Abstract
Background & Aims
Early fluid resuscitation is recommended to reduce morbidity and mortality among patients with acute pancreatitis (AP), although the impact of this intervention has not been quantified. We investigated the association between early fluid resuscitation and outcome of patients admitted to the hospital with AP.
Methods
Non-transfer patients admitted to our center with AP, from 1985 to 2009, were identified retrospectively. Patients were stratified into groups based on early (n=340) or late resuscitation (n=94). Early resuscitation was defined as receiving ≥ 1/3 of the total 72 h fluid volume within 24 hours of presentation, whereas late resuscitation was defined as receiving ≤ 1/3 of the total 72 h fluid volume within 24 hours of presentation. The primary outcomes were frequency of the systemic inflammatory response syndrome (SIRS), organ failure, and death.
Results
Early resuscitation was associated with decreased SIRS, compared with late resuscitation, at 24 h (15% vs. 32% P=0.001), 48 h (14% vs. 33%, P =0.001), and 72 h (10% vs. 23%, P =0.01), as well as reduced organ failure at 72 h (5% vs. 10%, P <0.05), a lower rate of admission to the intensive-care unit (6% vs. 17%, P< 0.001), and a reduced length of hospital stay (8 vs. 11 days, P=0.01). Subgroup analysis demonstrated that these benefits were more pronounced in patients with interstitial, rather than severe, pancreatitis at admission.
Conclusions
In patients with AP, early fluid resuscitation was associated with reduced incidence of SIRS and organ failure at 72 hours. These effects were most pronounced in patients admitted with interstitial, rather than severe, disease.
Keywords: pancreas, inflammation, treatment, efficacy
Introduction
Acute pancreatitis is an inflammatory process of the pancreas that leads to approximately 210,000 hospital admissions annually.1 Many of these admissions are associated with significant morbidity, leading to prolonged hospitalizations, and often require ICU admission. The estimated mortality rate for all patients with acute pancreatitis is approximately 5%.2 Additionally, the incidence of acute pancreatitis appears to be increasing in the United States with the direct medical costs of acute pancreatitis hospitalizations nationwide estimated to be greater than $2 billion dollars annually.3,4
The standard treatment of acute pancreatitis focuses on general supportive management; intravenous fluid resuscitation, pain control, correction of electrolyte disturbances, and provision of nutrition if prolonged fasting is expected.5 The diagnosis of acute pancreatitis should be made as early as possible to recognize disease severity and appropriately triage patients to higher levels of care.2,6,7
It is believed that intravenous fluid resuscitation is an important variable for improved outcomes in acute pancreatitis, although clinical evidence for this is limited. Historically recommendations for resuscitation have been based on expert opinion that urge “aggressive resuscitation,” and rely on clinical decision making to monitor for complications of the disease process or the resuscitation strategy itself. Most guidelines encourage targeting fluid resuscitation toward correcting hypotension, correcting hemoconcentration, and maintaining adequate urine output.2,5,8,9 The goal of fluid resuscitation is to improve patient outcomes, and prevent, or at least minimize, compromise of the microcirculation of the pancreas and prevent necrosis.6,10
The aim of our study was to determine the association between early fluid resuscitation on important clinical outcomes in patients admitted with acute pancreatitis. We hypothesized that early fluid resuscitation would be associated with reduced incidence of SIRS, organ failure, and mortality as compared to individuals resuscitated less aggressively.
Methods
The study was approved by the Committee for the Protection of Human Subjects #21847. Patients presenting directly to Dartmouth-Hitchcock Medical Center, an academic tertiary care hospital in Lebanon, NH, from 1985 to 2009 with the diagnosis of “acute pancreatitis” were identified retrospectively using ICD-9 codes. Only non-transferred patients were included in this study, and the primary diagnosis at admission had to be acute pancreatitis in order to be included. Acute pancreatitis was defined per the 1992 Atlanta classification, which required two of the following three features; abdominal pain characteristic of acute pancreatitis, elevated serum amylase and/or lipase greater than 3 times the upper limit of normal, and characteristic findings on transabdominal ultrasound or abdominal CT.11
Four individuals trained by the principal investigator reviewed electronic and paper medical records, and abstracted data regarding patient characteristics (age, gender, Charlson comorbidity score), process measures (admission antibiotics, total parenteral nutrition, need for surgery and/or endoscopic retrograde cholangiopancreatoscopy (ERCP)) and outcomes (presence of the systemic inflammatory response syndrome (SIRS), organ failure, presence of intra-abdominal bacterial or fungal infection, length of hospital stay, need for intensive care unit (ICU) admission, and death).12 SIRS was defined by the presence of > 2 of the following criteria: pulse > 90 beats per minute, respirations > 20 per minute or PaCO2 < 32 mmHg, temperature > > 90 beats per minute, respirations > 20 per minute or PaCO2 < 32 mmHg, temperature > 100.4°F or < 96.8°F, and white blood cell count > 12,000 or < 4,000 cells/mm3. Organ failure was defined per the 1992 Atlanta Classification as having at least one of the following: systolic blood pressure < 90 mmHg, PaO2 on room air < 60 mmHg, serum creatinine > 2 mg/dL, and gastrointestinal bleed > 500cc/hr. If not recorded, these values were assumed to be not present for purposes of the study. Severe acute pancreatitis was defined as having the presence of SIRS, developing organ failure present for greater than 48 hours, and/or having evidence of pancreatic necrosis on abdominal CT; all other patients were classified as having mild, or interstitial, pancreatitis.
The volume and type of IV fluid administered were recorded from initial presentation in the emergency department through 72 hours into the hospitalization using nursing administration documentation. At the time of data abstraction, abstracters were blinded to the outcomes being investigated. Oral fluid intake was not recorded, and patients who had incomplete IV fluid administration documentation were excluded from the study. This time period was then divided into 0–24, 24–48, and 48–72 hours, and early resuscitation was defined as receiving greater than 1/3 of the total 72 hour fluid volume within the first 24 hours of presentation to the emergency department. Late resuscitation was defined as receiving less than 1/3 of the total 72 hour fluid volume within the first 24 hours of presentation to the emergency department. This stratification schema was used because it allows for standardized comparisons between subjects by eliminating a reliance on absolute fluid volume.8
The study used a retrospective design. The primary study outcomes were the presence of SIRS and organ failure at 24, 48 and 72 hours, need for ICU admission, length of hospital stay, and death. Data were expressed as mean ± standard deviation and as percentages. Statistical significance was defined as p < 0.05. Categorical and continuous data were analyzed via standard one-tailed chi-square analysis and unpaired, two-tailed student’s t-tests. Univariate followed by multivariate logistic regression models was then performed to calculate odds ratios and 95% confidence intervals for the association between aggressive and non-aggressive IV fluid resuscitation (independent variables) and the primary outcomes of SIRS, organ failure and death (dependent variables). The odds ratios were then adjusted for age, gender and Charlson comorbidity score to account for confounding. Statistical analysis was performed using Graphpad (Graphpad Software, La Jolla, CA), Microsoft EXCEL (Microsoft, Corp, Redmond, WA), and A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna Austria).
Results
701 patients were admitted to our medical center from 1985 to 2009 with a primary diagnosis of acute pancreatitis. 222 patients were admitted in transfer and 45 had incomplete or missing fluid administration data, leaving 434 non-transferred patients who were included in the study. 340 patients were identified as “early resuscitation” and 94 patients were identified as “late resuscitation”. As shown in Table 1, there were no meaningful differences in baseline patient characteristics or Charlson score, but the late resuscitation group showed a greater number of acute pancreatitis attributed to post-ERCP etiology as compared to the early resuscitation group (p < 0.03).
Table 1.
Baseline Patient Characteristics
| Characteristic | Early Resuscitation | Late Resuscitation | p-value |
|---|---|---|---|
| Patients (n) | 340 | 94 | |
| Age (years) | 54 ± 20* | 49 ± 22* | 0.08 |
| Women (%) | 179 (53) | 50 (53) | 0.99 |
| Charlson Score | 2.51 ± 2.62* | 2.39 ± 2.68* | 0.70 |
| Etiology | |||
| Gallstone (%) | 130 (38) | 26 (28) | 0.06 |
| Alcohol (%) | 52 (15) | 18 (19) | 0.40 |
| Triglyceride (%) | 8 (2) | 2 (2) | 0.99 |
| Post-ERCP (%) | 18 (5) | 11 (12) | 0.03 |
| Medication (%) | 12 (4) | 1 (1) | 0.30 |
| Tumor (%) | 5 (1) | 2 (2) | 0.70 |
| Idiopathic (%) | 101 (30) | 27 (29) | 0.90 |
| Admission Antibiotics (%) | 18 | 18 | 0.99 |
| ERCP (%) | 19 | 23 | 0.50 |
| Parenteral Nutrition (%) | 26 | 46 | 0.01 |
| Intra-Abdominal Bacterial Infection (%) | 3 | 1 | 0.40 |
| Intra-Abdominal Fungal Infection (%) | 1 | 1 | 0.90 |
| Surgery | 50 (15) | 18 (19) | 0.40 |
| Cholecystectomy (%) | 37 (74) | 12 (67) | 0.70 |
| Necrosectomy (%) | 4 (8) | 2 (11) | 0.50 |
| Other (%) | 9 (18) | 4 (22) | 0.50 |
Mean ± standard deviation
Bold type indicates statistical significance
Fluid resuscitation volumes are shown in Table 2. There was a significant difference between the two groups in terms of volume of fluid administered for each of the major time periods analyzed. As expected, during the first 24 hours the early resuscitation group received more than the late resuscitation group (p < 0.0001), but from 24–48 hours, 48–72 hours, and in the total amount of fluid given in 72 hours, the late resuscitation group received greater amounts (p < 0.0001, p < 0.0001, and p < 0.0003 respectively). As nearly 85% of patients received normal saline, we did not note any differences in outcomes based on the type of fluids given.
Table 2.
Mean Intravenous Fluid Resuscitation Volumes
| Fluid Volume | Early Resuscitation (mL) | Late Resuscitation (mL) | p-value |
|---|---|---|---|
| 0–24 Hours | 3,493 ± 1,700 | 2,403 ± 1,216 | 0.0001 |
| 24–48 Hours | 2,571 ± 1,325 | 3,578 ± 2,490 | 0.0001 |
| 48–72 Hours | 1,841 ± 1,391 | 3,353 ± 1,615 | 0.0001 |
| Total | 7,600 ± 3,574 | 9,514 ± 4,469 | 0.0003 |
Bold type indicates statistical significance
Table 3 and Figure 1 highlight the primary and secondary outcomes. There was no difference in presence of SIRS between the two groups at time of admission (p < 0.15). However, at 0–24, 24–48, and 48–72 hours there was less SIRS in the early resuscitation group compared to the late resuscitation group (p < 0.001, p < 0.001, and p < 0.01). Additionally, there was no difference in the presence of organ failure between the two groups at time of admission, but at 72 hours less organ failure was observed in the early resuscitation group, as compared to the late resuscitation group (p < 0.05). Moreover, there were fewer ICU admissions (p < 0.001) and a shorter length of hospital stay (p < 0.01) in the early resuscitation group compared to the late group. There was no difference in mortality between the two groups. There was also no difference between the groups regarding the frequency of intraabdominal bacterial infection, intraabdominal fungal infection, and necrosis on CT. Regression analysis demonstrated limited confounding when controlling for Charlson comorbidity score, age, and gender (Table 4).
Table 3.
Primary and Secondary Clinical Outcomes
| Outcome | Early Resuscitation (%) | Late Resuscitation (%) | p-value | Relative Risk Reduction |
|---|---|---|---|---|
| SIRS | ||||
| Admission | 25 | 33 | 0.15 | - |
| 24 Hour | 15 | 32 | 0.001 | 2.1 |
| 48 Hour | 14 | 33 | 0.001 | 2.4 |
| 72 Hour | 10 | 23 | 0.01 | 2.3 |
| Organ Failure | ||||
| Admission | 10 | 9 | 0.80 | - |
| 24 Hour | 7 | 10 | 0.40 | 1.4 |
| 48 Hour | 6 | 9 | 0.40 | 1.5 |
| 72 Hour | 5 | 10 | 0.05 | 2.0 |
| Length of Stay | 8 ± 9.68* | 11 ± 10.2* | 0.01 | - |
| ICU | 6 | 17 | 0.001 | 2.8 |
| Mortality | 3 | 4 | 0.70 | 1.3 |
Mean ± standard deviation
Bold type indicates statistical significance
Figure 1.
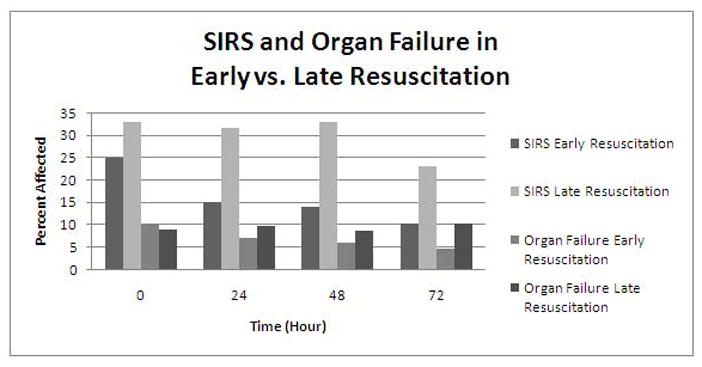
SIRS and Organ Failure in Early vs. Late Resuscitation
Table 4.
Primary and Secondary Outcomes Adjusted for Age, Gender, and Charlson Score
| Outcome | Adjusted Odds Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
| SIRS | |||
| 24 Hour | 0.39 | 0.22–0.67 | 0.0007 |
| 48 Hour | 0.32 | 0.19–0.56 | 0.0000 |
| 72 Hour | 0.40 | 0.21–0.76 | 0.0050 |
| Organ Failure | |||
| 24 Hour | 0.70 | 0.30–1.61 | 0.3976 |
| 48 Hour | 0.68 | 0.27–1.67 | 0.3966 |
| 72 Hour | 0.39 | 0.16–0.98 | 0.0460 |
| ICU | 0.30 | 0.15–0.63 | 0.0013 |
| Mortality | 0.80 | 0.24–2.64 | 0.7085 |
Bold type indicates statistical significance
Subgroup analysis was performed comparing patients with severe acute pancreatitis to those with interstitial disease at admission. 39 patients were identified as having severe acute pancreatitis, while 364 patients were identified as having interstitial disease. There were no major differences in baseline characteristics between the groups. In patients with severe disease, the early resuscitation group received more IV fluids from 0–24 hours (p < 0.006), but less from 24–48 hours, and 48–72 hours (p < 0.027; p < 0.002) than the late group respectively. There was no difference in the 72 hour total (p < 0.27) amount of fluid administered between the two groups. Table 5 reveals that the differences between the early and late resuscitation groups in terms of important clinical outcomes are observed in individuals with interstitial disease at admission, but not demonstrated in the severe subgroup. In patients with severe acute pancreatitis, the only difference between those resuscitated early and those resuscitated late was observed in the need for ICU admission (p < 0.02).
Table 5.
Subgroup Analysis: Interstitial vs. Severe Pancreatitis at Admission
| Outcome | Early Resuscitation (%) | Late Resuscitation (%) | p-value | Relative Risk Reduction |
|---|---|---|---|---|
| INTERSTITAL AT ADMISSION | ||||
| SIRS | ||||
| Admission | 23 | 30 | 0.20 | 1.3 |
| 24 Hour | 10 | 30 | 0.001 | 3.0 |
| 48 Hour | 10 | 31 | 0.001 | 3.1 |
| 72 Hour | 6 | 20 | 0.001 | 3.3 |
| Organ Failure | ||||
| Admission | 0 | 0 | - | |
| 24 Hour | 1 | 4 | 0.15 | 4.0 |
| 48 Hour | 1 | 2 | 0.30 | 2.0 |
| 72 Hour | 1 | 5 | 0.04 | 5.0 |
| Length of Stay | 7 ± 7.83* | 11 ± 10.6* | 0.001 | - |
| ICU | 3 | 12 | 0.01 | 4.0 |
| Mortality | 1 | 1 | 0.99 | 1.0 |
|
| ||||
| SEVERE AT ADMISSION | ||||
| SIRS | ||||
| Admission | 47 | 50 | 0.90 | 1.0 |
| 24 Hour | 62 | 50 | 0.60 | 0.8 |
| 48 Hour | 46 | 50 | 0.90 | 1.1 |
| 72 Hour | 50 | 50 | 0.99 | 1.0 |
| Organ Failure | ||||
| Admission | 100 | 100 | - | |
| 24 Hour | 67 | 75 | 0.70 | 1.1 |
| 48 Hour | 62 | 75 | 0.50 | 1.2 |
| 72 Hour | 43 | 63 | 0.40 | 1.5 |
| Length of Stay | 14 ± 17.5* | 13 ± 6.94* | 0.92 | |
| ICU Admission | 39 | 88 | 0.02 | 2.3 |
| Mortality | 23 | 38 | 0.40 | 1.7 |
Mean ± standard deviation
Bold type indicates statistical significance
Discussion
This study demonstrates that patients admitted with acute pancreatitis receiving early fluid resuscitation have lower rates of SIRS and organ failure, shorter hospitalizations, and less of a need for ICU admission than do patients who are not resuscitated as aggressively. This effect is observed only in patients with interstitial disease at admission, suggesting that in patients with severe disease, early intravenous fluid resuscitation is unlikely to substantially alter the patient’s clinical course. This effect was observed despite controlling for possible confounders; Charlson comorbidity score, age, and gender.
Patients presenting with acute pancreatitis are often hypovolemic due to vomiting, reduced oral intake, third spacing of fluids, and diaphoresis. In fact, one expert has written that the minimal intravenous fluid requirements of a 70 kg person during the first 48 hours after admission is already 6 liters without considering intravascular fluid sequestration loss.13 In addition, it is believed that the release of cytokines, chemokines, neutrophils, and macrophages lead to a pro-inflammatory state causing local and systemic inflammation. Such inflammation increases vascular permeability that can lead to hypoperfusion and third spacing of fluids. Often this is profound, as described by Greer and Burchard, “inflammation begets hypoperfusion and hypoperfusion begets inflammation,” leading to a self-propagating cycle that causes vascular dysfunction in both large vessels as well as the microcirculation of the pancreas.14 Early IV fluid resuscitation is essential in correcting hypovolemia, thereby supporting the macro and microcirculation of the pancreas to prevent serious complications such as pancreatic necrosis.
Despite recognition that intravenous fluid resuscitation is an essential component to the early treatment of acute pancreatitis, very few studies have been performed to qualify the effect of this intervention. Banks and colleagues have published numerous papers recognizing the detrimental effect of hemoconcentration on outcomes in acute pancreatitis.15,16,17 In one study of 39 patients, they found that while fluid resuscitation with crystalloid solution was not shown to prevent necrosis, all patients with inadequate fluid resuscitation as evidenced by persistence of hemoconcentration at 24 hours developed necrotizing pancreatitis.17 Eckerwall described in 99 patients admitted with severe acute pancreatitis in Sweden, those receiving 4000 ml or more of fluids during the first 24 hours (n=32) developed more respiratory complications (66% vs. 53%; P< 0.001) as compared to patients who received less than 4000 ml of fluid.18
In a series of patients admitted with severe acute pancreatitis at the Mayo Clinic Rochester using the same definitions of early and late resuscitation, patients in the late resuscitation group experienced significantly greater mortality than those in the early group (17.9% vs. 0%, p< 0.04) and demonstrated a trend toward higher rates of organ failure (42.9% vs. 35.3%) that did not reach statistical significance.8 Recently a multicenter study evaluating the impact of targeted fluid resuscitation volume to serial blood urea nitrogen (BUN) levels vs. standard of care fluid resuscitation was completed; the results have yet to be reported.
Given the paucity of human trials, it is not surprising that current guidelines for resuscitation are mostly vague and based almost exclusively on expert opinion.10 Some of the more specific recommendations include bolusing fluids to achieve “hemodynamic stability,” followed by 250–500cc/hr of crystalloid solutions, 250–300cc/hr for 48 hours, to 250–300cc/hr in non-volume depleted patients, 300–500 cc/hr for nonpancreatic fluid loss; and 500 to 1,000 cc/hr for severe depletion.19,13,6
The value of the current study is that it demonstrates the critical importance of early fluid resuscitation in acute pancreatitis, specifically in those with interstitial disease. The results support the dogma that in patients with less severe disease, early fluid resuscitation plays a significant role in preventing the development of severe disease. Conversely, the data suggest that in patients already presenting with severe disease, aggressive fluid resuscitation is unlikely to singularly reverse the clinical course.
There are weaknesses with this study, most importantly that it was retrospective and relied on having accurate measurements of IV fluid administration. However, meticulous attention was paid to eliminating patients with missing or incomplete data, and a number were excluded. Another limitation is the relatively small number of patients admitted with severe acute pancreatitis which limited our ability to draw conclusions in regard to mortality due to probable type II error. While we attempted to control for important confounders, we could not control for advances in care (infection control, improved enteral feedings, etc) that occurred over the course of the 24 year analysis. Adjustment for year or period of admission, which may have confounded the results assuming progressive advancements in volume resuscitation concepts and overall improved ICU care, was not performed.
In addition, due to the retrospective nature of the study, a circular argument can be forwarded – did patients develop a worse outcome due to limited early fluid resuscitation within the first 24 hours or was volume restriction due to certain circumstances associated with a worse outcome such as necrosis? Although efforts were made to establish that the groups were comparable at baseline, biases may have been introduced in this regard.
While the study does not allow for definitive recommendations in regards to the amount or type of fluid resuscitation, it helps qualify the importance of early fluid resuscitation on important clinical markers in acute pancreatitis, particularly those with interstitial disease. Until an effective pharmacologic agent is developed, optimization with intravenous fluids is one of the few interventions which is associated with improved clinically important outcomes in this disease.
Acknowledgments
Grant Support: Dr. Gardner is supported in part by NIH grant 1K23DK088832-01
Abbreviations
- CT
Computed Tomography
- ERCP
Endoscopic Retrograde Cholangiopancreatoscopy
- ICU
Intensive Care Unit
- OF
Organ Failure
- PaCO2
Partial Pressure of Carbon Dioxide
- PaO2
Partial Pressure of Oxygen
- SIRS
Systemic Inflammatory Response Syndrome
Footnotes
Financial Disclosures and Writing Assistance: None
Conflicts of Interest: None
Author Contributions: Study concept and design: All
Acquisition of data: All
Analysis and interpretation of data: Warndorf, Gardner, Gordon, Mackenzie
Drafting of the manuscript: Warndorf, Gardner, Gordon
Critical revision of the manuscript for important intellectual content: All
Statistical analysis: Warndorf, Gardner, Mackenzie
Obtained funding: NA
Administrative, technical, or material support: NA
Study supervision: Gardner and Gordon
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Swaroop VS, Chari ST, Clain JE. Severe acute pancreatitis. JAMA. 2004;291:2865–2868. doi: 10.1001/jama.291.23.2865. [DOI] [PubMed] [Google Scholar]
- 2.Banks PA, Freeman ML. Practice Parameters Committee of the American College of G. Practice guidelines in acute pancreatitis. American Journal of Gastroenterology. 2006;101(10):2379–2400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 3.Fagenholz PJ, Fernandez-Del Castillo C, Harris NS, Pelletier AJ, Camargo CA. Increasing United States Hospital Admissions for Acute Pancreatitis, 1988–2003. Annals of Epidemiology. 2007;17(7):491–498. doi: 10.1016/j.annepidem.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Fagenholz PJ, Fernandez-Del Castillo C, Harris NS, Pelletier AJ, Camargo CA. Direct medical costs of acute pancreatitis hospitalizations in the United States. Pancreas. 2007;35(4):302–307. doi: 10.1097/MPA.0b013e3180cac24b. [DOI] [PubMed] [Google Scholar]
- 5.Forsmark CE, Baillie J. AGA Institute technical review on acute pancreatitis. Gastroenterology. 2007;132(5):2022–2044. doi: 10.1053/j.gastro.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 6.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132(3):1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 7.Wu BU, Conwell DL. Acute Pancreatitis Part I: Approach to Early Management. Clinical Gastroenterology and Hepatology. 2010;8:410–416. doi: 10.1016/j.cgh.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 8.Gardner TB, Vege SS, Chari ST, Petersen BT, Topazian MD, Clain JE, Pearson RK, Levy MJ, Sarr MG. Faster rate of initial fluid resuscitation in severe acute pancreatitis diminishes in-hospital mortality. Pancreatology. 2010;9(6):770–776. doi: 10.1159/000210022. [DOI] [PubMed] [Google Scholar]
- 9.Wada K, Takada T, Hirata K, Mayumi T, Yoshida M, Yokoe M, Kiriyama S, Hirota M, Kimura Y, Takeda K, Arata S, Hirota M, Sekimoto M, Isaji S, Takeyama Y, Gabata T, Kitamura N, Amano H. Treatment strategy for acute pancreatitis. J Hepatobiliary Pancreat Sci. 2010;17:79–86. doi: 10.1007/s00534-009-0218-z. [DOI] [PubMed] [Google Scholar]
- 10.Gardner TB, Vege SS, Pearson RK, Chari ST. Fluid resuscitation in acute pancreatitis. Clinical Gastroenterology and Hepatology. 2008;6:1070–1076. doi: 10.1016/j.cgh.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Bradley EL. A clinically based classification system for acute pancreatitis. Arch Surg; Summary of the International Symposium on Acute Pancreatitis; Atlanta, GA. September 11 through 13, 1992; pp. 586–590. [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Tenner S. Initial management of acute pancreatitis: critical issues during the first 72 hours. American Journal of Gastroenterology. 2004;99:2489–2494. doi: 10.1111/j.1572-0241.2004.40329.x. [DOI] [PubMed] [Google Scholar]
- 14.Greer SE, Burchard KW. Acute Pancreatitis and Critical Illness: A Pancreatic Tale of Hypoperfusion and Inflammation. Chest. 2009;136:1413–1419. doi: 10.1378/chest.08-2616. [DOI] [PubMed] [Google Scholar]
- 15.Baillargeon JD, Orav J, Ramagopal V, Tenner SM, Banks PA. Hemoconcentration as an early risk factor for necrotizing pancreatitis. Am Journal Gastroenterol. 1998;93:2130–4. doi: 10.1111/j.1572-0241.1998.00608.x. [DOI] [PubMed] [Google Scholar]
- 16.Brown A, Orav J, Banks PA. Hemoconcentration is an early marker for organ failure and necrotizing pancreatitis. Pancreas. 2000;20:367–72. doi: 10.1097/00006676-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Brown A, Baillargeon JD, Hughes MD, Banks PA. Can fluid resuscitation prevent pancreatic necrosis in severe acute pancreatitis? Pancreatology. 2002;2:104–7. doi: 10.1159/000055899. [DOI] [PubMed] [Google Scholar]
- 18.Eckerwall G, Olin H, Andersson B, Andersson R. Fluid resuscitation and nutritional support during severe acute pancreatitis in the past: what have we learned and how can we do better? Clinical Nutrition. 2006;25:497–504. doi: 10.1016/j.clnu.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Whitcomb DC. Clinical practice. Acute pancreatitis. New England Journal of Medicine. 2006;354:2142–2150. doi: 10.1056/NEJMcp054958. [DOI] [PubMed] [Google Scholar]


