Abstract
Abnormal fat metabolism plays an important role in the pathogenesis of obesity-related type 2 diabetes mellitus. This study examined whether free fatty acid levels (FFAs), like insulin levels, oscillate rapidly in plasma. Peripheral and portal blood samples from dogs were assayed for FFA, glycerol, glucose, and insulin. FFA and glycerol showed correlated oscillatory profiles, with about 8 pulses/hour. Omental lipolysis was also pulsatile, with about 10 pulses/hour, and insulin levels oscillated rapidly in plasma with about 7 pulses/hour. We applied an insulin clamp, β-adrenergic blockade, or both together, to determine the driving force behind the FFA oscillation, and we analyzed our findings by approximate entropy (ApEn) for which lower values suggest regular pulses and higher values suggest disorder. Under basal conditions, ApEn was 0.3 ± 0.2. With insulin not oscillating, FFA still cycled at about 9 pulses/hour and the ApEn was 0.2 ± 0.1. In contrast, β-blockade, either in the presence or absence of an insulin clamp, removed the FFA oscillation in three of nine dogs. In the other six dogs, the oscillatory profile was unchanged, but ApEn was significantly higher than basal values, suggesting that the regularity of the profile was disrupted. These results suggest that the FFA oscillation is driven by the central nervous system, not by insulin.
Introduction
The metabolic abnormalities associated with obesity-related type 2 diabetes mellitus may have as much to do with abnormal fat metabolism as with defects in carbohydrate metabolism (1, 2). Elevated plasma FFA concentrations are associated with peripheral and hepatic insulin resistance (3–8). In normal subjects, resistance can be compensated by potentiation of glucose-stimulated insulin secretion, as well as reduced insulin clearance, either of which may be due to elevated FFAs (9–12). It has been hypothesized that in obesity, glucose tolerance and plasma glucose levels remain normal because the FFA-induced insulin resistance and elevated insulin are balanced. However, if FFA levels remain elevated or continue to rise, the islets can become incapable of further increases in insulin secretion and hyperglycemia may result (1, 2, 7, 10).
Because free fatty acids may play an important role in the pathogenesis of obesity-related type 2 diabetes mellitus, it is of interest and importance to know the associations between FFA and insulin in the normal basal state. In the basal state, plasma insulin oscillates rapidly with a period of 8–14 minutes (13–16). These oscillations appear to enhance the efficiency and stability of glucose disposal when compared with a continuous infusion of insulin (17–19). Also, patients with type 2 diabetes mellitus and first-degree relatives of patients with type 2 diabetes mellitus show abnormal basal insulin oscillations that are faster and more erratic than in healthy individuals (20, 21). Thus, it is apparent that the rapid insulin oscillation is an important indicator of glucose homeostasis.
Although insulin oscillations are well characterized, much less is known about cycles in FFAs. If a rapid FFA oscillation were to exist, it would also be of interest to know the driving force. Given that adipose tissue is extremely sensitive to small changes in insulin concentration, a rapid FFA oscillation could be driven by or modulated by the rapid insulin oscillation. However, due to the slow transendothelial transport of insulin and clearance of insulin from the interstitial fluid (22–24), the insulin signal seen in plasma is quite attenuated in the interstitial fluid (25). Thus, it is not clear whether the rapid plasma insulin oscillation would appear as a significant oscillation in the interstitium. Another possible driving force for FFA oscillations could be the central nervous system (CNS). Sympathetic adrenergic modulation of the FFA profile could result from catecholamines that reach adipose tissue via the general circulation or via direct sympathetic innervation.
There has been very little work done that examines basal FFA concentrations at frequent time points. In 1971, Court et al. reported 2-minute FFA fluctuations in both obese and normal children and that the oscillations in the obese children had a greater amplitude (26). In 1977, Smith et al. reported large fluctuations of FFA levels in dogs that appeared to be spontaneous and somewhat random, although sometimes they could be distinguished into periodic oscillations in the frequency range of one cycle per 18 minutes to one cycle per 7 hours (27). No correlation was found between the ultradian insulin and glucose oscillations and the FFA fluctuations. Thus, the presence of a rapid FFA oscillation and any relation to the rapid insulin oscillation still remains to be determined.
The present study examines the presence of a rapid plasma oscillation of FFA, its source of origination, and its driving force. This was accomplished by taking frequent blood samples from the carotid artery, jugular vein, and portal vein of conscious dogs and by measuring FFA, glycerol, glucose, and insulin. This enabled the determination of the basal, fasted plasma FFA profile, and the net release of FFA from the omental fat depot. The driving force of the FFA oscillation was examined by either removing the rapid insulin oscillation, blocking β-adrenergic modulation of the adipocyte, or examining the effect of both concurrently on the FFA profile.
Methods
Animals.
Experiments were performed on ten male mongrel dogs (average weight, 25.0 ± 3.0 kg). Dogs were housed under controlled kennel conditions in the University of Southern California Keck Medical School Vivarium. Dogs were given free access to standard chow (25% protein, 9% fat, 49% carbohydrate, 17% fiber; Wayne dog food; Alfred Mills, Chicago, Illinois, USA) and tap water. Food was withdrawn 24 hours before the experiments. Animals were used for experiments if judged to be in good health as determined by visual observation, weight stability, body temperature, and hematocrit. The University of Southern California Institutional Animal Care and Use Committee approved all surgical and experimental procedures.
Surgical procedures.
Chronic catheters (inner diameter = 0.13 cm; Tygon; Allegiance Health Care, McGaw Park, Illinois, USA) were surgically implanted 1 week before the experiments (28). One catheter was inserted into the jugular vein and advanced to the right atrium for sampling of central venous blood. A second catheter was inserted into the carotid artery for arterial blood sampling. A third catheter was placed in the portal vein 4 cm upstream from the porta hepatis for portal infusions of glucagon and insulin. A fourth catheter was placed in the portal vein just rostral to the junction with the splenic vein for portal sampling. A fifth catheter was placed in the femoral vein and advanced to the inferior vena cava for the infusion of somatostatin and propranolol.
Experimental protocol I: basal plasma profiles.
The presence of a rapid FFA oscillation in plasma was examined by two different methods: (a) venous blood samples were drawn from 24-hour fasted conscious dogs (n = 4) at 2-minute intervals for 3 hours or (b) arterial (n = 8) or central venous (n = 2) blood samples were drawn from 24-hour fasted conscious dogs at 1-minute intervals for 1 hour. In the first experiment, samples were measured for glucose, insulin, and FFA. In the second experiment, samples were measured for insulin, FFA, and glycerol.
Protocol II: omental lipolysis.
To investigate the source of the FFA oscillation, blood samples were drawn from 24-hour fasted conscious dogs (n = 6) simultaneously from the carotid artery and portal vein every minute for 1 hour. This allowed calculation of net FFA and glycerol release from the omental fat depot. Samples were assayed for insulin, FFA, and glycerol.
Protocol III: removal of the insulin oscillation.
To investigate a possible driving force of the FFA oscillation, blood samples were drawn from 24-hour fasted conscious dogs from either the carotid artery (n = 4) or the right atrium (n = 2) at 1-minute intervals for 1 hour, or from the right atrium (n = 3) at 2-minute intervals for 3 hours during (a) the basal, fasted state, and (b) elimination of the insulin oscillation (but not insulin itself) by suppression of exogenous secretion with basal hormone replacement. Somatostatin was infused into the femoral vein catheter (1 μg/min/kg; Bachem California, Torrance, California, USA); insulin was infused into the portal vein (0.3 mU/min/kg; regular insulin; Novo-Nordisk, Copenhagen, Denmark); and replacement glucagon was infused portally with insulin (1 ng/min/kg; porcine glucagon; Sigma Chemical Co., St. Louis, Missouri, USA). Glucose was clamped at basal by a variable glucose infusion. Infusions of insulin, glucagon, and somatostatin were started 1-hour before the start of sampling. Blood samples for online glucose assay were taken every 10 minutes throughout the experiment. Samples were measured for insulin and FFA.
Protocol IV: β-adrenergic blockade.
To investigate further a possible driving force of the FFA oscillation, blood samples were drawn from 24-hour fasted conscious dogs from either the carotid artery (n = 5) or the right atrium (n = 4) at 1-minute intervals for 1 hour during (a) the basal, fasted state, and (b) β-adrenergic blockade of the adipocyte by peripheral infusion of propranolol (5 μg/min/kg; Sigma Chemical Co.). The propranolol infusion was begun 1 hour before the start of sampling. The dogs’ heart rate and blood pressure were monitored throughout the experiment. Samples were measured for FFA, glucose, and insulin.
Protocol V: insulin clamp plus β-adrenergic blockade.
Blood samples were drawn from 24-hour fasted conscious dogs from either the carotid artery (n = 2) or the right atrium (n = 4) at 1-minute intervals for 1 hour during (a) the basal, fasted state, and (b) insulin clamp with concurrent peripheral infusion of propranolol. The insulin clamp and propranol infusion were performed as described in protocols III and IV. Both infusions were begun 1 hour before the start of sampling. The dogs’ heart rate and blood pressure were monitored throughout the experiment. Samples were measured for FFA, glucose, and insulin.
For all protocols, samples for glucose and insulin were collected in tubes coated with lithium fluoride and heparin. Samples for FFA and glycerol were collected in tubes containing EDTA and paraoxon (Sigma Chemical Co.) to suppress lipoprotein lipase (29). Samples for FFA, glycerol, and glucose were immediately centrifuged, and the plasma was separated and kept on ice for processing that day. Plasma for insulin assay was stored at –20°C.
Assays.
FFAs were measured using a kit from Wako (NEFA C; Wako Pure Chemical Industries, Richmond, Virginia, USA), that utilizes a colorometric assay based on the acylation of coenzyme-A. Glycerol was measured using a colorometric triglyceride kit from Sigma Chemical Co. (GPO-Trinder), which uses glycerol kinase and glycerol phosphate oxidase. Plasma glucose was analyzed by the glucose oxidase method on an automated analyzer (model 23A; YSI, Yellow Springs, Ohio, USA). To maximize the sensitivity and specificity of time series analysis (30), FFA were measured in triplicate (coefficient of variation [CV] = 2%), glycerol in duplicate (CV = 5%), and glucose in duplicate (CV = 2%). Insulin was measured in singlicate by an enzyme-linked immunospecific assay (ELISA, CV = 2%) originally developed for human serum or plasma by Novo-Nordisk and adapted for dog plasma in our laboratory (31). The method is based on two murine mAb’s that bind to different epitopes on the insulin molecule. The antibodies do not bind proinsulin. Novo-Nordisk (Bagsvaerd, Denmark) provided materials for the insulin assay.
Calculations.
Many hormones are secreted in an episodic manner (13–15, 32). Often, this episodic secretion appears as a sequence of irregular pulses or “bursts” instead of a regular oscillatory profile. Owing to this irregularity, there has been a proliferation of methods developed to quantify pulsatility. These methods are designed to characterize and recognize significant pulses that may be missed by more traditional methods (such as time series analysis or spectral analysis of the fast Fourier transform) in situations in which regularity may not exist (30, 32–35). In the present study, pulse analyses of the temporal profiles of FFA, glucose, glycerol, and insulin were performed using ULTRA software (provided by E. Van Cauter, University of Chicago, Chicago, Illinois, USA) (34). ULTRA eliminates all peaks of plasma concentration for which either the increment or the decrement does not exceed a certain threshold. The threshold is determined by parameters set by the user as the intra-assay CV and the number of CVs to be used as threshold significance. In this study, three times the CV of each assay was used as threshold (30, 34). However, two times the CV and four times the CV were also used as thresholds and did not show any difference in the resulting pulse data. ULTRA is largely insensitive to unstable baseline hormone concentrations and is not adversely affected by varying pulse amplitudes, widths, or configurations within the series. The effect of removing the insulin oscillation or β-adrenergic blockade of the adipocyte on FFA pulsatility was examined using the approximate entropy method as described by Pincus and colleagues (32, 35). This method, which evaluates system randomness, yields a parameter (ApEn) for which a lower value suggests regular pulses and higher values indicate disruption of cycles. For evaluation of the FFA profile, the parameters m = 1 and r = 5% of the SD of the individual data set were used. ApEn values were calculated using MATLAB (The MathWorks, Prentice-Hall Inc., Englewood Cliffs, New Jersey, USA). Cross-correlation analyses were used to determine any relationships between the rapid oscillation profiles of insulin, FFA, and glycerol. Given that slow trends in the data distort this sort of analysis, the best-fit linear trend was first removed from the oscillatory profiles. The detrended data were then cross correlated using MATLAB.
Omental lipolysis was defined as the omental net production or net release of FFA or glycerol and was determined using the following equation: (portal – arterial concentration) × portal flow rate. Portal plasma flow rate was assumed to be 500 mL/min determined by previous experiments performed in this laboratory (25). Portal and arterial FFA and glycerol concentrations were presmoothed using three-point moving average calculated in Microsoft Excel 97 (Microsoft Corp., Redmond, Washington, USA) to minimize the noise present in the time series. The noise could be due to the sampling method, assay error, and errors in the calculation. A-V methodology, although inherently noisy, is a relatively precise method for measuring substrate balance across a specific tissue bed, compared with tracer methodology.
Statistics.
Values are reported as mean ± SD. Comparisons of the basal state and insulin infusion experiments were made using the Student’s t test performed on Microsoft Excel 97. Cross-correlation peak significance was determined using 95% confidence intervals (CIs) determined in MATLAB.
Results
Protocol I: basal plasma profiles.
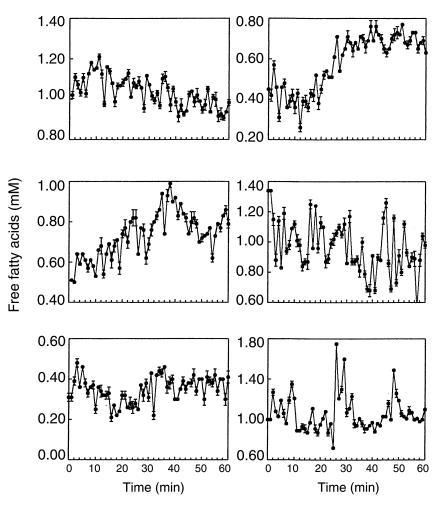
In all basal experiments (Figure 1, Table 1), FFA showed a rapid oscillation in plasma. There were an average of 8 ± 2 FFA pulses per 60 minutes (range, 7–11), with an average pulse length of 5 ± 1 minutes (range, 2–15) and average pulse amplitude of 0.06 ± 0.02 mM (range, 0.03–0.12). Insulin also showed a rapid oscillation in plasma in all basal experiments; there were an average of 7 ± 1 insulin pulses per 60 minutes (range, 5–7), with an average pulse length of 8 ± 1 minutes (range, 2–21) and average pulse amplitude of 33.3 ± 31.6 pM (range, 8.0–91.0). This rapid insulin oscillation is similar to that described previously (3, 14, 16). Plasma glycerol also oscillated at a similar frequency to FFA; there were an average of 8 ± 1 glycerol pulses per 60 minutes (range, 6–10), with an average pulse length of 5 ± 1 minutes (range, 2–17) and average pulse amplitude of 0.02 ± 0.01 mM (range, 0.014–0.04). The glucose profile appeared to have very small amplitude rapid oscillations; however, ULTRA analysis was unable to identify any significant glucose pulses.
Figure 1.
Rapid plasma FFA oscillations. Six representative dogs are shown during 1-minute sampling for 1 hour. Data are shown as mean ± SD.
Table 1.
Pulse data
Cross-correlation analysis of the systemic FFA and glycerol oscillations showed that the two oscillatory profiles are correlated with a lag time of approximately zero (95% CI). This result was expected since FFA and glycerol are released together during lipolysis. Because adipose tissue is extremely sensitive to small changes in insulin concentration, it is possible that the insulin oscillation is driving the FFA oscillation. This hypothesis was examined by cross correlating the systemic FFA and insulin oscillations. This analysis showed no correlation between the two profiles (CI < 95%), suggesting that it is not the insulin oscillation that is driving the FFA oscillation.
Protocol II: omental lipolysis.
For all six dogs (Table 1), portal FFA levels were significantly higher than arterial levels (0.78 ± 0.09 vs. 0.71 ± 0.19 mM, respectively; P < 0.0001), indicating net omental lipolysis. Similarly, portal glycerol levels were significantly higher than arterial levels (0.19 ± 0.04 vs. 0.14 ± 0.04 mM, respectively; P = 0.05). Pulse analysis confirmed oscillations in arterial FFA (8 ± 2 pulses/h; range, 7–11), portal FFA (10 ± 1 pulses/h; range, 8–11), arterial glycerol (8 ± 1 pulses/h; range, 7–10), portal glycerol (8 ± 1 pulses/h; range, 7–10 pulses/h), and arterial insulin (7 ± 1 pulses/h).
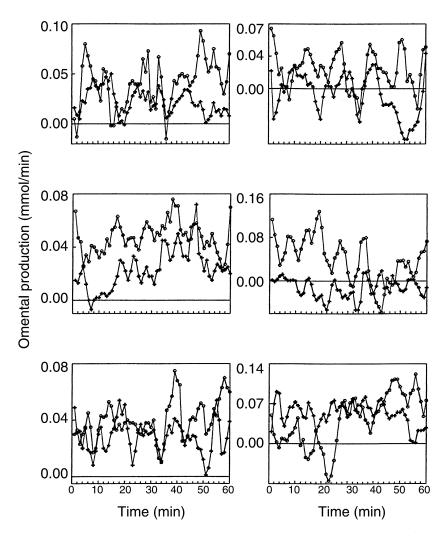
Omental lipolysis was calculated from the direct measurement of FFA and glycerol release from the omentum. For all six dogs (Figure 2, Table 1), there were an average of 10 ± 1 omental FFA pulses per 60 minutes (range, 8–11) and 9 ± 1 omental glycerol pulses (range, 7–10) per 60 minutes, with an average pulse length of 6 ± 1 minutes (range, 2–14). The omental FFA oscillation had an amplitude of 0.02 ± 0.01 mmol/min (range, 0.01–0.03), and the omental glycerol oscillation had an amplitude of 0.01 ± 0.003 mmol/min (range, 0.01–0.02). These results suggest that there is pulsatile lipolysis from the omentum. The average FFA/glycerol ratio calculated from the omental profiles was 2.5 ± 1.2 (range, 0.5–3.8), close to the expected ratio of 3 (three fatty acids and one glycerol are released during lipolysis). One dog had a FFA/glycerol ratio of 0.5, which was probably due to lower than expected basal FFA concentrations. This could have been due to increased FFA uptake due to stress, fasting, or increased insulin sensitivity on that day. Without that one dog, the average FFA/glycerol ratio was 2.9 + 0.6 (range, 2.1–3.8). To see whether the arterial insulin oscillation could be driving pulsatile omental lipolysis, the arterial insulin oscillation and the omental FFA profile were cross correlated. Cross correlation showed no relationship between the two profiles (CI < 95%), suggesting that insulin is not the driving force of pulsatile release of FFA from the omentum.
Figure 2.
Omental production of FFA (circles) and glycerol (plus symbols). Six representative dogs are shown during 1-minute sampling for 1 hour. Data are shown as mean ± SD. Omental production was calculated from direct measurements taken simultaneously from the portal vein and carotid artery (see the text).
Protocol III: removal of the insulin oscillation.
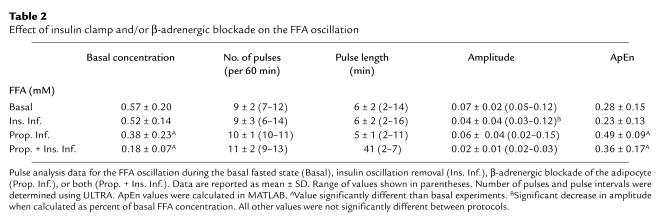
To examine further the hypothesis that insulin is driving the FFA oscillation, the insulin oscillation was removed by an insulin clamp. If insulin is the driving force of the FFA oscillation, then the FFA oscillation should be blunted, irregular, or removed during a constant insulin infusion. Figure 3 shows representative results of the insulin clamp experiments. In all nine dogs that received a constant insulin infusion, the FFA profile showed no difference in pulsatility when compared with the basal profile (Table 2). In the basal state, there were an average of 9 ± 2 FFA pulses per 60 minutes (range, 7–12), with an average pulse length of 6 ± 2 minutes (range, 2–14) and average pulse amplitude of 0.07 ± 0.02 mM (range, 0.05–0.12). When the insulin oscillation was removed by constant insulin infusion there were an average of 9 ± 3 FFA pulses per 60 minutes (range, 6–14), with an average pulse length of 6 ± 2 minutes (range, 2–16) and average pulse amplitude of 0.04 ± 0.02 mM (range, 0.03–0.07).
Figure 3.
Effect of insulin oscillation removal on the FFA oscillation. The top half shows one dog during sampling every 2 minutes for 3 hours, and the bottom half shows one dog with sampling every 1 minute for 1 hour. The left column shows the basal FFA and insulin profiles, and the right column shows the FFA and insulin profiles during insulin oscillation removal by insulin clamp.
Table 2.
Effect of insulin clamp and/or β-adrenergic blockade on the FFA oscillation
The number of pulses per 60 minutes and the average pulse length were not significantly different between the basal experiments and insulin clamp experiments (P = 0.4 and 0.3, respectively), indicating that the rapid insulin oscillation is not required for the FFA oscillation. However, the average FFA pulse amplitude was significantly lower during the insulin infusion protocol (P = 0.02). Given that the FFA concentration tended to be lower during the insulin clamp experiments, the FFA oscillation amplitude was recalculated as a percent of the average FFA concentration. During the basal state, the FFA oscillation amplitude was 13% of the average FFA concentration and 9% during the insulin clamp. This decrement is still significantly different (decrease of 30%; P = 0.04). The fall in FFA amplitude during the insulin clamp experiments was not due to overreplacement of insulin, as insulin was underreplaced (97.1 ± 79.8 pM ambient basal vs. 56.4 ± 25.7 pM during insulin infusion; P = 0.05). It is also possible that the drop in FFA amplitude is due to an under-replacement of glucagon or to the fact that growth hormone was not replaced.
The effect of removing the insulin oscillation on the FFA profile was also examined using the approximate entropy method (see Methods). During the basal state, the FFA profile had an average ApEn value of 0.28 ± 0.15. During constant insulin infusion, the FFA profile had an average ApEn value of 0.23 ± 0.13. These two ApEn values are not significantly different (P = 0.1), which further supports the assumption that the insulin oscillation is not the driving force of the FFA oscillation. In fact, the lower ApEn value and the decrease in amplitude of the FFA oscillation during removal of the insulin oscillation suggests that the FFA profile during the insulin infusion is less random than the basal FFA profile. This suggests that the insulin oscillation may be adding noise to the FFA oscillation.
Protocol IV: β-adrenergic blockade.
To examine the possible role of the CNS, adrenergic modulation of the adipocyte was blocked by peripheral infusion of propranolol. The dogs’ heart rate, blood pressure, and glucose showed no significant change from basal. Figure 4 shows representative results of the β-blockade experiments. In three of the nine dogs that received propranolol infusion, ULTRA was unable to identify any significant FFA pulses, suggesting that adrenergic blockade removed the FFA oscillation in these dogs. In the other six dogs, the FFA oscillation remained identifiable by ULTRA, and the number and length of pulses was unchanged when compared with the basal state (Table 2). During β-blockade, there were an average of 10 ± 1 FFA pulses per 60 minutes (range, 10–11), with an average pulse length of 5 ± 1 minutes (range, 2–11) and average pulse amplitude of 0.06 ± 0.04 mM (range, 0.02–0.15). Although the average pulse amplitude was lower during propranolol infusion, when calculated as percent of basal, the amplitudes were not significantly different (14% during basal state versus 14% during β-blockade; P = 0.4).
Figure 4.
Effect of β-adrenergic blockade or β-adrenergic blockade plus insulin oscillation removal on the FFA oscillation. The top row shows the basal FFA profiles. The bottom row shows the effect of propranolol infusion (Prop. Inf.) or propranolol infusion plus insulin clamp (Prop. + Ins. Inf.) on the FFA profile. The left half shows two dogs in which propranolol or propranolol plus insulin clamp seemed to remove the FFA oscillation, as determined by ULTRA (see the text). The right half shows two dogs in which propranolol or propranolol plus insulin clamp significantly disrupted the FFA oscillation, but did not remove it.
The effect of β-adrenergic blockade on the FFA profile was also examined using the approximate entropy method. During the basal state, the FFA profile had an average ApEn value of 0.28 ± 0.15. During β-adrenergic blockade, the FFA profile had an average ApEn value of 0.49 ± 0.09. The ApEn value during propranolol infusion was significantly higher than basal (P < 0.01). This suggests that β-adrenergic blockade disrupted the pulsatility of the FFA oscillation.
Protocol V: insulin clamp plus β-adrenergic blockade.
To see whether both the rapid insulin oscillation and the sympathetic nervous system play a role in regulating the FFA oscillation, experiments were performed in which the insulin oscillation was removed with an insulin clamp and adrenergic modulation of the adipocyte was blocked by peripheral infusion of the β-blocker propranolol. Figure 4 shows representative results of the insulin clamp plus β-blockade experiments. In three of the six dogs that received insulin clamp plus propranolol, ULTRA was unable to identify any significant FFA pulses, suggesting that the FFA oscillation was removed in these dogs. In the other three dogs, the FFA oscillation remained identifiable by ULTRA, and the number and length of pulses was unchanged when compared with the basal state (Table 2). There were an average of 11 ± 2 FFA pulses per 60 minutes (range, 9–13), with an average pulse length of 4 ± 1 minutes (range, 2–7) and average pulse amplitude of 0.02 ± 0.01 mM (range, 0.02–0.03). Although the average pulse amplitude was lower during insulin clamp plus propranolol infusion, when calculated as percent of basal, the amplitudes were not significantly different (12% during basal state versus 12% during β-blockade; P = 0.5).
The effect of insulin clamp plus β-adrenergic blockade on the FFA profile was also examined using the approximate entropy method. During the basal state, the FFA profile had an average ApEn value of 0.28 ± 0.15. During β-adrenergic blockade, the FFA profile had an average ApEn value of 0.36 ± 0.17. The ApEn value during insulin clamp plus propranolol infusion was significantly higher than basal (P < 0.01). Because these results are no different than those of propranol infusion alone, this suggests that the effect of β-adrenergic blockade on the FFA oscillation is independent of the rapid insulin oscillation.
Discussion
The results of this study have shown that a rapid FFA oscillation does exist in the basal, fasted state. These oscillations fluctuated about the mean by ± 8.5%. There were an average of 8 ± 2 FFA pulses per 60 minutes, with an average pulse length of 5 ± 1 minutes. Glycerol also showed a rapid oscillation in plasma at a similar frequency to and in phase with that of FFA. The FFA and glycerol oscillations were more frequent than those of insulin, which showed a rapid oscillation in plasma, with an average of 7 ± 1 insulin pulses per 60 minutes and an average pulse length of 8 ± 2 minutes.
The rapid FFA oscillations we report are likely generated by the cyclic modulation of FFA release. It is also possible that the FFA oscillation is due to cycles in FFA clearance; however, this seems unlikely. FFAs in plasma have a very short half-life of 3 minutes (36), and uptake of FFA behaves as a simple first-order clearance process proportional to FFA concentration (27). Uptake seems to follow FFA levels closely, linearly and passively, through the constant clearance parameter. The clearance coefficient for uptake of FFA has not been shown to be variable. Thus, it seemed highly likely that the fluctuations in plasma FFA are due to variations in FFA release from adipose tissue. This conclusion was fully supported by our arteriovenous measurements across the omental bed. We reported that there were approximately three FFA molecules produced for each glycerol (the ratio was 2.9, except for one unexplained deviation). We also reported that there is pulsatile lipolysis from the omentum, with an average of 10 ± 1 FFA pulses per 60 minutes that fluctuated about the mean by 58%. Although this measure of cycles in lipolysis from a single central adipose tissue bed does not rule out either oscillations in clearance by other adipose tissues or liver, they strongly support the concept that the FFA oscillations are due to regular cyclic variations in lipolysis.
An alternative possibility is that FFA oscillations result from rapid variations in total blood flow through the omental adipose tissue bed. In addition, FFA oscillations due to blood flow would require that local blood flow per se would be rate limiting for the delivery of FFA from the adipocyte. Possibly the rate-limiting step for FFA delivery is the cyclic provision of albumin binding sites in blood to adsorb FFA from the cells. Alternatively, there could be rhythmic variation in the parallel perfusion of adipocyte capillaries versus vessels that bypass the cells. Future research in this area using moment-to-moment assessment of blood flow distribution is justified. Although blood flow was not measured in the present study, portal blood flow has been monitored continuously in other experiments performed in our laboratory. These experiments were carried out in fasted, conscious dogs in which blood flow was measured using a perivascular ultrasonic flow probe (4-mm diameter; Transonic, Ithaca, New York, USA) that was surgically placed around the portal vein (8 cm caudal to the liver) 1 week before experimentation. Blood flow data were captured by computer every minute. The portal blood flow data of four dogs were analyzed using the methods described here, and none of the dogs showed any significant pulses within the rapid oscillatory range (data not shown). At present, whether sympathetic control reflects direct control of hormone sensitive lipase or reflects local blood flow variations is unknown. Regardless of the mechanism, FFA are supplied to the liver and other tissues in a cyclic pattern.
Because adipose tissue is exquisitely sensitive to small changes in insulin concentration, it is reasonable to ask whether the rapid insulin oscillation in blood drives the FFA oscillation. The present evidence does not support this hypothesis. The fact that the FFA oscillations are more rapid and therefore not synchronous with the insulin oscillation argues strongly that the rapid insulin oscillation is not the primary driving force. Also, cross-correlation analysis showed no evidence of a significant correlation between the rapid FFA oscillation and the rapid insulin oscillation, further suggesting that the FFA oscillation and the insulin oscillation have independent origins. The question of insulin as a driving force was further examined by removing the rapid insulin oscillation with an insulin clamp. If insulin were the driving force of the FFA oscillation, then the FFA oscillation should be blunted, irregular, or removed during a constant insulin infusion.
As shown in Figure 3 and Table 2, removing the rapid insulin oscillation did not remove the FFA oscillation. There was no significant difference in the number of pulses per 60 minutes or the average pulse length between the basal state experiments and insulin clamp protocol. However, the average FFA pulse amplitude was significantly lowered by 30% during the insulin clamp (P = 0.02). This suggests that although insulin does not appear to be the driving force of the FFA oscillation, it does have the ability to modulate the signal. The system randomness (determined by the approximate entropy method) of the FFA oscillation was also not significantly different between the basal state and insulin clamp experiments. During the basal state, the FFA profile had an average ApEn value of 0.28 ± 0.15. During the insulin clamp, the FFA profile had an average ApEn value of 0.23 ± 0.13. Although these two values are not significantly different, the lower ApEn value for the FFA oscillation during removal of the insulin oscillation suggests that the FFA profile during the insulin clamp is less random than the basal FFA profile. This suggests that the insulin oscillation is disrupting the FFA oscillation by adding noncorrelated fluctuations (i.e., “noise”) to the FFA profile.
One reason that the rapid insulin and rapid FFA oscillations are unrelated is the slow transport of insulin across the capillary endothelium (22–24). We have demonstrated marked attenuation of rapid insulin signals passing from the plasma to the interstitium (25). Thus, it is not known whether a rapid insulin oscillation would even appear in the interstitial fluid. If not, then the suppressive effect of insulin on lipolysis would depend only on the insulin concentration present in the interstitial fluid. Further studies of whether insulin variations can affect lipolysis dynamically are justified.
If insulin is not a dynamic stimulus to lipolysis, are oscillations in FFA a dynamic stimulus to the β-cells? One might expect that if FFA were a primary zeitgeber for cyclic insulin secretion, again there should be a relationship between the FFA and insulin cycles, and we did not see such a relationship. However, direct studies in which the effect of imposed changes in FFA cycles on insulin secretion are examined should be done, as we measured plasma insulin in this study, but not insulin secretion directly.
Our results suggest that something other than the plasma insulin oscillation is generating the FFA oscillation. Given that lipolysis is controlled largely by sympathetic activity and insulin concentration and that the rapid insulin oscillation does not seem to control the rapid FFA oscillation, it makes sense to hypothesize that either the oscillation of adipocyte lipolysis is independent of outside influences (i.e., autonomous), or the FFA oscillation is controlled by the CNS via adrenergic modulation. Because autonomous FFA oscillations have not been reported, and because paracrine entrainment of adipocytes remains to be demonstrated, we investigated the potential role of the CNS. CNS control could come from catecholamines that reach adipose tissue via the general circulation or via sympathetic innervation.
The hypothesis that the FFA oscillation is driven by the CNS was tested by blocking β-adrenergic modulation of the adipocyte with a peripheral infusion of the β-blocker propranolol. The propranolol dose used in this study was selected because it was known to be low enough to minimize side effects (37–39) that could have interfered with the interpretation of the results. In one third of the animals studied, propranolol did totally remove the FFA oscillation as determined by pulse detection. In the other dogs, the FFA oscillation was not removed, but appeared to be more disorderly as determined by approximate entropy. The failure of propranolol infusion to remove the FFA oscillation in all of the dogs may be due to the dosage of propranolol used and the sensitivity of the dogs to propranol. These data support the concept that the sympathetic nervous system may cause or modulate fluctuations in lipolysis.
We also examined the effect of both removing the insulin oscillation and adrenergic blockade of the adipocyte on the FFA oscillation. Similar to propranolol alone, insulin clamp plus β-adrenergic blockade totally removed the FFA oscillation in half of the animals studied. In the other dogs, the FFA oscillation appeared to be more disorderly as determined by approximate entropy. Because removal of the insulin oscillation alone did not remove the FFA oscillation in any dogs, it can be assumed that these results were due to propranolol infusion only. That β-adrenergic blockade disrupts the pulsatility of the FFA oscillation suggests that the sympathetic nervous system plays an important role in maintaining the regularity of the FFA oscillation. However, it remains unclear whether the sympathetic nervous system is the sole regulator of the FFA oscillation.
Mountains of evidence support the idea that the CNS regulates body adiposity and fuel utilization. It is hypothesized that leptin and NPY play an important role in the regulation of body weight and fat content. It has been shown that leptin is secreted in a pulsatile manner, with 32.0 ± 1.5 pulses every 24 hours and a pulse duration of 32.8 ± 1.6 minutes (40). Whether leptin shows a rapid oscillation similar to that of insulin or FFA is unknown. Because it has been shown that the adipose tissue is able to communicate with the CNS as to the state of relative adiposity, and this communication is pulsatile, it is possible to hypothesize that a rapid FFA oscillation could be driven by the CNS through pulsatile adrenergic modulation at the adipocyte. This would complete a feedback loop that allows communication from the adipocyte to the brain (through pulsatile leptin release) and from the brain to the adipocyte (through pulsatile signals from the CNS). However, it is not clear how the brain communicates back to the fat. Because there is evidence of sensory nerves innervating adipose tissue (41), it is possible that these nerves “sense” the FFA oscillation and that this information is sent to the CNS to provide information about nutritional status, relative adiposity, and lipolytic rate.
More studies need to be carried out to determine the driving force of the FFA oscillation and the physiological role this oscillation may play. Pulsatile sympathetic stimulation of lipolysis may have many beneficiary actions similar to those of pulsatile insulin delivery. Pulsatile adrenergic modulation may prevent the downregulation of β-adrenergic receptors on adipocytes. Also, pulsatile stimulation of the adipocyte may “prime” the system, enabling a quicker availability of energy in situations of stress. A putative pulsatile feedback loop between the CNS and the adipocyte would also allow the CNS to provide moment-to-moment regulation of the body’s energy balance and also provide immediate substrate availability when it is needed by the periphery.
Although the present study highly suggests that the CNS plays a critical role in maintaining the regularity of the FFA oscillation, it is entirely possible that the FFA oscillation originates within the adipocyte from an internal pacemaker similar to that seen in the β cell, and that sympathetic innervation aids in the synchronization of this oscillation. In the β cell, the exact identity of the intrinsic pancreatic pacemaker is unknown, but it is believed to involve oscillatory glucose metabolism within the β cell (42). Oscillations in glycolysis, the ATP/ADP ratio, and cytosolic Ca2+ levels have all been shown to exist within the β cell and are believed to play a role in the oscillation in insulin secretion (43, 44). Although the presence of oscillatory release of FFA from isolated adipocytes has previously been unexamined, Lipkin et al. have shown that glycolysis oscillates in isolated rat epididymal fat cells with a period of approximately 14 minutes (44). These results suggest that there may be oscillatory metabolism present within the adipocyte that leads to the oscillatory release of FFA.
An FFA oscillation may be beneficial. It is quite possible that an FFA oscillation provides the optimum balance between FFA and glucose uptake and metabolism by cells, thus contributing to the maintenance of glucose homeostasis. It is also possible that the FFA oscillation causes a rapid oscillation of glucose uptake (Rd) into cells. The FFA oscillation could also exert feedback on its own regulation in a fashion analogous to glucoregulation. This could be through modulation of the effects of insulin, the sympathetic nervous system, or on its own uptake, release, or reesterification. In this way, the FFA oscillation may also play an important role in setting the basal lipolytic rate. Given that FFAs account for 25–50% of energy metabolism in the rested, postabsorptive state in humans and in dogs (45), it is possible that an FFA oscillation also drives an oscillation in whole-body oxidation.
In summary, this study showed that a rapid oscillation of plasma FFA and glycerol exists in the basal, fasted state and there is also pulsatile lipolysis from the omentum. The FFA oscillation does not seem to be driven by the rapid insulin oscillation, as removal of the insulin oscillation does not remove the FFA oscillation. However, β-adrenergic blockade of the adipocyte either removes or disrupts the rapid FFA oscillation, suggesting that the sympathetic nervous system plays an important role in the maintenance of the rapid FFA oscillation. The presence of a rapid FFA oscillation suggests that the adipose tissue is a dynamic organ and supports the growing consensus that lipid metabolism plays an extremely important role not only in the regulation of glucose homeostasis, but in the regulation of whole-body energy fuel regulation, feeding behavior, and body composition.
Acknowledgments
This work was supported by research grants from the NIH (DK27619 and DK29867). The authors extend extreme gratitude to D.M. Moore, D.A. Davis, and E. Kirkman for their surgical and technical expertise and to M. Ader and J. Richey for their help, encouragement, and support.
References
- 1.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM: genetic and clinical implications. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 2.Milburn JL, et al. Pancreatic B-cells in obesity. J Biol Chem. 1995;270:1295–1299. doi: 10.1074/jbc.270.3.1295. [DOI] [PubMed] [Google Scholar]
- 3.Yki-Jarvinen H, Puhakainen I, Koivisto VA. Effect of free fatty acids on glucose uptake and nonoxidative glycolysis across human forearm tissues in the basal state and during insulin stimulation. J Clin Endocrinol Metab. 1991;72:1268–1277. doi: 10.1210/jcem-72-6-1268. [DOI] [PubMed] [Google Scholar]
- 4.Williamson JR, Kreisberg RA, Felts PW. Mechanism for the stimulation of gluconeogenesis by fatty acids in perfused rat liver. Proc Natl Acad Sci USA. 1966;56:247–254. doi: 10.1073/pnas.56.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Randle PJ, Hales CN, Garland PB, Newsholme EA. The glucose fatty-acid cycle: its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:787–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 6.Kim JK, Wi JK, Youn JH. Plasma free fatty acids decrease insulin-stimulated skeletal muscle glucose uptake by suppressing glycolysis in conscious rats. Diabetes. 1996;45:466–453. doi: 10.2337/diab.45.4.446. [DOI] [PubMed] [Google Scholar]
- 7.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1996;45:3–10. [PubMed] [Google Scholar]
- 8.Boden G. Fatty acids and insulin resistance. Diabetes Care. 1996;19:394–395. doi: 10.2337/diacare.19.4.394. [DOI] [PubMed] [Google Scholar]
- 9.Boden G, Chen X. Effects of fat on glucose uptake and utilization in patients with non-insulin-dependent diabetes. J Clin Invest. 1995;96:1261–1268. doi: 10.1172/JCI118160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou YP, Grill VE. Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J Clin Invest. 1994;93:870–876. doi: 10.1172/JCI117042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou YP, Berggren PO, Grill V. A fatty acid-induced decrease in pyruvate dehydrogenase activity is an important determinant of B-cell dysfunction in the obese diabetic db/db mouse. Diabetes. 1996;45:580–586. doi: 10.2337/diab.45.5.580. [DOI] [PubMed] [Google Scholar]
- 12.Crespin SR, Greenough WB, Steinberg D. Stimulation of insulin secretion by long-chain free fatty acids. J Clin Invest. 1973;52:1979–1984. doi: 10.1172/JCI107382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang DA, Matthews DR, Burnett M, Ward GM, Turner RC. Pulsatile, synchronous basal insulin and glucagon secretion in man. Diabetes. 1982;31:22–26. doi: 10.2337/diab.31.1.22. [DOI] [PubMed] [Google Scholar]
- 14.Lefebvre PJ, Paolisso G, Scheen AJ, Henquin JC. Pulsatility of insulin and glucagon release: physiological significance and pharmacological implications. Diabetologia. 1987;30:443–452. doi: 10.1007/BF00279610. [DOI] [PubMed] [Google Scholar]
- 15.Goodner CJ, et al. Insulin, glucagon, and glucose exhibit synchronous, sustained oscillations in fasting monkeys. Science. 1977;195:177–179. doi: 10.1126/science.401543. [DOI] [PubMed] [Google Scholar]
- 16.Jaspan JB, Lever E, Polonsky KS, Van Cauter E. In vivo pulsatility of pancreatic islet peptides. Am J Physiol. 1986;251:E215–E226. doi: 10.1152/ajpendo.1986.251.2.E215. [DOI] [PubMed] [Google Scholar]
- 17.Paolisso G, Scheen AJ, Albert A, Lefebvre PJ. Effects of pulsatile delivery of insulin and glucagon in humans. Am J Physiol. 1989;257:E686–E696. doi: 10.1152/ajpendo.1989.257.5.E686. [DOI] [PubMed] [Google Scholar]
- 18.Goodner CJ, Hom FG, Koerker DJ. Hepatic glucose production oscillates in synchrony with islet secretory cycle in fasting rhesus monkeys. Science. 1982;215:1257–1260. doi: 10.1126/science.7036347. [DOI] [PubMed] [Google Scholar]
- 19.Sturis J, Scheen AJ, Leproult R, Polonsky KS, Cauter EV. 24-hour glucose profiles during continuous or oscillatory insulin infusion. Demonstration of the functional significance of ultradian insulin oscillations. J Clin Invest. 1995;95:1464–1471. doi: 10.1172/JCI117817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang DA, Matthews DR, Burnett M, Turner RC. Brief, irregular oscillations of basal plasma insulin and glucose concentrations in diabetic man. Diabetes. 1981;30:435–439. doi: 10.2337/diab.30.5.435. [DOI] [PubMed] [Google Scholar]
- 21.O’Rahillly S, Turner RC, Matthew DR. Impaired pulsatile secretion of insulin in relatives of patients with non–insulin-dependent diabetes. N Engl J Med. 1988;318:1225–1230. doi: 10.1056/NEJM198805123181902. [DOI] [PubMed] [Google Scholar]
- 22.Bergman RN, Bradley DC, Ader M. On insulin action in vivo: the single gateway hypothesis. Adv Exp Med Biol. 1993;334:181–198. doi: 10.1007/978-1-4615-2910-1_13. [DOI] [PubMed] [Google Scholar]
- 23.Yang YJ, Hope ID, Ader M, Bergman RN. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest. 1989;84:1620–1628. doi: 10.1172/JCI114339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ader M, Bergman RN. Importance of transcapillary insulin transport to dynamics of insulin action after intravenous glucose. Am J Physiol. 1994;266:E17–E25. doi: 10.1152/ajpendo.1994.266.1.E17. [DOI] [PubMed] [Google Scholar]
- 25.Getty L, Hamilton-Wessler M, Ader M, Dea MK, Bergman RN. Biphasic insulin secretion during intravenous glucose tolerance test promotes optimal interstitial insulin profile. Diabetes. 1998;47:1941–1947. doi: 10.2337/diabetes.47.12.1941. [DOI] [PubMed] [Google Scholar]
- 26.Court JM, Dunlop ME, Leonard RF. High-frequency oscillation of blood free fatty acid levels in man. J Appl Physiol. 1971;31:345–347. doi: 10.1152/jappl.1971.31.3.345. [DOI] [PubMed] [Google Scholar]
- 27.Smith SW, Odell RH, Marsh DJ, Yates FE. Patterns of plasma free fatty acid concentrations in fasted, resting dogs. Am J Physiol. 1977;1:E25–E34. doi: 10.1152/ajpendo.1977.232.1.E25. [DOI] [PubMed] [Google Scholar]
- 28.Rebrin K, Steil GM, Getty L, Bergman RN. Free fatty acid as a link in the regulation of hepatic glucose output by peripheral insulin. Diabetes. 1995;44:1038–1045. doi: 10.2337/diab.44.9.1038. [DOI] [PubMed] [Google Scholar]
- 29.Zambon A, Hashimoto SI, Brunzell JD. Analysis of techniques to obtain plasma for measurement of levels of free fatty acids. J Lipid Res. 1993;34:1021–1028. [PubMed] [Google Scholar]
- 30.O’Meara NM, et al. Analytical problems in detecting rapid insulin secretory pulses in normal humans. Am J Physiol. 1993;264:E231–E238. doi: 10.1152/ajpendo.1993.264.2.E231. [DOI] [PubMed] [Google Scholar]
- 31.Andersen LB, Dinesen B, Jorgensen PN, Poulsen F, Roder ME. Enzyme immunoassay for intact human insulin in serum or plasma. Clin Chem. 1993;39:578–582. [PubMed] [Google Scholar]
- 32.Pincus SM, Keefe DL. Quantification of hormone pulsatility via an approximate entropy algorithm. Am J Physiol. 1992;262:E741–E754. doi: 10.1152/ajpendo.1992.262.5.E741. [DOI] [PubMed] [Google Scholar]
- 33.Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250:E486–E493. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- 34.Van Cauter E. Estimating false-positive and false-negative errors in analyses of hormonal pulsatility. Am J Physiol. 1988;254:E786–E794. doi: 10.1152/ajpendo.1988.254.6.E786. [DOI] [PubMed] [Google Scholar]
- 35.Pincus SM, Goldberger AL. Physiological time-series analysis: what does regularity quantify? Am J Physiol. 1994;266:H1643–H1656. doi: 10.1152/ajpheart.1994.266.4.H1643. [DOI] [PubMed] [Google Scholar]
- 36.Coppack SW, Jensen MD, Miles JM. In vivo regulation of lipolysis in humans. J Lipid Res. 1994;35:177–193. [PubMed] [Google Scholar]
- 37.Bjorkman O, Miles P, Wasserman D, Lickley L, Vranic M. Regulation of glucose turnover during exercise in pancreatectomized, totally insulin-deficient dogs. Effects of B-adrenergic blockade. J Clin Invest. 1988;81:1759–1767. doi: 10.1172/JCI113517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burns TW, Mohs JM, Langley PE, Yawn R, Chase GR. Regulation of human lipolysis. In vivo observation on the role of adrenergic receptors. J Clin Invest. 1973;53:338–341. doi: 10.1172/JCI107556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindsey CA, Faloona GR, Unger RH. Plasma glucagon levels during rapid exsanguination with and without adrenergic blockade. Diabetes. 1974;24:313–316. [PubMed] [Google Scholar]
- 40.Licinio J, et al. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat Med. 1997;3:570–579. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- 41.Fishman RB, Dark J. Sensory innervation of white adipose tissue. Am J Physiol. 1987;22:R944. doi: 10.1152/ajpregu.1987.253.6.R942. [DOI] [PubMed] [Google Scholar]
- 42.Coore HG, Randle PJ. Block of insulin secretion from the pancreas by D-mannoheptulose. Nature. 1963;197:1264–1266. doi: 10.1038/1971264a0. [DOI] [PubMed] [Google Scholar]
- 43.Bergsten P, Grapengiesser E, Gylfe E, Tengholm A, Hellman B. Synchronous oscillatons of cytoplasmic calcium and insulin release in glucose-stimulated pancreatic islets. J Biol Chem. 1994;269:8749–8753. [PubMed] [Google Scholar]
- 44.Lipkin EW, Teller DC, Haen C. Dynamic aspects of insulin action: synchronization of oscillatory glycolysis in isolated perifused rat fat cells by insulin and hydrogen peroxide. Biochemistry. 1983;22:792–799. doi: 10.1021/bi00273a013. [DOI] [PubMed] [Google Scholar]
- 45.Havel Rj, Naimark A, Borchgrevink CF. Turnover rate and oxidation of free fatty acids of blood plasma in man during exercise: studies during continuous infusion of palmitate-1-14C. J Clin Invest. 1963;42:1054–1063. doi: 10.1172/JCI104791. [DOI] [PMC free article] [PubMed] [Google Scholar]