblin CR, Cummins A, Jung M, Goldberg J, Raabe R, Tritton TR, Mossman BT. Asbestos-induced phosphorylation of epidermal growth factor receptor is linked to c-fos and apoptosis. Am J Physiol. 1999;277(4 Pt 1):L684–693. doi: 10.1152/ajplung.1999.277.4.L684. [DOI] [PubMed] [Google Scholar]
- Ziegler A, Seemayer CA, Hinterberger M, Vogt P, Bigosch C, Gautschi O, Tornillo L, Betticher DC, Moch H, Stahel RA. Low prevalence of SV40 in Swiss mesothelioma patients after elimination of false-positive PCR results. Lung Cancer. 2007;57(3):282–291. doi: 10.1016/j.lungcan.2007.03.025. [DOI] [PubMed] [Google Scholar]


