Abstract
The electroneutral Na/HCO3 cotransporter NBCn1 (SLC4A7) contributes to intracellular pH maintenance and transepithelial HCO3− movement. In this study, we expressed NBCn1 in Xenopus oocytes and examined the effect of NBCn1 on oocyte NH4+ transport by analyzing changes in membrane potential, current, and intracellular pH mediated by NH4Cl. In the presence of HCO3−/CO2, applying NH4Cl (20 mM) produced intracellular acidification of oocytes. The acidification was faster in oocytes expressing NBCn1 than in control oocytes injected with water. However, NH4Cl-mediated membrane depolarization was smaller in oocytes expressing NBCn1. In HCO3−/CO2-free solution, NH4Cl produced a smaller inward current in NBCn1-expressing oocytes (56% inhibition by 20 mM NH4Cl; measured at −60 mV), while minimally affecting intracellular acidification. The inhibition of the current by NBCn1 was unaffected when BaCl2 replaced KCl. Current-voltage relationships showed a positive and nearly linear relationship between NH4Cl-mediated current and voltage, which was markedly reduced by NBCn1. Large basal currents (before NH4Cl exposure) were produced in NBCn1-expressing oocytes due to the previously characterized channel-like activity of NBCn1. Inhibiting this channel-like activity by Na+ removal abolished NBCn1’s inhibitory effect on NH4Cl-mediated currents. The currents were progressively reduced over 72–120 h after NBCn1 cRNA injection, during which the channel-like activity was high. These results indicate that NBCn1 by its Na/HCO3 cotransport activity stimulates NH4+ transport, while reducing NH4+ conductance by its channel-like activity.
Keywords: bicarbonate, Xenopus, voltage-clamp
INTRODUCTION
The kidney regulates blood pH by reclaiming filtered acid and base equivalents or by releasing them into urine. Two major acid-base components that are regulated by the kidney are HCO3− and NH4+. HCO3− is filtered in the glomerulus, and filtered HCO3− is then reabsorbed in the proximal tubule (70–80%), thick ascending limb (10–15%), and cortical collecting duct (~5%) (Boron 1992). NH4+, which is the main component of urinary net acid excretion, is synthesized and secreted in the proximal tubule, reabsorbed in the thick ascending limb, and is secreted again into the lumen in the collecting duct (Knepper et al. 1989). The kidney makes adaptive changes in HCO3− and NH4+ absorption in response to acid-base disturbance, particularly to a low blood pH (Wagner 2007;Kraut & Kurtz 2005). Chronic metabolic acidosis stimulates HCO3− absorptive capacity and NH4+ production/secretion in the proximal tubule and increases the net HCO3− and NH4+ absorption in the thick ascending limb.
The absorption of HCO3− or NH4+ is primarily governed by ion transporters/channels that move these ions across the plasma membrane (Weiner & Verlander 2011;Cordat & Casey 2009;Sindic et al. 2007;Pushkin & Kurtz 2006). In particular, the electroneutral Na/HCO3 cotransporter NBCn1 (SLC4A7) plays an important role in transepithelial HCO3− movement in epithelia (Aalkjaer et al. 2004;Romero et al. 2004;Boron et al. 2009). NBCn1, which is a member of SLC4A bicarbonate transporters, normally moves HCO3− into the cytosol down the Na+ gradient in many different cells. Thus, the primary function of this transporter is to extrude acid (Pushkin et al. 1999;Lauritzen et al. 2010;Riihonen et al. 2010). In addition, the transporter has associated ionic conductance that is primarily governed by Na+ (Choi et al. 2000;Cooper et al. 2005). This channel-like activity can affect membrane potential and intracellular Na+ levels in heterologous expression systems (Choi et al. 2000;Cooper et al. 2005). The physiological role of this channel-like activity is unclear.
We recently reported that NBCn1 stimulates radiolabeled methylammonium uptake in Xenopus oocytes (Lee et al. 2010). Oocyte membranes have been considered to be less permeable to NH3 than to NH4+ (Burckhardt & Frömter 1992;Nakhoul et al. 2005). In response to NH4Cl, oocytes exhibit membrane depolarization and intracellular acidification without an initial rapid alkalinization (Cougnon et al. 1996;Burckhardt & Burckhardt 1997;Nakhoul et al. 2010). On the other hand, NH4+ currents are independent of NH4Cl concentrations at constant NH3 (Boldt et al. 2003), suggesting that oocytes possess two different pathways: an NH3 entry and an NH4+ conductance. It is thus unclear by what mechanism NBCn1 affects NH4+ transport and/or conductance in oocytes.
In this study, we examined the effect of NBCn1 activity and expression on oocyte NH4+ transport. We measured intracellular pH (pHi), membrane potential (Vm), and current (I) during NH4Cl application in both NBCn1-expressing oocytes and water-injected control oocytes and compared them. Our data show that NBCn1 inhibits oocyte NH4+ conductance by the Na/HCO3-independent mechanism, while stimulating NH4+-mediated pHi change by the intracellular buffering mechanism. A model for the effects of NBCn1 on oocyte NH4+ transport and conductance is proposed in the Discussion.
METHODS
Ethical Approval
All experiments in this study were conducted under the NIH guidelines for research on animals, and experimental protocols were approved by the Institutional Animal Care and Use Committee at Emory University.
NBCn1 expression in oocytes
Female frogs (Xenopus laevis) were purchased from Xenopus Express (Brooksville, FL, USA). A frog was anesthetized with fresh 0.1% 3-aminobenzoic acid ethyl ester for 30 min or until the animal was fully anesthetized. An incision in the abdomen, lateral to the midline, was made and oocytes were collected. After suture, the frog was returned to a recovery tank containing 0.1 M NaCl. Oocytes were agitated in Ca2+-free ND96 solution (in mM; 96 NaCl, 2 KCl, 1 MgCl2, and 10 Hepes, pH 7.5) five times for 20 min each. Follicles were removed by enzymatic digestion with collagenase type I (Sigma-Aldrich; St. Louis, MO, USA) twice for 20 min each at the concentration of 2 mg/ml. Oocytes were then washed with normal ND96 solution containing 1.8 mM CaCl2 for 20 min. Oocytes at stages V-VI were sorted and stored in OR3 medium (Chang et al. 2008) at 18°C overnight. For cRNA injection, rat splice variant NBCn1-E (Cooper et al. 2005) was in vitro transcribed with T7 RNA polymerase using the mMessage/mMachine transcription kit (Ambion; Austin, TX, USA). The injection was done with 25 ng RNA (in 46 nl). The control was injection with sterile water. Injected oocytes were maintained at 18°C for 3–5 days before use.
Two-electrode voltage clamp
Two glass capillaries with 1 mm outer diameter (World Precision Instruments; Sarasota, FL, USA) were filled with 3 M KCl and had a tip resistance of ~1 MΩ. One electrode was connected to the voltage headstage and the other to the current cable. The two probes were connected to the voltage-clamp amplifier OC-725C (Warner; Hamden, CT, USA). Input offset voltage was adjusted by placing electrodes in the chamber containing the nominally HCO3−/CO2-free recording solution (mM; 76 NaCl, 20 mM NMDG-Cl, 2 KCl, 1 MgCl2, 1.8 CaCl2, 5 Hepes, pH 7.4; 195–200 mOsm/kg). An oocyte was impaled with current and voltage electrodes and superfused with the recording solution. Signals from voltage and current electrodes were sampled by the digitizer Digidata 1322A (Molecular Devices; Sunnyvale, CA, USA) interfaced to a computer, and data were acquired using pClamp 8 (Molecular Devices). The membrane voltage of the oocyte was monitored before and after applying 10 and 20 mM NH4Cl, which replaced NMDG-Cl in the recording solution. For recording NH4Cl-mediated current, the oocyte was clamped at −60 mV using the OC-725C. Current-voltage relations were determined by commanding voltages from −120 mV to +60 mV with 20 mV increments for 100 msec before and after NH4Cl application. The voltage commands were imposed once the current value reached steady-state. For ionic conductance associated with NBCn1, current values were calculated by subtracting current values in control oocytes from values in NBCn1-expressing oocytes. Experiments were performed at room temperature.
Measurement of pHi
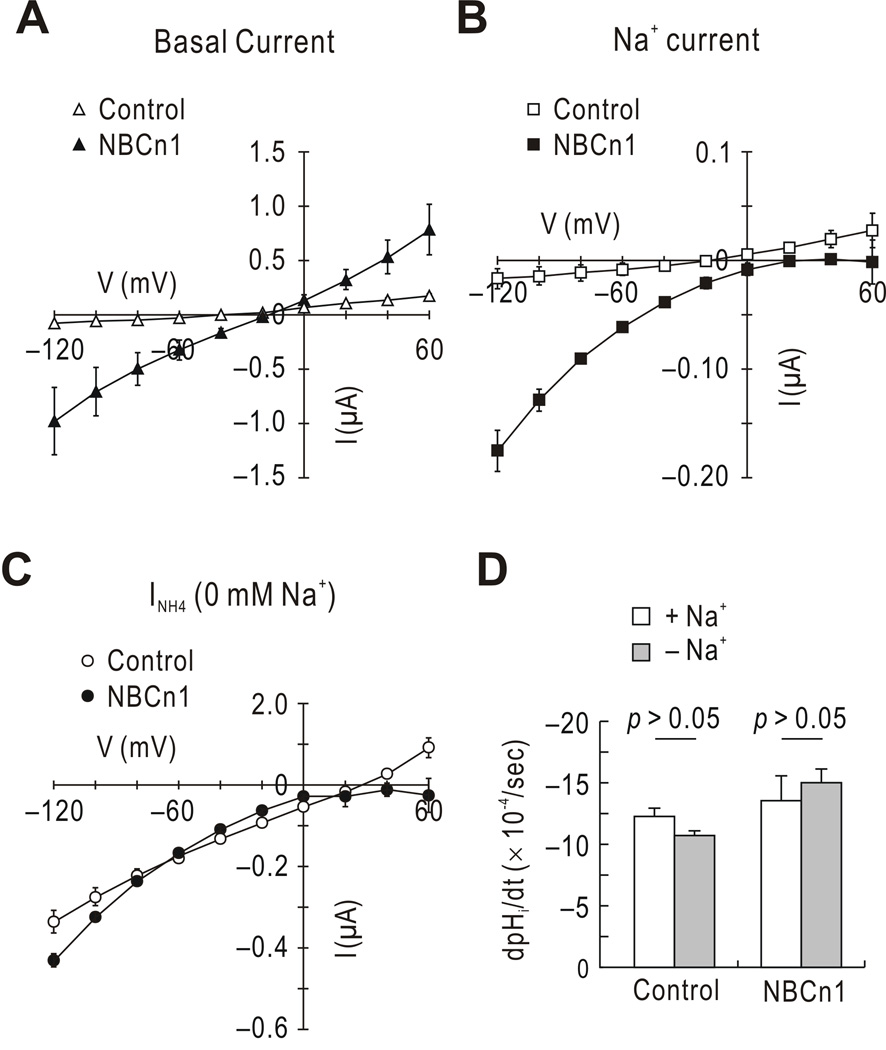
An oocyte was impaled with three microelectrodes: a pH electrode, a voltage electrode, and a current electrode. The pH electrode was baked at 200°C, silanized with bis-(methylamino)dimethylsilane (Sigma-Aldrich), and filled with the proton ionophore 1 cocktail B (Sigma-Aldrich). The electrode was then back-filled with the phosphate buffer at pH 7.0. The pH electrode was connected to the high-impedance electrometer FD-223 (World Precision Instruments; Sarasota, FL, USA) and the signal was then routed to a custom-made subtraction amplifier. The voltage and current electrodes filled with 3 M KCl were connected to the OC-725C. Signals from pH, voltage, and current electrodes were sampled by the Digidata 1322A, and data were acquired using pClamp 8. The signal in the voltage electrode was subtracted from the signal in the pH electrode to calculate the voltage for pH. The slope of voltage to pH was calibrated by placing all three electrodes in the chamber filled with pH 6.0 and 8.0 standards (Fisher Scientific; Pittsburgh, PA, USA). The slope was typically at the range of 53 ± 3 mV/pH. For recording in the presence of HCO3−/CO2, an oocyte clamped at −60 mV was superfused with a solution buffered with 25 mM HCO3−, 5% CO2 (pH 7.4) and then with 20 mM NH4Cl in the continued presence of HCO3−/CO2. NaCl was replaced mole for mole with NH4Cl. NH4Cl was applied ~2 min after pHi reached the lowest point. LiCl or N-methyl D-glucamine+(NMDG+) was used to replace NaCl in the Na+-free experiments, and BaCl2 was used to replace KCl in an effort to inhibit K+ channels. Oocyte NH4+ transport activity was measured from the rate of acidification after NH4Cl application. The rate of pHi change (i.e., dpHi/dt) was calculated by linear regression from the initial pHi value over the first 2 min after NH4Cl exposure. All experiments were performed at room temperature.
Statistical analysis
Data were reported as means ± standard error. The level of significance was assessed using the unpaired, 2-tailed Student t-test for comparison of dpHi/dt, Vm, and I between control oocytes and NBCn1-expressing oocytes. The one-way ANOVA with Bonferroni post test was used to analyze daily I values for 5 days. The p value of less than 0.05 was considered significant.
RESULTS
NBCn1 cotransport activity stimulates NH4Cl-mediated intracellular acidification in oocytes
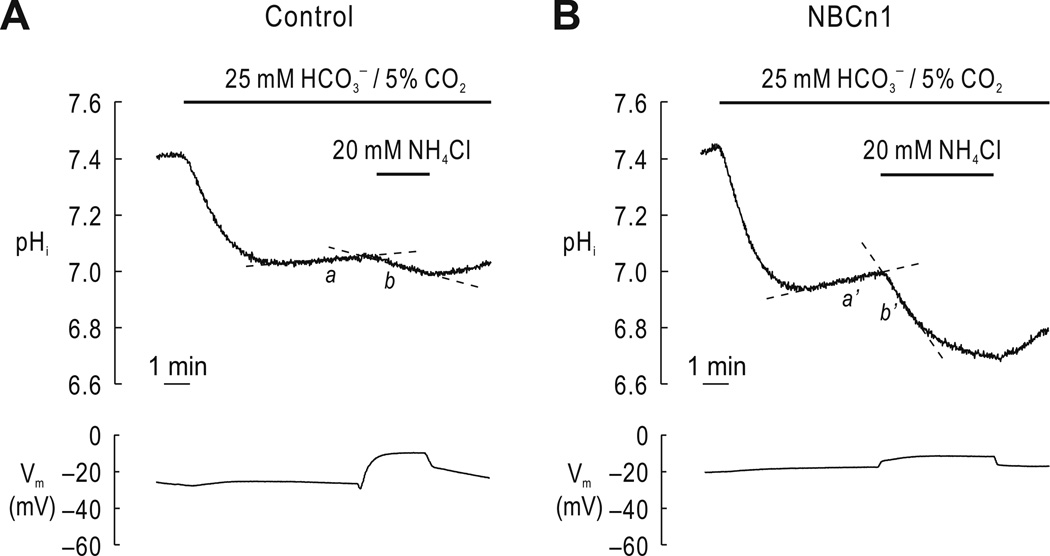
To assess the effect of NBCn1 cotransport activity on NH4+ transport, we expressed the rat renal splice variant NBCn1-E (hereafter, NBCn1) in Xenopus oocytes and simultaneously measured pHi and Vm during NH4Cl application in the presence of 25 mM HCO3−, 5% CO2 using the proton-selective pH microelectrode and the voltage electrode. Fig. 1A shows representative pHi and Vm traces in a water-injected control oocyte. The oocyte was bathed initially in the HEPES-buffered (HCO3−/CO2-free) solution to maintain stable pHi. Applying HCO3−/CO2-buffered medium caused pHi to decrease as CO2 entered the oocyte, hydrated, and produced H+. The pHi then reached steady-state as intracellular CO2 rose to equal extracellular CO2. A slight increase in pHi was often observed (a in the figure), possibly due to endogenous Na/H exchange (Busch 1997). Under this condition, subsequent exposure to 20 mM NH4Cl induced intracellular acidification (b in the figure). NH4Cl also caused the oocyte membrane to be depolarized. These responses to NH4Cl (i.e., intracellular acidification and membrane depolarization) are comparable to the previous observations by others (Cougnon et al. 1996;Burckhardt & Burckhardt 1997;Nakhoul et al. 2010).
Fig. 1.
pHi and Vm during NH4Cl application in the presence of HCO3−/CO2. A) Representative pHi and Vm traces in a control oocyte. The oocyte was superfused with a HEPES-buffered HCO3−/CO2-free solution and then exposed to a HEPES-free solution containing 25 mM HCO3−, 5% CO2. After pHi reached steady state, the oocyte was exposed to 20 mM NH4Cl in the continued presence of HCO3−/CO2. Intracellular acidification and membrane depolarization are shown. An initial rapid peak in Vm upon exposure to NH4Cl is a solution delivery artifact. One of 7 oocytes is shown. B) Representative pHi and Vm trances in an NBCn1-expressing oocyte. One of 6 oocytes is shown. dpHi/dt due to NH4+ transport was calculated by subtracting dpHi/dt in region b (or b’) from dpHi/dt in region a (or a’). dpHi/dt values and other values are summarized in Table 1.
In an NBCn1-expressing oocyte (Fig. 1B), the application of HCO3−/CO2 also caused pHi to decrease. However, the pHi recovered from an initial CO2-induced acidification (a’) as HCO3− transported via NBCn1 associated with intracellular H+. Applying NH4Cl in the continued presence of HCO3−/CO2 induced a rapid acidification (b’). NH4Cl-dependent intracellular acidification was evaluated by subtracting dpHi/dt values before NH4Cl application from values after NH4Cl application (i.e., b – a or b’ – a’). The acidification rate was −10.33 ± 0.47 × 10−4/sec (n = 6) for NBCn1-expressing oocytes, which was 2.8-fold higher than −3.73 ± 0.49 × 10−4/sec (n = 7) for controls (p < 0.05). Nonetheless, a smaller depolarization mediated by NH4Cl was observed in NBCn1-expressing oocytes (ΔVm = 13.4 ± 1.2 mV for controls and 10.3 ± 2.4 mV for NBCn1-expressing oocytes). pHi change rates and other values are summarized in Table 1.
Table 1.
pHi and Vm measurements of control oocytes and NBCn1-expressing oocytes. Recordings were performed in the presence of 20 mM NH4Cl, 25 mM HCO3−, 5% CO2.
| Control | NBCn1 | |
|---|---|---|
| Resting pHi | 7.36 ± 0.01 | 7.41 ± 0.03 |
| pHi in CO2/HCO3− | 6.94 ± 0.03 | 6.99 ± 0.02 |
| dpHi/dt before NH4Cl | 0.51 ± 0.12 | 1.11 ± 0.11* |
| dpHi/dt after NH4Cl | −3.22 ± 0.45 | −9.22 ± 0.37* |
| dpHi/dt due to NH4+ transport† | −3.73 ± 0.49 | −10.33 ± 0.47* |
| Resting Vm | −28.7 ± 1.6 | −22.4 ± 2.1* |
| ΔVm | 13.4 ± 1.2 | 10.3 ± 2.4 |
| n | 7 | 6 |
Calculated by subtracting dpHi/dt before NH4Cl from dpHi/dt after NH4Cl.
p < 0.05.
NBCn1 reduces NH4Cl-mediated inward current in oocytes
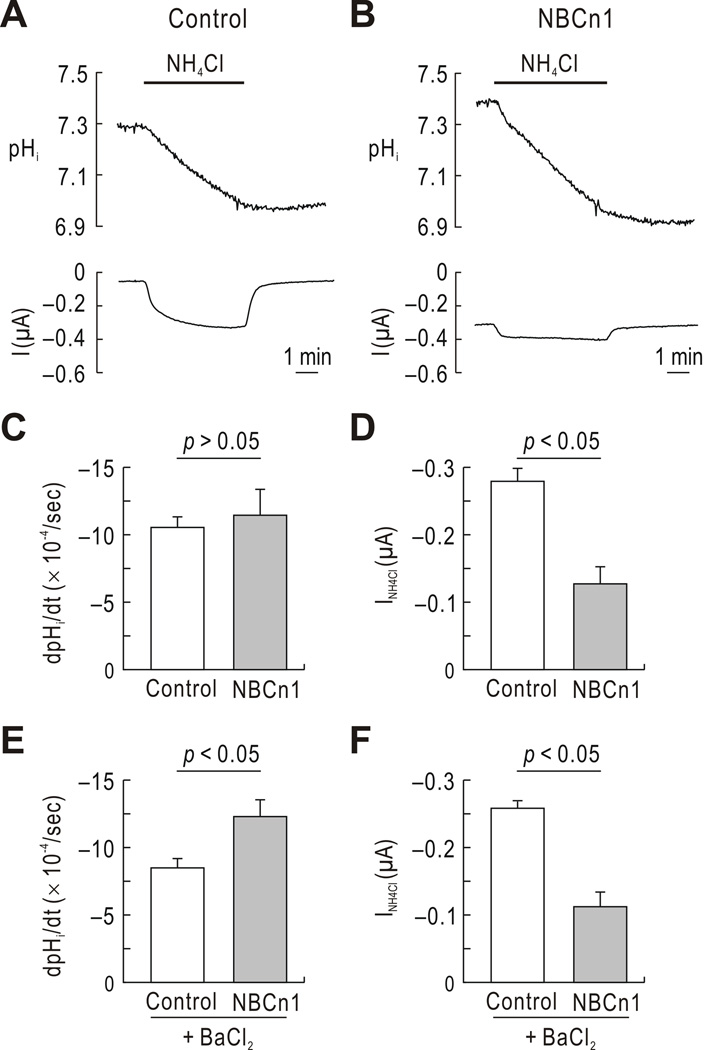
To further assess the effect of NBCn1 on NH4+ transport, we then applied NH4Cl to oocytes in the absence of HCO3−/CO2. For this analysis, oocytes were clamped at −60 mV, close to the mean resting potential of −61 mV for uninjected oocytes (Yang et al. 2009b), and pHi and I were simultaneously recorded. Fig. 2A shows a representative pHi trace in a control oocyte. A gradual intracellular acidification was observed in response to NH4Cl, reflecting that NH4+ transport is more favorable than NH3 entry in oocytes (Nakhoul et al. 2001). Consistent with this, there was no immediate pHi rise upon NH4Cl exposure. The pHi recovery after NH4Cl removal was relatively slow probably due to low levels of accumulated NH3 in the cells. An inward current was generated, which was abolished after NH4Cl removal.
Fig. 2.
pHi and I during NH4Cl application in the absence of HCO3−/CO2. A) Representative pHi and I in a control oocyte. Oocytes were exposed to 20 mM NH4Cl at the holding potential of −60 mV, and pHi and I were simultaneously measured using microelectrodes. One of 5 controls is shown. B) Representative pHi and I in an NBCn1-expressing oocyte. One of 6 NBCn1 oocytes is shown. C) Summary of pHi change rate. The pHi change rate (dpHi/dt) was calculated by linear regression analysis from the initial pHi over 2 min after NH4Cl application. D) Summary of I. NH4Cl-mediated current (INH4Cl) was obtained by subtracting I before NH4Cl application from I after NH4Cl application. E) Summary of dpHi/dt in the presence of BaCl2. Experiments were done in the presence of BaCl2, which replaced KCl mole for mole (n = 5 for each). F) Summary of INH4Cl in the presence of BaCl2. Measurements were done as described in D (n = 9 controls and 5 NBCn1).
Fig. 2B shows a representative pHi trace in an NBCn1-expressing oocyte. A gradual acidification in response to NH4Cl was observed without an immediate pHi rise. The mean dpHi/dt during NH4Cl application was similar between controls and NBCn1-expressing oocytes (Fig. 2C). The intracellular buffering power of Xenopus oocytes is reported to be similar at pHi ranging between 7.2 and 7.5 and estimated to be 24 mM/pH unit (Cougnon et al. 2002). Thus, the rate of NH4+ influx, which is equal to dpHi/dt × buffering power, was estimated to be similar between the two groups of oocytes. Nonetheless, the NBCn1-expressing oocyte produced a smaller current in response to NH4Cl. The mean I in these oocytes was −125.2 ± 25.8 nA, significantly smaller than −278.2 ± 16.7 nA for controls (p < 0.05) (Fig. 2D). NBCn1-expressing oocytes produced large holding currents before NH4Cl application due to channel-like activity of NBCn1.
NH4+ generally moves across the cell membrane by binding to the K+ site in K+ channels/transporters (Weiner & Hamm 2007). To test whether reduced NH4Cl-mediated current by NBCn1 involves K+, we repeated experiments in a solution containing BaCl2 instead of KCl (Fig. 2E and F). NBCn1-expressing oocytes had a slightly higher mean dpHi/dt (p < 0.05; n = 5 for each) probably due to higher initial pHi in these oocytes. Nonetheless, NBCn1-expressing oocytes had substantially smaller current mediated by NH4Cl (p < 0.05). The reduction was 57%, comparable to 55% in the presence of KCl (Fig. 2D). Thus, barium has a negligible effect on the transporter’s ability to inhibit NH4Cl-mediated current.
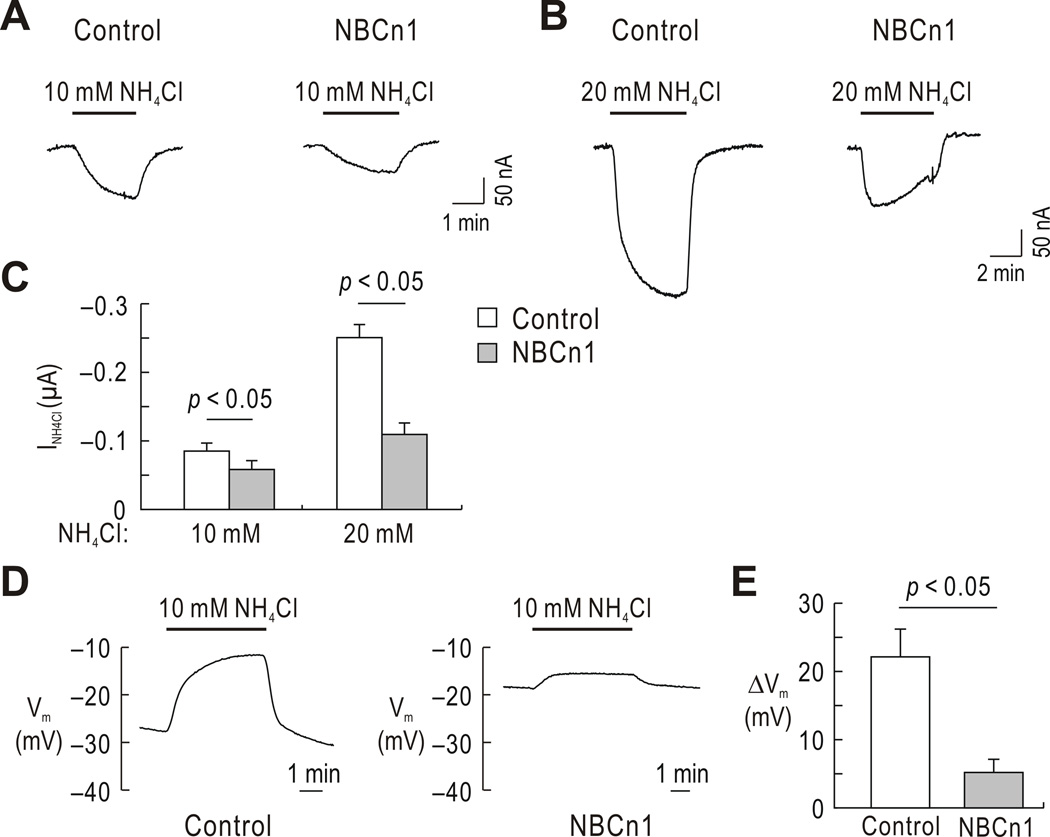
The inhibition of NH4Cl-mediated current by NBCn1 was further examined using two-electrode voltage clamp. Fig. 3A shows representative recordings of the current caused by 10 mM NH4Cl in oocytes (at −60 mV). NH4Cl-mediated current was induced in both a control oocyte and an NBCn1-expressing oocyte, but the current amplitude was smaller in the NBCn1-expressing oocyte. This difference was more evident at 20 mM NH4Cl (Fig. 3B), indicating that the inhibition depends on the amount of NH4Cl. As summarized in Fig. 3C, the inhibition was 33% at 10 mM NH4Cl and 56% at 20 mM NH4Cl (p < 0.05 for each). In other experiments, we measured Vm during NH4Cl application under the non-voltage clamp condition (Fig. 3D). NH4Cl-mediated membrane depolarization was smaller in NBCn1-expressing oocytes, measured on the same day (3–4 days after cRNA injection). The reduction was 72% (p < 0.05; Fig. 3E)
Fig. 3.
Inhibition of NH4Cl-mediated current by NBCn1. A) Representative inward currents during 10 mM NH4Cl application (clamped at −60 mV). Oocytes were superfused with HCO3−/CO2-free solution and then exposed to NH4Cl. Measurements were done on the same days after water or NBCn1 cRNA injection. One of 6 controls and one of 5 NBCn1-expressing oocytes are shown. B) Representative inward currents during 20 mM NH4Cl application. One of 6 controls and one of 8 NBCn1 oocytes are shown. C) Comparison of currents induced by 10 mM and 20 mM NH4Cl. D) Representative Vm traces during 10 mM NH4Cl application in a water-injected control oocyte and an NBCn1-expressing oocyte. One of 6 controls and one of 8 NBCn1-expressing oocytes are shown. E) Summary of NH4Cl-induced membrane depolarization (ΔVm).
NH4Cl-mediated current response to voltage is reduced by NBCn1
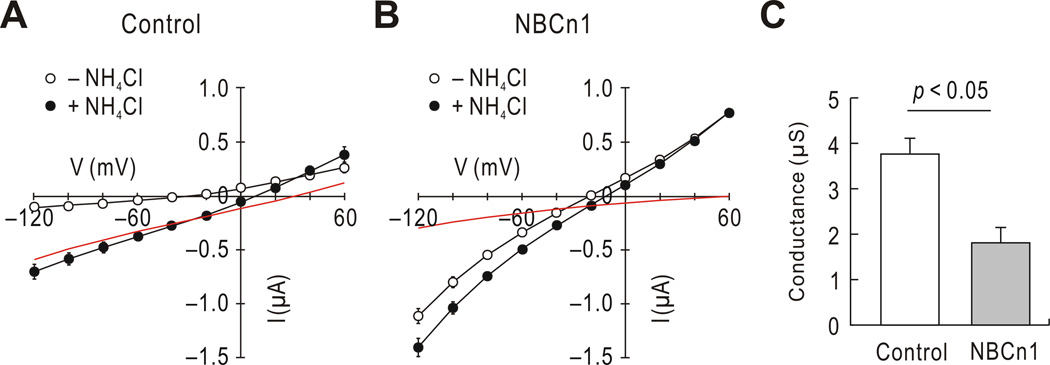
To analyze the NH4Cl-mediated current response to voltage, we determined current-voltage (I–V) relations by imposing voltage steps from −120 to +60 mV before and after NH4Cl exposure. The voltage command after exposure was made when the current reached steady-state (~2 min). In control oocytes (Fig. 4A), NH4Cl caused the I–V plot to shift down with a progressive increase in the inward direction at more negative voltages. The difference before and after NH4Cl application is a good estimate of the conductance for NH4Cl-mediated current (GNH4Cl; red line in the figure). The GNH4Cl was positive and almost linear with a reversal potential of 33.2 ± 2.2 mV (n = 5). In NBCn1-expressing oocytes (Fig. 4B), the I–V plot before NH4Cl application was already steeper. Upon the exposure to NH4Cl, the I–V plot shifted down. However, the change was small and almost negligible at more positive voltages. Thus, the reversal potential shifted positively. Fig. 4C shows that the GNH4 was reduced by 52% in NBCn1-expressing oocytes (measured between −80 and −40 mV; n = 5 for each).
Fig. 4.
NH4Cl-mediated currents at different voltages. A) Current–voltage (I–V) relationships before and after NH4Cl application in control oocytes (n = 5). Oocytes were clamped at −60 mV and voltages were stepped from −120 mV to +60 mM with 20 mV increments before (open circles) and 2 min after NH4Cl application (closed circles). The red line is the mean difference between the two I–V plots. B) I–V relationships in NBCn1-expressing oocytes (n = 5). Measurements were done using the protocol in A. Large basal currents before NH4Cl application (open circles) are due to NBCn1 channel-like activity. C) Average conductances for NH4Cl-mediated current under the two conditions. The slope conductance (GNH4Cl) was measured between −80 and −40 mV using the relationships between NH4Cl-mediated current and voltage
Channel-like activity of NBCn1 reduces oocyte NH4+ currents
In the above data, NBCn1 caused the oocyte resting Vm to shift positively, producing a high holding current. NBCn1 also caused a steeper slope in the I–V plot before NH4Cl application. These are hallmarks for channel-like activity of NBCn1 that occurs in the Na/HCO3-independent manner (Choi et al. 2000;Cooper et al. 2005). Fig. 5A shows another example of the channel-like activity. Inward currents at negative potentials and outward currents at positive potentials were elicited in NBCn1-expressing oocytes. The zero-current voltage shifted positively by ~25 mV and the slope measured at the zero-current voltage was 6.96 ± 2.3 µS, larger than 1.13 ± 0.06 µS for controls (n = 5 for each). This channel-like activity is primarily caused by a Na+ current and secondarily by outwardly rectifying anion currents (Choi et al. 2000;Cooper et al. 2005). Consistent with this, NBCn1-expressing oocytes produced Na+ currents (Fig. 5B).
Fig. 5.
Channel-like activity of NBCn1 and its effect on NH4Cl-mediated current. A) I–V relationships in HCO3−/CO2-free solution containing 96 mM Na+. Oocytes held at −60 mV were subjected to a step-voltage command from −120 to +60 mV. A large slope and a positive shift in the zero-current voltage are hallmarks for the channel-like activity of NBCn1 (n = 5 for each). B) Na+ component of NBCn1 channel-like activity. The Na+ component was measured by subtracting I in nominally Na+-free solution from I in 96 mM Na+ media (n = 4 controls and 5 NBCn1). NMDG+ was substituted for Na+. C) NH4Cl-mediated currents at different voltages in Na+-free condition. Currents were calculated using the protocol in Fig. 4 and plotted versus the voltage (n = 4 controls and 5 NBCn1). D) NH4Cl-mediated pHi changes mediated by NH4Cl in Na+-free solution. The dpHi/dt was determined in oocytes exposed to 76 mM Na+ (n = 4 for each) and in other oocytes exposed to 0 mM Na+ (n = 5 for each).
To determine whether this Na+ current component affects NH4Cl-mediated current, we performed experiments, in which oocytes were preincubated in Na+-free media for 20 min and subjected to the above I–V protocol. Fig. 5C shows the results. The two I–V plots corresponded to NH4Cl-mediated currents at different voltages in control oocytes and NBCn1-expressing oocytes. The two plots were nearly superimposed after preincubation in Na+-free media. The current was not reversed at more positive voltages in NBCn1-expressing oocytes, probably due to incomplete washout of intracellular Na+ after 20 min preincubation. Nevertheless, the values at the resting membrane potentials were similar between the two groups of oocytes (p > 0.05). Parallel experiments were then performed to examine pHi changes in Na+-free solution (Fig. 5D). The dpHi/dt values were similar before and after Na+ removal (p > 0.05).
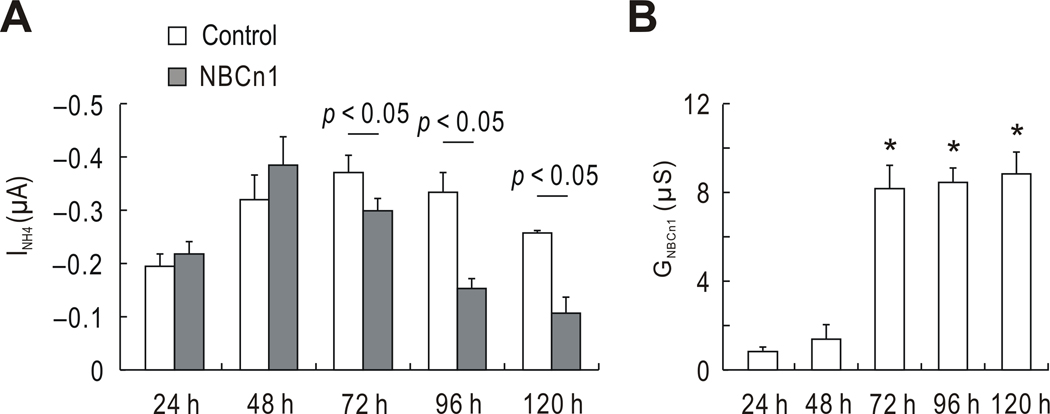
In other experiments, the time course of NH4Cl-mediated current at −60 mV was measured daily after water or NBCn1 cRNA injection (Fig. 6A). The current appeared to increase over the initial 48 h after injection, the reason for which is unclear. Nonetheless, no significant difference was observed between controls and NBCn1-expressing oocytes during this period (p > 0.05). At 72 h, the current began to decrease in NBCn1-expressing oocytes (p < 0.05). The decrease was more profound at 96–120 h. We then determined the channel-like activity of NBCn1 during these time periods by subtracting the slope in the I–V plot for control oocytes from the slope for NBCn1-expressing oocytes (Fig. 6B). The channel-like activity was negligible over the initial 48 h, but significantly increased at 72–120 h (p < 0.05; one-way ANOVA with Bonferroni post test; 4–6 oocytes for each group at different time points). Values were similar at these time points, comparable to the earlier report (Yang et al. 2009a) that NBCn1 expression in oocyte membranes is saturated after 72 h. These data demonstrate that there is an inverse relationship between channel-like activity and NH4Cl-mediated current in oocytes expressing NBCn1.
Fig. 6.
Time course of NH4Cl-mediated current and NBCn1 channel-like activity. A) Time course of NH4Cl-mediated currents after water or NBCn1 cRNA injection. Currents were daily measured in 4–6 oocytes from each group of oocytes. Measurements were done at −60 mV. B) Time course of NBCn1 channel-like activity. For this analysis, mean currents in control oocytes were first subtracted from currents of NBCn1-expressing oocytes at each voltage (n = 4–6 oocytes for each group at each time point). The subtracted values were then used to plot I–V relationships, from which the slope was calculated between −80 and −40 mV. The one-way ANOVA with Bonferroni post test was used to analyze the level of significance. Asterisks (*) represent p < 0.05 for comparison to the value at 48 h.
DISCUSSION
In our recent report (Lee et al. 2010), we demonstrated that NBCn1 increases HCO3−/CO2-dependent 14C-methylammonium uptake in oocytes. The goal of the present study was to further investigate the effect of NBCn1 on NH4+ transport by electrophysiological measurements of Vm, I, and pHi of oocytes. The major findings from this study are the following: 1) NBCn1 Na/HCO3 cotransport activity enhances intracellular acidification mediated by NH4Cl in oocytes; 2) In the absence of HCO3−/CO2, NBCn1 inhibits inward current mediated by NH4Cl, while producing a negligible effect on intracellular acidification; 3) NBCn1-dependent inhibition of NH4Cl current is unaffected by barium; 4) The inhibition is abolished by Na+ removal; and 5) NBCn1 channel-like activity appears to be responsible for inhibiting NH4Cl-mediated current. These findings are novel and provide new information on the effect of NBCn1 on oocyte NH4+ transport. In addition, the findings provide the first evidence for the functional significance of NBCn1’s channel-like activity. We propose that NBCn1 may play a key role in regulating NH4+ transport and conductance in cells, where HCO3− and NH4+ transport processes are tightly coupled.
In this study, we demonstrate that NBCn1 differentially affects pHi and I (or Vm) caused by NH4Cl in oocytes. The transporter stimulates intracellular acidification caused by NH4Cl in the presence of CO2/HCO3− (Fig. 1). NBCn1 moves Na+ and HCO3− into oocytes and causes pHi to recover from a CO2-induced acidification. NH4+ transport is stimulated in this condition because HCO3− moved via NBCn1 compensates intracellular H+ load. Thus, the intracellular buffering mechanism can account for increased rates of acidification during NH4Cl application in the presence of HCO3−/CO2. On the other hand, we observe a decrease in membrane depolarization under the same condition. Furthermore, NBCn1 inhibits NH4Cl-mediated current without significantly altering dpHi/dt in the absence of HCO3−/CO2 (Fig. 2 and Fig. 3). Therefore, the inhibition occurs regardless of Na/HCO3 cotransport activity of NBCn1.
The finding that NBCn1 differentially affects pHi and I leads us to revisit the mechanism of NH4+ transport in Xenopus oocytes. Our data show that NH4Cl induces membrane depolarization and intracellular acidification, consistent with previous reports by others (Cougnon et al. 1996;Burckhardt & Burckhardt 1997;Nakhoul et al. 2010). Given the reports that oocyte membranes are less permeable to NH3 than to NH4+ (Burckhardt & Frömter 1992), the changes in membrane depolarization and intracellular acidification during NH4Cl application have been considered to be due to carrier-mediated NH4+ transport. Upon NH4Cl application, NH4+ is preferentially transported into the oocyte cytosol and dissociates into NH3 and H+. The dissociation results in intracellular acidification. Thus, the movement of charged NH4+ into oocytes induces a depolarizing inward current. NH4+ has been proposed to enter the oocyte cytosol via a nonselective cation channel (Burckhardt & Burckhardt 1997) or a mechanism that exhibits a linear relationship between pHi change rate and NH4+ concentration (Nakhoul et al. 2010). Thus, according to this paradigm, the two events following NH4Cl application (i.e., intracellular acidification and inward current) are tightly coupled to each other.
In contrast, our data show that the cotransport activity of NBCn1 preferentially stimulates NH4+-mediated intracellular acidification, while reducing inward current. Thus, the two events can be separated from each other. Our data also show that NBCn1 can reduce the current during NH4Cl application, without altering intracellular acidification. This implies that oocyte NH4+ transport does not necessarily have to be electrogenic. The crystal structure of the bacterial ammonium transporter AmtB suggests translocation of NH3, but not NH4+, via the transporter (Zheng et al. 2004). Mammalian NH4+ transporter Rh glycoproteins have been proposed to be electroneutral (Weiner & Hamm 2007). Nonetheless, we are also aware that the Rh glycoproteins exhibit electrogenic NH4+ transport (Nakhoul et al. 2005;Nakhoul et al. 2010). In this sense, it is also interesting to mention that we often observe negligible electrogenicity of NH4+ transport in some preparations of oocytes, as shown in our previous report (Lee et al. 2010). The exact reason for this lack of electrogenicity in some preparations is unclear although oocytes are known to exhibit seasonal variances.
Our data show that NBCn1 exhibits channel-like activity (Fig. 4 and Fig. 5), consistent with previous reports (Choi et al. 2000;Cooper et al. 2005). Other HCO3− transporters also display channel-like conductance. The Cl/HCO3 exchanger AE1 in erythrocytes mediates a conductive anion flux. The DIDS-insensitive component of net ion efflux increases with increasing membrane hyperpolarization (Freedman & Novak 1997). The Cl/HCO3 exchangers Slc26a3 (DRA, CLD) and Slc26a6 (CFEX, PAT-1) also show channel-like activity, which mediates large NO3− and SCN− currents that are uncoupled to OH− or HCO3− transport (Ko et al. 2002;Shcheynikov et al. 2008). The channel-like activity of NBCn1 is known to increase intracellular Na+ levels and depolarize the membrane by 20–30 mV in Xenopus oocytes and HEK 293 cells (Choi et al. 2000;Cooper et al. 2005). Thus, Na+ influx via NBCn1 may lower the electrochemical Na+ gradient across the cell membrane, subsequently reducing the driving force for non-selective cation channels. Our finding that Na+ removal abolishes the capability of NBCn1 to inhibit NH4Cl current support this idea. Whether the channel-like activity affects nonselective cation channels needs further investigation.
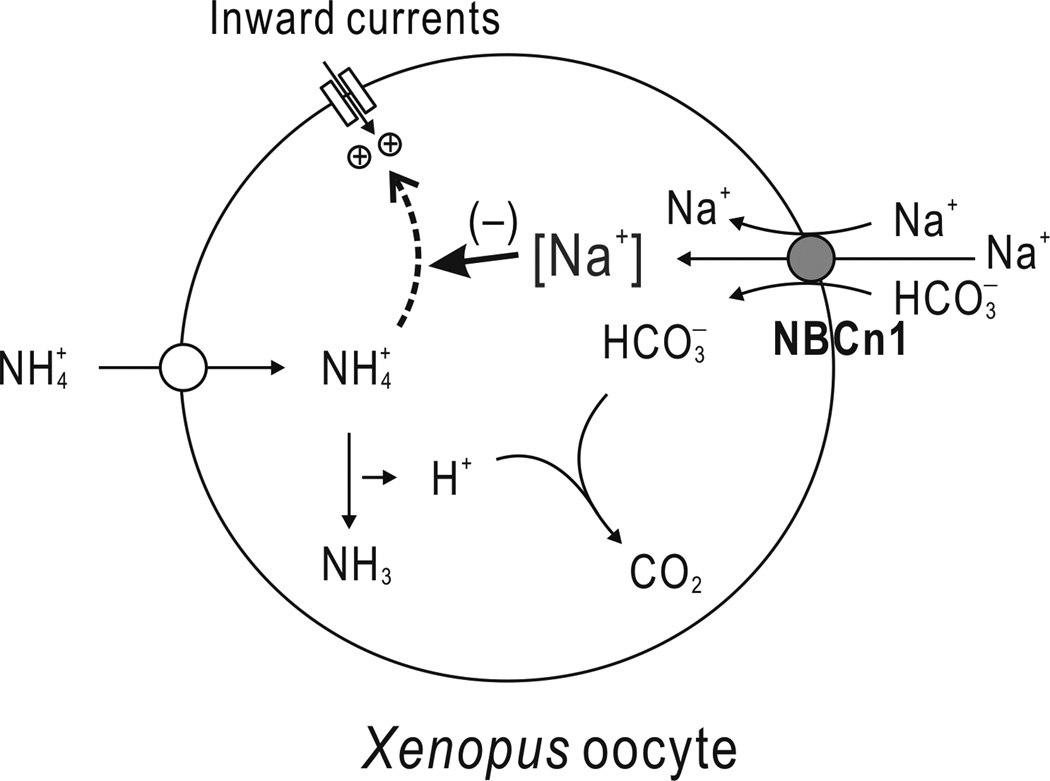
Based on the data obtained from this study, we propose a model for the effect of NBCn1 on oocyte NH4+ transport (Fig. 7). NH4+ moves to the cytosol via endogenous NH4+ transport and dissociates intracellularly into NH3 and H+. This process is stimulated by NBCn1 as HCO3− compensates H+. Intracellular NH4+ or NH3 then causes the oocytes to produce an inward current that can be affected by Na+ in the cytosol. The inward current can thus be inhibited by NBCn1 channel-like activity, which raises intracellular Na+. Our model assumes that oocyte NH4+ transport is mainly electroneutral because NBCn1 reduces NH4Cl-mediated current without significantly altering dpHi/dt in the absence of HCO3−/CO2.
Fig. 7.
A model for the effect of NBCn1 on oocyte NH4+ transport. NH4+ enters into the cytosol via the endogenous NH4+ transporter and dissociates into NH3 and H+. Intracellular NH4+ or NH3 secondarily causes the oocyte to induce an inward current that is affected by intracellular Na+ (dashed line). The Na/HCO3 cotransport activity of NBCn1 stimulates NH4+ transport by buffering H+ dissociated from NH4+. On the other hand, the channel-like activity of NBCn1 inhibits NH4+ (or NH3)-induced inward current by raising intracellular Na+ in the Na/HCO3-independent manner. The molecule responsible for the inward current is possibly a non-selective cation channel. The model assumes that oocyte NH4+ transport is mainly electroneutral.
What would be the physiological implication of the effect of NBCn1 on NH4+ transport? In the thick ascending limb, where NBCn1 is localized to the basolateral side of the cells, NH4+ is transported from the lumen mainly via the apical NKCC2 (Russell 2000;Kinne et al. 1986;Weiner & Hamm 2007). NH4+ translocates through the K+ binding site in NKCC2 and is inhibited by bumetanide (Kinne et al. 1986). In addition, the thick ascending limb has amiloride-sensitive NH4+ conductance (Attmane-Elakeb et al. 2001;Amlal et al. 1994). Together with verapamil-sensitive K+/NH4+ exchange, the NH4+ conductance contributes to apical NH4+ transport by 35–50% (Attmane-Elakeb et al. 2001). Leipziger’s groups (Jakobsen et al. 2004;Odgaard et al. 2004) reported that the basolateral NBCn1 affects the apical NH4+ transport by moving HCO3− into the cells and compensating H+ load. Our finding of NBCn1-mediated stimulation of intracellular acidification during NH4Cl application is in good agreement with this paradigm. Nonetheless, it is unclear what physiological significance there is in the inhibition of NH4+ conductance by NBCn1. One explanation is that the inhibition may help protect the tubules from membrane depolarization, which is deleterious to cells. This inhibition may be critical for cell function particularly during chronic metabolic acidosis, when NH4+ and HCO3− absorptive capacity significantly increases (Wagner 2007).
In summary, our study shows that NBCn1 inhibits oocyte NH4Cl-mediated current, while enhancing NH4+ uptake by the compensation of intracellular H+ load. We envision that NBCn1 not only serves as an acid extruder to maintain intracellular pH within the physiological range, but it also plays a critical role in regulating HCO3− and NH4+ transport. It will be interesting and important to test whether NBCn1 also inhibits NH4+ conductance in the thick ascending limb cells.
ACKNOWLEDGEMENTS
We appreciate Drs. John White and Christian Aalkjaer for helpful discussion about the data. This work was supported by Emory University Research Committee Grant and National Institutes of Health Grant GM078502 (I. C.).
REFERENCES
- Aalkjaer C, Frische S, Leipziger J, Nielsen S, Praetorius J. Sodium coupled bicarbonate transporters in the kidney, an update. Acta Physiol Scand. 2004;181:505–512. doi: 10.1111/j.1365-201X.2004.01324.x. [DOI] [PubMed] [Google Scholar]
- Amlal H, Paillard M, Bichara M. NH4+ transport pathways in cells of medullary thick ascending limb of rat kidney. NH4+ conductance and K+/NH4+(H+) antiport. J Biol Chem. 1994;269:21962–21971. [PubMed] [Google Scholar]
- Attmane-Elakeb A, Amlal H, Bichara M. Ammonium carriers in medullary thick ascending limb. Am J Physiol Renal Physiol. 2001;280:F1–F9. doi: 10.1152/ajprenal.2001.280.1.F1. [DOI] [PubMed] [Google Scholar]
- Boldt M, Burckhardt G, Burckhardt BC. NH4+ conductance in Xenopus laevis oocytes. III. Effect of NH(3) Pflügers Arch. 2003;446:652–657. doi: 10.1007/s00424-003-1122-z. [DOI] [PubMed] [Google Scholar]
- Boron WF. Control of intracellular pH: The Kidney: Physiology and Pathophysiology. New York: Raven Press; 1992. [Google Scholar]
- Boron WF, Chen L, Parker MD. Modular structure of sodium-coupled bicarbonate transporters. J Exp Biol. 2009;212:1697–1706. doi: 10.1242/jeb.028563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckhardt BC, Burckhardt G. NH4+ conductance in Xenopus laevis oocytes. Pflügers Archiv. 1997;434:306–312. doi: 10.1007/s004240050401. [DOI] [PubMed] [Google Scholar]
- Burckhardt BC, Frömter E. Pathways of NH3/NH4+ permeation across Xenopus laevis oocyte cell membrane. Pflügers Arch. 1992;420:83–86. doi: 10.1007/BF00378645. [DOI] [PubMed] [Google Scholar]
- Busch S. Cloning and sequencing of the cDNA encoding for a Na+/H+ exchanger from Xenopus laevis oocytes (X1-NHE) Biochim Biophys Acta. 1997;1325:13–16. doi: 10.1016/s0005-2736(97)00011-4. [DOI] [PubMed] [Google Scholar]
- Chang MH, Dipiero J, Sonnichsen FD, Romero MF. Entry to "Formula Tunnel" Revealed by SLC4A4 Human Mutation and Structural Model. J Biol Chem. 2008;283:18402–18410. doi: 10.1074/jbc.M709819200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi I, Aalkjaer C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature. 2000;405:571–575. doi: 10.1038/35014615. [DOI] [PubMed] [Google Scholar]
- Cooper DS, Saxena NC, Yang HS, Lee HJ, Moring AG, Lee A, Choi I. Molecular and functional characterization of the electroneutral Na/HCO3 cotransporter NBCn1 in rat hippocampal neurons. J Biol Chem. 2005;280:17823–17830. doi: 10.1074/jbc.M408646200. [DOI] [PubMed] [Google Scholar]
- Cordat E, Casey JR. Bicarbonate transport in cell physiology and disease. Biochem J. 2009;417:423–439. doi: 10.1042/BJ20081634. [DOI] [PubMed] [Google Scholar]
- Cougnon M, Benammou S, Brouillard F, Hulin P, Planelles G. Effect of reactive oxygen species on NH4+ permeation in Xenopus laevis oocytes. Am J Physiol Cell Physiol. 2002;282:C1445–C1453. doi: 10.1152/ajpcell.00410.2001. [DOI] [PubMed] [Google Scholar]
- Cougnon M, Bouyer P, Hulin P, Anagnostopoulos T, Planelles G. Further investigation of ionic diffusive properties and of NH4+ pathways in Xenopus laevis oocyte cell membrane. Pflügers Arch. 1996;431:658–667. doi: 10.1007/BF02191917. [DOI] [PubMed] [Google Scholar]
- Freedman JC, Novak TS. Electrodiffusion, barrier, and gating analysis of DIDS-insensitive chloride conductance in human red blood cells treated with valinomycin or gramicidin. J Gen Physiol. 1997;109:201–216. doi: 10.1085/jgp.109.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen JK, Odgaard E, Wang W, Elkjaer ML, Nielsen S, Aalkjaer C, Leipziger J. Functional up-regulation of basolateral Na+-dependent HCO3− transporter NBCn1 in medullary thick ascending limb of K+-depleted rats. Pflügers Arch. 2004;448:571–578. doi: 10.1007/s00424-004-1303-4. [DOI] [PubMed] [Google Scholar]
- Kinne R, Kinne-Saffran E, Schutz H, Scholermann B. Ammonium transport in medullary thick ascending limb of rabbit kidney: involvement of the Na+, K+, Cl−-cotransporter. J Membrane Biol. 1986;94:279–284. doi: 10.1007/BF01869723. [DOI] [PubMed] [Google Scholar]
- Knepper MA, Packer R, Good DW. Ammonium transport in the kidney. Physiol Rev. 1989;69:179–249. doi: 10.1152/physrev.1989.69.1.179. [DOI] [PubMed] [Google Scholar]
- Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO(3)(−) transport in cystic fibrosis. EMBO J. 2002;21:5662–5672. doi: 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraut JA, Kurtz I. Metabolic acidosis of CKD: diagnosis, clinical characteristics, and treatment. Am J Kidney Dis. 2005;45:978–993. doi: 10.1053/j.ajkd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Lauritzen G, Jensen MB, Boedtkjer E, Dybboe R, Aalkjaer C, Nylandsted J, Pedersen SF. NBCn1 and NHE1 expression and activity in DeltaNErbB2 receptor-expressing MCF-7 breast cancer cells: contributions to pHi regulation and chemotherapy resistance. Exp Cell Res. 2010;316:2538–2553. doi: 10.1016/j.yexcr.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Lee S, Lee HJ, Yang HS, Thornell IM, Bevensee MO, Choi I. Sodium-bicarbonate cotransporter NBCn1 in the kidney medullary thick ascending limb cell line is upregulated under acidic conditions and enhances ammonium transport. Exp Physiol. 2010;95:926–937. doi: 10.1113/expphysiol.2010.053967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhoul NL, Abdulnour-Nakhoul SM, Boulpaep EL, Rabon E, Schmidt E, Hamm LL. Substrate specificity of Rhbg: ammonium and methyl ammonium transport. Am J Physiol Cell Physiol. 2010;299:C695–C705. doi: 10.1152/ajpcell.00019.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhoul NL, Dejong H, Abdulnour-Nakhoul SM, Boulpaep EL, Hering-Smith K, Hamm LL. Characteristics of renal Rhbg as an NH4+ transporter. Am J Physiol Renal Physiol. 2005;288:F170–F181. doi: 10.1152/ajprenal.00419.2003. [DOI] [PubMed] [Google Scholar]
- Nakhoul NL, Hering-Smith KS, Abdulnour-Nakhoul SM, Hamm LL. Ammonium interaction with the epithelial sodium channel. Am J Physiol Renal Physiol. 2001;281:F493–F502. doi: 10.1152/ajprenal.2001.281.3.F493. [DOI] [PubMed] [Google Scholar]
- Odgaard E, Jakobsen JK, Frische S, Praetorius J, Nielsen S, Aalkjaer C, Leipziger J. Basolateral Na+-dependent HCO3− transporter NBCn1-mediated HCO3− influx in rat medullary thick ascending limb. J Physiol. 2004;555:205–218. doi: 10.1113/jphysiol.2003.046474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, Kurtz I. Cloning, tissue distribution, genomic organization, and functional characterization of NBC3, a new member of the sodium bicarbonate cotransporter family. J Biol Chem. 1999;274:16569–16575. doi: 10.1074/jbc.274.23.16569. [DOI] [PubMed] [Google Scholar]
- Pushkin A, Kurtz I. SLC4 base (HCO3−, CO32−) transporters: classification, function, structure, genetic diseases, and knockout models. Am J Physiol Renal Physiol. 2006;290:F580–F599. doi: 10.1152/ajprenal.00252.2005. [DOI] [PubMed] [Google Scholar]
- Riihonen R, Nielsen S, Vaananen HK, Laitala-Leinonen T, Kwon TH. Degradation of hydroxyapatite in vivo and in vitro requires osteoclastic sodium-bicarbonate co-transporter NBCn1. Matrix Biol. 2010;29:287–294. doi: 10.1016/j.matbio.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO3− transporters. Pflügers Arch. 2004;447:495–509. doi: 10.1007/s00424-003-1180-2. [DOI] [PubMed] [Google Scholar]
- Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev. 2000;80:211–276. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- Shcheynikov N, Yang D, Wang Y, Zeng W, Karniski LP, So I, Wall SM, Muallem S. The Slc26a4 transporter functions as an electroneutral Cl−/I−/HCO3− exchanger: role of Slc26a4 and Slc26a6 in I- and in regulation of CFTR in the parotid duct. J Physiol. 2008;586:3813–3824. doi: 10.1113/jphysiol.2008.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindic A, Chang MH, Mount DB, Romero MF. Renal physiology of SLC26 anion exchangers. Curr Opin Nephrol Hypertens. 2007;16:484–490. doi: 10.1097/MNH.0b013e3282e7d7d0. [DOI] [PubMed] [Google Scholar]
- Wagner CA. Metabolic acidosis: new insights from mouse models. Curr Opin Nephrol Hypertens. 2007;16:471–476. doi: 10.1097/MNH.0b013e3282a4a69c. [DOI] [PubMed] [Google Scholar]
- Weiner ID, Hamm LL. Molecular mechanisms of renal ammonia transport. Annu Rev Physiol. 2007;69:317–340. doi: 10.1146/annurev.physiol.69.040705.142215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner ID, Verlander JW. Role of NH3 and NH4+ transporters in renal acid-base transport. Am J Physiol Renal Physiol. 2011;300:F11–F23. doi: 10.1152/ajprenal.00554.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HS, Cooper DS, Rajbhandari I, Park HJ, Lee S, Choi I. Inhibition of rat Na+-HCO3− cotransporter (NBCn1) function and expression by the alternative splice domain. Exp Physiol. 2009a;94:1114–1123. doi: 10.1113/expphysiol.2009.048603. [DOI] [PubMed] [Google Scholar]
- Yang HS, Kim E, Lee S, Park HJ, Cooper DS, Rajbhandari I, Choi I. Mutation of aspartate 555 of the sodium/bicarbonate transporter SLC4A4/NBCe1 induces chloride transport. J Biol Chem. 2009b doi: 10.1074/jbc.M109.001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Kostrewa D, Berneche S, Winkler FK, Li XD. The mechanism of ammonia transport based on the crystal structure of AmtB of Escherichia coli. Proc Natl Acad Sci U S A. 2004;101:17090–17095. doi: 10.1073/pnas.0406475101. [DOI] [PMC free article] [PubMed] [Google Scholar]